Abstract
Background
Several case-control studies have suggested that passive smoking may increase the incidence of female breast cancer. However, the results of cohort studies have been inconsistent in establishing an association. The present study evaluated the association between passive smoking and incidence of female breast cancer through a meta-analysis of prospective cohort studies.
Methods
Relevant articles published before August 2012 were identified by searching the electronic databases PubMed, Embase, and Web of Science. Pooled relative risks (RRs) were determined with either a fixed or random effects model and were used to assess the strength of the association. Sensitivity and subgroup analyses according to ethnicity, menopausal status, and the period and place of exposure to passive smoking were also performed.
Results
Ten prospective cohort studies involving 782 534 female non-smokers were included in the meta-analysis and 14 831 breast cancer cases were detected. Compared with the women without exposure to passive smoking, the overall combined RR of breast cancer was 1.01 (95% confidence interval: 0.96 to 1.06, P = 0.73) among women with exposure to passive smoking. Similar results were achieved through the subgroup analyses. No evidence of publication bias was observed.
Conclusion
The results suggest that passive smoking may not be associated with increased incidence of breast cancer. However, the present conclusion should be considered carefully and confirmed with further studies.
Introduction
Breast cancer contributes significantly to physiological and psychological comorbidities among women worldwide. In 2011, an estimated 230,480 new cases of invasive breast cancer affected women in the US and breast cancer is expected to account for 29% (226,870) of all new cancer cases in 2012 in this group [1], [2]. Among the environmental or lifestyle factors involved in the etiology of breast cancer, tobacco smoking may be one of the leading preventable risk factors [3].
In the past two decades, the health risks associated with cigarette smoking and exposure to tobacco smoking have been widely investigated by both basic science researchers and epidemiologists. Tobacco smoke contains polycyclic aromatic hydrocarbons, aromatic amines, and N-nitrosamines [4], all of which potentially lead to breast cell carcinogensis. However, the antiestrogenic effect of smoking has also been associated with reduced risk of breast cancer [5]. The antiestrogenic effect of smoking has likewise been associated with both an increased risk of osteoporosis and early onset of natural menopause among female smokers [6], [7]. Compared with women who had never smoked, Luo and colleagues [8] found that the incidence of breast cancer was increased by 9% among former smokers and by 16% among current smokers; what’s the worse, the risk of breast cancer was increased by 35% among the active smokers who smoked at least 50 years.
Numerous observational studies [8]–[17] have been conducted in an attempt to find the association between passive smoking and incidence of breast cancer. Passive smoking, or exposure to environmental tobacco smoke (ETS), has been associated with significant physiological harm related to that experienced by active smokers [18]–[19]. Yet, the results from studies specifically considering the association between passive smoking and breast cancer incidence have been inconsistent and suggested positive [12], [15], [16], inverse [10], and null associations [9], [11], [13], [14], [16], [17]. This observation was the initial motivation of the current study. In addition, there were two reasons to conduct a meta-analysis that only included prospective cohort studies. First, case-control studies tend to be influenced by more confounding and bias than studies with a prospective cohort design, and a meta-analysis combining both study designs could be affected by these issues. Second, a previous meta-analysis [14] combined several cohort studies and reported that passive smoking had no significant effect on breast cancer risk. However, compared with women who never exposed to smoking, the results combined with retrospective studies (most of them were case-control studies) [14] showed that the incidence of breast cancer was increased by 21% (95%CI: 11%–32%, P<0.05) among women with exposure to smoking, which was different with the results of prospective studies. In that analysis, evidence was limited because one of the eight included studies [20] only reported on breast cancer mortality; one study was case-control in design [21]; and another two studies [22], [23] that were included have since been updated with extended follow-ups and larger samples [15], [17]. Therefore, with the recent accumulation of additional evidence [8], [11], [13], [16], our goal was to evaluate the association between passive smoking and incidence of breast cancer by conducting a meta-analysis of prospective cohort studies.
Materials and Methods
Search strategy
The current study was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement [24]. Relevant studies were identified through PubMed, Embase, and Web of Science databases by using the following search terms: “passive smoking” or “secondhand smoke” or “tobacco smoke pollution” or “environmental tobacco smoke” and “breast cancer” or “breast neoplasm” or “breast carcinoma.” We also sought additional studies by reviewing the reference lists of included articles, reviews, conference abstracts, and the bibliographies of expert advisors. All articles identified through this search strategy had been published on or before August 27, 2012.
Selection criteria
Titles and abstracts of all relevant papers were reviewed. The studies were chosen if they met all of the following criteria: (i) the study was designed as a prospective cohort study; (ii) the exposure was restricted to passive smoking; (iii) the articles provided relative risk (RR) estimates and the corresponding 95% confidence intervals, the size of the baseline samples, number of cases, follow-up years, confounders for adjustment, or other information that can help to infer the results; (iv) when multiple publications reported on the same or overlapping data, the study that was most recent or based on the largest population was selected; (v) the publication language was limited to English and Chinese. Reviews, meeting abstracts, editorials, and commentaries were excluded from our analysis.
