Alpha-toxin expression by CA-MRSA USA300, compromises human monocyte plasma membrane integrity, with a redundant role influencing overall cytokine expression during early human blood infection.
Keywords: Staphylococcus aureus, pathogenesis, virulence, monocyte, T cell, chemokine
Abstract
This investigation examines the influence of α-toxin (Hla) expression by CA-MRSA on host immune cell integrity and cytokine expression during infection of human blood. Flow cytometry analysis of human blood infected by Staphylococcus aureus PFGE type USA300 or a USA300Δhla demonstrated that Hla expression significantly increased plasma membrane permeability of human CD14+ monocytes. The increased susceptibility of human CD14+ monocytes to Hla toxicity paralleled the high cell-surface expression on these cell types of ADAM10. USA300 rapidly associated with PMNs and monocytes but not T cells following inoculation of human blood. Transcription analysis indicated a strong up-regulation of proinflammatory cytokine transcription following infection of human blood by USA300 and USA300Δhla. CBAs and ELISAs determined that IL-6, IL-10, TNF-α, IFN-γ, IL-1β, IL-8, and IL-4 are significantly up-regulated during the initial phases of human blood infection by USA300 relative to mock-infected blood but failed to distinguish any significant differences in secreted cytokine protein concentrations during infection by USA300Δhla relative to USA300. Collectively, these findings demonstrate that expression of Hla by USA300 has a significant impact on human CD14+ monocyte plasma membrane integrity but is not exclusively responsible for the proinflammatory cytokine profile induced by USA300 during the initial stages of human blood infection.
Introduction
S. aureus is a prominent Gram-positive bacterial pathogen capable of causing a wide range of disease in humans and other animals. Although the introduction of antibiotic therapy in the early 1940s significantly decreased mortality associated with invasive S. aureus infection, emerging antibiotic-resistant strains, such as MRSA, continually challenge our ability to successfully treat S. aureus disease [1]. The acquisition of antibiotic resistance has classically been associated with healthcare settings, but within the last 20 years, strains of CA-MRSA have been identified that are thought to have acquired antibiotic resistance from within the community [2]. In particular, CA-MRSA, identified by PFGE as USA300, is the major cause of soft-tissue infections in the United States [3] and has been noted for increased virulence relative to previously characterized hospital-associated-MRSA [2, 4, 5]. Alarmingly, USA300 is now established within some healthcare settings where it has become a significant cause of bloodstream infections [6]. The mechanisms driving the enhanced virulence observed for USA300 are not entirely clear, although increased expression of core S. aureus virulence factors, such as α-toxin (Hla), is thought to be important [4].
The heptameric pore-forming toxin Hla is strongly conserved between different S. aureus strains and is known to be active against numerous host cell types, including human monocytes, lymphocytes, and eosinophils [7, 8]. This toxin is critical for S. aureus virulence during animal models of infections [9, 10] and low concentrations of Hla are thought to induce programmed cell death in susceptible cell types that leads to subsequent host-cell plasma membrane permeability [7, 8]. Transcription of hla requires the S. aureus response regulatory system SaeR/S [11], while Hla expression is under the additional regulatory influence of the Agr quorum-sensing regulatory system [12]. Transcription of hla is up-regulated by S. aureus following exposure to human blood, in response to phagocytosis by human PMNs, by microbicides specifically produced by PMNs, and during infection of human tissue [5, 13–15]. Accumulating evidence suggests that increased expression of Hla by USA300, relative to other S. aureus, contributes to the enhanced virulence observed during infections caused by this strain [4, 16]. Indeed, the prominent impact of this toxin on S. aureus virulence has prompted investigations demonstrating the efficacy of therapies specifically targeting Hla to inhibit S. aureus disease [9, 10]. Recent work has also demonstrated that Hla specifically binds to ADAM10 and requires this host cell-surface protein for toxicity [17, 18]. Yet, the specific host cell types significantly impacted by S. aureus expression of Hla during human infection and how Hla influences these cell types to dictate the outcome of disease are just starting to be addressed.
To better understand which types of host cells are targeted by Hla during human blood pathogenesis and the relative influence of Hla on the host immune response during S. aureus infection, a model of human blood infection that examined host cell plasma membrane permeability and cytokine expression was used. Results from these findings indicate that Hla expression by USA300 causes significant monocyte plasma membrane damage during the initial stage of human blood infection, while having a relatively minimal impact on overall host cytokine expression in response to USA300 infection.
MATERIALS AND METHODS
Bacterial strains and culture
S. aureus strains were cultured in tryptic soy broth supplemented with 0.5% glucose and harvested at ME growth as described below and performed previously [5, 7, 11]. OD of S. aureus cultures was determined using a NanoDrop 2000c (Thermo Scientific, Wilmington, DE, USA). S. aureus PFGE-type USA300 (strain LAC) [19] was used to generate a USA300Δhla by temperature-sensitive allelic gene replacement as described previously [7]. USA300Δhla Comp was generated in previous reports [7]. USA300ΔsaeR/S was generated in previous studies [11]. For human blood infection assays, 1 mL S. aureus grown to ME growth phase (2×108 CFU/mL, as determined by plating on tryptic soy agar) was harvested by centrifugation (5000 g, 5 min, 4°C), washed twice with 1 mL DPBS, and resuspended in 1 mL DPBS. S. aureus was then diluted with DPBS to desired CFU/mL prior to inoculation.
Infection of human blood with S. aureus
Heparinized venous blood samples from healthy donors were collected in accordance with a protocol approved by the Institutional Review Board for Human Subjects at Montana State University. All donors provided written consent to participate in the study. Blood-infection assays were performed as described previously [7]. Briefly, freshly drawn heparinized human blood was inoculated with indicated concentrations of S. aureus (50 μl S. aureus in DPBS/mL human blood) or DPBS mock-infection control in a 5-mL culture tube and incubated at 37°C with end-over-end rotation (20 rpm). Pilot experiments for these studies were performed to determine the lowest inoculum that induced the measurable signal within several hours postinfection using the assays described. A lower inoculum produced similar trends to experimental results described, although the signal magnitude was decreased and experimental kinetics slowed.
