Abstract
We characterized HCV treatment knowledge, experience and barriers in a cohort of community-based injection drug users (IDUs) in Baltimore, MD. In 2005, a questionnaire on HCV treatment knowledge, experience and barriers was administered to HCV-infected IDUs. Self-reported treatment was confirmed from medical records. Of 597 participants, 71% were male, 95% African-American, 31% HIV co-infected and 94% were infected with HCV genotype 1; 70% were aware that treatment was available, but only 22% understood that HCV could be cured. Of 418 who had heard of treatment, 86 (21%) reported an evaluation by a provider that included a discussion of treatment of whom 30 refused treatment, 20 deferred and 36 reported initiating treatment (6% overall). The most common reasons for refusal were related to treatment-related perceptions and a low perceived need of treatment. Compared to those who had discussed treatment with their provider, those who had not were more likely to be injecting drugs, less likely to have health insurance, and less knowledgeable about treatment. Low HCV treatment effectiveness was observed in this IDU population. Comprehensive integrated care strategies that incorporate education, case-management and peer support are needed to improve care and treatment of HCV-infected IDUs.
Keywords: Hepatitis C virus, Injection drug use, Antiviral therapy, Health care access
Introduction
Injection drug use is the primary route of hepatitis C virus (HCV) transmission in industrialized countries, with more than two-thirds of new infections occurring among injection drug users (IDUs) [1]. In nearly all settings, the prevalence of HCV among IDUs exceeds 50%, approaching 95% in some populations [2-5]. Potentially curative treatment for HCV infection has been licensed for more than a decade with viral clearance rates of approximately 50% in controlled clinical trials [6,7]. However, active IDUs were excluded from nearly all early clinical trials of HCV treatment [8].
In 2002, the National Institutes of Health issued new HCV treatment guidelines which called for increasing availability of treatment to previously ineligible persons including IDUs on a case-by-case basis [9]. However, despite demonstrated efficacy of treatment among IDUs [10-13], overall effectiveness remains low [14-16]. The decision to initiate HCV treatment among IDUs involves considerations of risks and benefits which collectively determine a gradient of treatment eligibility, advisability and acceptability [17,18]. Treatment eligibility and advisability are determined chiefly by medical factors which impact the need for treatment, likelihood of success and anticipated tolerability. Acceptability of treatment, the focus of this paper, influences not only the decision to undergo treatment among medically eligible individuals who have been evaluated, but also, and perhaps more importantly, the likelihood of receiving an initial evaluation of medical eligibility.
We have previously observed low rates of treatment uptake among IDUs in Baltimore, MD. Among 845 HIV/HCV co-infected patients in routine care for HIV where cost and access were not barriers, less than one-quarter received a referral for an HCV evaluation and only 29 initiated HCV treatment [19]. In a community-based cohort of more than 1,500 IDUs, only one person as of 1998 had undergone treatment [20]. The objective of this study was to characterize the extent of HCV treatment knowledge and the impact of this knowledge and other barriers on uptake of HCV treatment in this same community-based cohort as of 2005.
Methods
Study Population
Between 1988 and 1989, 2,921 injection drug users (IDUs) from Baltimore, Maryland were enrolled into the AIDS Link to the IntraVenous Experience (ALIVE) study and followed at six-month intervals, as previously described [21]. All participants acknowledged injection drug use within the past 11 years, were 18 years of age or older and free of AIDS at entry into the study. An additional 765 were enrolled subsequently. At enrollment and at semi-annual follow-up visits, a standardized questionnaire collected information on demographics, medical care, illicit drug and alcohol use. At each visit, serum samples were collected and stored at −70°C. The study was approved by the Johns Hopkins University Committee on Human Research and all subjects provided written informed consent.
HCV infection was evaluated using a historical prospective study design as previously described [20,22]. HCV antibodies were assessed at enrollment and subsequent follow-up visits using a second- or later-generation enzyme immunoassay (Ortho Diagnostics, Raritan NJ). Greater than 90% of the cohort has antibodies to HCV. HCV RNA testing was done on a large subset of >1,000 patients using branched DNA technology (Chiron Corporation, Emeryville, CA) and for various substudies by using polymerase chain reaction (Roche Diagnostics, Branchburg, NJ) as previously described [20,23].
