Abstract
Periodontal diseases are a class of pathologies wherein oral microbes induce harmful immune responses in a susceptible host. Therefore, an agent which can both reduce microbial burden and lessen pathogenesis of localized inflammation would have beneficial effects in periodontal disease. 2,4,4-trichloro-2-hydroxydiphenyl-ether [triclosan] is currently used in oral care products due to broad spectrum anti-microbial and anti-inflammatory properties.
Objective
To determine effects of triclosan on the response of oral epithelial cells to stimulation with the inflammatory microbial product lipopolysaccharide [LPS], a ligand for toll-like receptor 4 [TLR4].
Materials/Methods
Primary human oral epithelial cells were stimulated with LPS in the presence and/or absence of triclosan after which expression of pro-inflammatory cytokines, β-defensins, micro-RNAs [miRNAs] or TLR signaling pathway proteins were evaluated.
Results
Here we demonstrate that triclosan is a potent inhibitor of oral epithelial cell LPS-induced pro-inflammatory responses by inducing miRNA regulation of the TLR-signaling pathway. Triclosan was not a pan-suppresser of oral epithelial cell responses as β-defensin 2 [βD2] and βD3 were upregulated by triclosan following LPS-stimulation.
Conclusions
These data demonstrate both a novel anti-microbial mechanism by which triclosan improves plaque control and an additional anti-inflammatory property which could have beneficial effects in periodontal disease resolution.
Keywords: oral epithelial cells, triclosan, inflammation, cytokines, chemokines, defensins
Introduction
Periodontal diseases are a class of inflammatory diseases of the oral cavity which if left untreated can result in soft and hard tissue destruction (Blinkhorn et al., 2009). While microbial factors are the etiological agents of periodontal disease, they do not directly cause disease, but rather induce harmful inflammatory responses in a susceptible host (Page & Kornman, 1997, Madianos et al., 2005, Gaffar et al., 1995, Blinkhorn et al., 2009). Research targeting periodontal diseases has focused on the host immune response triggered by microbes and microbial products, which are potent inducers of cytokines, chemokines and growth factors via innate immune receptors such as members of the toll-like receptor [TLR] family. Cells of the periodontium including epithelial cells, fibroblasts and monocytes express TLRs and produce immune and anti-microbial factors in response to stimulation (Sugawara et al., 2006, Mahanonda & Pichyangkul, 2007, Ford et al., 2010, Weinberg et al., 1998, Diamond et al., 2001, Dale et al., 2001, Kollisch et al., 2005). Oral epithelial cells are the first line of defense to potential infectious pathogens within the oral cavity. These key cells serve multiple immune roles along the mucosal surface functioning as: 1. a physical barrier to microbes; 2. a source of anti-microbial peptides which inhibit microbial growth; 3. inflammatory immune cells which produce cytokines to trigger anti-microbial immunity; and 4. tolerogenic immune cells which can produce immunoregulatory cytokines or remain quiescent in response to non-pathogenic commensal microorganisms (McCormick & Weinberg).
Dynamics of TLR signaling outcomes are attributable to several factors including the nature of the TLR ligand(s), concentration of the TLR ligand(s), duration of stimulation by the TLR ligand(s), and the cell type which is being engaged by the TLR ligand(s) (Erridge, 2010). Molecular responses to different TLR ligands under these variable conditions are regulated by redundant mechanisms including expression of TLR proteins, expression of TLR-inducible cell signaling proteins, or expression of regulatory micro-RNA [miRNA] molecules which in turn regulate TLR signaling protein expression (Liew et al., 2005). Within the periodontium, TLR-induced inflammation is a ‘two-edged-sword’ which initially aids in control of infection, but if left unresolved, tissue damage rather than repair occurs. If chronic inflammation of periodontal diseases can be controlled coincident with decreased bacterial load then it is possible that tissues may be driven towards repair rather than destruction (Blinkhorn et al., 2009).
2,4,4-trichloro-2-hydroxydiphenyl ether [triclosan] is a bisphenolic and non-catonic agent used in oral care products due to broad spectrum anti-microbial and anti-plaque activity (Blinkhorn et al., 2009). Triclosan decreases gingival inflammation and has direct antimicrobial effects upon bacteria and fungi (Blinkhorn et al., 2009). In vitro studies have found that triclosan modulates TLR-induced secretion of inflammatory factors by human gingival fibroblasts through inhibition of cyclo-oxygenase and lipoxygenase pathways resulting in decreased prostaglandin E2 [PGE2], leukotriene and interferon-γ [IFNγ] production (Blinkhorn et al., 2009, Gaffar et al., 1995, Barros et al., 2010). In addition, cDNA microarray analysis of LPS-stimulated whole blood demonstrated that triclosan mediates anti-inflammatory effects on a mixed cell population including neutrophils, monocytes and lymphocytes (Barros et al., 2010). While both in vivo and in vitro studies demonstrate that triclosan modulates inflammation, neither cell-specific effects nor associated mechanisms have been elucidated. Here the effects and mechanism of action of triclosan on TLR4-induced responses in human oral epithelial cells are evaluated.
