Human fetal lymphocyte lineage identified by CD27 and LIN28B expression during early B-cell development.
Keywords: human fetal liver, B cell development
Abstract
CD27, a member of the TNFR superfamily, is used to identify human memory B cells. Nonetheless, CD27+ B cells are present in patients with HIGM1 syndrome who are unable to generate GCs or memory B cells. CD27+IgD+ fetal B cells are present in umbilical cord blood, and CD27 may also be a marker of the human B1-like B cells. To define the origin of naïve CD27+IgD+ human B cells, we studied B cell development in both fetal and adult tissues. In human FL, most CD19+ cells coexpressed CD10, a marker of human developing B cells. Some CD19+CD10+ B cells expressed CD27, and these fetal CD27+ cells were present in the pro-B, pre-B, and immature/transitional B cell compartments. Lower frequencies of phenotypically identical cells were also identified in adult BM. CD27+ pro-B, pre-B, and immature/transitional B cells expressed recombination activating gene-1, terminal deoxynucleotidyl transferase and Vpre-B mRNA comparably to their CD27− counterparts. CD27+ and CD27− developing B cells showed similar Ig heavy chain gene usage with low levels of mutations, suggesting that CD27+ developing B cells are distinct from mutated memory B cells. Despite these similarities, CD27+ developing B cells differed from CD27− developing B cells by their increased expression of LIN28B, a transcription factor associated with the fetal lymphoid lineages of mice. Furthermore, CD27+ pro-B cells efficiently generated IgM+IgD+ immature/transitional B cells in vitro. Our observations suggest that CD27 expression during B cell development identifies a physiologic state or lineage for human B cell development distinct from the memory B cell compartment.
Introduction
Human B cell development begins in the FL at approximately 7 wk of gestation and continues through fetal development [1–3]. In adults, B cell development predominantly takes place in BM. B cell development is characterized by a coordinated series of gene and membrane antigen expression, with each stage of development defined by a unique combination of surface molecule, Ig, and gene expression. Standard developmental classifications include: pro-B, pre-B, immature B, transitional B, and mature B cells [4–6].
CD27 is generally used as a surface marker of post-GC human memory B cells [7–9] and plasmacytes [10]. However, CD27 expression on human B cells is not restricted to these compartments. Weller et al. [11] recovered a subset of IgM+IgD+CD27+ B cells that carry somatically mutated V(D)J rearrangements from the peripheral blood of patients with HIGM1 syndrome who cannot form GC and claimed that these B cells are precursors of circulating human MZ B cells [12, 13]. Although the origin(s) of human IgM+IgD+CD27+ B cells remains controversial [3, 7, 9, 11–13], evidence indicates that at least some IgM+IgD+CD27+ B cells enter mature B cell pools without T-cell help or antigen-driven clonal expansion [13]. Consistent with these observations and unlike post-GC memory B cells [3, 12, 13], Ig mutation patterns in IgM+IgD+CD27+ B cells appear not to be antigen selected [12, 13].
IgM+IgD+CD27+ B cells can also be detected in umbilical cord blood [11, 14, 15]. As few (approximately 3%) cord blood B lymphocytes are labeled by anti-CD27 mAbs, the initial conclusion was that the number of CD27+ B cells is negligible [14, 15]. Recently, however, this minor CD27+ cord blood B cell compartment was attributed to a distinct lineage of human B1-like B cells [16–18]. Griffin et al. [16] showed that CD20+CD27+CD43+CD70− human cord blood B cells exhibit crucial properties of mouse B-1 B cells, including spontaneous IgM secretion, efficient T-cell stimulation, and tonic BCR signaling. These potentially significant results, however, have been questioned [19, 20].
Nonetheless, these observations raise the possibility that CD27 expression marks a subset of newly formed B cells as well as mature antigen-experienced B cell populations. Consistent with this notion, developing subsets of CD19+ and nonmemory mature B cells have been reported to express CD27 [3, 21, 22]. Scheeren et al. [3] found CD19+CD27+IgD+/− cells in fetal tissues including liver, mesenteric lymph nodes, spleen, and BM. CD19+IgD−CD27+ cells from the FL and fetal BM were shown to lack surface Ig light chain expression but to have CD34 [3]. In pediatric BM samples, Nilsson et al. [21] found CD27 expression on CD19+CD10+ B cells as well as CD19+CD34+ cells. Vaskova et al. [22] also found CD27 expression on CD19+CD10+ B cells in the BM of children. The latter group showed that most of the CD27+CD19+CD10+ B cells expressed CD34 and that virtually all expressed TdT and VpreB [22].
We sought to identify and characterize the earliest human CD27+ B cells and to compare these cells with conventional CD27− developing B cells. Herein, we describe a population of CD27+ developing human B cells present in both FL and adult BM. Indeed, CD27+ cells are detected at each stage of B cell development, although they are significantly more abundant in FL than in adult BM. Gene expression profiles for TdT, RAG-1, and VpreB are comparable in both CD27+ and CD27− developing B cells. In contrast, whether recovered from FL or adult BM, CD27+ pre-B cells exhibited prolonged expression of LIN28B, a transcription factor that is enriched in FL cells and promotes the development of fetal lineage lymphocytes [23]. When placed in cultures that preferentially support fetal lineage human B cell development, CD27+ pro-B cells mature into surface IgM+ immature/transitional B cells significantly more efficiently than do CD27− pro-B cells. Our findings support the conclusion that CD27 expression by developing B cells marks a distinct pathway of human B-lymphocyte development that is most prominent in the fetus.
MATERIALS AND METHODS
Sample collection
Human FL (13 and 19 wk gestation), umbilical cord blood, and adult BM (age: 18–39 years, male or female) samples were obtained in accordance with Duke Institutional Review Board committee guidelines. The samples were obtained after elective terminations, in some cases for fetal defects not known to affect lymphopoiesis. Tissue was homogenized into a single-cell suspension, and leukocytes were isolated via Ficoll density gradient (Lymphoprep, Axis-Shield Laboratory Division, Dundee, Scotland). Samples were frozen and kept in liquid nitrogen until use.
Monoclonal antibodies
The following mAbs specific for human surface antigens were used. Anti-human CD10 PE-Cy7 (clone: HI10a), CD19-APC (HIB19), CD19-APC-Cy7 (SJ25C1), CD27-PE (L128), CD27-APC (M-T271), CD27-V450 (M-T271), CD34-Pacific Blue (581), CD43-FITC (1G10), CD45-Biotin (H130), IgM-FITC (G20-127), IgM-APC-Cy7 (MHM-88), IgD-FITC (IA6-2), IgD-PE (IA6-2), and mouse IgG1 isotype control-FITC (MOPC-21) were purchased from BD Biosciences (San Diego, CA, USA) or BioLegend (San Diego, CA, USA). Streptavidin-Pacific Orange was purchased from Invitrogen (Carlsbad, CA, USA).
Flow cytometry
Both analysis of B cell phenotypes and B cell isolation were performed by flow cytometry. Briefly, the cells were labeled with fluorochrome-conjugated mAb specific for the human surface antigens (listed above) in PBS containing 2% FBS. Bound biotin-conjugated mAbs were revealed by fluorochrome-conjugated streptavidin. Labeled cells were analyzed and sorted by FACS Canto or FACS Aria, with Diva software (BD Biosciences). In some experiments, labeled cells were fixed before analysis with Cytofix/Cytoperm reagent (BD Biosciences), according to the manufacturer's instructions. Doublets were excluded from our analysis and cell sorting by combination(s) of FSC-A vs. FSC-H, FSC-H vs. FSC-W, and SSC-H vs. SSC-W gatings. PI-positive cells (dead cells) were also excluded from our analysis. After exclusion of doublets and dead cells, labeled cells were gated on FSCloSSClo lymphocytes, and then B cell subsets were identified as follows: pro-B, CD45+CD19+CD10+IgM−IgD−CD34+; pre-B, CD45+CD19+CD10+IgM−IgD−CD34−; immature and transitional B, CD45+CD19+CD10+IgM+IgD+/−; and mature B, CD45+CD19+CD10−IgMintIgDhi cells [5, 6].
Gene expression analysis by quantitative PCR
Levels of human TdT, RAG-1, VpreB, and LIN28B mRNA in B cell subsets were determined by quantitative PCR [24, 25]. Briefly, total RNA was extracted from sorted pro-B, pre-B, pro-/pre-B, and immature/transitional B cells from human FL or adult BM and treated with DNase I (Invitrogen). cDNA was synthesized from the RNA by using Superscript III reverse transcriptase (Invitrogen) [24, 25]. The cDNA was used for quantitative PCR, and gene expression was calculated by the comparative threshold method relative to Igβ transcript levels [24, 25]. PCR conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 45 s. Primer sequences are as follows: TdT_F, 5′-TCCACCACGAGCGTCCCACT-3′; TdT_R, 5′-CTTTCCTGCGGGCCAGCTCC-3′; RAG1_F, 5′-TGGAGTGGCACCCCCACACA-3′; RAG1_R, 5′-GTGCTGACGGGCTTGTCTTGCT-3′; VpreB_F, 5′-TCAGCCGGTGCTGCATCAGC-3′; VpreB_R, 5′-GGGGGCCCTGGCTCTTGTCT-3′; LIN28B_F, 5′-ACCTACCACCAAGCTGGCTTCAAT-3′; LIN28B_R, 5′-GGGTTCACTTTGGTCTCTAGTACGGT-3′; Igβ_F, 5′-GTCATGGGATTCAGCACCTT-3′; Igβ_R, 5′-AGCCTTGCTGTCATCCTTGT-3′.
