Abstract
Background
Transient Receptor Potential (TRP) channels are a superfamily of broadly expressed ion channels with diverse physiological roles. TRPC1, 3 and 6 are believed to contribute to cardiac hypertrophy in mouse models. Human mutations in TRPM4 have been linked to progressive familial heart block. TRPM7 is a divalent-permeant channel and kinase of unknown function, recently implicated in the pathogenesis of atrial fibrillation, however its function in ventricular myocardium remains unexplored.
Methods and Results
We generated multiple cardiac-targeted knock-out mice to test the hypothesis that TRPM7 is required for normal ventricular function. Early cardiac Trpm7-deletion (<E9, TnT/Isl1-Cre) results in congestive heart failure and death by E11.5 due to hypo-proliferation of the compact myocardium. Remarkably, Trpm7-deletion late in cardiogenesis (~E13, αMHC-Cre) produces viable mice with normal adult ventricular size, function and myocardial transcriptional profile. TRPM7 deletion at an intermediate time-point results in 50% of mice developing cardiomyopathy associated with heart block, impaired repolarization and ventricular arrhythmias. Microarray analysis reveals elevations in transcripts of hypertrophy/remodeling genes and reductions in genes important for suppressing hypertrophy (Hdac9) and for ventricular repolarization (Kcnd2) and conduction (Hcn4). These transcriptional changes are accompanied by action potential prolongation and reductions in transient outward current (Ito; Kcnd2). Similarly, the pacemaker current (If; Hcn4), is suppressed in atrioventricular nodal cells, accounting for the observed heart block.
Conclusions
TRPM7 is dispensable in adult ventricular myocardium under basal conditions, but is critical for myocardial proliferation during early cardiogenesis. Loss of TRPM7 at an intermediate developmental time-point alters the myocardial transcriptional profile in adulthood, impairing ventricular function, conduction and repolarization.
Keywords: ion channel, electrophysiology, heart block, cardiomyocyte hypertrophy, potassium channels
INTRODUCTION
Transient receptor potential (TRP) channels are a loosely related family of ion channels that share the properties of cation permeability and minimal voltage sensitivity. With respect to cardiac physiology and disease, most interest to date has focused on the TRPC channels (1/3/4/6), which by permeating calcium can activate calcineurin-NFAT mediated cardiac hypertrophy1, 2. TRPC7 and TRPM2, as well, are proposed to mediate myocardial apoptosis3 and oxidative stress-induced cardiomyocyte death, respectively. Human mutations in TRPM4 have been linked to progressive familial heart block type I (PFHBI)4 and isolated cardiac conduction block5, due to impaired TRPM4 SUMOylation and enhanced membrane trafficking. Some forms of triggered cardiac arrhythmia are also postulated to arise from gain-of-function mutations in TRPM46. Interestingly, deletion of TRPM4 in mice exhibits sustained hypertension secondary to enhanced catecholamine release from enterochromaffin cells7, but is not associated with changes in murine cardiac function or electrical activity.
TRPM7, and its close homolog TRPM6, are unique in that they are ion channels containing a carboxyl terminal kinase. TRPM7 is divalent-permeant (e.g., Zn2+, Mg2+, Ca2+), inhibited by cytoplasmic Mg2+ 8, and proposed to be important for magnesium homeostasis9 (but see refs 10, 11). Although TRPM7 is ubiquitously expressed, in comparison to other TRPs, it is especially abundant in both human and murine heart12, 13, and is concentrated in myocardium during embryonic development10. Recently, TRPM7 was shown to be up-regulated in atrial fibroblasts harvested from patients with atrial fibrillation (AF). These authors hypothesized that it provided a calcium influx pathway that induced TGF-β1 mediated fibroblast proliferation and differentiation, thereby contributing to atrial fibrosis in the pathogenesis of AF14. In neurons, TRPM7 was also proposed to mediate calcium influx contributing to neuronal death in ischemic stroke15. Cardiac ventricular fibroblasts have a large TRPM7 current16 and a TRPM6/7-like current has been recorded from pig and guinea-pig cardiomyocytes17. Thus, TRPM7 represents a fascinating, relatively unexplored signaling molecule in cardiac biology that may participate in calcium and/or magnesium signaling via the channel domain and possibly activate downstream signaling pathways via the kinase domain under native conditions or in disease states.
In this study, we show that TRPM7 is a functional current in freshly dissociated adult murine ventricular cardiomyocytes. The physiological consequences of cardiac-targeted TRPM7 deletion depend on the timing of TRPM7 disruption during cardiogenesis. Early TRPM7 deletion (Trpm7 TnT/fl) induces congestive heart failure in utero and embryonic lethality by embryonic day 11.5 (E11.5). By contrast, TRPM7 deletion in late cardiogenesis (Trpm7 αMHC/fl) has no impact on adult ventricular function, conduction, repolarization, or on the myocardial transcriptional profile. TRPM7 deletion at an intermediate time-point (Trpm7 αMHC/−) disrupts atrioventricular conduction, ventricular repolarization and ventricular function in adulthood, predisposing to both spontaneous and induced ventricular arrhythmias. Transcriptional analysis reveals reduced expression of several ion channel genes including Kcnd2, encoding the important repolarizing transient outward current (Ito) in ventricular cells, and Hcn4, which encodes the pacemaker current (‘funny current’; If) in the atrioventricular node (AVN) and conduction system. Consistent with these findings, both If and Ito are significantly reduced in Trpm7 αMHC/− AVN and ventricular myocytes, respectively. Taken together, these findings suggest that TRPM7 is required for a critical period in embryonic cardiac development. The timing of perturbations in TRPM7 function during cardiogenesis variably affects cardiac function, ranging from arrested cardiac development in utero, to longstanding changes in the myocardial transcriptional profile into adulthood, impairing adult ventricular function, repolarization and atrioventricular conduction.
METHODS
See online Supplement for full methods
Cardiac-targeted Trpm7 knock out mice
All animal procedures have been reviewed and approved by the Institutional Animal Care and Use Committee at Children’s Hospital Boston. Cardiac-targeted knockout mice were generated by crossing Trpm7fl/fl and Trpm7−/fl mice described previously10 with TnT-Cre, Isl1-Cre mouse lines (provided by Dr. W. Pu) and αMHC-Cre (provided by Dr. M. Schneider). For simplicity, Trpm7fl/fl × (TnT-Cre) is denoted as Trpm7TnT/fl, Trpm7+/fl × (TnT-Cre) as Trpm7TnT/+, Trpm7fl/fl × (Isl1-Cre) as Trpm7Isl1/fl, Trpm7fl/fl × (αMHC-Cre) as Trpm7 αMHC/fl, and Trpm7−/fl × (αMHC-Cre) as Trpm7 αMHC/−. Mice were maintained on 129/SvEvTac genetic background.
Embryology
Embryo gestational age was determined from timed matings, with noon of the day of the vaginal plug defined as day 0.5. Embryos were removed from pregnant females euthanized by cervical dislocation between E9-12. For bromodeoxyuridine (BrdU) labeling, pregnant mice were injected intraperitoneally with BrdU (100 μg/g body weight) 1 h before sacrifice and embryo extraction/fixation.
Echocardiography
Echocardiography was performed on conscious mice using a Vevo 2100 ultrasound machine equipped with either a 30 MHz or 40 MHz ultrasound probe (Visual Sonics).
Immunostaining ventricular myocytes and frozen embryonic sections
Freshly dissociated ventricular myocytes and embryonic sections were immunostained using rabbit anti-Troponin-I polyclonal antibody (Santa Cruz, 1:1000 of 200 μg/ml). For BrdU immunostaining of embryonic sections we used rat anti-Brdu monoclonal antibody (Abcam 1:1000 of 1 mg/ml).
Electrocardiography (ECG)
ECGs were performed in supine mice sedated using Avertin anesthesia. Dissection pins were placed subcutaneously in the right arm and left leg. The ECG signal was amplified and then digitized using a Digidata 1320 board (Molecular Devices) acquired with Clampex (pClamp9, Molecular Devices).
Cellular Electrophysiology
TRPM7 current was measured in isolated cardiomyocytes in the whole-cell configuration The voltage protocol (shown in figure insets) held the myocytes at 0 mV, stepped to +100 mV for 40 ms and then ramped down to −80 mV over 500 ms, holding at −80 mV for 40 ms before stepping back up to 0 mV. This protocol was repeated every 4-6 s and recordings continued for 10-15 min per cell until a steady-state TRPM7 current was obtained. Action potentials and potassium currents were measured as previously described44.
Microarray Analysis
Microarray analysis was performed using Affymetrix Mouse 1.0ST arrays at the Microarray Core Facility at Children’s Hospital Boston. Four arrays were used for each group, WT (Trpm7fl/fl: 4 hearts and Trpm7 αMHC/+: 4 hearts), Trpm7 αMHC/fl (8 hearts) and Trpm7 αMHC/− (8 hearts), and total RNA from 2 hearts were pooled for each array to reduce inter-mouse variability.
qRT-PCR expression analysis
Microarray results were validated by qRT-PCR using the same pooled RNA samples used for microarray analysis (n = 4) from WT and Trpm7 αMHC/− +HB groups. For Trpm7 αMHC/− −HB qRT-PCR, RNA from one heart was used per run (n = 4) and compared to the same WT group. Real-time PCR reactions were run in triplicate using the SYBR Green method (ROX as passive reference dye; Affymetrix) as previously described10.
