Abstract
Rationale
Dopamine D2-like agonists maintain responding when substituted for cocaine in laboratory animals. However, these effects appear to be mediated by an interaction with stimuli that were previously paired with cocaine reinforcement (CS).
Objectives
To evaluate the extent to which the pramipexole-maintained and -induced responding are influenced by cocaine-paired stimuli.
Methods
Rats were trained to nosepoke for cocaine under fixed ratio 1 (FR1) or progressive ratio (PR) schedules of reinforcement. In FR1-trained rats, pramipexole was substituted for cocaine with injections either paired with CSs, or delivered in their absence. The capacity of experimenter-administered pramipexole to induce FR1 and PR responding for CS presentation was evaluated. The effects of altering stimulus conditions, as well as pretreatments with D2- (L-741,626) and D3-preferring (PG01037) antagonists on pramipexole-induced PR responding were also evaluated.
Results
When substituted for cocaine, pramipexole maintained responding at high rates when injections were paired with CSs, but low rates when CSs were omitted. Similarly, experimenter-administered pramipexole induced dose-dependent increases in FR1 or PR responding, with high rates of responding observed when the CS was presented, and low rates of responding when CS presentation was omitted. D2 and D3 antagonists differentially affected pramipexole-induced PR responding, with L-741,626 and PG01037 producing rightward, and downward shifts in the dose-response curve for CS-maintained responding, respectively.
Conclusions
These data indicate that pramipexole is capable of enhancing the reinforcing effectiveness of conditioned stimuli, and raise the possibility that similar mechanisms are responsible for the increased occurrence of impulse control disorders in patients being treated with pramipexole.
Introduction
Dopamine D2 and D3 receptors are thought to play important roles in drug-abuse-related and compulsive behaviors (e.g., Everitt et al. 2008; Heidbreder et al. 2005; Heidbreder and Newman 2010; Newman et al. 2005). For instance, positron emission tomography (PET) studies in humans, monkeys, and rats suggest that lower levels of striatal D2-like receptor availability are correlated with both the positive subjective (Volkow et al. 1999) and reinforcing effects (Morgan et al. 2002) of psychostimulants, such as cocaine, as well as with increased measures of impulsivity which may predispose individuals to abuse cocaine (Dalley et al. 2007). In addition D2-like agonists have been shown to reinstate previously extinguished responding for cocaine (De Vries et al. 2002; De Vries et al. 1999; Self et al. 1996), possess some cocaine-like discriminative stimulus effects (Barrett et al. 2001; Terry et al. 1994), and maintain responding at relatively high rates when substituted for cocaine in monkeys, rats, and mice (Caine and Koob 1993; Caine et al. 2002; Woolverton et al. 1984). Although these findings suggest that D2 and D3 receptors mediate, at least partially, the subjective and reinforcing effects of cocaine, D2-like agonists generally fail to produce cocaine-like discriminative stimulus effects in humans (Haney et al. 1998; Kumor et al. 1989), and are rarely abused (O'Sullivan et al. 2009). In fact, at clinically active doses pramipexole has been reported to increase sedation and nausea, and decrease positive mood and “like drug” effects (Hamidovic et al. 2008), again suggesting that a disconnect exists between preclinical laboratory animal models that are generally very good at predicting the potential for abuse in humans (for recent reviews see; Carter and Griffiths 2009; O'Connor et al. 2011), and the effects of D2-like agonists in patient populations.
In an attempt to elucidate the variables involved in the maintenance of self-administration behavior by D2-like agonists in rats, we have previously evaluated the influence of reinforcement history, operant history, and conditioned stimuli on the capacity of quinpirole to maintain responding. In rats, quinpirole maintained high rates of responding when substituted for cocaine or remifentanil, but failed to maintain responding in experimentally naïve rats, or rats with histories of either ketamine or food reinforcement (Collins and Woods 2007), suggesting that a relatively specific drug reinforcement history is necessary to establish the reinforcing effects of D2-like agonists. However, that quinpirole also failed to maintain responding in cocaine-trained rats when substituted on a previously unreinforced manipulandum, or when quinpirole injections were delivered without the previously cocaine paired stimuli suggests that simply providing an appropriate reinforcement history is not sufficient to establish the reinforcing effects of D2-like agonists (Collins and Woods 2009). When taken together with the finding that experimenter-administered quinpirole was equally effective at producing high rates of responding when responses resulted in the presentation of the cocaine-paired stimuli alone (i.e., no cocaine injection), these studies suggest that the response-maintaining effects of quinpirole are primarily mediated by an interaction between the activation of D2 and/or D3 receptors and the previously cocaine-paired stimuli, rather than a reinforcing effect of the quinpirole injection itself.
Although D2-like agonists do not appear to function as reinforcers in humans, they are known to induce a variety of compulsive behaviors, with an estimated 14% of patients who take these drugs displaying some form of an impulse control disorder (ICD) (Voon and Fox 2007; Weintraub et al. 2010). Originally described in Parkinson’s patients as a pramipexole-induced increase in the occurrence of pathological gambling (Driver-Dunckley et al. 2003), subsequent reports of compulsive eating, shopping, and sexual behavior in Parkinson’s, restless-leg, and fibromyalgia patients being treated with pramipexole or ropinirole (e.g., Driver-Dunckley et al. 2007; Holman 2009; Voon et al. 2006; Weintraub 2008) suggest that the pathophsyiologic changes associated with Parkinson’s are not necessary for the manifestation of ICDs. Although the mechanisms responsible for the development and maintenance of ICDs are currently unknown, it is important to note that these problematic behaviors typically resolve following dose reduction or cessation of treatment, indicating that D2-like agonists play a causal role in the expression of these compulsive behaviors.
The present studies were aimed at evaluating the capacity of the D3-preferring agonist pramipexole (∼30-fold selective for D3 over D2 in vivo; Collins et al. 2007; and ∼90-fold selective for D3 over D2 in vitro; Millan et al. 2002) to maintain responding when substituted for cocaine, and to enhance responding for the stimuli that were previously paired with cocaine-reinforcement (CS). First, the capacities of response-contingent pramipexole to maintain responding, and non-contingent pramipexole to induce responding were evaluated in cocaine-trained rats. Second, in order to determine whether pramipexole-induced responding represents an increase in the reinforcing effectiveness of the CS, pramipexole was administered prior to sessions in which rats were allowed to respond for CS presentation under a progressive ratio (PR) schedule of reinforcement. Third, since the CS was comprised of multiple components including a yellow light that served as the discriminative stimulus and signaled drug availability (CSD), as well as a 0.5-sec illumination of a green light followed by a 5-sec illumination of the house light that was paired with cocaine reinforcement (CSR), the relative importance of the CSD and CSR on the capacity of pramipexole to induce PR responding were systematically evaluated. Finally, D3- and D2-preferring antagonists were used to assess the relative contributions of the D3 and D2 receptors to pramipexole’s effects on CS-maintained responding.
Methods
Subjects
Male Sprague-Dawley rats (250–300 g) were obtained from Harlan (Indianapolis, IN), and individually housed for the duration of the study in a temperature- (21–23 °C), and humidity-controlled environment, on a 12-hr dark/light cycle with lights on at 7:00 AM. Rats had free access to tap water and ∼20g of Purina rat chow per day to maintain ∼80% of their free feeding weight. All studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Research 1996), as adopted and promulgated by the National Institutes of Health, and all experimental procedures were approved by the University of Michigan Committee on the Use and Care of Animals.
Surgery
Rats were surgically prepared with a chronic indwelling catheter in the left femoral vein under ketamine:xylazine (90:10 mg/kg; IP) anesthesia. Catheters were passed under the skin and attached to stainless steel tubing, exiting the back through a metal tether button positioned between the scapula. Rats were allowed 5–7 days to recover, and catheters were flushed with 0.2 ml of heparinized saline (100 U/ml) during recovery, as well as before and after sessions to insure patency.