Human pro-B cell culture
Human pro-B cells were placed in a culture known to support differentiation of human fetal pro-B cells [26] as well as mouse B-1 B cell progenitors [27], with modifications. Briefly, CD27− and CD27+ human pro-B cells were sorted from 19 wk FL or adult BM and cultured for 10 and 14 days on a monolayer of MS5 stromal cells [28] (gift of David Baltimore, Pasadena, CA, USA) in RPMI 1640 supplemented with 2-mercaptoethanol (55 μM), l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml) (all from Invitrogen); 10% FBS (Hyclone, Logan UT, USA) and recombinant human cytokines; SCF (20 ng/ml) (Invitrogen); IL-7 (10 ng/ml) (R&D, Minneapolis, MN, USA); TSLP (10 ng/ml) (R&D); and Flt3 ligand (10 ng/ml) (R&D). The culture medium was replaced every 4 days. After they were cultured, the cells were enumerated, labeled with fluorochrome-tagged mAb, and analyzed by flow cytometry.
Amplification of VHDJH rearrangements
Nested PCR was performed to amplify human VHDJH rearrangements from cDNA samples [29], with modifications. Briefly, synthesized cDNA from total RNA extracted from human B cell subsets and the Ramos cell line or total RNA libraries of human adult tonsil (Biochain Institute, Newark, CA, USA) and human FL (Biochain/Stratagene, La Jolla, CA, USA) was subjected to primary PCR with Pfu turbo DNA polymerase (Stratagene) and external primers that recognize VH1-6 and reverse primers that recognize IgM-, IgA-, or IgG-constant regions (listed below). One-twentieth (vol) of the primary PCR product was subjected to a second round of nested PCR, with Pfu turbo DNA polymerase with internal forward primers that recognize VH1-6 in separate reactions, with each reaction containing combined reverse primers specific for IgM-, IgA1-, or IgG-constant regions (listed below). PCR conditions (both primary and secondary PCR) were 95°C for 5 min, followed by 35 cycles at 94°C for 30 s, 55°C for 1 min, and 72°C for 1.5 min. VDJ amplicands were gel purified, ligated into plasmid vectors (Zero Blunt TOPO cloning kit, Invitrogen), and transformed into bacteria (Top10 chemically potent Escherichia coli, Invitrogen). DNA sequences were obtained at the Duke DNA sequencing facility. VDJ rearrangements and point mutations were identified with IMGT/V-QUEST (http://imgt.cines.fr) or the SoDA algorithm [30]. To estimate the PCR error rate, we amplified VDJ transcripts from a human Burkitt's lymphoma B cell line (Ramos [31]) that expresses a rearranged VH gene segment containing 6 point mutations compared with germline IGHV4-34*01. Additional point mutations were considered artifacts of the PCR. Primer sequences used in this study were as follows. External primers: VH1-Ext_F, 5′-CCATGGACTGGACCTGGAGG-3′; VH2-Ext_F, 5′-ATGGACATACTTTGTTCCA-3′; VH3-Ext_F, 5′-CCATGGAGTTTGGGCTGAGC-3′; VH4-Ext_F, 5′-ATGAAACACCTGTGGTTCTT-3′; VH5-Ext_F, 5′-ATGGGGTCAACCGCCATCCT-3′; VH6-Ext_F, 5′-ATGTCTGTCTCCTTCCTCAT-3′; IgM-Ext_R, 5′-CCGACGGGGAATTCTCACAG-3′; IgA-Ext_R, 5′-CGAYGACCACGTTCCCATCT-3′; IgG-Ext_R, 5′-TAGTCCTTGACCAGGCAGC-3′; Internal primers: VH1-Int_F, 5′-CAGGTGCAGCTGGTRCAGTCTGGG-3′; VH2-Int_F, 5′-CAGRGCACCTTGARGGAGTCTGGTCC-3′; VH3-Int_F, 5′-GAGGTKCAGCTGGTGGAGTCTGGG-3′; VH4-Int_F, 5′-CAGGTGCAGCTGCAGGAGTCGG-3′; VH5-Int_F, 5′-GARGTGCAGCTGGTGCAGTCTGGAG-3′; VH6-Int_F, 5′-CAGGTACAGCTGCAGCAGTCAGGTCC-3′; IgM-Int_R, 5′-GGAATTCTCACAGGAGACGAGG-3′; IgA1-Int_R, 5′-GCTGGTGCTGCAGAGGCTCAG-3′; IgG-Int_R, 5′-TCCARGAGCACCTCYGRGRG-3′.
Statistical analysis
The statistical significance of the results was determined by Student's t test, Mann-Whitney's U test, or χ2 test. P < 0.05 was deemed significant.
Online Supplemental Material
Supplemental Fig. S1 shows representative FACS plots for surface molecule expression in 13 wk human FL. Supplemental Fig. S2 shows the average CDR3 length of Ig heavy chain in 19 wk human FL B cell subsets. Supplemental Fig. S3 shows representative FACS histograms of CD43 expression on B cell subsets in 19 wk human FL.
RESULTS
CD27+CD19+CD10+ developing B cells in human FL
To determine the origins of CD27 expression on human B-lineage cells, we analyzed CD19+ human FL cells for expression of CD10, CD27, CD34, CD45, IgM, and IgD by flow cytometry. Consistent with a prior observation [1], the CD19+ B-lineage cells comprised 27.1 ± 14.8% of the CD45+ lymphocytes (FSCloSSClo) in 13 wk FL (Supplemental Fig. S1 and Table 1) and increased to 42.5 ± 5.4% in 19 wk FL (Fig. 1A and Table 1). Some 7.0–8.8% of the CD19+ FL cells expressed CD27 (Fig. 1A and Table 1), and the great majority (≥88%) of both the CD27−CD19+ and the CD27+CD19+ cells coexpressed CD10 (Fig. 1 and Table 1), a marker for developing human B cells (pro-B to transitional B cell stages) [32, 33]. Thus CD27 expression marks a significant fraction of developmentally immature human B cells in FL.
Table 1. Frequency of CD27+ B cell progenitors in FL, umbilical cord blood, adult BM, and peripheral blood.
| aCD19+ | (in CD19+) (%) |
CD27−CD10+ (in CD19+) (%) |
CD27+CD10+ (in CD19+) (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| bCD27+ | cCD10+ |
dIgM−IgD− CD34+ |
eIgM−IgD− CD34− |
fIgM+IgD+/− |
gIgM−IgD− CD34+ |
hIgM−IgD− CD34− |
iIgM+IgD+/− | ||
| FL (13 wk) n = 2 | 27.1 (±14.8) | 7.0 (±0.1) | 91.7 (±6.4) | 19.6 (±2.2) | 40.7 (±8.4) | 20.4 (±13.4) | 4.8 (±0.2) | 1.0 (±0.3) | 0.6 (±0.6) |
| FL (19 wk) n = 3 | 42.5 (±5.4) | 8.8 (±3.6) | 94.1 (±1.6) | 5.5 (±1.6) | 24.7 (±3.3) | 51.9 (±6.0) | 3.8 (±3.1) | 0.6 (±0.3) | 3.1 (±1.8) |
| UCB n = 3 | 3.8 (±2.5) | 2.6 (±0.5) | 33.1 (±5.3) | 0.2 (±0.1) | 0.3 (±0.2) | 20.7 (±5.3) | <0.1 | <0.1 | 0.5 (±0.3) |
| BM (Adult) n = 7 | 13.4 (±5.6) | 18.4 (±5.9) | 31.1 (±7.5) | 6.5 (±2.2) | 17.4 (±3.6) | 4.7 (±2.7) | 0.7 (±0.6) | 0.2 (±0.1) | 0.1 (±0.1) |
| Blood (Adult) n = 2 | 4.0 (±2.3) | 39.8 (±1.8) | 1.2 (±0.6) | <0.1 | <0.1 | 0.3 (±0.1) | <0.1 | 0.1 (±0.1) | 0.2 (±0.0) |
Frequencies of CD19+ cells in CD45+ lymphocytes are shown. Frequencies of
CD27+ cells,
CD10+ cells,
CD27−CD10+IgM−IgD−CD34+ cells,
CD27−CD10+IgM−IgD−CD34− cells,
CD27−CD10+IgM+IgD+/− cells,
CD27+CD10+IgM−IgD−CD34+ cells,
CD27+CD10+IgM−IgD−CD34− cells, and
CD27+CD10+IgM+IgD+/− cells in CD19+ cells (as in 1) are shown.
