BACKGROUND
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy, and while rare, is experiencing a rising incidence in North America and Europe (1). A malignancy of the intrahepatic biliary epithelium, ICC is rapidly fatal with a median survival of 3 to 8 months if left untreated (2, 3). This dismal prognosis stems from a clinically silent course, with many patients presenting with advanced disease not amenable to curative resection (3, 4). For these patients, several palliative therapies have been attempted to improve survival, with mixed results.
Systemic chemotherapy regimens do demonstrate a modest survival benefit over supportive care, but > 40% of patients experience significant clinical toxicities, and overall survival remains below 1 year (5–7). To minimize systemic toxicities, locoregional therapies have been employed with moderate success. The use of radiofrequency ablation (RFA) has been reported; however, most series involve small patient cohorts with limited survival data (8–10). Transarterial chemoembolization (TACE) has been demonstrated to improve overall survival over supportive care, but significant toxicities are observed in > 20% of patients (11, 12).
Given ICC’s relative radiosensitivity (13, 14), Yttrium-90 (Y90) radioembolization has promise as a locoregional treatment for this disease. Preliminary analyses of safety and efficacy have been reported in several small studies, with a reported median survival ranging from 9 to 14 months (2, 15, 16). The current study was undertaken to expand upon or intial proof-of-concept report (15), and further delineate the safety, antitumoral response, and survival following Y90 radioembolization of patients with unresectable intrahepatic ICC.
METHODS
Patients
We previously reported a pilot study in 24 patients with unresectable ICC (15). The current study expands upon the prior report, now including forty-six patients with unresectable ICC who were treated with Y90 radioembolization at a single institution from July 2003 – May 2011. We conducted a review of a prospectively collected database. Our Institutional Review Board approved this study, and all patients provided informed consent. Patients were referred for treatment by medical/surgical oncology and were discussed at multidisciplinary tumor board. Patient selection criteria included: 1) histologically proven ICC, 2) unresectable tumor, 3) an Eastern Cooperation Oncology Group (ECOG) performance status of 0–2, 4) adequate liver function with bilirubin <2.0 mg/dL, and 5) ability to undergo visceral angiography. Exclusionary criteria included: 1) flow to the gastrointestinal tract not correctable by coil embolization or 2) estimated radiation dose to the lungs > 30 Gray (Gy) in a single administration or 50 Gy cumulatively.
Treatment Protocol
Pretreatment mesenteric angiography and technetium-99m macroaggregated albumin scanning were performed according to previously published guidelines (17). The device used was TheraSphere (Ottawa, Ontario, Canada); the United States Food and Drug Administration (FDA) approved this brachytherapy device for hepatocellular carcinoma (HCC). This device was used off-label for this study.
Liver function tests, complete blood count, coagulation profiles, albumin and total bilirubin levels were obtained on the day of Y90 treatment for all patients. Y90 treatment was administered with a planned dose of 120 Gy. Patients with bilobar disease were treated in a sequential lobar fashion, treating the contralateral side 30–60 days after the first treatment. Patients were evaluated at 1 month, 3 months, and every 3 months on protocol after treatment. At each follow-up visit, patients were assessed for clinical and biochemical toxicities and imaging was obtained (either computed tomography (CT) or magnetic resonance imaging (MRI)). Patients were retreated if they demonstrated signs of incomplete tumor targeting, or progressive intra-hepatic disease as determined by imaging response.
Data Collection, Statistical Analysis, Outcome Measures
All medical, laboratory, clinical, and imaging data were acquired prospectively. The primary endpoint of this study was safety. Secondary endpoints included tumor response and overall survival (OS). Analyses were by intention to treat. The Common Terminology Criteria for Adverse Events of the National Cancer Institute (version 4.0) was used to categorize toxicities (18). Biochemical toxicities that occurred any time after treatment, without time cutoff, are reported.
Tumor response by CT/MRI was determined using the World Health Organization (WHO) classification for all measurable lesions (>1 cm) in which the sum of pretreatment and post-treatment cross products were calculated by multiplying the greatest lesion dimension and its maximum orthogonal distance. Definitions of responses were: 1) complete response (disappearance of all lesions); 2) partial response (>50% reduction in cross product); 3) progressive disease (>25% increase in cross product); and 4) stable disease (any tumor size between criteria for partial response and progressive disease). The European Association for the Study of Liver Disease (EASL) modification of WHO response criteria was implemented (19). Tumors that exhibited >50% necrosis on post-treatment imaging were categorized as EASL responders. Definitions of response were: 1) complete response (100% of treated lesion non-enhancing) and 2) partial response (between >50% and <100% of treated lesion non-enhancing).