Apparatus and Stimuli Conditions
All experimental sessions were conducted in operant conditioning chambers (30.5 cm W × 24 cm D × 21 cm H; Med Associates Inc., St. Albans, VT) placed inside sound attenuating cubicles. Each chamber was equipped with a nosepoke aperture located 6 cm above the grid floor and 1.5 cm from the left side of the wall, and a lever located 6.8 cm above the grid floor and 1.3 cm from the right side of the same wall (ENV-110M, ENV-114BM; Med Associates Inc.) A white house light (4.23 lux as measured from center of chamber) was located at the top center of the opposite wall. A yellow light inside the nosepoke aperture (0.11 lux as measured from center of chamber), and a set of green, yellow, and red LED lights (green, 0.11 lux; yellow, 0.17 lux; red, 0.05 lux; green, yellow and red, 0.33 lux as measured from center of chamber) above both the nosepoke aperture and lever could be illuminated. As summarized in Table 1, Illumination of the yellow light inside the nosepoke aperture served as the discriminative stimulus (CSD), whereas the 0.5-sec illumination of the green LED above the nosepoke aperture followed by the 5-sec illumination of the houselight served as the injection paired stimuli (CSR), and the 0.5-sec illumination of the three LEDs above the lever followed by a 5-sec period in which the houselight flashed at 0.5 Hz (1-sec on, 1-sec off) served as the novel stimuli (NvlS). The NvlS was included to control for non-specific effects of pramipexole on stimuli that had not been conditioned to cocaine, and the lever response was used so that the rats would have to make a topographically different response for the NvlS, thus reducing the possibility that they would make a cocaine-appropriate response (i.e., a nosepoke). Drug solutions were delivered by a pneumatic syringe pump (IITC, Woodland Hills, CA) through Tygon® tubing connected to a stainless steel fluid swivel (Instech Laboratories Inc, Plymouth Meeting, PA) and spring tether which was held in place by a counterbalanced arm.
Table 1.
Description of stimuli that comprise the designated stimulus conditions
| Stimulus Conditions | |
|---|---|
| CS | Yellow LED inside nosepoke aperture |
| 0.5-sec Green LED above nosepoke aperture followed by 5-sec illumination of houselight | |
| CSD | Yellow LED inside nosepoke aperture |
| CSR | 0.5-sec Green LED above nosepoke aperture followed by 5-sec illumination of houselight |
| NvlS | 0.5-sec Green, Yellow, and Red LED above lever followed by 5-sec flashing of houselight at 0.5 Hz |
Fixed Ratio: Training Procedures
Prior to experimental manipulations, 7 groups of rats (n=6/group) were trained to nosepoke for 0.56 mg/kg/inj cocaine under a fixed ratio (FR) 1 timeout (TO) 5.5-sec schedule of reinforcement during daily 90-min sessions. This dose of cocaine was chosen based on previous experience suggesting that a high percentage of rats would acquire responding, and to allow for a historical comparison between the effects of pramipexole and quinpirole (Collins and Woods 2007; Collins and Woods 2009). Illumination of the yellow light inside the nosepoke aperture served as the discriminative stimulus (CSD), and ratio completion resulted in an injection (100 µl/kg/0.5 sec) paired with a 0.5-sec illumination of a green LED located above the nosepoke aperture, and followed by a 5-sec illumination of the house light (CSR). During this 5.5-sec period all other stimuli were extinguished, and responding was recorded but had no scheduled consequence. Although lever presses were recorded they had no scheduled consequence. Upon acquisition of stable responding, defined as three consecutive sessions with less than a 20% difference, and no increasing or decreasing trend in responding, rats were randomly assigned to either substitution (6 groups of 6), or pretreatment studies (1 group of 6).
Fixed Ratio: Substitution Studies
To assess the capacity of pramipexole to maintain responding, one of four doses of pramipexole (0.01, 0.032, 0.1, or 0.32 mg/kg/inj) or saline was substituted for cocaine (1 group of 6 per dose) during 90-min sessions in which nosepoke and lever responses were reinforced under a concurrent FR1TO5.5-sec:FR1TO5.5-sec schedule of reinforcement. Although the contingencies and stimuli (i.e., CSD and CSR) associated with the nosepoke aperture were identical to the training conditions, a yellow LED above the lever was now illuminated concurrent with the CSD to signal that the lever was active, and that presses now resulted in a novel set of stimuli (NvlS) consisting of a 0.5-sec illumination of three LEDs above the lever and a 5-sec flashing (0.5 Hz) of the house light, but no injection. Substitutions were carried out for 7 consecutive sessions, after which the original contingencies (i.e., nosepoke for 0.56 mg/kg/inj cocaine under a FR1TO5.5-sec) were in place for 5 days.
The influence of the CS on nosepoke responding for pramipexole (0.1 mg/kg/inj) was evaluated in a separate group of 6 cocaine-trained rats, with substitutions conducted in three phases over 13 consecutive, 90-min sessions under a concurrent FR1TO5.5-sec:FR1TO5.5-sec schedule of reinforcement. The first phase of the substitution lasted three sessions, and was identical to the substitution studies above (i.e., CSs and pramipexole injections were scheduled on the nosepoke aperture, and NvlSs were scheduled on the lever). The second phase of the substitution lasted 7 sessions, with nosepoke and lever responses reinforced under a concurrent FR1TO5.5-sec:FR1TO5.5-sec schedule of reinforcement. During this phase, CSs and NvlSs were removed from the contingencies and nosepoke responses resulted in 0.1 mg/kg/inj pramipexole followed by an un-signaled 5.5-sec TO, and lever presses resulted in an un-signaled 5.5-sec TO. The CSs and NvlSs were reintroduced on the nosepoke and lever, respectively, during the third phase of the substitution, which lasted three sessions and was run under conditions identical to those described for the first phase of the substitution.
Fixed Ratio: Pretreatment Studies
To assess the direct effects of pramipexole on responding, a separate group of 6 cocaine-trained rats were pretreated with saline, or one of four doses of pramipexole (0.032, 0.1, 0.32, and 1.0 mg/kg; SC) immediately prior to the start of 90-min sessions in which nosepoking and lever pressing were reinforced under a concurrent FR1TO5.5-sec:FR1TO5.5-sec schedule of reinforcement. The stimuli conditions were identical to those described for the substitution sessions above (i.e., the CSD and yellow light above the lever signaled the active portion of the session, and the CSR and NvlS were presented upon ratio completion on the nosepoke and lever, respectively). However, unlike during the substitution studies no injections could be earned. Pramipexole doses were selected based on evidence that suggests the lower two doses (0.032 and 0.1) preferentially activate D3 receptors, whereas the higher two doses activate both D3 and D2 receptors in rats (Collins et al. 2007; Collins et al. 2009; Collins et al. 2005). Doses were presented in random order, with each rat receiving each dose for three consecutive sessions.
Upon completion of the dose-response, rats were pretreated with 0.32 mg/kg pramipexole for six additional sessions to test the importance of the CS on nosepoking, and the NvlS on lever pressing. Three of these sessions were conducted using stimulus conditions that were identical to those described above, however, all CSs and NvlSs were omitted from the contingencies during the other three sessions so that responses on the nosepoke or lever resulted in an un-signaled 5.5-sec TO. Each condition was in place for three consecutive sessions, with the order of the conditions counterbalanced across rats.
Progressive Ratio: Training Procedures
Two additional groups of 6 rats were trained to nosepoke for 0.56 mg/kg/inj cocaine under the identical FR1TO5.5-sec schedule of reinforcement used for the FR studies with the exception that the ratio requirements were gradually increased until responding stabilized at FR10. Rats were subsequently switched to a progressive ratio (PR) schedule of reinforcement, under which ratios incremented exponentially (1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, 737, 901, 1102, etc…) based on the equation (Ratio=[5e^(inj# * 0.2)]-5) from Richardson and Roberts (1996). Sessions lasted a maximum of 4 hrs, but terminated if a ratio was not completed within 45 min (i.e., 45-min limited hold). Upon stabilization of responding, defined as at least 7 sessions with less than a 20% difference and no increasing or decreasing trend in the number of ratios completed, rats were randomly assigned to either conditioned stimuli (1 group of 6), or antagonist studies (1 group of 6).
Progressive Ratio: Dose-Response Analysis and Conditioned Stimuli Studies
Pretreatments with saline and each of four doses of pramipexole (0.032, 0.1, 0.32, and 1.0 mg/kg; SC) occurred immediately prior to sessions in which the CSD signaled the active portion of the session, and ratio completion resulted in presentation of the CSR, but no longer resulted in cocaine injections. All five dosing conditions were evaluated in each rat, with the first dose always being 0.32 mg/kg pramipexole, and the remaining doses were presented in random order. Each dose was administered for at least three consecutive sessions.