Over time, various strategies were employed to notify subjects of their HCV status and to provide education regarding the meaning of their results. A full time HCV counselor was placed in the clinic and participants received personal counseling sessions, collective risk notification, as well as the opportunity to attend a series of weekly peer support meetings. Persons desiring medical evaluation were provided referral to the nearby Johns Hopkins Hospital clinics, and in some cases, this included access to free care through clinical trials.
Data Collection
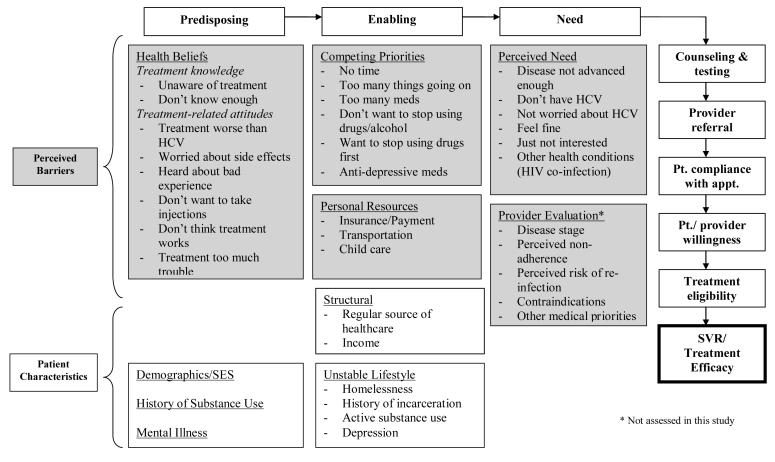
Between February and December 2005, a questionnaire on HCV treatment knowledge, barriers and experience was administered. In terms of treatment knowledge, participants were asked about the mechanism, efficacy and side effects of treatment. Andersen’s behavioral model for healthcare utilization was the conceptual model used to help understand HCV treatment uptake through predisposing, enabling and need factors [24]. Factors considered for inclusion in the conceptual model included self-reported perceived barriers to HCV treatment and characteristics related to treatment uptake (Figure 1). With respect to perceived barriers, survey questions were grouped into three broad categories: 1) individual attitudes/beliefs (e.g. health beliefs including treatment-related knowledge and perceptions, competing priorities and perceived health), 2) experience with providers, and 3) personal resources.
Figure 1.
Conceptual framework of predisposing factors, enabling factors and need for HCV treatment [24]
All self-reports of treatment were confirmed by medical record review and only reports of treatment confirmed through this process were considered as treatment in analysis.
Statistical Analysis
Characteristics of persons who did versus did not discuss treatment with their provider and who were versus were not treated were compared using chi-square and exact tests for categorical variables and Mann Whitney tests for continuous variables. For those treated, characteristics were taken from the study visit closest to the date where treatment was started and for those who were not treated, characteristics were taken from the visit where the questionnaire was administered.
Results
Characteristics of the 597 HCV-infected participants interviewed are in Table 1. Overall, the median age was 50 years, 71% were male, 95% African-American, 31% were HIV-coinfected, 29% were actively injecting drugs and 41% reported current alcohol use. The median duration of HCV infection (estimated by year of 1st drug injection) was 29 years and 97% were infected with HCV genotype 1. 83% had detectable HCV RNA in 1995 when HCV RNA testing was done. Everyone was included in the analysis even if they were HCV RNA negative in 1995 because we have previously demonstrated that re-infection occurs frequently in this cohort.[23]
Table 1.