Median survival was calculated using the Kaplan-Meier method from the time of first treatment and was stratified by: 1) performance status, 2) portal vein thrombosis (PVT), 3) extrahepatic metastases, 4) prior systemic chemotherapy, 5) tumor morphologic variant (peripheral, well-defined mass-like versus infiltrative, ill-defined); and 6) tumor distribution. Patients were censored if they were alive at the end of the study period. Additionally, patients who underwent either resection or transplantation were also censored. The log-rank test was used to assess differences in survival estimates between groups. A P value < 0.05 was considered significant.
RESULTS
Baseline Patient Characteristics
Table 1 summarizes baseline patient characteristics. During an 8-year period, 46 patients (25 men and 21 women) were treated. The median patient age was 68 years (range: 44–86). Eighteen patients (39%) were treatment naïve. The majority of patients were ECOG 0 (n=24, 52%), exhibited solitary (n=29; 65%), peripheral ICC (n=26; 57%) without portal vascular invasion (n=34; 74%) or extrahepatic metastases (n=30; 65%).
Table 1.
Baseline Characteristics
| Characteristic | Category | N (%) |
|---|---|---|
| Age (years) | <65 | 25 (54) |
| ≥65 | 21 (46) | |
| Gender | Male | 25 (54) |
| Female | 21 (46) | |
| Tumor Burden | <25% | 36 (78) |
| 25–50% | 8 (17) | |
| >50% | 2 (4) | |
| Distribution | Unilobar | 30 (65) |
| Bilobar | 16 (35) | |
| Number of lesions | Solitary | 30 (65) |
| Multifocal | 16 (35) | |
| Portal Vein Invasion | No | 35 (76) |
| Yes | 11 (24) | |
| Extrahepatic Metastases | No | 30 (65) |
| Yes | 16 (35) | |
| Pattern | Peripheral | 26 (57) |
| Infiltrative | 20 (43) | |
| ECOG | 0 | 24 (52) |
| 1 | 21 (46) | |
| 2 | 1 (2) | |
| Previous chemotherapy | No | 30 (65) |
| Yes | 16 (35) | |
| Previous Liver-directed Therapy | None | 39 (85) |
| Resection | 5 (11) | |
| RFA | 1 (2) | |
| Transplant | 1(2) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group; RFA, Radiofrequency ablation; TACE, Trans-arterial chemoembolization,
patients may exhibit 1 or more symptom
Treatment
All patients were treated on an outpatient basis and were discharged 2–6 hours following the procedure. The study population underwent a total of 92 treatments (average: 2/patient). Fourteen patients received 1 treatment, 8 patients received 2 treatments, and 24 patients received ≥3 treatments. Table 2 summarizes treatment data for all patients.
Table 2.
Yttrium 90 Dosimetry
| Treatment | # Patients | Treatment | Median Dose (95% CI), Gy |
|---|---|---|---|
| Overall | 46 | ||
| Right | 25 | 70 | 95.4, 73.8–115.0 |
| Left | 8 | 23 | 114.7, 97.7–129.8 |
| Bilobar | 13 | 60 | 90.9, 68.0–103.7 |
| Lung, Overall | 46 | 90 | 3.9, 3.4–4.9 |
Gy indicates Gray; 95% CI, 95% confidence interval.
Biochemical and Clinical Toxicity
Clinical toxicities included fatigue in 25 patients (54%); transient, vague abdominal pain in 13 patients (28%); nausea in 6 patients (13%); vomiting in 4 patients (9%) and anorexia in 2 patients (4%). Four patients (9%) developed grade 3 albumin toxicity, and three patients (7%) experienced grade 3 bilirubin toxicity. No other serologic toxicities were observed. One patient (2%) developed a gastroduodenal ulcer that was refractory to medical management and necessitated an antrectomy and gastrojejunostomy. This was secondary to non-target microsphere administration to the lesser curvature of the stomach via an unrecognized small right gastric artery. Postprocedural imaging findings (available in 45 patients) of ascites and pleural effusions were demonstrated in 7 (15%) and 2 patients (4%), respectively.
