Abstract
Assessing current Hg pools in forest soils of the northeastern U.S. is important for monitoring changes in Hg cycling. The forest floor, upper and lower mineral horizons were sampled at 17 long-term upland forest sites across the northeastern U.S. in 2011. Forest floor Hg concentration was similar across the study region (274 ± 13 μg kg−1) while Hg amount at northern sites (39 ± 6 g ha−1) was significantly greater than at western sites (11 ± 4 g ha−1). Forest floor Hg was correlated with soil organic matter, soil pH, latitude and mean annual precipitation and these variables explained approximately 70% of the variability when multiple regressed. Mercury concentration and amount in the lower mineral soil was correlated with Fe, soil organic matter and latitude, corresponding with Bs horizons of Spodosols (Podzols). Our analysis shows the importance of regional and soil properties on Hg accumulation in forest soils.
Keywords: Spodosols, Inceptisols, regional mercury, forest floor, mineral soil, Vermont, New Hampshire
1. Introduction
Mercury (Hg) is a global pollutant and is of particular concern to humans in the northeastern U.S. who consume local fish, as most states in the region have fish consumption advisories due to their tissue exceeding levels deemed hazardous by the U.S. Environmental Protection Agency (USEPA 2010). Mercury is also a threat to wildlife in general; piscivorous birds, for example, have shown multiple signs of chronic Hg poisoning (Evers et al., 2004; Driscoll et al., 2007). Forest soils are a large, potentially mobile reservoir that may supply Hg to aquatic ecosystems (Aastrup et al., 1991; Lorey and Driscoll, 1999; Schwsig et al., 1999; Grigal et al., 2000; Driscoll et al., 2007). This is important for the northeastern U.S., as forest soils receive comparatively elevated deposition rates of Hg and are widely forested (Rea et al., 2002; Miller et al., 2005). The transfer of Hg from forest soils to aquatic ecosystems in this region may be altered by future shifts in regional emissions and changes in climate. Mercury deposition rates to this region may be reduced as a result of new measures adopted by the United States Environmental Protection Agency (USEPA) to reduce coal-fired power plant Hg emissions to 30% of current values by 2025 (Han et al., 2008; USEPA, 2011). The forest soil Hg pools must first be characterized in order to establish a baseline upon which to compare with future assessments.
The forest floor, the organic horizons overlying the mineral soil, is a characteristic feature of forest soils and is dominated by decomposing litter and woody debris. The forest floor is a strong accumulator of certain atmospherically-deposited metals and has been used to assess the distribution and deposition of other metals such as lead (Johnson et al., 1982; Kaste et al., 2006; Steinnes and Friedland, 2006; Kaste et al., 2011). However, the mineral soil often contains greater quantities of metals than the forest floor due to its greater volume (Grigal, 2003; Gabriel and Williamson, 2004; Stankwitz et al., 2012). Mercury distribution in the forest floor and mineral soil has been shown to be dependent on both regional abiotic factors and soil properties (McNeal and Rose, 1974; Nater and Grigal, 1992; Lorey and Driscoll, 1999; Grigal, 2003; Demers et al., 2007; Obrist et al., 2011; Stankwitz et al., 2012; Shi et al., 2013). It is unclear if the distribution of Hg in the forest floor across the northeastern U.S. is dependent on the same regional factors and soil properties as identified by previous Hg studies in other regions, at different scales. In addition, few studies have conducted a spatial approach to study Hg in the mineral soil.
At the regional scale, Hg distribution has been reported to be dependent on soil temperature and precipitation (Nater and Grigal, 1992; Smith-Downey et al., 2010; Obrist et al., 2011; Tipping et al., 2011). Soil temperature may affect Hg accumulation in soils by controlling soil organic matter (SOM) dynamics among other processes. The dependence of Hg accumulation on SOM in forest soils has been described by Obrist et al. (2011) as the ‘legacy effect’. In the legacy effect, SOM at colder climates has a slower decomposition rate and is exposed to a longer duration of Hg deposition, and accumulates more Hg. Another temperature dependent process is Hg0 volatilization, which is greater at higher soil temperatures (Schlüter, 2000). Furthermore, precipitation may also influence Hg accumulation because wet deposition is a major pathway by which Hg reaches the forest soil (Rea et al., 2002; Demers et al., 2007; Obrist et al., 2011; Juillerat et al., 2012; Stankwitz et al., 2012). In addition, physical and chemical soil properties have also been shown to influence Hg accumulation in soils (Grigal, 2003; Gabriel and Williamson, 2004; Demers et al., 2007). Soil organic matter, soil pH, clay-sized particle abundance and metal oxides can influence the surface sorption of Hg in soils (Schuster, 1991; Yin et al., 1996; Roulet et al., 1998; Han et al., 2003; Grigal, 2003; Gabriel and Williamson, 2004; Liao et al., 2009; Obrist et al., 2011; Shi et al., 2013).
It is unclear which regional factors (latitude, longitude, climate, mean annual precipitation) or soil properties (SOM, pH, Fe, clay) are important for Hg accumulation in the forest soils of the northeastern U.S. The objectives of this study were to determine the spatial and vertical distribution of Hg in the forest floor and mineral soil at seventeen long-term forest research sites across the northeastern U.S. and identify the regional and soil properties that explain the pattern of Hg accumulation.
2. Materials and Methods
2.1 Site Descriptions
Twenty-five long-term, upland forest research sites were established in 1980 as part of a larger study on metals in the forest floor (Johnson et al., 1982). Sites were situated in undisturbed forests, mostly in higher elevation regions, with many located within National and State forests. Seventeen of the twenty-five original sites were relocated using GPS and resampled in 2011 and are shown in Fig. 1. Sites were grouped into three sub-regions: western, central and northern (Fig. 1). The range of elevations at each sub-region was not significantly different for the three sub-regions. Site elevation ranged from 305 to 820 m (Table 1) with a mean of 562 ± 35 m. The soil temperature regime for each site was determined from mapped soils on Web Soil Survey (Soil Survey Staff). Soils were from the frigid (mean annual soil temperature < 8 °C) and mesic (mean annual soil temperature > 8 °C) temperature regime. Northern sites were primarily frigid while western sites were all mesic (Table 1). Precipitation for each site was interpolated from the mean annual precipitation (1981 – 2010) spatial model from the PRISM climate group (Prism database; Prism Climate Group 2012).
Fig. 1.
Location of the upland forest, long term research sites and their sub-region across the northeastern United States.
Table 1.
