Abstract
Objectives
In this study we investigate the relationships between placental size and neonatal bone mass and body composition, in a population based cohort.
Study design
914 mother-neonate pairs were included. Placental dimensions were measured via ultrasound at 19 weeks gestation. Dual X-ray absorptiometry (DXA) was performed on the neonates within the first two weeks of life.
Results
We observed positive relationships between placental volume at 19 weeks, and neonatal bone area (BA; r=0.26, p<0.001), bone mineral content (BMC; r=0.25, p<0.001) and bone mineral density (BMD; r=0.10, p=0.001). Thus placental volume accounted for 6.25% and 1.2% of the variation in neonatal BMC and BMD respectively at birth. These associations remained after adjustment for maternal factors previously shown to be associated with neonatal bone mineral accrual (maternal height, smoking, walking speed in late pregnancy, serum 25(OH) vitamin D and triceps skinfold thickness).
Conclusions
We found that placental volume at 19 weeks gestation was positively associated with neonatal bone size and mineral content. These relationships appeared independent of those maternal factors known to be associated with neonatal bone mass, consistent with notion that such maternal influences might act through modulation of aspects of placental function, e.g. utero-placental blood flow or maternal nutrient concentrations, rather than placental size itself. Low placental volume early in pregnancy may be a marker of a reduced postnatal skeletal size and increased risk of later fracture.
Introduction
Osteoporosis is a major cause of morbidity and mortality through its association with age-related fractures. Bone size and density appear to track throughout childhood to peak in early adulthood, and this peak bone mass has been shown to be a major determinant of osteoporosis risk in later life (1). We have previously demonstrated that growth in utero and early childhood predict adult bone mineral content (BMC) (2;3) and risk of hip fracture in later life (4). Maternal factors such as smoking, body build, physical activity, diet (5) and circulating 25(OH) vitamin D status (6) during pregnancy are associated with offspring bone mineral accrual. During the period of a normal human pregnancy the fetus accumulates approximately 30g of calcium (7). This fetal demand is met through placental calcium transport, which results in a higher calcium concentration in fetal than maternal blood (8). We have previously demonstrated that the expression of a placental calcium transporter (PMCA3) gene predicts neonatal bone mineral content (9), but it remains unclear whether the maternal influences described act on fetal bone development via placental size or function.
In this study we investigate the relationships between placental dimensions, measured by ultrasound scanning, and offspring body composition, bone size and density assessed by dual x-ray absorptiometry (DXA) at birth, using a longitudinal population-based mother-offspring cohort, the Southampton Women’s Survey (SWS).
Methods
Participants
Details of the SWS have been published previously (10). In brief, 12,583 non-pregnant women aged 20-34 years were recruited via their general practitioners. Assessments of lifestyle, diet (by validated food frequency questionnaire) and anthropometry were performed at study entry and then in early (11 weeks) and late (34 weeks) gestation in those women who became pregnant. Maternal social class was also measured via questionnaire. Maternal height was measured pre-pregnancy with a stadiometer (Seca, Birmingham, UK), weight with calibrated digital scales (Seca, Birmingham, UK) and skin folds (biceps, triceps, subscapular and supra-iliac) with Harpenden callipers (Baty International, Sussex, UK). Serum 25(OH) Vitamin D was measured in late pregnancy (Diasorin Liaison RIA, Minnesota, USA). Women were asked to characterise their walking speed into one of five categories (very slow, stroll at an easy pace, normal speed, fairly brisk or fast) as a marker of overall physical activity. The women’s own birth-weight was recorded (by recall, checked, where possible, by asking her to consult her own parents).
Prenatal Ultrasound Scanning
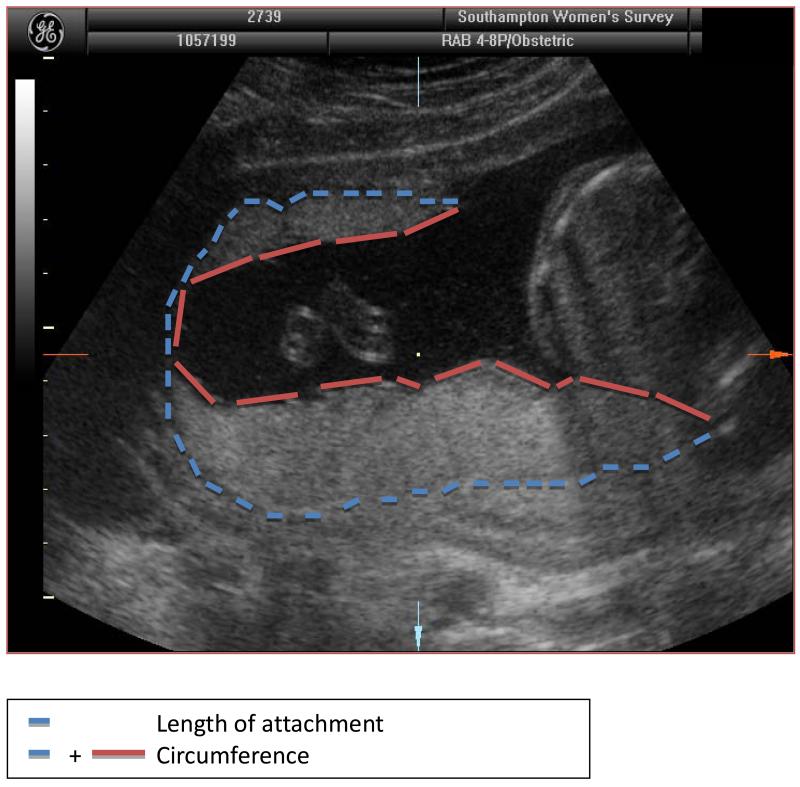