Response and Imaging Findings
Figure 1 illustrates response as a waterfall plot. Follow-up imaging demonstrated an overall objective tumor response (any decrease in size) in 44 patients (98%); mean tumor reduction was 35%. By WHO criteria, a partial response was seen in 11 patients (25%), stable disease in 33 patients (73%), and disease progression in 1 patient (2%). According to EASL criteria, a complete response was observed in 4 lesions (9%), and a partial response was observed in 28 lesions (64%). No patient had disease progression by EASL.
Figure 1.
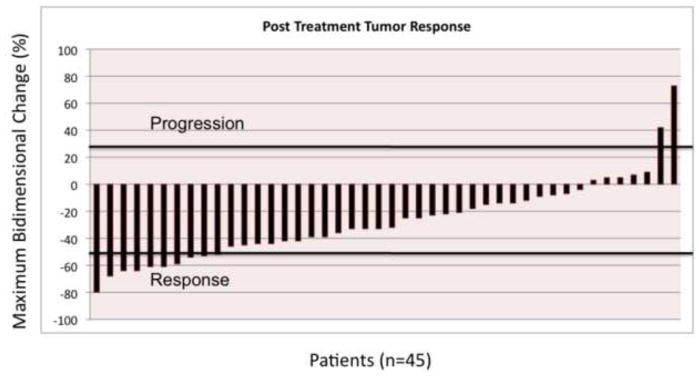
Tumor response using World Health Organization criteria are presented here as a waterfall plot. Bar values demonstrate the maximum change in tumor size from baseline in 45 patients after yttrium-90 radioembolization. Thresholds for disease progression and response are marked.
Post Y90 resection
Five patients (11%) were downstaged to resection after treatment. Within this subset of patients, all were ECOG 0 with solitary peripheral tumors without portal vascular invasion. All patients within this subset were treatment naïve prior to radioembolization, and deemed unresectable by a multidisciplinary tumor board including surgical oncology/transplant surgery. Three patients underwent right lobectomy, and 2 patients underwent trisegmentectomy (Table 3). The median time from radioembolization to surgical resection was 113 days, with a median follow-up after resection of 979 days. All patients were alive at the conclusion of the study. One patient (2%) with a known history of ulcerative colitis and primary sclerosing cholangitis underwent orthotopic liver transplantation after demonstrating a favorable response to radioembolization.
Table 3.
Y90 Resection Cohort
| Patient | Resection Type | Time Y90 to Resection (days) | Follow-up (days) |
|---|---|---|---|
| 1 | Right lobectomy | 155 | 979 |
| 2 | Trisegmentectomy | 78 | 1412 |
| 3 | Right lobectomy | 77 | 1344 |
| 4 | Trisegmentectomy | 113 | 169 |
| 5 | Right lobectomy | 262 | 905 |
All patients in the resection cohort were alive at the conclusion of the study
Survival
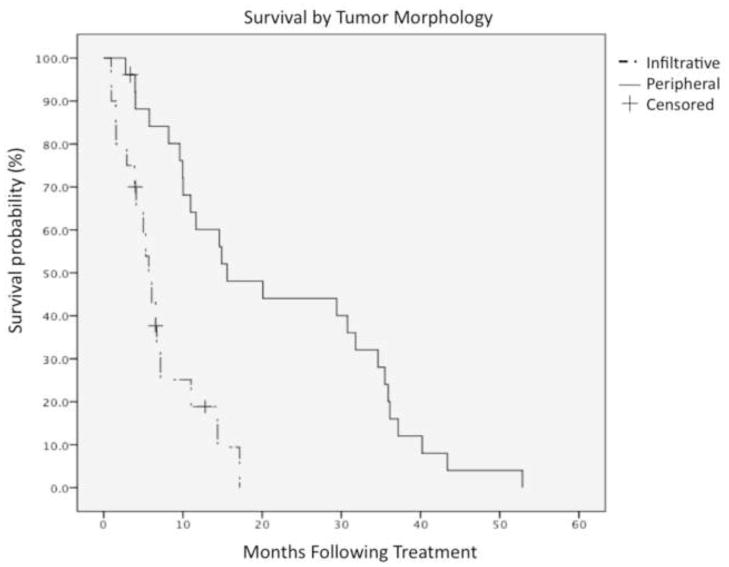
At final survival analysis (May 2012), 7 patients (15%) remained alive, and 39 patients (85%) had succumbed to their disease. The median follow-up was 29 months. Figure 2 displays survival stratified by tumor morphology using a Kaplan-Meier survival curve.