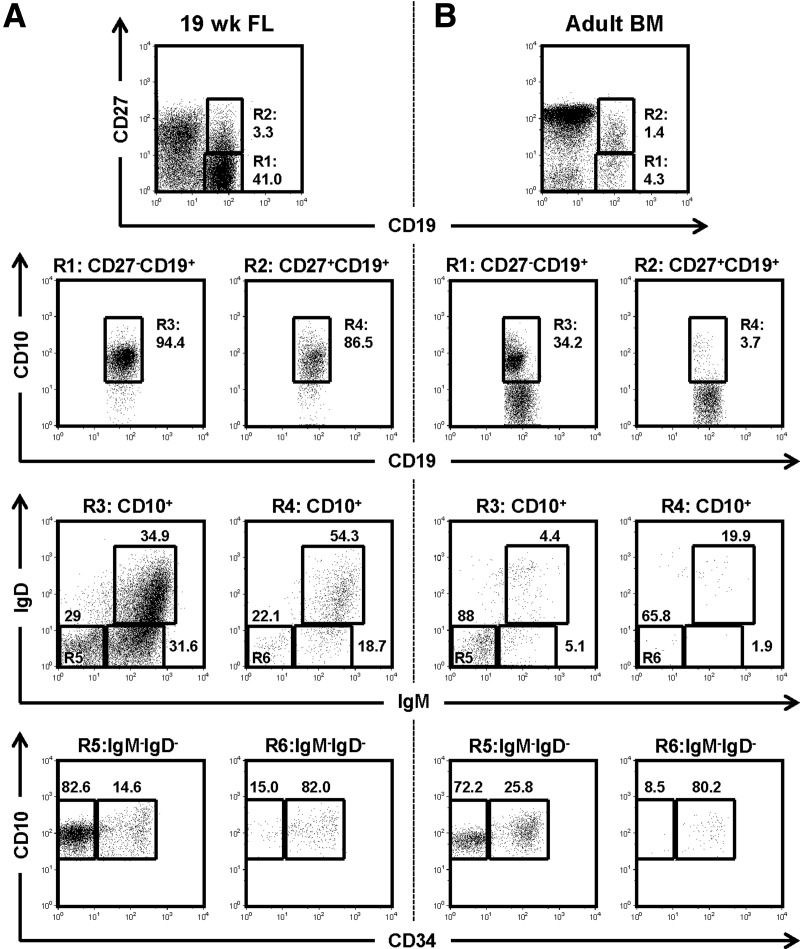
Figure 1. Subsets of human developing B cells in FL and adult BM expressed low levels of CD27.
Typical flow cytometry diagrams of human developing B cell subsets in 19 wk FL (A) and adult BM (B) are shown. After exclusion of doublets and PI+ cells, CD19 and CD27 expression on CD45+FSCloSSClo lymphocytes are shown (top row). CD19+CD27− cells (R1) and CD19+CD27+ cells (R2) were further gated on CD19+CD10+ cells (R3 and R4, respectively) to identify developing B cells (second row). Those developing B cells were analyzed for surface IgM and IgD expression status to identify pro-/pre-B (IgM−IgD−; R5 and R6), immature B (IgM+IgD−), and transitional B (IgM+IgD+) cells (third row). Surface IgM−IgD− cells (R5 and R6) were then analyzed by CD10 and CD34 expression to identify pro-B (CD10+CD34+) and pre-B cells (CD10+CD34−; bottom row). Figures near gating squares indicate frequencies of gated population within each diagram.
The fetal CD27− and CD27+ CD19+CD10+ B cell compartments were further analyzed for surface IgM and IgD expression, to identify IgM−IgD− (pro-/pre-B) and IgM+IgD+/− (immature/transitional B) cells. In 13 wk FL, a quarter (23.7±14.2%) of the CD27−CD10+ B cells expressed surface IgM, whereas a 10th (9.0±9.2%) of the CD27+ developing B cells were IgM positive (Supplemental Fig. S1). The frequency of IgM+ cells in the CD19+CD10+ B cells was higher in the 19 wk FL; surface IgM+ cells were present in 61.6 ± 4.5% and 47.7 ± 35.9% of the CD27− and CD27+ developing B cells, respectively (Fig. 1A).
To delineate human pro-B and pre-B cells, we analyzed CD19+CD10+IgM−IgD− pro-/pre-B cells for CD34 expression [33]. Similar to human adult BM [33], the CD27− pro-/pre-B cell compartment in 19 wk FL comprised a smaller CD34+ pro-B cell compartment (17.9±2.6%) and a larger CD34− pre-B cell compartment (82.1±2.6%) (Fig. 1A). In contrast, The CD27+CD34+CD19+CD10+IgM−IgD− pro-B cell compartment was significantly larger than the CD27+CD34− pre-B cell compartment (85.5±4.0 vs. 14.5±4.0%) (Fig. 1A). CD27 expression on human B-lineage cells was detected as early as the pro-B cell stage in FL, and all developing B cell compartments contained a subset of CD27+ B cells.
Low frequencies of CD27+CD19+CD10+ B cells in adult BM
To determine whether CD27+ developing B cells are present only in FL, we performed similar analyses of adult BM, the postnatal site of primary B-lymphopoiesis. The frequency of CD19+ cells among the CD45+ FSCloSSClo lymphocytes from adult BM was lower (13.4±5.6%) than in FL (Fig. 1 and Table 1). We found that approximately 20% of CD19+ cells were positive for CD27 expression (Fig. 1B and Table 1). However, unlike FL samples in which ≥88% of the CD19+ cells expressed CD10 (Fig. 1A), the majority of the CD19+ cells (63.3±8.5% and 93.0±6.2% for the CD27− and CD27+ subsets, respectively) did not express CD10 (Fig. 1B). These CD10−CD19+ cells comprised CD27−IgMintIgDhi mature B and CD27+IgM−IgD− class-switched memory B cells (data not shown). In the CD10+CD19+ B cell compartments, IgM−IgD− pro-/pre-B cells were the most abundant (Fig. 1B). As in the FL samples, CD34+ pro-B cells were predominant (77.8±13.2%) in the CD27+ pro-/pre-B cell compartment, whereas CD34− pre-B cells were the dominant subpopulation (73.6±6.1%) (Fig. 1B) in the CD27− pro-/pre-B cell pool.
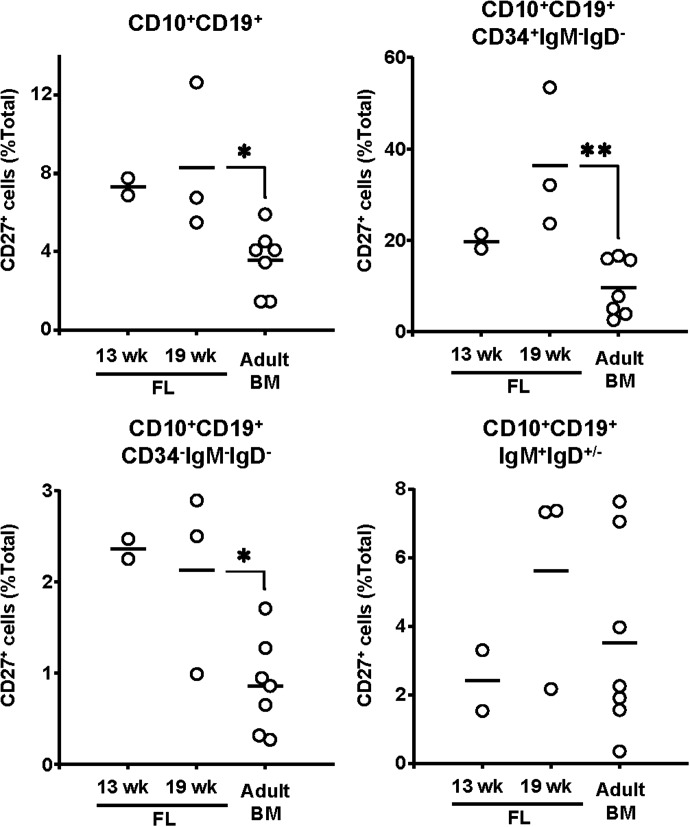
In general, the frequency of CD27+ cells in each compartment of developing B cells was higher in FL than in adult BM (Fig. 2). In FL, frequencies of CD27+ cells among the CD19+CD10+ B cells was about 8% (13 wk, 7.3±0.6%; 19 wk, 8.3±3.8%). The frequency of CD27+ cells was highest, approximately 25%, in the pro-B cell compartment (13 wk, 19.7±2.3%; 19 wk, 36.4±15.4%) and declined in the pre-B (13 wk, 2.4±0.2%; 19 wk, 2.1±1.0%) and the immature/transitional B cell (13 wk, 2.4±1.3%; 19 wk, 5.6±3.0%) compartments (Fig. 2). In adult BM, the frequency of CD27+ cells in these B cell compartments followed a similar pattern, albeit at significantly lower frequencies. About 4% (3.6±1.6%; P≤0.02 when compared with the FL samples) of the CD19+CD10+ adult BM B cells expressed CD27, whereas only 10% (9.6±6.2%; P≤0.07 and P≤0.01, when compared to 13 and 19 week FL, respectively) of the pro-B cells did (Fig. 2). Similarly, the frequency of CD27+ pre-B cells in BM (0.9±0.5%; P≤0.03) and immature/transitional B cells (3.5±2.8%; P>0.32) was about half that of FL (Fig. 2).
Figure 2. CD27+ developing B cells were present in FL at a higher frequency than in adult BM.
Frequencies of CD27+ cells in CD19+CD10+ total developing B cells, CD19+CD10+IgM−IgD−CD34+ pro-B cells, CD19+CD10+IgM−IgD−CD34− pre-B cells, and CD19+CD10+IgM+IgD+/− immature/transitional B cells in 13 wk FL (n=2), 19 wk FL (n=3), and adult BM (n=7) are shown. Vertical bars, the mean; (○), individual samples. *P < 0.05; **P < 0.01, two-tailed Student's t test.