Materials and Methods
Cell culture
Primary human oral keratinocytes (HOK), primary human periodontal ligament fibroblasts (HPLF) and a human acute monocytic leukemia cell line (THP1) were purchased from ScienCell Research Laboratories, (Carlsbad, CA ) and ATCC (Manassas, VA) respectively.
HOK are cultured in a serum free HEPES and bicarbonate buffered complete keratinocyte medium (KM) which contains proprietary essential and non-essential amino acids, vitamins, organic and inorganic compounds, hormones, growth factors, trace minerals, keratinocyte growth supplements, and penicillin/streptomycin (ScienCell Research Labs). HPLF are cultured in a HEPES and bicarbonate buffered fibroblast medium (FM) which contains proprietary essential and non-essential amino acids, vitamins, organic and inorganic compounds, hormones, growth factors, trace minerals fibroblast growth supplements, penicillin/ streptomycin and 2% fetal bovine serum (ScienCell Research Labs). THP1 cells are cultured in RPMI1640 complete media which contains 10% fetal bovine serum, 0.005% β-2 mercaptoethanol, and 1% penicillin/ streptomycin (Fisher Scientific).
1×106 HOK, HPLF or THP1 were left untreated or stimulated with 100ng/ml of an ultra-pure TLR4 agonist [E. coli LPS] (InVivogen, SanDiego, CA) in the presence or absence of triclosan (Sigma-Aldrich, St. Louis, MO) for 0, 6 or 24hrs. Culture supernatants, mRNA and protein were collected for analysis. All assays were performed 3–5 times using primary cells passaged less than 5 times
Cell viability
Viability was assessed using the colorimetric MTT Cell Growth Assay assay (Millipore, Billerica, MA) following stimulation described above. MTT assay was performed according to manufacturer’s instructions and absorbance was quantified with a spectrophotometer set at a dual wavelength reading of 570nm with a reference of 630nm. Culture media alone was used as a negative control while cells lysed with 1.0% triton were used as a positive control.
Soluble Factors
A Milliplex Human Cytokine Chemokine 14-multiplex, multiplex assay 9 Millipore, Billerica, MA) was used to simultaneously quantify 14-cyto/chemokines [GM-CSF, INFγ, IL1α, IL2, IL4, IL5, IL6, IL7, IL8, Il10, IL12(p70), IL13, MCP1, TNFα] in culture supernatants, while hβD2 and hβD3 levels were quantified using an ELISA (Alpco, Salem, NH). For soluble mediators, concentrations were determined using either the Luminex 200 multi-laser detection system (Millipore), standard curves, 5-parameter logistics, and Milliplex Analyst Software (VigeneTech Inc, Billerica, MA) or a spectrophotometer and standard curves.
real time PCR [qPCR]
Total RNA from HOK cells was harvested using an RNAeasy extraction Kit (Qiagen, Valencia, CA). RNA was reverse transcribed to generate cDNA using reverse transcription master mix [5X buffer, 1mM DTTs, 2.5mM dNTPs, SuperScript III (Invitrogen, Carlsbad, CA), and oligonucleotides]. Primers specific for GAPDH (Integrated DNA Technologies, Coraville, IA) or hTLR-4 RT2 qPCR primers (SABiosciences, Frederick, MD) and Cyber Green 2x Master Mix, (BioRad, Hercules, CA) were used for qPCR reactions. Standard curves were used to determine mRNA transcript copy number. Each gene was detected in independent reactions. Data are expressed copy number normalized to GAPDH. Normalized mRNA copy number = [raw transcript copy number] × [GAPDH corrective ratio]. GAPDH corrective ratio = [lowest GAPDH copy number within sample set] / [GAPDH copy number for cell of interest]. Similarly, total RNA, TaqMan MicroRNA Reverse Transcription Universal PCR Master Mix, (Applied Biosystems, Foster City, CA) and miR146a primers (Applied Biosystems, Foster City, CA ) were used for miRNA qRT-PCR analysis. miRNA expression values were expressed as fold change over miR146a expression in un-stimulated cells at 0hrs. The cycle threshold [Ct] values = PCR cycle number at which fluorescence emission reaches a threshold above baseline emission. Normalized miRNA fold change = [[corrected Ct value of sample of interest]/ [corrected Ct value of unstimulated cells at 0hrs] −1]. Corrected Ct value = [raw Ct value] × [RNU6 corrective ratio]. RNU6 corrective ratio = [lowest RNU6 Ct within sample set]/[RNU6 copy number for cell of interest].