Site location, vegetation type, and soil taxonomy
| Sub-region† | Site | Site Name | Elev.(m) | mean annual precipitation‡ (mm yr−1) | Latitude | Longitude | Vegetation | Soil order | Soil temperature regime |
|---|---|---|---|---|---|---|---|---|---|
| W | 1 | Heart’s Content, PA | 580 | 1170 | 41.689 | −79.252 | Pine/Hemlock | Ultisol | Mesic |
| W | 2 | Cook’s Forest, PA | 430 | 1163 | 41.347 | −79.212 | Pine/Hemlock | Inceptisol | Mesic |
| W | 3 | Tionesta, PA | 520 | 1141 | 41.477 | −79.379 | Oak | Ultisol | Mesic |
| C | 4 | Balsam Lake, NY | 820 | 1268 | 42.067 | −74.574 | N. Hardwood | Inceptisol | Frigid |
| C | 5 | Mohonk, NY | 366 | 1288 | 41.770 | −74.158 | N. Hardwood | Inceptisol | Mesic |
| C | 6 | Mt Tremper, NY | 305 | 1365 | 42.071 | −74.312 | Pine/Hemlock | Inceptisol | Mesic |
| C | 7 | Windham, NY | 580 | 1215 | 42.301 | −74.170 | N. Hardwood | Inceptisol | Frigid |
| C | 8 | Mohawk, CT | 503 | 1192 | 41.820 | −73.297 | Oak | Inceptisol | Frigid |
| C | 9 | Mt. Everett, MA | 790 | 1276 | 42.102 | −73.431 | Oak/Pitch Pine | Inceptisol | Frigid |
| N | 10 | Appalachian Gap, VT | 778 | 1533 | 44.211 | −72.931 | Spruce/Fir | Spodosol | Frigid |
| N | 11 | Sherburne Pass, VT | 671 | 1467 | 43.662 | −72.833 | N. Hardwood | Spodosol | Frigid |
| N | 12 | Bromley, VT | 625 | 1465 | 43.214 | −72.967 | N. Hardwood | Spodosol | Frigid |
| N | 13 | Bristol Cliffs, VT | 555 | 1112 | 44.140 | −73.064 | Pine/Hemlock | Spodosol | Frigid |
| N | 14 | Mt Cardigan, NH | 579 | 1306 | 43.645 | −71.933 | Spruce/Hemlock | Spodosol | Frigid |
| N | 15 | Valley Way, NH | 433 | 1217 | 44.369 | −71.287 | Spruce/N. Hardwood | Spodosol | Frigid |
| N | 16 | Gale River, NH | 440 | 1181 | 44.232 | −71.608 | N. Hardwood | Inceptisol | Frigid |
| N | 17 | Wildcat Mt, NH | 590 | 1527 | 44.266 | −71.238 | Spruce/N. Hardwood | Inceptisol | Frigid |
W = western region, C = central region, N= northern region
precipitation values interpolated from PRISM database (Prism Climate Group, 2012)
Vegetation at each site was mixed; sites ranged from primarily deciduous vegetation such as oak (Quercus spp.), beech (Fagus spp.), maple (Acer spp.), and birch (Betula spp.), to coniferous such as pine (Pinus spp.), spruce (Picea spp.), and hemlock (Tsuga spp.) (Table 1). Northern sites were generally more conifer-dominated while western and central sites were deciduous-dominated. The soils were developed from glacial till, outwash deposits, or outcrops of weathered bedrock (c.f. Siccama, 1974; Kaste et al., 2006). The soils were well-drained and on level to shallow slopes (< 12%). Soils were primarily classified as either Spodosols (Podzols in FAO/UNESCO) or Inceptisols (Cambisols in FAO/UNESCO) (Table 1), except Site #1 and 3, which were classified as Ultisols (Acrisol in FAO/UNESCO). In general, western sites were Ultisols, central sites were Inceptisols and northern sites were Spodosols (Table 1).
2.2 Soil collection
Soils were sampled in a 30 m by 30 m plot at each site between July and September 2011 (Fig. 1). Sites were relocated using GPS and instructions from previous investigators. Five forest floor (Oi + Oe + Oa) and upper mineral soil (A or E horizons) were collected from all sites. Lower mineral soil (Bw or Bs horizons) was sampled at most sites except sites 4, 6, 7, 10, and 17 due to hardpan layer, extreme rock content, or lithic contact. The forest floor sampling technique was the same as those described by Johnson et al. (1982). In brief, five 15 × 15 cm square sections of forest floor were separated from the underlying mineral horizons and collected. In an adjacent location, a pit was excavated to allow access to the mineral soil. To avoid mixing or contaminating samples, the lower mineral horizon was sampled from the soil pit face using hand trowels first, followed by the upper mineral soil. Upper mineral soils were classified as either an A or E horizon and lower mineral soils were classified as either Bw or Bs horizon following the USDA Soil Taxonomy guidelines (Soil Survey Staff, 2010). High-density polypropylene tubes were used to collect intact soil cores samples for bulk density measurements.
2.4 Soil processing
Forest floor and mineral soil samples were air-dried to a constant weight and roots > 5 mm in diameter were removed. The forest floor total mass was calculated using oven-dried sub-samples and the volume was calculated using the area of the template and measured depths. Soil mass was was calculated using the bulk density and the mass of the sieved, oven-dried 100 ° C sample. Forest floor and mineral soil samples were milled and sieved, respectively, to ≤ 2 mm. Mineral soil mass was corrected for rock and coarse fragments using bulk cores and visual soil pit estimates. It should be noted that both methods may underestimate the rock fraction because soil pits and soil cores could not include cobbles and boulders, leading to an overestimation of total soil mass (Huntington et al., 1988). To determine the soil pH, 4 g of soil was added to 10 g of water for a 2:5 soil–water gravimetric ratio and shaken for 1 hr using a wrist-action shaker and allowed to settle for 10 min. The soil pH of the supernatant was measured using a pH meter (8015 VWR) (Table 2). Loss on ignition was used to estimate the %SOM present in the samples (Table 2). For loss on ignition, 4 g of soil was held at 475 °C for 8 hours and %SOM was determined from change in mass from thermal oxidation. Soil particle size distribution was determined using a modified Bouyocous hydrometer method (Gee and Bauder, 1986). For the process, 30 g of soil were treated with 30% w/w hydrogen peroxide to oxidize SOM aggregates and dispersed with 100 mL of 0.08 M sodium hexametaphosphate overnight. Soil pH, %SOM, and %Clay are given for each sub region and soil horizon in Table 2.
Table 2.
Selected chemical properties of the forest soils by sub-region for the three depths sampled (± 1 standard error). The value n is number of sites.