Data extraction
Data were extracted independently by two reviewers (Zhang F. and Yang Y.). Consensus was reached by discussion. A third party (Liu S.) was involved when necessary. The following information was extracted from each article: first author, year of publication, study location, ethnicity of subjects, study period, duration of follow-up, source population, mean age of the baseline sample, number of cases, assessment of passive smoking, ascertainment of cases, adjustment for covariates, relative risk (RR) and the corresponding 95% confidence interval.
Assessment of risk of bias
Two authors (Zhang F, Yang Y) independently assessed the quality of each study based on the Newcastle-Ottawa-Scale (NOS). The NOS was developed for quality assessment of non-randomized observational studies, including both case-control studies and cohort studies [25]. A “star system” was developed under the NOS and was applied in the present analysis to judge each included study on the following: selection of the study groups, between-group comparability, and choice of outcome. Using the scale, we assigned a number of stars to each study. A study was awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars were assigned for Comparability. The total NOS star count ranged from zero to nine. Decisions were compared and consensus was reached, with a third party (Liu S) participating in quality assessment when necessary.
Statistical analyses
Relative risk (RR) was used to measure the association between passive smoking and incidence of breast cancer. Two studies [13], [17] reported stratified risk estimates by place of exposure to passive smoking; one study [10] reported stratified risk estimates by number of household smokers; and one study [8] reported stratified risk estimates by husband's smoking status. We combined these estimates using the method reported by Hamling [26] and then used the pooled estimates for the overall meta-analysis. In the study by Lin et al [13], the control groups in the subgroup analyses of exposures to smoking at home and in public places were different, according to this, we treated this two subgroup analyses as two studies.
Heterogeneity of RR across studies was assessed using the Cochran's χ2-based Q test and the I-squared test. Heterogeneity was not considered as significant when P>0.10 or I2<50%. If no significant heterogeneity was found, the pooled RR estimate of each study was calculated using the fixed effects (Mantel-Haenszel) model [27]. Otherwise, the random effects (DerSimonian and Laird) model was used. Stratification analyses by ethnicity, menstrual status and place of passive smoking were conducted to reduce the heterogeneity and get more accurate results. We also performed additional meta-analyses that included only those studies with subjects experiencing passive smoking in childhood or adulthood, and then only those studies with follow-up of more than 10 years. The sensitivity analysis was conducted by excluding (i) the studies followed up less than five years, (ii) the studies with less than 100 cases, and (iii) the studies with NOS score less than 7. It was also conducted by using a leave-one-out approach to take into account variations in the assessed study quality.
Egger's linear regression test [28] and the Begg's rank correlation test [29] were used to assess potential publication bias. All statistical tests were conducted using STATA software (Version 11). A P-value of 0.05 for any test or model was considered to be statistically significant, except where otherwise specified.
Results
Literature search
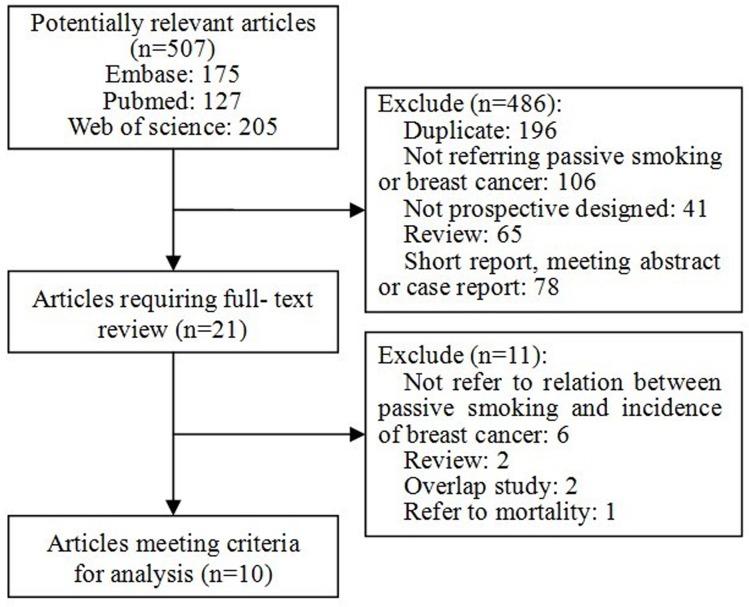
We initially retrieved 507 citations from PubMed, Web of Science and Embase databases. After title and abstract screening, most of the publications were excluded, mainly because they were duplicate records, reviews, cross-sectional or case-control studies, or not relevant to our study. Among the 21 studies included for full-text review, six [20], [30]–[35] were excluded from the analysis because they did not refer to the association between passive smoking and incidence of breast cancer. Two more articles [22], [23] were excluded since we were using articles that provided more recent data on the same studies but with extended follow-up [15], [17]. An additional two reviews [36], [37] and one study [20] referring to breast cancer mortality were excluded. A total of 10 studies were ultimately included in the analyses and all of them were published in English language. ( Figure 1 )
Figure 1. Flow chart of literatures selection.
Flow chart shows literature search for prospective cohort studies of passive smoking in relation to incidence of breast cancer.
