Chronic exposure to sphingosine-1-phosphate within, or outside, mast cells causes gene expression alterations that modulate FcεRI-mediated mast cell responses, and the onset of allergic reaction.
Keywords: FcεRI, mast cell degranulation, cytokine production, anaphylaxis
Abstract
Both genes and the environment are determinants in the susceptibility to allergies and may alter the severity of the disease. We explored whether an increase in the levels of the lipid mediator S1P in vivo, a condition found during allergic asthma, could affect the sensitivity or the response of MCs to IgE/Ag and the onset of allergic disease. We found that increasing S1P levels by genetic deletion of S1P lyase, the enzyme catabolizing S1P, led to elevated activity of circulating tryptase. Accordingly, MCs of S1P lyase-deficient mice were mostly degranulated in the tissues and showed enhanced calcium levels, degranulation, and cytokine production in response to IgE/Ag in vitro. Th 1-skewed mice (C57BL/6) had lower levels of S1P in circulation and histamine responses than did Th 2-skewed (129/Sv) mice. However, when S1P levels were increased by pharmacologic inhibition of S1P lyase, the C57BL/6 mice showed increased histamine release into the circulation and anaphylactic responses similar to those in the 129/Sv mice. Culturing of MCs in the presence of S1P enhanced their degranulation responses, and when the S1P-treated MCs were used to reconstitute MC-deficient (KitW-sh) mice, they caused enhanced anaphylaxis. Gene expression arrays in S1P lyase-deficient MCs and MCs treated with S1P continuously revealed increased expression of numerous genes, including the adhesion molecule CNTN4,which contributed to the enhanced responses. Our findings argue that dysregulation in the metabolism of S1P is a contributing factor in modulating MC responsiveness and the allergic response.
Introduction
MCs are both innate effector and immunomodulatory cells with important roles in inflammatory and allergic reactions. A variety of stimuli from innate or adaptive immune cells, the nervous system, or the environment can trigger MCs to release stored mediators immediately or to synthesize cytokines and lipid mediators de novo for delayed release [1, 2]. The combination of all these substances can initiate, enhance, or suppress tissue or immune responses [3, 4]. In addition to this functional plasticity, MCs show a phenotypic plasticity. The MC phenotype can be shaped by the combined effects of microenvironmental factors on committed MC precursors or, potentially, on progenitors in the bone marrow and other tissues. These epigenetic or phenotypic alterations, specific to each microenvironment, may be further modified by additional genetic or environmental challenges that can contribute to the susceptibility of an individual to particular immune or allergic reactions.
S1P is a lipid mediator known to play a central role in immune cell trafficking and function [5–7]. In MCs, S1P that is produced after engagement of a high-affinity receptor, FcεRI, plays a significant part in degranulation, cytokine production, and chemotaxis after Ag stimulation [8, 9]. Other stimuli, such as SCF and IL-3, which are critical in the differentiation of MCs in vitro, can also activate the enzymes that synthesize S1P, namely SphK1 and −2 [10]. The specific role of S1P produced by MCs (under stimuli other than IgE/Ag) and the function of S1P in MCs, other than in acute stimulation conditions, are not known. In general, the cellular localization or microdomain in which S1P is produced may be a determinant of its function. S1P is a ligand for 5 related G-protein-coupled receptors, S1PR-1 to -5, which are ubiquitously expressed [7, 11]. However, S1P has also long been described as an intracellular messenger, a role that has been highlighted by the finding that it can directly interact with signaling proteins, such as TRAF2, and activate its E3 ligase activity, which is critical for NF-κB activation [12]. Furthermore, S1P can be produced in the nucleus by SphK2 and binds and inhibits HDAC1 and -2, resulting in increased gene transcription [13]. In addition, alterations in S1P metabolism caused, for example, by deletion of the enzyme Sgpl1,which terminally degrades S1P, result in elevated concentrations of S1P in tissues, blood, and cells [14, 15], as well as in the nucleus, where it reduces HDAC activity and increases histone H3 acetylation [16]. These examples illustrate that changes in the metabolism of S1P can regulate gene expression by either modifying cellular signaling or directly affecting chromatin structure.
Beyond the aforementioned intracellular role of S1P in transcriptional regulation, changes in the concentration of S1P in the blood (or in the environment surrounding cells in tissues) can induce cell differentiation, mostly via S1PR [5, 17–23]. Normally, the levels of S1P are low in tissues (nanomolar range) and high in blood (micromolar range), and the maintenance of this gradient is tightly regulated by the enzymes involved in S1P metabolic pathways [24]. However, changes in this homeostasis may occur under certain physiological or pathologic circumstances (such as inflammation or cancer) [24], leading to elevated levels of S1P in the tissue microenvironment, which may affect the differentiation of hematopoietic progenitors. Our studies in mice deficient in SphK1 or −2 have indicated an association between S1P levels in circulation (low and high, respectively) and their histamine responses to a systemic IgE/Ag challenge. We hypothesized that the level of S1P in the circulation of these mice influences the responsiveness of MCs and the onset of anaphylaxis in vivo by a mechanism that differs from the intracellular role of newly generated S1P inside MCs following FcεRI engagement [9, 25].
The current study was undertaken to further examine the influence of long-term S1P exposure on the MC phenotype and responsiveness, both in vivo and in vitro. Using various genetic and pharmacologic mouse models that alter S1P levels, we found that increased levels of S1P were associated with increased histamine release and enhanced systemic anaphylaxis, regardless of the mechanism by which S1P metabolism is dysregulated. The hyper-responsive phenotype was also seen in vitro in MCs with altered catabolism of S1P (S1P lyase-deficient MCs) or when BMMCs were differentiated in the presence of S1P in the culture medium. This enhanced MC responsiveness differed from the effects of acute stimulation of MCs with S1P, because it was brought about by changes in gene expression and in gene functional pathways after long-term exposure to S1P. Among the genes with enhanced expression, we identified CNTN4, a GPI-linked membrane protein [26], as a gene product that contributes to the enhanced calcium and degranulation responses in Sgpl1-deficient MCs but not in WT MCs. Thus, alterations in the metabolism of S1P locally, systemically, or inside MCs can modify the pattern of gene expression in these cells, resulting in a gain of function for certain gene products, such as CNTN4, that may not normally play a role in MC degranulation, leading to hyper-reactivity and heightened histamine responses to a systemic allergic challenge.
MATERIALS AND METHODS
Mice, bone marrow isolation, and BMMC cultures
Mice were maintained and used in accordance with NIH guidelines and animal study proposals approved by NIAMS or the NIDDK Animal Care and Use Committee. C57BL/6 and 129/Sv mice were from Jackson Laboratories (Bar Harbor ME,USA). MC-deficient mice (KitW-sh/W-sh, C57BL/6, Jackson Laboratories) were generated inhouse from heterozygous matings. Sgpl1−/− mice [27] were derived by gene trap mutagenesis and maintained by heterozygous matings. The pups were genotyped as described elsewhere [14] and the Sgpl1−/− and WT littermates were used for experiments at about 18 days after birth, because they die after weaning, as a result of alterations in lipid metabolism, the presence of inflammatory cytokines in circulation, and organ failure [14]. Sphk2-deficient mice with a conditional deletion in Sphk1 were generated by crossing Sphk2−/− mice with mice carrying a myxovirus-resistant 1-Cre (Mx1-Cre) transgene (Tg[Mx1-Cre]1Cgn/J, stock no. 002527; Jackson Laboratories) [28] and conditional Sphk1 alleles (Sphk1fl/fl) (denoted as S1P-less mice) [29]. Cre expression was induced by poly(I:C) treatment of Mx1-Cre Tg+/Sphk1fl/fl:Sphk2−/− mice [29]. WT mice were Sphk1fl/fl, but not the Mx1-Cre Tg+. CNTN4-deficient mice were as described elsewhere [30].
BMMCs were isolated from 5–6-wk-old mice (or 18 days in the case of the Sgpl1−/− and WT counterparts) and cultured in RPMI medium supplemented with 20 ng/ml IL-3 and 20 ng/ml SCF [10]. The BMMCs were generally grown for 4–8 wk. Peritoneal MCs were obtained from peritoneal lavages after culturing of lavaged cells in the same medium as described for BMMCs for about 2–3 wk [31]. All MC cultures were used when greater than 95% of the population expressed FcεRI and c-Kit [32]. Usually, by 4 wk in culture, no c-Kit− cells (corresponding to IL-3-dependent basophils) were found, and more than 98% of the population was positive for FcεRI and c-Kit and expressed the proteases characteristic of MCs [31, 33]. S1P (range, 0.1–2 μM) was added to some of the cultures at the time of isolation from the bone marrow and was replenished every time the medium was replaced. Experiments with S1P-treated cells were performed at least 3 days after the last change of medium. S1P (BioMol, Plymouth Meeting, PA, USA) was prepared as a 1 mM solution in methanol, divided into aliquots, and dried under an N2 stream. Lipid aliquots were resuspended in growth medium with a water bath sonicator and were added to the cells at the indicated concentrations (see Figs. 1, 4, and 5).
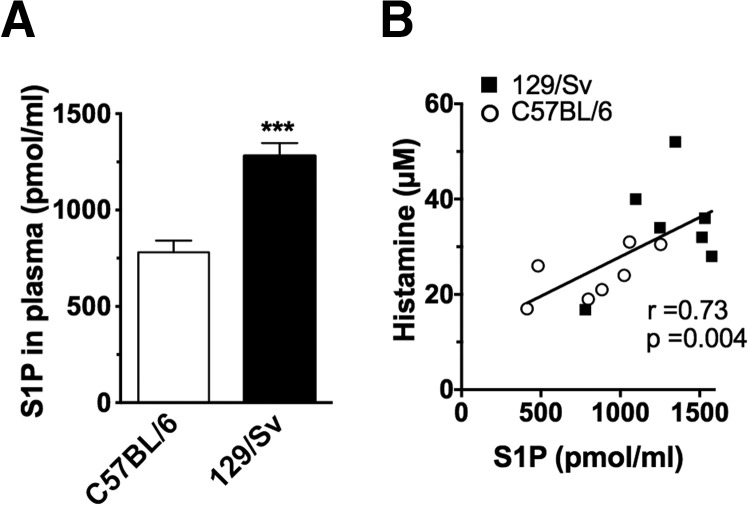
Figure 1. Higher levels of S1P in Th2-skewed genetic backgrounds correlated with heightened histamine responses to an Ag challenge.