Western blot
Total protein was collected using NP40 Lysis Buffer (Invitrogen, Carlsbad, CA) and Complete Mini protease inhibitor mixture (Roche Diagnostic, Indianapolis, IN). 50ug of total protein was separated by SDS-PAGE and electroblotted to a polyvinylidene difluoride membrane. Membranes were blocked with 5% nonfat milk in 1xTBS/0.05% Tween 20 and probed with rabbit anti-IRAK-1 or anti-TRAF6 (Cell Signaling, Boston, MA) followed by goat anti-rabbit IgG-horseradish peroxidase. Bands were visualized by incubation with SuperSignal West Dura (ThermoScientific, Rockford, IL) and photosensitive film development. Band intensity was quantified using a densitometer.
Statistics
Statistical analyses were performed using GraphPad Software (GraphPad Software Inc. La Jolla, CA) where one-way ANOVA with Bonferroni’s multiple comparisons test was used to determine significance (p value <0.05 was considered significant). Kinetics of TRAF6 or IRAK1 expression were calculated using a one-phase decay model and kinetics of mir146a expression were calculated using a one-phase association model.
Results
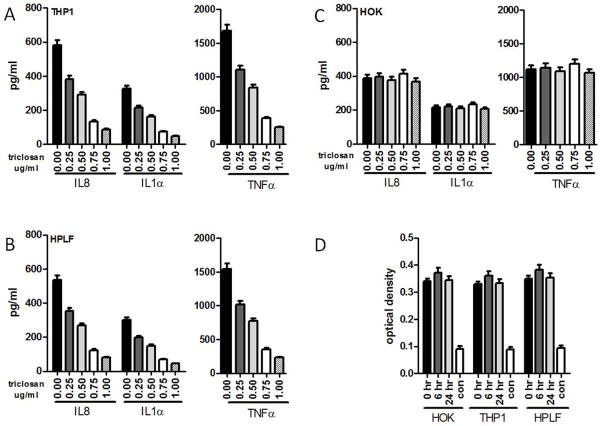
To evaluate efficacy of triclosan for inhibition of LPS-induced inflammatory cytokine responses by cells typically found within the oral mucosa, human monocytic cells [THP1], human periodontal ligament fibroblasts [HPLF] and human oral epithelial cells [HOK] were LPS-stimulated in the absence or presence of triclosan. LPS induced expression of interleukin-8 [IL-8], IL-1α, and TNFα in THP1, HPLF and HOK cells (Fig. 1A–C). Triclosan caused dose-dependent inhibition of LPS-induced cytokines that was similar for THP1 and HPLF cells (Fig. 1A, B). In contrast, triclosan did not affect LPS-induced cytokine secretion of HOK even at the highest doses tested (Fig. 1C). To determine if cellular toxicity of triclosan was responsible for effects observed, cell viability was assessed following treatment with 0.75ug/ml of triclosan. Following 24 or 48 hours of triclosan exposure, cell viability was not affected in THP1, HPLF or HOK (Fig. 1D).
Figure 1. Simultaneous treatment with triclosan inhibits LPS-induced inflammatory cytokine secretion by monocytes and fibroblasts but not by oral epithelial cells.
(A) THP1, (B) HPLF, and (C) HOK were simultaneously treated with 0.25, 0.50, 0.75 or 1.0ug/ml of triclosan and 100ng of LPS for 24hrs after which supernatants were used to quantify IL8, IL1α, and TNFα. Data are presented as stimulated concentrations minus un-stimulated concentrations. (D) Cell viability of THP1, HPLF, and HOK was evaluated 0, 6 and 24hrs following treatment with 0.75ug/ml of triclosan. 1.0% Triton-X-100 was used to induce lysis in all cell types as a positive control. All assays were performed five times.
Keratinized cells may have reduced membrane permeability relative to monocytes or fibroblasts delaying the kinetics of intracellular bioavailability of triclosan in HOK cells. To determine if prolonged exposure of HOK to triclosan can cause effects similar to those observed in THP1 and HPLF, HOK cells were pretreated with 0.75ug/ml of triclosan for 24 hours prior to LPS stimulation. Here triclosan inhibited LPS-induced IL8, IL1α and TNFα production by HOK (Fig. 2A) to a degree that was similar to what was observed for THP1 and HPLF cells with simultaneous triclosan and LPS treatment (Fig. 1A, B). In fact, pre-treatment with triclosan significantly inhibited LPS-induced secretion of 6 of 7 additional cytokines that were detected by a multiplex assay (Table 1). It is important to note that suppression of cytokine production by THP1 nor HPLF was unaltered following pretreatment with triclosan (data not shown).