TdT, RAG-1, and VpreB mRNA expression in CD27+ developing B cells replicated that of CD27− developing B cells
TdT, RAG-1, and Vpre-B are specifically expressed by the pro-B cells, pre-B cells, or both [34–36]. To confirm our identification of CD27+ developing B cells, we quantified and compared these transcripts in the CD27− and CD27+ pro-B (CD19+CD10+IgM−IgD−CD34+), pre-B (CD19+CD10+IgM−IgD−CD34−), pro-/pre-B (CD19+CD10+IgM−IgD−), and immature/transitional B cells (CD19+CD10+IgM+IgD+/−) from human FL and adult BM by quantitative PCR.
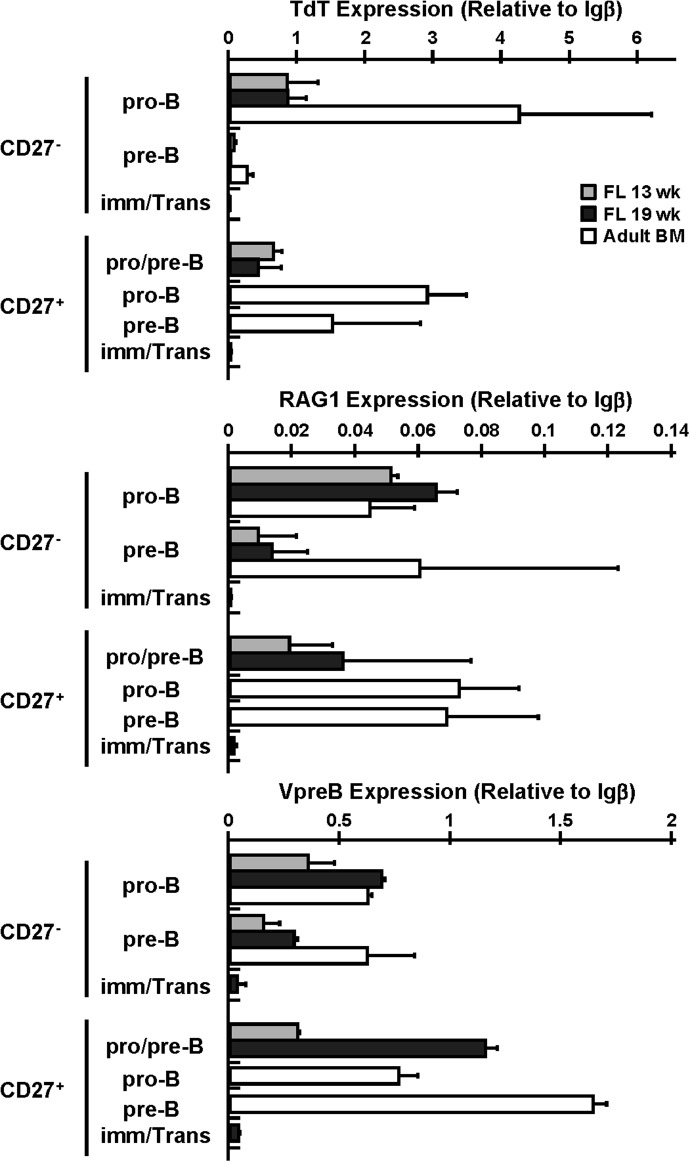
The expression pattern and levels of TdT, RAG-1, and VpreB mRNA in the CD27+ developing B cell compartments were similar to those observed in the conventional CD27− developing B cell compartments (Fig. 3). Consistent with previous observations [34–36], TdT expression by the FL CD27− subsets was highest in the pro-B (0.84±0.30; relative to Igβ), fell in the pre-B (0.03±0.03; relative to Igβ), and was virtually absent in the immature/transitional B cells. The CD27+ pro-/pre-B cells expressed levels of TdT (0.53±0.25; relative to Igβ) comparable to those in the CD27− pro-B cells. As pro-B cells were predominant (85.5 ± 4.0%) (Fig. 1A) in the CD27+ pro-/pre-B cells, these data suggest that CD27+ and CD27− pro-B cells express comparable levels of TdT in FL, although we cannot exclude the possibility that some pre-B cells in the CD27+ pro-/pre-B cell compartment express TdT. TdT expression was negligible in the CD27+ immature/transitional B cells. As expected [37], TdT expression in adult BM was higher than in FL. Expression was very high in the CD27− pro-B cells (4.24±1.95; relative to Igβ) and fell precipitously in the pre-B cells (0.25±0.10; relative to Igβ). The CD27+ pro-B cells in adult BM also expressed high levels (2.90±0.58; relative to Igβ) of TdT, but unlike the CD27− pre-B cells, substantial levels of TdT expression (1.50±1.29; relative to Igβ) were maintained in the CD27+ pre-B cells (Fig. 3).
Figure 3. TdT, RAG-1, and VpreB mRNA expression in CD27+ developing B cells.
Levels of TdT, RAG-1, and VpreB mRNA expression in CD27− and CD27+ developing B cell compartments were determined by quantitative PCR. CD19+CD10+IgM−IgD−CD34+ pro-B, CD19+CD10+IgM−IgD−CD34− pre-B, and CD19+CD10+IgM−IgD−CD34+/− pro-/pre-B cells were sorted from 13 wk FL, 19 wk FL, and adult BM and CD19+CD10+IgM+IgD+/− immature/transitional B cells were sorted from 19 wk FL. TdT, RAG1, and VpreB mRNA levels were normalized to Igβ mRNA levels. Histograms represent mean results ± sd (n=2–4).
RAG-1 transcript levels in the CD27+ pro-/pre-B cells (0.03±0.03; relative to Igβ) were within the range of those in the CD27− pro-B (0.06±0.01; relative to Igβ) and pre-B (0.01±0.01; relative to Igβ) cells, and few or no RAG-1 transcripts were recovered from the CD27+ immature/transitional B cells in FL (Fig. 3). Similarly, RAG-1 expression was comparable among the CD27− and CD27+ pro-B and pre-B cells in adult BM (range: CD27−, 0.02-0.10; CD27+, 0.05-0.09; relative to Igβ) (Fig. 3).
VpreB mRNA was detected in the pro-B, pre-B and pro-/pre-B cells in FL and adult BM, with higher levels in the CD27+ pro-/pre-B cells from 19 wk FL and the CD27+ pre-B cells from adult BM compared with their CD27− counterparts; the CD27+ pro-/pre-B cells in 19 wk FL expressed 1.7- and 4.0-fold higher levels of VpreB mRNA than did the CD27− pro-B and pre-B cells, respectively (Fig. 3). In adult BM, the CD27+ pre-B cells expressed 2.5-fold higher levels of VpreB mRNA when compared to levels in the CD27− pre-B cells (CD27+ pre-B, 1.64±0.06; CD27− pre-B, 0.62±0.22; relative to Igβ) (Fig. 3).
We concluded that the characteristic patterns of surface molecules and gene expression that define each developmental stage of conventional CD27− B lymphopoiesis are applicable to CD27+ developing B cells.
CD27− and CD27+ developing B cells showed similar VH gene usage with infrequent point mutations
To compare VH gene usage, HCDR3 lengths, and VH point mutations in CD27− and CD27+ developing B cell subsets, we amplified cDNA from VHDJH transcripts from 19 wk FL developing B cell subsets and cloned and sequenced them.
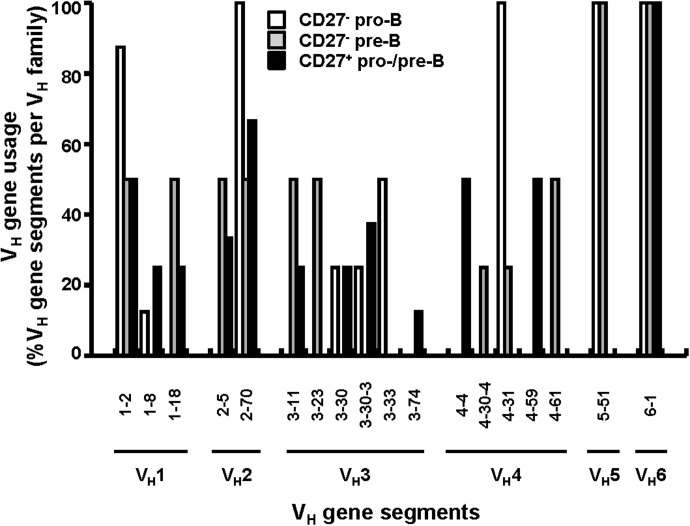
Our amplifications revealed similar VH gene family (VH1–VH6) usage in the CD27− pro-B and pre-B cells (n=47) and in the CD27+ pro-/pre-B cell compartment (n=28), although some differences in the frequencies of specific VH gene segments were noted (Fig. 4). Consistent with results in a previous study of human fetal BM pro-B cell libraries [38], the VH3-30 gene segment represented 25% of all VH3 gene rearrangements in both the CD27− pro-B cells (1/4) and the CD27+ pro-/pre-B cells (2/8), although one must consider the contribution of pre-B cells (14.5±4.0%) in the CD27+ population. VDJ rearrangements from other VH gene families were also recovered from the CD27+ and the CD27− pro-/pre-B cells, with no obvious differences in their frequencies (P≥0.14) (Fig. 4).
Figure 4. CD27− and CD27+ developing B cell compartments showed equally diversified VH gene usage.