Upon completion of the dose-response, the effects of 0.32 mg/kg pramipexole were re-evaluated under four distinct stimulus conditions: CSD-CSR: both the CSD (yellow light inside the nosepoke aperture) and CSR (0.5-sec green LED + 5-sec house light) were scheduled, No Stim: neither the CSD, nor the CSR were scheduled, CSD: only the CSD was scheduled, and CSR: only the CSR was scheduled. Conditions were in place for three consecutive sessions and presented in random order, with the CSD-CSR condition always evaluated twice, once at the beginning, and once at the end of the testing cycle.
Progressive Ratio: Antagonist Studies
As described above, saline or pramipexole (0.1, 0.32, 1.0, and 3.2 mg/kg; SC) was administered immediately before sessions in which the CSD signaled the active portion of the session and ratio completion resulted CSR presentation, but no longer resulted in cocaine injections. All five dosing conditions were evaluated in each of the 6 rats, with the 0.32 mg/kg dose always assessed first, and the remaining doses evaluated in random order. Each dose of pramipexole was administered for at least three consecutive sessions prior to assessing a 1.0 mg/kg dose of the D2-preferring antagonist L-741,626 (∼13-fold selective for D2 over D3 receptors in vitro; Millan et al. 2000), and a 32.0 mg/kg dose of the D3-preferring antagonist PG01037 (∼133-fold selective for D3 over D2 receptors in vitro; Grundt et al. 2005; Grundt et al. 2007). Antagonists were administered as 30-min pretreatments to pramipexole, with the doses chosen based on their capacity to selectivity antagonize in vivo effects that have been linked to the activation of D2 (hypothermia) or D3 (yawning) receptors (Collins et al. 2007; Collins et al. 2009; Collins et al. 2005). The order of antagonist administration was randomized across rats, and separated by least three sessions.
Upon completion of all antagonist × pramipexole dosing combinations, the dose-response curve for pramipexole alone (saline, 0.1, 0.32, 1.0, and 3.2 mg/kg; SC) was reevaluated prior to assessing the effects of cocaine (3.2, 10.0, and 32.0 mg/kg; IP) on PR responding for the CS. The doses of pramipexole and cocaine were administered for at least three consecutive sessions, and were presented in random order.
Drugs
Cocaine was obtained from the National Institute on Drug Abuse (Bethesda, MD). PG01037 was synthesized by Ms. J. Cao (Medicinal Chemistry Section-NIDA, Baltimore, MD) according to published procedures (Grundt et al. 2005). Pramipexole was obtained from APAC Pharmaceutical, LLC (Columbia, MD), and L-741,626 was obtained from Tocris Chemical Company (Ellisville, Mo). Cocaine and pramipexole were dissolved in physiologic saline. L-741,626 was dissolved in 5% ethanol and sterile water, and PG01037 was dissolved in 10% β-cyclodextrin. All pretreatments were administered subcutaneously in a volume of 1.0 ml/kg.
Data analysis
All data are presented as the mean ± SEM, n=6 for each endpoint. Significant differences in responding during pramipexole substitutions were determined using two-way (Dose × Session) repeated measures ANOVA with post-hoc Bonferroni tests (GraphPad Prism). Significant effects of CS presentation on pramipexole-maintained responding were determined using one-way (Session) repeated measures ANOVA with post-hoc Newman-Keuls. Significant effects of CS on pramipexole-induced FR1 responding were determined by two-tailed paired t-tests. Significant effects of pramipexole or cocaine pretreatment on responding (and other PR endpoints) were determined using a one-way (Dose) repeated measures ANOVA with post-hoc Newman-Keuls tests and compared the respective endpoints observed during cocaine-maintained responding, or following saline pretreatments. A two-way (Antagonist Dose × Pramipexole Dose) repeated measures ANOVA with post-hoc Bonferroni tests was used to determine if antagonists significantly altered the effects of pramipexole on PR endpoints, as well as to determine if the effects of pramipexole on PR responding were significantly altered upon reevaluation (Time × Pramipexole Dose).
Results
Fixed Ratio: Substitution Studies
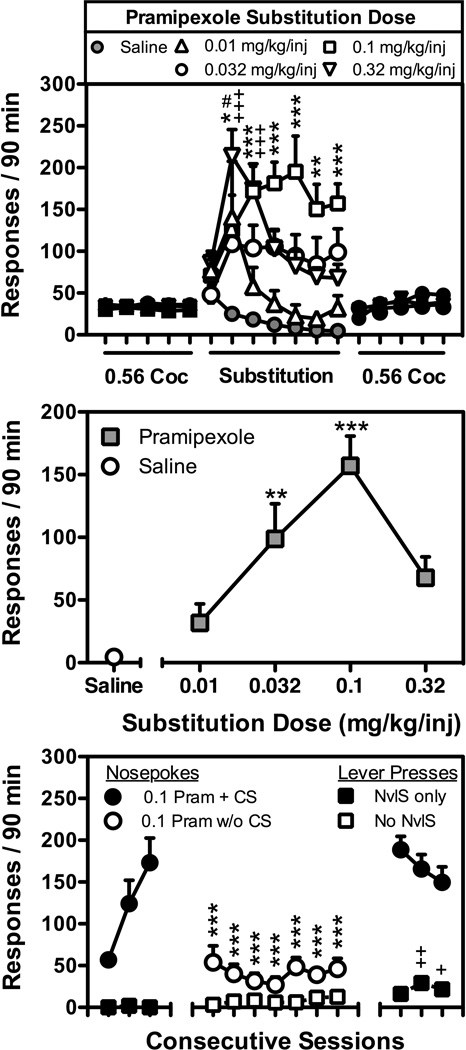
As shown in Figure 1, pramipexole maintained FR1TO5.5-sec responding in a dose- and session-dependent manner when substituted for 0.56 mg/kg/inj cocaine (Dose: F[4,150]=9.7; p<0.001; Session: F[6,150]=6.0; p<0.001). High rates of responding were maintained by doses of 0.032 to 0.32 mg/kg/inj pramipexole over the last 6 days of the substitution, with a dose of 0.1 mg/kg/inj maintaining significantly more responding than saline on each of the last 6 days (Fig. 1; top panel), and doses of 0.032, and 0.1 mg/kg/inj pramipexole maintaining significantly more responding than saline on day 7 (Fig. 1; middle panel). Although slight increases in lever responding were observed following the introduction of the NvlS contingency, lever pressing during pramipexole substitutions was no different (Dose: F[4,150]=0.6; p=0.66; Session: F[6,150]=1.4; p=0.2) than when saline was available for injection (data not shown). These high rates of pramipexole-maintained responding were dependent upon the injection-CS pairing (F[10,50]=20.1; p<0.001) as significantly lower rates of nosepoking were observed throughout the 7-day period when CS (i.e., CSD and CSR) presentations were omitted (Fig. 1; bottom panel). Following the reintroduction of the injection-CS pairing, nosepoking occurred at rates that were no different than those observed prior to removal of the CS from the contingency. Although removal of the NvlS contingency from the lever did not significantly alter rates of lever pressing, a slight but significant increase lever pressing was observed when the rats were returned to the original substitution contingencies in which nosepoking resulted in pramipexole paired with the CS, and lever pressing resulted in the presentation of the NvlS (Fig. 1; bottom panel).
Figure 1.
Top Panel) Responding maintained by pramipexole (0.01, 0.032, 0.1, or 0.32 mg/kg/inj), or saline under an FR1TO5.5-sec:FR1TO5.5-sec schedule of reinforcement during a 7-day substitution from cocaine (0.56 mg/kg/inj). Data represent the mean (± S.E.M.), n=6, number of nosepoke responses that resulted in cocaine injections paired with stimuli, or pramipexole injections paired with the stimuli that were previously paired with cocaine delivery (CS) during daily 90 min sessions. #, p<0.05; *, p<0.05; **, p<0.01; ***,p<0.001; +++, p<0.001. Significant differences in nosepoke responding maintained by pramipexole [0.01(#), 0.1(*), or 0.32(+) mg/kg/inj] and saline were determined by two-way ANOVA with post-hoc Bonferroni tests. Middle Panel) Dose-response curve for pramipexole-maintained responding. Data represent the mean (± S.E.M.), n=6, number of reinforced responses made during the 7th day of substitution from cocaine (0.56 mg/kg/inj). **, p<0.01; ***,p<0.001. Significant differences in nosepoke responding from saline were determined by one-way ANOVA with post-hoc Newman-Keuls tests. Bottom Panel) Responding maintained by 0.1 mg/kg/inj pramipexole during a 13-day substitution from 0.56 mg/kg/inj cocaine. Filled symbols represent nosepoke responses (filled circles) that resulted in the delivery of pramipexole in conjunction with presentation of the CS and lever presses (filled squares) that resulted in presentation of the NvlS. Open symbols represent nosepoke responses (open circles) that resulted in the delivery of pramipexole and an un-signaled 5.5-sec TO, and lever presses (open squares) that resulted in an un-signaled 5.5-sec TO. **, p<0.01; ***,p<0.001; ++, p<0.01; +++,p<0.001. Significant differences in nosepoke (*) or lever (+) responding as compared with nosepoke or lever responding during day 3 of the substitution were determined by one-way ANOVA with post-hoc Newman-Keuls tests.