Study Population*
| N (%) | |
|---|---|
| HCV-infected | 597 |
| Median age (interquartile range [IQR]) | 50 (45-54) |
| Male gender | 423 (71) |
| African-American race | 570 (95) |
| HCV/HIV co-infected | 188 (31) |
| HCV genotype 1† | 415 (97) |
| HCV RNA positive‡ | 470 (83) |
| Median duration of HCV infection (years) | 29 (22-35) |
| Any drug use | 253 (42) |
| Injection drug use | |
| None | 421 (71) |
| <daily | 80 (13) |
| ≥daily | 93 (16) |
| Methadone maintenance | 138 (23) |
| Alcohol use | 242 (41) |
| Depression§ | |
| <16 | 124 (69) |
| 16-23 | 21 (12) |
| ≥ 23 | 34 (19) |
| Homeless | 66 (11) |
| Incarcerated | 59 (10) |
| Health insurance | |
| None | 219 (37) |
| Medicaid | 211 (36) |
| Other | 164 (28) |
Except where otherwise indicated, characteristics refer to the six month-period prior to the survey. Data are presented as n(%) except where otherwise indicated
Genotype results not available for 169 persons; Includes 1 individual with genotype 1a/2
HCV RNA testing was performed in 1994-1995; Available for 567 persons
Depression scores based on Centers for Epidemiologic Studies Depression (CES-D) scale; Only available for 179 persons
Treatment Knowledge
Overall, 418 of 597 (70%) participants were aware that treatment for HCV was available. Of the 418, only 90 (22%) correctly answered that treatment could cure HCV infection. The majority responded either that treatment could not cure HCV infection (39%) or that they did not know (35%). In terms of treatment safety, 130 (32%) reported that treatment was safe, but could cause side effects. However, 130 (32%) participants reported that they did not know if treatment was safe or not. Treatment-related knowledge did not differ by HIV serostatus.
Barriers to HCV treatment
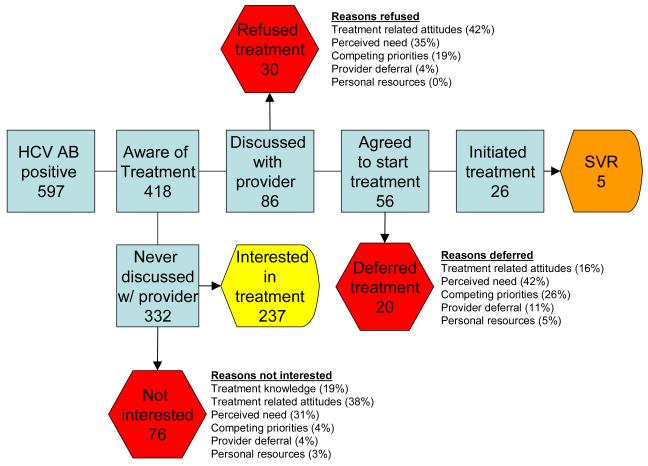
Of the 418 participants who had heard of treatment, 86 (21%) reported an evaluation by a provider that included a discussion of treatment of whom 30 refused treatment, 20 chose to defer treatment and 36 reported initiating treatment (6% overall) (Figure 2). The most common reasons for refusal of treatment were related to treatment-related perceptions (42%) followed by low perceived need for treatment (35%). The most common reasons for deferral were lack of perceived need for treatment (42%) followed by competing priorities (26%).
Figure 2.
Flow diagram: Of 597 HCV-infected ALIVE participants, 418 had heard of treatment, 86 had an evaluation by a provider that included a discussion of treatment of whom 56 agreed to initiate treatment, 26 initiated treatment and 5 had a sustained virologic response (SVR). Reasons for refusal, deferral and not being interested in treatment are listed.
309 (79%) reported never having a discussion related to HCV treatment with their provider. Compared to those who had discussed treatment with their provider (n=86), these individuals were significantly more likely to report any substance use (including alcohol and injection drug use), less likely to have health insurance or to have had a recent outpatient visit or a liver biopsy outside of the ALIVE study and less knowledgeable about HCV (Table 2). When asked about their interest in treatment, 237 (78%) reported that they were interested in treatment and 70 (21%) reported that they were not or did not know whether they were interested in treatment. Thirty eight percent of participants stated that a treatment-related perception or fear was the primary reason for not wanting treatment (Table 3). Those who were interested in treatment (n=237) were asked about services that would help with initiating treatment. The main service need reported was help with cost (reported by 47%), which was more frequently reported by HCV mono-infected compared with HIV/HCV co-infected participants (51% vs 39%).