Figure 2.
Overall survival as stratified by tumor morphology, peripheral versus infiltrative. Median survival with peripheral tumor morphology was 15.6 months, versus 6.1 months for infiltrative morphology (P=0.006).
Table 4 summarizes univariate/multivariate analyses according to baseline characteristics. Univariate analyses revealed portal vein thrombosis, multifocal disease, infiltrative pattern, and tumor burden >25% as negative prognosticators of survival. Of these variables, however, portal vein thrombosis was not found to be significant in a multivariate model. The median survival was 14.6 months for patients with solitary tumors (n=29), versus those with multifocal tumors (P<0.005). The median survival for patients with peripheral tumor morphology (n=26) versus infiltrative tumor morphology (n =20), was 15.6 months and 6.1 months, respectively (P=0.006). The median survival for patients (n=36) with <25% disease burden was 14.4 months, versus 5.3 months in those with >25% disease burden (P=0.028).
Table 4.
Uni/Multivariate Analysis
| Univariate analysis (Survival) | Multivariate analysis (Cox Proportional Hazards Model)** | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Category | Median Survival in months | Hazard Ratio (CI) | P value | Adjusted P* Value | CI | P |
| ECOG | 0 | 14.3 | 0.56 (0.07 – 4.27) | 0.804 | |||
| 1 | 7.2 | 0.68 (0.08 – 5.01) | |||||
| 2 | 9.9 | 1.00 | |||||
| Number of lesions | Solitary | 14.6 | 0.37 (0.17 – 0.81) | 0.0016 | 0.014 | 0.28 (0.12 – 0.65) | 0.0031 |
| Multifocal | 5.7 | 1.00 | 1.00 | ||||
| Prior Chemotherapy | No | 14.6 | 0.48 (0.22 – 1.05) | 0.0262 | 0.235 | 0.62 (0.25 – 1.49) | 0.28 |
| Yes | 5.7 | 1.00 | 1.00 | ||||
| PVT | No | 14.4 | 0.32 (0.11– 0.86) | 0.0007 | 0.0063 | 0.96 (0.38 – 2.38) | 0.919 |
| Yes | 5.3 | 1.00 | 1.00 | ||||
| Tumor Distribution | Unilobar | 11.7 | 0.72 (0.36 – 1.44) | 0.370 | |||
| Bilobar | 10.9 | 1.00 | |||||
| Tumor Type | Peripheral | 15.6 | 0.35 (0.16 – 0.78) | 0.0005 | 0.0045 | 0.24 (0.09 – 0.66) | 0.006 |
| Infiltrative | 6.1 | 1.00 | 1.00 | ||||
| Extrahepatic | No | 11.7 | 0.78 (0.40 – 1.53) | 0.448 | |||
| Yes | 10.0 | 1.00 | |||||
| Central Lesion | No | 10.0 | 1.19 (0.62 – 2.27) | 0.605 | |||
| Yes | 14.6 | 1.00 | |||||
| Tumor Burden | <25% | 14.4 | 0.01 (0.001–0.13) | <0.0001 | 0.0009 | 0.46 (0.17 – 1.23) | 0.124 |
| 25–50% | 5.3 | 0.03 (0.002–0.35) | 0.05 (0.004 – 0.72) | 0.028 | |||
| >50% | 1.23 | 1.00 | 1.00 | ||||
Abbreviations: CI: Confidence Interval; ECOG: Eastern Cooperative Oncology Group; PVT: Portal Venous Thrombosis
Adjusted for multiple comparison (correction factor=9)
Factors were included in multivariate analysis if P <.25 in univariate analysis (unadjusted for multiple comparisons)
DISCUSSION
ICC accounts for 10–20% of all primary liver cancers, and presents with a clinically silent course, portending a poor prognosis (20). If left untreated, median survival ranges from 3–8 months (3). Over 60% of patients are not candidates for curative therapy due to advanced disease and comorbidities (4). For those with non-operative disease, multiple palliative therapies exist to control tumor growth and prolong survival, with no consensus on a standard of care.