Eligible studies
All of the included 10 studies were published between 1999 and 2011. Three studies were conducted in the United States, three in Japan, one in the United Kingdom, one in Korea, and two were multi-country studies. Six studies enrolled Caucasian participants and four studies' participants were of Asian background. At the study baseline, a total of 782 534 female non-smokers were involved. 14 831 cases were detected during the follow-up period. The length of follow-up ranged from 3.5 to 24 years (mean 10.2 years). The ascertainment of passive smoking varied across studies, with the majority basing exposure on self-report or interviewer-administered questionnaires. Most of the studies confirmed disease status according to ICD-9, ICD-O-2, medical records or pathology reports. The time of exposure to smoking were reported mainly by childhood [8], [13], [15], [16], adulthood [8], [13], [15], or the lifetime [9]–[12], [14], [17]. However, the kind of exposure included at passive smoking in the home [8]–[11], [13], [14], [17], a public place [13], or the workplace [8], [15], [17]. Several studies [12], [14], [15] were concerned with the premenopausal women, and some [8], [12], [14], [15] on postmenopausal women, and the rest included studies were not analyzed separately according to menstrual status. ( Table 1 )
Table 1. The characteristics of the included prospective cohort studies.
| Studies | Country | Period | Ethnicity | Duration (years) | Population# | Number of all cases | Age (years) | NOS score | Menstrual status | Exposure | Adjustment for Covariates | |
| Time | Place | |||||||||||
| 1999, Jee | Korea | 1994–1997 | Asian | 3.5 | 160,130 | 138 | 40–88 | 8 | NA | NA | Home | Age, husband's age, husband's occupation, husband's vegetable consumption, residency, socioeconomic status |
| 2001,Nishino | Japan | 1984–1992 | Asian | 9.0 | 9,675 | 67 | >40 y | 7 | NA | NA | Home | Age |
| 2005, Gram | Norway, Sweden | 1991–1992 | Caucasian | 9.3 | 102,098 | 1130 | 30–50 | 7 | NA | NA | Home | Age, age at menarche, age at first birth, alcohol, BMI, family history of breast cancer, hormonal contraceptive use, menopausal status, number of children |
| 2005,Hanaoka | Japan | 1990–1999 | Asian | 10.0 | 20,191 | 162 | 40–59 y | 8 | Premenopausal or postmenopausal | NA | NA | Age, employment status, education level, BMI, family history of breast cancer, history of past benign breast disease, age at menarche, number of births, menopausal status, hormone use and alcohol consumption. |
| 2008,Lin | Japan | 1988–1990 | Asian | 12.0 | 32,023 | 196 | 40–79 y | 8 | NA | Childhood or adulthood | Home or public place | Age, area, BMI, family history of breast cancer, alcohol, daily walking, age at menarche, age at the first birth, menopausal status, number of births and use of sex hormones. |
| 2008,Pirie | UK | 1996–2001 | Caucasian | 3.5 | 224,917 | 2,344 | 50–64 y | 7 | Premenopausal or postmenopausal | Childhood or adulthood | NA | Age, BMI, age at first birth, age at menarche, region of residence, socio-economic group, physical activity, alcohol consumption, menopausal status, parity, hormone use. |
| 2009,Reynolds | US | 1997 | Caucasian | 10.0 | 57,523 | 1,754 | NA | 6 | Premenopausal or postmenopausal | Childhood or adulthood | Home or workplace | Age, race, family history of breast cancer, age at menarche, pregnancy history, lifetime duration of breastfeeding, physical activity, alcohol consumption, BMI, menopausal status with use of hormone therapy. |
| 2011,Chuang | Multi-countries* | 1992–1998 | Caucasian | 10.0 | 98,938 | 3,411 | 25–70 y | 8 | NA | Childhood | NA | Age, sex, and study center, and adjusted for education, baseline alcohol drinking, BMI, physical activity, vegetable intake, fruit intake, non-alcoholic energy intake, and adulthood passive smoking, age at menarche, parity, ever use of oral contraceptives, and menopausal status |
| 2011,Luo | US | 1993–1998 | Caucasian | 10.3 | 41,022 | 3,520 | 50–79 y | 8 | Postmenopausal | Childhood or adulthood | Home or workplace | Age, race, parity, education, BMI, physical activity, alcohol intake, family history of breast cancer, hormone use |
| 2011,Xue | US | 1982–2006 | Caucasian | 24.0 | 36,017 | 2,109 | 30–55 y | 7 | NA | NA | Home or workplace | Age, family history of breast cancer, history of benign breast disease, BMI, BMI at age 18 years, height, alcohol consumption, age at menarche, parity, age at first birth, physical activity, oral contraceptive use, menopausal status, hormone use, and age at menopause. |
BMI: Body mass index; CI: Confidence interval; NA: Not available; NOS: Newcastle-Ottawa-Scale; RR: Relative risk; US: United States.
Multi-countries contain Sweden, Denmark, Norway, the Netherlands, UK, France, Germany, Spain, Italy, and Greece.
Number of non-smoking women.
All 10 studies included adjustment for more than three variables, such as age, ethnicity, body mass index (BMI), menstrual status, family history of breast cancer, hormone use, socioeconomic status, alcohol, etc. The details were shown in the Table 1 . After assessment of risk of bias using the NOS, all studies received from six to eight stars which means the quality of the studies were moderate to high.