Table 2.
Characteristics of persons by whether or not they had a provider evaluation that included a discussion of treatment*
| No discussion of treatment with provider (n=309) |
Discussed treatment with provider (n=86) |
P-value | |
|---|---|---|---|
| Median age (yrs) | 54 (50-58) | 50 (46-55) | 0.25 |
| Male | 221 (72) | 66 (77) | 0.34 |
| African-American | 291 (94) | 82 (95) | 0.68 |
| HIV/HCV co-infected | 90 (29) | 32 (37) | 0.15 |
| HCV genotype 1 | 205 (96) | 68 (97) | 0.46 |
| Any drug use | 143 (46) | 26 (30) | <0.01 |
| Injection drug use | 96 (31) | 16 (19) | 0.02 |
| Methadone maintenance | 75 (24) | 15 (17) | 0.18 |
| Alcohol use | 128 (41) | 25 (29) | 0.04 |
| Homeless | 38 (12) | 9 (10) | 0.64 |
| Incarcerated | 33 (11) | 8 (9) | 0.70 |
| Health insurance | 182 (59) | 71 (83) | <0.0001 |
| Depression† | 15 (16) | 5 (22) | 0.54 |
| On medication for depression | 44 (14) | 22 (26) | 0.01 |
| Inpatient hospitalization | 39 (13) | 12 (14) | 0.74 |
| Outpatient visit | 155 (50) | 62 (72) | <0.001 |
| Emergency room visit | 80 (26) | 24 (28) | 0.71 |
| Liver biopsy | |||
| No | 202 (66) | 30 (35) | <0.0001 |
| Only through the ALIVE study | 62 (20) | 25 (29) | |
| Received biopsy outside the ALIVE study | 40 (13) | 31 (36) | |
| Self-perceived disease severity‡ | |||
| Low | 93 (36) | 28 (38) | 0.90 |
| Moderate | 136 (52) | 36(49) | |
| High | 33 (13) | 9 (12) | |
| Aware that treatment can cure HCV | 60 (19) | 28 (33) | 0.01 |
The sample includes 418 individuals who reported that they had heard of treatment and who had data to characterize whether they had been offered treatment (e.g. 23 have missing data) P values from chi square tests for categorical variables and Mann-Whitney tests for continuous variables
Depression characterized as CES-D Scale ≥23; Only available for 116 participants
Participants were asked to estimate the likelihood of developing cirrhosis in the next 10 years
Treatment Experience
Of the 36 who reported receiving treatment, 30 described actual courses of HCV treatment (e.g. 5 reported receiving hepatitis A and B vaccines as treatment). The medical charts of these 30 persons plus an additional 7 persons who reported receiving treatment after the initial visit were reviewed. Thirty two of 37 had a confirmed evaluation for HCV, among whom treatment was not recommended in 4, not initiated in 2 and initiated in 26. Of the 26, 13 received treatment through a clinical trial or research study. Five of 26 (19%) achieved sustained virologic response (SVR). The rate of treatment for HCV among this cohort did not increase significantly from 1998 to 2005 (data not shown) remaining relatively stable at <1% per year.
Compared to participants not previously treated, treated participants were more often male, significantly less likely to report injection drug or alcohol use and significantly more likely to have health insurance at the time of treatment. Treatment initiation was not associated with methadone maintenance or HIV serostatus (data not shown).
Discussion
In 1998, we reported that only one person in our large urban cohort of near universally HCV-infected IDUs had received treatment [20]. Since then, we enhanced universal counseling and testing efforts already ongoing in the clinic with provision of additional brochures on treatment as well as weekly peer support groups that were open to everyone. While not a formal intervention, the goal was to increase care-seeking behavior. However, knowledge remained poor and overall effectiveness of HCV treatment was even lower. Prior studies have similarly observed low treatment rates among IDUs [25-27]. Treatment effectiveness among IDUs is important because they comprise the leading risk group for HCV infection worldwide.