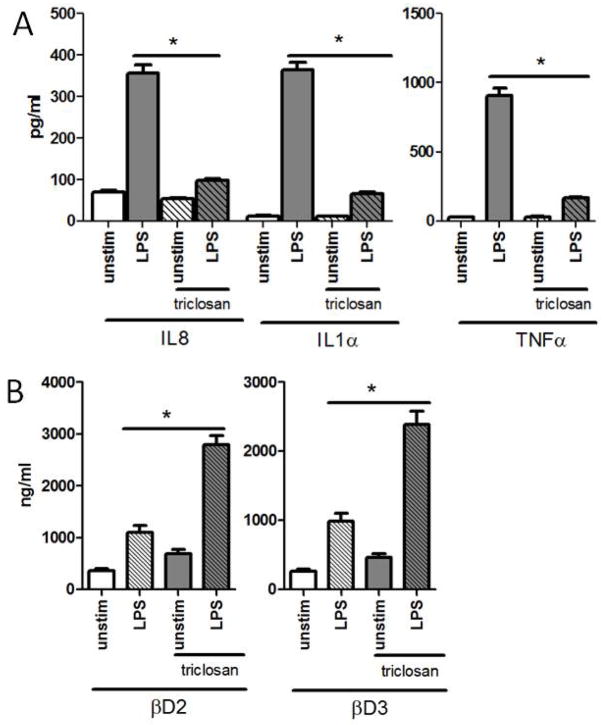
Figure 2. Pre-exposure of HOK to triclosan perturbs LPS-induced inflammation while augmenting LPS-induced anti-microbial responses.
HOK were pretreated with 0.75ug/ml of triclosan for 24hrs after which they were left un-stimulated or were stimulated with 100ng of LPS for an additional 24hrs. Supernatants were used to quantify (A) IL8, IL1α, and TNFα, along with (B) βD2 and βD3 *p value <0.05. ANOVA with Bonferroni’s Multiple Comparisons. All assays were performed five times.
Table 1.
LPS-induced cytokine expression1 of HOK following triclosan pre-treatment.
| unstim | LPS | unstim + triclosan | LPS + triclosan | p value* | p value^ | |
|---|---|---|---|---|---|---|
| IL6 (pg/ml+/− STD) | 108.31 +/− 5.747 |
557.36 +/− 29.573 |
82.58 +/− 4.381 |
150.94 +/− 8.009 |
ns | <0.0001 |
| MCP1 | 15.71 +/− 0.833 |
491.36 +/− 26.071 |
16.77 +/− 0.843 |
88.67 +/− 4.705 |
ns | <0.0001 |
| GM-CSF | 6.71 +/− 0.356 |
196.55 +/− 10.428 |
6.28 +/− 0.333 |
35.47 +/− 1.882 |
ns | <0.0001 |
| IFNγ | 10.37 +/− 0.550 |
324.30 +/− 17.207 |
11.07 +/− 0.556 |
58.53 +/− 3.105 |
ns | <0.0001 |
| IL12(p70) | 6.60 +/− 0.350 |
206.37 +/− 10.950 |
7.04 +/− 0.354 |
37.24 +/− 1.976 |
ns | <0.0001 |
unstim vs. unstim+triclosan,
LPS vs. LPS+triclosan
In addition to secretion of cytokines, oral epithelial cells secrete bioactive anti-microbial molecules such as β-defensins in response to infection (Diamond & Ryan, 2011). Therefore, the effect of triclosan pretreatment on induction of β-defensin-2 [βD2] and β-defensin-3 [βD3] was evaluated in HOK. In contrast to the observed suppression of cytokine secretion, pretreatment of HOK with triclosan enhanced hβD2 and hβD3 production in response to LPS (Fig. 2B).
