Abstract
Brain tissue being rich in polyunsaturated fatty acids, is very susceptible to lipid peroxidation. Iron is well known to be an important initiator of free radical oxidations. We propose that the principal route to iron-mediated lipid peroxidations is via iron-oxygen complexes rather than the reaction of iron with hydrogen peroxide, the Fenton reaction. To test this hypothesis, we enriched leukemia cells (K-562 and L1210 cells) with docosahexaenoic acid (DHA) as a model for brain tissue, increasing the amount of DHA from approximately 3 mole % to 32 mole %. These cells were then subjected to ferrous iron and dioxygen to initiate lipid peroxidation in the presence or absence of hydrogen peroxide. Lipid-derived radicals were detected using EPR spin trapping with α-(4-pyridyl-1-oxide)-N-t-butylnitrone (POBN). As expected, lipid-derived radical formation increases with increasing cellular lipid unsaturation. Experiments with Desferal demonstrate that iron is required for the formation of lipid radicals from these cells. Addition of iron to DHA-enriched L1210 cells resulted in significant amounts of radical formation; radical formation increased with increasing amount of iron. However, the exposure of cells to hydrogen peroxide before the addition of ferrous iron did not increase cellular radical formation, but actually decreased spin adduct formation. These data suggest that iron-oxygen complexes are the primary route to the initiation of biological free radical oxidations. This model proposes a mechanism to explain how catalytic iron in brain tissue can be so destructive.
Keywords: Free radicals, iron, docosahesaenioc acid, lipid peroxidation, EPR, hydrogen peroxide
INTRODUCTION
Brain tissue is rich in polyunsaturated fatty acids (PUFAs). These PUFAs are especially vulnerable to oxidation (Wagner, 1994). Free radical-mediated lipid peroxidation has three major components: initiation, propagation, and termination reactions (Gardner, 1989).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
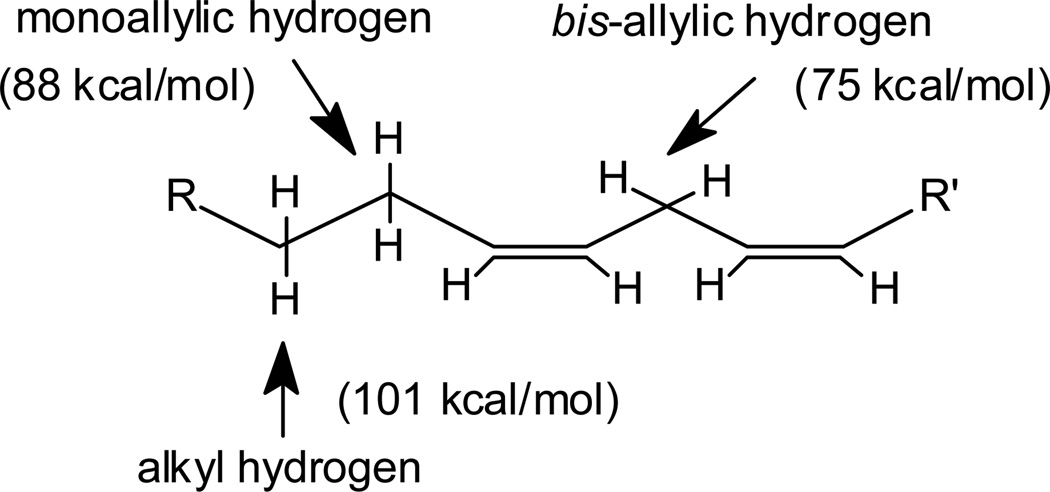
L-H represents a lipid, generally a polyunsaturated fatty acid moiety. The rate of propagation is governed by the various carbon-hydrogen bond dissociation energies in the lipid chain. The weakest carbon-hydrogen bonds are those of the bis-allylic methylene positions, which have carbon-hydrogen bond energies of approximately 75 kcal/mol compared to 101 kcal/mol for typical alkyl C-H bonds (Gardner, 1989, Koppenol, 1990) (Fig 1).
Fig. 1. The types of C-H bonds in lipids.
Previous studies by our group have demonstrated that the rate of cellular lipid peroxidation increases exponentially with the total number of bis-allylic positions contained in cellular lipids (Wagner, 1994).