3,159 singleton pregnancies were followed. At 19 weeks gestation, the women underwent high-resolution ultrasound scanning using an Acuson Sequoia 512 system (Siemens, Malvern, PA, USA) with a 5-8MHz curvelinear transducer. After establishing correct positioning according to standard anatomical landmarks, measurements of placental circumference, length of attachment to the uterine wall and cross-sectional area were made on the frozen two-dimensional images using electronic callipers by an experienced sonographer (either PM or CN) – (Figure 1). The sonographer measured the placenta in two planes, initially along the longest edge of attachment to the uterine wall (length) and then at 90 degrees to this, so that the image plane bisected the longitudinal axis (breadth). Each measurement was performed in triplicate and the mean value used for analysis. Placental volume was later calculated using the two-dimensional ultrasound measurements. To estimate the volume of the placenta we assumed that it was an ellipsoid. The two measured circumferences and two areas were expressed as functions of the three ellipsoid radii. Estimates of the radii, obtained by least squares, were then combined to estimate the volume. This method demonstrated good correlation with placental volume measured by 3D ultrasound (Kretz Voluson 730 (r=0.64, p<0.0001)) in a subset of 28 pregnancies at mean 19.9 (0.4) weeks gestation.
Figure 1. A 19 week ultrasound scan showing placental size measurements in the longitudinal plane.
Neonatal DXA Assessment
Mothers registered with specific general practices were invited to participate in the bone component of the SWS. These practices were selected to avoid the mothers participating in more than one sub-study, and were representative of the population Southampton as a whole. At birth, the babies were weighed on calibrated digital scales (Seca, UK). The mother was asked to give written informed consent for her baby to undergo assessment of bone mass and body composition within 2 weeks of birth, using a DXA scanner with specific paediatric software (Lunar DPX-L paediatric small scan mode v 4.7c, GE Corporation, Madison, Wisconsin, USA). The instrument underwent daily quality assessment and was calibrated against a water phantom weekly.
At the visit to the scan room, the baby was pacified and fed if necessary, undressed completely, and then swaddled in a standard towel. Measurement of whole body bone area (BA), bone mineral content (BMC), areal bone mineral density (aBMD) and body composition were performed (total and proportionate fat and lean). The short-term and long-term coefficients of variation (CV) for adult whole body BMD for the DXA instrument were 0.8% and 1.4% respectively. The radiation exposure to the baby was estimated as a maximum of 8.9 microsieverts for whole body measurement, which is equivalent to 3 days’ exposure to normal background radiation. All DXA scans were reviewed and those with movement artefact (41 scans) were excluded from the study.
Statistical Analysis
Gestational age was determined using an algorithm combining last menstrual period and early ultrasound data. All variables were checked for normality. Neonatal total fat mass, proportionate lean mass and proportionate fat mass were not normally distributed and were transformed using a Fisher-Yates transformation (standard deviate units). Unpaired t-tests were used to compare unstandardized neonatal characteristics by sex. A Mann-Whitney U test was used if assumptions of normality were not met.
Pearson correlation and linear regression were used to relate placental measurements to neonatal body composition and bone size and density. We then explored whether previously identified maternal determinants of neonatal bone mass might be mediated via placental measurements using multivariate linear regression. Stata V11.1 (StataCorp LP, College Station, TX) was used for all analyses. Bone outcomes used included whole body BA, BMC and aBMD. To adjust for body size we used size-corrected BMC (BMC adjusted for BA, and the baby’s length and weight (scBMC)). DXA measurements were associated with the square of offspring age at the scan, consistent with the known tendency of infants to lose weight transiently over the first week of postnatal life. Thus, all neonatal outcomes were adjusted for gestational age, sex and the square of the age at DXA; birthweight was adjusted for gestational age. The ultrasound-derived placental measures were also adjusted for gestational age at which the measurement was taken using the method of Royston and subsequently standardized to z-scores (11).
The study had full ethical approval from the Southampton and Southwest Hampshire Local Research Ethics Committee and all participants gave written informed consent.
Results
Characteristics of the mothers and neonates
Of the 3,159 participants, 914 had complete 19 week ultrasound and neonatal DXA data, and delivered after 37 weeks gestation. Baseline characteristics of the women are shown in Table 1(a). The median (IQR) age of the mothers at the birth of their babies was 31.1 (28.0-33.8) years. Their mean (SD) height was 163.4 (6.3) cm and median (IQR) BMI pre-pregnancy was 24.2 (22.0-27.5) kg/m2.