Analysis of host cell plasma membrane permeability
To analyze plasma membrane permeability of PMN, CD3+ lymphocyte, and CD14+ monocyte cells during S. aureus infection of human blood, these cell types were isolated postinfection using protocols described previously [7, 20], with the following modifications. Blood (1.5 mL) treated with S. aureus (1×106 CFU/mL blood) or DPBS control, as described above, was collected at 1 h or 3 h postinfection and combined with 7.5 mL room-temperature DPBS in 15 mL conical centrifuge tubes. Samples were underlayed gently with 3 mL Histopaque-1070 (Sigma, St. Louis, MO, USA) and centrifuged (400 g, 4°C, 30 min). For analysis of CD3+ and CD14+ PBMCs, the buffy-coat layer was transferred to a 1.5-mL microcentrifuge tube and centrifuged (400 g, 4°C, 5 min). The supernatant was carefully aspirated and pelleted cells resuspended in 300 μl DPBS. Aliquots (100 μl) of resuspended PBMCs were then incubated with mouse anti-human CD3-APC clone UCHT1 or mouse anti-human CD14-APC clone M5E2 (BD PharMingen, San Diego, CA, USA) for at least 10 min on ice in 5 mL polystyrene, round-bottom flow cytometry tubes. Following incubation, each sample was combined with 1 mL cold DPBS and centrifuged (400 g, 4°C, 5 min). The supernatant was then carefully aspirated and samples resuspended in 400 μl DPBS with 5 μl PI (Invitrogen, Molecular Probes, Carlsbad, CA, USA), a fluorescent DNA-intercalating molecule used to assess plasma membrane permeability. CD3+ lymphocytes or CD14+ monocytes were then analyzed using FACSCalibur (BD Biosciences, San Jose, CA, USA). For analysis of PMNs, the cell pellet produced following Histopaque-1070 gradient centrifugation described above was resuspended in 7 mL sterile H2O and gently mixed for 45 s to lyse red blood cells. Samples were then combined with 7 mL 1.7% NaCl and centrifuged (300 g, 4°C, 10 min). Following centrifugation, the supernatant was removed and PMNs resuspended in 500 μl ice-cold DPBS. Resuspended PMNs were then transferred to 5 mL polystyrene, round-bottom flow cytometry tubes (100 μl aliquots), combined with 400 μl DPBS containing 5 μl PI, and analyzed using FACSCalibur (BD Biosciences) to determine the geometric mean PI+ signal.
Defining human PMN, CD3+, and CD14+ cell-surface expression of ADAM10
To quantify cell-surface expression of ADAM10 on human PMN, CD3+ lymphocytes, and CD14+ monocytes, 1.5 mL human blood was combined with 7.5 mL room-temperature erythrocyte lyses buffer (Buffer EL; Qiagen, Valencia, CA, USA) in a 15-mL conical tube and incubated on ice for 15 min with gentle mixing every 5 min. Samples were centrifuged (800 g, 4°C, 5 min), the supernatant was carefully aspirated and the cell pellet resuspended in 3 mL ice-cold Buffer EL. Samples were centrifuged again (800 g, 4°C, 5 min), supernatant was removed, and cells were resuspended in 400 μl ice-cold DPBS. Cells were labeled with mouse anti-human ADAM10-FITC clone 163,003 (R&D Systems, Minneapolis, MN, USA) or control mouse IgG clone X40 (BD Biosciences), as well as anti-CD3-APC or anti-CD14-APC, as described above, and the geometric mean ADAM10-FITC+ signal was then determined using FACSCalibur (BD Biosciences).
Examining the association of S. aureus with host cells during human blood infection
To examine the association of S. aureus with PMN, CD3+ lymphocyte, and CD14+ monocyte cells during human blood infection, S. aureus harvested at ME, as described above, was incubated with FITC, as described previously [21]. Following incubation, FITC-labeled S. aureus was washed twice, resuspended in DPBS, and used to inoculate freshly drawn human blood, as described above. At 1 h and 3 h postinfection, 1.5 mL samples were collected and red blood cell lysed using EL buffer (Qiagen). For flow cytometry analysis, cells were labeled using anti-CD3-APC or anti-CD14-APC, as described above, and the geometric mean FITC+ signal of PMN, CD3+ lymphocyte, and CD14+ monocyte cells was determined using a FACSCalibur (BD Biosciences). For immunofluorescence microscopy, cells were incubated with anti-CD14-PE clone HCD14 (BioLegend, San Diego, CA, USA) on ice for at least 20 min. Following incubation, each sample was combined with 1 mL cold DPBS and centrifuged (400 g, 4°C, 5 min). The supernatant was then carefully aspirated and samples resuspended in DPBS and spun onto glass plates using Cytospin 4 (Thermo Scientific; 700 rpm, 7 min). Cell smears were allowed to dry for 10 min at room temperature before the addition of ProLong Gold Antifade mounting media with DAPI (Invitrogen, Molecular Probes) and coverslip. Samples were then visualized using a Nikon Eclipse 80i and images captured using a Nikon DS-Ri1 camera.
Cytokine transcription analysis
For cytokine transcription analysis, RNA from human blood components, infected with S. aureus (1×105 CFU/mL), as described above, was purified at 3 h postinfection using RNeasy kit (Qiagen). Purified RNA was analyzed using a human inflammatory cytokine and receptor RT2 Profiler PCR Array Data Analysis (SABiosciences, Qiagen; Catalog #PAHS-011), following the manufacturer's instructions and analysis software (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php) using a 7500 Fast Real-Time PCR system (Applied Biosystems, Life Technologies, Carlsbad, CA, USA).
Cytokine expression analysis
To assess differences in cytokine concentrations that may reflect changes in cytokine transcription defined at 3 h postinfection, cytokine concentrations were determined at 5 h postinfection using ELISAs and CBAs. Human blood was infected with S. aureus (1×105 CFU/mL), as described above, and at 5 h postinfection, samples were centrifuged (500 g, 24°C, 5 min) and plasma stored at <−20°C. Analysis of IL-1β and IL-8 expression was performed using the BD OptEIA Human IL-1β ELISA Kit II and Human IL-8 ELISA Kit II (BD Biosciences), following the manufacturer's protocol. For samples with heat-treated S. aureus, USA300 harvested at ME growth was incubated at 98°C for 25 min. Following incubation, heat-treated USA300 was plated on tryptic soy agar to verify heat inactivation. Heat-treated USA300 was combined with freshly drawn human blood (1×105 CFU heat-treated USA300/mL human blood) and analyzed for IL-1β and IL-8 expression, as described above. Further cytokine expression was determined using the human inflammatory cytokine (IL-6, IL-10, TNF, and IL-12p70) and human Th1/Th2/Th17 cytokine (IL-2, IL-4, IL-6, IL-10, TNF, IFN-γ, and IL-17A) BD CBAs (BD Biosciences), following the manufacturer's protocol and analyzed using a BD LSR II flow cytometer.
Statistical procedures
Unless otherwise noted, statistical analysis was performed using GraphPad Prism version 5 with figures presenting the standard mean signal and error bars indicating the sem.
RESULTS
Hla increases CD14+ monocyte cell membrane permeability significantly during ex vivo infection of human blood
Previous research has established that Hla expression by USA300 promotes programmed cell death of human monocytes, T cells, B cells, and eosinophils that is associated with significantly increased plasma membrane permeability of these cell types [7, 8]. However, these experiments were performed on isolated PBMCs or eosinophils and in the absence of other blood components, leaving the relative influence of Hla expression on these cell types in the context of whole-blood infection by USA300 uncertain. Furthermore, it has been shown that the abundance of the different toxins produced by S. aureus is largely determined by the type of growth media used [4, 22], undermining the validity of in vitro experiments examining the impact of Hla on host cell integrity and function relative to other toxins expressed by USA300. To better understand the influence of Hla expression by S. aureus on different cell types during infection of whole human blood, we used the CA-MRSA PFGE-type USA300 strain LAC, USA300Δhla, and USA300Δhla Comp in an ex vivo model of human blood infection followed by flow cytometry analysis of plasma membrane permeability (Fig. 1).