Systemic chemotherapy regimens incorporating gemcitabine demonstrate marginal survival benefit, with a median overall survival of 8 months, at the cost of Grade 3–4 toxicities in > 40% of patients (6, 7). With limited response and significant toxicities associated with systemic approaches, locoregional therapies have been explored for these patients.
Several studies have examined the use of TACE in the setting of unresectable ICC (21). A prospective study by Kiefer et al involved 62 patients (11). Tumor response was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST), demonstrating partial response in 11%, stable disease in 64%, and progression in 24% of patients. Five patients experienced major complications. Post-embolization syndrome (CTCAE grade 1 or higher of post-procedural pain, fever, nausea or vomiting) occurred in 65% of patients. Median OS was 15 months from time of first treatment. Park et al (12) compared the benefits of TACE over supportive care in the management of ICC in 155 patients. By RECIST criteria, 23% experienced a partial response, 66% had stable disease, and 11% had progressive disease. Major hematologic and non-hematologic toxicities (CTCAE>grade 3) were observed in 13% and 24% of patients, respectively. TACE exhibited a survival benefit over supportive care, with a median OS of 12.2 vs. 3.3 months (p<0.001). These results corroborate prior smaller studies, with median OS ranging from 9–12 months (22, 23). More recently, 2 small studies from Aliberti et al (24) and Poggi et al (25) have revealed a potential therapeutic role for drug-eluting microspheres, however sample size and survival data is very limited.
Prior to the current study, three studies have examined the utility of Y90 radioembolization in patients with unresectable ICC (2, 16), including one preliminary study from our own institution (15). Hoffman et al (16) retrospectively reported on 33 patients who underwent Y90 radioembolization as inpatients. These patients had largely failed other therapies, with 79% of patients receiving prior chemotherapy, 37% receiving prior surgery, and 18% receiving prior RFA, TACE, or external beam radiation. Using RECIST criteria, disease control (partial response or stable disease) was noted in 85% of patients. Median OS was 10 months from time of first treatment. Patients with favorable functional status (ECOG=0) and limited tumor burden (<50%) experienced significantly better prognosis. A study by Saxena et al (2) in 25 patients revealed similar results, demonstrating disease control in 74% of patients by RECIST, with a median OS of 9.3 months. The majority of this cohort had received prior chemotherapy (68%), with many having had prior liver resections (40%). The presence of favorable functional status (ECOG=0) and non-infiltrative tumors improved survival in these patients. Our original pilot study (15), represented a proof-of-concept, and demonstrated safety in a limited patient cohort. The current study confirms our initial results, and represents a more mature cohort of these patients, with long-term follow-up. As such, the current survival data does not demonstrate early censoring bias. In our current study 35% of patients had previous systemic chemotherapy, while 15% had previous liver-directed therapies. By WHO imaging criteria, disease control was evident in 98% of patients, and by EASL guidelines, 73% of patients demonstrated >50% necrosis on follow-up imaging. The most common post treatment clinical symptoms were fatigue (54%) and abdominal pain (28%), which is in line with other studies utilizing radioembolization for hepatic tumors (26). After multivariate analyses, several prognostic factors were noted to negatively affect survival: multifocal disease, infiltrative disease, and tumor burden >25%.
Our current study demonstrates a subset of patients with peripheral solitary tumors may be downstaged to surgical resection. The ability to downstage offers a clear advantage with regard to survival, as resection offers the only potentially curative therapeutic option (20). Five-year survival following resection ranges from 27–48% (4, 20). Eleven percent of our patients (5/46) initially presented with localized unresectable tumors, which were downstaged to surgical resection. All patients within this subset were treatment naïve. None of these patients received cytotoxic therapies following radioembolization, and therefore maintained good performance status. Three patients had right lobe tumors abutting the middle hepatic vein. None had adequate future liver remnant to allow for a right trisegmentectomy. Following Y90 radioembolization, two of these patients experienced tumor retraction from the middle hepatic vein, allowing for a right hepatectomy. The third patient also experienced tumor retraction from the middle hepatic vein but required portal vein embolization prior to right hepatectomy. Two patients were deemed unresectable due to a central tumor abutting both the right and left hepatic veins. Following radioembolization, tumor retraction from the left hepatic vein permitted right trisegmentectomy. The fifth patient had a mass abutting the IVC precluding surgical resection. Following radioembolization, a clear margin between the tumor and IVC was evident on CT and confirmed with intravascular ultrasound.