Statistics
All data are represented as means ± S.E.M, unless otherwise specified. All p-values were calculated using the non-parametric Mann-Whitney test when comparing two groups. For multiple comparisons among multiple groups, as in Figure 3 and Supplemental Figure 3, we used the non-parametric version of the ANOVA or Kruskal-Wallis (K-W) test. If the p value from the K-W test was lower than 0.05 we performed Dunn’s multiple comparisons test. A p-values less than 0.05 were considered statistically significant.
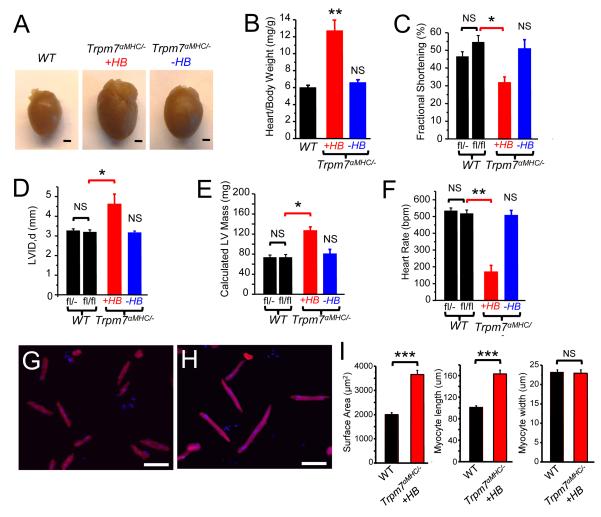
Figure 3.
Dilated cardiomyopathy in Trpm7 αMHC/− mice. (A) Hearts extracted from Trpm7fl/fl(WT) and Trpm7 αMHC/− mice with heart block (+HB) and without heart block (−HB). Scale bar, 1mm. (B) Heart weight to body weight ratios of WT (n = 15), Trpm7 αMHC/− +HB (n= 7) and Trpm7 αMHC/− −HB (n = 10). Fractional shortening (C), left ventricular dimension in diastole (LVID, d) (D), calculated corrected left ventricular mass (E), and heart rate (F), on transthoracic echocardiography in 4 week old WT controls (Trpm7fl/−, n = 10; Trpm7flfl, n=9, black bars) compared to litter-mate Trpm7 αMHC/− mice with HB (red bar, n = 4) and without HB (blue bar, n = 5) . (G) WT and (H) Trpm7 αMHC/− +HB ventricular cardiomyocytes enzymatically dissociated, fixed (4% PFA) and immunostained for cardiac troponin (TnI: red). Nuclei stained with DAPI (blue). Scale bar, 100 μm. (I) Quantification of cardiomyocyte surface area (n = 46), length (n = 49) and width (n = 52) from WT and Trpm7 αMHC/− +HB (n = 49/35/35). * p < 0.05, ** p < 0.01, *** p < 0.001 compared to age matched WT (Trpm7fl/fl) NS; not statistically significant.
RESULTS
TRPM7 current in adult mouse ventricular myocytes
We previously showed that TRPM7 is predominantly expressed in the heart of E9.5 embryos10, and then becomes ubiquitously expressed in later embryonic development10 and into adulthood12, 13 . TRPM6/7–like currents have been recorded in pig, guinea pig and rat ventricular cardiomyocytes 17 but the molecular identity of this current was not determined. To confirm that this current arises from Trpm7, we first recorded TRPM7-like current from isolated murine adult ventricular cardiomyocytes by applying ramps from +100 to −80 mV over 500 ms from a holding potential of 0 mV. TRPM7-like current on “break-in” is initially absent, but then runs-up over the course of 10-15 minutes to a maximum amplitude at steady state (Figure 1A). The initial currents measured immediately after patch rupture were largely background currents, as these were not inhibited by 10 mM MgCl2 (TRPM7 is inhibited by internal Mg2+; KD~0.1-0.6 mM18). To eliminate the contribution of these background currents from the TRPM7-like current, either Mg2+-inhibited, or “break-in” traces were subtracted from net current (Figure 1B), revealing the outwardly rectifying current-voltage relationship characteristic of TRPM7.
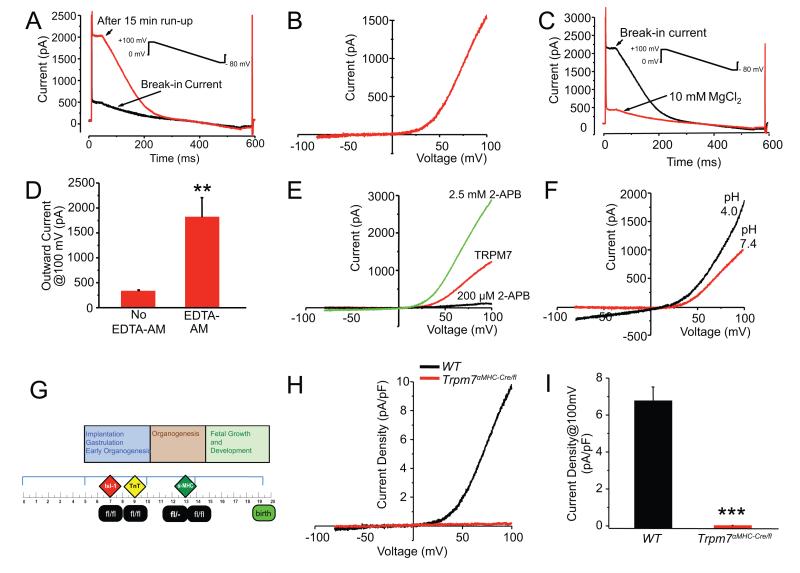
Figure 1.
Trpm7 current in freshly isolated mouse ventricular myocytes. (A) Current vs. time in response to 500 ms voltage ramp from +100 mV to −80 mV (after the initial 40 ms at +100 mV). Black trace; initial current immediately after establishing whole-cell configuration (break-in current). Over the course of 15 min, an outwardly rectifying current develops (red trace). (B) Characteristic outwardly-rectifying TRPM7 current trace derived from subtraction of break-in current (or current in 10 mM MgCl2, as in C) from the steady-state current that develops after ~15 min. (C) Break-in current measured from a mouse ventricular myocyte after incubation in 30 μM EDTA-AM for 30 min (black trace) and after application of 10 mM MgCl2 (red trace). (D) Mean outward current at +100 mV on break-in with (1813 ± 394 pA, n = 5) and without (327 ± 28 pA, n = 7) 30 μM EDTA-AM incubation. (E) Response of TRPM7-like current (red) to 200 μM (black) and 2.5 mM (green) 2-APB. (F) Potentiation of TRPM7-like current by low extracellular pH. (G) A panel of Cre-expressing alleles was used to drive recombination at varying times in cardiac development: Islet1 (Isl1-Cre), Troponin-T (TnT-Cre), and α-myosin heavy chain (αMHC-Cre). (H, I) Trpm7 current is eliminated in all Trpm7 αMHC/fl ventricular myocytes compared to WT. Mean current densities at +100 mV, in Trpm7 αMHC/fl and WT myocytes. ** p < 0.01, *** p < 0.001.
The gradual run-up of TRPM7 after patch rupture is a feature attributed to either gradual chelation of internal inhibitory Mg2+ 18 or dialysis-mediated removal of a diffusible inhibitor. To test whether Mg2+ chelation alone would be sufficient to activate TRPM7 in the absence of patch rupture, cardiomyocytes were incubated in 30 μM ethylenediaminetetraacetic acid-aminoester (EDTA-AM), a membrane-permeant form of the divalent cation chelator, EDTA, for 30 min prior to patch-clamp studies. Under these conditions, EDTA-AM treated myocytes yield large outwardly rectifying currents immediately upon break-in (no ‘run-up’ period required) and these currents are fully inhibited by 10 mM MgCl2 (Figure 1C, D) leaving only the background currents typically seen on break-in under standard whole-cell patch-clamp conditions (Figure 1A). These results suggest that dialysis and chelation of intracellular Mg2+ alone is sufficient to activate TRPM7 under whole-cell patch clamp conditions19.
In addition to inhibition by Mg2+, TRPM7 is potently inhibited by μM concentrations of 2-Aminoethyl diphenylborinate (2-APB) and potentiated by mM concentrations of 2-APB; in contrast, 2-APB only potentiates TRPM620. Consistent with this pharmacology, TRPM7-like currents measured from mouse cardiomyocytes were noted to be substantially inhibited by 200 μM 2-APB, but potentiated by 2.5 mM 2-APB (Figure 1E). Also, consistent with TRPM7, these currents were noted to exhibit the same dependence on external pH as previously described21 (Figure 1F). Collectively, these data strongly suggest that the outwardly rectifying TRPM7-like current measured in mouse ventricular cardiomyocytes is indeed TRPM7.