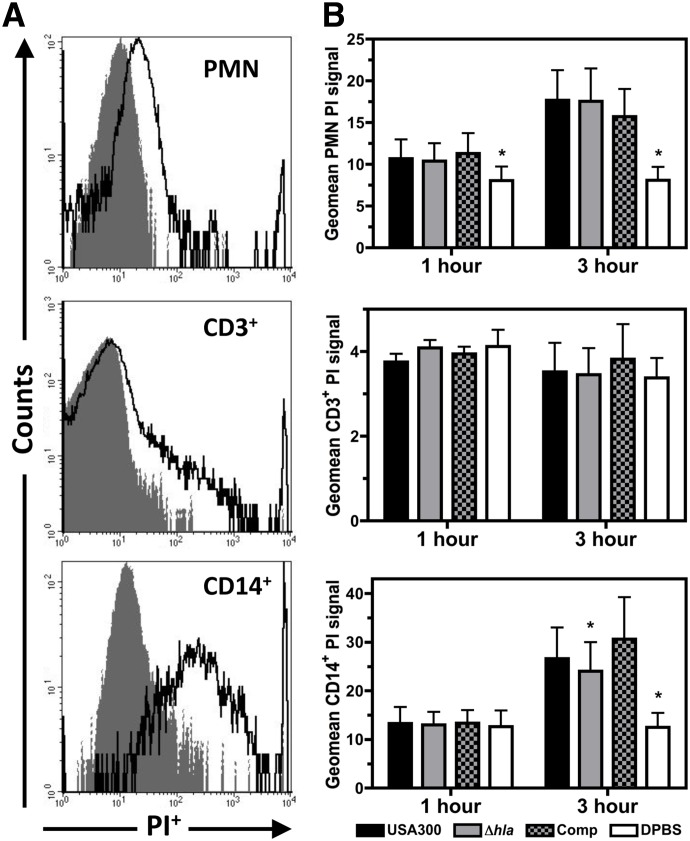
Figure 1. Flow cytometry analysis of PMN, CD3+ lymphocyte, and CD14+ monocyte cells following infection of human blood with USA300, USA300Δhla, USA300Δhla Comp, or DPBS control.
(A) Representative flow cytometry histograms for PI-stained human PMN, CD3+ lymphocyte, and CD14+ monocyte cells following infection with USA300 (dark, solid lines) relative to the uninfected DPBS control (shaded gray areas) at 3 h postinfection with 1 × 106 CFU/mL human blood. (B) Geometric mean PI+ signal of PMN, CD3+ lymphocyte, and CD14+ monocyte cells at 1 h and 3 h postinfection of human blood with 1 × 106 CFU/mL USA300, USA300Δhla (Δhla), USA300Δhla Comp (Comp), or uninfected DPBS control. Data represent four separate experiments using at least three different blood donors, with *P ≤ 0.05, as determined by paired two-tailed t-test relative to samples infected with USA300.
Isolated PMNs and CD14+ monocytes from human blood infected with USA300 exhibited a significantly increased geometric mean PI+ signal relative to PMNs and CD14+ monocytes isolated from DPBS mock-infected blood (Fig. 1A and B), while no significant difference in PI+ signal was detected for CD3+ lymphocytes. In congruence with previous reports [7], Hla expression by USA300 did not influence the PI+ signal of PMNs isolated from infected blood, while the PI+ signal of CD14+ monocytes was significantly decreased in blood infected with USA300Δhla relative to USA300 at 3 h postinfection. No significant difference in PI+ signal of CD14+ monocytes was observed between blood infected with USA300 relative to USA300Δhla Comp. These findings indicate that within 3 h postinfection of human blood by USA300, the plasma membrane permeability of human PMNs and monocytes but not T cells is significantly increased, and Hla expression by USA300 at least partially promotes monocyte plasma membrane permeability while having no significant impact on PMN plasma membrane permeability.
Cell-surface expression of ADAM10 is significantly higher on monocytes relative to PMNs or T cells
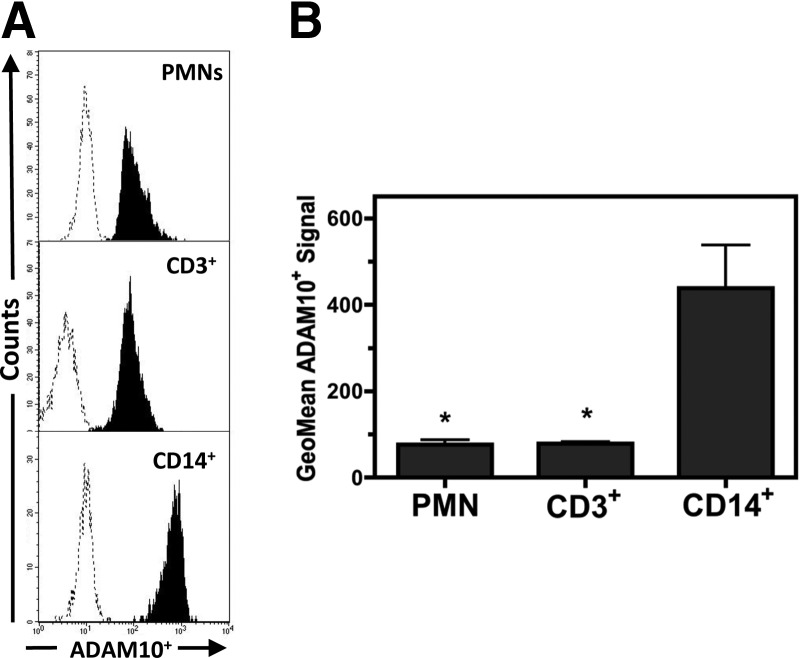
Others have shown that ADAM10 is the specific host cell-surface receptor recognized by Hla and is required for the engagement of this toxin on susceptible host cell types [17, 18]. To determine if different levels of cell-surface ADAM10 expression on PMNs, monocytes, and T cells may be responsible for the varying degrees of susceptibility to Hla observed for these cell types, human blood cells were stained with anti-ADAM10-FITC and examined using flow cytometry (Fig. 2). An increased geometric mean ADAM10-FITC+ signal relative to a nonspecific IgG-FITC control antibody was observed for human PMNs, CD3+ lymphocytes, and CD14+ monocytes (Fig. 2A). However, a significantly higher ADAM10-FITC+ signal was observed for CD14+ monocytes relative to PMNs or CD3+ lymphocytes (Fig. 2B). These findings demonstrate that ADAM10 is expressed on human PMNs, T cells, and monocytes, although significantly higher ADAM10 expression was observed on the surface of monocytes.
Figure 2. Flow cytometry analysis of host cell-surface ADAM10 expression.
(A) Representative flow cytometry histograms of human PMN, CD3+ lymphocyte, and CD14+ monocyte cells stained with anti-ADAM10 (shaded areas) relative to staining with an IgG control (dotted lines). (B) Compiled results comparing the geometric mean ADAM10+ signal of human PMN, CD3+ lymphocyte, and CD14+ monocyte cells. Data represent three separate experiments using different blood donors, with *P ≤ 0.05, as determined by one-way repeated-measures ANOVA with Tukey's post-test relative to CD14+ monocytes.