There are multiple interrelated factors that contribute to low levels of treatment effectiveness and it is difficult to disentangle their relative contributions. Individuals need access to HCV testing and counseling that is coupled with referral to a provider. In turn, the individual needs to attend an initial evaluation and comply with additional appointments to ensure a complete blood workup and a liver biopsy to stage disease and determine treatment need. There are strict eligibility criteria for HCV treatment, some absolute and others modifiable. Eligible individuals must further be willing to take a medication that is associated with side effects including depression and must remain in follow-up long enough to complete a potential one-year course of treatment with limited efficacy. The objective of this analysis was not to comprehensively evaluate treatment eligibility or efficacy, but rather to understand the barriers to successful treatment of hepatitis C for the individual at each step of the process.
The major barrier to receiving treatment in this population was getting to the initial evaluation as only 20% made it past this initial hurdle. We cannot determine whether the low rate of evaluation reflects lapses in referral or attendance, but prior studies suggest that both contributed [28]. Provider referral rates for HCV have been low even for persons in regular health care especially for those actively injecting drugs. What is not known is whether drug use itself impedes referral or if drug use is a marker for another factor such as unstable lifestyle that providers perceive to reduce the appropriateness of referral [16,26,28-32]. Attendance rates have been less frequently examined, but in one study less than 50% of HCV infected persons referred for an evaluation attended even one appointment [28]. In addition to substance use, we also observed that markers of health care access and treatment-related knowledge were strong predictors of having an evaluation. These same factors played a role downstream as well, reducing not only the likelihood of an initial evaluation, but of initiating treatment once evaluated.
Beyond these factors, the major barrier to treatment initiation appeared to be fear of the treatment itself, which was strongly associated with treatment refusal and to a lesser extent treatment deferral. Also important was a low perceived need for treatment. Whether these beliefs are driven by awareness of low disease stage or lack of knowledge of the risks of progression cannot be determined from this analysis because we did not have biopsy results for the majority of persons. Interestingly and perhaps not surprisingly, reasons for deferral were slightly different from refusal. Whereas refusal appeared to be driven by fear and low perceived need, deferral was driven by low perceived need and competing priorities. More contact with a provider appeared to alleviate some fears related to treatment, but moving further through the process raised other issues related to social stability. In fact actual treatment initiation was less common among individuals who were incarcerated, homeless, drinking alcohol and injecting drugs. Surprisingly lack of personal resources including health insurance was rarely reported as a reason for deferral or refusal. However, care and treatment were more common among those with health insurance and regular contact with a provider.
Among those who initiated treatment, treatment efficacy was limited but the SVR rate was not substantially different from other populations with high rates of HIV co-infected, genotype 1-infected African-Americans [6,33-36]. New therapies including protease and polymerase inhibitors are on the horizon and appear promising [37]. However, given that only 28 of 579 participants initiated treatment, even a therapy that is 100% efficacious would only have cured 5% of the persons in this cohort.
At the other extreme, increased access to counseling and testing in and of itself is also not likely to have an impact on treatment utilization. Knowledge of HCV infection itself was not a barrier at least in this setting. Given the low rates of evaluation even after extensive counseling, more intensive efforts are needed to get people from the step of learning their test results to having an evaluation by a physician. Interventions should address fears related to treatment as well as the importance of understanding disease stage and factors such as alcohol which accelerate progression of disease [38]. It is important to note that not all IDUs in this cohort likely needed treatment. Since we used HCV antibody results, approximately 15% would have cleared infection naturally [39] and prior studies in this cohort have suggested that disease stage is relatively low with less than 20% having significant fibrosis [40]. Deferment of treatment in the setting of no or mild fibrosis especially in light of other comorbidities and psychosocial issues until more efficacious, less toxic and potentially shorter therapies are available may be the most reasonable decision.