VHDJH rearrangements were amplified by nested PCR with cDNA obtained from CD19+CD27− and CD19+CD27+ developing B cell compartments in 19 wk FL. After PCR, the amplicands were cloned into bacteria and sequenced, and the VH gene sequences were compared to reference sequences to obtain VH gene segment identity. The percentage of VH gene segments within each VH gene family from CD19+CD27− pro-B (n=30), CD19+CD27− pre-B (n=17) and CD19+CD27+ pro/pre-B cells (n=28) are shown.
Average HCDR3 lengths in VHDJH rearrangements recovered from CD27+ pro-/pre- B cells (13.0±3.1 amino acids) (Supplemental Fig. S2) were comparable to those from the CD27− pro-B cells (11.6±3.9 amino acids, P=0.14) (Supplemental Fig. S2) and the CD27− pre-B cells (13.0±3.9 amino acids, P=0.97) (Supplemental Fig. S2). CD27+ developing B cells and conventional CD27− developing B cells in human FL had similarly diverse repertoires of VH gene rearrangements.
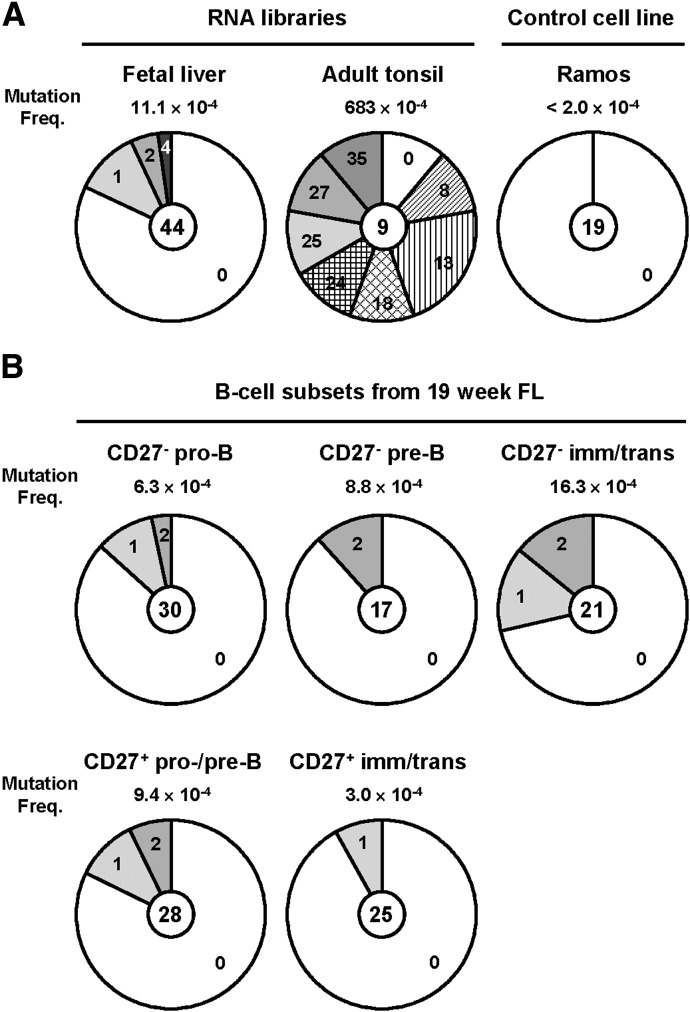
To determine whether CD27− and CD27+ developing B cells can be distinguished by their IgH mutation status, we compared frequencies of VH mutations in the CD27− and CD27+ FL B cell subsets to those in a FL RNA library and an adult tonsil RNA library known to contain high frequencies of mutated VH rearrangements (683×10−4 mutations per bp) (Fig. 5A). We also amplified VHDJH rearrangements from the Ramos cell line to determine the background mutation frequency of our assay (≤2.0×10−4 mutations/bp) (Fig. 5A). The VH mutation frequency in the FL library (11.1×10−4 mutations/bp; 0–4 point mutations/VH gene segment) was ≈50-fold lower than that in the adult tonsil library but significantly higher (P≤0.05) than background (Fig. 5A). Similarly, low (3.0–16.3×10−4 mutations/bp) VH mutations were observed in both the CD27− and CD27+ developing B cell compartments (Fig. 5B). In the CD27− developing B cells, the VH mutations increased with B cell maturation (pro-B, 6.3 × 10−4 mutations/bp, P=0.10; pre-B, 8.8 × 10−4 mutations/bp, P=0.12; immature/transitional B cells, 16.3 × 10−4 mutations/bp, P=0.013) (Fig. 5B). The CD27− pro-B, pre-B, and immature/transitional B cells carried 0–2 point mutations/VH gene segment (Fig. 5B). Comparably low VH mutations were observed in the CD27+ pro-/pre-B cells (9.4×10−4 mutations/bp, P=0.054; 0–2 point mutations/VH gene segment) (Fig. 5B) and CD27+ immature/transitional B cells (3.0×10−4 mutations/bp; 0–1 point mutations/VH gene segment) (Fig. 5B). This low mutation frequency is consistent with the observation that CD27+IgM+IgD+ B cells in umbilical cord blood carry few or no V(D)J mutations but accumulate a substantial number of mutations over time [11]. These findings suggest that CD27+ immature and transitional B cells in FL are precursors to the mutated CD27+IgM+IgD+ MZ B cells present in older children and patients with HIGM1 [11]. In contrast, CD27+ FL B cells are distinct from bona fide CD27+ mutated memory B cells [7].
Figure 5. CD27− and CD27+ developing B cells have infrequent point mutations.
VHDJH rearrangements were amplified by nested PCR with cDNA synthesized from commercially available FL and adult tonsil RNA libraries (A), RNA extracted from the Ramos cell line (A), and the CD19+CD27− and CD19+CD27+ developing B cell compartments from 19 wk FL (B). PCR amplicands were cloned into bacteria and sequenced, and the VH gene sequences were compared to reference sequences to obtain VH mutation frequencies and the number of point mutations per VH gene segment. Mutation frequencies (numbers of point mutations per bp sequenced) in each sample are indicated, and distributions of the number of point mutations (0–35) in each sequence is shown as a proportion of total sequences analyzed (numbers in center). Levels of background mutation due to the assay were established from the Ramos cell line. All VHDJH rearrangements recovered from that cell line contained identical 6 nucleotide substitutions compared with germline IGHV4-34*01 sequence, but no additional VH point mutation was observed in 5015 bp of nucleotides sequenced. We estimated the background mutation frequency in our assay to be lower than 1 mutation per 5015 bp (≤ 2.0×10−4 mutations/bp).
Human CD27+ pro- and pre-B cells expressed elevated levels of LIN28B message
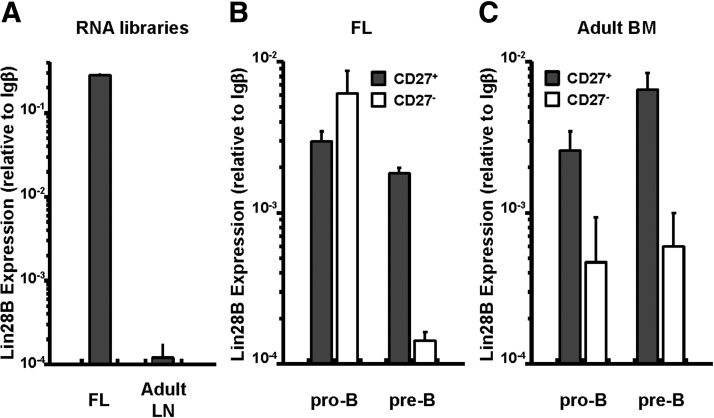
LIN28B, a transcription factor normally restricted to fetal and neonatal hematopoietic cells, is capable of biasing adult lymphopoietic output toward “fetal lineage” lymphocytes: B-1 and MZ B cells and γδ T cells [23]. To compare LIN28B expression in developing CD27+ and CD27− B cells, we sorted CD27+ and CD27− pro-B and pre-B cells from human FL and adult BM and quantified LIN28B expression by quantitative PCR. As expected [23], the LIN28B transcripts were abundant in a FL library, but rare in an adult LN library (Fig. 6A). The CD27+ and CD27− pro-B cells recovered from FL expressed comparably elevated levels of LIN28B. In contrast, whereas the CD27+ pre-B cells retained high levels of LIN28B expression, LIN28B message in the CD27− pre-B cells fell significantly (Fig. 6B). LIN28B expression in the CD27+ pro-B and pre-B cells from adult BM was similar to that in FL (Fig. 6C). The CD27− pro- and pre-B cells in adult BM expressed much lower levels of LIN28B (Fig. 6C), characteristic of the adult LN library (Fig. 6A). CD27 expression by pro-B and pre-B cells, whether in FL or adult BM correlates strongly with LIN28B transcript levels.
Figure 6. CD27+ pre-B cells from FL expressed higher levels of LIN28B.
Levels of LIN28B mRNA expression in FL and adult lymph node RNA libraries (A) and CD27+ pro-B and pre-B and CD27− pro-B and pre-B cells sorted from FL (B) or adult BM (C) are shown. LIN28 mRNA levels were normalized to Igβ mRNA levels (mean ± sd, 4 independent measurements). Samples: n=1 for RNA libraries and FL; n=2–3 for adult BM.
Human CD27+ pro-B cells generated IgM+ B cells more efficiently in vitro
To define their differentiation potential, we sorted CD27− and CD27+ pro-B cells from 19 wk human FL and cultured them independently on MS-5 stromal cells in media supplemented with cytokines (SCF, IL-7, TSLP, and Flt3 ligand) that support the differentiation of human fetal pro-B cells [26] and mouse B-1 B cell progenitors [27].