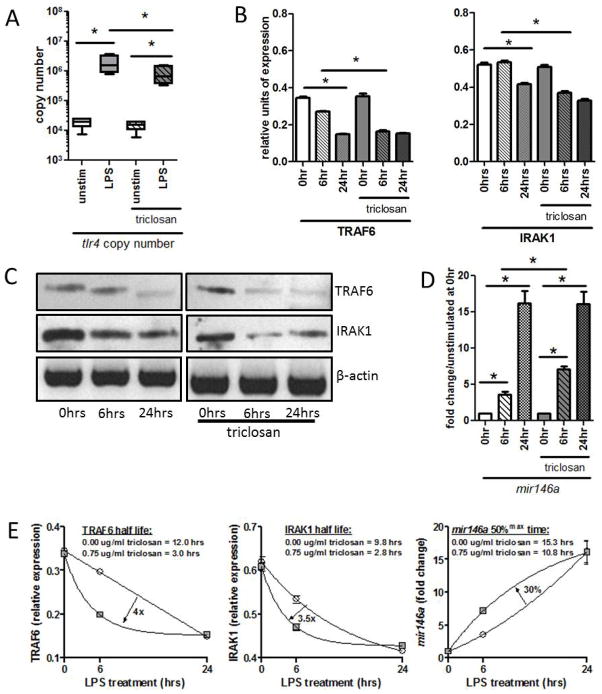
LPS-induced cellular activation is regulated at multiple levels (Liew et al., 2005). Expression of the LPS receptor, TLR4, or expression of intracellular signaling proteins downstream of TLR4 can be altered to enhance or lessen reactivity to LPS (Liew et al., 2005). To determine the mechanism by which triclosan inhibits LPS-induced HOK activation, expression of TLR4 and two key signaling proteins TRAF6 and IRAK1 were evaluated by qPCR and Western Blot respectively. HOK cells were pretreated with 0.75ug/ml triclosan for 24 hours then activated with LPS. LPS stimulation increased expression of tlr4 significantly above baseline levels (Fig. 3A). Pretreatment of HOK with triclosan did not entirely ablate LPS-induced tlr4 expression; however tlr4 expression was significantly lower upon triclosan/LPS treatment compared to LPS alone (Fig. 3A). Consistent with previous studies (Nahid et al., 2009, Nahid et al., 2011), LPS treatment resulted in decreased levels of TRAF6 and IRAK1 protein after 24 hours, although little difference was detected after only 6 hours (Fig. 3B,C). In contrast IRAK1 and TRAF6 protein levels were both significantly decreased in triclosan pretreated HOK as early as 6 hours following LPS stimulation, with a more prominent decrease in TRAF6 expression observed (Fig. 3B,C). The effect of triclosan pre-treatment on TLR signaling proteins was a net decrease in the half-lives of TRAF6 and IRAK1 by 4 fold and 3.5 fold, respectively (Fig. 3E).
Figure 3. Triclosan regulates TLR signaling by down regulation of TRAF6 and IRAK1 expression and de novo synthesis of regulatory miRNA 146a [mir146a].
HOK were pretreated with 0.75ug/ml of triclosan for 24hrs after which they were stimulated with 100ng of LPS for an additional 0, 6 or 24hrs. (A) mRNA was used to quantify tlr4 copy number (B–C) cellular protein was used to determine IRAK1 and TRAF6 expression and (D) total RNA was probed for miR146a expression. (E) Kinteics of TRAF6 and IRAK1 expression were determined by one-phase decay and kinetics of mir146a accumulation was determined by one-phase association. circle = 0.00ug/ml triclosan; square = 0.75ug/ml triclosan *p value <0.05. ANOVA with Bonferroni’s Multiple Comparisons. All assays were performed 3–4 times.
The microRNA miR146a regulates TLR4 signaling by controlling translation and subsequent expression of IRAK1 and TRAF6 proteins (Taganov et al., 2006). Therefore, to determine if miR146a was associated with the effects observed in protein expression, levels were evaluated by qPCR. Here, expression of mir146a was inversely related to TRAF6 and IRAK1 protein levels. Specifically, mir146a was only marginally elevated after 6 hours, but significantly up-regulated 24 hours post LPS-treatment (Fig. 3D). Triclosan pretreatment significantly accelerated the kinetics of mir146a expression where 6 hours post-LPS-treatment, up-regulation of mir146a was observed to be 2-fold greater than that in the absence of triclosan (Fig. 3D). The overall rate of LPS-induced mir146a accumulation was 30% faster for triclosan pre-treated HOK, reflected by a reduction in the time required to reach 50% maximal expression [50%max] from 15.3 hours without triclosan to 10.8 hours with triclosan (Fig. 3E).
Discussion
Periodontal diseases are common where treatment can be expensive and time-consuming (Blinkhorn et al., 2009). Because plaque is the main cause of periodontal diseases, its control has long been the cornerstone in the management of periodontal disease (Blinkhorn et al., 2009). Over the past decade, supplemental treatments, including triclosan/copolymer toothpaste, have been developed which claim a therapeutic effect beyond conventional periodontal therapy (Blinkhorn et al., 2009). Results from scientific and clinical studies together with two systematic reviews and a meta-analysis provide strong evidence that twice daily use of triclosan significantly improves clinical plaque control and slows periodontal disease progression (Rosling et al., 1997a, Rosling et al., 1997b, Furuichi et al., 1999, Ellwood et al., 1998, Cullinan et al., 2003a, Cullinan et al., 2003b, Papas et al., 2007, Kerdvongbundit & Wikesjo, 2003). For many years it has been recognized that triclosan has anti-bacterial activity, however in the early 1990s it was suggested that the anti-inflammatory properties of triclosan provide an additional benefit in the management of the inflammatory periodontal disease (Blinkhorn et al., 2009).