Table 1. Characteristics of mothers and neonates.
| A) Maternal Characteristics | |
|---|---|
| Maternal Characteristics n= 914 | |
| Age at child’s birth (yr) | 31.1 (28.0-33.8) |
| Height (cm) | 163.4 (6.3) |
| BMI pre-pregnancy (kg/m2) | 24.2 (22.0-27.5) |
| Triceps skin fold at 34 weeks (mm) | 20.6 (16.7-25.6) |
| Parity | |
| 0 | 480 (52.5%) |
| 1 or more | 434 (47.5%) |
| Smoking before pregnancy | |
| No | 665 (72.8%) |
| Yes | 249 (27.2%) |
| Smoking at 34 weeks | |
| No | 760 (86.6%) |
| Yes | 118 (13.4%) |
| Walking speed at 34 weeks | |
| Very slow | 139 (16.2%) |
| Stroll | 433 (50.5%) |
| Normal speed | 230 (26.5%) |
| Fairly brisk | 52 (6.1%) |
| Fast | 3 (0.4%) |
| Serum 25(OH) Vitamin D at 34 weeks (nmol/l) | 63.9 (44.0-87.0) |
| Placental circumference1 at 19 weeks (cm) | 29.5 (27.2-32.2) |
| Placental circumference2 at 19 weeks (cm) | 29.2 (26.7-38.9) |
| Placental length of attachment1 at 19 weeks (cm) | 15.6 (14.1-17.4) |
| Placental length of attachment2 at 19 weeks (cm) | 15.6 (14.0-22.4 |
| Placental cross-sectional area1 at 19 weeks (cm2) | 24.4 (21.0-28.5) |
| Placental cross-sectional2 at 19 weeks (cm2) | 24.3 (20.6-28.9) |
| Placental volume at 19 weeks (cm3) | 230.1 (192.7-277.9) |
| Data are mean (SD), median (IQR) or number (%) | |
| B) Neonatal characteristics | |||
|---|---|---|---|
| Boys n=474 | Girls n=440 | P Diff | |
| Birth weight (kg) | 3.59 (0.5) | 3.49 (0.5) | 0.002 |
| Gestational age (weeks) | 40.1 (1.2) | 40.3 (1.2) | 0.01 |
| Gestational age at time of scan (weeks) | 19.6 (0.6) | 19.6 (0.5) | 0.7 |
| Birth crown-heel length (cm) | 50.5 (1.9) | 49.7 (1.9) | <0.001 |
| Age at DXA (days) | 6.4 (2-11) | 6.5 (2-12) | 0.69 |
| Whole body bone area (cm2) | 121.4 (25.3) | 118.0 (24.9) | 0.001 |
| Whole body BMC (g) | 65.0 (15.6) | 61.3 (15.1) | <0.001 |
| Whole body aBMD (g/cm2) | 0.5 (0.03) | 0.5 (0.3) | <0.001 |
| Size corrected BMC (g) | 62.4 (2.9) | 61.8 (2.9) | 0.003 |
| Total lean mass (g) | 3026.6 (358.8) | 2884.9 (323.7) | <0.001 |
| Total fat mass (g) | 507.7 (382.4-655.6) | 533.3 (403.5-694.6) | 0.01 |
| %lean mass (%) | 84.2 (81.2-87.3) | 83.0 (79.6-85.6) | <0.001 |
| % fat mass (%) | 13.9 (11-16.9) | 15.3 (12.7-18.5) | <0.001 |
| Data are mean (SD) or median (IQR) | |||
measured along the longest edge of attachment to the uterine wall (length)
Placenta measured perpendicular to the longest edge of attachment to the uterine wall (breadth)
The baseline characteristics of the 914 (474 male) neonates are shown in Table 1(b). The boys tended to be heavier at birth, with higher BA, BMC and aBMD. All outcome measures were therefore adjusted for infant’s sex.
Compared with mothers of children born to the SWS cohort during the same time frame but who did not have placental measurements at 19 weeks or a neonatal DXA scan, the mothers in this study were more highly educated (24.5% versus 21.2% achieving a higher degree, p=0.10) and were less likely to smoke in pregnancy, although this did not achieve statistical significance (17.6% vs. 21.7%, p=0.06). There were no differences in maternal age at child’s birth, maternal height, BMI or smoking before pregnancy between the two groups.
Placental ultrasound measurements at 19 weeks and neonatal body composition
Table 2 summarises the relationships between placental measurements and offspring body composition and bone size and density. We observed strong positive relationships between each of placental section perimeter, length of attachment to the uterine wall and cross-sectional area at 19 weeks and neonatal BA and BMC (all p<0.001). However, there was some disparity in the relationship between neonatal aBMD and these placental measurements depending on the plane of placental measurement; a positive association was seen between placental measurements and aBMD when the placenta was measured along its breadth (p all<0.01), but no association was seen when the placenta measured along its longest axis (length).