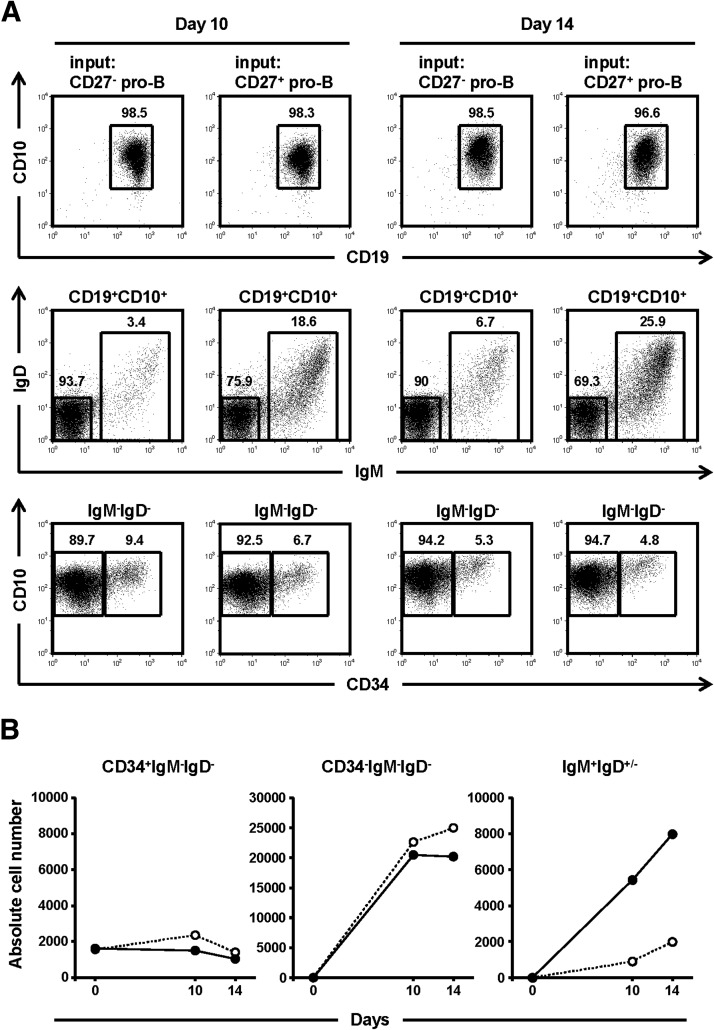
In culture, the absolute number of both CD27+ and CD27− pro-B cells remained relatively constant, indicating similar rates of proliferation/survival, and both pro-B cell compartments gave rise to a similar number (2.0–2.5×104) of pre-B cells over 14 days in vitro (Fig. 7). Virtually all (≥96%) differentiated progeny of the CD27+ or CD27− pro-B cells retained CD19 and CD10 expression, confirming them as B lymphocytes (Fig. 7). CD27+ pro-B cells, however, generated IgM+IgD+/− immature/transitional B cells far more efficiently than did the CD27− progenitors; the number of immature/transitional B cells in the cultures of CD27+ pro-B cells was 4- to 6-fold greater than in the cultures of FL derived, CD27− pro-B cells (Fig. 7). Indeed, approximately 20% (day 10, 19%; day 14, 26%) of all CD19+CD10+ cells recovered from the CD27+ pro-B cell cultures exhibited the IgM+IgD+/− immature/transitional phenotype, whereas only about 5% (day 10, 3%; day 14, 7%) of cells from the CD27− pro-B cell culture did (Fig. 7).
Figure 7. CD27+ pro-B cells from FL efficiently gave rise to immature/transitional B cells in vitro.
Differentiation potential of CD27+ and CD27− pro-B cells were compared in culture. Sort-purified CD27+ or CD27− pro-B cells from 19 wk FL were cultured on an MS5 monolayer [28] in the presence of SCF, IL-7, TSLP, and Flt3 ligand for 10 and 14 days. (A) Flow diagrams of CD19 and CD10 expression on cultured CD45+FSCloSSClo FL lymphocytes are shown in the top row. Those CD19+CD10+ cells were analyzed for surface IgM and IgD expression (middle row), and the IgM−IgD− pro-/pre-B cell compartments were further plotted based on CD34 and CD10 expression (bottom row). The number near each gating square indicates the frequency of gated cells within each flow diagram. (B) Absolute cell numbers of input CD19+CD10+IgM−IgD−CD34+ pro-B, and postcultured CD19+CD10+IgM−IgD−CD34+ pro-B, CD19+CD10+IgM−IgD−CD34− pre-B, and CD19+CD10+IgM+IgD+/− immature/transitional B cells from CD27− pro-B (○) and CD27+ pro-B cells (●) are shown. Representative data are shown from similar results obtained from 2 independent experiments.
CD27+ pro-B cells are unquestionably functional progenitors of B lymphocytes, and their distinctive behavior in vitro suggests that CD27+ and CD27− pro-B cells have distinguishable differentiation potentials in vivo.
DISCUSSION
CD27 expression is an accepted marker for most post-GC human memory B cells [7–9] and plasmacytes [10]. A more controversial notion [3, 7, 9, 11–13] is that CD27 may also identify additional human B cell subsets, including MZ B cells [11–13], developing B cells [3, 21, 22], and B1-like B cells [16, 17]. We determined the origins of nonmemory CD27+ B cells, by analyzing CD27 expression on developing B cells from human FL and adult BM and observed significant CD27 expression by subsets of pro-B, pre-B, and immature/transitional B cells (Figs. 1 and 2). CD27+ developing B cells were most abundant in FL and especially so within the pro-B cell compartment (Fig. 2). LIN28B expression was prolonged in CD27+ pre-B cells whether recovered from FL or adult BM (Fig. 6)—an indication, we think, of the distinctive physiology of these cells (Figs. 3, 6, and 7). Finally, in vitro, CD27+ pro-B cells exhibited a higher capacity to generate IgM+ immature/transitional B cells than did their CD27− counterparts when cultured in cytokines that optimally support the development of human fetal pro-B cells (Fig. 7) [26]. We propose that in humans, CD27 also marks constituents of the fetal lymphocyte lineage [23].
Consistent with the role of LIN28B in the specification of fetal lineage lymphocytes [23], CD27+CD10+ developing B cells were most numerous in FL and were enriched especially in the pro-B cell compartment (Fig. 2). Indeed, the average frequency of CD27+ pro-B cells in FL (13- and 19 wk) was 6 times higher than that observed in adult BM (Table 1).
CD27+ pre-B and immature B cell frequencies were also higher in FL than in adult BM (Table 1) and remarkably so (31-fold) for immature/transitional B cells in 19 wk FL (3.1 vs. 0.1%; Table 1). This increase is, at least in part, due to expanded B-lymphopoiesis in FL (frequencies of CD10+ cells are 94 and 31% in FL and adult BM, respectively). We note, however, that the increased number of CD27+ pre- and immature/transitional B lymphocytes mirrors the abundance of mouse B1 B cell precursors in FL and neonates [39–42]. The enrichment of both mouse B1 B cell precursors and human CD27+ developing B cells in FL may be the result of developmental cues specific to the fetal environment or cell-intrinsic developmental programming [43]. In this regard, it was recently reported that Lin28b is expressed by mouse hematopoietic progenitors, including B cell progenitors, in FL, by human fetal tissues and by CD34+ human umbilical cord blood cells, but not by their adult-derived counterparts [23]. Transduction of Lin28b into adult mouse hematopoietic progenitors confers the properties of fetal lineage cells (i.e., transduced progenitors support biased lymphopoiesis for B-1a and MZ B cells, γδ T cells, and NKT cells) [23].
In our hands, LIN28B expression levels were equivalent in CD27− and CD27+ pro-B cells from FL, comparable in CD27+ pro-B cells from adult BM, but significantly lower in CD27− pro-B cells recovered from adult BM (Fig. 6). Given the linkage between CD27 and LIN28B expression by adult BM pro-B cells (Fig. 6), we conclude that, whereas the human fetal environment may promote LIN28B expression, at least some component of CD27/LIN28B expression by human CD10+ B cells is intrinsic. The low frequency of CD27+ pro-B cells in human adult BM (Figs. 1 and 2, Table 1) most likely precluded Yuan et al. [23] from identifying this adult LIN28B compartment.
In FL and adult BM, the frequency of CD27+ cells was highest in the pro-B cell stage and subsequently fell with maturation to the pre-B and immature/transitional B cell stages (Fig. 2). One explanation for this maturation-associated decline is reduced proliferation by CD27+ large pre-B cells. Indeed, the CD27+ pro-/pre-B cell compartment was characterized by the predominance of CD34+ pro-B cells; in contrast, CD34− pre-B cells dominated the CD27− pro-/pre-B cell pools (Fig. 1). Proper pre-BCR assembly/signaling is crucial for the proliferation of large pre-B cells (pre-BI) [44], and it may be that CD27+ human pre-BI cells proliferate less than CD27− pre-BI cells, owing to suboptimal pre-BCR assembly and/or signaling [44]. The inefficient downregulation of TdT in CD27+ pre-B cells (Fig. 3) is consistent with impaired pre-BCR signaling [44, 45].