Generation of cardiac-restricted TRPM7 deletion in myocardium
To circumvent the embryonic lethality associated with global deletion of Trpm79, 10, we generated mice with cardiac-restricted Trpm7 deletion using Cre-loxP technology. The conditional Trpm7 allele contained loxP sites flanking exon 1710 (Trpm7fl/fl). Cre-mediated recombination induces a frameshift that prevents expression of the ion channel and kinase domains of TRPM710. We used a panel of Cre-expressing alleles that drive recombination at varying times in cardiac development (Figure 1G, see Methods): Islet1 (Isl1-Cre, ~E722, Trpm7 Isl1/fl), Troponin-T (TnT-Cre, ~E923, Trpm7 TnT/fl), and α-myosin heavy chain (αMHC-Cre, ~E12.5 24, Trpm7 αMHC/fl and Trpm7 αMHC/−).
We first evaluated α-MHC-Cre recombinase expression patterns by crossing αMHC-Cre mice with the mT/mG reporter mouse line (ROSA26mTmG), in which membrane-targeted green fluorescent protein expression (mG) is induced only after Cre-mediated recombination25. Cre recombinase was expressed throughout the ventricular myocardium in a cardiomyocyte-restricted manner (Supplemental Figure 1A). Effective deletion of Trpm7 exon 17 in myocardium is evident by PCR at postnatal day 1 in Trpm7 αMHC/fl mice (Supplemental Figure 1B) based on the presence of the expected size of the deletion product in cardiac genomic DNA. TRPM7 current is absent in enzymatically dissociated adult Trpm7 αMHC/fl cardiomyocytes (0.003 ± 0.03 pA/pF, n = 19 vs. 6.8± 0.8 pA/pF at +100 mV, n = 10 in Trpm7fl/fl (WT) myocytes; Figure 1H, I). These data confirm that TRPM7 protein is eliminated in Trpm7 αMHC/fl ventricular cardiomyocytes and establishes the identity of this Mg2+-inhibitable outwardly rectifying current as TRPM7.
Trpm7 is required for early embryonic cardiac development but dispensable in adult myocardium
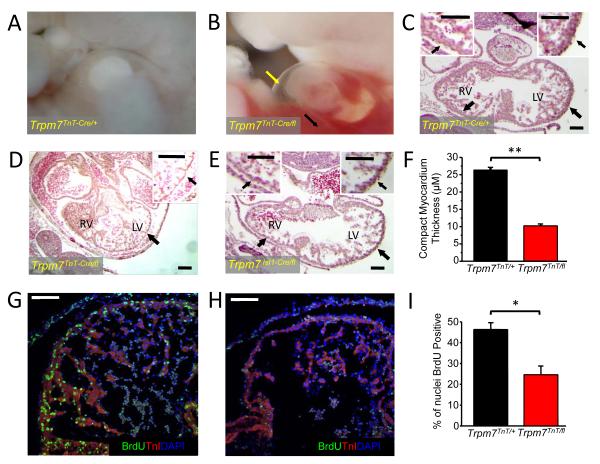
Based on our previous findings that TRPM7 was largely expressed in early embryonic myocardium10, we examined the effects of Trpm7 deletion at early (<E9, Trpm7 Isl1/fl, Trpm7 TnT/fl) and late (> E12.5, Trpm7 αMHC/fl) stages of cardiac development. TnT-Cre recombines in the ventricle at approximately E923 (Supplemental Figure 1C) while Isl1-Cre recombines in the precursors of the secondary heart field (E7) that give rise to the right ventricle (RV) and right ventricular outflow tract22 (Supplemental Figure 1D). αMHC-Cre deletes in ventricle at after E12.524. We find no Trpm7 TnT/fl or Trpm7 Isl1/fl mice survive to birth. Deletion of Trpm7 exon 17 in genomic DNA isolated from E9 Trpm7 TnT/fl embryos confirms deletion at this time point (Supplemental Figure 1E). By embryonic day 10.5-11.5, both Trpm7 TnT/fl and Trpm7 Isl1/fl develop large pericardial effusions and blood congestion in the liver (Figure 2A, B; TnT-Cre shown), and die in utero of congestive heart failure by E11.5. Histological examination of E10.5-11 embryos reveals severe thinning of the compact myocardium and impaired ventricular septation in both genotypes (Figure 2C-F), with RV compact myocardium thinning predominant in Trpm7 Isl1/fl embryos (Figure 2E). As thinned compact myocardium may result from either reduced proliferation or increased apoptosis, we next assessed cardiomyocyte proliferation by bromodeoxyuridine uptake (BrdU) and apoptosis by TUNEL assay. Immuno-stained Trpm7 TnT/fl embryos show significantly reduced nuclear BrdU uptake in the compact myocardial layer as compared to Trpm7 TnT/+ controls (Figure 2G-I). In contrast, we detect no differences in myocyte apoptosis in Trpm7 TnT/fl hearts compared to Trpm7 TnT/+ controls (Supplemental Figure 2), suggesting that impaired myocardial proliferation is the primary mechanism responsible for thinned myocardium and congestive heart failure upon early embryonic cardiac-targeted Trpm7 deletion.
Figure 2.
Trpm7TnT/fl and Trpm7Isl1/fl mice develop severe thinning of the compact myocardium and die of congestive heart failure in utero. E10.5 WT embryo (A) compared with age-matched Trpm7TnT/fl embryo (B). Note pericardial effusions (yellow arrow) and hepatic congestion (black arrow) consistent with congestive heart failure in E10.5 Trpm7TnT/fl mice. Hematoxylin-Eosin stain of Trpm7 Trpm7TnT/+ (C), Trpm7TnT/fl (D) and Trpm7Isl1/fl (E) heart sections. Trpm7TnT/fl and Trpm7Isl1/fl hearts show severe thinning of the compact myocardium (D, E, black arrows). Insets in C-E show expanded views of right and left ventricular walls (RV and LV). (F) Mean data of compact myocardium thickness in Trpm7TnT/+ (n = 188, from 3 hearts) and Trpm7TnT/fl (n = 300, from 3 hearts). Heart cryosections from (G) Trpm7TnT/+ and (H) Trpm7TnT/fl embryos immunostained with cardiac Troponin-I (TnI: red) and bromodeoxyuridine (BrdU: green). Nuclei stained with DAPI (blue). (I) Percent of BrdU positive nuclei are significantly reduced in Trpm7TnT/fl compared to Trpm7TnT/+ controls (n = 4 hearts each). Scale bar, 100 μm. * p < 0.05, ** p < 0.01.
Remarkably, we find that all Trpm7 αMHC/fl mice survive to adulthood and exhibit entirely normal ventricular morphology and function, under basal conditions, as assessed in 4 to 6 week-old mice (Supplemental Table 1). These data suggest that Trpm7 is critical for early events in embryonic cardiac development but is dispensable in late embryonic or adult myocardium.
The timing of cardiac TRPM7 deletion variably disrupts adult ventricular function, conduction and repolarization
To further probe the importance of Trpm7 in the developmental period E9-E12.5, we generated an additional line of mice: Trpm7 αMHC/−. This line is expected to delete Trpm7 slightly earlier in embryonic development than Trpm7 αMHC/fl, as there exists only one allele for Cre-mediated excision. Interestingly, this subtle difference in the timing of TRPM7 disruption results in significantly different cardiac phenotypes. Trpm7 αMHC/− mice exhibit a bimodal phenotype in which a subset of mice (12/30; 40%) develop a dilated cardiomyopathy (Figure 3A). This cardiomyopathy is associated with heart block (HB), as noted in the ECG during the echocardiogram (see Movies 1 and 2), and is independent of age. Heart weight: body weight ratios (Figure 3B), systolic function (Figure 3C), left ventricular size (Figure 3D) and calculated LV mass (Figure 3E) are all significantly abnormal in those Trpm7 αMHC/− mice with HB (Trpm7 αMHC/− +HB) as early as 3-4 weeks of age. Trpm7 αMHC/− mice without heart block (Trpm7 αMHC/− −HB) exhibit normal heart size, ventricular function and morphology (Figure 3AF, Movie 3). Isolation of ventricular myocytes from TRPM7fl/fl (WT) and Trpm7 αMHC/− +HB hearts confirm that cardiomegaly is due to cardiac myocyte hypertrophy (Figure 3G-H) as Trpm7 αMHC/− +HB myocytes are nearly two-fold larger by surface area (AreaWT = 2002 ± 82 μm2, n = 46; Area Trpm7αMHC/− +HB = 3652 ± 176 μm2, n = 49, p < 0.001; Figure 3I), and this increase in size is mediated primarily by an increase in myocyte length (LengthWT = 101 ± 3.0 μm, n = 49; Length Trpm7αMHC/− +HB = 163 ± 7.0 μm, n = 35, p < 0.001), while myocyte width is unchanged. This eccentric myocyte hypertrophy is consistent with the phenotype of dilated cardiomyopathy in Trpm7 αMHC/− hearts. In addition to cardiomyocyte hypertrophy, we also note significant interstitial extracellular matrix deposition and interstitial fibrosis on histological examination of Trpm7 αMHC/− hearts at 6-8 months of age (Supplemental Figure 3). Despite this pathology in Trpm7 αMHC/− mice, there is no significant difference in mortality observed out to 1 year of age as compared to littermate controls, including Trpm7 αMHC/fl, Trpm7 αMHC/+, and Trpm7fl/fl mice.