(A) Blood was collected from 7 to 9 naïve C57BL/6 (Th1-skewed) and 129/Sv (Th2-skewed) mice, and plasma S1P was measured. Data represent the mean ± se (n=14–17 mice for each strain). ***P<0.001. (B) Association between S1P levels in the plasma and histamine released in the circulation of the corresponding mouse 90 s after induction of anaphylaxis. Systemic anaphylaxis was initiated by injecting IgE (15 μg/ml) i.v. 24 h before a challenge with anti-IgE or -IgG as a control (60 μg). The mice were euthanized after 90 s, and blood was withdrawn by cardiac puncture. Histamine levels in plasma were measured by competitive ELISA. r and P, by Spearman test.
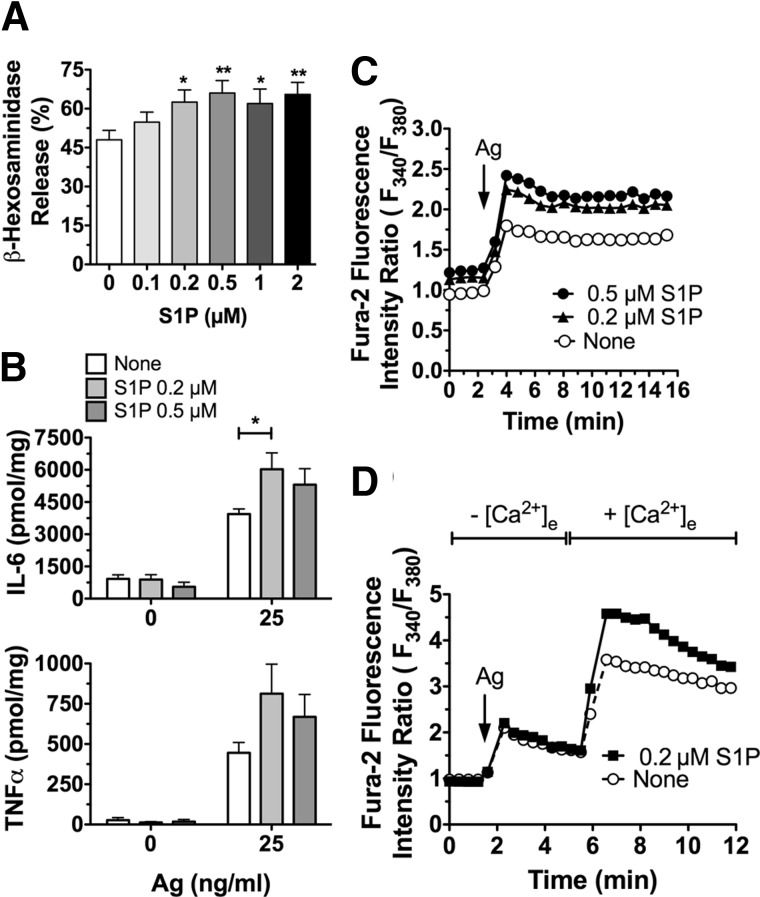
Figure 4. Enhanced responses to IgE/Ag in MCs differentiated in the presence of S1P.
BMMC progenitors were cultured in SCF and IL-3-containing medium, including the indicated concentrations of S1P. After 4–6 wk in these culture conditions, degranulation (A), cytokine production (B), and calcium responses (C, D) were assessed as in Fig. 3. All experiments were conducted in Tyrodes buffer (A, C, D) or medium without serum or cytokines (B) 3 to 5 days after the last change of medium. Results are the average of 3–8 independent cultures. *P < 0.05, **P < 0.01.
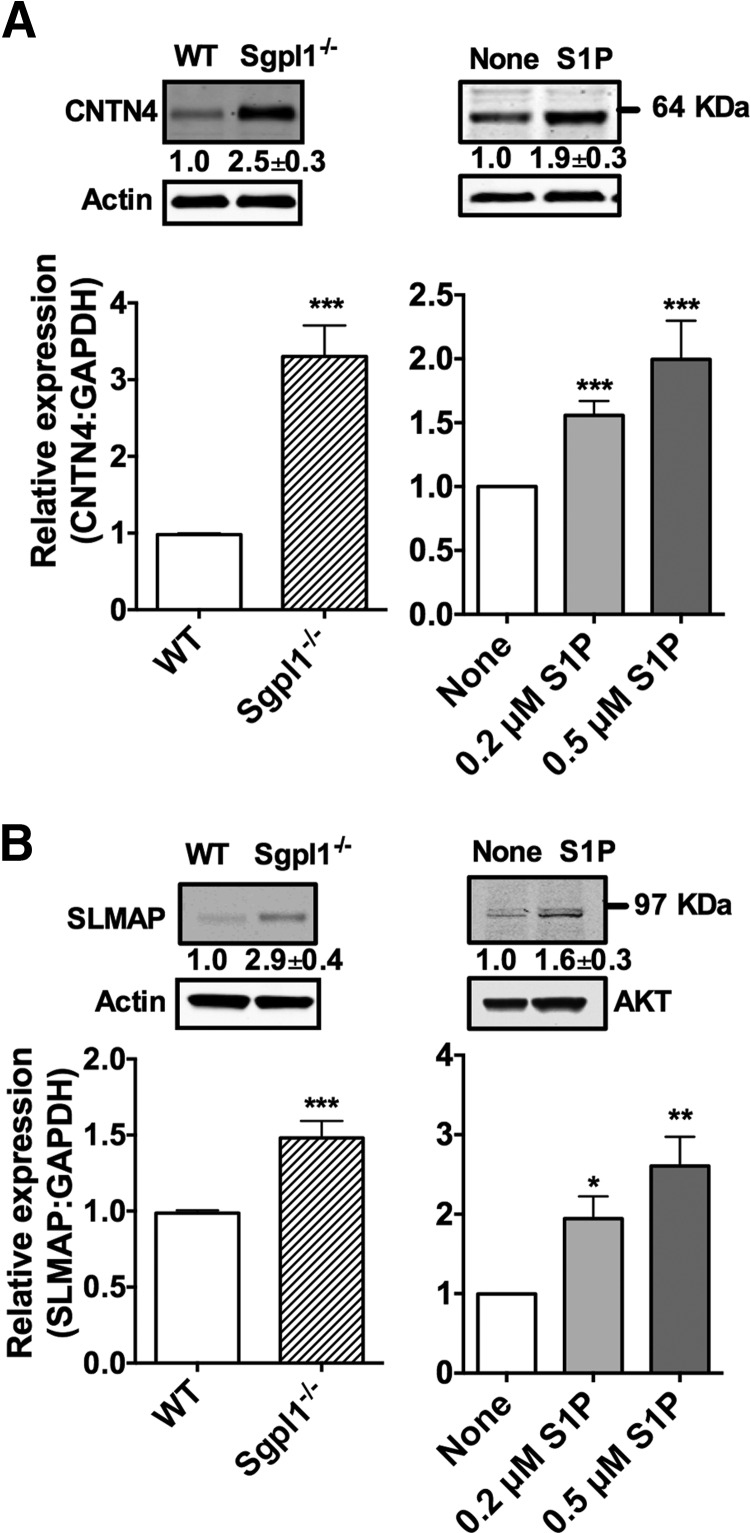
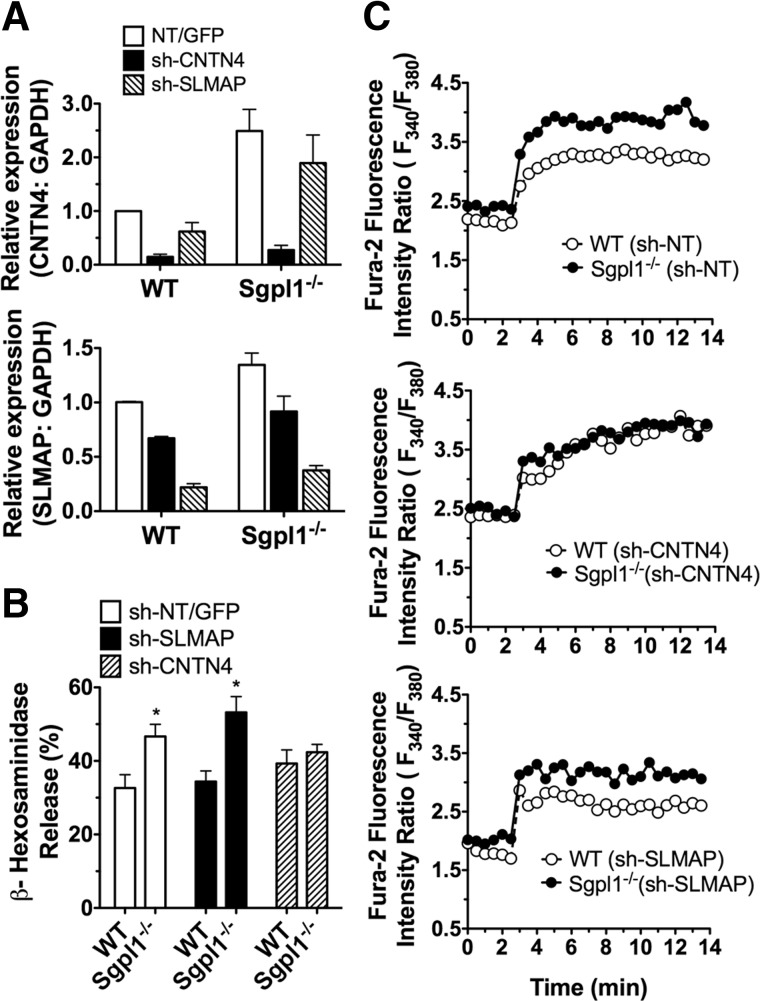
Figure 5. The mRNA levels of CNTN4 and SLMAP were upregulated in S1P lyase-deficient MCs and in MCs differentiated in the presence of S1P.