As an alternative to signaling differences, CD27 expression in B cell progenitors may be determined by an intrinsic developmental program that serially reduces expression of this molecule [22]. Vaskova et al. [22] identified pro-B, pre-BI, pre-BII (small pre-B), and immature B cells in pediatric BM as CD27+CD44−, CD27+CD44+, CD27−CD44+/−, and CD27−CD44−IgM+ cells (all CD19+CD10+), respectively. The predominance of CD34+ cells in the CD27+ pro-/pre-B cell compartment (Fig. 1) is consistent with their claim. However, we found CD27−CD34+ pro-B cells to be substantially more numerous than CD27+ pro-B cells both in FL and adult BM (Figs. 1 and 2, Table 1). More significantly, we demonstrated functional differences between CD27+ and CD27− pro-B cells as determined by their different capacities to generate immature/transitional B cells in vitro (Fig. 7). If Vaskova et al. [22] are correct and all human pro-B and pre-BI cells express CD27, this expression pattern must be intrinsically programmed, and all CD27− pro-B and pre-BI cells must descend from CD27+ populations. We think this possibility unlikely.
The pre-BCR signaling and intrinsic program models are neither mutually exclusive nor exhaustive. Other pathways may lead to reduced frequencies of CD27+ B cell progenitors and precursors. For example, we note that LIN28B repression of let-7 micro (mi)RNA suggests the possibility that unknown transcriptional regulators play decisive roles in this developmental pathway. Additional work is needed, to characterize fully the development of this interesting, early B cell population.
It remains unclear whether CD27+ developing B cells retain CD27 expression throughout their development and maturation or whether CD27 is expressed in subsets of cells in the various stages of B cell development. The former possibility implies a distinct lineage, whereas the latter is consistent with alternative differentiation potentials. In vivo, we observed CD27+ developing B cells at all developmental stages, including pro-B, pre-BI/II, and immature/transitional B cells (Fig. 2). In vitro, however, immature/transitional B cells generated from sorted CD27+ pro-B cells did not retain surface CD27 expression (data not shown), perhaps due to the absence of unknown developmental signals in our culture system. As expected for a fetal lymphocyte lineage, the frequencies of CD27+ pro-B and pre-B cells in adult BM showed a strong, inverse correlation with donor age; in contrast, the ratios of CD27+ IgM− (pro-B or pre-B) and IgM+ immature/transitional B cell compartment correlated poorly (data not shown). These results provide equivocal evidence, then, for a progenitor–progeny relationship for CD27+ pro-B, pre-B, and immature B cells. Nonetheless, it is interesting to consider the possible relationship between our CD27+ developing B cells and distinct subsets of mature CD27+ B cells, such as human B1 B cells and circulating MZ B cells.
Recently, Griffin et al. [16] reported that a small population of CD20+CD27+CD43+CD70− human B cells, presented both in umbilical cord and adult peripheral blood, represents the counterpart of mouse B1 B cells. Consistent with their report [16], we observed that 3.8 ± 2.5% of cord blood B cells were CD27+ (Table 1). Most of these CD27+ B cells displayed the IgMhiIgD+CD10low/− phenotype of transitional or MZ B cells; approximately 80% of the transitional/MZ B cells also expressed CD43 (data not shown). We could not, however, directly connect the CD27+ developing B cells in FL to the human B1 populations described by Griffin et al. [16], because CD43 is expressed by virtually all CD19+CD10+ B cells in FL and adult BM, regardless of whether they are CD27+ or CD27− (Supplemental Fig. S3).
Similarly, our CD27+ developing B cells could be related to circulating MZ B cells, which are defined as CD19+CD27+IgM+IgD+ [11–13]. Circulating MZ B cells are present in human cord blood [11], suggesting fetal/prenatal development. In this regard, Scheeren et al. [3] have reported IgM+IgD+CD27+ mature B cells in human fetal spleen and liver (14- to 18 wk gestation) with the surface phenotype of circulating MZ B cells. The splenic IgM+IgD+CD27+ fetal B cells carried remarkably high frequencies of IgH mutations [3] normally present in older children and adults [11–13]. In our hands, virtually all CD19+IgM+IgD+CD27+ FL B cells expressed CD10 and high levels of IgM (Fig. 1A), a phenotype consistent with transitional, but not mature human B cells [6]. Although, it is unclear whether Scheeren et al. evaluated CD10 expression, their IgM+IgD+CD27+ splenic B cells and our CD19+CD10+IgM+IgD+CD27+ FL B cells are clearly distinguishable by their mutational status.
We cannot conclusively link the CD27+ developing B cells of human FL to the B1-like [16, 17] and circulating MZ [11–13] B cells described by other groups. Nonetheless, enrichment of CD27+ B cells in FL (Figs. 1 and 2, Table 1) and the strong correlation between CD27 and LIN28B expression in pro-B and pre-B cells (Fig. 6) support this possibility. Adult mouse BM hematopoietic progenitors transduced with Lin28b preferentially generate so-called fetal lineage lymphocytes, including B1a and MZ B cells [23]. It is reasonable, we think, to conclude that these B cell lineages have a common physiologic progenitor that expresses Lin28b [23]. It is equally plausible to propose that the human FL CD27+ pro- and pre-B cells that express higher levels of LIN28B are the precursors of both B1-like and circulating MZ B cells in humans. It is interesting that unlike mouse, human adult BM contains a small but significant population of CD27+ pro- and pre-B cells that express LIN28B (Fig. 6). This finding suggests that the generation of fetal lineage lymphocytes may continue, albeit on a reduced scale, even in adult humans. If LIN28B is expressed by committed B1 B cell progenitors, then both FL and adult BM contain B1 B cell progenitors, albeit at 10-fold lower frequencies. In contrast, LIN28B could be expressed at basal levels by uncommitted, CD27− progenitors and only respond to signals uniquely present in the FL pro-B cell niche. In either case, CD27− pro-B cells that express higher levels of LIN28B (Fig. 6) would be more likely to generate B1 B cells. Unfortunately, however, LIN28B expression may not be useful as a lineage marker, in that mature B-1a B cells in mice do not express Lin28b [23]. Clearly, additional investigation is necessary to elucidate any connections between CD27+ developing B cells in human FL and the B1-like and circulating MZ B cells.
Our work characterizing CD27 expression in the earliest stages of human B cell development expands the accepted understanding of this cell-surface marker, suggests an alternate pattern of expression associated with fetal lineage B cells, and points to a common origin for B1-like and circulating MZ B cells that is established in part by unique features of fetal development.
Supplementary Material
ACKNOWLEDGMENTS
G.K. was supported in part by U.S. National Institutes of Health-National Institute of Allergy and Infectious Disease grants AI056363 and AI24335. L.M. was a recipient of a Eunice Kennedy Shriver National Institute of Child Health and Human Development Award (K12HD000850). L.M. is a Fellow of the Pediatric Scientist Development Program.
We thank Dr. J. Kurtzberg and the Carolinas Cord Blood Bank at Duke University for anonymous umbilical cord blood samples. We gratefully acknowledge Sally Howland, Nicholas Tofolo, Jr., Julie Smith, and Thaddeus C. Gurley for assistance in procuring the samples.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- BM
- bone marrow
- F
- forward
- FL
- fetal liver
- FSC
- forward scatter (FSC-A=area, -H=height, -W = width) FSC-A=FSC-area
- FSC-H
- FSC-height
- FSC-W
- FSC-width
- GC
- germinal center
- HCDR3
- heavy chain complementarity determining region 3
- HIGM
- hyper IgM syndrome
- MZ
- marginal zone
- PI
- propidium iodide
- R
- reverse
- RAG-1
- recombination activating gene-1
- SCF
- stem cell factor
- SHM
- somatic hypermutation
- SSC
- side scatter (SSC-H=height, -W=width)
- TSLP
- thymic stromal lymphopoietin
- T1
- transitional 1
- T2
- transitional 2
AUTHORSHIP
L.M. performed the experiments, analyzed the results, and wrote the paper. K.S. performed the experiments and analyzed the results. X.L. and D.L. contributed to the studies of VH gene cloning/sequencing and expression. S.F. provided the human FL samples. J.A. processed the human BM and peripheral blood samples. M.A.M. provided the human BM and peripheral blood samples. G.K. planned and directed the study, designed the experiments, analyzed and interpreted the results, and wrote the paper. M.K. designed and performed the experiments, analyzed and interpreted the results, and wrote the paper.
DISCLOSURES
The authors declare no financial conflicts of interest.