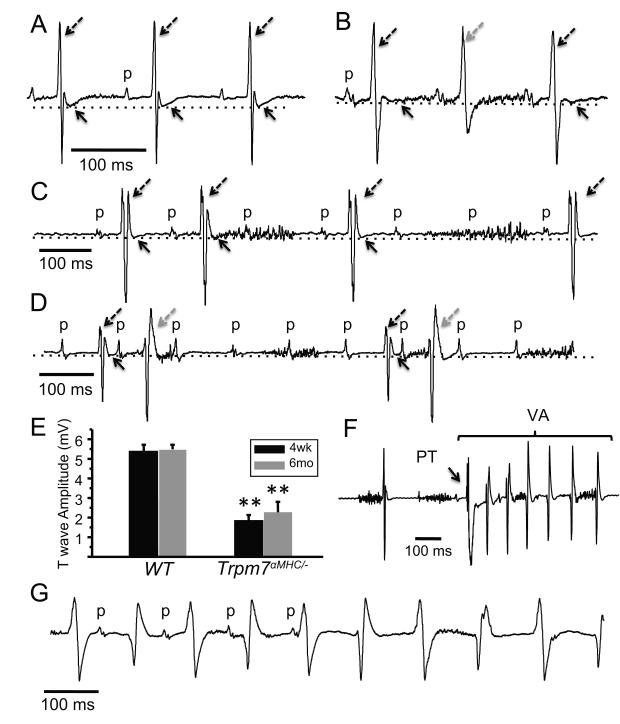
To better examine the electrophysiological defects in Trpm7 αMHC/− mice noted on echocardiography, we next performed sedated ECG’s (Figure 4). We observe atrioventricular block in the same subset of mice with cardiomyopathy, ranging from second degree Mobitz Type 2 block with 2:1 conduction (Figure 4B), to 3:2/3:1 conduction (Figure 4C), to 5:1 conduction with coupled premature ventricular complexes (Figure 4D). Such severe heart block did not develop progressively, as it was evident in Trpm7 αMHC/− mice as early as 3-4 weeks of age with similar frequency (9/18; 50%). T waves are significantly flattened, and consequently QT intervals appear prolonged in normally conducted beats of the affected subpopulation of Trpm7 αMHC/− mice (Figure 4A, B-D, E). Furthermore, bidirectional ventricular arrhythmia can be reproducibly induced by precordial ‘thump’ in sedated Trpm7 αMHC/− mice with severe AV block and impaired ventricular repolarization (Figure 4F). Finally, ventricular arrhythmia is observed to occur spontaneously in some severely affected Trpm7 αMHC/− mice (Figure 4G, 4/12 mice; 33%). However, no sudden cardiac deaths occur in even the most severely affected mice.
Figure 4.
Trpm7 αMHC/− mice exhibit high-grade atrioventricular block, T wave flattening, and ventricular arrhythmias. (A) Normal sinus rhythm (p denote P waves; atrial depolarization) with intact atrioventricular conduction and normal ventricular repolarization (T wave amplitude) (dotted line, solid arrows) in the ECG of a sedated WT mouse. (B) 2:1 (C) 3:2/3:1 and (D) 5:1 second degree atrioventricular block and reduced T wave amplitude (dotted line, solid arrows) in ECGs of sedated Trpm7 αMHC/− mice: broken black arrow = conducted beat, broken grey arrow = ectopic ventricular beat/fusion beat. (E) T wave amplitude is depressed in Trpm7 αMHC/− (n = 8) compared to WT (n = 6) mice at both 4 weeks- and 6 months-of-age. (F) Ventricular arrhythmia (VA) induced by mechanical stimulation (PT) on a dispersed T wave in a Trpm7 αMHC/− mouse. (G) Sustained bidirectional ventricular arrhythmia (VA) captured in 30% of affected Trpm7 αMHC/− mice. VA episodes typically lasted 30-100 beats and spontaneously terminate. ** p < 0.01.
Myocardial Mg2+ and Zn2+ are unchanged after Trpm7 ablation
Since TRPM7 is known to permeate divalent cations and has been proposed to regulate intracellular magnesium9 and zinc26 homeostasis, it is possible that the phenotypes in Trpm7 αMHC/− mice arise from myocardial deficiencies of these divalents. To examine this possibility, we measured total cellular magnesium and zinc content (using Inductively-Coupled Mass Spectrometry; ICP-MS) in ventricular tissue from Trpm7fl/fl (WT), Trpm7fl/− and Trpm7 αMHC/− mice. We detect no differences in total cellular Mg2+ or Zn2+ between these groups (Supplemental Figure 4), confirming that neither myocardial hypomagnesemia nor hypozincemia occurs after cardiac-deletion of TRPM7, and thus are not associated with the cardiomyopathy or electrophysiological disorders observed in Trpm7 αMHC/− mice.
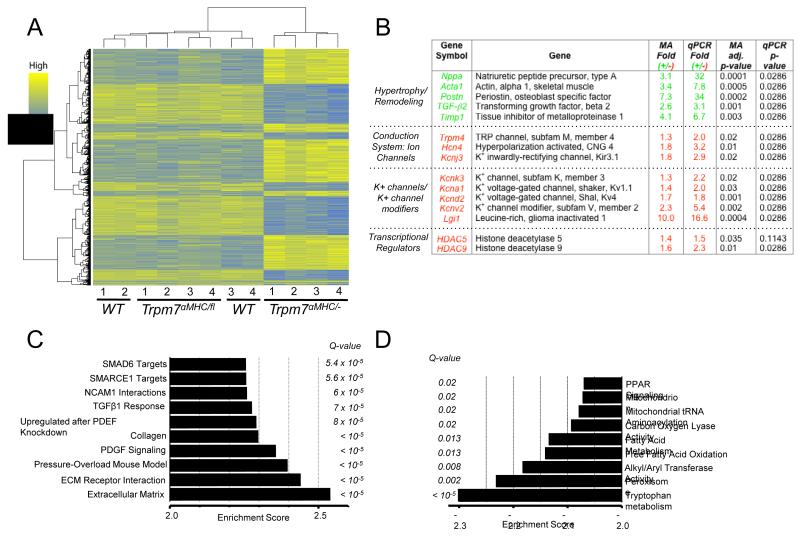
Microarray analysis of Trpm7 αMHC/− ventricular tissue
To search for putative pathways and biological processes induced by deletion of Trpm7, we performed microarray analysis on 3-5 week old WT, Trpm7 αMHC/fl and Trpm7 αMHC/− hearts. Strikingly, we found no significant transcriptional changes between WT and the later cardiac Trpm7 knockout, Trpm7 αMHC/fl (Figure 5A). Conversely, we found that ~1300 genes were differentially expressed based on a criterion of at least a 1.3-fold change and an adjusted p-value < 0.05 when comparing Trpm7 αMHC/− to WT (Figure 5A, Supplemental Table 2). Fifteen differentially expressed genes of interest were then chosen to validate the microarray by qRTPCR (Figure 5B). Significant changes in gene expression are consistent with the phenotype of cardiac hypertrophy/ventricular remodeling (Nppa, Acta1, Timp1, Postn), as well as electrical remodeling of both conduction system (Trpm4, Hcn4, Kcnj3), and ventricular cells (Kcnv2, Kcnd2, Kcnk3, Kcna1, Lgi1). Interestingly, pivotal transcriptional regulators of myocyte differentiation and cardiac hypertrophy (Hdac5, Hdac9) are also noted to be down-regulated. Using Gene Set Enrichment Analysis (GSEA, using http:// www.broadinstitute.org/gsea) we found Trpm7 αMHC/− hearts to be enriched in gene expression patterns pertaining to embryonic development, cellular proliferation and cellular differentiation pathways (Figure 5C; TGF-β1 response, SMAD6 targets, SMARCE1 target, PDGF signaling). Consistent with the phenotype of cardiomyopathy, we also observed the expected enrichment in genes up-regulated in the extracellular matrix (ECM), ECM receptor interactions, and pressure-overload murine heart disease. Genes downregulated in Trpm7 αMHC/− hearts compared to WT (or negative enrichments) clustered to metabolic pathways (tryptophan, fatty acid, lipid, peroxisome proliferator-activated receptor (PPAR) signaling) and mitochondrial function (Figure 5D).
Figure 5.