Iron can be a detrimental catalyst in lipid peroxidation because it can both initiate and amplify lipid peroxidation. The initiation step can be induced by two different iron -dependent mechanisms. The hydroxyl radical-dependent mechanism has been adopted by most researchers. An alternate mechanism, hydroxyl radical-independent, proposes that iron-oxygen complexes rather than HO• radical initiates lipid peroxidation. In the first mechanism, iron serves as a reagent for the Fenton reaction, forming HO•, which eventually initiates lipid peroxidation. In the second mechanism, however, it has been proposed that lipid peroxidation is not initiated by HO• but rather by iron in the form of iron-oxygen complexes, such as perferryl ion or ferryl ion1. Because of the high physiological ratio of [O2]/[H2O2] (≥ 103), we have proposed that the Fenton reaction with pre-existing H2O2 is only a minor initiator of free radical oxidations such as lipid peroxidation and that the major initiators of biological free radical oxidations are the oxidizing species formed by the reaction of loosely bound Fe2+ with dioxygen (Qian, 1999).
In the present study we have used leukemia cells enriched with docosahexaenoic acid (DHA, 22:6ω) to examine the ability of these two mechanisms to initiate free radical oxidations.
Brain contains high amounts of unsaturated fatty acids, especially DHA, thus our cells enriched with DHA are a good model to study lipid peroxidation in brain. It has been shown that homogenates of isolated brain tissue can have high rates of lipid peroxidation, and a direct correlation between iron content and the rate of peroxidation was found (Zaleska, 1995). We find that all that is required to initiate lipid peroxidation in our model system is ferrous iron and dioxygen.
MATERIALS AND METHODS
Chemicals
Photofrin® (porfimer sodium, QLT Phototherapeutics, Inc., Vancouver, BC, Canada) was dissolved in 5% dextrose (pH 7.4) sterile filtered and frozen at −20°C. α-(4-Pyridyl-1-oxide)-N-tert-butylnitrone (POBN) (Aldrich Chemical Co., Milwaukee, WI) was prepared as a 1.0 M stock solution in distilled water. Docosahexaenoic acid (DHA) from Cayman Chemical Co., (Ann Arbor, MI, USA) was prepared as a 32 µM solution in 10% FBS-RPMI medium. Desferal (Ciba Pharmaceutical Co, Summit. NJ) was dissolved in phosphate buffered-saline (PBS) (pH 7.5) to a final concentration of 100 µM. To make phosphate buffered-saline, 210 mg potassium dihydrogen phosphate, 407 mg sodium monohydrogen phosphate and 9 g NaCl (0.9%) were dissolved in 1 L of water and adjusted to pH 7.4 with 1 M HCl. Both the NaCl and the PBS solution were sterile filtered and stored over chelating resin (Sigma Chemical Co., St. Louis, MO) to minimize the level of adventitious transition metals (Buettner, 1988). Stock solutions of ascorbic acid (10 mM) and ferrous iron, Fe(NH4)2(SO4)2•6 H2O, (1 mM) in distilled water were freshly prepared before each experiment.
Cell culture
K-562 cells, a human leukemia cell line, and L1210 cells, a murine leukemia cell line, were acquired from American Type Culture Collection (ATCC, Rockville, MD). All cells were grown in medium consisting of RPMI 1640 medium (Gibco/Life Technologies, Grand Island, NY) and 10% fetal bovine serum. Experiments were performed when cells were in exponential growth phase.
Fatty acid modification
K-562 and L1210 cells were grown in 10% FBS-RPMI medium supplemented with 32 µM (DHA), for 48 h. Cells were washed twice, first in RPMI-1640 medium then in PBS by centrifugation (300 g). Upon re-suspension, cell density (6–8×106 cells/mL) was adjusted for EPR experiments.
Photofrin uptake
Cells were exposed to Photofrin (9 µg/mL) at 37°C for 45 min in PBS pH 7.4. After incubation with Photofrin, cells were centrifuged (300 × g) and resuspended in 0.9% NaCl.