Association of USA300 with monocytes during ex vivo infection of human blood
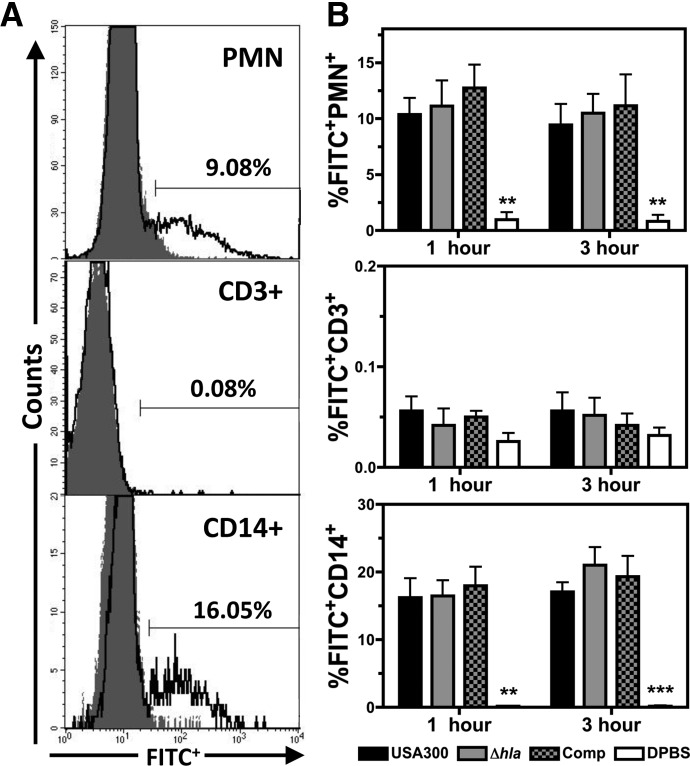
Previous reports demonstrate that S. aureus is ingested by human PMNs in vitro [5, 23]. To determine if USA300 associates with PMNs as well as monocytes during infection of human blood and if Hla expression influences this interaction, human blood was infected ex vivo with FITC-labeled USA300, USA300Δhla, USA300Δhla Comp, or a DPBS control and then analyzed via flow cytometry and immunofluorescence microscopy (Fig. 3). Approximately 10% of PMNs and 15% of CD14+ monocytes were FITC+ during blood infection with FITC-labeled USA300 at 1 h and 3 h postinfection (Fig. 3A and B). In contrast, no significant FITC+ signal was observed for CD3+ lymphocytes following infection of human blood with FITC-labeled USA300. Immunofluorescence microscopy further illustrated the association of CD14+ monocytes with FITC-labeled USA300 during infection of human blood (data not shown). At 1 and 3 h postinfection, no significant difference in FITC+ signal was noted for PMNs or CD14+ monocytes infected with FITC-labeled USA300Δhla relative to FITC-labeled USA300 (Fig. 3B). Together these results indicate that USA300 directly associates with PMNs and monocytes but not T cells during infection of human blood and suggest that Hla expression does not impact the initial interaction of USA300 with human monocytes or PMNs.
Figure 3. The association of USA300 with PMN, CD3+ lymphocyte, and CD14+ monocyte cells following infection of human blood.
(A) Representative flow cytometry histograms of PMNs, CD3+ lymphocyte, and CD14+ monocyte cells at 3 h postinfection with FITC-labeled USA300 (solid lines) relative to a DPBS control (shaded areas), with the percentage of FITC+ human cells indicated. (B) Compiled results illustrating the percentage of FITC+ PMN, CD3+ lymphocyte, and CD14+ monocyte cells at 1 h and 3 h postinfection with 1 × 106 CFU/mL FITC-labeled USA300, USA300Δhla, USA300Δhla Comp, or DPBS control. Data represent five separate experiments using at least three different blood donors, with **P ≤ 0.01, and ***P ≤ 0.001, as determined by paired two-tailed t-test relative to samples infected with USA300.
Hla expression by USA300 during ex vivo infection of human blood reduces transcript abundance of monocyte-associated cytokines
Previous research has shown that Hla transcription is up-regulated during superficial and invasive human infections [13], upon exposure to human blood [14], human PMNs [5], or antimicrobial PMN components [15], and this toxin has been shown to be a major virulence determinant of USA300 during animal models of superficial and systemic infection [9, 10]. However, Hla expression by USA300 did not significantly influence bacterial survival during early ex vivo infection of human blood or during infection of purified human PMNs in vitro [7]. As Hla and other pore-forming toxins generated by S. aureus have been shown to significantly influence host cytokine expression [24–29], we first examined ex vivo transcription of select proinflammatory cytokines in human blood at 3 h postinoculation with USA300, USA300Δhla, or DPBS control (Fig. 4). For USA300 and USA300Δhla infected blood, a significant increase in transcript abundance of numerous cytokines relative to a DPBS mock-infected control was observed. These included the IL-1 superfamily (IL-1α, IL-1β, IL-1RN, and IL-1F9), CC chemokines (CCL3, CCL4, CCL18, CCL20, and CCL23), CXC chemokines (CXCL1, CXCL10, and CXCL11), TNF-α (TNF), complement component 3 (C3), and IL-17C (IL-17C). A significant decrease in the CCR2 (CCR2) for USA300 and USA300Δhla infected blood was also noted while transcript abundance of IL-5 (IL-5) was significantly decreased only in USA300Δhla-infected blood. The proinflammatory cytokine transcriptional response during USA300 infection of human blood was similar to that observed during murine and rat models of S. aureus infection [25, 30, 31]. As opposed to previous reports demonstrating that the expression of Hla by USA300 during murine models of lung infection promotes substantial increases in proinflammatory cytokine transcription at 24 h postinfection [25], the proinflammatory cytokine transcription profile of human blood at 3 h postinfection ex vivo with USA300Δhla was noticeably, although not significantly, enhanced relative to blood infected by USA300-expressing Hla. In particular, the overall transcript abundance of chemokines and cytokines, known to be secreted by monocytes upon stimulation by bacterial components—namely CCL3, CCL4, CCL18, CCL20, the IL-1 superfamily, and TNF [32–35]—was increased in blood infected with USA300Δhla relative to USA300. Monocyte cell-membrane damage caused by Hla might at least partially explain the overall reduction in monocyte-associated proinflammatory cytokine transcript abundance during ex vivo human blood infection; compromised monocyte cell permeability could promote mRNA degradation while having only a minor effect on protein secretion. However, it is possible that other factors may be important for the Hla-dependent modulation of host cytokine transcription observed following inoculation of human blood with USA300. Together, these findings demonstrate that S. aureus infection promotes host proinflammatory cytokine transcription and imply that the expression of Hla by USA300 reduces the abundance of monocyte-associated cytokine transcripts during ex vivo infection of human blood.
Figure 4. Hla expression modulates proinflammatory cytokine transcript abundance during USA300 infection of human blood.