RNA and cell lysates for Western blot were prepared from BMMCs of WT and S1P lyase-deficient (Sgpll−/−) mice (left panels) or BMMCs cultured with the indicated concentrations of S1P (right panels). Gene expression assays specific for CNTN4 (A) and SLMAP (B) were used to determine the relative mRNA levels in the indicated cells, and commercially available antibodies were used for the detection of CNTN4 (A) and SLMAP (B). Each measurement for CNTN4 and SLMAP was normalized to GAPDH (ΔCt), and measurements were then compared to those for WT (left panels) or untreated (right panels) BMMCs (ΔΔCt). The baseline Ct numbers in WT for CNTN4 were 30.2 ± 0.4, for SLMAP: 26.1 ± 0.4, and 19.5 ± 0.8 for GAPDH (n=9). The numbers under the blots indicate the quantitation of the band intensities of CNTN4 and SLMAP, corrected by the intensity found in the loading control. Results are the average of 4–8 independent cultures. *P < 0.05, **P < 0.01,***P < 0.001.
Reconstitution of KitW-sh/W-sh mice
BMMCs, cultured and differentiated as just described in the presence or absence of 0.2 or 0.5 μM S1P for 4–6 wk, were washed with RPMI and injected intravenously into 6–8 wk MC-deficient KitW-sh/W-sh mice (107 cells/mouse in 200 μl RPMI) [34]. After 10–12 wk, the mice were subjected to passive systemic anaphylaxis. Successful engraftment was verified in mice not subjected to anaphylaxis, by determining the presence of MCs in the spleen, stomach, lungs, and lymph nodes. In mice subjected to anaphylaxis, the MCs engrafted in the spleen appeared degranulated after anaphylaxis, and degranulated MCs were more variably observed in the other tissues; however, all animals showed MC engraftment.
Passive systemic anaphylaxis and S1P lyase inhibitor treatment
Mice were sensitized (i.v.) with 3 μg of DNP-specific IgE (murine hybridoma IgE H1 DNP-ε-26) in 0.2 ml PBS, and after 24 h, the mice were challenged with either Ag (DNP-HSA, 250 μg), with 15–60 μg rat anti-mouse IgE (R35-92 clone, BD PharMingen, San Diego, CA, USA), or with rat IgG1 isotypic control (BD PharMingen). After 90 s, the mice were euthanized with CO2, and blood was immediately withdrawn by cardiac puncture. Plasma histamine concentration was determined with a competitive histamine immunoassay kit (Beckman Coulter, Fullerton CA, USA). Alternatively, body temperature was measured with an implantable electronic transponder inserted under the dorsal neck skin fold [35]. In these experiments, the mice were challenged with 0.2–1 μg of rat anti-mouse IgE (depending on the preparation), a dose that in preliminary studies elicited responses similar to those induced by 250 μg of Ag (DNP36-HSA), with no extensive changes in vascular extravasation, or with a bolus of histamine (5 μmol in 0.2 ml saline) [35]. Unless otherwise indicated, injections were conducted on anesthetized mice (isoflurane 2%:oxygen 98% mix for 2–3 min) in a closed chamber. The mice were euthanized at the completion of the experiment.
Some mice were given THI, an inhibitor of S1P lyase, in acidified drinking water (100 μg/L) with 5 g/L glucose [36]. The treated water was administered ad libitum for 5 consecutive days. Control mice drank acidified water with glucose alone. The mice were sensitized with IgE on the fourth day and challenged on the fifth day after the initiation of THI administration.
Tissue MC staining and tryptase activity assays
Samples of the dorsal skin and stomach from euthanized WT or Sgpl−/− mice were fixed in 10% buffered formalin and embedded in paraffin, and sections were stained with toluidine blue at low pH and counterstained with eosin. Metachromatic-stained MCs were counted in each field under the microscope. Tryptase activity present in the serum of mice (18 days old) was evaluated by colorimetric assay (Mast Cell Degranulation Assay Kit; Chemicon International, Temecula, CA, USA), according to the manufacturer's instructions.
S1P measurements
For measurement of S1P in the plasma, lipids were extracted from plasma or MCs (15–30 million cells) under alkaline conditions [10]. The aqueous phase containing S1P was treated with alkaline phosphatase to dephosphorylate S1P to sphingosine. After lipid extraction in acid conditions, the generated sphingosine was isolated from the organic phase and quantified by an enzymatic assay by using cell lysates from HEK293 cells overexpressing SphK1 [10].
β-Hexosaminidase and cytokine release assays
Cells were sensitized for 1–3 h with 0.5 μg IgE per million cells, washed, and stimulated with the indicated concentration of Ag (see Figs. 3, 4, and 6). The enzymatic activity of the granule marker β-hexosaminidase, released into the extracellular medium after 10 min of stimulation, was measured [32]. For cytokine measurements, IgE-sensitized cells were challenged with Ag in serum-free medium, SCF, IL-3 containing 0.05% fatty acid-free BSA and an EDTA-free protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN, USA) for 4 h at 37°C in a CO2 incubator. To normalize for any variability in cell counts during the period of incubation, we measured protein concentration for each collected sample. TNFα and IL-6 were measured by specific ELISA and the results expressed as the amount of cytokine released per milligram total protein in each sample (∼1 mg/ml).
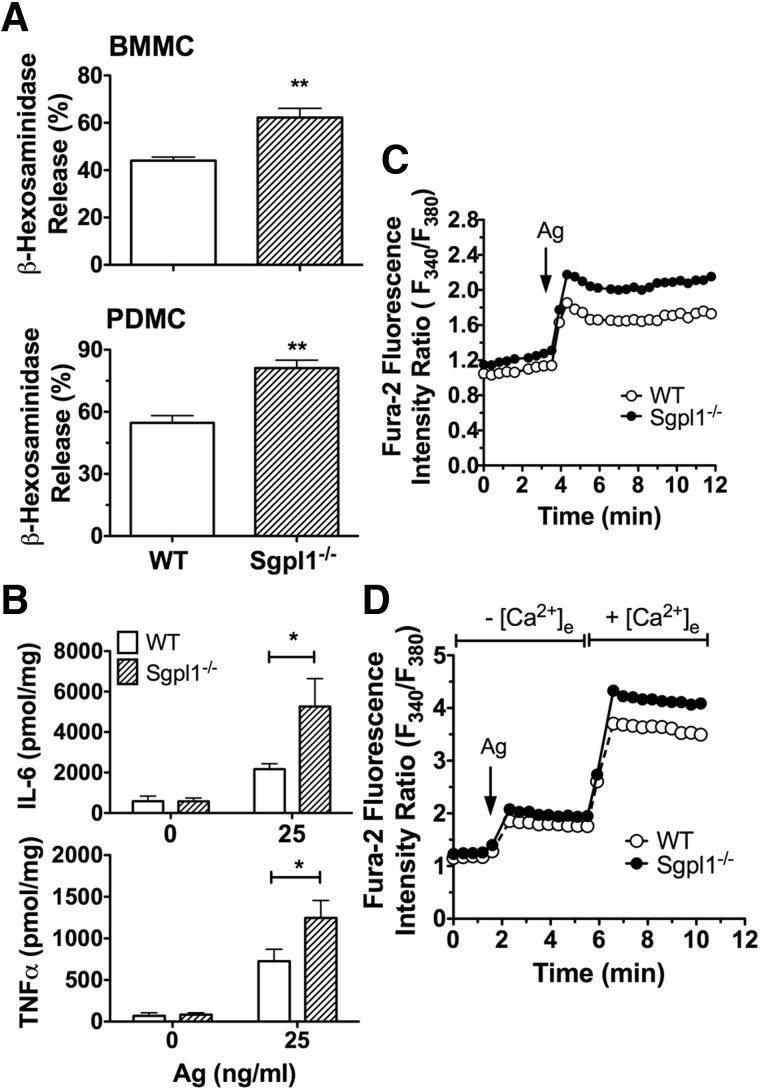
Figure 3. Enhanced responses to IgE/Ag in S1P lyase-deficient MCs.
Degranulation (A) and cytokine production (B) in response to IgE and Ag (25 ng/ml) in BMMCs (A, top panel, and B) or PDMCs (A, bottom panel) from WT and S1P lyase-deficient (Sgpl1−/−) 18–20-day-old mice. β-Hexosaminidase release was measured from supernatants of cells challenged with 25 ng/ml Ag (DNP-HSA) for 10 min (A) or cytokines (B) for 3 h. (C) Intracytosolic calcium changes were monitored after IgE/Ag stimulation of FURA2-AM-loaded BMMCs. Changes are reported as the ratio of fluorescence emitted by excitation at 340 or 380 nm, with emission captured at 510 nm for each time point. The cells were challenged with Ag (20 ng/ml of DNP-HSA) at the time point indicated. (D) The cells were stimulated with Ag in the absence of extracellular calcium. At the time point indicated, CaCl2 (1 mM) was added to the medium to promote calcium influx, and changes in intracytosolic calcium concentration were monitored as in (C). Results are the average of 4–9 independent cultures. *P < 0.05; **P < 0.01.
Figure 6. Knockdown of CNTN4, but not SLMAP, abrogated the enhanced degranulation and calcium responses of Sgpl1−/− BMMCs.
MCs were transduced with lentivirus containing shRNA sequences specific for CNTN4, SLMAP, or NT shRNA control. (A) Efficient shRNA silencing of CNTN4 (top panel) and SLMAP (bottom panel) was determined by qRT-PCR with gene expression assays specific for these transcripts. A probe specific for GAPDH was used as an internal control. Each measurement was normalized to GAPDH (ΔCt) and measurements were then compared to the NT values (ΔΔCt). (B) Degranulation in response to IgE and Ag (25 ng/ml) was calculated as the net release of β-hexosaminidase induced by Ag relative to the total cellular content of β-hexosaminidase for each treatment. *P<0.05. (C) Intracellular calcium changes after Ag stimulation (20 ng/ml of DNP-HSA) initiated at 2min were monitored as described in Fig. 3. Results are the average of 3–5 independent cultures. Each culture was subjected to at least 2 separate lentiviral transductions, and for each response, a triplicate of each sample was used.
Microarray analysis
Purified RNAs from duplicate samples of WT and Sgpl1−/− BMMCs or from BMMCs treated with 0.5 μM S1P or left untreated during their differentiation were used for the hybridization of target-labeled samples in the GeneChip Mouse Genome 430 2.0 Array (Affymetrix, Santa Clara, CA, USA). Hybridization and the microarray analysis were performed in the NIDDK Microarray Core Facility. Analysis of the signals was performed in accordance with an Affymetrix RMA algorithm. Genes with a significant difference in expression between WT and Sgpl1-deficient BMMCs or S1P-treated and untreated BMMCs were selected on the basis of P < 0.05 and equal or above absolute values of 1.5-fold change, as assessed by ANOVA and Gen Set-ANOVA (GO-ANOVA) obtained with Partek Pro software (St. Charles, MO, USA).