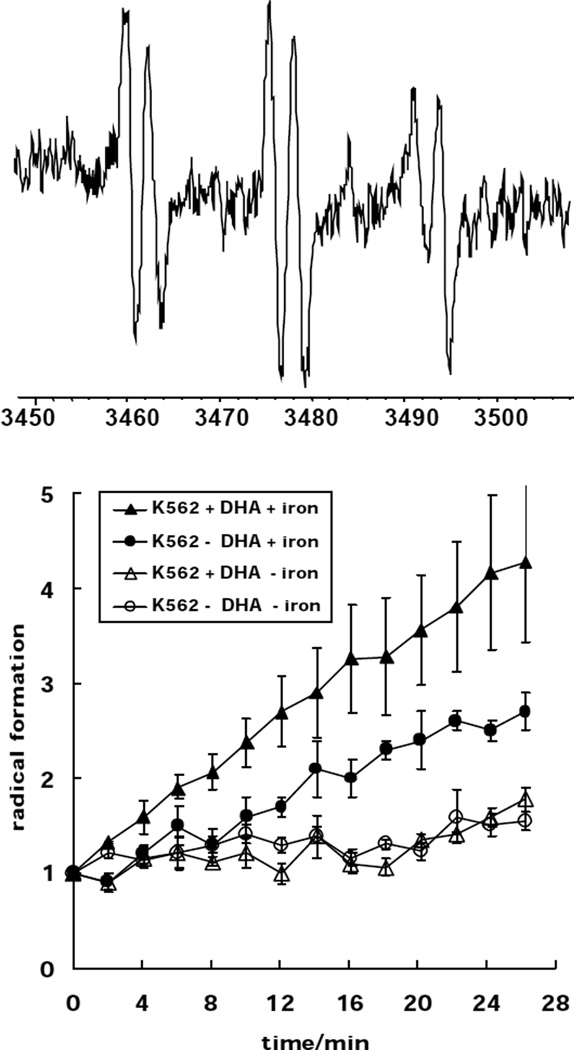
EPR experiment (Fig. 3)
Fig. 3. Enrichment of K-562 cells with DHA enhances lipid peroxidation.
TOP) A representative EPR spectrum of a carbon-centered POBN spin adduct derived from lipid peroxidation in K-562 cells, aN = 15.6 G, aH = 2.6 G. This spectrum was collected after the addition of 10 µM ferrous iron to DHA-enriched cells that had been exposed to Photofrin and light. Bottom) K-562 cells (+/− DHA) were incubated with/without 100 µM Desferal (during Photofrin uptake) and then exposed to light in PBS (pH 6.5). Solid symbols: Ferrous iron (5 µM) was added to K-562 cells (●) and K-562 cells enriched with DHA (♦) in PBS pH 6.5 at the beginning of the experiment; Desferal was not present. Open Symbols: K-562 cells (○) and K-562 cells enriched with DHA (△) were incubated with Desferal during Photofrin uptake. Cells were exposed to light in PBS pH 6.5 containing 100 µM Desferal. No ferrous iron was added to the cell samples.
K-562 cells were enriched with DHA. Cells (8×106/mL) were then incubated with Photofrin (9 µg/mL) for 45 min, washed and resuspended in 1 mL 0.9 % NaCl. An aliquot of 500 µL was mixed with POBN (25 mM), ascorbate (100 µM) and Fe(NH4)2(SO4)2•6 H2O (5 µM) and placed into a TM110 EPR quartz flat cell. Ferrous iron and ascorbic acid were needed to initiate radical formation from lipid hydroperoxides. Cells were exposed to visible light (tungsten, 180 J/m2 s) directly in the EPR cavity and the EPR signal intensity of the POBN radical adduct was monitored versus time. Each data point represents the signal-averaged result of 5 scans of the low field doublet of the POBN/lipid-derived radical adduct spectrum. The first five scans were performed in the dark. The first data point representing the POBN/Ld• spin adduct concentration was below the limit of detection for the instrument parameters used. Thus, “1” represents the instrument noise level. EPR experiments were performed with a Bruker ESR-300 EPR spectrometer (Karlsruhe, Germany).
Desferal experiments
Cells were exposed to Photofrin in 100 µM Desferal in PBS (pH 7.5) for 45 min at 37°C. After centrifugation (300 × g) cells were resuspended in 100 µM Desferal in 0.9% NaCl and used for EPR experiments.