| n | Thickness cm | soil pH† | %SOM‡ g g−1 | %Clay§ g g−1 | Fe mg kg−1 | Fe kg ha−1 | |
|---|---|---|---|---|---|---|---|
| Western Region | |||||||
| Forest floor | 3 | 3.2 ± 0.5 | 4.48 ± 0.35 | 52 ± 5 | n.a. | 9 ± 3 | 0.2 ± 0.1 |
| Upper mineral soil | 3 | 4.1 ± 0.6 | 4.43 ± 0.37 | 9 ± 2 | 11 ± 2 | 12 ± 6 | 6 ± 3 |
| Lower mineral soil | 3 | 5.9 ± 0.5 | 4.38 ± 0.32 | 5 ± 1 | 12 ± 3 | 15± 6 | 12 ± 6 |
| Central Region | |||||||
| Forest floor | 6 | 6.9 ± 1.4 | 4.24 ± 0.10 | 67 ± 5 | n.a. | 6 ± 1 | 0.6 ± 0.2 |
| Upper mineral soil | 6 | 5.9 ± 0.4 | 4.10 ± 0.06 | 15 ± 4 | 12 ± 3 | 14 ± 4 | 6 ± 1 |
| Lower mineral soil | 4 | 9.7 ± 1.2 | 4.12 ± 0.19 | 9 ± 3 | 6 ± 2 | 16 ± 3 | 20 ± 1 |
| Northern Region | |||||||
| Forest floor | 9 | 8.7 ± 1.8 | 3.97 ± 0.04 | 73 ± 3 | n.a. | 4 ± 1 | 0.7 ± 0.2 |
| Upper mineral soil | 9 | 6.4 ± 0.9 | 3.69 ± 0.03 | 9 ± 2 | 14 ± 2 | 12 ± 5 | 7 ± 3 |
| Lower mineral soil | 7 | 7.5 ± 0.6 | 4.13 ± 0.14 | 11 ± 1 | 12 ± 2 | 28 ± 2 | 25 ± 2 |
measured in a 2:5 soil water extract
estimated from loss on ignition
determined by particle size distribution
2.3 Metal analyses
The U.S. EPA method 3051A was used to quantify Hg through direct digestion. Homogenized 2 g sub-samples of organic and mineral soil samples were dried to a constant weight at 45 °C for 5 days. 250 mg (± 1 mg) sub-samples were digested with 5 mL of a 1:9 ratio of trace metal grade hydrochloric acid:nitric acid (HNO3, 70%; HCl, 70%). The digest solutions were allowed to de-gas overnight in lightly sealed 50 mL polypropylene centrifuge tubes. The solutions were then heated using a CEM MARS digestion system at 90 °C for 45 minutes (CEM Mathews, NC). After cooling, the digested samples were brought to a 50 ml final volume with DI water. Digested O horizon samples were filtered using 0.45 μm polypropylene Whatman syringe filters. The digests were diluted a further 10X with deionized water and then analyzed by an Agilent 7500 series ICP-MS (Agilent Technologies Santa Clara, CA). With every 20 digested samples we included: one randomly spiked sample with 50 μL of 1 ppm HgCl2 (3.7 nM HgCl2); one replicate; one preparation blank; and one standard reference material (SRM). Peach leaves SRM 1547 and Montana soil SRM 2711 from the National Institute of Standards and Technology (National Insitute of Standards and Technology Gaithersburg, MD) were used as reference Hg values for forest floor and mineral soil samples, respectively. All measured Hg concentrations for SRM materials were within 7% of their certified values. Recovery rates for spiked samples were > 95%. Hg concentration in the preparation blank samples was below detection limits. The digests were also analyzed for extractable Fe using an IRIS Intrepid II XSP ICP-OES (Thermo Electron Franklin, MA). Iron concentrations were within 8% of the Peach leaves SRM 1547 and Montana soil SRM 2711 reported values.
2.6 Data Analyses
Descriptive statistics for soil Hg and soil properties were calculated using Matlab (Matlab INC Natick, MA). Correlative relations between Hg concentration and amount with regional factors (mean annual precipitation, latitude and longitude) and soil properties (% SOM, pH, Fe concentration, % clay) were investigated using stepwise regressions and multiple regression in Matlab. Non-continuous data (e.g. soil temperature regime) were not included in the stepwise regression models. Variables from the stepwise regressions were linearly regressed with each other and the variables with R2 values greater than 0.25 and a causal relationship were determined to covary and not included in the multiple regressions. The independent variables found to be significantly correlated with Hg concentration or Hg amount in the stepwise regression were used in multiple regression models and shown in Table 3. The variation in Hg with soil order, and sub-region, were determined with the Kruskal-Wallis test. Post-hoc tests utilized include Student’s two-sample t-tests and Wilcoxon rank signed tests. An alpha-level of 0.05 was used for all tests and mean values given in text and figures show ± 1 standard error.
Table 3.
Calculated multiple-regression model outputs for Hg concentration and amount with significant site characteristics and soil properties from stepwise-regressions for all 17 sites
| Variables | R2 | P | |
|---|---|---|---|
| Forest floor | |||
| Mercury concentration | % SOM, pH and latitude | 0.77 | < 0.01 |
| Mercury amount | mean annual precipitation, pH and % SOM | 0.65 | < 0.01 |
| Upper mineral soil horizon | |||
| Mercury concentration | Fe concentration | 0.35 | 0.06 |
| Mercury amount | Fe amount | 0.21 | 0.38 |
| Lower mineral soil horizon | |||
| Mercury concentration | Latitude, % SOM and Fe concentration | 0.80 | < 0.01 |
| Mercury amount | Latitude, % SOM and Fe amount | 0.73 | 0.02 |
3. Results and Discussion
3.1 Mercury in the forest floor
Forest floor Hg concentration ranged from 118 to 373 with a mean of 274 ± 13 μg kg−1 and did not vary significantly at the forested sites across the study region as shown in Fig. 2. These concentrations are similar to those reported by Evans et al. (2005), Obrist et al. (2011), and Juillerat et al. (2012) for forest floor samples from the northeastern U.S. Forest floor Hg amount ranged from 4 to 51 g ha−1 with a mean of 30 ± 4 g. The observed Hg amounts were similar to those reported by Evans et al. (2005) and Demers et al. (2007), with their values ranging from 26 to 46 g ha−1. The total mass of Hg in the forest floor was significantly greater at northern sites (39 ± 6 g ha−1) compared to western sites (11 ± 4 g ha−1) (P < 0.05). The elevated concentration and amount of Hg in the forest floor in the northeastern U.S. has been considered to be largely anthropogenic, as human activities have significantly increased Hg deposition rates to forests (Fitzgerald et al., 1998; Grigal, 2003; Seigneur et al., 2003; Driscoll et al., 2007). Although increased deposition due to human activity has been documented (e.g. Fitzgerald et al., 1998), the sources of Hg in this study were not distinguished and should be assumed to be both geogenic and anthropogenic.
Fig. 2.
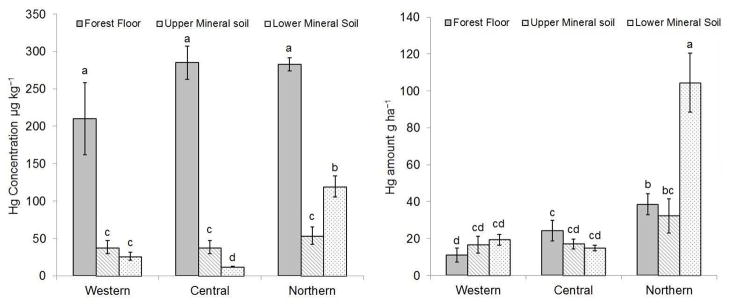
The means ± 1 standard errors for Hg concentrations (μg kg−1) and Hg amounts (g ha−1) are shown for the three subregions. Letters group values by statistical significance (P < 0.05).
The spatial distribution of Hg in the forest floor has been hypothesized to be dependent on both regional (latitude, longitude, climate, precipitation) and soil properties (SOM, pH, Fe, clay), with temperature and precipitation being the two dominant regional factors (Evans et al., 2005; Obrist et al., 2011). Soil temperature may influence Hg accumulation in the forest floor by controlling kinetics of chemical processes and SOM dynamics (Nater and Grigal, 1992; Grigal, 2003). When sites were grouped by their soil temperature regime, forest floor Hg concentration was not significantly different between mesic and frigid soils. However, frigid soils had greater forest floor Hg amount (33 ± 5 g ha−1) compared to mesic forest floor (18 ± 5 g ha−1) (P < 0.05). Further, frigid soils had a greater mean forest floor depth (10 ± 2 cm) compared to mesic soils (5 ± 1 cm) (P < 0.05).