Fixed Ratio: Pretreatment Studies
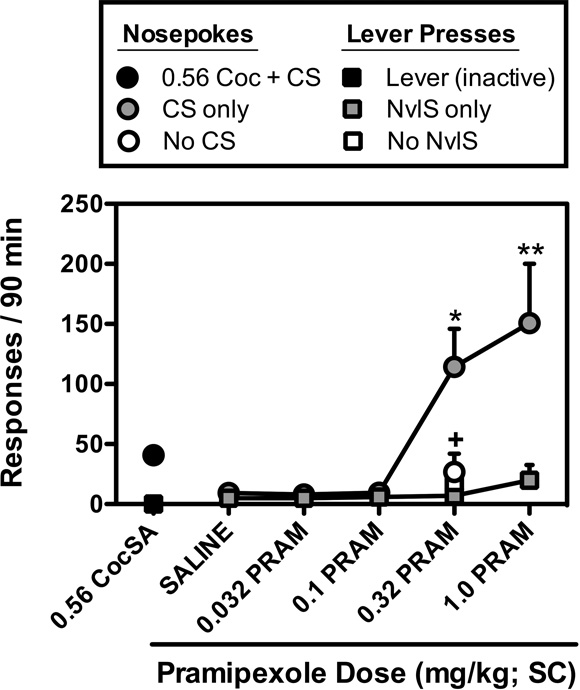
Dose-dependent (F[4,15]=6.9; p<0.01) increases in nosepoke responding were also observed when rats were pretreated with pramipexole prior to sessions in which nosepoke responses resulted in CS presentation and lever presses resulted in NvlS presentation (Fig. 2). Although significant increases in nosepoke responding were observed following pretreatment with doses of 0.32 and 1.0 mg/kg pramipexole, no increases in lever pressing were observed following administration of any dose of pramipexole. In addition, these pramipexole-induced increases in nosepoke responding were dependent upon the nosepoke-CS contingency as responding occurred at significantly lower, saline-like rates when CS presentations were omitted (p<0.05).
Figure 2.
Effects of pretreatments with saline or pramipexole (0.032, 0.1, 0.32, and 1.0 mg/kg; SC) on nosepoke responding (gray circles) for the presentation of stimuli that were previously paired with 0.56 mg/kg/inj cocaine reinforcement (CS), and lever pressing (gray squares) for a set of novel stimuli (NvlS). The effects of 0.32 mg/kg dose of pramipexole on nosepoke responses that failed to produce the CS (open circles), and lever presses failed to produce the NvlS (open squares) are also shown. Data represent the mean (± S.E.M.), n=6, number of responses that resulted in ratio completion during the final session for each pretreatment dose. *, p<0.05; **, p<0.01. Significant increases in responding for CS or NvlS presentation were determined by one-way repeated measures ANOVA with post-hoc Newman-Keuls tests. +, p<0.05. Significant differences between the amounts of responding observed during sessions in which nosepoke and lever responding resulted in the presentation of the CS and NvlS, and sessions in which CS and NvlS presentations were omitted were determined by two-tailed t-tests.
Progressive Ratio: Conditioned Stimuli Studies
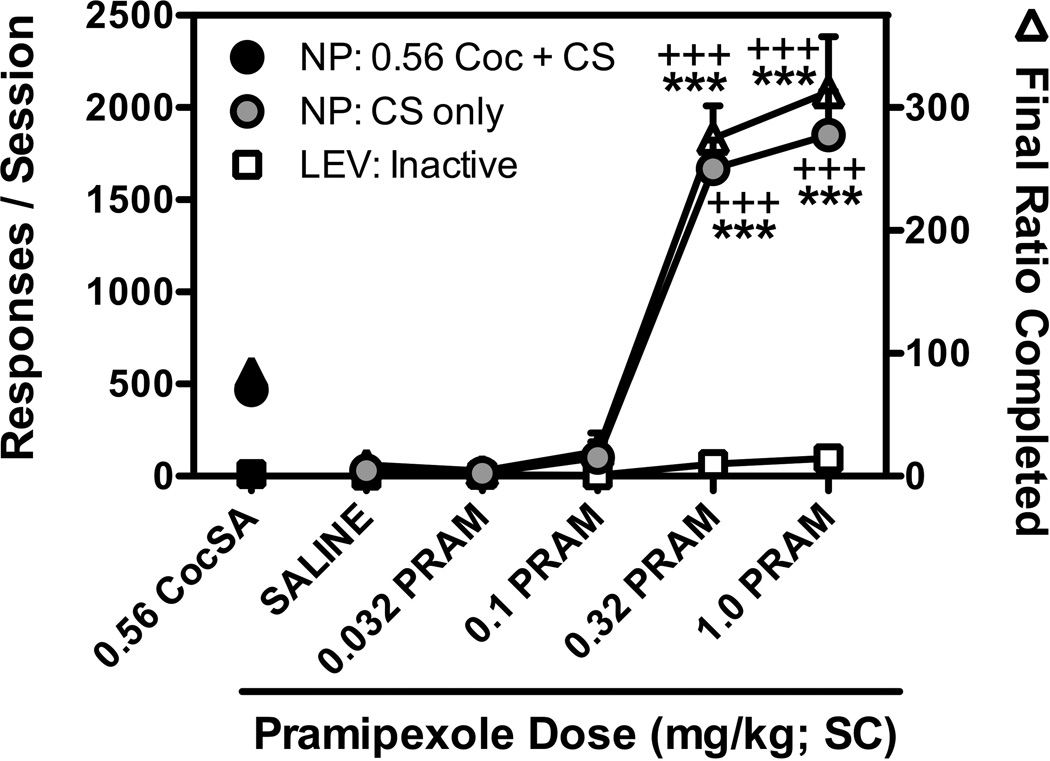
Similar to the effects observed when pramipexole was administered prior to sessions in which CSs were presented for nosepoking on an FR1TO5.5-sec schedule of reinforcement, dose-dependent increases (F[4,20]=46.1; p<0.001) in CS-maintained responding were also observed when pramipexole was administered prior to sessions in which CS presentations were delivered under a PR schedule of reinforcement. As shown in Figure 3, pretreatment with doses of 0.32 and 1.0 mg/kg pramipexole resulted in significantly more responding than was observed after saline pretreatment or during cocaine reinforced sessions. Pramipexole did not alter lever (inactive) responding at any dose tested. Pramipexole pretreatments also resulted in dose-dependent increases in the number of CS presentations earned (F[4,20]=55.3; p<0.001), final ratio completed (F[4,20]=38.5; p<0.001), response rates (F[4,20]=53.2; p<0.001), and total session duration (F[4,20]=29.2; p<0.001) as compared with when saline was administered prior to the session.
Figure 3.
Effects of pretreatment with saline or pramipexole (0.032, 0.1, 0.32, and 1.0 mg/kg; SC) prior to sessions in which nosepoke responding was reinforced under a progressive ratio (PR) schedule of reinforcement by presentation of the previously cocaine-paired stimuli (CS). Black symbols represent the mean ± SEM, n=6, of each endpoint during the last day of PR responding for 0.56 mg/kg/inj cocaine for each endpoint. The total number of CS-reinforced nosepoke responses (gray circles) and inactive lever responses (open squares) are shown on the left y-axis, and represent the mean ± SEM, n=6, number of responses emitted during the final session for each pretreatment dose. The final ratio completed (open triangles) are shown on the right y-axis, and represent the mean ± SEM, n=6, value of the final ratio that was completed prior to session termination due to the 45-min limited hold, or 240-min session limit, whichever came first. +++,p<0.001; ***,p<0.001. Significant differences in each endpoint compared to saline pretreatment (*) or 0.56 mg/kg/inj cocaine-reinforced responding (+) were determined by one-way repeated measures ANOVA with post-hoc Newman-Keuls tests.