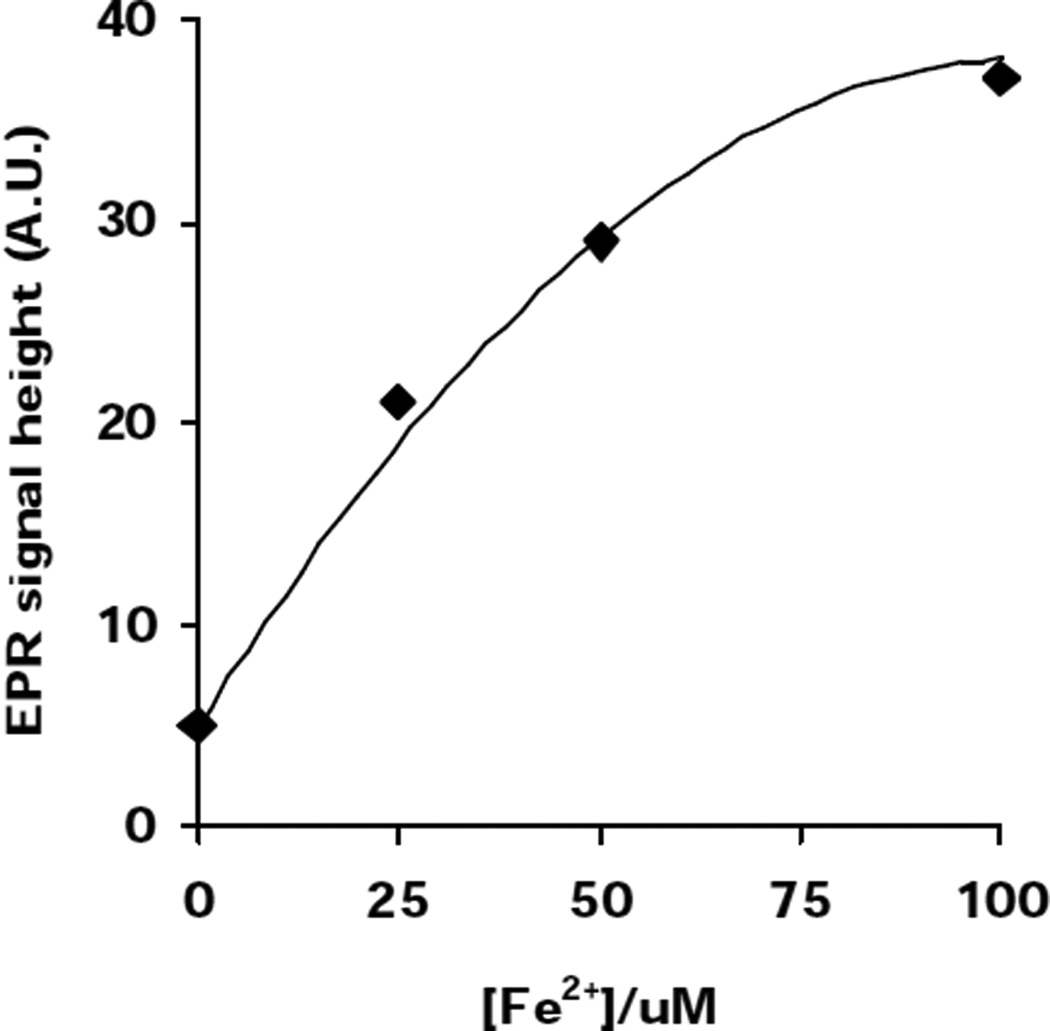
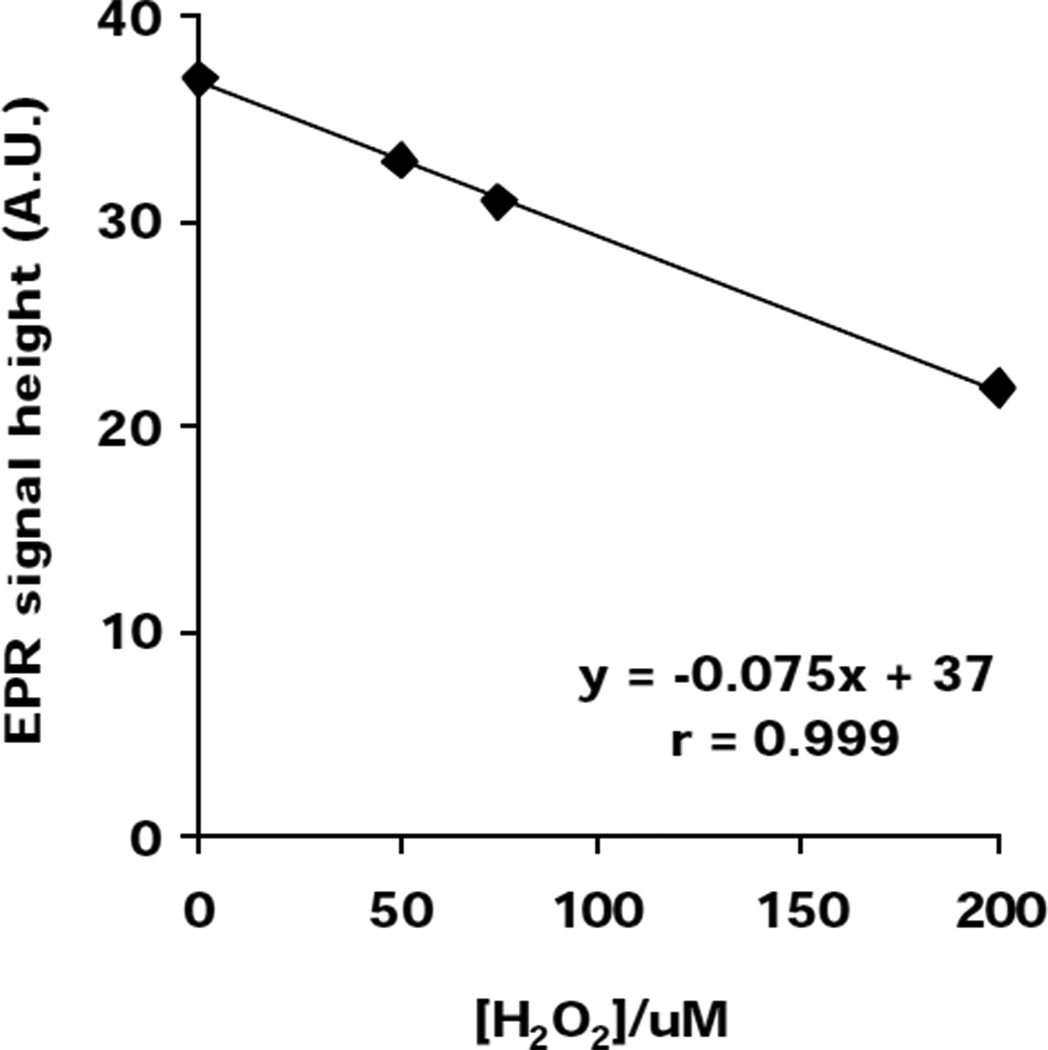
EPR experiment Fig. (4,5)
Fig. 4. Lipid-derived radical formation in L1210 cells enriched with DHA is Fe2+-dependent.
POBN (50 mM) and Fe2+ were added to DHA-enriched L1210 cells (6×106 cells /mL) in 0.9% NaCl. Cells were then placed into a TM110 EPR quartz flat cell and the POBN radical adduct EPR signal intensity was monitored for 2 min. Each data point represents the signal-averaged result of 5 scans of the POBN/lipid-derived radical adduct spectrum. These data are representative of at least 3 independent experiments.
Fig. 5. Lipid-derived radical formation initiated by iron in L1210 cells enriched with DHA is decreased by H2O2.
POBN (50 mM), H2O2 and Fe2+ were added to DHA-enriched L1210 cells (6×106 cells /mL) in 0.9% NaCl. Cells were then placed into a TM110 EPR quartz flat cell and the POBN radical adduct EPR signal intensity was monitored for 2 min. Each data point represents the signal-averaged result of 5 scans of the POBN/lipid-derived radical adduct spectrum. These data are representative of at least 3 independent experiments.
L1210 cells were enriched with DHA; then 6×106 cells /mL were suspended in 0.9% NaCl mixed with POBN (50 mM), H2O2 (0–200 µM) and Fe(NH4)2(SO4)2•6 H2O (0–100 µM) and placed into a TM110 EPR quartz flat cell. The POBN radical adduct EPR signal intensity was monitored for 2 min. Each data point represents the signal-averaged result of 5 scans of the POBN/lipid-derived spin adduct spectrum.
Statistics
Experiments were done in triplicates; standard errors are shown.