The dependence of Hg on SOM dynamics has been observed in previous spatial studies (Nater and Grigal, 1992; Obrist et al., 2011; Stankwitz et al., 2012; Shi et al., 2013). The cooler frigid soil temperature regime may have promoted the accumulation of Hg through multiple mechanisms. Cooler forest floor temperatures generally lead to longer turnover times due to slower decomposition rates of SOM (Berg et al., 1993; Moore et al., 1999; Schwesig and Matzner, 2001; Obrist et al., 2011; Stankwitz et al., 2012). This legacy effect was further supported by Stankwitz et al. (2012), who observed greater Hg accumulation in soils with longer 210Pb residence times. The forest floor at northern sites may have a corresponding longer turnover time than at western sites and may be exposed to longer periods of Hg deposition. Assuming that forest floor depth is roughly proportional to turnover rate regionally, then our results also may be a result of the legacy effect. Thus, it is hypothesized that the forest floor at northern sites has a longer turnover rate, was exposed to a longer duration of Hg deposition and has accumulated greater amounts of Hg.
Soil temperature may also influence Hg accumulation in the forest floor by controlling the kinetics of Hg0 re-emission or volatilization (Schlüter, 2000; Schwesig and Matzner, 2001; Demers et al., 2007; Tipping et al., 2011). Volatilization has been recognized as an unconstrained pathway of Hg loss from soils (Johnson and Lindeberg, 1995; Grigal et al., 2000; Grigal, 2003; Obrist et al., 2011; Demers et al., 2013). Estimates for Hg volatilization using mass balances have varied widely and ranged from 0 – 300 mg ha−1 yr−1 (Carpi and Lindberg, 1998; Grigal et al., 2000; Demers et al., 2007). Volatilization rates for Hg in northeastern U.S. soils are poorly understood with few empirical studies in North America and volatilization may control local accumulation of Hg in soil (Demers et al., 2013). Demers et al. (2007) reported 95% of atmospherically deposited Hg was retained in forest soils of New York. The METALLICUS project in the Experimental Lakes Area of Ontario, Canada reported losses of 8% of Hg amended to soil (Hintelmann et al., 2002). Lindqvist et al. (1991) and Driscoll et al. (1994) both estimated that approximately 5 % of the annual atmospherically deposited Hg to Spodosols in southern Sweden would be lost via volatilization. From these estimates, the influence of re-emission and volatilization of Hg may be considered limited but requires further attention to determine the effect of soil temperature on Hg accumulation in the forest soils.
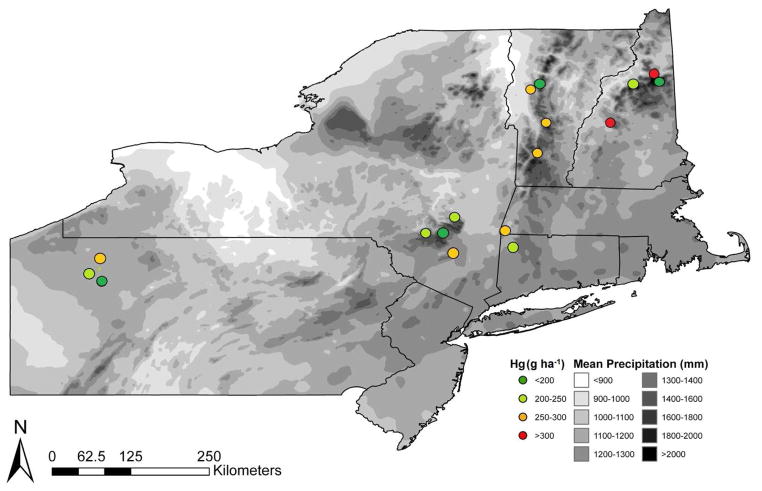
Wet deposition is a major pathway in which Hg reaches forest soils and may explain the spatial variation of Hg in the forest floor (Tipping et al., 2011). Miller et al. (2005) has concluded that rural, upland forests of the northeastern U.S. receive elevated rates of Hg in precipitation, with estimates > 120 mg ha−1 yr−1. Further, total Hg deposition projected for these sites by data from Miller et al. (2005) suggest values from 200 – 350 mg ha−1 yr−1. It must be noted that this study and Miller et al. (2005) have considered only Hg deposition estimates for forested ecosystems; land-use and vegetation cover significantly affects Hg deposition to soils and was not considered extensively in this study (Smith-Downey et al., 2010). For a rough estimate of wet deposition of Hg, the interpolated mean annual precipitation from the PRISM database was assumed to be proportional to the wet deposition of Hg (Prism database; Prism Climate Group 2012). The PRISM database does not account for many site-specific Hg deposition fluxes for total deposition such as litterfall (Hall and St. Louis, 2004), but may be used as a general estimate for wet Hg deposition (Tipping et al., 2011). The PRISM database is used by the National Atmospheric Deposition Program and Mercury Deposition Network for this purpose. When regressed, forest floor Hg concentration was found to be poorly correlated with mean annual precipitation. However, forest floor Hg amount was moderately correlated with interpolated mean annual precipitation amount with an R2 value of 0.46 (P < 0.05). There are advantages and disadvantages with using interpolated mean annual precipitation amounts; 30 year averaged data is less susceptible to the problems associated with annual variations and error from sample collection. The primary disadvantage of using the interpolated values to estimate the mean annual precipitation at each site is the lack of comprehensive measurements for validation (Miller et al., 2005). Despite this drawback, a general association can be observed between forest floor Hg amount and mean annual precipitation, suggesting sites that receive greater precipitation have a greater forest floor Hg mass.
Although only wet deposition was evaluated in this study, dry deposition may be an equally important but complex flux of Hg to forest soils (Miller et al., 2005; Demers et al., 2013). The dry deposition of Hg in forests of the northeastern U.S. has been estimated to be equal to or greater than Hg wet deposition (Miller et al., 2005). Rea et al. (2002) estimated dry deposition of Hg was nearly 33% of the total Hg entering the Lake Champlain watershed in northern Vermont. Dry deposition of Hg at our study sites was not estimated because of its complex variance with parameters at the landscape scale to leaf stomata scale (Ericksen et al., 2003; Miller et al., 2005; Smith-Downey et al. 2011). Furthermore, dry deposited Hg is readily washed off during precipitation making it difficult to quantify and generally it is integrated into throughfall (Rea et al. 2002; Miller et al., 2005). Dry deposition may explain variation in Hg concentration and amount in the forest floor and should be considered in future studies.
Winter fluxes of Hg are a less-understood mechanism that may influence Hg accumulation in the forest floor. Mean annual precipitation includes all forms of precipitation. However, Hg in snow may not permeate into the forest floor and mineral soil during winter months as effectively as rainfall (Schwesig and Matzner, 2001). In addition, Hg has been observed to transfer from the soil into overlying snow (Susong et al. 2003). The precipitation type, snowpack and snowmelt across the northeastern U.S. are likely different in total amount and temporally. This may influence the amount of Hg reaching the forest floor and entering the mineral soil (Schwesig and Matzner, 2001; Demers et al., 2007).