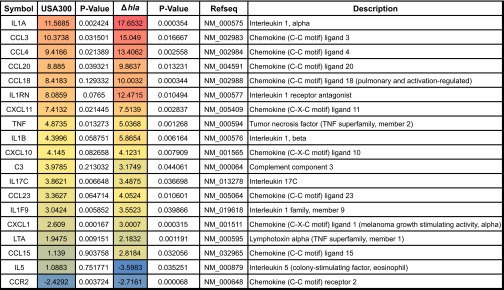
Cytokine transcription profiles at 3 h postinfection of human blood with 1 × 105 CFU/mL USA300 or USA300Δhla relative to DPBS mock-infected blood. Only genes that exhibited at least a twofold change in transcript abundance and a P value < 0.05 for USA300- or USA300Δhla-infected blood relative to the DPBS mock-infected control are shown. Data represent compiled results from three separate experiments using different blood donors. Fold-changes and P values were calculated using SABiosciences web-based software, as described in Materials and Methods.
Proinflammatory cytokine concentrations are not significantly influenced by Hla during ex vivo infection of human blood by USA300
Prior studies have illustrated that Hla facilitates caspase-1 activation through the NLRP3 inflammasome induced by S. aureus lipoproteins to promote IL-1β secretion [27, 36]. Others have suggested that Hla promotes detection of muramyl dipeptide by the NOD2 resulting in secretion of IL-1β and IL-8 by THP-1 monocytes [26].
As Hla increased monocyte plasma membrane permeability and influenced IL-1β transcript abundance during infection of human blood, we examined the influence of Hla on IL-1β and IL-8 concentrations in human blood at 5 h postinfection with USA300, USA300Δhla, USA300Δhla Comp, DPBS control, and heat-treated USA300 (Fig. 5A), using ELISAs, which elucidated significant increases in IL-1β and IL-8 cytokine levels in human blood following infection with USA300 relative to a mock-infected blood or inoculation with heat-inactivated USA300.
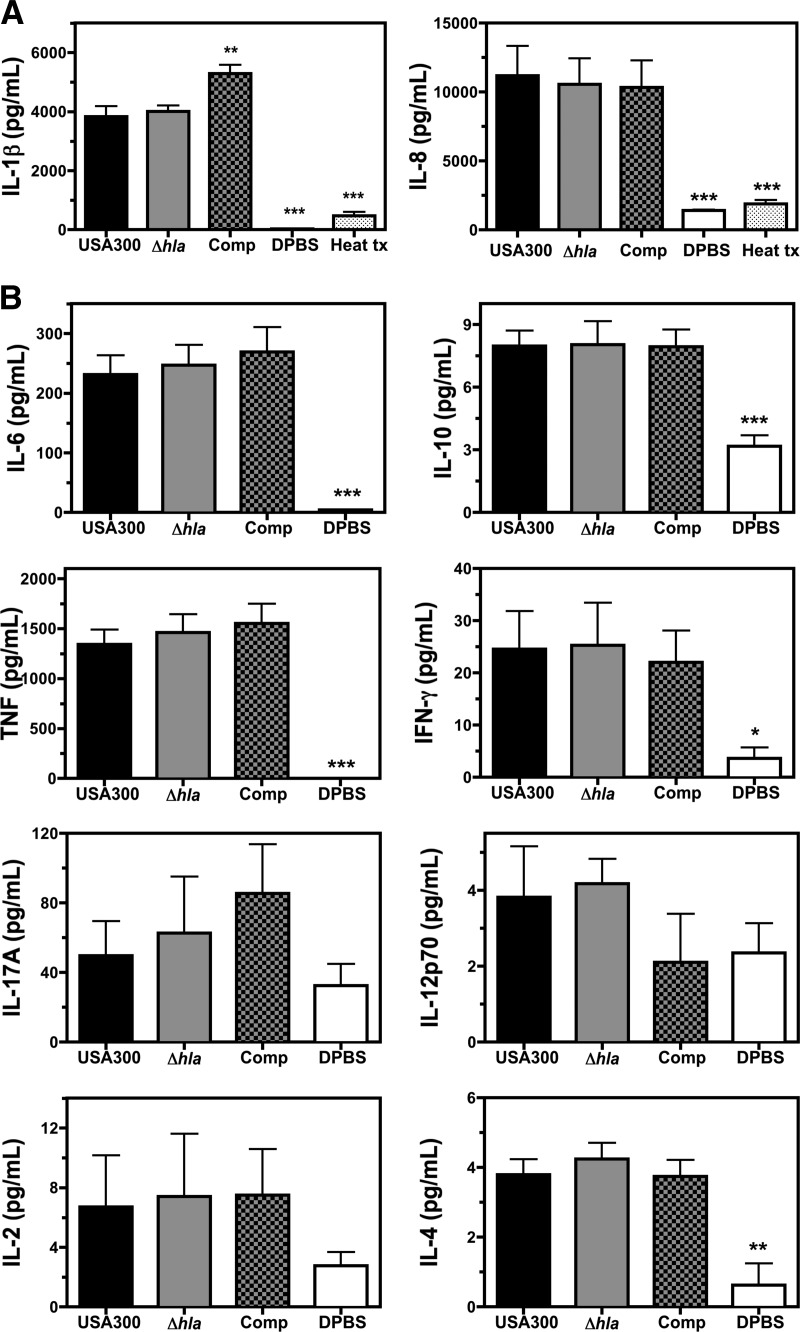
Figure 5. The influence of Hla on cytokine expression following infection of human blood with USA300.
(A) Concentrations of IL-1β and IL-8, as determined by ELISA at 5 h postinfection of human blood with 1 × 105 CFU/mL USA300, USA300Δhla (Δhla), USA300Δhla Comp (Comp), DPBS control, or USA300 incubated at ≥98°C for 20 min prior to infection (Heat tx). (B) CBA analysis of IL-6, IL-10, TNF-α, IFN-γ, IL-17A, IL-12p70, IL-2, and IL-4 expression in human blood at 5 h postinfection with 1 × 105 CFU/mL USA300, USA300Δhla, USA300Δhla Comp, or DPBS control. Data represent at least four separate experiments using at least three different blood donors, with *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001, as determined by one-way repeated-measures ANOVA with Tukey's post-test relative to samples infected with USA300.
No differences in IL-1β or IL-8 cytokine concentrations could be detected in blood infected with USA300Δhla relative to USA300. However, the concentration of IL-1β was significantly higher in blood infected with USA300Δhla Comp relative to USA300, suggesting that artificial overexpression of Hla by USA300 promotes IL-1β secretion during infection of human blood.
To further explore the influence of Hla expression by USA300 on host cytokine expression, CBAs were used to quantify IL-6, IL-10, TNF-α, IFN-γ, IL-17, and IL-12p70 cytokine abundance at 5 h postinfection of human blood with USA300, USA300Δhla, USA300Δhla Comp, or DPBS mock-infected control (Fig. 5B). Increases in concentrations of the proinflammatory cytokines IL-6, TNF-α, and IFN-γ were observed following infection with USA300 relative to a DPBS control. Levels of the anti-inflammatory cytokines IL-10 and IL-4 were also increased during infection, although concentrations of these cytokines were relatively low. No significant differences in IL-6, IL-10, TNF-α, IFN-γ, or IL-4 concentrations were detected in blood inoculated with USA300, USA300Δhla, or USA300Δhla Comp. No significant differences in IL-17, IL-12p70, and IL-2 were detected between infected and uninfected samples. These findings demonstrate a substantial increase in the abundance of the proinflammatory cytokines IL-6, TNF-α, IL-1β, and IL-8 following ex vivo infection of human blood with USA300 and suggest that Hla plays a redundant role with other USA300 components in promoting the proinflammatory host response to S. aureus infection as indicated by others [27].