RESULTS AND DISCUSSION
Unsaturated lipids in cell membranes increase susceptibility to lipid peroxidation
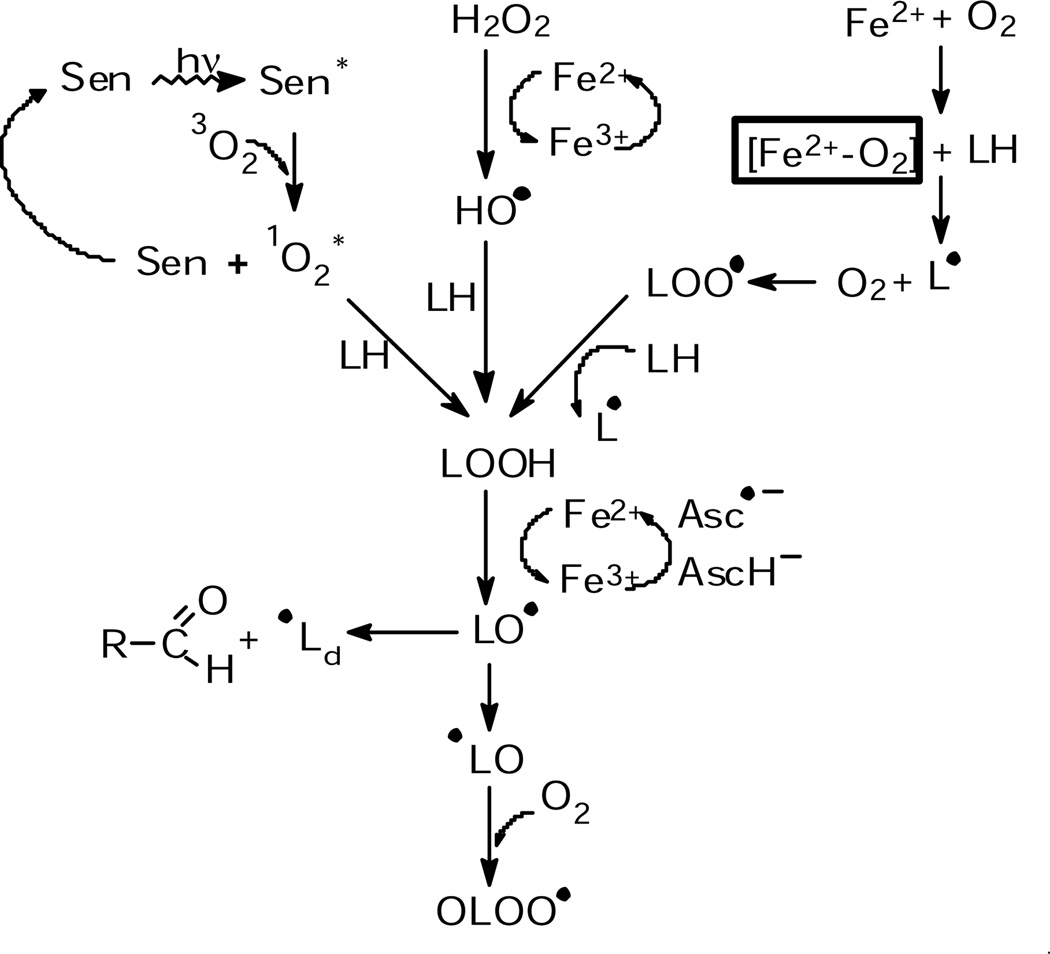
The lipids of a cell contain both saturated and unsaturated fatty acids. Unsaturated fatty acids are highly susceptible to lipid peroxidation, fig. 2 shows the various reactions. A hydrogen atom in a bis-allylic position can be abstracted by oxygen -centered radicals such as peroxyl (LOO•), alkoxyl (LO•), and hydroxyl radicals (HO•); the unsaturated region in the lipid chain can also react with singlet oxygen to form lipid hydroperoxides. The higher the number of double bonds, the greater the oxidizability of a lipid. The average number of bis-allylic positions per fatty acid chain in a lipid ensemble (such as a cell membrane) can be expressed as the Methylene Bridge Index (MBI); the average number of double bonds in a lipid ensemble as Double Bond Index (DBI) (Wagner et al., 1994). Cell lines vary greatly in their MBI and DBI (Schafer and Buettner, 1999); therefore, their susceptibility to lipid peroxidation is different. Supplementation of cell culture media with docosahexaenoic acid (DHA), a fatty acid with six double bonds, increases the MBI of K -562 cells 7 fold (K-562 cells MBI = 0.24, K-562 enriched with DHA, MBI = 1.77). The amount of DHA incorporated into the cells increased from approximately 3 mole % to 32 mole %.