Quantitative synthesis
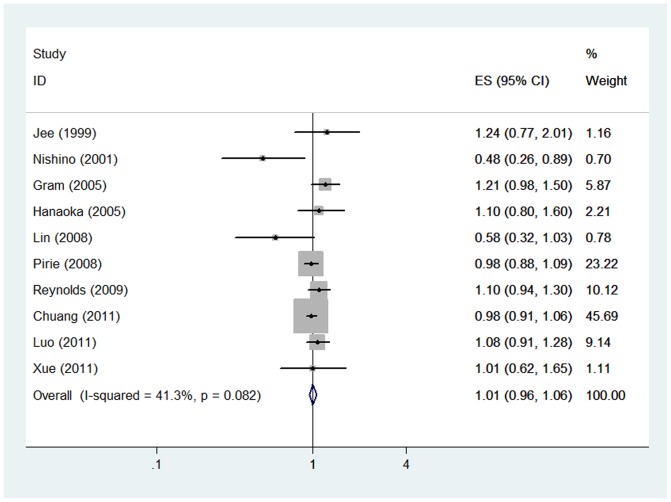
Based on the combined results of the 10 cohort studies, among women who had never smoked, compared with those who had never been exposed to passive smoking, women exposed to passive smoking were not significantly associated with increased incidence of breast cancer (RR = 1.01, 95%CI: [0.96, 1.06], P = 0.73) under the fixed effect model (Heterogeneity: I2 = 41.3%). ( Figure 2 )
Figure 2. Forest plot of overall pooled RR.
Forest plot shows association between passive smoking and incidence of breast cancer. CI: confidence interval; RR: relative risk.
A subgroup analysis was conducted according to ethnicity and no significant associations were found in either Asian populations (RR = 0.82, 95%CI: [0.54, 1.27], P = 0.38) or Caucasian populations (RR = 1.02, 95%CI: [0.96, 1.07], P = 0.58). Subgroup analysis according to menopausal status further revealed that passive smoking was not associated with the incidence of breast cancer (Premenopausal: RR = 1.11, 95%CI: [0.55, 2.24], P = 0.78; Postmenopausal: RR = 1.01, 95%CI: [0.85, 1.20], P = 0.90). Whether the period of exposure occurred in childhood or adulthood did not affect the findings of a null association between passive smoking and risk of breast cancer (Childhood: RR = 1.09, 95%CI: [0.99, 1.20], P = 0.10; Adulthood: RR = 1.03, 95%CI: [0.91, 1.17], P = 0.63). No association was observed upon combining the results of studies grouped according to whether exposure occurred at home or in the outside (Home: RR = 0.96, 95%CI: [0.81, 1.14], P = 0.67; Workplace or public place: RR = 1.01, 95%CI: [0.93, 1.10], P = 0.76). Furthermore, when we combined the studies with follow-up of at least 10 years, no statistically significant relationship was observed between passive smoking and the incidence of breast cancer (RR = 1.01, 95%CI: [0.95, 1.07], P = 0.80). ( Table 2 )
Table 2. Subgroup analyses of meta-analysis.
| Groups | No. of studies | RR | 95% CI | P values | I2 | Analysis models |
| Asian | 4 | 0.82 | [0.54, 1.23] | 0.38 | 67.3% | Random effects |
| Caucasian | 6 | 1.02 | [0.96, 1.07] | 0.58 | 5.6% | Fixed effects |
| Premenopausal | 3 | 1.11 | [0.55, 2.24] | 0.78 | 82.4% | Random effects |
| Postmenopausal | 4 | 1.01 | [0.85, 1.20] | 0.90 | 64.6% | Random effects |
| Childhood | 4 | 1.09 | [0.99, 1.20] | 0.10 | 0.0% | Fixed effects |
| Adulthood | 2 | 1.03 | [0.91, 1.17] | 0.63 | 0.0% | Fixed effects |
| Home | 7 | 0.96 | [0.81, 1.14] | 0.67 | 55.5% | Random effects |
| Workplace | 4 | 1.01 | [0.93, 1.10] | 0.76 | 0.0% | Fixed effects |
| Follow-up≥10 y | 6 | 1.01 | [0.95, 1.07] | 0.80 | 15.9% | Fixed effects |
CI: Confidence interval; RR: Relative risk.
Sensitivity analysis
Sensitivity analyses were conducted to examine the influence of various exclusion criteria on the overall risk estimate and to explore potential sources of heterogeneity in the association between passive smoking and risk of breast cancer. Exclusion of the two studies that had a follow-up time of less than five years yielded a pooled RR of 1.02 (95%CI: [0.91, 1.15], P = 0.71, I2 = 51.5%). Restricting the analysis to studies that had more than 100 breast cancer cases (only the study by Nishino et al was excluded) yielded a pooled RR of 1.02 (95%CI: [0.96, 1.07], P = 0.58), without evidence of between-study heterogeneity (I2 = 17.5%; P = 0.29). The pooled RR was 1.00 (95%CI: [0.95, 1.05], P = 0.99, I2 = 43.4%) when the study conducted by Reynolds et al [14] was excluded, which got lowest NOS score. Furthermore, exclusion of any single study did not materially alter the overall combined RR, which then ranged from 1.00 (95% CI: [0.95, 1.05], P = 0.94) to 1.04 (95% CI: [0.97, 1.11], P = 0.34).