Many investigators have observed a strong correlation between Hg with % SOM and soil pH in the forest floor (Schuster, 1991; Gabriel and Williamson, 2004; Szopka et al., 2011; Obrist et al., 2011; Shi et al., 2013). Mercury accumulation is influenced by SOM and pH as they control sorption capacity of soil (Schuster, 1991; Gabriel and Williamson, 2004). Northern sites had significantly higher % SOM and lower pH compared with western sites (P < 0.05) (Table 2). The higher % SOM may have increased the sorption capacity for Hg (Gabriel and Williamson, 2004). Further, our results suggest lower pH promoted higher Hg concentration and amount, which does not agree with other studies (e.g. Gabriel and Williamson, 2004). The mechanism responsible for the negative correlation between forest floor Hg and pH is unclear but may be due to a soil properties not investigated in this study such as biological activity of fungi or annelid. Greater % SOM did not correlate with lower pH (R2 = 0.19) and is presumed not to be the masking mechanism for greater pH correlating with greater Hg.
A stepwise regression was used to rank the relative importance of each regional factor (mean annual precipitation, latitude and longitude) and soil property (pH, SOM, and Fe concentration and amount) on forest floor Hg concentration and amount. Forest floor Hg concentration was significantly correlated with % SOM, pH, and latitude while forest floor Hg amount was correlated with % SOM, pH and precipitation (Table 2). These regressions correspond with the variables used in spatial Hg models by Smith-Downey et al. (2010) and Obrist et al. (2011). In addition, the stepwise regressions also suggest the importance of soil pH in the northeastern US, a variable not used in models by Obrist et al. (2011) or Smith-Downey et al. (2010).
A multiple regression of Hg concentration in the forest floor was calculated using %SOM, pH, and latitude, with an R2 value of 0.77 (P < 0.01) (Table 3). Similarly, a multiple regression to describe forest floor Hg amount was determined using mean annual precipitation, % SOM and pH with an R2 value of 0.65 (P < 0.01) (Table 3). The dependence of forest floor Hg on latitude, precipitation, pH and % SOM demonstrate that regional and soil properties are both integral to Hg accumulation in forest soils. Regional factors (latitude and precipitation) likely control Hg deposition to the forest floor while soil properties (SOM and pH) may dictate the retention of Hg in the mineral soil.
3.2 Mercury in the mineral soil
The mean Hg concentrations in the upper and lower mineral horizons were 45 ± 6 and 71 ± 13 μg kg−1, respectively. The Hg concentrations are similar to those reported by previous studies in the northeastern U.S. (McNeal and Rose, 1974; Rea et al., 2002; Obrist et al., 2011; Juillerat et al., 2012). Mercury concentration in the upper mineral horizon was similar across the study region (Fig. 2). In the lower mineral horizon, Hg concentration was significantly higher at the northern sites (Fig. 2). Mercury concentration in the upper and lower mineral horizons were significantly lower compared with the forest floor (P < 0.01) (Fig. 2). The mean Hg amount in the upper and lower mineral horizons were 23 ± 5 and 64 ± 12 g ha−1, respectively. These values are similar with Evans et al. (2005) but are much lower than those reported by Demers et al. (2007), likely due to site-specific characteristics of the soil profiles (i.e. profile depth and rock content). Mercury amount in the lower mineral horizon at northern sites was significantly higher than all other soil horizons at all sub-regions (Fig. 2).
Many of the soil properties of the upper and lower mineral soil also varied significantly across the study region. Soil horizons were thinner at western sites compared to central and northern sites (Table 2). Soil pH was highest in the upper mineral soil of the western sites and lowest in the upper mineral soil of the northern sites (Table 2). % SOM in the upper mineral was similar across the study sites with an average of 12 % and a median of 10 %. However, the lower mineral horizons at western sites were significantly lower in % SOM than central and northern sites (P < 0.05) (Table 2). The clay content of the soil was generally low and ranged from 6 to 23 % with a mean of of 13 ± 1.4 %. Iron concentrations in the upper mineral soil were similar across sites with a mean of 13 ± 3 mg kg−1. In the lower mineral horizons, northern sites contained greater Fe concentrations than western and central sites (P < 0.01) (Table 2). Similarly, Fe amounts in the upper mineral horizons were comparable, with a mean of 7 ± 2 but, northern sites were significantly greater than western and central sites (P < 0.05) (Table 2).
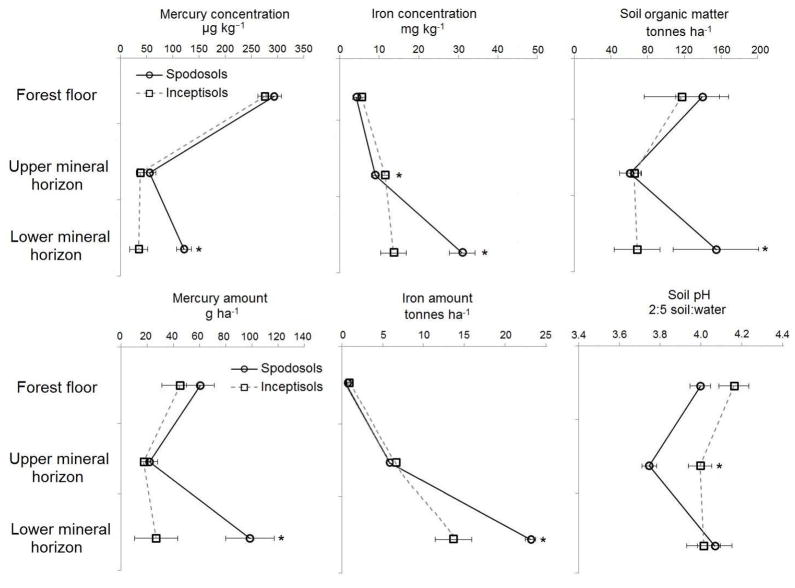
The accumulation of SOM and Fe in the lower mineral horizon at northern sites is indicative of Spodosols and their presence may explain the higher Hg concentration and amount at northern sites. Central and northern sites were grouped by soil order, to determine if Hg concentration and amount in the soil profile varied substantially between the two dominant soil orders: Inceptisols and Spodosols (Fig. 4). For the Inceptisols, Hg concentration followed the SOM distribution and decreased with depth (Fig. 4). Mercury amount however, was nearly uniform through the profile of Inceptisols. Mercury concentration and amount in the Bs horizons of the Spodosols were significantly higher compared to all other mineral horizons (P < 0.01, Fig. 4). Maximum accumulation of Hg in the Bs horizon is consistent with other studies of Hg in Spodosols in other regions (e.g. Ma et al., 1997; do Valle et al., 2005; Grimaldi et al., 2008) and explains the significantly higher Hg in the lower mineral horizons at the northern sites. The importance of spodic horizons on lead accumulation in the mineral soil has been observed in previous studies in the northeastern U.S. (e.g. Johnson and Petras, 1998; Kaste et al., 2011) and in warmer climates (e.g. Ma et al., 1997). This is a finding that has not been reported for Hg in other regional studies in the northeastern U.S.
Fig. 4.
Mercury and soil properties displayed by depth for three horizons for Spodosols and Inceptisols at central and northern sites. (*) designates a significant difference (P < 0.05) using the Wilcoxon rank signed test.