Fig. 2. Initiation of lipid-derived radical chain reactions through different pathways.
Reactive oxygen species, such as singlet oxygen, hydrogen peroxide and iron-oxygen complexes, produce lipid hydroperoxides from unsaturated lipids. Catalytic iron can then initiate lipid-derived radical formation.
We used Photofrin, a photosensitizer used in photodynamic treatment of cancer, as a tool to produce singlet oxygen. Because Photofrin is lipophilic, it accumlates in membranes where it produces singlet oxygen upon light exposure. Singlet oxygen can add to the double bonds of the unsaturated lipids forming lipid hydroperoxides. In the absence of catalytic metal, these LOOHs accumulate in the cell membranes. Upon introduction of ferrous iron, lipid-derived radical chain reactions are initiated. To investigate the role of iron and PUFAs in lipid peroxidation, we supplemented K-562 cells with DHA and exposed them to Photofrin, ferrous iron and light. Using POBN as a spin trap, radical formation was monitored via EPR. A significant increase in radical formation is observed in DHA-enriched K-562 cells compared to control cells, (Fig 3) When cells were incubated with Desferal, a metal chelator, no detectable lipid peroxidation was observed in either DHA-enriched cells or control cells. These data demonstrate that increased unsaturation of lipids increases their susceptibility to lipid peroxidation and that catalytic metals, such as ferrous iron, are needed to induce lipid-derived radical formation. In fact, lipid-derived radical formation in DHA-enriched L1210 cells increases as the Fe2+ concentration increases, (Fig. 4).
Fe-O2 complexes enhance cellular free radical oxidations; H2O2 does not
Iron-mediated cellular lipid peroxidation is thought to be one of the key factors in cell injury. The initiation step of lipid peroxidation can be induced by two different mechanisms, a HO•-dependent (Fenton reaction) or independent mechanism ('Fe+O2’ chemistry). To determine if 'Fe+O2’ chemistry is more important than the Fenton reaction in initiating membrane lipid peroxidation in cells, we enriched the membranes of L1210 murine leukemia cells with DHA and subjected them to either Fe2+ + O2 or Fe2+ + H2O2 + O2. We observed that Fe2+ and dioxygen will yield lipid-derived radicals in L1210 cells, (Fig.4). Rather than enhancing lipid-derived radical formation, addition of H2O2 to this system, actually decreases POBN adduct formation, (Fig. 5). This decrease has a remarkable inverse correlation with [H2O2], suggesting that cellular oxidation is mediated predominantly by Fe2+-dioxygen chemistry. This is consistent with the observations of Hempel et al. (1996) that iron can protect cells from H2O2. In these in vitro experiments, this protection was proposed to result from the removal of ferrous iron and hydrogen peroxide via the Fenton reaction. Even though the hydroxyl radical would be produced, much of this hydroxyl radical resulted in unspecific oxidations of little consequence. Iron-oxygen complexes are proposed to be more specific and thereby bring about more cell damage. Our observation that Fe2+-dioxygen chemistry is important in cellular lipid peroxidation is in agreement with work on models of lipid systems that have demonstrated that the autoxidation of ferrous iron can initiate lipid peroxidation (Yin, 1992; Tien, 1982).
SUMMARY
Unsaturated lipids are the target for free radical-mediated lipid peroxidation. Our data show that catalytic iron and dioxygen initiate lipid peroxidation in cells. We found that Fe2+-dioxygen chemistry is more important than the Fenton reaction with pre-existing H2O2 in initiating the formation of lipid-derived radicals. Some areas of the brain have a high iron content (Beard, 1993) and oxidative damage could lead to iron leakage. Because brain tissue has high levels of unsaturated lipids, catalytic iron would be especially detrimental. Thus, iron needs to be carefully controlled to protect brain tissue that is high in PUFAs and low in antioxidant enzymes (Marklund 1982). The data presented here support the proposal that Fe2+-dioxygen chemistry is an important route in the initiation of lipid peroxidation. This oxidative chemistry is proposed to be a major player in deleterious free radical-mediated biological oxidations in brain injury and brain pathology.