We also conducted the analysis by combining those six studies in which, passive smoking was more comprehensively assessed and defined specifically as exposure to smoking by parents, partners, or coworkers [8], [13]–[17]. However, the result was not materially changed with pooled RR of 1.00 (95% CI: [0.95, 1.05], P = 0.96, I2 = 14.5%).
Publication bias
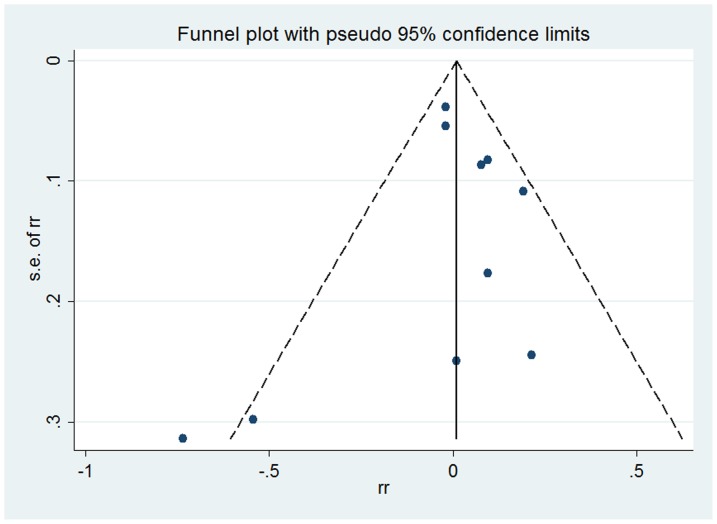
Funnel plot was generated to assess the possibility of publication bias. As shown in Figure 3 , the shape of funnel plot was relatively symmetrical thus suggesting there was no significant publication bias. Begg's rank correlation test and Egger's linear regression test also supported no obvious publication bias (P Begg = 0.37, P Egger = 0.79).
Figure 3. Funnel plot of included studies.
Funnel plot shows association between passive smoking and incidence of breast cancer.
Discussion
There is rapidly growing interest in the public health implications of passive smoking. Our study focused on the risk of breast cancer in female non-smokers with passive exposure. This meta-analysis found no association between passive smoking and incidence of female breast cancer. This finding was consistent in all analyses, including subgroup analyses separately investigating the association in Asian versus Caucasian populations, premenopausal versus postmenopausal women, individuals with childhood versus adulthood exposure, and individuals with household versus workplace exposure. This null association was also observed in our meta-analysis considering the six studies with extended follow-up of 10 years or longer.
Previous original studies investigating this association have generated a variety of results. For instance, the California Environmental Protection Agency (CalEPA) [38] reported that exposure to tobacco smoke at younger ages, specifically in the premenopausal years, was associated with a 68% increased risk of breast cancer, compared to women without exposure to passive smoking. However, most of the studies included in CalEPA research were retrospective in design. Recently, Luo and colleagues [8] suggested that postmenopausal women with the most extensive exposure to passive smoking (10 or more years of exposure in childhood, 20 or more years of exposure as an adult at home, and/or 10 or more years exposure as an adult at work) experienced a 32% increase in the risk of breast cancer compared to postmenopausal women without exposure. The California Teachers Study (CTS) [15] and the Nurses' Health Study [17] both failed to find a positive association between passive smoking and incidence of breast cancer. Due to limitations in the data available from original studies, our study could not include a dose-response analysis by passive smoking exposure metrics.
Several possible reasons may explain the lack of statistically significant findings in the present review. First, underlying toxicological and biological mechanisms of tobacco smoke are likely responsible for any association between passive smoking and increased incidence of breast cancer. A study by Petrakis et al detected carcinogens in breast fluid obtained through standard nipple aspiration techniques among nonlactating women [39] and thus provided evidence for a relationship between passive smoking and increased incidence of breast cancer. Other studies, however, have found that oestriol excretion was significantly lower in smokers than in non-smokers [5], [40], which indicated that smoking has an antiestrogenic effect. Since use of estrogen replacement therapy has been shown to result in a significantly higher risk of breast cancer [41], [42] and higher estrogen levels have been associated with increased incidence of breast cancer, the antiestrogenic effect of smoking could be treated as a rational possible explanation for the results of our meta-analysis.