Podzolization, the process of SOM complexing and eluviating Fe and other metals from the E horizon to the Bs horizon (Lundstöm et al., 2000), is likely responsible for the elevated Hg accumulation in the Bs horizon (do Valle et al., 2005; Kaste et al., 2011; Bushey et al., in review). The accumulation of SOM and Fe oxides in the Bs horizon promote greater Hg accumulation due to increased surface binding sites (Schuster, 1991; Yin et al., 1996; Roulet et al., 1998; Han et al., 2003; Grigal, 2003; Gabriel and Williamson, 2004; Liao et al., 2009; Obrist et al., 2011). The mean SOM amount in the Bs horizons was significantly greater compared to the all other mineral soil horizons (P < 0.05) (Fig. 4). Similarly, the mean extractable Fe concentration in the Bs horizons (31 ± 2 kg ha−1) was higher compared to the E horizons (9 ± 3 kg ha−1) (P < 0.05) (Fig. 4). The Bs horizons have greater Fe oxides than the other soil horizons, assuming that the extracted Fe concentration is roughly representative of Fe oxide abundance.
Regional factors (mean annual precipitation, latitude and longitude) and soil properties (pH, % SOM, Fe content, and clay content) were stepwise regressed with Hg concentration and amount in the mineral soil. Mercury concentration and amount in the upper mineral horizon were only correlated with Fe concentration and amount, respectively. The correlation between Fe concentration and amount in the upper mineral horizons with Hg are arbitrary because they only explained 35% and 21% of the variation in Hg in the upper mineral horizon, respectively (Table 3). For the lower mineral horizon, Hg was correlated with latitude, % SOM, and Fe. Soil pH and % SOM may not have been correlated with mineral soil Hg concentration and amount due to the small range in values. Iron was correlated with Hg concentration and amount in the soil for two possible reasons: 1) factors that promote the sorption of Fe and Hg in the lower mineral horizons are similar (e.g. similar affinity for SOM); or 2) Fe, present in the lower mineral horizon as an oxide or hydroxide, may promote Hg sorption, with the assumption that Fe concentration is roughly representative of Fe oxide abundance. The % clay may not have been correlated with Hg in the soil due to the mineralogy of the clay-sized particles. Soils of the northeastern US, especially at northern sites, generally lack clay minerals known to strongly sorb metals (Coen and Arnold, 1972; April and Newton, 1983). Mean annual precipitation was not correlated with Hg concentration or amount in the upper and lower mineral soil horizons, suggesting that deposition processes may be less important to the spatial distribution of Hg in the mineral soil. This finding agrees with the conclusion by Obrist et al. (2011) that Hg distribution in the mineral soil does not coincide with variables associated with Hg deposition.
The significantly correlated variables from the stepwise regressions were used in multiple regressions to estimate Hg concentration and amount in the mineral soil. As described earlier, Hg concentration and amount in the upper mineral horizon was inadequately explained by Fe concentration and amount, respectively (Table 3). For the lower mineral horizons, Hg concentration and amount were well explained using latitude, % SOM and Fe concentration and amount with R2 values of 0.80 and 0.73, respectively (Table 3). The significant correlation of SOM, Fe, and latitude in the lower mineral horizon is suggestive of the importance of Spodosols, which only occurred in northern sites and had greater % SOM and Fe concentrations in the lower mineral horizons. The strong correlation among Hg concentration, Fe concentration, and % SOM in the Bs horizon, coupled with the greater accumulation of Hg in the Bs horizon compared with Bw horizons in Inceptisols, suggests that podzolization promoted Hg accumulation. We hypothesize that podzolization has transferred Hg bound to SOM in the forest floor through the E horizon and into the Bs horizon. Based upon the strong contrast in the vertical distribution of Hg, soil orders were more indicative of the spatial pattern of Hg in the mineral soil than a multiple regression of soil properties (e.g. % SOM). This is a finding that has not been elaborated on by previous studies modeling Hg in soils (e.g. Obrist et al., 2011). Future studies on Hg in forest soils should consider incorporating soil orders into spatial estimates of Hg as a contrast to regressions with only regional factors and soil properties.
4. Conclusions
The findings of this study indicated that Hg accumulation in the forest floor varied with regional factors: soil temperature and precipitation; and physicochemical properties: pH, SOM, and depth. Soil temperature influenced Hg accumulation in the forest floor through multiple possible mechanisms: forest floor turnover rate, Hg volatilization, or winter Hg flux. The variables that correlated with Hg in other studies were also found to be important for multiple regressions in this region, with the addition of soil pH. The variation in Hg distribution in the mineral soil horizons was best explained by comparing the two dominant soil orders rather than by multiple regressions of regional factors and soil properties. The Spodosols had greater Hg in their Bs horizons compared to all other mineral horizons, as they occurred at higher latitudes with significant accumulations of SOM and Fe oxides. This result suggests the importance of soil order on Hg accumulation in the mineral soil and may provide an alternative approach to spatial modeling of Hg in forest soils. With this assessment of current Hg levels in forest soils of the northeastern U.S., the effect of future changes in Hg deposition or cycling at these sites may be monitored.
Fig. 3.
Forest floor Hg amount at 17 sites (in circles) displayed with mean annual precipitation (1981–2011) map graphics from the PRISM Climate Group. Copyright © 2011, PRISM Climate Group, Oregon State University, http://prism.oregonstate.edu, Map created 10 21 2012.
Mercury in the forest floor and mineral soil was quantified at 17 sites.
Concentrations and amounts were regressed with regional factors and soil properties.
Forest floor Hg was most explained by soil organic matter, pH, and precipitation.
Mineral soil Hg was explained by latitude, Fe concentration, and soil organic matter.
Mineral soil Hg was greatest in Bs horizons of Spodosols due to podzolization.