Table 2. Relationship between placental size and neonatal bone mass and body composition.
| BA (cm2) |
BMC (g) |
aBMD (g/cm2) |
BA adjusted for crown-heel length (cm2) |
Size corrected BMC (g/cm2) |
Total lean (g) |
Total fat (z) |
%lean (z) |
%fat (z) |
|
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| r | r | r | r | r | r | r | r | r | |
| Placental measurements at 19 weeks | |||||||||
| Circumference1(z) | 0.15*** | 0.13*** | 0.04 | 0.07* | −0.03 | 0.14*** | 0.12*** | −0.10*** | 0.09** |
| Length of attachment1 (z) | 0.12*** | 0.11*** | 0.04 | 0.06 | −0.03 | 0.11** | 0.09** | −0.08* | 0.07* |
| Cross sectional area1 (z) | 0.18*** | 0.17*** | 0.05 | 0.09* | −0.01 | 0.18*** | 0.18*** | −0.15*** | 0.15*** |
|
| |||||||||
| Circumference2 (z) | 0.20*** | 0.19*** | 0.11** | 0.14*** | 0.006 | 0.16*** | 0.16*** | −0.14*** | 0.14*** |
| Length of attachement2 (z) | 0.16*** | 0.16*** | 0.09** | 0.13*** | 0.008 | 0.10** | 0.13*** | −0.13*** | 0.13*** |
| Cross sectional area2 (z) | 0.22*** | 0.21*** | 0.11*** | 0.14*** | 0.02 | 0.18*** | 0.19*** | −0.18*** | 0.17*** |
|
| |||||||||
| Volume (z) | 0.26*** | 0.25*** | 0.11** | 0.16*** | −0.001 | 0.23*** | 0.23*** | −0.20*** | 0.19*** |
Table shows Pearson’s correlation coefficients (r) from univariate regression analyses
measured along the longest edge of attachment to the uterine wall (length)
measured perpendicular to the longest edge of attachment to the uterine wall (breadth)
P <0.05
P<0.01
P<0.001
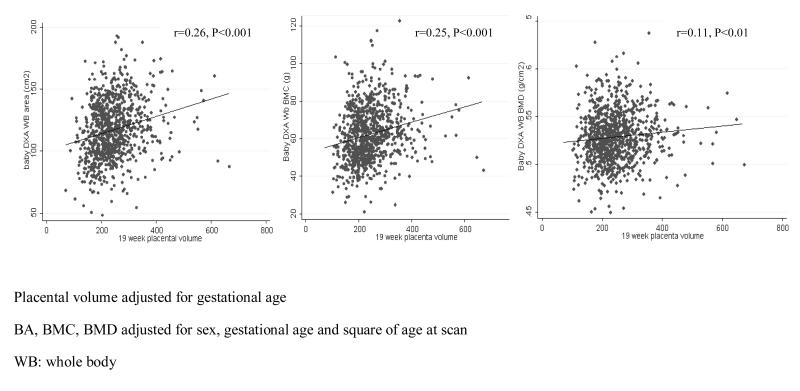
Placental volume correlated positively with neonatal BA, BMC and aBMD (p all <0.01) and neonatal BA adjusted for crown-heel length (r=0.15, p<0.001). Thus, for every 1 SD increase in placental volume, BA increased by 6.2cm2, BMC increased by 3.6g, aBMD increased by 0.0029g/cm2 and BA adjusted for crown-heel length increased by 2.9 cm2 (Figure 2). No significant association was observed between placental size and neonatal size-corrected BMC (all P>0.36).
Figure 2. Association between placental volume at 19 weeks and neonatal bone mass.
Scatter-plots illustrating the relationship between placental volume, measured by ultrasound at 19 weeks, and neonatal bone mass measured by dual x-ray absorptiometry (DXA). Placental volume correlated positively with neonatal BA, BMC and aBMD (p all <0.01). For every 1 SD increase in placental volume, BA increased by 6.2cm2, BMC increased by 3.6g, aBMD increased by 0.0029g/cm2 and BA adjusted for crown-heel length increased by 2.9 cm2.
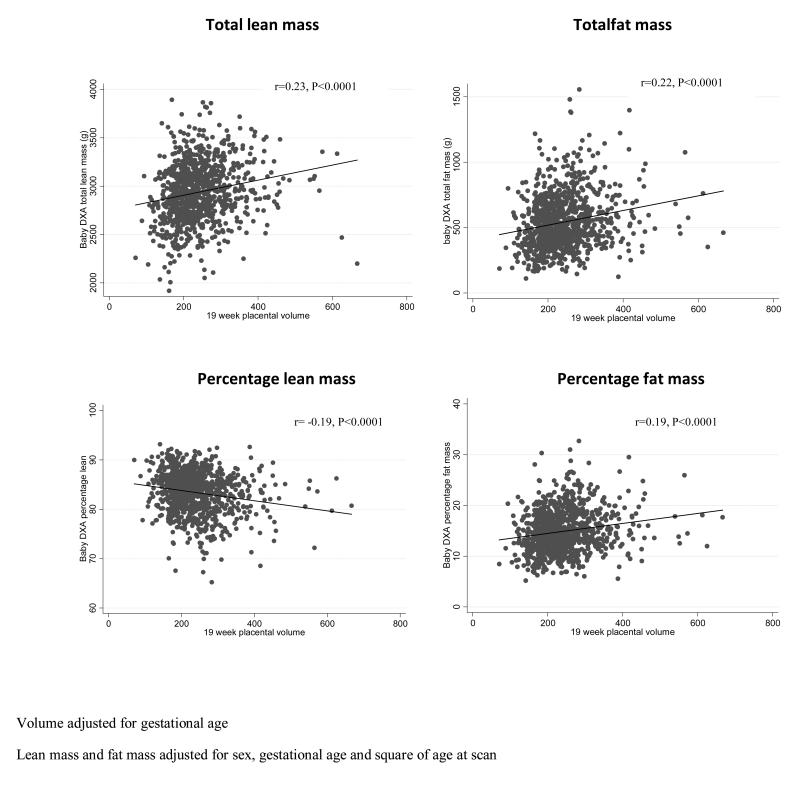
Placental volume at 19 weeks was positively associated with neonatal total lean mass (r=0.23, p<0.0001) and fat mass (r=0.23, p<0.0001). There was a different pattern with proportionate body composition. Thus, placental volume was positively related to percent fat (r=0.19, p<0.0001) but negatively to percent lean (r=−0.20, p<0.0001), indicating that as placental volume increased, total neonatal size increased, but with an increase in percentage fat and a reduction in percentage lean within the overall size envelope (Figure 3). We performed a sensitivity analysis by removing extreme values (excluding those participants with placental volume >500 mm3, total lean mass < 2200g, percent lean < 70 and percent fat >30) and the strongly significant relationships remained with little or no change in r value (Pearson correlation coefficient for association with placental volume: total lean mass, r=0.2, p<0.00001; total fat mass, r=0.23, p<0.00001; percent lean, r=−0.17, p<0.00001; percent fat, r=0.18, p<0.00001).