Myocardial transcriptional profile is altered in Trpm7 αMHC/− ventricular tissue. (A) Heat map displaying 2-way hierarchical clustering and expression levels of differentially expressed genes in WT, Trpm7 αMHC/fl and Trpm7 αMHC/− hearts (each column 1-4 represents RNA pooled from 2 hearts). ~1300 genes were differentially expressed in Trpm7 αMHC/− hearts based on an adjusted p-value < 0.05 and a > 1.3-fold change relative to WT. (B) Selected up-regulated and down-regulated genes from microarray (MA) analysis and validation by qPCR. Green (+): fold upregulated and Red (−): fold down regulated. (C-D). Gene Set Enrichment Analysis (GSEA) revealing pathways and processes enriched in Trpm7 αMHC/− compared to WT (C) and negatively enriched (D). Q-value: False Discovery Rate (FDR) adjusted p-value. Q < 0.05 is statistically significant.
Taken together, these findings show that cardiac phenotypes resulting from disruption of Trpm7 arise primarily from perturbations in early embryonic ventricular development, not from altered global cellular Mg2+ or Zn2+ levels in adulthood, nor from a requirement of Trpm7 in normal adult cardiomyocyte physiology, under basal conditions.
Trpm7 deletion silences Hcn4 expression and If in atrioventricular node
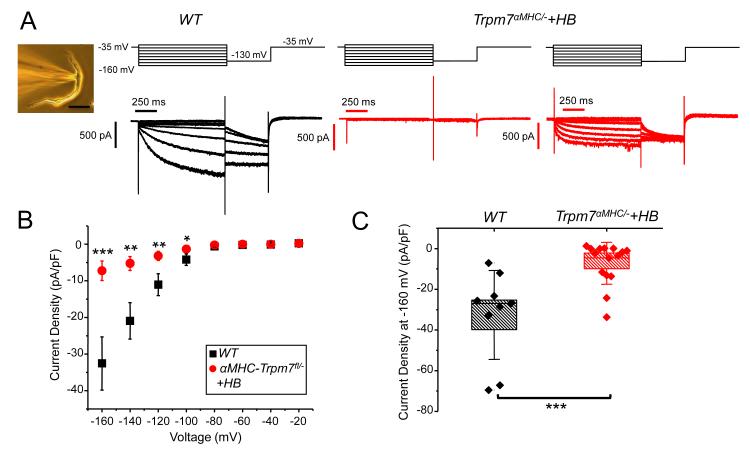
To elucidate the molecular mechanism for atrioventricular block induced by cardiac Trpm7 deletion we focused on ion channels downregulated in Trpm7 αMHC/− hearts that are enriched in atrioventricular node and are thought to be important for the normal activity of the cardiac conduction system. Hcn4 encodes a hyperpolarization-activated cyclic nucleotide gated channel and is the predominant Hcn isoform in the cardiac conduction system. It is the primary molecular determinant of If and is important for pacemaking and atrioventricular node function. Indeed, inducible cardiac-targeted deletion of Hcn4 in mouse results in severe heart block27. We measured the If current in freshly isolated atrioventricular node (AVN) cells from WT and Trpm7 αMHC/− +HB hearts by whole-cell patch clamp (Figure 6, inset) to determine whether reductions in Hcn4 mRNA from whole ventricle reflect reductions in If current in AVN. We find robust If currents in all AVN cells isolated from WT mice (If, WT = −32.6 ± 7.3 pA/pF at −160 mV, n = 9) (Figure 6A, left, B,C). In Trpm7 αMHC/− +HB hearts, If is on average significantly diminished in AVN cells (If, Trpm7αMHC/− +HB = −7.3 ± 2.7 pA/pF at −160 mV, n = 15; Figure 6B, C), and remarkably absent in some (6/15) (Figure 6A, center). Notably, of the subset of AVN cells from Trpm7 αMHC/− +HB with measurable If, a few have significantly increased activation kinetics (Figure 6A, right), which is suggestive of If encoded by Hcn2 and/or Hcn128. Since Hcn1, Hcn2 and Hcn4 all contribute to If in heart, down regulation of Hcn4 is anticipated to uncover the component of If provided by Hcn1 and Hcn2, as seen previously in an Hcn4 knockout model29. Taken together these data suggest that deletion of Trpm7 in heart suppresses Hcn4 expression and If current in AVN cells resulting in atrioventricular block.
Figure 6.
Trpm7 deletion significantly diminishes If in isolated atrioventricular nodal cells. (A) Inset: patch-clamped atrioventricular nodal cell (AVN): AVN were identified by their characteristic spindle-like morphology and lower cell capacitance, ranging from 20-40 pF (CapAVN-WT = 31 ± 2 pF, n= 9; CapAVN−KO+HB = 36 ± 4, n = 15, p = 0.4). Hyperpolarization-activated current traces, If, from WT (black: left) and Trpm7 αMHC/− +HB (red: center, right). Voltage protocols are shown above current traces. If was abolished in several AVN cells from Trpm7 αMHC/− +HB (6/15, center). In some AVN cells from Trpm7 αMHC/− +HB, If had more rapid activation kinetics consistent with If contributed by Hcn2 (right). (B) If current-voltage relationship from AVN of WT (■, n = 9) and Trpm7 αMHC/− +HB (●, n = 15). If is significantly diminished in Trpm7 αMHC/− +HB AVN compared to WT AVN at multiple membrane potentials. Error bars represent standard error of the mean. (C) Box plots with overlying data points showing the distribution of If current densities at −160 mV from WT (black, n = 9) and Trpm7 αMHC/− +HB (red, n = 15) AVN cells. In box plots, error bars represent the standard deviation of the mean. Box height represents the standard error. * p < 0.05, ** p < 0.01, *** p < 0.001.
Trpm7 deletion prolongs action potentials in ventricular cells via reductions in transient outward current
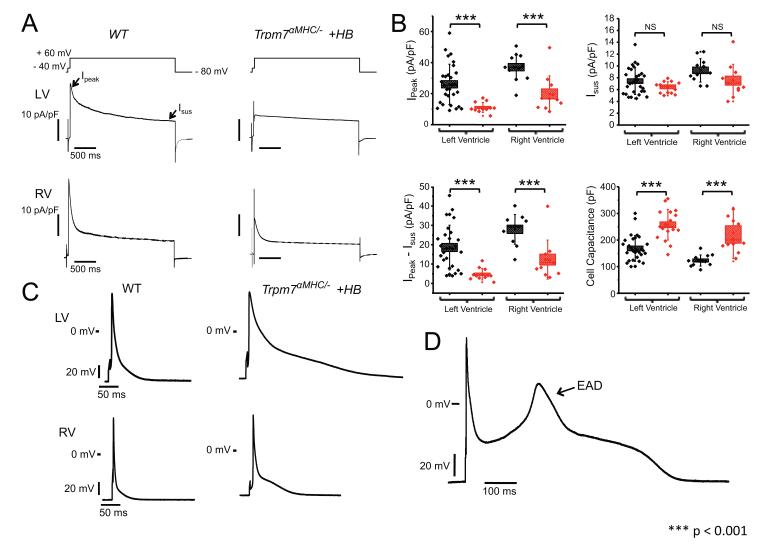
The T wave flattening on sedated ECGs of Trpm7 αMHC/− mice suggests a defect in ventricular repolarization. Transcriptional analysis of Trpm7 αMHC/− ventricular tissue revealed significant down-regulation of numerous potassium channel genes (Kcnd2, Kcnk3, Kcna1) and potassium channel modifier genes (Kcnv2, Lgi1) 30, 31. Of these genes, Kcnd2, encoding the transient outward current (Ito), is the most prominent outward current in murine ventricular cells32, dominating ventricular repolarization33. Indeed, eliminating Ito or its transmural gradient in mouse is sufficient to induce QT prolongation, T wave flattening and triggered ventricular tachycardia34. Accordingly, we measured Ito in freshly dissociated ventricular myocytes from WT and Trpm7 αMHC/− +HB. We find that total peak outward potassium current (Ipeak) and Ito (Ipeak − Isus, see Methods) measured at +60 mV is significantly diminished in Trpm7 αMHC/− +HB ventricular cells (Ipeak-LV, Trpm7αMHC/− +HB = 11 ± 0.9 pA/pF, n = 12; Ito-LV, Trpm7αMHC/− +HB = 5 ± 0.9 pA/pF, n = 12) compared to WT (Ipeak-LV, WT = 26 ± 2.4 pA/pF, n = 29; Ito-LV, WT = 19 ± 2.1, n = 29, p <0.001) (Figure 7A&B). The sustained component, Isus, contributed by delayed rectifiers32 is not different (Isus-LV, WT = 7 ± 0.4 pA/pF, n = 29; Isus-LV, Trpm7αMHC/− +HB = 6 ± 0.3 pA/pF, n = 12, p = 0.1), supporting the notion that the transient outward currents specifically, and not all outward potassium currents, are diminished in Trpm7 αMHC/− +HB myocytes. Furthermore, the broad distribution of Ipeak and Ito current densities observed in ventricular myocytes from WT hearts, reflecting the normal transmural gradient of Ito, appears to be abolished in Trpm7 αMHC/− +HB hearts (Figure 7B). As a result of Ito down regulation, action potentials (AP) measured in Trpm7 αMHC/− +HB myocytes from both LV and RV are significantly prolonged compared to WT (Figure 7C, Table 1). Furthermore, in a number of Trpm7 αMHC/− +HB myocytes with prolonged APs, early afterdepolarizations (EAD) are observed (Figure 7D). These EADs may provide triggers for spontaneous ventricular arrhythmias observed in Trpm7 αMHC/− +HB mice (Figure 3G). As TRPM7 mediates an outward current at depolarized membrane potentials (0 to +50 mV), it is possible that this may contribute directly to AP repolarization. However, in Figure 1 we showed that prior to myocyte dialysis, TRPM7 current (“break-in” current) is inactive under basal conditions. Indeed, consistent with this notion, under physiological conditions, we find that Trpm7 αMHC/fl ventricular AP properties are not different from WT (Table 1). These data suggest that TRPM7 contributes minimally in a direct fashion to AP repolarization under basal conditions. Instead, the pronounced AP changes seen in Trpm7 αMHC/− myocytes result indirectly from reductions in Kcnd2 expression and Ito. Taken together, these results establish a cellular mechanism for both the impairment in ventricular repolarization and ventricular tachycardia observed in Trpm7 αMHC/− mice (Figure 3).