As demonstrated in prior studies (4, 27, 28), patients who undergo curative (R0) resection have the best long-term survival, which was seen in our small subset. Similar to HCC, progression-free survival following radioembolization for ICC may yield insight into tumor biology (29, 30). These patients may possess less biologically aggressive tumors, and may benefit from adjuvant curative resection. Based on the published literature, and our current report, adjuvant resection rates are higher following radioembolization (2, 16) than TACE (11, 12, 22, 23). These results, taken together, suggest that therapeutic and patient selection can be further refined to improve the efficacy of downstaging.
There are several limitations to this study. This is a single institution study at a tertiary-level center reporting on a small patient cohort, thereby making definitive treatment recommendations difficult. Second, the majority of the patients (61%) had undergone prior therapy. Isolating any survival effect achieved by the radioembolization is not possible. In order to investigate a survival benefit with radioembolization, randomized studies will be necessary.
This study confirms that in patients with unresectable ICC, radioembolization is safe, and demonstrates a significant anti-tumoral response and survival benefit. These survival benefits are dependent on baseline patient characteristics, especially disease burden. Results are most pronounced in patients with solitary tumors, who can be downstaged to curative resection. For patients with limited disease burden, our multi-disciplinary team prefers first line therapy with Y90 radioembolization; systemic chemotherapy is utilized in the adjuvant setting. Progression-free survival following radioembolization may be indicative of less biologically aggressive tumors, and may be used to expand the criteria for curative resection. As many patients present with advanced disease outside resection criteria, the possibility of downstaging disease burden via radioembolization should be further explored in future studies.
Acknowledgments
Role of Funding: There was no funding provided for this study. RS is supported in part by NIH grant CA126809.
The authors would like to thank Dr. Ahsun Riaz, MD, and Vanessa L. Gates, MS, from the Department of Radiology, Northwestern Memorial Hospital, for their assistance in the preparation of this manuscript. The authors also wish to thank Dr. Michael Abecassis, MD, from the Department of Surgery, Northwestern Memorial Hospital, for his commentary and critiques in the preparation of this manuscript.
Abbreviations
- ICC
Intrahepatic cholangiocarcinoma
- CR
Complete Response
- CI
95% Confidence Interval
- CT
triphasic contrast-enhanced computerized tomography
- ECOG
Eastern Cooperative Oncology Group
- EASL
European Association for the Study of the Liver
- HCC
Hepatocellular Carcinoma
- MRI
gadolinium-enhanced magnetic resonance imaging
- OS
Overall Survival
- PVT
Portal venous thrombosis
- PD
Progressive Disease
- PR
Partial Response
- RCT
RECIST, Response Evaluation Criteria in Solid Tumors
- SD
Stable Disease
- TACE
Transarterial Chemoembolization
- WHO
World Health Organization
- 90Y
Yttrium-90 radioembolization
Footnotes
Conflict of Interest: ABB, MFM, RS and RJL are advisors to Nordion. MFM and RS receive research funding from Nordion.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 2.Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17:484–491. doi: 10.1245/s10434-009-0777-x. [DOI] [PubMed] [Google Scholar]
- 3.Park J, Kim M-H, Kim K-p, et al. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut and Liver. 2009;3:298–305. doi: 10.5009/gnl.2009.3.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan JCC, Coburn NG, Baxter NN, Kiss A, Law CHL. Surgical management of intrahepatic cholangiocarcinoma--a population-based study. Ann Surg Oncol. 2008;15:600–608. doi: 10.1245/s10434-007-9627-x. [DOI] [PubMed] [Google Scholar]
- 5.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 6.Harder J, Riecken B, Kummer O, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. British Journal of Cancer. 2006;95:848–852. doi: 10.1038/sj.bjc.6603334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang J-S, Lim HY, Hwang IG, et al. Gemcitabine and oxaliplatin in patients with unresectable biliary cancer including gall bladder cancer: a Korean Cancer Study Group phase II trial. Cancer Chemother Pharmacol. 2010;65:641–647. doi: 10.1007/s00280-009-1069-7. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Won HJ, Shin YM, Kim K-A, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;196:W205–209. doi: 10.2214/AJR.10.4937. [DOI] [PubMed] [Google Scholar]