Another possible explanation for the observed null association was that all of the included cohort studies did not consider genetic confounders. Several breast cancer susceptibility genes, such as BRAC1, BRAC2 [43] and CHEK2 [44], [45], have been detected in recent decades. Additionally, Conlon et al [46] suggested that the effect of smoking on breast cancer may be differentially modified by the N-acetyltransferase 2 (NAT2) phenotype. Among NAT2 fast acetylators, subjects with a history of more than 20 pack-years were nearly two times as likely (OR: 1.93, 95%CI: 1.01–3.69) to develop breast cancer as compared to individuals with a history of less than 20 pack-years; however, no association was detected among NAT2 slow acetylators. Another recent meta-analysis [47] further suggested that NAT2 polymorphisms contribute to the risk of breast cancer when smoking history is taken into account. NAT1 genetic polymorphisms were also considered to be sensitive to smoking history in the etiology of breast cancer. A previous study [48] revealed that the relationship between smoking and risk of breast cancer was modified by the NAT1 or NAT2 genotype among postmenopausal women. Zhang et al [49] also found that the association between NAT1*10 allele and risk of breast cancer was mainly limited to former smokers (OR = 3.30, 95% CI: 1.20–9.50). Several conventional confounders—such as age, age at menarche, age at first birth, parity, family history of breast cancer, body mass index (BMI), oral contraceptive use, menopausal status, hormone use, and age at menopause—were included for analysis in our study, which were adjusted in most of the included studies for eliminating the potential bias. However, genetic distributions, especially for genes with expression affected by tobacco smoke should be considered in the future research.
Subgroup analysis showed that passive smoking could not increase/decrease the incidence of breast cancer in premenopausal women, which was different from the findings of Hanaoka et al [12] and Pirie et al [14]. Hanaoka and colleagues [12] found that passive smoking increased breast cancer risk (RR = 2.6; 95%CI: [1.3, 5.2]) in premenopausal women. However, in the Million Women Study [14], inverse association was shown in premenopausal women (RR = 0.54, 95%CI: [0.30, 0.99]). No significant association was found in the study conducted by Reynolds et al [15]. Maybe this was the potential explanation why the heterogeneity was high in this subgroup. Combining those results, the association between passive smoking and incidence of breast cancer was not statistically significant (RR = 1.11, 95%CI: [0.55, 2.24], P = 0.78). The possible explanation was that the genetic factor may impact the effect of tobacco smoking on breast cancer [47]–[49]. In the subgroup analysis of postmenopausal women, although Hanaoka et al [12] found that passive smoking may decrease the incidence of breast cancer (RR = 0.6, 95%CI: [0.4, 1.0]), this study did not support this point by combining 4 studies (RR = 1.01, 95%CI: [0.85, 1.20], P = 0.90). So, future studies should consider other potential impact factors, not only the menstrual status.
Substantial heterogeneity was observed among the included studies, which was not surprising given the differences in study population characteristics, assessment of passive smoking, duration of follow-up, and adjustment for confounding factors. Our sensitivity analyses suggest that studies with fewer than 100 cases probably contributed to the observed heterogeneity. The study [10] with the smallest numbers of both participants and cases increased the possibility that chance accounted for their results; the RR reported in this study was evidently lower than that reported in others, thus indicating that passive smoking could decrease the risk of breast cancer. Exclusion of any single study in the sensitivity analysis revealed that the overall combined RR ranged from 1.00 to 1.02 which indirectly indicated that the result of our study was robust.
A major strength of our study is that all the included original studies used a prospective cohort design, which minimizes selection bias. Moreover, the lack of an association between passive smoking and risk of breast cancer did not change with sensitivity analyses based on rigorous exclusion criteria and various subgroup analyses. Additionally, by pooling evidence from multiple studies and generating a large overall sample size, we have enhanced statistical power to provide more precise and reliable risk estimates than provided by individual studies.
In this meta-analysis, several limitations may have influenced our findings. One potential limitation involved variations in the how passive smoking was measured in the included studies. Most studies assessed passive smoking through self-completed questionnaires containing various items. Three [9]–[11] of them assessed active smoking among the non-smokers' family members—husband, parents, or other cohabitating partners—and they defined a passive smoker as someone living with an active smoker. One study [12] focused on exposure to passive smoking not only at home, but also at the workplace. In the other six studies [8], [13]–[17], passive smoking was measured more accurately, and the combined result of those studies was not substantially changed (RR = 1.00, 95% CI: [0.94, 1.05], P = 0.89, I2 = 6.3%). A second limitation is the substantial heterogeneity among studies. However, through the sensitivity analysis, we were able to identify the major source of heterogeneity and demonstrate through a leave-one-out procedure that our findings are robust despite it. Thirdly, a dose-response analysis could not be conducted due to limitations in the data of original studies. Addressing the level of exposure may result in different findings. For instance, Luo and colleagues [8] found that the postmenopausal women with most extensive exposure to passive smoking had a 32% increased risk of breast cancer compared with those who had never been exposed to passive smoking. In an attempt to consider length of exposure, we combined the studies with 10 or more years of follow up in an additional, separate analysis; however, we found no significant association between passive smoking and incidence of breast cancer in this analysis. Finally, language bias was introduced since our search included studies written in English or Chinese. According to the above limitations, our conclusion should be considered carefully and confirmed with further studies that concentrate on questionnaire design and a dose-response analysis of passive smoking.
Conclusions
The meta-analysis of prospective cohort studies suggests that passive smoking may not be associated with increased incidence of breast cancer. However, the present conclusion should be considered carefully and confirmed with further studies.
Supporting Information
PRISMA Checklist.