Figure 3. Association between placental volume at 19 weeks and neonatal fat and lean mass.
Scatter-plots illustrating the relationship between placental volume, measured by ultrasound at 19 weeks, and neonatal bone mass measured by dual x-ray absorptiometry (DXA). Placental volume at 19 weeks was positively associated with neonatal total lean mass (r=0.23, p<0.0001) and fat mass (r=0.23, p<0.0001). There was a different pattern with proportionate body composition. Thus, placental volume was positively related to percent fat (r=0.19, p<0.0001) but negatively to percent lean (r=−0.20, p<0.0001).
All associations remained after adjustment for maternal factors previously shown to affect neonatal bone mineral accrual (parity, smoking, walking speed, maternal serum 25(OH) vitamin D and maternal triceps skinfold thickness in pregnancy). In addition, the relationship between placental size and offspring bone mass was adjusted for maternal height, as maternal body build tends to be collinear with placental size, and although this attenuated some results, the relationships remained statistically significant (Table 3).
Table 3. Relationship between placental size and neonatal bone mass and body composition, adjusting for potentially confounding maternal influences.
| BA (cm2) |
BMC (g) |
aBMD (g/cm2) |
BA adjusted for crown-heel length (cm2) |
Size corrected BMC (g/cm2) |
Total lean (g) |
Total fat (z) |
%lean (z) |
%fat (z) |
|
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| r | r | r | r | r | r | r | r | r | |
| Circumference1(z) | 0.15 *** | 0.13 *** | 0.03 | 0.07* | −0.05 | 0.14 *** | 0.14 *** | −0.12 *** | 0.12 ** |
| Length of attachment1 (z) | 0.15 *** | 0.13 *** | 0.04 | 0.08* | −0.04 | 0.11 ** | 0.12 ** | −0.10** | 0.09** |
| Cross sectional area1 (z) | 0.15 *** | 0.14 *** | 0.03 | 0.08 * | −0.01 | 0.15 *** | 0.16 *** | −0.14 *** | 0.14 *** |
|
| |||||||||
| Circumference2 (z) | 0.22 *** | 0.22 *** | 0.12*** | 0.15*** | 0.02 | 0.19 *** | 0.20 *** | −0.17*** | 0.17 *** |
| Length of attachement2 (z) | 0.20 *** | 0.20 *** | 0.12*** | 0.13*** | 0.03 | 0.16 *** | 0.18 *** | −0.16 *** | 0.16 *** |
| Cross sectional area2 (z) | 0.18 *** | 0.18 *** | 0.10 ** | 0.10** | 0.04 | 0.18 *** | 0.16 *** | −0.15 *** | 0.14 *** |
|
| |||||||||
| Volume (z) | 0.24 *** | 0.23 *** | 0.10 ** | 0.14*** | 0.003 | 0.22 *** | 0.23 *** | −0.20 *** | 0.19 *** |
Table shows Pearson’s correlation coefficients (r) from multiple regression analyses taking account of maternal height, smoking in late pregnancy, walking speed in late pregnancy, triceps skinfold thickness in late pregnancy and serum 25(OH) Vitamin D in late pregnancy as confounders.
measured along the longest edge of attachment to the uterine wall (length)
measured perpendicular to the longest edge of attachment to the uterine wall (breadth)
P <0.05
P<0.01
P<0.001
Relationships between placental and DXA measurements were similar in boys and girls, with all placental measurement /sex interactions on bone outcomes failing to achieve statistical significance (p>0.05).
We calculated the ratio of placental volume: birth weight as a marker of placental “efficiency”. This was positively associated with neonatal BA and BMC (p<0.01), however after adjustment for maternal factors known to affect neonatal bone mineral accrual, significant associations were no longer seen.
Maternal characteristics and placental size
Several maternal factors were positively correlated with placental volume at 19 weeks (Table 4). Placental volume was positively associated with maternal height (r=0.09, p=0.007), body fat pre-pregnancy (r=0.11, p=0.001) and age at child’s birth (r=0.07, p=0.03). Smoking, 25(OH) vitamin D status, parity, social class and walking speed pre-pregnancy were not statistically significantly associated with placental volume.