Real-time PCR and Western blot analysis
RNA was extracted from BMMCs (1–4×106 cells) with RNeasy mini kits (Qiagen, Valencia, CA, USA), digested with DNAse I, and reversed transcribed with the Superscript III First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, USA), as specified by the manufacturers. CNTN4 and SLMAP mRNA was determined by quantitative real-time PCR performed with TaqMan Gene Expression Assays (CNTN4, Mm00476065 or Mm01188640; SLMAP, Mm01218825 or Mm00473504; Applied Biosystems, Foster City, CA, USA) on an ABI/PRISM 7700 Sequence Detector System (Applied Biosystems). Relative levels of mRNA were calculated based on ΔCt with GAPDH used as the endogenous control gene. For immunoblots, cells were solubilized and separated by electrophoresis, transferred to membranes, and probed [33] with anti-CNTN4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-SLMAP (Sigma-Aldrich, St. Louis, MO, USA).
shRNA-mediated knockdown of CNTN4 and SLMAP
A lentivirus gene transduction system (Sigma-Aldrich) was used for shRNA-mediated stable knockdown. Viral supernatants were produced by transfection of the packaging cells 293LTV with 3.9 μg of shRNA constructs (TRCN0000113631 or TRCN0000113633 for CNTN4, and TRCN0000175889 or TRCN0000176237 for SLMAP; Sigma-Aldrich) using Fugene 6 (Roche Applied Science, Indianapolis, IN) or Lipofectamine 2000 (Invitrogen). Various NT shRNAs (SHC001, SHC002, or SHC004) were used as the control. BMMCs were infected according to a published procedure [33]. After infection, the cells were washed and allowed to recover in growth medium for 2 days before selection of transduced cells with 3 μg/ml puromycin (Sigma-Aldrich). After a week of selection, the cells were grown for an additional week in growth medium without puromycin and then used for functional assays.
Intracellular calcium measurements
For intracellular calcium measurements, the cells were sensitized with IgE for 1 h and loaded with 1 μM FURA-2-AM (Invitrogen) for 20 min, washed, and divided into 96-well plates (9×104 cells/well). After 20 min, the cells were challenged with Ag (25 ng/ml), and changes in intracellular calcium were monitored with a microplate fluorescence reader (Wallac Victor2; Perkin Elmer, Wellesley, MA, USA). FURA-2-AM emission at 510 nm during rapid excitation between 340 and 380 nm at 37°C was measured. Background fluorescence was determined from unloaded cells. The ratio of fluorescence at 340 and 380 nm (R) after subtracting the respective background values was calculated for each measurement.
Statistical analysis
Statistical analysis was performed with Prism GraphPad Software (San Diego, CA, USA). For most comparisons, a 2-tailed unpaired t test was used. A 2-way ANOVA test was used for comparing responses of the various mutant cells over time. P < 0.05 indicated statistical significance. For all experiments (with exceptions as indicated in the figure legends) a minimum of 3 independent experiments were performed. Data shown are expressed as the mean ± se.
Online Supplemental Material
Supplemental Table S1 and Supplemental Figs. S1–S3 are provided.
RESULTS
Elevated levels of S1P enhance MC responsiveness in vivo
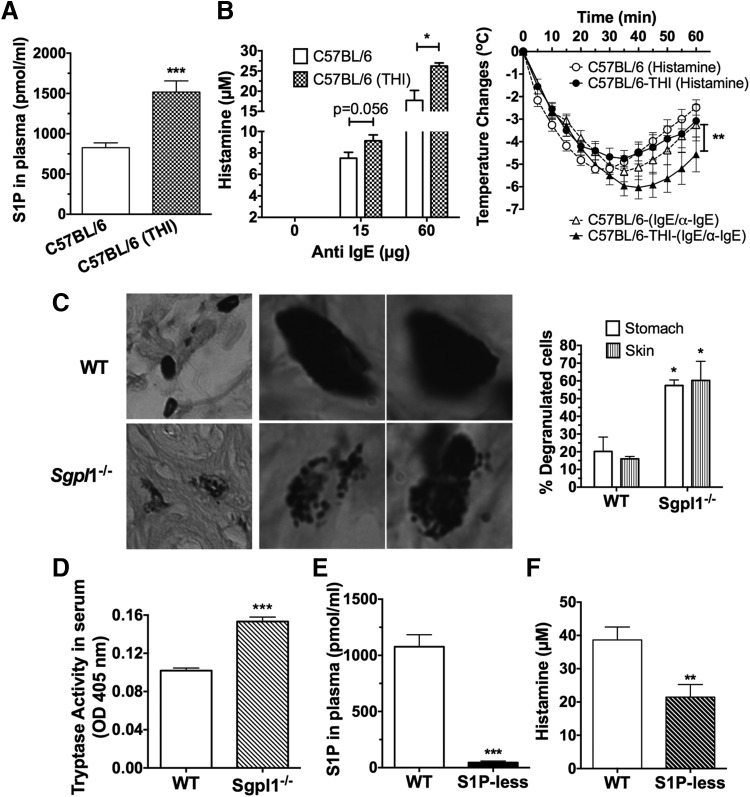
To study whether the levels of S1P in the in vivo environment are associated with MC responsiveness in models other than in the SphK1- or -2-deficient mice [25], we first measured S1P in the plasma of mice from 2 genetic backgrounds with known Th (Th)1- and -2-skewed phenotypes, C57BL/6 and 129/Sv, respectively. The levels of S1P were higher in mice from the 129/Sv background (Fig. 1A), which are known for more robust Th2 immune responses than are C57BL/6 mice [37]. To verify that MCs are more responsive in the 129/Sv mice, we systemically challenged both the C57BL/6 and the 129/Sv mice with anti-IgE. We first injected the mice with IgE to make certain that the MCs were equivalently saturated with IgE, making the possible differences in receptor occupancy with IgE irrelevant to the subsequent challenge. Histamine responses to the anti-IgE challenge were significantly higher in the 129/Sv mice (Fig. 1B), which correlated with the higher levels of S1P in these mice. To further explore a possible cause–effect relationship, we induced a chronic elevation of S1P levels in the C57BL/6 mice (Fig. 2A) to levels consistent with a 129/Sv strain (Fig. 1A) by administering an inhibitor of S1P lyase, THI, in the drinking water for several days before induction of anaphylaxis. THI treatment dramatically increases S1P levels in tissues, ablates the gradient of S1P between tissues and blood, and impairs lymphocyte egress from secondary lymph nodes, causing lymphopenia [36]. Accordingly, the THI treatment caused increases in cellular S1P levels in the C57BL/6 mice (for example, in bone marrow cells) by more than 80-fold (data not shown) and caused acute lymphopenia (treated, 1.81±0.23 K/μl, n=21; untreated 6.25±0.49 K/μl, n=18), whereas no differences in red blood cells were observed (untreated, 10.15±0.22 M/μl, n=17; THI-treated, 9.66±0.24 M/μl, n=18). Concomitant with the increased S1P levels in plasma (Fig. 2A), the THI-treated C57BL/6 mice had higher histamine responses to IgE crosslinking by anti-IgE than did their untreated C57BL/6 counterparts (Fig. 2B, left panel). These responses were remarkably similar to those in the 129/Sv genetic strain (Fig. 1B). The THI-treated mice also had more severe hypothermia during the course of anaphylaxis, compared with the untreated ones (Fig. 2B, right panel), consistent with increased levels of circulating histamine. This effect is not likely to be the result of a contribution of basophils, because the total content of histamine in blood after ex vivo cell lysis was only 0.6 μM [31]; and, after IgE/Ag challenge in the KitW-sh/W-sh MC-deficient mice (which have a normal number of basophils), the histamine release into plasma ranged from 3 to 30 nM, well below the observed differences after THI treatment. When anaphylaxis of similar severity was induced independent of MC FcεRI triggering, by injection of histamine [35], the THI-treated mice responded similarly to the control mice (Fig. 2B, right panel), supporting the notion that the effects of S1P in IgE-induced anaphylaxis are due to changes in MC responsiveness to Ag, not to other, unrelated effects of THI. It is important to note, however, that under more severe anaphylactic conditions induced by higher concentrations of anti-IgE, the protective role of S1P in recovery from anaphylaxis, through regulation of the blood pressure, the rate of histamine clearance, and vascular permeability [29, 35], became dominant over its effects at the onset, with the THI-treated mice showing a faster recovery (data not shown).
Figure 2. Interference of S1P catabolism in C57BL/6 mice enhanced MC responsiveness in vivo, and reduced S1P production impaired histamine release during anaphylaxis.
(A) C57BL/6 mice were given an inhibitor of S1P lyase, THI (100 μg/L), in acidified glucose (1g/L) in the drinking water or acidified glucose water alone (control group, C57BL/6) for 5 days. S1P levels were measured from plasma with an enzymatic assay. (B) Anaphylaxis was induced as in Fig. 1B with the indicated concentrations of anti-IgE (left panel). Alternatively, anaphylaxis was induced with IgE/anti-IgE (n=9–14 mice) or with histamine (n=5), and body temperature changes were recorded over 1 h with implantable transponders (right panel). **P < 0.01, 2-way ANOVA test. (C, D) Tissues and blood were collected from 20-day-old WT or S1P lyase (Sgpl1)-deficient mice. (C) MCs in sections from the skin or stomach were stained with toluidine blue. Examples of skin mast cells showing a degranulated morphology are shown at 2 different magnifications (left panel, column 1 vs. columns 2 and 3). The average number of degranulated or intact MCs per field is shown for 3 mice (right panel). (D) Tryptase activity in the serum from WT or Sgpl1−/− mice (n=20) was measured in a colorimetric enzymatic assay. *P < 0.05, **P < 0.01; ***P < 0.001, Student's t test. (E) S1P concentration in the plasma of the indicated mice was determined with an enzymatic assay. S1P-less mice refers to Sphk2-deficient mice with a conditional deletion in Sphk1, and WT mice were Sphk1fl/fl, without Mx1-Cre Tg+. (F) Histamine released 90 s after induction of anaphylaxis in S1P-less mice. Systemic anaphylaxis was induced by injecting IgE (15 μg/ml) i.v. 24 h before an i.v. challenge with DNP-HSA (250 μg). Histamine levels in the plasma were measured by ELISA.