(DOC)
Funding Statement
This work was supported by the National Natural Science Foundation of China (NSFC 81272265). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62(1): 10–29. [DOI] [PubMed] [Google Scholar]
- 2. DeSantis C, Siegel R, Bandi P, Jemal A (2011) Breast cancer statistics, 2011. CA Cancer J Clin 61(6): 409–418. [DOI] [PubMed] [Google Scholar]
- 3. Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, et al. (2008) Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 100(23): 1672–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. el-Bayoumy K (1992) Environmental carcinogens that may be involved in human breast cancer etiology. Chem Res Toxicol 5(5): 585–590. [DOI] [PubMed] [Google Scholar]
- 5. MacMahon B, Trichopoulos D, Cole P, Brown J (1982) Cigarette smoking and urinary estrogens. N Engl J Med 307(17): 1062–1065. [DOI] [PubMed] [Google Scholar]
- 6. Jensen J, Christiansen C, Rødbro P (1985) Cigarette smoking, serum estrogens, and bone loss during hormone-replacement therapy early after menopause. N Engl J Med 313(16): 973–975. [DOI] [PubMed] [Google Scholar]
- 7. Baron JA, La Vecchia C, Levi F (1990) The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol 162(2): 502–514. [DOI] [PubMed] [Google Scholar]
- 8. Luo J, Margolis KL, Wactawski-Wende J, Horn K, Messina C, et al. (2011) Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. BMJ 342: d1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jee SH, Ohrr H, Kim IS (1999) Effects of husbands' smoking on the incidence of lung cancer in Korean women. Int J Epidemiol 28(5): 824–828. [DOI] [PubMed] [Google Scholar]
- 10. Nishino Y, Tsubono Y, Tsuji I, Komatsu S, Kanemura S, et al. (2001) Passive smoking at home and cancer risk: a population-based prospective study in Japanese nonsmoking women. Cancer Causes Control 12(9): 797–802. [DOI] [PubMed] [Google Scholar]
- 11. Gram IT, Braaten T, Terry PD, Sasco AJ, Adami HO, et al. (2005) Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol Biomarkers Prev 14(1): 61–66. [PubMed] [Google Scholar]
- 12. Hanaoka T, Yamamoto S, Sobue T, Sasaki S, Tsugane S (2005) Japan Public Health Center-Based Prospective Study on Cancer and Cardiovascular Disease Study Group (2005) Active and passive smoking and breast cancer risk in middle-aged Japanese women. Int J Cancer 114(2): 317–322. [DOI] [PubMed] [Google Scholar]
- 13. Lin Y, Kikuchi S, Tamakoshi K, Wakai K, Kondo T, et al. (2008) Active smoking, passive smoking, and breast cancer risk: findings from the Japan Collaborative Cohort Study for Evaluation of Cancer Risk. J Epidemiol 18(2): 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pirie K, Beral V, Peto R, Roddam A, Reeves G, et al. (2008) Passive smoking and breast cancer in never smokers: prospective study and meta-analysis. Int J Epidemiol 37(5): 1069–1079. [DOI] [PubMed] [Google Scholar]
- 15. Reynolds P, Goldberg D, Hurley S, Nelson DO, Largent J, et al. (2009) Passive smoking and risk of breast cancer in the California teachers study. Cancer Epidemiol Biomarkers Prev 18(12): 3389–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chuang SC, Gallo V, Michaud D, Overvad K, Tjønneland A, et al. (2011) Exposure to environmental tobacco smoke in childhood and incidence of cancer in adulthood in never smokers in the European prospective investigation into cancer and nutrition. Cancer Causes Control 22: 487–494. [DOI] [PubMed] [Google Scholar]
- 17. Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB (2011) Cigarette smoking and the incidence of breast cancer. Arch Intern Med 171(2): 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glantz SA, Parmley WW (1995) Passive Smoking and Heart Disease: Mechanisms and Risk. JAMA 273(13): 1047–1053. [PubMed] [Google Scholar]
- 19. Kiechl S, Werner P, Egger G, Oberhollenzer F, Mayr M, et al. (2002) Active and passive smoking, chronic infections, and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Stroke 33: 2170–2176. [DOI] [PubMed] [Google Scholar]
- 20. Wartenberg D, Calle EE, Thun MJ, Heath CW, Lally C, et al. (2000) Passive smoking exposure and female breast cancer mortality. J Natl Cancer Inst 92(20): 1666–1673. [DOI] [PubMed] [Google Scholar]
- 21. Delfino RJ, Smith C, West JG, Lin HJ, White E, et al. (2000) Breast cancer, passive and active cigarette smoking and N-acetyltransferase 2 genotype. Pharmacogenetics 10: 461–469. [DOI] [PubMed] [Google Scholar]
- 22. Egan KM, Stampfer MJ, Hunter D, Hankinson S, Rosner BA, et al. (2002) Active and passive smoking in breast cancer: prospective results from the Nurses' Health Study. Epidemiology 13(2): 138–145. [DOI] [PubMed] [Google Scholar]
- 23. Reynolds P, Hurley S, Goldberg DE, Anton-Culver H, Bernstein L, et al. (2004) Active smoking, household passive smoking, and breast cancer: evidence from the California Teachers Study. J Natl Cancer Inst 96(1): 29–37. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J (2009) Altman DG; PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, et al. (2012) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 2012 Oct 19.