DISCUSSION
Although Hla expression did not influence human PMN plasma membrane integrity, this toxin significantly increased human monocyte plasma membrane permeability following USA300 infection in accordance with previous results [7]. The relatively high susceptibility of monocytes to Hla following the initial exposure of USA300 to human blood corresponds to the high cell-surface expression of ADAM10 found on human monocytes compared with human PMNs and T cells. Recent work has demonstrated that Hla directly interacts with ADAM10 on the host cell surface and manipulates the activity of this metalloprotease to advance S. aureus pathogenesis during murine models of pathogenesis [17, 18]. Together with previous reports, findings within this investigation suggest that the high abundance of ADAM10 on the surface of human monocytes is responsible for the relative high toxicity of Hla toward these cell types during infection of human blood. Although Hla significantly influenced human monocyte permeability under the conditions tested within this investigation, future studies are needed to confirm the biological relevance of these findings.
No difference in CD3+ lymphocyte permeability to PI was noted following S. aureus infection of human blood at the doses tested, indicating that T cell plasma membrane integrity is relatively uninfluenced by S. aureus infection at early time-points during bloodstream infection. These findings appear to contradict previous studies indicating that Hla is the primary toxin produced by USA300 responsible for causing human T cell plasma membrane damage [7]. However, this investigation examined T cell plasma membrane integrity in the context of whole-blood infection ex vivo while prior research examined partially purified human PBMCs under in vitro conditions. The relatively low levels of T cell-surface ADAM10 expression suggests the concentration of Hla produced by USA300 under the conditions tested within this report are insufficient for inducing T cell plasma membrane damage.
Results from this investigation show that S. aureus rapidly associates with PMNs following infection of human blood and suggest a subsequent loss of host cell plasma membrane integrity as indicated by previous research [5, 23]. S. aureus was also closely associated with human monocytes within 1 h of inoculation of human blood and these cell types exhibited significantly increased cell membrane damage within 3 h postinoculation, again implying that the close association of host cell types with S. aureus leads to host cell destruction. It should be noted that this investigation does not address the long-term impact of host cell internalization by USA300 as emerging evidence indicates that some PMNs and monocytes that survive following internalization of S. aureus may act as an intercellular reservoir that can further bacterial dissemination during infection in vivo [37–41].
The observed increase of IL-1β, IL-6, IL-8, IL-10, TNF-α, and IFN-γ cytokine concentrations during USA300 infection of human blood is in agreement with previous research demonstrating that S. aureus components are recognized by different host receptors including TLRs, the host cytoplasmic receptor NOD2, and the NLRP3 inflammasome to influence expression of specific host cytokines [42–47]. Hla is thought to facilitate the intracellular recognition of S. aureus components by NLRP3 inflammasome and NOD2 [26, 27, 36], yet host cytokine concentrations during USA300 infection of human blood were independent of Hla expression under the conditions tested in this investigation. It has been demonstrated that S. aureus β-toxin (Hlb) can play a redundant role with Hla in facilitating NLRP3 inflammasome-dependent secretion of IL-1β [27], while others have recently shown that the PVL can also promote IL-1β secretion via the NLRP3 inflammasome [48]. In addition to Hla, Hlb, and PVL, USA300 expresses a number of other pore-forming toxins that may influence cytokine expression in human blood including lukG/H, γ-hemolysins (HlgA, HlgB, HlgC), phenol-soluble modulin peptides, and lukE/D [19].
Infection of human blood by USA300 appeared to promote an early Th17-like host immune response as indicated by increased IL-1β and IL-6 protein concentrations and an up-regulation in host cytokine transcription of IL-17C, CXCL1, CXCL10, CCL3, and CCL20. Expression of IL-1β and IL-6 during infection of human blood is thought to foster a Th17 host immune response as opposed to a Th1 response largely driven by IL-12 expression [49–52]. USA300 infection also induced significantly increased protein concentrations of the anti-inflammatory cytokines IL-4 and IL-10, although their concentrations were much lower than IL-1β or IL-6 concentrations, and their expression may be part of a regulatory feedback loop tempering the robust host inflammatory response to S. aureus infection. Host cytokine transcription of IL-17C as well as numerous cytokines and chemokines associated with Th17 cell polarization such as CXCL1, CXCL10, CCL3, and CCL20 further indicates a Th17-biased immune response toward USA300 infection of human blood [49, 50]. Despite prior reports showing an up-regulation of IL-17 expression during S. aureus infection in humans and in mice [53–55] that is thought to be influenced by expression of Hla [25, 28], no significant increase in IL-17 cytokine concentration was noted during the initial exposure of human blood to USA300 under the conditions tested in this study. However, IL-17 protein concentrations were measured relatively early during the course of infection (5 h postinoculation) and previous studies indicate that longer infection times are required to induce substantial IL-17 expression in human PBMCs [28]. Collectively, human blood cytokine expression analysis suggests that USA300 infection prompts a Th17 host immune response corresponding to other investigations identifying IL-17 as important to host defense against S. aureus infection in humans [54].
Using a model of human blood infection by USA300, this investigation demonstrates that CA-MRSA increases monocyte plasma membrane permeability that is partially dependent on Hla expression. Given that acute S. aureus infections in children increase CD14+ monocyte numbers [56] while chronic S. aureus infections are associated with an impoverished monocyte/macrophage cytokine response [57], the significant impact of Hla on human monocytes observed in this investigation suggests that this toxin plays an important role in determining the outcome of invasive S. aureus disease in humans. Collectively, these findings further our understanding of S. aureus pathogenesis by elucidating the relative impact of Hla produced by CA-MRSA on host immune cell plasma membrane permeability and cytokine expression during infection of human blood.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health grants 5R01AI090046-02, 5R21AI088041-02, GM103500, and P20RR16455-07, as well as Montana State University Agriculture Experiment Station and an equipment grant from the M. J. Murdock Charitable Trust. We thank Drs. Agnieska Rynda-Apple and Steve Swain from the Department of Immunology and Infectious Diseases (Montana State University) for their assistance and support and Dr. Alexander Horswill from the Department of Microbiology (University of Iowa) for providing the plasmid overexpressing Hla used to generate USA300Δhla Comp.