Genetic deletion of S1P lyase results in the elevation of S1P levels in the circulation and tissues and induces marked lymphopenia, neutrophilia, and elevated circulating proinflammatory cytokines [14, 15, 38, 39]. Because some of these cytokines are abundantly produced by MCs, we reasoned that activated MCs in the mice might be a contributory factor to the pronounced inflammatory phenotype. Although the number of MCs was normal in the S1P lyase-deficient mice, a higher percentage of them showed a degranulated phenotype in their tissues (Fig. 2C, left and right panels). In agreement, serum tryptase activity, a measurement of MC activity, was elevated in the mice (Fig. 2D), suggesting a constitutive activation of MCs that is consistent with the hyper-reactivity of the cells by chronic exposure to S1P.
One might hypothesize that the inverse is also true (i.e., that in the absence of S1P in circulation, MC responses are dampened). SphK1/SphK2 double-deficient mice (S1P-less mice), in which SphK1 is deleted after birth, mostly in the hematopoietic compartment to avoid the lethality of deleting both kinases embryonically, have no circulating S1P (Fig. 2E) [29]. An IgE/Ag challenge of these mice resulted in a significantly reduced histamine response (Fig. 2F), consistent with our previous results in mice with triple allele mutations (SphK1−/−/SphK2+/−), which had reduced levels of S1P and histamine responses [25]. Our data support the model that S1P in circulation can influence MC responsiveness. Despite the reduced MC histamine release, the lack of S1P in circulation was found to be detrimental to the recovery from anaphylaxis, resulting in a high incidence of lethality (ref. [29] and data not shown). The increased lethality in the absence of S1P was shown to be a consequence of an increase in vascular permeability [29] and deregulated vascular tone [35]. These effects on anaphylaxis recovery were independent of MC-mediated initiation of anaphylaxis [35].
Thus, both the genetic models and pharmacologic tools used to manipulate S1P levels in vivo, strongly support the hypothesis that circulating S1P levels contribute to determining MC responsiveness in vivo.
S1P lyase-deficient MCs or MCs chronically exposed to S1P showed enhanced responsiveness
To better characterize which MC responses are affected by an S1P-rich environment and to explore the underlying mechanisms, we investigated whether prolonged exposure of MCs to S1P during differentiation in vitro might mimic the observed in vivo enhancement of MC responses. We used 2 different experimental approaches: MCs from S1P lyase-deficient mice, which produce and secrete high levels of S1P, even when not stimulated (Supplemental Fig. S1), and from their WT counterparts; and MCs from the bone marrow of WT mice cultured in vitro in the presence of exogenous S1P during differentiation.
In vitro-differentiated S1P lyase-deficient BMMCs and PDMCs showed increased degranulation responses to IgE/Ag when compared to the WT cells (Fig. 3A). Furthermore, production of cytokines such as IL-6 and TNFα was increased (Fig. 3B). We next investigated whether the enhanced effector responses were associated with changes in calcium mobilization, an important mechanism controlling MC responses [40, 41]. As shown in Fig. 3C, calcium mobilization was enhanced in S1P lyase-deficient MCs in response to IgE/Ag. In the absence of extracellular calcium, however, there were no differences between WT and S1P lyase-deficient cells in the calcium mobilized from intracellular stores (Fig. 3D). Replenishment of calcium 4 min after Ag stimulation demonstrated that the influx of calcium was enhanced in S1P lyase-deficient cells relative to WT MCs (Fig. 3D). These findings show that S1P lyase-deficient MCs had an enhanced calcium influx in response to IgE/Ag stimulation, a property known to enhance MC responsiveness [42, 43].
Although S1P lyase-deficient BMMCs had high intracellular levels of S1P, they also secreted more S1P into the medium (Supplemental Fig. S1A). Thus, to determine whether the enhanced MC responsiveness after chronic exposure to S1P is a consequence of intracellular or extracellular exposure, we treated BMMCs with different concentrations of S1P during their differentiation from bone marrow progenitors. The BMMCs that differentiated in the presence of various concentrations of S1P showed increased degranulation (Fig. 4A), and their cytokine release was also enhanced by stimulation with IgE/Ag, particularly at lower concentrations of S1P (Fig. 4B). Similar to S1P lyase-deficient BMMCs, calcium mobilization by IgE/Ag stimulation was augmented in these cells (Fig. 4C); this effect was also found to be a consequence of increased calcium influx (Fig. 4D).
These findings suggested that the changes in MC calcium mobilization and effector responses induced by chronic exposure to S1P differ from those induced by acute S1P treatment (compare Figs. 3 and 4 with Supplemental Fig. S1B and the supplemental figure S1A in ref. 25). To determine whether the enhanced effector responses were maintained in the absence of S1P, treatment with S1P was discontinued for a week or more, and MC degranulation was measured. As shown in Supplemental Fig. 2A, MCs weaned from S1P still had an enhanced degranulation for at least 2 wk after suspension of treatment, suggesting that treatment of S1P during the differentiation process induced stable phenotypic changes. We hypothesized that if such changes were long lasting, then MCs that were chronically treated with S1P should maintain a hyper-reactive phenotype in vivo. To test this possibility, MC-deficient KitW-sh/W-sh mice were reconstituted with BMMCs grown in the presence or absence of S1P, and, after approximately 12 wk were allowed for engraftment, the mice were subjected to an anaphylactic challenge. As shown in Supplemental Fig. 2B, MC-deficient KitW-sh/W-sh mice reconstituted with BMMCs grown in the presence of S1P had stronger anaphylactic responses to IgE/Ag than did those reconstituted with untreated BMMCs. MC engraftment was similar between these 2 groups, in that similar numbers of MCs were detected in the stomach, skin, lungs, and lymph nodes (data not shown).
These findings showed that the long-term exposure of MCs to higher concentrations of S1P (whether by treatment of MCs during differentiation or exposure due to an inability to catabolize S1P) induced changes in the responsiveness of MCs to IgE/Ag stimulation. This hyper-responsive phenotype was maintained for at least 12 wk (as seen in vivo), demonstrating stability in the newly acquired MC phenotype.
S1P lyase-deficiency or continuous exposure to S1P caused gene expression changes in MCs
To gain a better understanding of the stable changes induced by prolonged exposure to S1P, we profiled gene expression patterns by using Affymetrix microarrays with mRNA from WT, Sgpl1−/−, and WT MCs cultured with S1P during their differentiation. Identification of the major pathways affected by prolonged exposure to increased S1P levels by Gene Ontology (GO) pathway analysis (Gen Set-ANOVA) demonstrated that, similar to that described for mRNA from the liver of S1P lyase-deficient mice [14], sphingolipid, glycosphingolipid, and membrane lipid catabolic processes were markedly affected in the S1P lyase-deficient MCs (P<0.0001) but not in the WT MCs differentiated in the presence of S1P (Supplemental Table S1). This finding argues that the intrinsic changes in the catabolism of sphingolipids were unlikely to be responsible for the differences in MC responsiveness, since these pathways were not significantly altered in S1P-treated MCs (Supplemental Table S1). Comparisons of S1P lyase-deficient to WT BMMCs and S1P-treated to untreated BMMCs indicated that genes associated with the biological processes of cholesterol and phospholipid efflux and transport, lipoprotein biosynthetic processes, protein amino acid lipidation, and secretory processes were significantly upregulated (Supplemental Table S1). The genes most affected in these pathways included those for lipid transporters (particularly ABCA1), proteins that exchange lipids between membranes (such as STARD5 and PITPNC1), proteins involved in vesicular trafficking of lipids (VPS4B), and proteins involved in exocytosis processes (such as synapsin 2, syntaxin-binding protein 1, and neurexin 1), suggesting that lipid transfer between cellular compartments and vesicle trafficking within those cells may be enhanced, consistent with their increased sensitivity to exocytosis. In general, the results of this analysis suggest that changes in the metabolism of S1P or exposure of MCs to S1P during their differentiation induce vast changes in gene expression that may affect the enhanced effector function of these cells.
A comparison of the affected genes (by >1.5-fold) revealed a change in expression of 443 of them in S1P lyase-deficient versus WT BMMCs, whereas 1935 were differentially affected in S1P-treated WT BMMCs relative to untreated WT cells. Of note, no differences were detected in the expression of the various S1PR in the microarray or by qRT-PCR (data not shown). The expression of 31 genes was significantly enhanced or reduced, commonly in the S1P lyase-deficient and the S1P-treated BMMCs. Because several genes showed elevated expression in an Affymetrix analysis, we narrowed our focus to 5 putative candidate genes linked to ion channel regulation or degranulation. These genes included (Table 1) NPY1R, a receptor that has been linked to the modulation of calcium channels in the rat hippocampus [44]; Rock2, which, along with its other family members, is known to regulate acrosomal exocytosis and actin polymerization in sea urchin sperm [45]; MT2, a protein that governs the intracellular levels of metals such as zinc that are known to regulate MC degranulation [46]; SLMAP, which has been linked to excitation–contraction coupling in the heart and has a mutation that has been shown to impair the trafficking of hNav 1.5 [47, 48], a cardiac sodium channel; and CNTN4 or BIG-2, a GPI-anchoring glycoprotein belonging to the Ig superfamily, which plays an important role in neural circuit formation [30, 49] and could associate with CNTN-associated protein 2, a protein that regulates the localization of the voltage-gated potassium channel Kv1 [50, 51]. The expression of NPYR1, MT2, and Rock2, determined by either qRT-PCR or Western blot, was not consistently elevated in both models of chronic S1P exposure (data not shown). We confirmed a reproducible increase in mRNA expression and protein, respectively, by qRT-PCR and Western blot for CNTN4 (Fig. 5A) and SLMAP (Fig. 5B) in both Sgpl−/− MCs (left panels) and MCs treated with S1P (right panels). Available antibodies against CNTN4 and SLMAP were neither sensitive nor highly specific, but showed protein bands (64–70 kDa for CNTN4 and 80–90 kDa for SLMAP) that were upregulated in Sgpl1-deficient MCs and S1P-treated cells by approximately 2–3-fold as shown in Fig. 5. However, the major CNTN4 isoform (∼135 kDa in the olfactory bulb; data not shown and ref. [30]) was not detectable in BMMCs. These findings show that regulation of CNTN4 and SLMAP expression was associated with prolonged exposure of MCs to S1P.