- 26. Hamling J, Lee P, Weitkunat R, Ambuhl M (2008) Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Statist Med 27: 954–970. [DOI] [PubMed] [Google Scholar]
- 27. Overton, Randall C (1998) A comparison of fixed-effects and mixed (random-effects) models for meta-analysis tests of moderator variable effects. Psychological Methods 3(3): 354–379. [Google Scholar]
- 28. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 30. Kabat GC, Kim M, Phipps AI, Li CI, Messina CR, et al. (2011) Smoking and alcohol consumption in relation to risk of triple-negative breast cancer in a cohort of postmenopausal women. Cancer Causes Control 22(5): 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rushton L, Bagga S, Bevan R, Brown TP, Cherrie JW, et al. (2010) Occupation and cancer in Britain. Br J Cancer 102(9): 1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kabat GC, Kim M, Kakani C, Tindle H, Wactawski-Wende J, et al. (2010) Cigarette smoking in relation to risk of ductal carcinoma in situ of the breast in a cohort of postmenopausal women. Am J Epidemiol 172(5): 591–599. [DOI] [PubMed] [Google Scholar]
- 33. Pukkala E, Martinsen JI, Lynge E, Gunnarsdottir HK, Sparén P, et al. (2009) Occupation and cancer - follow-up of 15 million people in five Nordic countries. Acta Oncol 48(5): 646–790. [DOI] [PubMed] [Google Scholar]
- 34. Cui Y, Miller AB, Rohan TE (2006) Cigarette smoking and breast cancer risk: update of a prospective cohort study. Breast Cancer Res Treat 100(3): 293–299. [DOI] [PubMed] [Google Scholar]
- 35. Al-Delaimy WK, Cho E, Chen WY, Colditz G, et al. (2004) A prospective study of smoking and risk of breast cancer in young adult women. Cancer Epidemiol Biomarkers Prev 13(3): 398–404. [PubMed] [Google Scholar]
- 36. Johnson KC, Miller AB, Collishaw NE, Palmer JR, Hammond SK, et al. (2011) Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009). Tob Control 20(1): e2. [DOI] [PubMed] [Google Scholar]
- 37. Johnson KC, Glantz SA (2008) Evidence secondhand smoke causes breast cancer in 2005 stronger than for lung cancer in 1986. Prev Med 46(6): 492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee PN, Hamling J (2006) Environmental Tobacco Smoke Exposure and Risk of Breast Cancer in Nonsmoking Women: A Review with Meta-Analyses. Inhal Toxicol 18: 1053–1070. [DOI] [PubMed] [Google Scholar]
- 39. Petrakis NL, Gruenke LD, Beelen TC, Castagnoli N, Craig JC (1978) Nicotine in breast fluid of nonlactating women. Science 199(4326): 303–305. [DOI] [PubMed] [Google Scholar]
- 40.Key TJ, Pike MC, Brown JB, Hermon C, Allen DS, et al. (1996) Cigarette smoking and urinary oestrogen excretion in premenopausal and post-menopausal women. Br J Cancer 74: ; 1313–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steinberg KK, Thacker SB, Smith SJ, Stroup DF, Zack MM, et al. (1991) A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA 265(15): 1985–1990. [PubMed] [Google Scholar]
- 42. Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, et al. (2003) Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA 289(24): 3243–3253. [DOI] [PubMed] [Google Scholar]
- 43. Chen S, Parmigiani G (2007) Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 10 25(11): 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang Y, Zhang F, Wang Y, Liu S (2012) CHEK2 1100delC Variant and Breast Cancer Risk in Caucasians: An Updated Meta-analysis Based on 25 Studies with 29 154 Cases and 37 064 Controls. Asian pac j cancer prev 13(7): 3501–3505. [DOI] [PubMed] [Google Scholar]
- 45. Brennan P, McKay J, Moore L, Zaridze D, Mukeria A, et al. (2007) Uncommon CHEK2 mis-sense variant and reduced risk of tobacco-related cancers: case control study. Hum Mol Genet 16(15): 1794–1801. [DOI] [PubMed] [Google Scholar]
- 46. Conlon MS, Johnson KC, Bewick MA, Lafrenie RM, Donner A (2010) Smoking (active and passive), N-acetyltransferase 2, and risk of breast cancer. Cancer Epidemiol 34(2): 142–149. [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, Qiu LX, Wang ZH, Wang JL, He SS, et al. (2010) NAT2 polymorphisms combining with smoking associated with breast cancer susceptibility: a meta-analysis. Breast Cancer Res Treat 123(3): 877–883. [DOI] [PubMed] [Google Scholar]
- 48. Millikan RC, Pittman GS, Newman B, Tse CK, Selmin O, et al. (1998) Cigarette smoking, N-acetyltransferases 1 and 2, and breast cancer risk. Cancer Epidemiol Biomarkers Prev 7(5): 371–378. [PubMed] [Google Scholar]
- 49. Zheng W, Deitz AC, Campbell DR, Wen WQ, Cerhan JR, et al. (1999) N-acetyltransferase 1 genetic polymorphism, cigarette smoking, well-done meat intake, and breast cancer risk. Cancer Epidemiol Biomarkers Prev 8(3): 233–239. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)