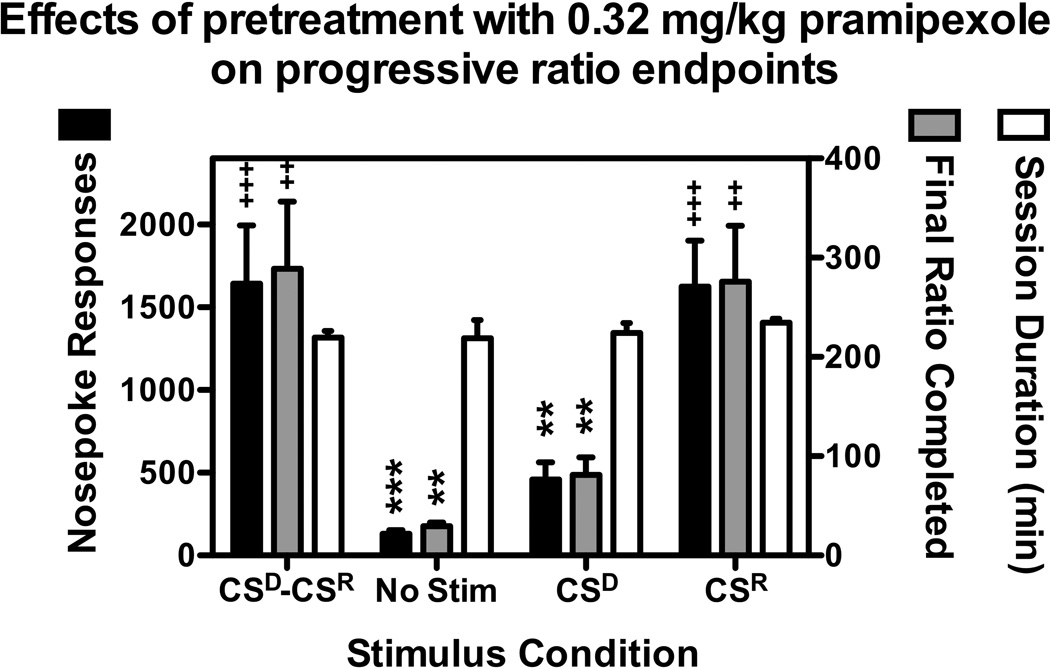
As was observed with FR1 responding for CS presentation, altering the stimulus conditions significantly affected the capacity of pramipexole to induce responding under a PR schedule of reinforcement (F[3,15]=14.2; p<0.001). As shown in Figure 4, significantly lower levels of responding were observed when 0.32 mg/kg pramipexole was administered prior to sessions in which the CSD and CSR were omitted from the contingency, as well as when the CSD was scheduled, but ratio completion failed to produce CSR presentation. Conversely, when the CSD was not presented, but ratio completion resulted in CSR presentation, responding occurred at levels no different than those observed in the CSD-CSR condition. Altering the stimulus conditions also significantly reduced the number of ratios completed (F[3,15]=27.5; p<0.001), final ratio completed (F[3,15]=10.6; p<0.001), and response rates (F[3,15]=16.4; p<0.001) However, the total session duration was unaffected by manipulation of the stimulus conditions, with all sessions lasting approximately 230 minutes.
Figure 4.
Effects of pretreatment with 0.32 mg/kg pramipexole on PR responding under four distinct stimulus conditions: CSD-CSR -- both the CSD (yellow light inside the nosepoke aperture) and CSR (green LED + house light) were scheduled, No Stim -- neither the CSD, nor the CSR were scheduled, CSD -- only the CSD was scheduled, and CSR -- only the CSR was scheduled. Data represent the mean ± SEM, n=6, for each endpoint during the final session that each condition was in place. Total nosepoke responses (black bars) are shown on the left y-axis and represent the mean ± SEM, n=6, number of nosepoke responses that resulted in ratio completion. The final ratio completed (gray bars) is shown on the right y-axis and represent the mean ± SEM, n=6, value of the final ratio that was completed prior to session termination due to the 45-min limited hold, or 240-min session limit, whichever came first. The session duration (open bars) is shown on the right y-axis and represents the mean ± SEM, n=6, time in minutes that elapsed prior to session termination due to the 45-min limited hold, or 240-min session limit, whichever came first. **,p<0.01; ***,p<0.001; ++,p<0.01; +++,p<0.001. Significant differences from CSR-CSD (*) and No Stim (+) as determined by one-way repeated measures ANOVA with post-hoc Newman-Keuls tests.
Progressive Ratio: Antagonist Studies
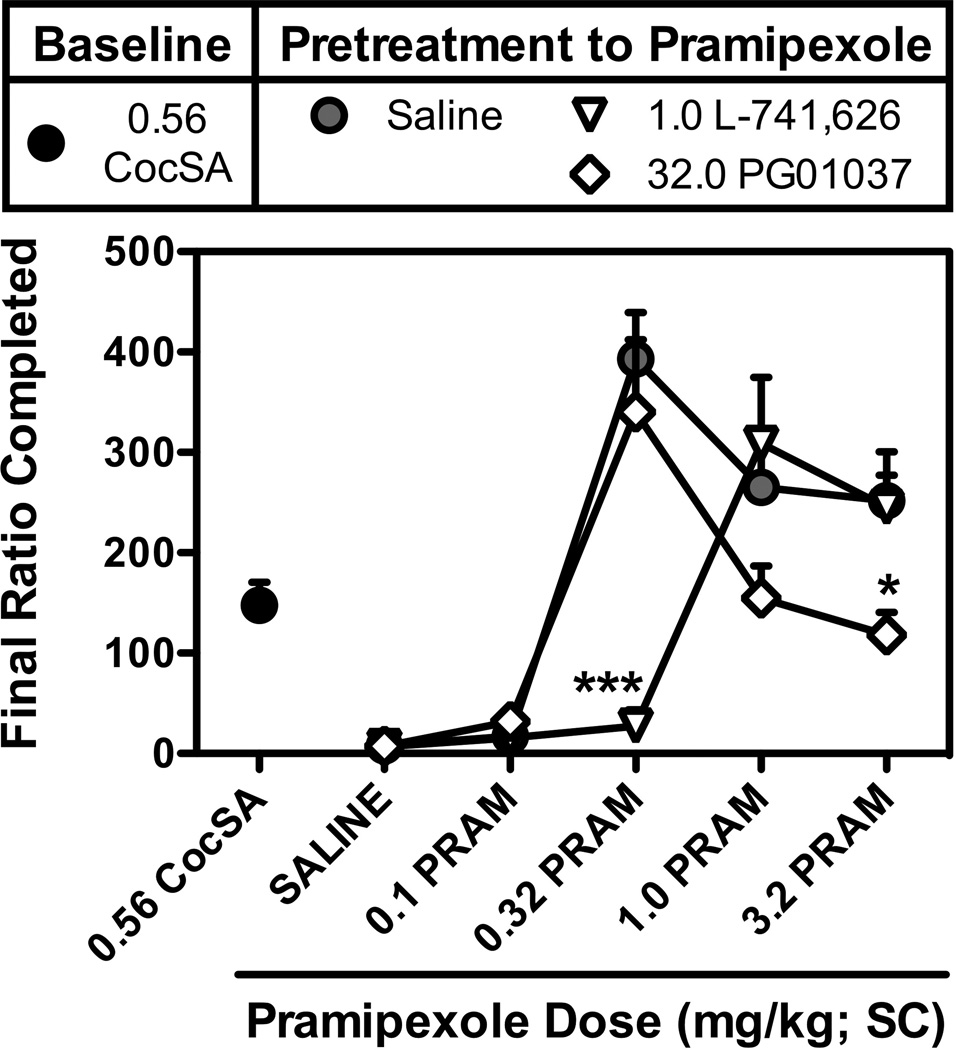
As shown in Figure 5, pramipexole induced dose-dependent increases in CS-maintained responding (F[4,20]=25.0; p<0.001) and final ratio completed (F[4,20]=24.0; p<0.001) over a wide range of doses with significant increases observed following doses of 0.32, 1.0, and 3.2 mg/kg pramipexole. Similar dose-dependent increases were also observed with respect to the number of ratios completed, response rate, and session duration (data not shown). Pretreatment with a D2 dose of 1.0 mg/kg L-741,626 produced a significant rightward shift in the dose-response curve, with significantly lower levels of responding (F[1,25]=17.5; p<0.001) and final ratio completed (F[1,25]=17.0; p<0.001) observed at the 1.0 mg/kg L-741,626 × 0.32 mg/kg pramipexole dose combination. Unlike the effects of L-741,626, pretreatment with a D3 dose of 32.0 mg/kg PG01037 produced a downward shift in dose-response curve for pramipexole-induced responding (F[1,25]=7.8; p<0.01) and the final ratio completed (F[1,25]=7.4; p<0.05), with significant reductions in responding and the final ratio completed observed at the 3.2 mg/kg dose of pramipexole (Fig. 5).