Table 4. Relationship between maternal characteristics and placental volume.
| Placental Volume adjusted for gestation (z) | ||||
|---|---|---|---|---|
|
| ||||
| β (95% CI) | P | Mutually adjusted β (95% CI) |
P | |
| Age (SD) | 0.07 (0.008-0.13) |
0.03 | 0.01 (−0.008-0.12) |
0.09 |
| Height (SD) | 0.09 (0.02-0.15) |
0.01 | 0.08 (0.01-0.14) |
0.02 |
| Parity, 2 groups | 0.12 (−0.01-0.24) |
0.07 | 0.10 (−0.03-0.23) |
0.14 |
|
Walking speed pre-pregnancy
(5 groups) |
−0.003 (−0.09-0.08) |
0.95 | −0.03 (−0.12-0.06) |
0.50 |
| Body fat pre-pregnancy (SD) | 0.11 (0.05-0.17) |
0.001 | 0.09 (0.03-0.16) |
0.004 |
|
Smoking in pregnancy
(Yes/No) |
0.11 (−0.07-0.29) |
0.23 | 0.12 (−0.06-0.30) |
0.19 |
| Social class (6 groups) | 0.01 (−0.02-0.05) |
0.45 | 0.03 (−0.03-0.09) |
0.29 |
Table shows regression coefficient (95% confidence interval) and mutually adjusted regression coefficient from univariate and multiple regression analyses respectively.
Discussion
To our knowledge, this is the first study to relate ultrasound measurements of mid-pregnancy placental size to postnatal infant bone mass and body composition measured by DXA. Placental section perimeter, length of attachment and volume were positively associated with offspring intrauterine bone mineral accrual. An association between mid-pregnancy placental size and neonatal body composition was also observed; thus greater placental volume was associated with greater birth weight, higher percentage fat and lower percentage lean. These relationships appeared independent of maternal height and other factors (parity, smoking, walking speed, maternal serum 25(OH) vitamin D and maternal triceps skinfold thickness in pregnancy) known to influence neonatal bone mass, suggesting that these maternal factors may act through modulation of aspects of placental function, such as utero-placental blood flow or maternal nutrient concentrations, rather than size alone.
This was a large prospective study, with detailed characterization of the mothers and babies, using a validated measure of bone mineral. However, several limitations should be considered in the interpretation of our results. First, intrauterine ultrasound measurements are prone to reproducibility error; however the scans were performed by two experienced sonnographers following standard guidelines. Second, although the use of DXA is well established in adults, there are limitations to its use in neonates due to their tendency to move and their low absolute BMC. Specific paediatric software was used to minimise loss of edge detection, and movement artefact was modest and uniform across the cohort. Babies with excessive movement were excluded from the analysis. It was not possible to perform repeat DXA assessments on the neonates to determine values for the coefficient of variation of DXA in children. However, DXA measurements of bone mass have been shown to correlate well with whole body calcium content in studies of small animals such as piglets (12). We have reported on a sub-set of the original SWS births. On average the mothers included in this study were better educated and were slightly less likely to smoke in pregnancy compared with SWS mothers of babies who were not assessed by ultrasound at 19 weeks nor had neonatal DXA measurements. However, as the observations we report are based on internal comparisons, there is no reason to suppose that this would have biased the results. The use of DXA does not allow for true measurement of volumetric bone density, making it difficult to distinguish relationships with bone size from those with bone density. Finally, as this was an observational study it is not possible to deduce the direction of co-linearity between placental size and offspring bone mass, and to be certain about the distinction between effects of placental size and placental function. Further basic science studies are needed to examine these relationships further.
Several studies have investigated the association between birth weight and placental size at various points in gestation. Placental volume measured by 3D ultrasound scanning in the first trimester was positively associated with birth weight in one study of 199 women (13). Likewise, placental volume derived from ultrasound measurements in mid-pregnancy and by direct measurement at birth have been shown to correlate positively with birth weight (14-17). The rate of placental growth appears to be an important determinant of birth weight, with the rate between 17 to 20 weeks gestation being a predictor of fetal abdominal and head circumference, femoral length and biparietal diameter; weaker associations are observed for placental growth earlier in pregnancy (at 14-17 weeks) (18).
The size, weight and shape of the placenta are all subject to wide variations (19), and growth along one axis may be controlled differently from that along the other axis (20). Growth of the placenta may be polarised from the time of implantation, and it has been suggested that growth along the major axis (maximal diameter or placental length) is aligned with the rosto-caudal axis of the embryo, whereas tissue along the minor axis (breadth) may be more important for transfer of nutrient to the fetus and may be more sensitive to the mother’s nutrition (20). Thus, the human foetus may attempt to compensate for under-nutrition by expansion of the placenta along its minor axis. Pre-eclampsia, which is associated with reduced placental size, is an example of this. Placentas from pregnancies complicated by pre eclampsia have increased thickness due to a reduction in breadth, with no significant association with length (21). In our study, length of attachment to the uterine wall, section perimeter and cross-sectional area measured along both axis, were equally associated with offspring BA and BMC. These placental measurements, when taken transversely along the minor axis, were also associated with neonatal aBMD; in contrast, no association with aBMD was seen when the placenta was measured along its longest axis (length). This may suggest that growth along the minor axis may be more important for nutrient transfer and capacity for mineralisation.