However, progression can occur even among those with low disease stage and strategies should focus on more cost-effective ways to find those who need treatment the most and get them into care. Even determination of disease stage requires a physician evaluation. In this regard, widespread adoption of non-invasive strategies to stage disease including serum fibrosis markers or liver elastography will prove especially beneficial for IDUs given that the current standard of care, liver biopsy, is expensive and rarely obtained on IDUs outside of research settings [41,42]. Further, intervention efforts need to be tailored to smaller groups or even individuals as the broader strategies used thus far have not reached the majority of our participants.
While individuals who make the first step are likely to face barriers downstream after completing an initial evaluation, efforts need to begin here in order to impact the largest number of persons and be sustainable. Comprehensive care programs which include HIV care, substance abuse treatment and counseling, psychiatric care, social and peer support among other services can address not only the initial barriers, but those that manifest as individuals get further through the process [25,43,44]. Finally, issues of cost appear to be overwhelmed by limited treatment-related knowledge and competing priorities. However, there remains a need for financial assistance for HCV mono-infected patients that is comparable to the availability of third party reimbursement through the Ryan White care act for HIV co-infected patients especially given that 50% of the small number who received treatment received it through a research study.
We were limited in this study because we used discussing treatment with a provider as a proxy for evaluation. Because all participants receive counseling and testing and some receive biopsies as part of this study, it is difficult to query them about a physician evaluation because many view the study as clinical care. Thus we may have missed some additional persons who received an evaluation from their physician but did not discuss treatment options. We do not expect that this was a substantial bias since only 40 of those who did not discuss treatment with their provider received a biopsy outside of the ALIVE study. One additional issue is that weekly peer support groups have been ongoing for the past few years. Although attended by only a small group of participants and intended to educate and alleviate fears regarding treatment, it is possible that some negative experiences of participants with treatment that are discussed in these support groups may have contributed to the high levels of treatment fear observed here again reinforcing the need for separate tailored groups.
In conclusion, we observed low rates of HCV treatment uptake among community-based IDUs in Baltimore, MD despite changes in NIH treatment guidelines, universal counseling and testing for HCV and high levels of awareness of the availability of treatment. Although treatment efficacy, particularly among genotype 1 infected African-Americans remains a concern, the more daunting issue is the low rate of pre-treatment evaluation that was impacted by a number of factors chief among which were limited knowledge related to treatment and fears of the treatment itself. Efforts need to focus on getting IDUs over this initial hurdle if they are to have an impact on overall treatment effectiveness.
Acknowledgements
The authors acknowledge Lisa McCall for project management, Charles Spoler for HCV counseling, Melody Schaeffer for chart abstraction, and the ALIVE study staff and participants without whom this would not have been possible. This work was supported in part by Public Health Service Grants, National Institute on Drug Abuse, DA16078, DA04334 and DA12568.
Contributor Information
Shruti H. Mehta, Johns Hopkins Bloomberg School of Public Health Department of Epidemiology 615 N. Wolfe Street, E6537 Baltimore, MD 21205.
Becky L. Genberg, Johns Hopkins Bloomberg School of Public Health Department of Epidemiology 615 N. Wolfe Street, E6537 Baltimore, MD 21205.
Jacquie Astemborski, Johns Hopkins Bloomberg School of Public Health Department of Epidemiology 615 N. Wolfe Street, E6537 Baltimore, MD 21205.
Ravi Kavasery, Johns Hopkins Bloomberg School of Public Health Department of Epidemiology 615 N. Wolfe Street, E6537 Baltimore, MD 21205.
Gregory D. Kirk, Johns Hopkins Bloomberg School of Public Health Department of Epidemiology 615 N. Wolfe Street, E6537 Baltimore, MD 21205; Johns Hopkins School of Medicine Division of Infectious Diseases Baltimore, MD.
David Vlahov, Center for Urban Epidemiologic Studies New York Academy of Medicine New York, NY.
Steffanie A. Strathdee, Division of International Health and Cross-Cultural Medicine University of California San Diego, School of Medicine San Diego, CA.