Table 1. Candidate Genes Linked to Ion Fluxes or Degranulation that were Similarly Affected in S1P Lyase (Sgpl1)-deficient MCs or MCs Cultured in the Presence of S1P.
| Gene name | Gene symbol |
Sgpl1−/− vs. WT |
S1P treated vs.nontreated |
||
|---|---|---|---|---|---|
| P | Fold change | P | Change | ||
| Contactin 4 | Cntn4 | 0.017 | 1.60 | 0.024 | 1.52 |
| Metallothionein 2 | Mt2 | 0.002 | 1.74 | 0.001 | 2.15 |
| Neuropeptide Y receptor Y1 | Npy1r | 0.001 | 1.56 | 0.001 | 1.73 |
| Regulator of G-protein signaling 13 | Rgs-13 | 0.002 | 1.61 | 0.001 | 1.94 |
| Rho-associated coiled-coil containing protein kinase 2 | Rock2 | 0.005 | 1.51 | 0.041 | 1.53 |
| Rho-GTPase activating protein 5 | Arhgap5 | 0.001 | 1.68 | 0.0001 | 2.13 |
| Sarcolema associated protein | Slmap | 0.014 | 1.55 | 0.016 | 1.53 |
Affymetrix microarray gene expression analysis of purified RNA corresponding to the indicated types of BMMCs. P < 0.05 was considered significant.
Increased expression of CNTN4 in S1P lyase-deficient MCs contributed to enhanced calcium and hyperdegranulation responses
SLMAP and CNTN4 and their family members have been linked to ion flux regulation in cardiac myocytes [47, 48, 52, 53] and brain cells [26, 51, 54], respectively. Thus, we investigated whether their increased expression could influence MC degranulation and calcium responses by IgE/Ag stimulation. Using lentiviral-based shRNA silencing, we reduced the expression of SLMAP and CNTN4 in both WT and S1P lyase-deficient BMMCs (Fig. 6A, top and bottom panels, and Supplemental Fig. S3A–C).
Silencing of CNTN4, but not of SLMAP, expression eliminated the enhanced degranulation of S1P lyase-deficient BMMCs, when compared to WT cells (Fig. 6B). Consistent with the reversal of the hyperdegranulation phenotype, silencing of CNTN4 in S1P lyase-deficient BMMCs also reversed the increased calcium responses in these cells (Fig. 6C, compare top and middle panels). However, silencing of SLMAP did not change the calcium responses in S1P lyase-deficient versus WT BMMCs (Fig. 6C, compare top and bottom panels). Silencing of CNTN4 did not affect degranulation or calcium responses in WT BMMCs, a result that was confirmed in BMMCs from CNTN4-deficient mice, which showed no differences with respect to their WT counterparts in these responses (Supplemental Fig. S3). These findings argue for a gain of function induced by overexpression of CNTN4 in MCs following prolonged S1P exposure. They suggest that CNTN4 upregulation, in the context of changes in the expression of other genes induced by an alteration in S1P metabolism, enhances calcium mobilization and degranulation in MCs.
DISCUSSION
The worldwide incidence of allergic disorders has been on the rise for more than a decade, and although much is known about the triggers and the events that occur during the allergic response, there is still a paucity of information on many aspects of the regulation of the immune response and on the mechanisms underlying these complex disorders [55]. Part of the challenge in understanding the causes of the qualitative and quantitative variations in allergic disease, as manifested in an individual, is the identification of factors that may enhance the susceptibility or severity of disease in genetically predisposed individuals. Herein, we describe a function for the sphingolipid mediator S1P in promoting an enhanced immediate and delayed hypersensitivity response through its ability to phenotypically reshape the MC. Prolonged exposure of MCs to S1P markedly changed the gene expression profile of these cells and the increased expression of a GPI-anchored adhesion molecule, CNTN4, which, in this new S1P-induced gene product landscape, acquired an additional function contributing to the upregulation of calcium mobilization and degranulation in MCs.
S1P generated in the nucleus was found to bind and inhibit HDAC activity and increase histone acetylation, a process that has been linked to epigenetic gene regulation [13, 16]. These observations raise the possibility that a variety of stimuli found in an altered microenvironment (i.e., in individuals susceptible to type-2 immune responses) can activate SphK or inhibit S1P catabolism, increasing the levels of S1P in the nucleus of MCs or other cells, thus affecting transcriptional regulation. S1P itself could be one of the deregulated stimuli found in the blood or tissues of susceptible individuals. S1P may be partly transported from the extracellular environment into cells by a mechanism involving dephosphorylation into sphingosine and subsequent rephosphorylation into S1P by SphK2 [56], which can be localized or translocated to the nucleus [57, 58]. S1P outside of cells can also act through its receptors (S1PR1 to −5), which can use SphKs as part of their signaling repertoire and induce changes in the nuclear content of S1P. Given that the changes in expression of CNTN4 (and other genes) were similar between S1P lyase-deficient MCs (which have high levels of intracellular S1P) and S1P-treated MCs, one might argue that changes in gene expression are more likely due to exogenous S1P engagement of S1PRs and subsequent localized signals that cause enhancement of certain genes. It is well documented that specific S1PRs affect the differentiation of immune cells and can promote a shift in the type of immune response, both in vitro and in vivo (reviewed in ref. [5]). Although our microarray and qRT-PCR did not show significant differences in the expression of the most abundant MC S1PR between S1P lyase-deficient MCs or S1P-treated MCs compared with their respective controls, several regulators of small G-proteins and the cytoskeleton were upregulated in those cells (Table 1), which may suggest enhanced activity of G-protein-coupled receptors, such as S1PRs. However, S1PR2, the receptor with the most abundant message in the MCs, did not appear to be involved, because S1PR2-deficient BMMCs chronically exposed to S1P during differentiation showed enhanced degranulation responses, when compared with the nontreated S1PR2-deficient BMMCs (data not shown). Nevertheless, this does not exclude the potential involvement of other S1PRs alone or in combination, a subject that merits further investigation.
Regulation of the levels of S1P in the microenvironment, together with a regulation of S1PR expression in DCs and T cells during the generation of the immune response [5, 19, 59, 60], can contribute to a shift toward a dominant Th2 response. In this context, mice with highly elevated S1P levels caused by deficiency in S1P lyase showed high levels of inflammatory cytokines, some of them of the Th2 type, such as IL-6. The marked neutrophilia in the blood of these mice contributes to the proinflammatory phenotype [39]; however, our findings of a high number of degranulated MCs in the tissues and of elevated tryptase levels in the blood argue that MCs, which abundantly secrete TNFα and IL6, may contribute to the initiation or amplification of the proinflammatory response, particularly considering that these mice have a low number of lymphocytes and neutrophils in their tissues [15, 39]. Moreover, the findings argue that the dysregulation of S1P levels in these mice lead to a changed MC phenotype, whereby these cells are more easily susceptible to degranulation than are MCs in WT mice.
Our results show that MCs exposed exogenously to S1P during differentiation or MCs with an S1P lyase deficiency possessed altered expression of a group of genes involved in exocytosis and secretion, coupling of excitation to secretion, or formation of stable fusion pores in neuronal cells (Supplemental Table S1). This, together with the enhanced pathways of Ag processing (MHC type II gene loci), which require Golgi sorting and endosomal transport [61], and pathways for cholesterol and phospholipid transport, including proteins involved in vesicular lipid exchange and trafficking, strongly suggests active vesicular trafficking, both endo- and exocytic, which is consistent with the hyper-reactive phenotype and the presence of tryptase in the serum of S1P lyase-deficient mice (Supplemental Table S1). An analogous upregulation of gene types related to vesicular trafficking and release was observed after CRH stimulation of MCs [62]. Stimulation with CRH has been associated with increased vascular permeability in skin disorders exacerbated by stress [63, 64]. This gene profile was found to be distinct from an upregulation of mostly inflammation-related genes in MCs stimulated with IgE/Ag. The gene array profile of MCs with prolonged exposure to S1P is also suggestive of a broader functional shift. These MCs are not only more responsive to IgE/Ag stimulation, but may be more supportive of processing and presentation of Ags via MHC class II [65] and of neuronal-like exocytosis (Supplemental Table S1).
Along those lines, CNTN4, an axonal adhesion protein that affects neuronal function [26] and has a mutation that is linked to autism spectrum disorders [66], was also upregulated (Table 1 and Fig. 5A). In IgE/Ag-stimulated S1P lyase-deficient MCs, CNTN4 acquired a function in regulating calcium mobilization and degranulation that is not observed in WT cells. CNTNs, a subfamily of the Ig superfamily [49], have been shown to interact with multiple types of adhesion molecules [26, 49, 67] and with voltage-gated sodium channels and potassium channels through Caspr [51, 68], regulating the surface expression and localization of these channels and, thus, their physiological function [54, 69, 70]. The mechanisms by which increased expression of CNTN4 causes a gain of function and increases calcium influx in MCs is unclear. However, it is clear that the modulation of other ion channels such as potassium and sodium channels plays an important role in the activation of MCs, because they regulate cell membrane potential and thus can influence calcium influx [71]. The K+ channel iKCA1 has been found to potentiate calcium influx and degranulation of human lung MCs [72]. Given that CNTN4 has the potential to be in a complex with Caspr and seemingly regulates the localization of ion channels, including K+ channels such as Kv1 [50, 51], it seems plausible that overexpression of CNTN4 in MCs may cause a gain of function through regulation of expression or function of such Ca2+-activated K+ currents, which are known to regulate calcium influx [71, 73].