Figure 7.
Transient outward current is diminished and action potentials prolonged in ventricular cells from Trpm7 αMHC/− mice with heart block. (A) Outward potassium current recorded from WT (left) and Trpm7 αMHC/− +HB (right) ventricular myocytes from left ventricle (LV: top) and right ventricle (RV: bottom) in response to a 2.5 s voltage step to +60 mV after a 50 ms step to −40 mV from a holding potential of −80 mV. Peak outward potassium current (Ipeak) and sustained outward potassium current (Isus) are indicated (arrows). (B) Box plots with overlying data points showing the distribution of Ipeak (top left), Isus (top right), Ipeak − Isus (Ito, bottom left) current densities at +60 mV and cell capacitance (bottom right) from WT (black, n = 29, LV; n = 11, RV) and αMHC-Cre-Trpm7fl/− +HB (red, n = 12-17, LV; n = 11, RV)) left and right ventricular myocytes. (C) Representative action potentials measured in current-clamp mode from WT (left) and Trpm7 αMHC/− +HB (right) from LV (top) and RV (bottom). (D) Representative action potential with early after-depolarization (EAD) in a Trpm7 αMHC/− +HB myocyte. Error bars on box plots represent the standard deviation of the mean. Box height represents the standard error. *** p < 0.001.
Table 1.
Action potential (AP) parameters of isolated ventricular myocytes from WT, Trpm7 MHC/fl, Trpm7 MHC/− +HB and Trpm7 MHC/− −HB hearts. APDx indicates AP duration to x% repolarization from the peak of the AP. RMP is myocyte resting membrane potential. Peak is the maximum AP membrane potential.
| Left Ventricle | |||||
|---|---|---|---|---|---|
| WT | Trpm7 MHC/fl | Trpm7 MHC/− +HB |
Trpm7 MHC/− −HB |
n | |
| APD30 (ms) | 2.4±0.3 | 2.6±0.3 | 6.5±1.6*** | 7.1±3.0** | 33/24/18/19 |
| APD50 (ms) | 5.6±0.8 | 6.2±0.9 | 22.2±7.0*** | 20.0±8.0** | 33/24/18/19 |
| APD70 (ms) | 10.8±1.4 | 11.3±1.6 | 100.3±41.1*** | 34.4±11** | 33/24/18/19 |
| APD90 (ms) | 35.8±3.7 | 43.4±5.1 | 207.6±50.5*** | 81.4±13.8*** | 33/24/18/19 |
| RMP (mV) | −71.6±0.7 | ND | −70.3±0.6 | −74.0±0.7* | 33/24/18/19 |
| Peak (mV) | 55.3±1.0 | ND | 57.9±0.6 | 51.7±2.7 | 33/24/18/19 |
| Right Ventricle | |||||
| WT | Trpm7αMHC/fl |
Trpm7αMHC/−
+HB |
Trpm7αMHC/−
−HB |
n | |
| APD30 (ms) | 1.4±0.1 | ND | 3.0±0.5** | ND | 13/13 |
| APD50 (ms) | 2.7±0.3 | ND | 7.0±1.4** | ND | 13/13 |
| APD70 (ms) | 5.6±0.8 | ND | 14.8±3.0** | ND | 13/13 |
| APD90 (ms) | 16.9±2.4 | ND | 71.8±15.6** | ND | 13/13 |
| RMP (mV) | −71.9±0.9 | ND | −72.8±0.3 | ND | 13/13 |
| Peak (mV) | 55.6±1.4 | ND | 56.1±0.8 | ND | 13/13 |
P<0.05;
P<0.01;
P<0.001; compared to WT. ND, not determined. n, indicates number of myocytes in each group in order of columns from left to right.
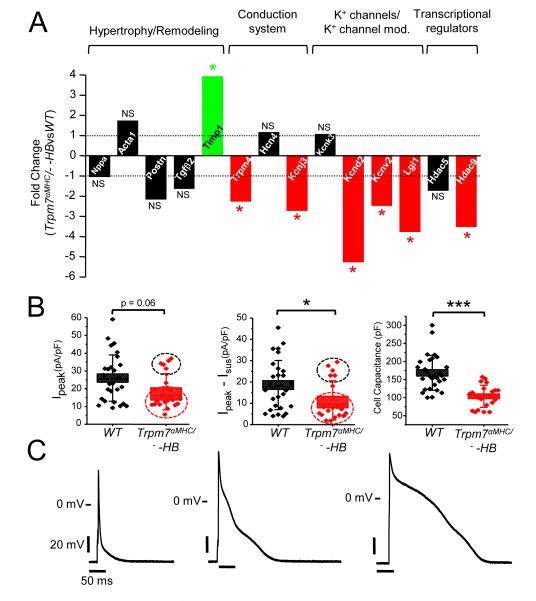
Since down regulation of Kcnd2 and Ito are well known to occur in the setting of cardiac hypertrophy33, it is possible that these effects of Trpm7 deletion on Kcnd2 expression, Ito current and myocyte repolarization may be secondary to the hypertrophic response. To dissect out the contributions of cardiac hypertrophy from the effects of Trpm7 deletion on myocardial gene expression we next examined, by qRT-PCR, expression levels of the same subset of differentially expressed genes in Trpm7 αMHC/− mice without heart block (Trpm7 αMHC/− −HB) since these hearts are not hypertrophied (Figure 8A). Consistent with the absence of cardiac hypertrophy in these Trpm7 αMHC/− −HB mice, hypertrophy/remodeling genes previously increased in Trpm7 αMHC/− +HB are largely unchanged, with the exception of Timp1, which remains significantly elevated. Also, Hcn4 expression in Trpm7 αMHC/− −HB hearts is unchanged, consistent with the absence of AV block in these hearts. Remarkably, many of the potassium channel genes (Kcnj3, Kcnd2), channel modifier genes (Kcnv2, Lgi1), Trpm4, and Hdac9 remain significantly down regulated.
Figure 8.
Kcnd2 expression and Ito are diminished and action potentials prolonged in Trpm7 αMHC/− hearts, independent of cardiac hypertrophy. (A) qRT-PCR assessment of mRNA from Trpm7 αMHC/− −HB ventricular tissue (n = 4 hearts) compared to WT of the selected gene set examined in Trpm7 αMHC/− +HB hearts. Fold change significantly (p < 0.05) increased (green), decreased (red) or unchanged (black). P-values are indicated above or below the bars. (B) Box plots with overlying data points showing the distribution of Ipeak (left), Ipeak − Isus (Ito, center) current densities at +60 mV and cell capacitance (right) from WT (black, n = 29) and Trpm7 αMHC/− −HB (red, n = 20-22) left ventricular myocytes. A bimodal distribution of Ipeak and Ito current densities are observed (black and red dashed circles) suggesting a mosaic effect on Ito reduction. (C) Representative action potentials measured in current-clamp mode from Trpm7 αMHC/− −HB ventricular myocytes, ranging from short WT-like (left) to moderately (center) and severely prolonged (right). * p < 0.05, *** p < 0.001.