Acknowledgments
We would like to thank Robbie Meyers and Eirik Melbye-Buraas for field support. We are grateful for the technical and laboratory assistance provided by Paul Zeitz, R. Arthur Baker, and Janet Towse. The manuscript was greatly improved by comments from an anonymous reviewer and suggestions from Claire ‘Taylor’ Hornig. This research was funded by a United States Department of Agriculture, Forest Service Northeastern State Research Cooperative grant to Andrew Friedland. Brian Jackson and the Dartmouth Trace Element Analysis Laboratory are partially supported by United States National Institute of Health grant P42 ES007373.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aastrup M, Johnson J, Bringmark E, Bringmark I, Iverfeldt Å. Occurrence and transport of mercury within a small catchment area. Water Air and Soil Pollution. 1991;56:155–167. [Google Scholar]
- April RH, Newton RM. Mineralogy and chemistry of some Adirondack Spodosols. Soil Science. 1983;135:301–307. [Google Scholar]
- Berg B, Berg M, Bottner P, Box E, Breymeyer A, Calvo de Anta R, Couteaux M, Gallardo A, Escudero A, Kratz W, Madeira M, Mälkönen E, Meentemeyer V, Muñoz F, Piussi P, Remacle J, Virzo De Santo A. Litter mass loss in pine forests of Europe and Eastern United States as compared to actual evapotranspiration on a European scale. Biogeochemistry. 1993;20:127–153. [Google Scholar]
- Bushey JT, Driscoll CT, Selvendiran P, Mason EF, Jr, Fuss CB, Page BD, Mitchell MJ, Montesdeoca MR. The transport and fate of mercury in a northern deciduous upland forest soil. (in revision) Submitted to Biogeochemistry. [Google Scholar]
- Carpi A, Lindberg SE. Aplication of a teflon™ dynamic flux chamber for quantifying soil mercury flux: Tests and results over background soil. Atmospheric Environment. 32:873–882. [Google Scholar]
- Coen GM, Arnold RW. Clay mineral genesis of some New York Spodosols. Soil Science Society of America Proceedings. 1972;36:342–350. [Google Scholar]
- Demers JD, Driscoll CT, Fahey TJ, Yavitt JB. Mercury cycling in litter and soil in different forest types in the Adirondack region, New York, USA. Ecological Applications. 2007;17:1341–1351. doi: 10.1890/06-1697.1. [DOI] [PubMed] [Google Scholar]
- Demers JD, Blum JD, Zak DR. Mercury isotopes in a forested ecosystem: Implications for air-surface exchange dynamics and the global mercury cycle. Global Biogeochemical Cycles. 2013;27:222–238. doi: 10.1002/gbc.20021. [DOI] [Google Scholar]
- do Valle CM, Santana GP, Augusti R, Egreja Filho FB, Windmüller CC. Speciation and quantification of mercury in Oxisol, Ultisol, and Spodosol from Amazon (Manaua, Brazil) Chemosphere. 2005;58:779–792. doi: 10.1016/j.chemosphere.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Otton JK, Iverfeldt Å. Trace metals speciation and cycling. In: Moldan B, Cerry J, editors. Biogeochemistry of small catchments. J Wiley; New York: 1994. [Google Scholar]
- Driscoll CT, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK. Mercury contamination in forest and freshwater ecosystems in the northeastern United States. BioScience. 2007;57:17–28. [Google Scholar]
- Ericksen JA, Gustin MS, Schorran DE, Johnson DW, Lindberg SE, Coleman JS. Accumulation of atmospheric mercury in forest foliage. Atmospheric Environment. 2003;37:1613–1622. [Google Scholar]
- Evans GC, Norton SA, Fernandez IJ, Kahl JS, Hanson D. Changes in concentrations of major elements and trace metals in northeastern U.S.-Canadian sub-alpine forest floors. Water Air and Soil Pollution. 2005;163:245–267. [Google Scholar]
- Evers DC, Lane OP, Savoy L, Goodale W. Assessing the Impacts of Methylmercury on Piscivorous Wildlife Using a Wildlife Criterion Value Based on the Common Loon, 1998–2003. Gorham (ME): Maine Department of Environmental Protection, BioDiversity Research Institute; 2004. Report no. BRI 2004–05. [Google Scholar]
- Fitzgerald WF, Engstrom DR, Mason RP, Nater E. The case for atmospheric mercury contamination in remote areas. Environmental Science and Technology. 1998;32:1–7. [Google Scholar]
- Gabriel MC, Williamson DG. Principal biogeochemical factors affecting the speciation and transport of mercury through the terrestrial environment. Environmental Geochemistry and Health. 2004;26:421–434. doi: 10.1007/s10653-004-1308-0. [DOI] [PubMed] [Google Scholar]
- Gee GW, Bauder JW. Particle-size analysis. In: Klute A, et al., editors. Methods of soil analysis, part 1. 2. ASA and SSSA; Madison, WI: 1986. pp. 404–408. Monogr. 9. [Google Scholar]
- Grigal DF, Kolka RK, Fleck JA, Nater EA. Mercury budget of an upland-peatland watershed. Biogeochemistry. 2000;50:95–109. [Google Scholar]
- Grigal DF. Mercury Sequestration in Forests and Peatlands: A Review. Journal of Environmental Quality. 2003;32:393–405. doi: 10.2134/jeq2003.3930. [DOI] [PubMed] [Google Scholar]
- Grimaldi C, Grimaldi M, Guedron S. Mercury distribution in tropical soil profiles related to origin of mercury and soil processes. Science of the Total Environment. 2008;401:121–129. doi: 10.1016/j.scitotenv.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Hall BD, St Louis VL. Methylmercury and total mercury in plant litter decomposing in upland forests and flooded landscapes. Environmental Science and Technology. 2004;38:5010–5021. doi: 10.1021/es049800q. [DOI] [PubMed] [Google Scholar]
- Han Y, Kingston HM, Boylan HM, Rahman GMM, Shah S, Richter RC, Link DD, Bhandari S. Speciation of mercury in soil and sediment by selective solvent and acid extraction. Analytical and Bioanalytical Chemistry. 2003;375:428–436. doi: 10.1007/s00216-002-1701-4. [DOI] [PubMed] [Google Scholar]
- Han Y, Holsen TM, Evers DC, Driscoll CT. Reduced mercury deposition in New Hampshire from 1996 to 2002 due to changes in local sources. Environmental Pollution. 2008;156:1348–1356. doi: 10.1016/j.envpol.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Hintelmann H, Harris R, Heyes A, Hurley JP, Kelly CA, Krabbenhoft DP, Lindberg SE, Rudd JWM, Scott KJ, St Louis VL. Reactvity and Mobility of New and Old Mercury Deposition in a Boreal Forest Ecosystem during the First Year of the METAALICUS study. Environmental Science and Technology. 2002;36:5034–5040. doi: 10.1021/es025572t. [DOI] [PubMed] [Google Scholar]
- Huntington TG, Ryan DF, Hamburg SP. Estimating Soil Nitrogen and Carbon Pools in a Northern Hardwood Forest Ecosystem. Soil Science Society of America Journal. 1988;52:1162–1167. [Google Scholar]
- Johnson DW, Lindberg SE. The biogeochemical cycling of Hg in forests: alternative methods for quantifying total deposition and soil emission. Water Air and Soil Pollution. 1995;80:1069–1077. [Google Scholar]
- Johnson AH, Siccama TG, Friedland AJ. Spatial and temporal patterns of lead accumulation in the forest floor in the northeastern United States. Journal of Environmental Quality. 1982;11:577–580. [Google Scholar]
- Johnson CE, Petras RJ. Distribution of Zinc and Lead fractions within a Spodosol. Soil Science Society of America Journal. 1998;62:782–789. [Google Scholar]
- Juillerat JI, Ross DS, Bank MS. Mercury in Litterfall and Upper Soil Horizons in Forested Ecosystems in Vermont, USA. Environmental Toxicology and Chemistry. 2012;31:1720–1729. doi: 10.1002/etc.1896. [DOI] [PubMed] [Google Scholar]