In summary, our study revealed a previously unappreciated aspect of the complex regulation of the allergic response by S1P: modification of the MC phenotype toward a hyperresponsive phenotype by prolonged exposure of the cells to this sphingolipid. This effect differs from that of the acute exposure of MCs to S1P or of the newly formed S1P on IgE/Ag stimulation of MCs, in that the changes described herein required the gene expression modification that occurs after prolonged exposure to S1P, to manifest an enhanced MC responsiveness. One of the changes was the enhanced expression levels of the neuronal adhesion protein CNTN4, which mediates the enhanced calcium influx and degranulation in cells with disturbed S1P metabolism. Our findings further suggest that prolonged S1P in the cellular microenvironment also enhances MC Ag presentation, potentially affecting the adaptive immune system. S1P may also enhance the MCs ability to interact with neuronal cell types via adhesion molecules such as CNTN4. This result could be of relevance in allergic skin disorders, where cutaneous nerves and MCs establish significant interactions that may affect certain aspects of the disease [74], but also in other pathologic conditions involving MCs and neuronal cells, such as in proximity to the brain–blood barrier during multiple sclerosis or during stress-induced allergic inflammation [75]. Ultimately, this work has shown that prolonged exposure of MCs to S1P results in a new gene product landscape, possibly epigenetic, that modifies the phenotypic responses of these cells and influences the allergic response.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the NIAMS and the NIDDK of the U.S. National Institutes of Health. We are grateful for the support of the Laboratory Animal Care and Use Section of the Office of Science and Technology, NIAMS.
We are also grateful to Dr. Barbara Dema for help with statistical analysis.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ABCA1
- ATP-binding cassette, subfamily A1
- BMMC
- bone marrow mast cell
- Caspr
- contactin-associated proteins
- CNTN4
- contactin-4
- CRH
- corticotropin releasing hormone
- DNP-2
- 4-dinitrophenyl
- FcεRI
- high-affinity receptor for IgE
- GPI
- glycosylphosphatidylinositol
- HDAC
- histone deacetylase
- MC
- mast cell
- MT2
- metallothionein 2
- NIAMS
- National Institute of Arthritis and Musculoskeletal and Skin Diseases
- NPY1R
- neuropeptide Y receptor 1
- NIDDK
- National Institute of Diabetes and Digestive and Kidney Diseases
- NT
- nontarget
- PDMC
- peritoneal derived mast cell
- PITPNC1
- phosphatidyl-inositol transfer protein, cytoplasmic 1
- qRT-PCR
- quantitative RT-PCR
- RMA
- robust multichip average
- Rock2
- rho-associated coiled-coil—containing protein kinase 2
- RPMI
- Rose Park Memorial Institute
- SCF
- stem cell factor
- Sgpl1
- sphingosine-1-phosphate lyase gene
- S1P
- sphingosine-1-phosphate
- S1PR
- sphingosine-1-phosphate receptor
- shRNA
- small hairpin RNA
- SLMAP
- sarcolemma associated protein
- SphK
- sphingosine kinase
- STARD5
- StAR-related lipid transfer domain containing 5
- THI
- 2-acetyl-4(5)-[1(R),2(S),3(R),4-tetrahydroxybutyl]-imidazole
- TRAF2
- tumor necrosis factor-receptor—associated factor 2
- VPS4B
- vacuolar protein-sorting 4, homolog B
- WT
- wild type
AUTHORSHIP
A.O., Y.K., L.D.W., and M.L.A. conducted experiments and generated the necessary reagents, cells, and mice. A.O., J.R., and R.L.P. were involved in summarizing the data and writing the manuscript and contributed to the conceptual development of this work. W.C. conducted the microarrays and their analysis. T.K.G. and Y.Y. provided the bone marrow of the CNTN4-deficient mice and the CNTN4 antibody.
DISCLOSURE
The authors declare no financial conflicts of interest.
REFERENCES
- 1. Galli S. J., Grimbaldeston M., Tsai M. (2008) Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 8, 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rivera J., Olivera A. (2007) Src family kinases and lipid mediators in control of allergic inflammation. Immunol. Rev. 217, 255–268 [DOI] [PubMed] [Google Scholar]
- 3. Galli S. J., Kalesnikoff J., Grimbaldeston M. A., Piliponsky A. M., Williams C. M., Tsai M. (2005) Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 23, 749–786 [DOI] [PubMed] [Google Scholar]
- 4. Galli S. J., Borregaard N., Wynn T. A. (2011) Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat. Immunol. 12, 1035–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rivera J., Proia R. L., Olivera A. (2008) The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8, 753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwab S. R., Cyster J. G. (2007) Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8, 1295–1301 [DOI] [PubMed] [Google Scholar]
- 7. Spiegel S., Milstien S. (2011) The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olivera A. (2008) Unraveling the complexities of sphingosine-1-phosphate function: the mast cell model. Prostaglandins Other Lipid Mediat. 86, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olivera A., Rivera J. (2011) An emerging role for the lipid mediator sphingosine-1-phosphate in mast cell effector function and allergic disease. Adv. Exp. Med. Biol. 716, 123–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olivera A., Urtz N., Mizugishi K., Yamashita Y., Gilfillan A. M., Furumoto Y., Gu H., Proia R. L., Baumruker T., Rivera J. (2006) IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J. Biol. Chem. 281, 2515–2525 [DOI] [PubMed] [Google Scholar]
- 11. Sanchez T., Hla T. (2004) Structural and functional characteristics of S1P receptors. J. Cell Biochem. 92, 913–922 [DOI] [PubMed] [Google Scholar]
- 12. Alvarez S. E., Harikumar K. B., Hait N. C., Allegood J., Strub G. M., Kim E. Y., Maceyka M., Jiang H., Luo C., Kordula T., Milstien S., Spiegel S. (2010) Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465, 1084–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., Spiegel S. (2009) Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bektas M., Allende M. L., Lee B. G., Chen W., Amar M. J., Remaley A. T., Saba J. D., Proia R. L. (2010) Sphingosine 1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J. Biol. Chem. 285, 10880–10889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vogel P., Donoviel M. S., Read R., Hansen G. M., Hazlewood J., Anderson S. J., Sun W., Swaffield J., Oravecz T. (2009) Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS One 4, e4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ihlefeld K., Claas R. F., Koch A., Pfeilschifter J. M., Meyer Zu Heringdorf D. (2012) Evidence for a link between histone deacetylation and Ca(2)+ homoeostasis in sphingosine-1-phosphate lyase-deficient fibroblasts. Biochem. J. 447, 457–464 [DOI] [PubMed] [Google Scholar]
- 17. Pantoja M., Fischer K. A., Ieronimakis N., Reyes M., Ruohola-Baker H. (2013) Genetic elevation of sphingosine 1-phosphate suppresses dystrophic muscle phenotypes in Drosophila. Development 140, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Donati C., Marseglia G., Magi A., Serrati S., Cencetti F., Bernacchioni C., Nannetti G., Benelli M., Brunelli S., Torricelli F., Cossu G., Bruni P. (2011) Sphingosine 1-phosphate induces differentiation of mesoangioblasts towards smooth muscle: a role for GATA6. PLoS One 6, e20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Idzko M., Panther E., Corinti S., Morelli A., Ferrari D., Herouy Y., Dichmann S., Mockenhaupt M., Gebicke-Haerter P., Di Virgilio F., Girolomoni G., Norgauer J. (2002) Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 16, 625–627 [DOI] [PubMed] [Google Scholar]
- 20. Loh K. C., Leong W. I., Carlson M. E., Oskouian B., Kumar A., Fyrst H., Zhang M., Proia R. L., Hoffman E. P., Saba J. D. (2012) Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through an S1PR2/STAT3 signaling pathway. PLoS One 7, e37218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Price M. M., Kapitonov D., Allegood J., Milstien S., Oskeritzian C. A., Spiegel S. (2009) Sphingosine-1-phosphate induces development of functionally mature chymase-expressing human mast cells from hematopoietic progenitors. FASEB J. 23, 3506–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sato C., Iwasaki T., Kitano S., Tsunemi S., Sano H. (2012) Sphingosine 1-phosphate receptor activation enhances BMP-2-induced osteoblast differentiation. Biochem. Biophys. Res. Commun. 423, 200–205 [DOI] [PubMed] [Google Scholar]
- 23. Kihara A., Ikeda M., Kariya Y., Lee E. Y., Lee Y. M., Igarashi Y. (2003) Sphingosine-1-phosphate lyase is involved in the differentiation of F9 embryonal carcinoma cells to primitive endoderm. J. Biol. Chem. 278, 14578–14585 [DOI] [PubMed] [Google Scholar]
- 24. Olivera A., Allende M. L., Proia R. L. (2012) Shaping the landscape: metabolic regulation of S1P gradients. Biochim. Biophys. Acta 183, 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olivera A., Mizugishi K., Tikhonova A., Ciaccia L., Odom S., Proia R. L., Rivera J. (2007) The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity 26, 287–297 [DOI] [PubMed] [Google Scholar]
- 26. Zuko A., Bouyain S., van der Zwaag B., Burbach J. P. (2011) Contactins: structural aspects in relation to developmental functions in brain disease. Adv. Protein. Chem. Struct. Biol. 84, 143–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmahl J., Raymond C. S., Soriano P. (2007) PDGF signaling specificity is mediated through multiple immediate early genes. Nat. Genet. 39, 52–60 [DOI] [PubMed] [Google Scholar]
- 28. Kuhn R., Schwenk F., Aguet M., Rajewsky K. (1995) Inducible gene targeting in mice. Science 269, 1427–1429 [DOI] [PubMed] [Google Scholar]
- 29. Camerer E., Regard J. B., Cornelissen I., Srinivasan Y., Duong D. N., Palmer D., Pham T. H., Wong J. S., Pappu R., Coughlin S. R. (2009) Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest. 119, 1871–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaneko-Goto T., Yoshihara S., Miyazaki H., Yoshihara Y. (2008) BIG-2 mediates olfactory axon convergence to target glomeruli. Neuron 57, 834–846 [DOI] [PubMed] [Google Scholar]
- 31. Charles N., Watford W. T., Ramos H. L., Hellman L., Oettgen H. C., Gomez G., Ryan J. J., O'Shea J. J., Rivera J. (2009) Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity 30, 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saitoh S., Arudchandran R., Manetz T. S., Zhang W., Sommers C. L., Love P. E., Rivera J., Samelson L. E. (2000) LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity 12, 525–535 [DOI] [PubMed] [Google Scholar]