REFERENCES
- 1. Gathings W. E., Lawton A. R., Cooper M. D. (1977) Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur. J. Immunol. 7, 804–810 [DOI] [PubMed] [Google Scholar]
- 2. Nunez C., Nishimoto N., Gartland G. L., Billips L. G., Burrows P. D., Kubagawa H., Cooper M. D. (1996) B cells are generated throughout life in humans. J. Immunol. 156, 866–872 [PubMed] [Google Scholar]
- 3. Scheeren F. A., Nagasawa M., Weijer K., Cupedo T., Kirberg J., Legrand N., Spits H. (2008) T cell-independent development and induction of somatic hypermutation in human IgM+IgD+CD27+ B cells. J. Exp. Med. 205, 2033–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lemmers B., Gauthier L., Guelpa-Fonlupt V., Fougereau M., Schiff C. (1999) The human (PsiL+mu-) proB complex: cell surface expression and biochemical structure of a putative transducing receptor. Blood 93, 4336–4346 [PubMed] [Google Scholar]
- 5. LeBien T. W. (2000) Fates of human B cell precursors. Blood 96, 9–23 [PubMed] [Google Scholar]
- 6. Sims G. P., Ettinger R., Shirota Y., Yarboro C. H., Illei G. G., Lipsky P. E. (2005) Identification and characterization of circulating human transitional B cells. Blood 105, 4390–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein U., Rajewsky K., Kuppers R. (1998) Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188, 1679–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agematsu K., Hokibara S., Nagumo H., Komiyama A. (2000) CD27: a memory B cell marker. Immunol. Today 21, 204–206 [DOI] [PubMed] [Google Scholar]
- 9. Seifert M., Kuppers R. (2009) Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J. Exp. Med. 206, 2659–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jung J., Choe J., Li L., Choi Y. S. (2000) Regulation of CD27 expression in the course of germinal center B cell differentiation: the pivotal role of IL-10. Eur. J. Immunol. 30, 2437–2443 [DOI] [PubMed] [Google Scholar]
- 11. Weller S., Faili A., Garcia C., Braun M. C., Le Deist F. F., de Saint Basile G. G., Hermine O., Fischer A., Reynaud C. A., Weill J. C. (2001) CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc. Natl. Acad. Sci. U. S. A. 98, 1166–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weller S., Braun M. C., Tan B. K., Rosenwald A., Cordier C., Conley M. E., Plebani A., Kumararatne D. S., Bonnet D., Tournilhac O., Tchernia G., Steiniger B., Staudt L. M., Casanova J. L., Reynaud C. A., Weill J. C. (2004) Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 104, 3647–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weller S., Mamani-Matsuda M., Picard C., Cordier C., Lecoeuche D., Gauthier F., Weill J. C., Reynaud C. A. (2008) Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+IgD+CD27+ B cell repertoire in infants. J. Exp. Med. 205, 1331–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maurer D., Holter W., Majdic O., Fischer G. F., Knapp W. (1990) CD27 expression by a distinct subpopulation of human B lymphocytes. Eur. J. Immunol. 20, 2679–2684 [DOI] [PubMed] [Google Scholar]
- 15. Agematsu K., Nagumo H., Yang F. C., Nakazawa T., Fukushima K., Ito S., Sugita K., Mori T., Kobata T., Morimoto C., Komiyama A. (1997) B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur. J. Immunol. 27, 2073–2079 [DOI] [PubMed] [Google Scholar]
- 16. Griffin D. O., Holodick N. E., Rothstein T. L. (2011) Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J. Exp. Med. 208, 67–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffin D. O., Rothstein T. L. (2011) A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J. Exp. Med. 208, 2591–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Griffin D. O., Rothstein T. L. (2012) Human “orchestrator” CD11b(+) B1 cells spontaneously secrete IL-10 and regulate T cell activity. Mol. Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Descatoire M., Weill J. C., Reynaud C. A., Weller S. (2011) A human equivalent of mouse B-1 cells? J. Exp. Med. 208, 2563–2564; author reply 2566–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reynaud C. A., Weill J. C. (2012) Gene profiling of CD11b(+) and CD11b(−) B1 cell subsets reveals potential cell sorting artifacts. J. Exp. Med. 209, 433–434; author reply 434–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nilsson A., de Milito A., Mowafi F., Winberg G., Bjork O., Wolpert E. Z., Chiodi F. (2005) Expression of CD27-CD70 on early B cell progenitors in the bone marrow: implication for diagnosis and therapy of childhood ALL. Exp. Hematol. 33, 1500–1507 [DOI] [PubMed] [Google Scholar]
- 22. Vaskova M., Fronkova E., Starkova J., Kalina T., Mejstrikova E., Hrusak O. (2008) CD44 and CD27 delineate B-precursor stages with different recombination status and with an uneven distribution in nonmalignant and malignant hematopoiesis. Tissue Antigens 71, 57–66 [DOI] [PubMed] [Google Scholar]
- 23. Yuan J., Nguyen C. K., Liu X., Kanellopoulou C., Muljo S. A. (2012) Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 335, 1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ueda Y., Liao D., Yang K., Patel A., Kelsoe G. (2007) T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J. Immunol. 178, 3593–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuraoka M., Liao D., Yang K., Allgood S. D., Levesque M. C., Kelsoe G., Ueda Y. (2009) Activation-induced cytidine deaminase expression and activity in the absence of germinal centers: insights into hyper-IgM syndrome. J. Immunol. 183, 3237–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Namikawa R., Muench M. O., de Vries J. E., Roncarolo M. G. (1996) The FLK2/FLT3 ligand synergizes with interleukin-7 in promoting stromal-cell-independent expansion and differentiation of human fetal pro-B cells in vitro. Blood 87, 1881–1890 [PubMed] [Google Scholar]
- 27. Montecino-Rodriguez E., Dorshkind K. (2006) Stromal cell-dependent growth of B-1 B cell progenitors in the absence of direct contact. Nat. Protoc. 1, 1140–1144 [DOI] [PubMed] [Google Scholar]
- 28. Luo X. M., Maarschalk E., O'Connell R. M., Wang P., Yang L., Baltimore D. (2009) Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood 113, 1422–1431 [DOI] [PubMed] [Google Scholar]
- 29. Liao H. X., Levesque M. C., Nagel A., Dixon A., Zhang R., Walter E., Parks R., Whitesides J., Marshall D. J., Hwang K. K., Yang Y., Chen X., Gao F., Munshaw S., Kepler T. B., Denny T., Moody M. A., Haynes B. F. (2009) High-throughput isolation of immunoglobulin genes from single human B cells and expression as monoclonal antibodies. J. Virol. Methods 158, 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Volpe J. M., Cowell L. G., Kepler T. B. (2006) SoDA: implementation of a 3D alignment algorithm for inference of antigen receptor recombinations. Bioinformatics 22, 438–444 [DOI] [PubMed] [Google Scholar]
- 31. Zhang W., Bardwell P. D., Woo C. J., Poltoratsky V., Scharff M. D., Martin A. (2001) Clonal instability of V region hypermutation in the Ramos Burkitt's lymphoma cell line. Int. Immunol. 13, 1175–1184 [DOI] [PubMed] [Google Scholar]
- 32. Ryan D., Kossover S., Mitchell S., Frantz C., Hennessy L., Cohen H. (1986) Subpopulations of common acute lymphoblastic leukemia antigen-positive lymphoid cells in normal bone marrow identified by hematopoietic differentiation antigens. Blood 68, 417–425 [PubMed] [Google Scholar]
- 33. Loken M. R., Shah V. O., Dattilio K. L., Civin C. I. (1987) Flow cytometric analysis of human bone marrow, II: normal B lymphocyte development. Blood 70, 1316–1324 [PubMed] [Google Scholar]
- 34. Li Y. S., Hayakawa K., Hardy R. R. (1993) The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J. Exp. Med. 178, 951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghia P., ten Boekel E., Sanz E., de la Hera A., Rolink A., Melchers F. (1996) Ordering of human bone marrow B lymphocyte precursors by single-cell polymerase chain reaction analyses of the rearrangement status of the immunoglobulin H and L chain gene loci. J. Exp. Med. 184, 2217–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghia P., ten Boekel E., Rolink A. G., Melchers F. (1998) B cell development: a comparison between mouse and man. Immunol. Today 19, 480–485 [DOI] [PubMed] [Google Scholar]
- 37. Gregoire K. E., Goldschneider I., Barton R. W., Bollum F. J. (1979) Ontogeny of terminal deoxynucleotidyl transferase-positive cells in lymphohemopoietic tissues of rat and mouse. J. Immunol. 123, 1347–1352 [PubMed] [Google Scholar]
- 38. Rao S. P., Riggs J. M., Friedman D. F., Scully M. S., LeBien T. W., Silberstein L. E. (1999) Biased VH gene usage in early lineage human B cells: evidence for preferential Ig gene rearrangement in the absence of selection. J. Immunol. 163, 2732–2740 [PubMed] [Google Scholar]
- 39. Hardy R. R., Hayakawa K. (1991) A developmental switch in B lymphopoiesis. Proc. Natl. Acad. Sci. U. S. A. 88, 11550–11554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Solvason N., Lehuen A., Kearney J. F. (1991) An embryonic source of Ly1 but not conventional B cells. Int. Immunol. 3, 543–550 [DOI] [PubMed] [Google Scholar]
- 41. Kantor A. B., Stall A. M., Adams S., Herzenberg L. A. (1992) Differential development of progenitor activity for three B cell lineages. Proc. Natl. Acad. Sci. U. S. A. 89, 3320–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Montecino-Rodriguez E., Leathers H., Dorshkind K. (2006) Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 7, 293–301 [DOI] [PubMed] [Google Scholar]
- 43. Barber C. L., Montecino-Rodriguez E., Dorshkind K. (2011) Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc. Natl. Acad. Sci. U. S. A. 108, 13700–13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Melchers F. (2005) The pre-B cell receptor: selector of fitting immunoglobulin heavy chains for the B cell repertoire. Nature Rev. Immunol. 5, 578–584 [DOI] [PubMed] [Google Scholar]
- 45. Wasserman R., Li Y. S., Shinton S. A., Carmack C. E., Manser T., Wiest D. L., Hayakawa K., Hardy R. R. (1998) A novel mechanism for B cell repertoire maturation based on response by B cell precursors to pre-B receptor assembly. J. Exp. Med. 187, 259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.