Nutrient transport is one of the many functions of the placenta. Up to 30g of calcium crosses to the fetus in a successful pregnancy; in the third trimester calcium transport quadruples to around 140mg/kg per day to sustain adequate mineralisation of the fetal skeleton (7). Placental calcium transport occurs in the synctiotrophoblast (22); calcium crosses the placenta bound to calcium transport proteins including calbindin-D9K and calnexin before being actively extruded from the basal plasma membrane of the trophoblast layer to the fetal circulation via a number of pumps and exchangers, such as Na+/Ca2+ exchanger and plasma membrane Ca2+-ATPase. This last group of transport proteins includes four individual isoforms (PMCA 1-4) (22). It has previously been demonstrated in animal models that a 2-3 fold increase in PMCA gene expression is associated with a 72-fold increase in calcium transport across the placenta in late gestation (23).
Maternal 25 (OH) vitamin D concentration appears to influence offspring bone mineral accrual though effects on the concentration of umbilical venous calcium (6), and it is possible that regulation of placental transport may be important in the relationships between placenta and offspring bone mass. Indeed we have previously shown that expression of PMCA 3 was positively related to neonatal whole body BMC (9), and several studies have demonstrated the importance of nutrient transport across the placenta, even after adjustment for overall size. Maternal vitamin D concentration may exert its effects on offspring bone mass through PMCA 3 expression (9), however mechanistic confirmation is required to determine whether the effects are due to altered presentation of nutrient to the placenta (substrate dependent) or a direct action on transport processes. It is difficult to distinguish between the two potential mechanisms, and our results would be consistent with either, rather than an effect purely on placental size per se.
The strongest associations detected in our study were between placental size and neonatal skeletal size. Placental volume predicted neonatal BA and BMC more strongly than aBMD and scBMC. This finding is consistent with previous studies showing that skeletal size, rather than volumetric density is influenced by early life factors. Density tends to be more dependent on environmental influences later in the life course, such as loading and nutrition (2;24). However, in adult studies, bone size and BMC perform well as predictors for fracture risk suggesting that the overall size of the skeletal envelope will have longer term implications.
Thus, recent work has suggested that peak bone mass is a critically important determinant of osteoporosis in later life (1) and we have previously demonstrated that early growth predict adult bone mass (2;3) and risk of hip fracture in later life (4). We have found that for every 1 SD increase in placental volume, neonatal BMC increases by 3.6 grams. Placental volume accounted for 6.25% of the variation in BMC and 1.2% in the variation of BMD at birth. Although these associations appear modest, they are potentially biologically significant: the difference in mean BMC or BMD for those individuals who were in the top compared with bottom quartile of placental volume at 19 weeks was 0.7 SD and 0.3 SD respectively. If these differences were to be sustained into adulthood, they might equate to a 15% difference in risk of fracture (25). This figure is similar to the 13% increased risk of vertebral fractures in women who smoke [a risk factor incorporated into the standard international method of risk stratification (FRAX™) (26)] compared to women who do not. Therefore our findings may well be relevant in terms of later bone health.
Conclusions
In conclusion, we have demonstrated that placental size measured in mid pregnancy by ultrasound predicts neonatal bone mass and body composition, independently of maternal factors known to influence neonatal bone mass (parity, smoking, walking speed, maternal serum 25(OH) vitamin D and maternal triceps skinfold thickness in pregnancy). This suggests that these factors may act through modulation of aspects of placental function, such as utero-placental blood flow or maternal nutrient concentrations, rather than placental size. Low placental volume early in pregnancy may be a marker of a smaller postnatal skeletal size and potentially increased risk of fracture in older age.
Acknowledgements
We thank the mothers who gave us their time; and a team of dedicated research nurses and ancillary staff for their assistance. CRH and NCH are joint first author. This work was supported by grants from the Medical Research Council, Arthritis Research UK, National Osteoporosis Society and the International Osteoporosis Foundation. KG is supported by the National Institute for Health Research through the Southampton NIHR Nutrition, Diet & Lifestyle Biomedical Research Unit. We thank Mrs G Strange and Mrs R Fifield for helping prepare the manuscript.