A number of in vitro studies have addressed anti-inflammatory properties of triclosan on human gingival fibroblasts and peripheral blood leukocytes where diminished inflammatory cytokine production and decreased TLR-induced signaling was observed (Blinkhorn et al., 2009, Gaffar et al., 1995, Barros et al., 2010). Epithelial cells of the periodontium are also TLR-responsive and secrete soluble factors which contribute to host-tissue destruction as well as host-tissue repair, although the effects of triclosan on oral epithelial responses have not been evaluated. The current study evaluated the effects of triclosan on TLR-induced monocyte, fibroblast and epithelial cell responses. We identified unique effects of triclosan whereby monocyte or fibroblast activation was perturbed by co-treatment with triclosan and LPS, whereas epithelial cells required pretreatment with triclosan to prevent inflammation. While the interactions of triclosan with mammalian cell membranes have yet to be described, it has been suggested that triclosan may alter lipid membrane function (James et al., 2010). Interaction of lipids within the membrane, including those occurring in lipid rafts, regulate many signaling processes including TLRs (Takeda & Akira, 2004). Therefore the vastly different makeup of proteins and lipids in the membrane of epithelial cells compared to those of fibroblasts and monocytes might account for the difference in exposure necessary for triclosan to have an effect on TLR-induced responses.
Triclosan binds to and inactivates kinases that are necessary for endocrine and hormone processes (James et al., 2010, Zorrilla et al., 2009, Gee et al., 2008). Together with our data, this supports that triclosan has pleiotropic effects, where different mechanisms of action result in divergent biological outcomes. Here we describe a selective positive effect of triclosan on epithelial cell responses where pretreatment of HOK with triclosan induces a more rapid induction of NFkB-responsive mir146a following LPS-stimulation. Enhanced mir146a expression occurs coincident to an accelerated decrease in expression of TLR signaling intermediates IRAK1 and TRAF6 known to be regulated by miR146a (Nahid et al., 2009, Nahid et al., 2011, Taganov et al., 2006). Together these data suggest that triclosan promotes accelerated LPS tolerance through regulation of the TLR-induced signaling cascade thus preventing over-activation of HOK. An additional regulatory mechanism involved in TLR-induced signaling is cell surface availability of the TLR. While our qPCR mRNA expression data does not suggests a change in the amount of TLR4 mRNA expression, the protein levels of TLR4 on the surface of the epithelium were not evaluated and thus regulation at this level of cannot be ruled out. In addition, we demonstrate that while triclosan decreased pro-inflammatory cytokine production in epithelial cells, it significantly enhanced LPS-induced expression of anti-microbial and immune-modulatory hβD2 and hβD3 proteins. This is in contrast to the observation that induction of β-defensins in epithelial cells can be mediated by the IRAK-TRAF6 pathway(Lee et al., 2008), On the other hand, others have observed, similar to our findings with Triclosan, that low molecular weight hyaluronic acid (LMW-HA) is able to induce hβD2 production in the absence of pro-inflammatory cytokines induction (and NFkB translocation) yet in a TLR4-specific manner (Dusio et al., 2011). Together these data suggest the presence of a TLR-dependent signaling pathway which is divergent from the conical TLR-induced NFkB mediated pro-inflammatory cascade to be involved in the up-regulation of hβD2. β-defensins act as both natural antibiotics as well as chemo-attractants for cells of the adaptive immune response (Diamond & Ryan, 2011). Specifically, βD2 recruits immature dendritic cells [DCs] and memory lymphocytes, while βD3 recruits immature DCs and monocytes (Diamond & Ryan, 2011). We propose a novel antimicrobial mechanism by which triclosan improves plaque control through enhancement of host-derived anti-microbial proteins. In addition, our model demonstrates that triclosan is not a pan-suppressor of immune activation, but rather interferes with select processes of TLR-induced signaling. This finding is important because immune cell responses are necessary for wound repair/healing processes and pan-suppression of cellular responses may slow periodontal disease resolution.
In summary, triclosan potently inhibits LPS-induced pro-inflammatory responses in HOK cells by inducing miRNA regulation of the TLR signaling pathway while simultaneously enhancing LPS-induced hβD2 and hβD3 secretion. Although triclosan is used in a multitude of household products, the pleiotropic actions of triclosan including controlling microbial growth/colonization, inflammation as well as hormonal and endocrine function are still being uncovered (James et al., 2010, Zorrilla et al., 2009, Gee et al., 2008). Because triclosan has many beneficial properties, it is important that the mechanism(s) of action continue to be investigated to better understand the role triclosan plays in controlling cellular and biological processes.