- 9.Carrafiello G, Laganà D, Cotta E, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33:835–839. doi: 10.1007/s00270-010-9849-3. [DOI] [PubMed] [Google Scholar]
- 10.Fu Y, Yang W, Wu W, et al. Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. Journal of vascular and interventional radiology: JVIR. 2012;23:642–649. doi: 10.1016/j.jvir.2012.01.081. [DOI] [PubMed] [Google Scholar]
- 11.Kiefer MV, Albert M, Mcnally M, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2011;117:1498–1505. doi: 10.1002/cncr.25625. [DOI] [PubMed] [Google Scholar]
- 12.Park S-Y, Kim JH, Yoon H-J, et al. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66:322–328. doi: 10.1016/j.crad.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Nathan H, Pawlik TM, Wolfgang CL, et al. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg. 2007;11:1488–1496. doi: 10.1007/s11605-007-0282-0. discussion 1496-1487. [DOI] [PubMed] [Google Scholar]
- 14.Grove MK, Hermann RE, Vogt DP, Broughan TA. Role of radiation after operative palliation in cancer of the proximal bile ducts. Am J Surg. 1991;161:454–458. doi: 10.1016/0002-9610(91)91111-u. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim SM, Mulcahy MF, Lewandowski RJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008;113:2119–2128. doi: 10.1002/cncr.23818. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann RT, Paprottka PM, Schon A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35:105–116. doi: 10.1007/s00270-011-0142-x. [DOI] [PubMed] [Google Scholar]
- 17.Salem R, Lewandowski RJ, Sato KT, et al. Technical aspects of radioembolization with 90Y microspheres. Tech Vasc Interv Radiol. 2007;10:12–29. doi: 10.1053/j.tvir.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Cirillo M, Venturini M, Ciccarelli L, et al. Clinician versus nurse symptom reporting using the National Cancer Institute-Common Terminology Criteria for Adverse Events during chemotherapy: results of a comparison based on patient’s self-reported questionnaire. Ann Oncol. 2009;20:1929–1935. doi: 10.1093/annonc/mdp287. [DOI] [PubMed] [Google Scholar]
- 19.Forner A, Ayuso C, Varela M, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–623. doi: 10.1002/cncr.24050. [DOI] [PubMed] [Google Scholar]
- 20.Poultsides GA, Zhu AX, Choti MA, Pawlik TM. Intrahepatic cholangiocarcinoma. Surg Clin North Am. 2010;90:817–837. doi: 10.1016/j.suc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Hong K, Geschwind J-FH. Locoregional intra-arterial therapies for unresectable intrahepatic cholangiocarcinoma. Semin Oncol. 2010;37:110–117. doi: 10.1053/j.seminoncol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Gusani NJ, Balaa FK, Steel JL, et al. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experience. J Gastrointest Surg. 2008;12:129–137. doi: 10.1007/s11605-007-0312-y. [DOI] [PubMed] [Google Scholar]
- 23.Burger I, Hong K, Schulick R, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol. 2005;16:353–361. doi: 10.1097/01.RVI.0000143768.60751.78. [DOI] [PubMed] [Google Scholar]
- 24.Aliberti C, Benea G, Tilli M, Fiorentini G. Chemoembolization (TACE) of unresectable intrahepatic cholangiocarcinoma with slow-release doxorubicin-eluting beads: preliminary results. Cardiovasc Intervent Radiol. 2008;31:883–888. doi: 10.1007/s00270-008-9336-2. [DOI] [PubMed] [Google Scholar]
- 25.Poggi G, Quaretti P, Minoia C, et al. Transhepatic arterial chemoembolization with oxaliplatin-eluting microspheres (OEM-TACE) for unresectable hepatic tumors. Anticancer Res. 2008;28:3835–3842. [PubMed] [Google Scholar]
- 26.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18:651–658. doi: 10.1245/s10434-010-1325-4. [DOI] [PubMed] [Google Scholar]
- 28.Ribero D, Pinna AD, Guglielmi A, et al. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-Institutional Analysis of 434 Patients. Arch Surg. 2012:1–7. doi: 10.1001/archsurg.2012.1962. [DOI] [PubMed] [Google Scholar]
- 29.Kulik L, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere®) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. Journal of surgical oncology. 2006;94:572–586. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 30.Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]