David L. Thomas, Johns Hopkins Bloomberg School of Public Health Department of Epidemiology 615 N. Wolfe Street, E6537 Baltimore, MD 21205; Johns Hopkins School of Medicine Division of Infectious Diseases Baltimore, MD.
References
- 1.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 2.Thomas DL, Vlahov D, Solomon L, et al. Correlates of hepatitis C virus infections among injection drug users. Medicine. 1995;74:212–220. doi: 10.1097/00005792-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Bell J, Batey RG, Farrell GC, Crewe EB, Cunningham AL, Byth K. Hepatitis C virus in intravenous drug users. Med J Aust. 1990;153:274–276. doi: 10.5694/j.1326-5377.1990.tb136900.x. [DOI] [PubMed] [Google Scholar]
- 4.van Ameijden EJ, Van den Hoek JA, Mientjes GH, Coutinho RA. A longitudinal study on the incidence and transmission patterns of HIV, HBV and HCV infection among drug users in Amsterdam. Eur J Epidemiol. 1993;9:255–262. doi: 10.1007/BF00146260. [DOI] [PubMed] [Google Scholar]
- 5.Mathei C, Buntinx F, van DP. Seroprevalence of hepatitis C markers among intravenous drug users in western European countries: a systematic review. J Viral Hepat. 2002;9:157–173. doi: 10.1046/j.1365-2893.2002.00339.x. [DOI] [PubMed] [Google Scholar]
- 6.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 7.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health Consensus Development Conference Panel statement: management of hepatitis C. Hepatology. 1997;26:2S–10S. doi: 10.1002/hep.510260701. [DOI] [PubMed] [Google Scholar]
- 9.NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1–46. [PubMed] [Google Scholar]
- 10.Sylvestre DL. Approaching treatment for hepatitis C virus infection in substance users. Clin Infect Dis. 2005;41(Suppl 1):S79–S82. doi: 10.1086/429501. [DOI] [PubMed] [Google Scholar]
- 11.Backmund M, Meyer K, Von Zielonka M, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:188–193. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- 12.Van Thiel DH, Anantharaju A, Creech S. Response to treatment of hepatitis C in individuals with a recent history of intravenous drug abuse. Am J Gastroenterol. 2003;98:2281–2288. doi: 10.1111/j.1572-0241.2003.07702.x. [DOI] [PubMed] [Google Scholar]
- 13.Mauss S, Berger F, Goelz J, Jacob B, Schmutz G. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004;40:120–124. doi: 10.1002/hep.20279. [DOI] [PubMed] [Google Scholar]
- 14.Fleming CA, Craven DE, Thornton D, Tumilty S, Nunes D. Hepatitis C virus and human immunodeficiency virus coinfection in an urban population: low eligibility for interferon treatment. Clin Infect Dis. 2003;36:97–100. doi: 10.1086/344907. [DOI] [PubMed] [Google Scholar]
- 15.Doab A, Treloar C, Dore GJ. Knowledge and attitudes about treatment for hepatitis C virus infection and barriers to treatment among current injection drug users in Australia. Clin Infect Dis. 2005;40(Suppl 5):S313–20. doi: 10.1086/427446. [DOI] [PubMed] [Google Scholar]
- 16.Stoove MA, Gifford SM, Dore GJ. The impact of injecting drug use status on hepatitis C-related referral and treatment. Drug Alcohol Depend. 2005;77:81–86. doi: 10.1016/j.drugalcdep.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 18.Mehta SH, Thomas DL, Sulkowski MS, Safaein M, Vlahov D, Strathdee SA. A framework for understanding factors that affect access and utilization of treatment for hepatitis C virus infection among HCV-mono-infected and HIV/HCV-co-infected injection drug users. AIDS. 2005;19(Suppl 3):S179–S189. doi: 10.1097/01.aids.0000192088.72055.90. [DOI] [PubMed] [Google Scholar]
- 19.Mehta SH, Lucas GM, Mirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361–2369. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- 20.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 21.Vlahov D, Anthony JC, Munoz A, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 22.Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol. 1997;35:3274–3277. doi: 10.1128/jcm.35.12.3274-3277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–1483. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 24.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36:1–10. [PubMed] [Google Scholar]