Acknowledgments
This study was supported by both the American Diabetes Association [ADA] 1-08-JF and Colgate-Palmolive Corporation A-2008-634-OC. MAW was supported by NIH T32AR007603. DLC was supported by NIH T32DE007200.
Footnotes
Author Contributions
Mark A. Wallet –execution and interpretation of experiments presented in the manuscript, along with manuscript preparation.
Nadia L. Calderon –execution of soluble mediator assays.
Tess R. Alonso –execution of miRNA experiments.
Christina Choe –execution of defensin assays.
Dana L. Catalfamo –execution of cell culture assays.
Charles J. Lalane –execution of protein assays.
Kathleen G. Nevia –interpretation of experiments presented in the manuscript, along with manuscript preparation.
Foti Panagakos - collaborator overseeing and contributing to the design, execution and interpretation of the experiments presented in the manuscript, along with manuscript preparation and editing.
Shannon M. Wallet –Principle Investigator overseeing and contributing to the design, execution and interpretation of the experiments presented in the manuscript, along with manuscript preparation and editing.
Conflicts of Interest
No conflicts of interest are reported by any of the authors.
References
- Barros SP, Wirojchanasak S, Barrow DA, Panagakos FS, Devizio W, Offenbacher S. Triclosan inhibition of acute and chronic inflammatory gene pathways. J Clin Periodontol. 2010;37:412–8. doi: 10.1111/j.1600-051X.2010.01548.x. [DOI] [PubMed] [Google Scholar]
- Blinkhorn A, Bartold PM, Cullinan MP, Madden TE, Marshall RI, Raphael SL, Seymour GJ. Is there a role for triclosan/copolymer toothpaste in the management of periodontal disease? Br Dent J. 2009;207:117–25. doi: 10.1038/sj.bdj.2009.669. [DOI] [PubMed] [Google Scholar]
- Cullinan MP, Hamlet SM, Westerman B, Palmer JE, Faddy MJ, Seymour GJ. Acquisition and loss of Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Prevotella intermedia over a 5-year period: effect of a triclosan/copolymer dentifrice. J Clin Periodontol. 2003a;30:532–41. doi: 10.1034/j.1600-051x.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- Cullinan MP, Westerman B, Hamlet SM, Palmer JE, Faddy MJ, Seymour GJ. The effect of a triclosan-containing dentifrice on the progression of periodontal disease in an adult population. J Clin Periodontol. 2003b;30:414–9. doi: 10.1034/j.1600-051x.2003.20030.x. [DOI] [PubMed] [Google Scholar]
- Dale BA, Kimball JR, Krisanaprakornkit S, Roberts F, Robinovitch M, O’Neal R, Valore EV, Ganz T, Anderson GM, Weinberg A. Localized antimicrobial peptide expression in human gingiva. J Periodontal Res. 2001;36:285–94. doi: 10.1034/j.1600-0765.2001.360503.x. [DOI] [PubMed] [Google Scholar]
- Diamond DL, Kimball JR, Krisanaprakornkit S, Ganz T, Dale BA. Detection of beta-defensins secreted by human oral epithelial cells. J Immunol Methods. 2001;256:65–76. doi: 10.1016/s0022-1759(01)00442-2. [DOI] [PubMed] [Google Scholar]
- Diamond G, Ryan L. Beta-defensins: what are they REALLY doing in the oral cavity? Oral Dis. 2011 doi: 10.1111/j.1601-0825.2011.01799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusio GF, Cardani D, Zanobbio L, Mantovani M, Luchini P, Battini L, Galli V, Diana A, Balsari A, Rumio C. Stimulation of TLRs by LMW-HA induces self-defense mechanisms in vaginal epithelium. Immunology and cell biology. 2011;89:630–9. doi: 10.1038/icb.2010.140. [DOI] [PubMed] [Google Scholar]
- Ellwood RP, Worthington HV, Blinkhorn AS, Volpe AR, Davies RM. Effect of a triclosan/copolymer dentifrice on the incidence of periodontal attachment loss in adolescents. J Clin Periodontol. 1998;25:363–7. doi: 10.1111/j.1600-051x.1998.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989–99. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- Ford PJ, Gamonal J, Seymour GJ. Immunological differences and similarities between chronic periodontitis and aggressive periodontitis. Periodontol 2000. 2010;53:111–23. doi: 10.1111/j.1600-0757.2010.00349.x. [DOI] [PubMed] [Google Scholar]
- Furuichi Y, Rosling B, Volpe AR, Lindhe J. The effect of a triclosan/copolymer dentifrice on healing after non-surgical treatment of recurrent periodontitis. J Clin Periodontol. 1999;26:63–6. doi: 10.1034/j.1600-051x.1999.260201.x. [DOI] [PubMed] [Google Scholar]