Acknowledgements
This work was supported by NIH Grant CA-66081 and CA-81090.
Abbreviations
- A.U.
arbitrary units
- DBI
double bond index
- DHA
docosahexaenoic acid
- EPR
electron paramagnetic resonance
- HO•
hydroxyl radical
- H2O2
hydrogen peroxide
- L-H
unsaturated lipid
- L•
carbon-centered lipid radical
- oxidant•
a radical that has oxidizing properties
- POBN
α-(4-pyridyl-1-oxide)-N-t-butylnitrone
- PUFA
polyunsaturated fatty acid(s)
Footnotes
- Fe2+ + O2 ↔ [Fe2+-O2 ↔ Fe3+-O2•−] ↔ Fe3+ + O2•−
- Perferryl ion
- Ferryl ion is thought to be formed by two routes.
- Fe2+ + H2O2 → Fe2+O + H2O
- Ferryl ion
- or
- Fe2+ + Fe2+-O2 → Fe2+-O2-Fe2+
- Perferryl ion
- then,
- Fe2+-O2-Fe2+ → 2Fe2+-O
- Ferryl ion
- Due to their high electron affinities, perferryl and ferryl ions are proposed to be important oxidants in detrimental biological oxidations, with reactivities approaching that of HO•.
REFERENCES
- Beard JL, Connor JR, Jones BC. Iron in the brain. Nutr. Rev. 1993;51:157–170. doi: 10.1111/j.1753-4887.1993.tb03096.x. [DOI] [PubMed] [Google Scholar]
- Buettner GR. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: Ascorbate as a test for catalytic metals. J. Biochem. Biophys. Meth. 1988;16:20–40. doi: 10.1016/0165-022x(88)90100-5. [DOI] [PubMed] [Google Scholar]
- Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic. Biol. Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- Hempel SL, Buettner GR, Wessels DA, Galvan GM, O'Malley YQ. Extracellular iron (II) can protect cells from hydrogen peroxide. Arch. Biochem. Biophys. 1996;330:401–408. doi: 10.1006/abbi.1996.0268. [DOI] [PubMed] [Google Scholar]
- Koppenol WH. Oxyradical reactions: from bond-dissociation energies to reduction potentials. FEBS Lett. 1990;264:165–167. doi: 10.1016/0014-5793(90)80239-f. [DOI] [PubMed] [Google Scholar]
- Marklund SL, Westman NG, Lundgren E, Roos G. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res. 1982;42:1955–1961. [PubMed] [Google Scholar]
- Qian SY, Buettner GR. Iron and dioxygen chemistry is an important route to initiation of biological free radical oxidations: An electron paramagnetic resonance spin trapping study. Free Radic. Biol. Med. 1999;26:1447–1456. doi: 10.1016/s0891-5849(99)00002-7. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Singlet oxygen toxicity is cell line-dependent: A study of lipid peroxidation in nine leukemia cell lines. Photochem. Photobiol. 1999;70:858–867. [PubMed] [Google Scholar]
- Tien M, Morehouse LA, Bucher JR, Aust SD. The multiple effects of ethylenediaminetetraacetate in several model lipoid peroxidation systems. Arch. Biochem. Biophys. 1982;218:450–458. doi: 10.1016/0003-9861(82)90367-8. [DOI] [PubMed] [Google Scholar]
- Wagner BA, Buettner GR, Burns CP. Free radical-mediated peroxidation in cells: Oxidizability is a function of cell lipid bis-allylic hydrogen content. Biochemistry. 1994;33:4449–4453. doi: 10.1021/bi00181a003. [DOI] [PubMed] [Google Scholar]
- Yin D, Lingnert H, Ekstrand B, Brunk UT. Fenton reagents may not initiate lipid peroxidation in emulsified linoleic acid model system. Free Radic. Biol. Med. 1992;13:543–556. doi: 10.1016/0891-5849(92)90149-b. [DOI] [PubMed] [Google Scholar]
- Zaleska MM, Floyd RA. Regional lipid peroxidation in rat brain in vitro: possible role of endogenous iron. Neurochem. Res. 1985;10:397–410. doi: 10.1007/BF00964608. [DOI] [PubMed] [Google Scholar]