- Kaste JM, Bostick BC, Friedland AJ, Schroth AW, Siccama TG. Fate and speciation of gasoline-derived lead in organic horizons of the northeastern USA. Soil Science Society of America Journal. 2006;70:1688–1698. [Google Scholar]
- Kaste JM, Bostick BC, Heimsath AM, Steinnes E, Friedland AJ. Using atmospheric fallout to date organic horizon layers and quantify metal dynamics during decomposition. Geochimica et Cosmochimica Acta. 2011;75:1642–1661. [Google Scholar]
- Liao L, Selim HM, DeLaune RD. Mercury adsorption-desportion and transport in soils. Journal of Environmental Quality. 2009;38:1608–1616. doi: 10.2134/jeq2008.0343. [DOI] [PubMed] [Google Scholar]
- Lindqvist O, Johansson K, Aastrup M, Andersson A, Bringmark L, Hovsenius G, Håkanson L, Iverfeldt Å, Meili M, Timm B. Mercury in the Swedish environment: recent research on causes, consequences and corrective methods. Water Air and Soil Pollution. 1991;55:23–36. [Google Scholar]
- Lorey P, Driscoll CT. Historical trends of mercury deposition in Adirondack lakes. Environmental Science and Technology. 1999;33:718–722. [Google Scholar]
- Lundstöm US, van Breeman N, Bain D. The podzolization process. A review. Geoderma. 2000;94:91–107. [Google Scholar]
- Ma LQ, Tan F, Harris WG. Concentrations and distributions of eleven metals in Florida soils. Journal of Environmental Quality. 1997;26:769–775. [Google Scholar]
- McNeal JM, Rose AW. The geochemistry of mercury in sedimentary rocks and soils in Pennsylvania. Geochimica et Cosmochimica Acta. 1974;3:1759–1784. [Google Scholar]
- Miller EK, Vanarsdale A, Keeler GJ, Chalmers A, Poissant L, Kamman NC, Brulotte RF. Estimation and Mapping of Wet and Dry Deposition Across Northeastern North America. Ecotoxicology. 2005;14:53–70. doi: 10.1007/s10646-004-6259-9. [DOI] [PubMed] [Google Scholar]
- Moore TR, Trofymow JA, Taylor B, Prescott CE, Camiré C, Duschene L, Fyles J, Kozak L, Kranabetter M, Morrison I, Siltanen M, Smith S, Titus B, Visser S, Wein R, Zoltai S. Litter decomposition rates in Canadian forests. Global Change Biology. 1999;5:75–82. [Google Scholar]
- [NADP] National Atmospheric Deposition Program. Illinois State Water Survey. University of Illinois; Urbana-Champaign, IL: 2011. National Atmospheric Deposition Program 2010 Annual Summary. NADP Data Report 2011-01. [Google Scholar]
- Nater EA, Grigal DF. Regional trends in mercury distribution across the Great Lakes states, north central USA. Nature. 1992;358:139–141. [Google Scholar]
- Obrist D, Johnson DW, Lindberg SE, Luo Y, Hararuk O, Bracho R, Battles JJ, Dail DB, Edmons RL, Monson RK, Ollinger SV, Pallardy SG, Pregitzer KS, Todd DE. Mercury distribution across 14 U.S. forests. Part 1: Spatial patterns of concentrations in biomass, litter, and soils. Environmental Science and Technology. 2011;45:3974–3981. doi: 10.1021/es104384m. [DOI] [PubMed] [Google Scholar]
- PRISM Climate Group. Prism database. PRISM Climate Group, Oregon State University; 2012. Map created 14 October 2012, http://prism.oregonstate.edu. [Google Scholar]
- Rea AW, Lindberg SE, Scherbatskoy T, Keeler GJ. Mercury accumulation in foliage over time in two northern mixed-hardwood forests. Water Air and Soil Pollution. 2002;133:49–67. [Google Scholar]
- Roulet M, Lucotte M, Saint-Aubin A, Tran S, Rhéault I, Farella N, De Jesus Da silva E, Dezencourt J, Sousa passos CJ, Santos Soares G, Guimarães JRD, Mergler D, Amorim M. The geochemistry of mercury in central Amazonian soils developed on the Alter-do-Chão formation of the lower Tapajós River Valley, Pará state, Brazil. Science of the Total Environment. 1998;223:1–24. doi: 10.1016/s0048-9697(98)00265-4. [DOI] [PubMed] [Google Scholar]
- Schlüter K. Review: evaporation of mercury from soils. An integration and synthesis of current knowledge. Environmental Geology. 2000;39:249–271. [Google Scholar]
- Schuster E. The behavior of mercury in soil with special emphasis on complexation and adsoprtion processes – a review of the literature. Water Air and Soil Pollution. 1991;56:667–680. [Google Scholar]
- Schwesig D, Matzner E. Dynamics of mercury and methylmercury in forest floor and runoff of a forested watershed in Central Europe. Biogeochemistry. 2001;53:181–200. [Google Scholar]
- Seigneur C, Lohman K, Vijayaraghavan K, Shia R. Contributions of global and regional sources to mercury deposition in New York State. Environmental Pollution. 2003;123:365–373. doi: 10.1016/s0269-7491(03)00027-7. [DOI] [PubMed] [Google Scholar]
- Shi J, Meng M, Shao J, Zhang K, Zhang Q, Jian G. Spatial distribution of mercury in topsoil from five regions in China. Environmental Science and Pollution Research. 2013;20:1756–1761. doi: 10.1007/s11356-012-1115-6. [DOI] [PubMed] [Google Scholar]
- Siccama TG. Vegetation, soil, and climate on green mountains of Vermont. Ecological Monographs. 1974;44:325–349. [Google Scholar]
- Smith-Downey NV, Sunderland EM, Jacob DJ. Anthropogenic impacts on global storage and emissions of mercury from terrestrial soils: Insights from a new global model. Journal of Geophysical Research. 2010;115:G03008. doi: 10.1029/2009JG001124. [DOI] [Google Scholar]
- Soil Survey Staff. Web Soil Survey. Natural Resources Conservation Service, United States Department of Agriculture; [Accessed [10/18/2012]]. Available online at http://websoilsurvey.nrcs.usda.gov/ [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy. 11. USDA-Natural Resources Conservation Service; Washington, DC: 2010. [Google Scholar]
- Steinnes E, Friedland AJ. Metal contamination of natural surface soils from long-range atmospheric transport: Existing and missing knowledge. Environmental Reviews. 2006;14:169–186. [Google Scholar]
- Stankwitz C, Kaste JM, Friedland AJ. Threshold increases in soil lead and mercury from tropospheric deposition across an elevational gradient. Environmental Science and Technology. 2012;46:8061–8068. doi: 10.1021/es204208w. [DOI] [PubMed] [Google Scholar]
- Susong DD, Abbott ML, Krabbenhoft DP. Mercury accumulation in snow on the Idaho National Engineering and Environmental Laboratory and surrounding region, Southeast Idaho, USA. Environmental Geology. 2003;43:357–363. [Google Scholar]
- Szopka K, Karczewksa A, Kabała C. Mercury accumulation in surface layers of mountain soils: A case study from the Karkonosze Mountains, Poland. Chemosphere. 2011;83:1507–1512. doi: 10.1016/j.chemosphere.2011.01.049. [DOI] [PubMed] [Google Scholar]
- Tipping E, Wadworth RA, Norris DA, Hall JR, Ilyin I. Long-term mercury dynamics in UK soils. Environmental Pollution. 2008;159:3474–3483. doi: 10.1016/j.envpol.2011.08.019. [DOI] [PubMed] [Google Scholar]
- [USEPA] US Environmental Protection Agency. National Emission Standards for Hazardous Air Pollutants from Coal- and Oil-fired Electric Utility Steam Generating Units and Standards of Performance for Fossil-Fuel-Fired Electric Utility. Industrial-Commercial-Institutional, and Small Industrial-Commercial-Institutional Steam Generating Units; 2011. EPA-HQ-OAR-2009-0234; EPA-HQ-OAR-2011-0044. [Google Scholar]
- Yin Y, Allen HE, Li Y, Huang CP, Sanders PF. Adsorption of mercury(II) by soil: Effects of pH, chloride, and organic matter. Journal of Environmental Quality. 1996;25:837–844. [Google Scholar]