Figure 5.
Effects of the pretreatment with the D2-preferring antagonist L-741,626 (1.0 mg/kg; SC), or the D3-selective antagonist PG01037 (32.0 mg/kg; SC) on the response-inducing effects of pramipexole (0.1, 0.32, 1.0, and 3.2 mg/kg; SC) or saline. Data represent the final ratio completed during sessions in which nosepoke responding was reinforced under a progressive ratio (PR) schedule of reinforcement by presentation of the stimuli that were previously paired with 0.56 mg/kg/inj cocaine reinforcement (CS). Saline data (gray circles) represent the mean ± SEM, n=6, of final ratios completed for sessions in which saline was administered 30-min prior to the administration of pramipexole (or saline). The effects of 1.0 ,g/kg L-741,626 (open inverted triangles) and 32.0 mg/kg PG01037 (open diamonds) represent the mean ± SEM, n=6, final ratio completed during sessions in which antagonists were administered 30-min prior to the administration of pramipexole (or saline). *,p<0.05; ***,p<0.001. Significant differences in the final ratio completed during sessions that were preceded by antagonist pretreatment as compared to those that were preceded by saline were determined by two-way repeated measures ANOVA with post-hoc Bonferroni tests.
Table 2 shows the effects of pretreatment with pramipexole or cocaine on PR responding for CS presentation. Pramipexole induced dose-dependent increases in nosepoke responding (F[5,20]=18.1; p<0.001), final ratio (F[5,20]=18.7; p<0.001), CSs earned (F[5,20]=82.7; p<0.001), response rate (F[5,20]=11.1; p<0.001), and session duration (F[5,20]=41.9; p<0.001) when PR responding resulted in CS presentation, and there were no significant differences between the initial dose-response and the subsequent redetermination of pramipexole-induced PR responding for CS presentation obtained approximately 45 to 60 sessions after the termination of cocaine self-administration. Although cocaine produced dose-dependent increases in nosepoke responding (F[3,15]=4.7; p<0.05), final ratio completed (F[3,15]=4.6; p<0.05), CSs earned (F[3,15]=7.3; p<0.01), response rate (F[3,15]=3.6; p<0.05), and session duration (F[3,15]=3.8; p<0.05) when administered prior to sessions in which nosepoke responses resulted in CS presentation these effects were much smaller in magnitude than those observed following pretreatment with pramipexole.
Table 2.
Effects of pretreatment with saline, pramipexole, and cocaine on progressive ratio responding for CS presentation
| Nosepoke Responses |
Final Ratio Completed |
CSs Earned | Response Rate Resp / min |
Session Duration (min) |
|
|---|---|---|---|---|---|
| Saline PT | |||||
| Saline a | 22.7 (8.1) | 6.9 (2.0) | 4.1 (0.7) | 0.4 (0.1) | 60.9 (4.9) |
| Saline b | 16.3 (2.3) | 6.5 (0.5) | 3.7 (0.3) | 0.3 (0.04) | 50.8 (1.6) |
| Pramipexole PT Dose | |||||
| 0.1 mg/kg a | 94.7 (47.5) | 19.5 (8.1) | 6.1 (1.2) | 0.7 (0.2) | 86.9 (17.4) |
| 0.1 mg/kg b | 80.7 (52.5) | 15.7 (7.2) | 6.0 (1.5) | 1.5 (1.2) | 95.8 (16.9) |
| 0.32 mg/kg a | 2243.3 (312.8)*** | 380.9 (52.8)*** | 21.3 (0.6)*** | 9.3 (1.3)*** | 235.2 (3.6)*** |
| 0.32 mg/kg b | 1937.7 (230.6)*** | 337.7 (37.7)*** | 21.0 (0.5)*** | 8.5 (1.1)*** | 230.2 (6.3)*** |
| 1.0 mg/kg a | 1496.4 (257.5)*** | 261.7 (45.9)*** | 19.4 (0.9)*** | 6.5 (1.0)*** | 229.9 (8.4)*** |
| 1.0 mg/kg b | 1368.5 (149.1)** | 226.2 (26.5)** | 19.0 (0.6)*** | 6.1 (0.6)** | 223.3 (7.3)*** |
| 3.2 mg/kg a | 1471.4 (150.6)*** | 251.8 (25.5)*** | 19.5 (0.6)*** | 6.8 (0.6)*** | 216.8 (11.3)*** |
| 3.2 mg/kg b | 1789.3 (449.8)*** | 297.0 (72.5)*** | 19.8 (1.1)*** | 9.3 (2.1)*** | 198.7 (19.4)*** |
|
Cocaine PT Dose | |||||
| 3.2 mg/kg b | 28.8 (5.1) | 8.7 (1.3) | 2.5 (0.5) | 0.5 (0.1) | 66.4 (8.8) |
| 10.0 mg/kg b | 77.3 (35.3) | 18.3 (6.4) | 7.0 (1.6) | 1.0 (0.4) | 77.3 (6.3) |
| 32.0 mg/kg b | 182.0 (63.5)* | 36.5 (11.6)* | 9.0 (2.0)* | 1.7 (0.5)* | 88.9 (13.4)* |
Effects determined during the evaluation of antagonist pretreatments of PR responding for CSs
Effects determined after the evaluation of antagonist pretreatments of PR responding for CSs
p<0.05
p<0.01
p<0.001.
Significant difference in endpoint from the respective saline data as determined by one-way ANOVA with post-hoc Newman-Keuls tests.
Discussion
Previous studies have demonstrated that the response-maintaining effects quinpirole, a D3-preferring agonist, appear to be dependent upon relatively specific reinforcement histories, as well as an interaction between quinpirole and the stimuli that were previously paired with cocaine reinforcement (Collins and Woods 2007; Collins and Woods 2009). The results of the current studies confirm these general findings and extend them in several ways. First, when substituted for cocaine, the clinically used D3-preferring agonist pramipexole maintained responding in a dose-dependent and selective manner, with high rates of responding observed when pramipexole injections were paired with stimuli that were previously paired with cocaine reinforcement (i.e., the CS), and significantly lower rates of responding observed when CS presentation was omitted. Second, dose-dependent and selective increases in nosepoke responding were also observed when cocaine-trained rats were pretreated with pramipexole and allowed to respond under an FR1 for CS presentation alone, with peak rates of responding similar to those observed when pramipexole injections were paired with the CS and delivered contingent upon responding. Third, pramipexole induced dose-dependent and selective increases in nosepoke responding when CSs were presented under a PR schedule of reinforcement, with maximal increases in responding approximately 10 times greater than those produced by pretreatment with cocaine. Fourth, these large increases in responding were dependent upon an interaction between pramipexole and the stimuli that were previously paired with cocaine injections (CSR), rather than those that predicted cocaine availability (CSD). Finally, D2 and D3 antagonists differentially affected pramipexole-induced PR responding for CS presentation, with the D2-preferring antagonist L-741,626 producing a rightward shift, and the D3-preferring antagonist PG01037 producing a downward shift in the dose-response curve. Together, these studies suggest that although pramipexole injections are capable of maintaining responding when substituted for cocaine, the high rates of responding are being controlled by the contingency between the response and the CS presentation rather than by the injection of pramipexole itself.
Similar to previous reports with quinpirole (Collins and Woods 2009), the capacity of pramipexole injections to maintain responding appeared to be dependent upon their being paired with the CS, as these high rates of pramipexole-maintained responding were not only significantly reduced when the CS was removed from the contingency, but also recovered following the reintroduction of the injection-CS pairing. Importantly, a previous study failed to show similar decreases in FR1 responding maintained by 0.56 mg/kg/inj cocaine following removal of the CS from the contingency (Collins and Woods 2009), suggesting that CS presentations may be more important for drugs that function as weak reinforcers, such as nicotine (Donny et al. 2003), or when low doses of drugs that are typically thought of as powerful reinforcers, such as cocaine, are available for injection (Schenk and Partridge 2001). In addition to enhancing pramipexole’s response-maintaining effects, the CS was also important for pramipexole’s response-inducing effects. Not only were the dose-dependent increases in responding produced by experimenter-administered pramipexole selective for conditions in which nosepoke responses were reinforced by the CS, but they also occurred in the absence of any increase in lever responding which resulted in a similar, but novel stimulus change (NvlS), and at doses that lack locomotor stimulatory effects (Collins et al. 2011). Together, these findings suggest that the response-maintaining and response-inducing effects of pramipexole are similarly mediated by an interaction between the direct effects of pramipexole and the conditioned effects of the CS, rather than a more general increase in responding for neutral stimuli (i.e., NvlS) as has been shown for other drugs, such as nicotine (Donny et al. 2003).