- Gaffar A, Scherl D, Afflitto J, Coleman EJ. The effect of triclosan on mediators of gingival inflammation. J Clin Periodontol. 1995;22:480–4. doi: 10.1111/j.1600-051x.1995.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Gee RH, Charles A, Taylor N, Darbre PD. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J Appl Toxicol. 2008;28:78–91. doi: 10.1002/jat.1316. [DOI] [PubMed] [Google Scholar]
- James MO, Li W, Summerlot DP, Rowland-Faux L, Wood CE. Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environ Int. 2010;36:942–9. doi: 10.1016/j.envint.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdvongbundit V, Wikesjo UM. Effect of triclosan on healing following non-surgical periodontal therapy in smokers. J Clin Periodontol. 2003;30:1024–30. doi: 10.1046/j.0303-6979.2003.00417.x. [DOI] [PubMed] [Google Scholar]
- Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, Bauer S, Jakob T, Mempel M, Ollert M. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114:531–41. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Takeshita T, Shimada J, Akopyan A, Woo JI, Pan H, Moon SK, Andalibi A, Park RK, Kang SH, Kang SS, Gellibolian R, Lim DJ. Induction of beta defensin 2 by NTHi requires TLR2 mediated MyD88 and IRAK-TRAF6-p38MAPK signaling pathway in human middle ear epithelial cells. BMC infectious diseases. 2008;8:87. doi: 10.1186/1471-2334-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Madianos PN, Bobetsis YA, Kinane DF. Generation of inflammatory stimuli: how bacteria set up inflammatory responses in the gingiva. J Clin Periodontol. 2005;32(Suppl 6):57–71. doi: 10.1111/j.1600-051X.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- Mahanonda R, Pichyangkul S. Toll-like receptors and their role in periodontal health and disease. Periodontol 2000. 2007;43:41–55. doi: 10.1111/j.1600-0757.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- McCormick TS, Weinberg A. Epithelial cell-derived antimicrobial peptides are multifunctional agents that bridge innate and adaptive immunity. Periodontology. 2000;54:195–206. doi: 10.1111/j.1600-0757.2010.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahid MA, Pauley KM, Satoh M, Chan EK. miR-146a is critical for endotoxin-induced tolerance: IMPLICATION IN INNATE IMMUNITY. J Biol Chem. 2009;284:34590–9. doi: 10.1074/jbc.M109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahid MA, Satoh M, Chan EK. Mechanistic role of microRNA-146a in endotoxin-induced differential cross-regulation of TLR signaling. J Immunol. 2011;186:1723–34. doi: 10.4049/jimmunol.1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol 2000. 1997;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Papas A, He T, Martuscelli G, Singh M, Bartizek RD, Biesbrock AR. Comparative efficacy of stabilized stannous fluoride/sodium hexametaphosphate dentifrice and sodium fluoride/triclosan/copolymer dentifrice for the prevention of periodontitis in xerostomic patients: a 2-year randomized clinical trial. J Periodontol. 2007;78:1505–14. doi: 10.1902/jop.2007.060479. [DOI] [PubMed] [Google Scholar]
- Rosling B, Dahlen G, Volpe A, Furuichi Y, Ramberg P, Lindhe J. Effect of triclosan on the subgingival microbiota of periodontitis-susceptible subjects. J Clin Periodontol. 1997a;24:881–7. doi: 10.1111/j.1600-051x.1997.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Rosling B, Wannfors B, Volpe AR, Furuichi Y, Ramberg P, Lindhe J. The use of a triclosan/copolymer dentifrice may retard the progression of periodontitis. J Clin Periodontol. 1997b;24:873–80. doi: 10.1111/j.1600-051x.1997.tb01205.x. [DOI] [PubMed] [Google Scholar]
- Sugawara Y, Uehara A, Fujimoto Y, Kusumoto S, Fukase K, Shibata K, Sugawara S, Sasano T, Takada H. Toll-like receptors, NOD1, and NOD2 in oral epithelial cells. J Dent Res. 2006;85:524–9. doi: 10.1177/154405910608500609. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Krisanaprakornkit S, Dale BA. Epithelial antimicrobial peptides: review and significance for oral applications. Crit Rev Oral Biol Med. 1998;9:399–414. doi: 10.1177/10454411980090040201. [DOI] [PubMed] [Google Scholar]
- Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, Stoker TE. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci. 2009;107:56–64. doi: 10.1093/toxsci/kfn225. [DOI] [PubMed] [Google Scholar]