Funding Sources: Arthritis Research UK, Medical Research Council
Footnotes
Conflict of interest: all authors report no conflict of interest
Reference List
- (1).Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003 Oct;14(10):843–7. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- (2).Cooper C, Cawley M, Bhalla A, Egger P, Ring F, Morton L, et al. Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res. 1995 Jun;10(6):940–7. doi: 10.1002/jbmr.5650100615. [DOI] [PubMed] [Google Scholar]
- (3).Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr Res. 2005 Apr;57(4):582–6. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- (4).Cooper C, Eriksson JG, Forsen T, Osmond C, Tuomilehto J, Barker DJ. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporos Int. 2001;12(8):623–9. doi: 10.1007/s001980170061. JID - 9100105. [DOI] [PubMed] [Google Scholar]
- (5).Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, et al. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. 2001 Sep;16(9):1694–703. doi: 10.1359/jbmr.2001.16.9.1694. JID - 8610640. [DOI] [PubMed] [Google Scholar]
- (6).Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006 Jan 7;367(9504):36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- (7).Hosking DJ. Calcium homeostasis in pregnancy. Clin Endocrinol (Oxf) 1996 Jul;45(1):1–6. [PubMed] [Google Scholar]
- (8).Schauberger CW, Pitkin RM. Maternal-perinatal calcium relationships. Obstet Gynecol. 1979 Jan;53(1):74–6. [PubMed] [Google Scholar]
- (9).Martin R, Harvey NC, Crozier SR, Poole JR, Javaid MK, Dennison EM, et al. Placental calcium transporter (PMCA3) gene expression predicts intrauterine bone mineral accrual. Bone. 2007 May;40(5):1203–8. doi: 10.1016/j.bone.2006.12.060. [DOI] [PubMed] [Google Scholar]
- (10).Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. Cohort profile: The Southampton Women’s Survey. Int J Epidemiol. 2005 Sep 29; doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Royston P. Calculation of unconditional and conditional reference intervals for foetal size and growth from longitudinal measurements. Stat Med. 1995 Jul 15;14(13):1417–36. doi: 10.1002/sim.4780141303. [DOI] [PubMed] [Google Scholar]
- (12).Koo WW, Walters J, Bush AJ. Technical considerations of dual-energy X-ray absorptiometry-based bone mineral measurements for pediatric studies. J Bone Miner Res. 1995 Dec;10(12):1998–2004. doi: 10.1002/jbmr.5650101220. [DOI] [PubMed] [Google Scholar]
- (13).Antsaklis A, Anastasakis E, Komita O, Theodora M, Hiridis P, Daskalakis G. First trimester 3D volumetry. Association of the gestational volumes with the birth weight. J Matern Fetal Neonatal Med. 2011 Aug;24(8):1055–9. doi: 10.3109/14767058.2010.545915. [DOI] [PubMed] [Google Scholar]
- (14).Kabir N, Kawser CA, Rahman F, Kabir ML, Rahman A. The relationship of placental weight with birth weight. Mymensingh Med J. 2007 Jul;16(2):177–80. [PubMed] [Google Scholar]
- (15).Kinare AS, Natekar AS, Chinchwadkar MC, Yajnik CS, Coyaji KJ, Fall CH, et al. Low midpregnancy placental volume in rural Indian women: A cause for low birth weight? Am J Obstet Gynecol. 2000 Feb;182(2):443–8. doi: 10.1016/s0002-9378(00)70237-7. [DOI] [PubMed] [Google Scholar]
- (16).Wolf H, Oosting H, Treffers PE. Second-trimester placental volume measurement by ultrasound: prediction of fetal outcome. Am J Obstet Gynecol. 1989 Jan;160(1):121–6. doi: 10.1016/0002-9378(89)90102-6. [DOI] [PubMed] [Google Scholar]
- (17).Clapp JF, 3rd, Rizk KH, S. K, Crass JR. Second-trimester placental volumes predict birth weight at term. J Soc Gynecol Investig. 1995 Jan;2(1):19–22. [PubMed] [Google Scholar]
- (18).Thame M, Osmond C, Bennett F, Wilks R, Forrester T. Fetal growth is directly related to maternal anthropometry and placental volume. Eur J Clin Nutr. 2004 Jun;58(6):894–900. doi: 10.1038/sj.ejcn.1601909. [DOI] [PubMed] [Google Scholar]
- (19).Hamilton WJ, Boyd Jd, Mossman HW. Human Embryology. W. Heffer & Sons; Cambridge: 1945. [Google Scholar]
- (20).Barker DJP, Eriksson JG, Kajantie E, Alwasel SH, Fall CHD, Roseboom TJ, et al. The maternal and placental origins of chronic disease. In: Burton GJ, Barker DJP, Moffett A, Thornburg K, editors. The Placenta And Human Developmental Programming. 1st ed Cambridge University Press; Cambridge: 2011. pp. 5–16. [Google Scholar]
- (21).Kajantie E, Thornburg KL, Eriksson JG, Osmond C, Barker DJ. In preeclampsia, the placenta grows slowly along its minor axis. Int J Dev Biol. 2010;54(2-3):469–73. doi: 10.1387/ijdb.082833ek. [DOI] [PubMed] [Google Scholar]
- (22).Belkacemi L, Bedard I, Simoneau L, Lafond J. Calcium channels, transporters and exchangers in placenta: a review. Cell Calcium. 2005 Jan;37(1):1–8. doi: 10.1016/j.ceca.2004.06.010. [DOI] [PubMed] [Google Scholar]
- (23).Glazier JD, Atkinson DE, Thornburg KL, Sharpe PT, Edwards D, Boyd RD, et al. Gestational changes in Ca2+ transport across rat placenta and mRNA for calbindin9K and Ca(2+)-ATPase. Am J Physiol. 1992 Oct;263(4 Pt 2):R930–R935. doi: 10.1152/ajpregu.1992.263.4.R930. [DOI] [PubMed] [Google Scholar]
- (24).Gale CR, Martyn CN, Kellingray S, Eastell R, Cooper C. Intrauterine programming of adult body composition. J Clin Endocrinol Metab. 2001 Jan;86(1):267–72. doi: 10.1210/jcem.86.1.7155. [DOI] [PubMed] [Google Scholar]
- (25).Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996 May 18;312(7041):1254–9. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Ward KD, Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int. 2001 May;68(5):259–70. doi: 10.1007/bf02390832. [DOI] [PMC free article] [PubMed] [Google Scholar]