We patch-clamped freshly isolated ventricular myocytes from Trpm7 αMHC/− −HB hearts to determine whether these reductions in Kcnd2 expression in whole heart translated to reduced Ito and impaired AP repolarization. We find Ito to be significantly reduced in Trpm7 αMHC/− −HB ventricular myocytes (Ito-LV, Trpm7αMHC/− −HB = 11 ± 2.1 pA/pF, n = 20) compared to WT (Ito-LV, WT = 19 ± 2.1, n = 29, P <0.05; Figure 8B, center), but to a lesser extent than observed in Trpm7 αMHC/− +HB myocytes (Ito-LV, Trpm7αMHC/− +HB = 5 ± 0.9 pA/pF, n = 12). Importantly, cell capacitance measurements (Figure 8B, right), which are proportional to cell surface area, confirm that Trpm7 αMHC/− −HB myocytes are not hypertrophied (Cap Trpm7αMHC/− −HB = 103 ± 7 pF, n = 22), but are instead significantly smaller than WT myocytes (CapWT = 168 ± 9 pF, n = 29, p < 0.001). Examining the distribution of Ipeak and Ito amplitudes in Trpm7 αMHC/− −HB myocytes (Figure 8B, dotted black and red circles) reveals a bimodal distribution suggestive of a mosaic effect on Ito down regulation in these hearts. The distribution of Ito current densities from Trpm7 αMHC/− −HB ventricular cells is best fit by two Gaussian distributions (R2 = 1), with one population centered at 5 pA/pF and another centered at 25 pA/pF (Supplemental Figure 5). On the other hand, the distribution of WT Ito current densities is best fit by a single Gaussian (R2 = 0.99). The mean Ito current density in the subgroup of ventricular myocytes from Trpm7 αMHC/− −HB hearts with lower Ito (Ito-red circle, = 7 ± 1.1 pA/pF, n = 15) is significantly different from the other group (Ito-black circle = 25 ± 1.6, n = 6, P < 0.001) and comparable to Ito from Trpm7 αMHC/− +HB myocytes (Ito-LV, Trpm7αMHC/− +HB = 5 ± 0.9 pA/pF, n = 12, p = 0.2). This mosaic effect is also reflected in the APs measured from Trpm7 αMHC/− −HB ventricular myocytes as these ranged from WT-like in a small subset (Figure 8C, left) to markedly prolonged (Figure 8C, right). Nonetheless, the mean AP durations measured in Trpm7 αMHC/− −HB myocytes (APD50 = 20 ± 8.0 ms, n = 18) are comparable to Trpm7 αMHC/− +HB (APD50 = 22 ± 7.0 ms, n = 19) and significantly longer than WT (APD50, WT = 6 ± 0.8 ms, n = 33, p < 0.01; Table 1).
DISCUSSION
We sought to determine the contribution of TRPM7 to cardiac function by studying the consequences of cardiac-targeted Trpm7 deletion. By examining phenotypes resulting from Trpm7 deletion at different time-points in cardiogenesis using a panel of cardiac-targeted Cre lines (Isl1-Cre, ~E7; TnT-Cre, ~E9; αMHC-Cre, ~E12.5) we show that the timing of Trpm7 deletion variably perturbs cardiac development and function. Late embryonic deletion (αMHC-Cre) results in normal ventricular function, while early embryonic deletion (Isl1-Cre/TnT-Cre), leads to impaired compact myocardium development with consequent congestive heart failure and embryonic death. Deletion of Trpm7 between these two time points in cardiogenesis results in a ~50% penetrant adult cardiomyopathy characterized by ventricular dysfunction, disrupted atrioventricular conduction, dispersed ventricular repolarization and spontaneous/inducible ventricular arrhythmias (Supplemental Figure 6). This cardiomyopathy is associated with up-regulated hypertrophy/remodeling genes (Nppa/Acta1/Postn/TGF-β2/Timp1) while repolarizing potassium channel genes (Kcnk3, Kcna1, Kcnd2, Kcnj3, Kcnv2) and genes expressed in the conduction system (Trpm4, Hcn4, Kcnj3) are down regulated. This phenotype does not arise from dysregulated myocardial magnesium levels, as we observe no changes in total intracellular magnesium (or zinc) content in TRPM7-deficient myocardium, consistent with our previous findings in Trpm7 deleted T-lymphocytes10, or in other tissues11. These data further support the contention that, in adult tissue, TRPM7 is not required for magnesium homeostasis10, 11. Alternative magnesium influx pathways (e.g., MagT1, TUSC3, ACDP/CNNM, other TRPM channels, CNG channels) are likely more important or able to compensate loss of TRPM7 to determine global intracellular magnesium levels in adult tissue35, 36.
The dilated cardiomyopathy that occurs with cardiac-targeted TRPM7 deletion appears associated with heart block and therefore suggests that this cardiomyopathy may arise from hemodynamic stresses secondary to chronic bradycardia rather than from primary effects of Trpm7 deletion. On the other hand, it is notable that both heart block and cardiomyopathy are observed concomitantly in Trpm7 αMHC/− mice as early as 3 weeks of age, and the extent of left ventricular remodeling/dysfunction does not correlate with the severity of heart block. This argues against a mechanism of chronic bradycardia-induced cardiomyopathy and suggests, instead, that this phenotype is congenital in nature or very rapidly progressing. The finding that histone deacetlyase 9 (Hdac9) and to a lesser extent histone deacetlyase 5 (Hdac5) are downregulated in all in Trpm7 αMHC/− mice could provide a mechanism for a rapidly progressive cardiomyopathy in the context of only mild heart block. HDAC5/9 have been shown to act as transcriptional repressors of myocyte enhancing factor 2c (Mef2c), a critical transcription factor mediating gene expression in cardiac development37 and hypertrophy 38. Indeed, HDAC9−/− mice develop exaggerated cardiac hypertrophy when subjected to pressure-overload or chronic calcineurin activation 38. It is possible that HDAC5/9 down regulation in Trpm7 αMHC/− mice may sensitize the heart to cardiac hypertrophy such that a normally innocuous hemodynamic challenge (mild heart block) can induce a significant cardiomyopathy.
Atrioventricular (AV) block observed with cardiac-targeted Trpm7 deletion is associated with significant down regulation of a hyperpolarization-activated cyclic nucleotide gated channel gene (Hcn4) and a transient receptor potential channel gene (Trpm4). Both Hcn4 and Trpm4 are expressed in the murine and human atrioventricular node and conduction system 4, 39. Mutations in Trpm4 have been associated with progressive familial heart block type 1 (PFHBI) in humans4 and inducible cardiac-targeted deletion of Hcn4 in mouse results in severe heart block27. Indeed, we find that the If current encoded by Hcn4 is significantly depressed and often entirely silenced in AVN cells from Trpm7 αMHC/− +HB mice, providing a cellular mechanism for HB in these mice.
In addition to heart block, Trpm7 αMHC/− mice exhibit T wave flattening suggestive of impaired ventricular repolarization, associated with reduced expression of numerous potassium channel genes, including Kcnd2. Kcnd2 encodes the transient outward current (Ito), which is the primary repolarizing current in rodents33, and when selectively eliminated in mice results in QT prolongation, atrioventricular block, early-after-depolarizations and ventricular tachycardia40, 41. Similarly, elimination of Ito by deleting Kv Channel Interacting Protein 2 (KChiP2) also confers susceptibility to ventricular tachycardia42, as does loss of the transmural Ito gradient due to knock-out of the homeodomain transcription factor Irx534. We find that Ito is significantly diminished in patch-clamped Trpm7 αMHC/− ventricular cells and consequently action potentials are considerably prolonged compared to WT.
In summary, we show that the mechanisms responsible for TRPM7-mediated effects on cardiac function are complex. They result from changes in myocardial transcription that depends on the level of TRPM7 in myocardium during early developmental events, rather than on a direct role of TRPM7 in cardiac function (Supplemental Figure 6). The notion that altered cardiac function in adult mice arise from perturbations in Trpm7 activity during embryonic development is a novel and unique concept among TRP channels and is also consistent with our findings in other tissues11. Our efforts are now focused on elucidating how this ion channel-kinase is able to transduce changes in gene expression to influence cellular differentiation/development in a timing-dependent fashion. One important direction centers on our recently published finding that TRPM7 carboxy-terminal kinase can be cleaved from the ion channel in lymphocytes and activate down-stream signaling events43. Indeed, we find that a significant amount of TRPM7 is cleaved in embryonic myocardium compared to adult myocardium (RS, GK, DEC, unpublished observations). We speculate that these cleavage fragments may be interacting, either directly, or indirectly, with key cardiac transcription factors important for myocardial development and cardiac hypertrophy (e.g. Mef2). Furthermore, given the extent of cardiac pathology that ensues from disruptions in TRPM7 function, it is possible that patients with unexplained structural or electrophysiological heart disease may harbor previously unidentified disease causing mutations in Trpm7.
Supplementary Material
Acknowledgements
We thank Janice L. Robertson for assistance with data analysis and her thoughtful review of the manuscript.
Funding Sources: This work was supported by the Howard Hughes Medical Institute (HHMI, DEC), the Leadership Council in Cardiovascular Care (LCIC, RS) and the American Heart Association (AHA) Fellow-to-Faculty transition award (RS). Microarray studies were performed by the Molecular Genetics Core Facility at Children’s Hospital Boston (NIH-P50-NS40828, and NIH-P30-HD18655). Mice generated by Neurodevelopmental Behavioral Core and the Gene Manipulation Facility of the Boston Children’s Hospital Mental Retardation and Developmental Disabilities Research Center (NIH grant P30-HD18655). DC was supported by the NIH grant F32HL107021. ANR-2010-BLAN-1128-01 and ANR-09-GENO-034 (MEM). The IGF group is a member of the Laboratory of Excellence, Ion Channel Science and Therapeutics and is supported by a grant from ANR.