Although the effects of pramipexole on responding maintained by conditioned stimuli have not been studied in rats, quinpirole has been shown to dose-dependently increase responding for stimuli previously paired with water (Wolterink et al. 1993), or cocaine (Collins and Woods 2009), suggesting that D2-like agonists may be psychostimulant-like in their capacity to enhance the reinforcing effects of conditioned stimuli (e.g., Beninger et al. 1981; Robbins 1976; 1978; Robbins and Koob 1978). Consistent with this notion, experimenter-administered pramipexole also produced dose-dependent increases in responding when CSs were available under a PR schedule of reinforcement which is commonly used to assess the relative reinforcing effectiveness of drug and non-drug reinforcers. Importantly, even though rats earned approximately 8 times fewer CS presentations, they emitted 10 times more responses, and responded 4 times faster when pramipexole was administered prior to PR as compared to FR1 sessions, suggesting that pramipexole-induced, CS-maintained responding was occurring in a schedule appropriate manner. Moreover, these effects of pramipexole were not only persistent, lasting for at least 60 sessions, but the high rates of responding corresponded to CSs maintaining substantially higher final ratios (greater than 300) than when either cocaine (∼37), or saline (∼7) was administered prior to the session.
Whilst these findings suggest that CSs were functioning as powerful reinforcers when rats were under the influence of pramipexole, it should be noted that D2-like agonists, such as quinpirole, have also been shown to induce perseverative responding in a variety of operant procedures (Boulougouris et al. 2009; Joel et al. 2001; Kurylo 2004; Kurylo and Tanguay 2003), raising the possibility that these increases in responding were mediated by a mechanism(s) distinct from alterations in the reinforcing effectiveness of the CS. To this end, the effectiveness of pramipexole to induce PR responding was evaluated under a variety different stimulus conditions. In agreement with a pramipexole-induced enhancement of conditioned reinforcement, the stimuli that were previously paired with cocaine injection (CSR) maintained equally high rates of responding regardless of whether the stimulus that predicted cocaine availability (CSD) was scheduled or not. Curiously, although removal of either the CSR alone, or both the CSR and the CSD resulted in significantly lower levels of responding, pretreatment with pramipexole continued to induce low rates of responding that were sufficient to extend the sessions for approximately 230 minutes despite the fact that there was no scheduled consequence for responding. Given than pretreatments with saline or low doses of pramipexole resulted in low levels of responding over relatively short periods of time (∼50–95 min), these findings suggest that pramipexole’s main effect may have been to induce low levels of perseverative, unreinforced responding, with high rates of responding only observed when responses resulted in the presentation of the CSR.
Alternatively, since D2-like agonists are known to possess cocaine-like discriminative stimulus effects (e.g., Barrett et al. 2001; Terry et al. 1994), it is possible that increases in nosepoke responding (i.e., cocaine-appropriate responding) resulted from a cocaine-like enteroceptive stimulus produced by pramipexole that “suggested” cocaine availability. Although the current data suggest that any potential overlap between the enteroceptive effects of pramipexole and cocaine were insufficient to produce high rates of responding in the absence of the CSR, this is nonetheless an intriguing possibility that warrants further study to more fully elucidate the degree to which the enteroceptive effects of pramipexole are involved in its response-maintaining and response-inducing effects.
Although the doses of pramipexole that were active in the current studies are known to have effects at both D3 and D2 receptors in rats (Collins et al. 2008; Collins et al. 2007; Collins et al. 2009; Collins et al. 2005), the parallel rightward shift of the dose-response curve for pramipexole-induced, CS-maintained responding produced by the D2-preferring antagonist L-741,626 suggests that these effects are mediated by an agonist activity at the D2 receptor. While the lack of D2-like agonist self-administration in D2 receptor knockout mice (Caine et al. 2002) supports this notion, the downward shift in the dose-response curve produced by the D3 antagonist PG01037 raises the possibility that these effects are also modulated by D3 receptor activation. That PG01037 was not more effective at altering CS-maintained responding was somewhat surprising given reports that D3 antagonists effectively inhibit responding that is either induced (e.g., cue-induced reinstatement) or maintained (e.g., second-order schedules of reinforcement) by stimuli conditioned to a variety of drug reinforcers (e.g., Cervo et al. 2007; Di Ciano et al. 2003; Higley et al. 2010; Khaled et al. 2009; Pilla et al. 1999). However, since similar studies have not been performed with antagonists that possess even modest degrees of selectivity for the D2 receptor, such as L-741,626, it is difficult to determine the relative contributions of these two receptor subtypes in other cue-related behaviors. In addition, although the relative ineffectiveness of PG01037 may be due to a prepotent stimulation of D2 receptors by pramipexole that does not occur in cue-induced reinstatement experiments, reports that PG01037 equipotently decreases PR responding for methamphetamine and sucrose (Higley et al. 2010) raise the possibility that the current decreases in PR responding may have resulted from a non-specific effect of PG01037 on relatively high rates of responding.
In summary, the results of these studies provide convergent evidence to suggest that the CSR was capable of functioning as a powerful reinforcer in its own right when rats were under the influence of pramipexole. Importantly, these pramipexole-induced increases in CS-maintained responding were not only dose-dependent, and sensitive changes in the stimulus condition, but they were also blocked by L-741,626 suggesting that they are primarily mediated by a D2 receptor mechanism. Moreover, when taken together with the capacity of pramipexole to induce persistent, low rates of unreinforced responding these results suggest that pramipexole’s primary effect may be to induce low levels of perseverative, or “habitual” responding, with high rates of responding only occurring in situations in which pramipexole-treated rats come into contact stimuli that have taken on conditioned reinforcing properties.
Interestingly, these effects appear to be similar to those observed in a subset of patients being treated with dopamine replacement therapies (e.g., l-DOPA, pramipexole, and ropinirole). In addition to an increased occurrence of impulse control disorders (ICDs; Driver-Dunckley et al. 2003; Driver-Dunckley et al. 2007; Holman 2009; Voon et al. 2007; Weintraub et al. 2010; Weintraub and Potenza 2006), these drugs have also been associated with the development of punding (Evans et al. 2004; Voon et al. 2007). Although ICDs involve increases in goal-oriented behaviors (e.g., gambling, eating, shopping, and sex), whereas punding involves prolonged, stereotyped patterns of behavior that are habitual in nature and often related to the patients’ occupations, hobbies, or pastimes, both of these effects are typically described as compulsive in nature, and are likely influenced to a great degree by an individual’s history, and/or the environmental stimuli that have been associated with the particular activity. Furthermore, just as the pramipexole-induced increases in responding described in the current studies were highly dose-dependent, these aberrant behaviors are more common in patients taking high doses of D2-like agonists, and generally resolve upon dose reduction, or the cessation of treatment (e.g., Evans et al. 2004; Weintraub 2008). Finally, although the current studies were limited to studying the effects of pramipexole in animals that were trained to respond for cocaine, there is evidence to suggest that this may be a more general effect of D2-like agonists to enhance conditioned reinforcing effects as similar increases in responding have also been observed for water-paired stimuli (Wolterink et al. 1993). Nevertheless, the degree to which the observed effects were influenced by the overlapping enteroceptive effects of pramipexole and cocaine and/or the specific reinforcement history is not known. Further studies will be required to more fully examine the generality of pramipexole’s conditioned reinforcement enhancing effects, as well as how these effect may relate to clinical observations of pramipexole-induced impulse control disorders specifically, and drug abuse-related behaviors more generally.
Acknowledgments
This research was supported by NIDA grants DA 024897, DA 020669, as well as the NIDA-IRP.