- 33. Dillahunt S. E., Sargent J. L., Suzuki R., Proia R. L., Gilfillan A., Rivera J., Olivera A. (2013) Usage of sphingosine kinase isoforms in mast cells is species and/or cell type determined. J. Immunol. 190, 2058–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grimbaldeston M. A., Chen C. C., Piliponsky A. M., Tsai M., Tam S. Y., Galli S. J. (2005) Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 167, 835–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olivera A., Eisner C., Kitamura Y., Dillahunt S., Allende L., Tuymetova G., Watford W., Meylan F., Diesner S. C., Li L., Schnermann J., Proia R. L., Rivera J. (2010) Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J. Clin. Invest. 120, 1429–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwab S. R., Pereira J. P., Matloubian M., Xu Y., Huang Y., Cyster J. G. (2005) Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 [DOI] [PubMed] [Google Scholar]
- 37. Yamashita Y., Charles N., Furumoto Y., Odom S., Yamashita T., Gilfillan A. M., Constant S., Bower M. A., Ryan J. J., Rivera J. (2007) Cutting edge: genetic variation influences Fc epsilonRI-induced mast cell activation and allergic responses. J. Immunol. 179, 740–743 [DOI] [PubMed] [Google Scholar]
- 38. Weber C., Krueger A., Munk A., Bode C., Van Veldhoven P. P., Graler M. H. (2009) Discontinued postnatal thymocyte development in sphingosine 1-phosphate-lyase-deficient mice. J. Immunol. 183, 4292–4301 [DOI] [PubMed] [Google Scholar]
- 39. Allende M. L., Bektas M., Lee B. G., Bonifacino E., Kang J., Tuymetova G., Chen W., Saba J. D., Proia R. L. (2011) Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 286, 7348–7358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baba Y., Nishida K., Fujii Y., Hirano T., Hikida M., Kurosaki T. (2008) Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat. Immunol. 9, 81–88 [DOI] [PubMed] [Google Scholar]
- 41. Vig M., Kinet J. P. (2009) Calcium signaling in immune cells. Nat. Immunol. 10, 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suzuki R., Liu X., Olivera A., Aguiniga L., Yamashita Y., Blank U., Ambudkar I., Rivera J. (2010) Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J. Leukoc. Biol. 88, 863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoshimaru T., Suzuki Y., Inoue T., Ra C. (2009) L-type Ca2+ channels in mast cells: activation by membrane depolarization and distinct roles in regulating mediator release from store-operated Ca2+ channels. Mol. Immunol. 46, 1267–1277 [DOI] [PubMed] [Google Scholar]
- 44. Silva A. P., Carvalho A. P., Carvalho C. M., Malva J. O. (2003) Functional interaction between neuropeptide Y receptors and modulation of calcium channels in the rat hippocampus. Neuropharmacology 44, 282–292 [DOI] [PubMed] [Google Scholar]
- 45. De la Sancha C. U., Martinez-Cadena G., Lopez-Godinez J., Castellano L. E., Nishigaki T., Darszon A., Garcia-Soto J. (2007) Rho-kinase (ROCK) in sea urchin sperm: its role in regulating the intracellular pH during the acrosome reaction. Biochem. Biophys. Res. Commun. 364, 470–475 [DOI] [PubMed] [Google Scholar]
- 46. Yamasaki S., Sakata-Sogawa K., Hasegawa A., Suzuki T., Kabu K., Sato E., Kurosaki T., Yamashita S., Tokunaga M., Nishida K., Hirano T. (2007) Zinc is a novel intracellular second messenger. J. Cell Biol. 177, 637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen X., Ding H. (2011) Increased expression of the tail-anchored membrane protein SLMAP in adipose tissue from type 2 Tally Ho diabetic mice. Exp. Diabetes Res. 2011, 421982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ishikawa T., Sato A., Marcou C. A., Tester D. J., Ackerman M. J., Crotti L., Schwartz P. J., On Y. K., Park J. E., Nakamura K., Hiraoka M., Nakazawa K., Sakurada H., Arimura T., Makita N., Kimura A. (2012) A novel disease gene for Brugada syndrome: sarcolemmal membrane-associated protein gene mutations impair intracellular trafficking of hNav1.5. Circ. Arrhythm. Electrophysiol. 5, 1098–1107 [DOI] [PubMed] [Google Scholar]
- 49. Yoshihara Y., Kawasaki M., Tamada A., Nagata S., Kagamiyama H., Mori K. (1995) Overlapping and differential expression of BIG-2, BIG-1, TAG-1, and F3: four members of an axon-associated cell adhesion molecule subgroup of the immunoglobulin superfamily. J. Neurobiol. 28, 51–69 [DOI] [PubMed] [Google Scholar]
- 50. Dityatev A., Bukalo O., Schachner M. (2008) Modulation of synaptic transmission and plasticity by cell adhesion and repulsion molecules. Neuron. Glia. Biol. 4, 197–209 [DOI] [PubMed] [Google Scholar]
- 51. Poliak S., Gollan L., Martinez R., Custer A., Einheber S., Salzer J. L., Trimmer J. S., Shrager P., Peles E. (1999) Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron 24, 1037–1047 [DOI] [PubMed] [Google Scholar]
- 52. Guzzo R. M., Salih M., Moore E. D., Tuana B. S. (2005) Molecular properties of cardiac tail-anchored membrane protein SLMAP are consistent with structural role in arrangement of excitation-contraction coupling apparatus. Am. J. Physiol. Heart Circ. Physiol. 288, H1810–H1819 [DOI] [PubMed] [Google Scholar]
- 53. Nader M., Westendorp B., Hawari O., Salih M., Stewart A. F., Leenen F. H., Tuana B. S. (2012) Tail-anchored membrane protein SLMAP is a novel regulator of cardiac function at the sarcoplasmic reticulum. Am. J. Physiol. Heart Circ. Physiol. 302, H1138–H1145 [DOI] [PubMed] [Google Scholar]
- 54. Swanwick R. S., Pristera A., Okuse K. (2010) The trafficking of Na(V)1.8. Neurosci. Lett. 486, 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pulendran B., Artis D. (2012) New paradigms in type 2 immunity. Science 337, 431–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sensken S. C., Bode C., Nagarajan M., Peest U., Pabst O., Graler M. H. (2010) Redistribution of sphingosine 1-phosphate by sphingosine kinase 2 contributes to lymphopenia. J. Immunol. 184, 4133–4142 [DOI] [PubMed] [Google Scholar]
- 57. Wattenberg B. W., Pitson S. M., Raben D. M. (2006) The sphingosine and diacylglycerol kinase superfamily of signaling kinases: localization as a key to signaling function. J. Lipid Res. 47, 1128–1139 [DOI] [PubMed] [Google Scholar]
- 58. Spiegel S., Milstien S. (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4, 397–407 [DOI] [PubMed] [Google Scholar]
- 59. Bajwa A., Huang L., Ye H., Dondeti K., Song S., Rosin D. L., Lynch K. R., Lobo P. I., Li L., Okusa M. D. (2012) Dendritic cell sphingosine 1-phosphate receptor-3 regulates Th1-Th2 polarity in kidney ischemia-reperfusion injury. J. Immunol. 189, 2584–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schulze T., Golfier S., Tabeling C., Rabel K., Graler M. H., Witzenrath M., Lipp M. (2011) Sphingosine-1-phospate receptor 4 (S1P(4)) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 25, 4024–4036 [DOI] [PubMed] [Google Scholar]
- 61. Stumptner-Cuvelette P., Benaroch P. (2002) Multiple roles of the invariant chain in MHC class II function. Biochim. Biophys. Acta 1542, 1–13 [DOI] [PubMed] [Google Scholar]
- 62. Theoharides T. C., Kempuraj D., Tagen M., Conti P., Kalogeromitros D. (2007) Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 217, 65–78 [DOI] [PubMed] [Google Scholar]
- 63. Donelan J., Boucher W., Papadopoulou N., Lytinas M., Papaliodis D., Dobner P., Theoharides T. C. (2006) Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc. Natl. Acad. Sci. U. S. A. 103, 7759–7764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Theoharides T. C., Donelan J. M., Papadopoulou N., Cao J., Kempuraj D., Conti P. (2004) Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol. Sci. 25, 563–568 [DOI] [PubMed] [Google Scholar]
- 65. Kambayashi T., Allenspach E. J., Chang J. T., Zou T., Shoag J. E., Reiner S. L., Caton A. J., Koretzky G. A. (2009) Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J. Immunol. 182, 4686–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shimoda Y., Watanabe K. (2009) Contactins: emerging key roles in the development and function of the nervous system. Cell Adh. Migr. 3, 64–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rutishauser U. (2000) Defining a role and mechanism for IgCAM function in vertebrate axon guidance. J. Cell Biol. 149, 757–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Falk J., Bonnon C., Girault J. A., Faivre-Sarrailh C. (2002) F3/contactin, a neuronal cell adhesion molecule implicated in axogenesis and myelination. Biol. Cell 94, 327–334 [DOI] [PubMed] [Google Scholar]
- 69. Boyle R. (2000) Morphology of lumbar-projecting lateral vestibulospinal neurons in the brainstem and cervical spinal cord in the squirrel monkey. Arch. Ital. Biol. 138, 107–122 [PubMed] [Google Scholar]
- 70. Poliak S., Salomon D., Elhanany H., Sabanay H., Kiernan B., Pevny L., Stewart C. L., Xu X., Chiu S. Y., Shrager P., Furley A. J., Peles E. (2003) Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J. Cell Biol. 162, 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bradding P. (2005) Mast cell ion channels. Chem. Immunol. Allergy 87, 163–178 [DOI] [PubMed] [Google Scholar]
- 72. Duffy S. M., Berger P., Cruse G., Yang W., Bolton S. J., Bradding P. (2004) The K+ channel iKCA1 potentiates Ca2+ influx and degranulation in human lung mast cells. J. Allergy Clin. Immunol. 114, 66–72 [DOI] [PubMed] [Google Scholar]
- 73. McCloskey C., Jones S., Amisten S., Snowden R. T., Kaczmarek L. K., Erlinge D., Goodall A. H., Forsythe I. D., Mahaut-Smith M. P. (2010) Kv1.3 is the exclusive voltage-gated K+ channel of platelets and megakaryocytes: roles in membrane potential, Ca2+ signalling and platelet count. J. Physiol. 588, 1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kakurai M., Monteforte R., Suto H., Tsai M., Nakae S., Galli S. J. (2006) Mast cell-derived tumor necrosis factor can promote nerve fiber elongation in the skin during contact hypersensitivity in mice. Am. J. Pathol. 169, 1713–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Theoharides T. C., Alysandratos K. D., Angelidou A., Delivanis D. A., Sismanopoulos N., Zhang B., Asadi S., Vasiadi M., Weng Z., Miniati A., Kalogeromitros D. (2012) Mast cells and inflammation. Biochim. Biophys. Acta 1822, 21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.