Footnotes
Conflict of Interest Disclosures: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. Trpc6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu X, Eder P, Chang B, Molkentin JD. Trpc channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci U S A. 2010;107:7000–7005. doi: 10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satoh S, Tanaka H, Ueda Y, Oyama J, Sugano M, Sumimoto H, Mori Y, Makino N. Transient receptor potential (trp) protein 7 acts as a g protein-activated ca2+ channel mediating angiotensin ii-induced myocardial apoptosis. Mol Cell Biochem. 2007;294:205–215. doi: 10.1007/s11010-006-9261-0. [DOI] [PubMed] [Google Scholar]
- 4.Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Brink P, Pongs O. Impaired endocytosis of the ion channel trpm4 is associated with human progressive familial heart block type i. J Clin Invest. 2009;119:2737–2744. doi: 10.1172/JCI38292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, Bozio A, Kurtbay G, Megarbane A, Ohmert I, Blaysat G, Villain E, Pongs O, Bouvagnet P. Gain-of-function mutations in trpm4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet. 2010;3:374–385. doi: 10.1161/CIRCGENETICS.109.930867. [DOI] [PubMed] [Google Scholar]
- 6.Guinamard R, Demion M, Magaud C, Potreau D, Bois P. Functional expression of the trpm4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension. 2006;48:587–594. doi: 10.1161/01.HYP.0000237864.65019.a5. [DOI] [PubMed] [Google Scholar]
- 7.Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londono JE, Uhl S, Voets T, Hummel B, van den Bergh A, Herijgers P, Nilius B, Flockerzi V, Schweda F, Freichel M. Increased catecholamine secretion contributes to hypertension in trpm4-deficient mice. J Clin Invest. 2010;120:3267–3279. doi: 10.1172/JCI41348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates-Withers C, Sah R, Clapham DE. Trpm7, the mg(2+) inhibited channel and kinase. Adv Exp Med Biol. 2011;704:173–183. doi: 10.1007/978-94-007-0265-3_9. [DOI] [PubMed] [Google Scholar]
- 9.Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG. Trpm7 is essential for mg(2+) homeostasis in mammals. Nat Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of trpm7 disrupts embryonic development and thymopoiesis without altering mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin J, Wu LJ, Jun J, Cheng X, Xu H, Andrews NC, Clapham DE. The channel kinase, trpm7, is required for early embryonic development. Proc Natl Acad Sci U S A. 2012;109:E225–233. doi: 10.1073/pnas.1120033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of trp channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human trpm cation channel family. J Recept Signal Transduct Res. 2006;26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 14.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L. Trpm7-mediated ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010;106:992–1003. doi: 10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for trpm7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 16.Runnels LW, Yue L, Clapham DE. The trpm7 channel is inactivated by pip(2) hydrolysis. Nat Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 17.Gwanyanya A, Sipido KR, Vereecke J, Mubagwa K. Atp and pip2 dependence of the magnesium-inhibited, trpm7-like cation channel in cardiac myocytes. Am J Physiol Cell Physiol. 2006;291:C627–635. doi: 10.1152/ajpcell.00074.2006. [DOI] [PubMed] [Google Scholar]
- 18.Demeuse P, Penner R, Fleig A. Trpm7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol. 2006;127:421–434. doi: 10.1085/jgp.200509410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langeslag M, Clark K, Moolenaar WH, van Leeuwen FN, Jalink K. Activation of trpm7 channels by phospholipase c-coupled receptor agonists. J Biol Chem. 2007;282:232–239. doi: 10.1074/jbc.M605300200. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases trpm6 and trpm7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang J, Li M, Yue L. Potentiation of trpm7 inward currents by protons. J Gen Physiol. 2005;126:137–150. doi: 10.1085/jgp.200409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JW, Zhou B, Yu QC, Shin SJ, Jiao K, Schneider MD, Baldwin HS, Bergelson JM. Cardiomyocyte-specific deletion of the coxsackievirus and adenovirus receptor results in hyperplasia of the embryonic left ventricle and abnormalities of sinuatrial valves. Circ Res. 2006;98:923–930. doi: 10.1161/01.RES.0000218041.41932.e3. [DOI] [PubMed] [Google Scholar]
- 24.Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor alk3. Proc Natl Acad Sci U S A. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 26.Inoue K, Branigan D, Xiong ZG. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem. 2010;285:7430–7439. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baruscotti M, Bucchi A, Viscomi C, Mandelli G, Consalez G, Gnecchi-Rusconi T, Montano N, Casali KR, Micheloni S, Barbuti A, DiFrancesco D. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene hcn4. Proc Natl Acad Sci U S A. 2011;108:1705–1710. doi: 10.1073/pnas.1010122108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: From molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann S, Stieber J, Stockl G, Hofmann F, Ludwig A. Hcn4 provides a ‘depolarization reserve’ and is not required for heart rate acceleration in mice. Embo J. 2007;26:4423–4432. doi: 10.1038/sj.emboj.7601868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czirjak G, Toth ZE, Enyedi P. Characterization of the heteromeric potassium channel formed by kv2.1 and the retinal subunit kv8.2 in xenopus oocytes. J Neurophysiol. 2007;98:1213–1222. doi: 10.1152/jn.00493.2007. [DOI] [PubMed] [Google Scholar]
- 31.Schulte U, Thumfart JO, Klocker N, Sailer CA, Bildl W, Biniossek M, Dehn D, Deller T, Eble S, Abbass K, Wangler T, Knaus HG, Fakler B. The epilepsy-linked lgi1 protein assembles into presynaptic kv1 channels and inhibits inactivation by kvbeta1. Neuron. 2006;49:697–706. doi: 10.1016/j.neuron.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 32.Guo W, Xu H, London B, Nerbonne JM. Molecular basis of transient outward k+ current diversity in mouse ventricular myocytes. J Physiol. 1999;521(Pt 3):587–599. doi: 10.1111/j.1469-7793.1999.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oudit GY, Kassiri Z, Sah R, Ramirez RJ, Zobel C, Backx PH. The molecular physiology of the cardiac transient outward potassium current (i(to)) in normal and diseased myocardium. J Mol Cell Cardiol. 2001;33:851–872. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- 34.Costantini DL, Arruda EP, Agarwal P, Kim KH, Zhu Y, Zhu W, Lebel M, Cheng CW, Park CY, Pierce SA, Guerchicoff A, Pollevick GD, Chan TY, Kabir MG, Cheng SH, Husain M, Antzelevitch C, Srivastava D, Gross GJ, Hui CC, Backx PH, Bruneau BG. The homeodomain transcription factor irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell. 2005;123:347–358. doi: 10.1016/j.cell.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou H, Clapham DE. Mammalian magt1 and tusc3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc Natl Acad Sci U S A. 2009;106:15750–15755. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Second messenger role for mg2+ revealed by human t-cell immunodeficiency. Nature. 2011;475:471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang S, McKinsey TA, Zhang CL, Richardson JA, Hill JA, Olson EN. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol. 2004;24:8467–8476. doi: 10.1128/MCB.24.19.8467-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class ii histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herrmann S, Layh B, Ludwig A. Novel insights into the distribution of cardiac hcn channels: An expression study in the mouse heart. J Mol Cell Cardiol. 2011;51:997–1006. doi: 10.1016/j.yjmcc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Guo W, Li H, London B, Nerbonne JM. Functional consequences of elimination of i(to,f) and i(to,s): Early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking kv1.4 and expressing a dominant-negative kv4 alpha subunit. Circ Res. 2000;87:73–79. doi: 10.1161/01.res.87.1.73. [DOI] [PubMed] [Google Scholar]
- 41.London B, Baker LC, Petkova-Kirova P, Nerbonne JM, Choi BR, Salama G. Dispersion of repolarization and refractoriness are determinants of arrhythmia phenotype in transgenic mice with long qt. J Physiol. 2007;578:115–129. doi: 10.1113/jphysiol.2006.122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, Nguyen-Tran VT, Gu Y, Ikeda Y, Chu PH, Ross J, Giles WR, Chien KR. A defect in the kv channel-interacting protein 2 (kchip2) gene leads to a complete loss of i(to) and confers susceptibility to ventricular tachycardia. Cell. 2001;107:801–813. doi: 10.1016/s0092-8674(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 43.Desai BN, Krapivinsky G, Navarro B, Krapivinsky L, Carter BC, Febvay S, Delling M, Penumaka A, Ramsey IS, Manasian Y, Clapham DE. Cleavage of trpm7 releases the kinase domain from the ion channel and regulates its participation in fas-induced apoptosis. Dev Cell. 2012;22:1149–1162. doi: 10.1016/j.devcel.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sah R, Oudit GY, Nguyen TT, Lim HW, Wickenden AD, Wilson GJ, Molkentin JD, Backx PH. Inhibition of calcineurin and sarcolemmal ca2+ influx protects cardiac morphology and ventricular function in k(v)4.2n transgenic mice. Circulation. 2002;105:1850–1856. doi: 10.1161/01.cir.0000014211.47830.4d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.