Footnotes
- ADAM10
- a disintegrin and metalloproteinase domain-containing protein 10
- APC
- allophycocyanin
- CA-MRSA
- community-associated methicillin-resistant Staphylococcus aureus
- Hla/Hlb
- α/β-hemolysin
- lukG/H/E/D
- leukocidin G/H/E/D
- ME
- midexponential
- MRSA
- methicillin-resistant Staphylococcus aureus
- NLRP3
- nucleotide-binding oligomerization domain-like receptor, pyrin domain-containing 3
- NOD2
- nucleotide-binding oligomerization domain 2
- PFGE
- pulsed-field gel electrophoresis
- PMN
- polymorphonuclear leukocyte
- PVL
- Panton-Valentine leukocidin
- USA300Δhla
- isogenic deletion mutant of hla in USA300
- USA300Δhla Comp
- isogenic deletion mutant of hla in USA300, complemented with a plasmid overexpressing Hla
AUTHORSHIP
T.K.N. contributed to project design and experimental procedures, analyzed data and figure presentation, and wrote the manuscript. K.B.P. and O.W.Z. contributed to project design, experimental procedures, and data analysis. J.M.V. contributed to project design, data analysis, figure presentation, and manuscript writing.
DISCLOSURES
The authors have declared that there are no competing financial interests.
REFERENCES
- 1. Klevens R. M., Morrison M. A., Nadle J., Petit S., Gershman K., Ray S., Harrison L. H., Lynfield R., Dumyati G., Townes J. M., Craig A. S., Zell E. R., Fosheim G. E., McDougal L. K., Carey R. B., Fridkin S. K., Active Bacterial Core Surveillance (ABCs) MRSA Investigators (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298, 1763–1771 [DOI] [PubMed] [Google Scholar]
- 2. Nygaard T. K., DeLeo F. R., Voyich J. M. (2008) Community-associated methicillin-resistant Staphylococcus aureus skin infections: advances toward identifying the key virulence factors. Curr. Opin. Infect. Dis. 21, 147–152 [DOI] [PubMed] [Google Scholar]
- 3. Moran G. J., Krishnadasan A., Gorwitz R. J., Fosheim G. E., McDougal L. K., Carey R. B., Talan D. A., EMERGEncy ID Net Study Group (2006) Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355, 666–674 [DOI] [PubMed] [Google Scholar]
- 4. Li M., Cheung G. Y., Hu J., Wang D., Joo H. S., Deleo F. R., Otto M. (2010) Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J. Infect. Dis. 202, 1866–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voyich J. M., Braughton K. R., Sturdevant D. E., Whitney A. R., Said-Salim B., Porcella S. F., Long R. D., Dorward D. W., Gardner D. J., Kreiswirth B. N., Musser J. M., DeLeo F. R. (2005) Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175, 3907–3919 [DOI] [PubMed] [Google Scholar]
- 6. Seybold U., Kourbatova E. V., Johnson J. G., Halvosa S. J., Wang Y. F., King M. D., Ray S. M., Blumberg H. M. (2006) Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 42, 647–656 [DOI] [PubMed] [Google Scholar]
- 7. Nygaard T. K., Pallister K. B., Dumont A. L., Dewald M., Watkins R. L., Pallister E. Q., Malone C., Griffith S., Horswill A. R., Torres V. J., Voyich J. M. (2012) α-Toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One 7, e36532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prince L. R., Graham K. J., Connolly J., Anwar S., Ridley R., Sabroe I., Foster S. J., Whyte M. K. (2012) Staphylococcus aureus induces eosinophil cell death mediated by α-hemolysin. PLoS One 7, e31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bubeck Wardenburg J., Schneewind O. (2008) Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 205, 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kennedy A. D., Bubeck Wardenburg J., Gardner D. J., Long D., Whitney A. R., Braughton K. R., Schneewind O., DeLeo F. R. (2010) Targeting of α-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J. Infect. Dis. 202, 1050–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nygaard T. K., Pallister K. B., Ruzevich P., Griffith S., Vuong C., Voyich J. M. (2010) SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J. Infect. Dis. 201, 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montgomery C. P., Boyle-Vavra S., Daum R. S. (2010) Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS One 5, e15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loughman J. A., Fritz S. A., Storch G. A., Hunstad D. A. (2009) Virulence gene expression in human community-acquired Staphylococcus aureus infection. J. Infect. Dis. 199, 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malachowa N., Whitney A. R., Kobayashi S. D., Sturdevant D. E., Kennedy A. D., Braughton K. R., Shabb D. W., Diep B. A., Chambers H. F., Otto M., DeLeo F. R. (2011) Global changes in Staphylococcus aureus gene expression in human blood. PLoS One 6, e18617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palazzolo-Ballance A. M., Reniere M. L., Braughton K. R., Sturdevant D. E., Otto M., Kreiswirth B. N., Skaar E. P., DeLeo F. R. (2008) Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J. Immunol. 180, 500–509 [DOI] [PubMed] [Google Scholar]
- 16. Montgomery C. P., Boyle-Vavra S., Adem P. V., Lee J. C., Husain A. N., Clasen J., Daum R. S. (2008) Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J. Infect. Dis. 198, 561–570 [DOI] [PubMed] [Google Scholar]
- 17. Inoshima I., Inoshima N., Wilke G. A., Powers M. E., Frank K. M., Wang Y., Bubeck Wardenburg J. (2011) A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med. 17, 1310–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilke G. A., Bubeck Wardenburg J. (2010) Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus α-hemolysin-mediated cellular injury. Proc. Natl. Acad. Sci. USA 107, 13473–13478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diep B. A., Gill S. R., Chang R. F., Phan T. H., Chen J. H., Davidson M. G., Lin F., Lin J., Carleton H. A., Mongodin E. F., Sensabaugh G. F., Perdreau-Remington F. (2006) Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367, 731–739 [DOI] [PubMed] [Google Scholar]
- 20. Boyum A. (1968) Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 97, 77–89 [PubMed] [Google Scholar]
- 21. Voyich J. M., DeLeo F. R. (2002) Host-pathogen interactions: leukocyte phagocytosis and associated sequelae. Methods Cell. Sci. 24, 79–90 [DOI] [PubMed] [Google Scholar]
- 22. Graves S. F., Kobayashi S. D., Braughton K. R., Diep B. A., Chambers H. F., Otto M., Deleo F. R. (2010) Relative contribution of Panton-Valentine leukocidin to PMN plasma membrane permeability and lysis caused by USA300 and USA400 culture supernatants. Microbes Infect. 12, 446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kobayashi S. D., Braughton K. R., Palazzolo-Ballance A. M., Kennedy A. D., Sampaio E., Kristosturyan E., Whitney A. R., Sturdevant D. E., Dorward D. W., Holland S. M., Kreiswirth B. N., Musser J. M., DeLeo F. R. (2010) Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J. Innate Immun. 2, 560–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhakdi S., Muhly M., Korom S., Hugo F. (1989) Release of interleukin-1 β associated with potent cytocidal action of Staphylococcal α-toxin on human monocytes. Infect. Immun. 57, 3512–3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frank K. M., Zhou T., Moreno-Vinasco L., Hollett B., Garcia J. G., Bubeck Wardenburg J. (2012) Host response signature to Staphylococcus aureus α-hemolysin implicates pulmonary Th17 response. Infect. Immun. 80, 3161–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hruz P., Zinkernagel A. S., Jenikova G., Botwin G. J., Hugot J. P., Karin M., Nizet V., Eckmann L. (2009) NOD2 contributes to cutaneous defense against Staphylococcus aureus through α-toxin-dependent innate immune activation. Proc. Natl. Acad. Sci. USA 106, 12873–12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Munoz-Planillo R., Franchi L., Miller L. S., Nunez G. (2009) A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J. Immunol. 