- 25.Taylor LE. Delivering care to injection drug users coinfected with HIV and hepatitis C virus. Clin Infect Dis. 2005;40(Suppl 5):S355–61. doi: 10.1086/427453. [DOI] [PubMed] [Google Scholar]
- 26.Fishbein DA, Lo Y, Reinus JF, Gourevitch MN, Klein RS. Factors Associated With Successful Referral For Clinical Care of Drug Users With Chronic Hepatitis C Who Have or Are At Risk For HIV Infection. J Acquir Immune Defic Syndr. 2004;37:1367–1375. doi: 10.1097/01.qai.0000131932.21612.49. [DOI] [PubMed] [Google Scholar]
- 27.Broers B, Helbling B, Francois A, et al. Barriers to interferon-alpha therapy are higher in intravenous drug users than in other patients with acute hepatitis C. J Hepatol. 2005;42:323–328. doi: 10.1016/j.jhep.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Mehta SH, Lucas GM, Mirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361–2369. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- 29.Restrepo A, Johnson TC, Widjaja D, et al. The rate of treatment of chronic hepatitis C in patients co-infected with HIV in an urban medical centre. J Viral Hepat. 2005;12:86–90. doi: 10.1111/j.1365-2893.2005.00548.x. [DOI] [PubMed] [Google Scholar]
- 30.Davis GL, Rodrigue JR. Treatment of chronic hepatitis C in active drug users. N Engl J Med. 2001;345:215–217. doi: 10.1056/NEJM200107193450312. [DOI] [PubMed] [Google Scholar]
- 31.Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754–758. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews G, Kronborg IJ, Dore GJ. Treatment for hepatitis C virus infection among current injection drug users in Australia. Clin Infect Dis. 2005;40(Suppl 5):S325–9. doi: 10.1086/427448. [DOI] [PubMed] [Google Scholar]
- 33.Chung RT, Andersen J, Volberding P, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 35.Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]
- 36.Jeffers LJ, Cassidy W, Howell CD, Hu S, Reddy KR. Peginterferon alfa-2a (40 kd) and ribavirin for black American patients with chronic HCV genotype 1. Hepatology. 2004;39:1702–1708. doi: 10.1002/hep.20212. [DOI] [PubMed] [Google Scholar]
- 37.Lawitz EJ, Rodriguez-Torres M, Muir A, et al. 28 days of the hepatitis C protease inhibitor VX-950 in combination with PEG-interferon-ALFA-2a and Ribavirin, is well-tolerated and demonstrates robust antiviral effects. Gastroenterology. 2006;131:950–951. [Google Scholar]
- 38.Strathdee SA, Latka M, Campbell J, et al. Factors associated with interest in initiating treatment for hepatitis C Virus (HCV) infection among young HCV-infected injection drug users. Clin Infect Dis. 2005;40(Suppl 5):S304–S312. doi: 10.1086/427445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. 1999;29:908–914. doi: 10.1002/hep.510290311. [DOI] [PubMed] [Google Scholar]
- 40.Rai R, Wilson LE, Astemborski J, et al. Severity and correlates of liver disease in hepatitis C virus-infected injection drug users. Hepatology. 2002;35:1247–1255. doi: 10.1053/jhep.2002.33151. [DOI] [PubMed] [Google Scholar]
- 41.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 42.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Sylvestre DL, Litwin AH, Clements BJ, Gourevitch MN. The impact of barriers to hepatitis C virus treatment in recovering heroin users maintained on methadone. J Subst Abuse Treat. 2005;29:159–165. doi: 10.1016/j.jsat.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Kresina TF, Eldred L, Bruce RD, Francis H. Integration of pharmacotherapy for opioid addiction into HIV primary care for HIV/hepatitis C virus-co-infected patients. AIDS. 2005;19(Suppl 3):S221–S226. doi: 10.1097/01.aids.0000192093.46506.e5. [DOI] [PubMed] [Google Scholar]