Contributor Information
Gregory T. Collins, Department of Pharmacology, 1301 MSRB III, 1150 W. Medical Center Drive, University of Michigan Medical School, Ann Arbor, MI 48109-0632, USA, collinsg@umich.edu, Tel.: +1-734-764-2307, Fax: +1-734-764-7118
Alyssa R. Cunningham, Department of Pharmacology, 1301 MSRB III, 1150 W. Medical Center Drive, University of Michigan Medical School, Ann Arbor, MI 48109-0632, USA
Jianyong Chen, Departments of Internal Medicine and Medicinal Chemistry, University of Michigan Medical School, Ann Arbor, MI 48109-0934, USA.
Shaomeng Wang, Department of Pharmacology, 1301 MSRB III, 1150 W. Medical Center Drive, University of Michigan Medical School, Ann Arbor, MI 48109-0632, USA; Departments of Internal Medicine and Medicinal Chemistry, University of Michigan Medical School, Ann Arbor, MI 48109-0934, USA.
Amy H. Newman, Medicinal Chemistry Section, National Institutes on Drug Abuse-Intramural Research Program, National Institutes of Health, 333 Cassell Drive, Baltimore, MD 21224-0180, USA
James H. Woods, Department of Pharmacology, 1301 MSRB III, 1150 W. Medical Center Drive, University of Michigan Medical School, Ann Arbor, MI 48109-0632, USA
References
- Barrett AC, Morgan D, Izenwasser S, Picker MJ. Cocaine-like discriminative stimulus effects and [3H]dopamine uptake inhibition produced by selected partial opioid agonists. Behav Pharmacol. 2001;12:225–235. doi: 10.1097/00008877-200107000-00001. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Hanson DR, Phillips AG. The acquisition of responding with conditioned reinforcement: effects of cocaine, (+)-amphetamine and pipradrol. Br J Pharmacol. 1981;74:149–154. doi: 10.1111/j.1476-5381.1981.tb09967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Castane A, Robbins TW. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacology (Berl) 2009;202:611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 2009;1(105 Suppl):S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int J Neuropsychopharmacol. 2007;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters N’-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacol Exp Ther. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Truccone A, Haji-Abdi F, Newman AH, Grundt P, Rice KC, Husbands SM, Greedy BM, Enguehard-Gueiffier C, Gueiffier A, Chen J, Wang S, Katz JL, Grandy DK, Sunahara RK, Woods JH. Proerectile effects of dopamine D2-like agonists are mediated by the D3 receptor in rats and mice. J Pharmacol Exp Ther. 2009;329:210–217. doi: 10.1124/jpet.108.144048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Truong YN, Levant B, Chen J, Wang S, Woods JH. Behavioral sensitization to cocaine in rats: evidence for temporal differences in dopamine D(3) and D (2) receptor sensitivity. Psychopharmacology (Berl) 2011;215:609–620. doi: 10.1007/s00213-010-2154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine Agonist-Induced Yawning in Rats: A Dopamine D3 Receptor-Mediated Behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Drug and Reinforcement History as Determinants of the Response-Maintaining Effects of Quinpirole in the Rat. J Pharmacol Exp Ther. 2007;323:599–605. doi: 10.1124/jpet.107.123042. [DOI] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Influence of conditioned reinforcement on the response-maintaining effects of quinpirole in rats. Behav Pharmacol. 2009;20:492–504. doi: 10.1097/FBP.0b013e328330ad9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Raaso H, Vanderschuren LJ. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Vanderschuren LJ. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology (Berl) 1999;143:254–260. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Driver-Dunckley E, Samanta J, Stacy M. Pathological gambling associated with dopamine agonist therapy in Parkinson’s disease. Neurology. 2003;61:422–423. doi: 10.1212/01.wnl.0000076478.45005.ec. [DOI] [PubMed] [Google Scholar]
- Driver-Dunckley ED, Noble BN, Hentz JG, Evidente VG, Caviness JN, Parish J, Krahn L, Adler CH. Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clin Neuropharmacol. 2007;30:249–255. doi: 10.1097/wnf.0b013e31804c780e. [DOI] [PubMed] [Google Scholar]
- Evans AH, Katzenschlager R, Paviour D, O’Sullivan JD, Appel S, Lawrence AD, Lees AJ. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19:397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Kang UJ, de Wit H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. J Clin Psychopharmacol. 2008;28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology (Berl) 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZZ, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. J Psychopharmacol. 2010;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman AJ. Impulse Control Disorder Behaviors Associated with Pramipexole Used to Treat Fibromyalgia. J Gambl Stud. 2009;25:425–431. doi: 10.1007/s10899-009-9123-2. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research CoLS, National Research Council. 7th Edition. The National Academies Press, The National Academies Press; 1996. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Joel D, Avisar A, Doljansky J. Enhancement of excessive lever-pressing after post-training signal attenuation in rats by repeated administration of the D1 antagonist SCH 23390 or the D2 agonist quinpirole, but not the D1 agonist SKF 38393 or the D2 antagonist haloperidol. Behav Neurosci. 2001;115:1291–1300. doi: 10.1037//0735-7044.115.6.1291. [DOI] [PubMed] [Google Scholar]
- Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, Gaal J, Le Foll B. The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacol. 2009:1–10. doi: 10.1017/S1461145709991064. [DOI] [PubMed] [Google Scholar]
- Kumor K, Sherer M, Jaffe J. Effects of bromocriptine pretreatment on subjective and physiological responses to i.v. cocaine. Pharmacol Biochem Behav. 1989;33:829–837. doi: 10.1016/0091-3057(89)90478-4. [DOI] [PubMed] [Google Scholar]
- Kurylo DD. Effects of quinpirole on operant conditioning: perseveration of behavioral components. Behav Brain Res. 2004;155:117–124. doi: 10.1016/j.bbr.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Kurylo DD, Tanguay S. Effects of quinpirole on behavioral extinction. Physiol Behav. 2003;80:1–7. doi: 10.1016/s0031-9384(03)00218-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Dekeyne A, Rivet JM, Dubuffet T, Lavielle G, Brocco M. S33084, a novel, potent, selective, and competitive antagonist at dopamine D(3)-receptors: II. Functional and behavioral profile compared with GR218,231 and L741,626. J Pharmacol Exp Ther. 2000;293:1063–1073. [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN. The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev. 2011;35:912–938. doi: 10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SS, Evans AH, Lees AJ. Dopamine dysregulation syndrome: an overview of its epidemiology, mechanisms and management. CNS Drugs. 2009;23:157–170. doi: 10.2165/00023210-200923020-00005. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1976;264:57–59. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The acquisition of responding with conditioned reinforcement: effects of pipradrol, methylphenidate, d-amphetamine, and nomifensine. Psychopharmacology (Berl) 1978;58:79–87. doi: 10.1007/BF00426794. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Koob GF. Pipradrol enhances reinforcing properties of stimuli paired with brain stimulation. Pharmacol Biochem Behav. 1978;8:219–222. doi: 10.1016/0091-3057(78)90308-8. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Influence of a conditioned light stimulus on cocaine self-administration in rats. Psychopharmacology (Berl) 2001;154:390–396. doi: 10.1007/s002130000608. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Terry P, Witkin JM, Katz JL. Pharmacological characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther. 1994;270:1041–1048. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Voon V, Fox SH. Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol. 2007;64:1089–1096. doi: 10.1001/archneur.64.8.1089. [DOI] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, de Souza M, Thomsen T, Fox S, Lang AE, Miyasaki J. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67:1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- Voon V, Potenza MN, Thomsen T. Medication-related impulse control and repetitive behaviors in Parkinson’s disease. Curr Opin Neurol. 2007;20:484–492. doi: 10.1097/WCO.0b013e32826fbc8f. [DOI] [PubMed] [Google Scholar]
- Weintraub D. Dopamine and impulse control disorders in Parkinson’s disease. Ann Neurol. 2008;2(64 Suppl):S93–S100. doi: 10.1002/ana.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Potenza MN. Impulse control disorders in Parkinson’s disease. Curr Neurol Neurosci Rep. 2006;6:302–306. doi: 10.1007/s11910-006-0022-y. [DOI] [PubMed] [Google Scholar]
- Wolterink G, Phillips G, Cador M, Donselaar-Wolterink I, Robbins TW, Everitt BJ. Relative roles of ventral striatal D1 and D2 dopamine receptors in responding with conditioned reinforcement. Psychopharmacology (Berl) 1993;110:355–364. doi: 10.1007/BF02251293. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ. Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther. 1984;230:678–683. [PubMed] [Google Scholar]