183, 3942–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niebuhr M., Gathmann M., Scharonow H., Mamerow D., Mommert S., Balaji H., Werfel T. (2011) Staphylococcal α-toxin is a strong inducer of interleukin-17 in humans. Infect. Immun. 79, 1615–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walev I., Weller U., Strauch S., Foster T., Bhakdi S. (1996) Selective killing of human monocytes and cytokine release provoked by sphingomyelinase (β-toxin) of Staphylococcus aureus. Infect. Immun. 64, 2974–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Montgomery C. P., Daum R. S. (2009) Transcription of inflammatory genes in the lung after infection with community-associated methicillin-resistant Staphylococcus aureus: a role for Panton-Valentine leukocidin? Infect. Immun. 77, 2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kielian T., Bearden E. D., Baldwin A. C., Esen N. (2004) IL-1 and TNF-α play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J. Neuropathol. Exp. Neurol. 63, 381–396 [DOI] [PubMed] [Google Scholar]
- 32. Auffray C., Sieweke M. H., Geissmann F. (2009) Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27, 669–692 [DOI] [PubMed] [Google Scholar]
- 33. Menten P., Wuyts A., Van Damme J. (2002) Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 13, 455–481 [DOI] [PubMed] [Google Scholar]
- 34. Schutyser E., Richmond A., Van Damme J. (2005) Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J. Leukoc. Biol. 78, 14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schutyser E., Struyf S., Van Damme J. (2003) The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 14, 409–426 [DOI] [PubMed] [Google Scholar]
- 36. Kebaier C., Chamberland R. R., Allen I. C., Gao X., Broglie P. M., Hall J. D., Jania C., Doerschuk C. M., Tilley S. L., Duncan J. A. (2012) Staphylococcus aureus α-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 205, 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gresham H. D., Lowrance J. H., Caver T. E., Wilson B. S., Cheung A. L., Lindberg F. P. (2000) Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164, 3713–3722 [DOI] [PubMed] [Google Scholar]
- 38. Kubica M., Guzik K., Koziel J., Zarebski M., Richter W., Gajkowska B., Golda A., Maciag-Gudowska A., Brix K., Shaw L., Foster T., Potempa J. (2008) A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One 3, e1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prajsnar T. K., Hamilton R., Garcia-Lara J., McVicker G., Williams A., Boots M., Foster S. J., Renshaw S. A. (2012) A privileged intraphagocyte niche is responsible for disseminated infection of Staphylococcus aureus in a zebrafish model. Cell. Microbiol. 14, 1600–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thwaites G. E., Gant V. (2011) Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat. Rev. Microbiol. 9, 215–222 [DOI] [PubMed] [Google Scholar]
- 41. Koziel J., Maciag-Gudowska A., Mikolajczyk T., Bzowska M., Sturdevant D. E., Whitney A. R., Shaw L. N., DeLeo F. R., Potempa J. (2009) Phagocytosis of Staphylococcus aureus by macrophages exerts cytoprotective effects manifested by the upregulation of antiapoptotic factors. PLoS One 4, e5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller L. S., Cho J. S. (2011) Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 11, 505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang J. E., Jorgensen P. F., Almlof M., Thiemermann C., Foster S. J., Aasen A. O., Solberg R. (2000) Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor α, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infect. Immun. 68, 3965–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frodermann V., Chau T. A., Sayedyahossein S., Toth J. M., Heinrichs D. E., Madrenas J. (2011) A modulatory interleukin-10 response to Staphylococcal peptidoglycan prevents Th1/Th17 adaptive immunity to Staphylococcus aureus. J. Infect. Dis. 204, 253–262 [DOI] [PubMed] [Google Scholar]
- 45. Van Beelen A. J., Zelinkova Z., Taanman-Kueter E. W., Muller F. J., Hommes D. W., Zaat S. A., Kapsenberg M. L., de Jong E. C. (2007) Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27, 660–669 [DOI] [PubMed] [Google Scholar]
- 46. Shimada T., Park B. G., Wolf A. J., Brikos C., Goodridge H. S., Becker C. A., Reyes C. N., Miao E. A., Aderem A., Gotz F., Liu G. Y., Underhill D. M. (2010) Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1β secretion. Cell Host Microbe 7, 38–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watkins R. L., Pallister K. B., Voyich J. M. (2011) The SaeR/S gene regulatory system induces a pro-inflammatory cytokine response during Staphylococcus aureus infection. PLoS One 6, e19939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holzinger D., Gieldon L., Mysore V., Nippe N., Taxman D. J., Duncan J. A., Broglie P. M., Marketon K., Austermann J., Vogl T., Foell D., Niemann S., Peters G., Roth J., Löffler B. (2012) Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J. Leukoc. Biol. 92, 1069–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reynolds J. M., Angkasekwinai P., Dong C. (2010) IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 21, 413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilson N. J., Boniface K., Chan J. R., McKenzie B. S., Blumenschein W. M., Mattson J. D., Basham B., Smith K., Chen T., Morel F., Lecron J. C., Kastelein R. A., Cua D. J., McClanahan T. K., Bowman E. P., de Waal Malefyt R. (2007) Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8, 950–957 [DOI] [PubMed] [Google Scholar]
- 51. Zielinski C. E., Mele F., Aschenbrenner D., Jarrossay D., Ronchi F., Gattorno M., Monticelli S., Lanzavecchia A., Sallusto F. (2012) Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518 [DOI] [PubMed] [Google Scholar]
- 52. Acosta-Rodriguez E. V., Napolitani G., Lanzavecchia A., Sallusto F. (2007) Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8, 942–949 [DOI] [PubMed] [Google Scholar]
- 53. Cho J. S., Pietras E. M., Garcia N. C., Ramos R. I., Farzam D. M., Monroe H. R., Magorien J. E., Blauvelt A., Kolls J. K., Cheung A. L., Cheng G., Modlin R. L., Miller L. S. (2010) IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma C. S., Chew G. Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D. A., Tangye S. G., Cook M. C. (2008) Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205, 1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schmaler M., Jann N. J., Ferracin F., Landmann R. (2011) T and B cells are not required for clearing Staphylococcus aureus in systemic infection despite a strong TLR2-MyD88-dependent T cell activation. J. Immunol. 186, 443–452 [DOI] [PubMed] [Google Scholar]
- 56. Ardura M. I., Banchereau R., Mejias A., Di Pucchio T., Glaser C., Allantaz F., Pascual V., Banchereau J., Chaussabel D., Ramilo O. (2009) Enhanced monocyte response and decreased central memory T cells in children with invasive Staphylococcus aureus infections. PLoS One 4, e5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Szkaradkiewicz A., Karpinski T. M., Zeidler A., Szkaradkiewicz A. K., Masiuk H., Giedrys-Kalemba S. (2012) Cytokine response in patients with chronic infections caused by Staphylococcus aureus strains and diversification of their Agr system classes. Eur. J. Clin. Microbiol. Infect. Dis. 31, 2809–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]