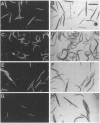
Abstract
Nematodes can alter their surface coat protein compositions at the molts between developmental stages or in response to environmental changes; such surface alterations may enable parasitic nematodes to evade host immune defenses during the course of infection. Surface antigen switching mechanisms are presently unknown. In a genetic study of surface antigen switching, we have used a monoclonal antibody, M37, that recognizes a surface antigen on the first larval stage of the free-living nematode Caenorhabditis elegans. We demonstrate that wild-type C. elegans can be induced to display the M37 antigen on a later larval stage by altering the growth conditions. Mutations that result in nonconditional display of this antigen on all four larval stages fall into two classes. One class defines the new gene srf-6 II. The other mutations are in previously identified dauer-constitutive genes involved in transducing environmental signals that modulate formation of the dauer larva, a developmentally arrested dispersal stage. Although surface antigen switching is affected by some of the genes that control dauer formation, these two process can be blocked separately by specific mutations or induced separately by environmental factors. Based on these results, the mechanisms of nematode surface antigen switching can now be investigated directly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. S., Brown S. J., Riddle D. L. Sensory control of dauer larva formation in Caenorhabditis elegans. J Comp Neurol. 1981 May 20;198(3):435–451. doi: 10.1002/cne.901980305. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hartwieg E., Horvitz H. R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993 Aug 13;74(3):515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Horvitz H. R. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991 Mar 8;251(4998):1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- Blaxter M. L., Page A. P., Rudin W., Maizels R. M. Nematode surface coats: actively evading immunity. Parasitol Today. 1992 Jul;8(7):243–247. doi: 10.1016/0169-4758(92)90126-m. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada R. C., Russell R. L. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975 Oct;46(2):326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Cox G. N., Kusch M., Edgar R. S. Cuticle of Caenorhabditis elegans: its isolation and partial characterization. J Cell Biol. 1981 Jul;90(1):7–17. doi: 10.1083/jcb.90.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N. Molecular and biochemical aspects of nematode collagens. J Parasitol. 1992 Feb;78(1):1–15. [PubMed] [Google Scholar]
- Estevez M., Attisano L., Wrana J. L., Albert P. S., Massagué J., Riddle D. L. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993 Oct 14;365(6447):644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- Ferguson E. L., Horvitz H. R. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics. 1985 May;110(1):17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto D., Kanaya S. Cuticlin: a noncollagen structural protein from Ascaris cuticle. Arch Biochem Biophys. 1973 Jul;157(1):1–6. doi: 10.1016/0003-9861(73)90382-2. [DOI] [PubMed] [Google Scholar]
- Gems D., Maizels R. M. An abundantly expressed mucin-like protein from Toxocara canis infective larvae: the precursor of the larval surface coat glycoproteins. Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1665–1670. doi: 10.1073/pnas.93.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi L. L., Albert P. S., Riddle D. L. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell. 1990 May 18;61(4):635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982 Nov 5;218(4572):578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L. A pheromone-induced developmental switch in Caenorhabditis elegans: Temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proc Natl Acad Sci U S A. 1984 Feb;81(3):819–823. doi: 10.1073/pnas.81.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984 Apr;102(2):368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- Hemmer R. M., Donkin S. G., Chin K. J., Grenache D. G., Bhatt H., Politz S. M. Altered expression of an L1-specific, O-linked cuticle surface glycoprotein in mutants of the nematode Caenorhabditis elegans. J Cell Biol. 1991 Dec;115(5):1237–1247. doi: 10.1083/jcb.115.5.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R., Brenner S., Hodgkin J., Herman R. K. A uniform genetic nomenclature for the nematode Caenorhabditis elegans. Mol Gen Genet. 1979 Sep;175(2):129–133. doi: 10.1007/BF00425528. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993 May;9(5):178–183. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- Jones S. J., Baillie D. L. Characterization of the let-653 gene in Caenorhabditis elegans. Mol Gen Genet. 1995 Oct 25;248(6):719–726. doi: 10.1007/BF02191712. [DOI] [PubMed] [Google Scholar]
- Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993 Dec 2;366(6454):461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Khoo K. H., Maizels R. M., Page A. P., Taylor G. W., Rendell N. B., Dell A. Characterization of nematode glycoproteins: the major O-glycans of Toxocara excretory-secretory antigens are O-methylated trisaccharides. Glycobiology. 1991 Mar;1(2):163–171. doi: 10.1093/glycob/1.2.163. [DOI] [PubMed] [Google Scholar]
- Larsen P. L., Albert P. S., Riddle D. L. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995 Apr;139(4):1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone E. A., Inoue T., Thomas J. H. Genetic analysis of the roles of daf-28 and age-1 in regulating Caenorhabditis elegans dauer formation. Genetics. 1996 Jul;143(3):1193–1205. doi: 10.1093/genetics/143.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- Perkins L. A., Hedgecock E. M., Thomson J. N., Culotti J. G. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986 Oct;117(2):456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Philipp M., Rumjaneck F. D. Antigenic and dynamic properties of helminth surface structures. Mol Biochem Parasitol. 1984 Mar;10(3):245–268. doi: 10.1016/0166-6851(84)90025-2. [DOI] [PubMed] [Google Scholar]
- Politz S. M., Chin K. J., Herman D. L. Genetic analysis of adult-specific surface antigenic differences between varieties of the nematode Caenorhabditis elegans. Genetics. 1987 Nov;117(3):467–476. doi: 10.1093/genetics/117.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politz S. M., Philipp M. Caenorhabditis elegans as a model for parasitic nematodes: a focus on the cuticle. Parasitol Today. 1992 Jan;8(1):6–12. doi: 10.1016/0169-4758(92)90302-i. [DOI] [PubMed] [Google Scholar]
- Politz S. M., Philipp M., Estevez M., O'Brien P. J., Chin K. J. Genes that can be mutated to unmask hidden antigenic determinants in the cuticle of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2901–2905. doi: 10.1073/pnas.87.8.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot L., Kusel J. R., Smith H. V., Harnett W., Worms M. J., Kennedy M. W. Rapid changes in the surface of parasitic nematodes during transition from pre- to post-parasitic forms. Parasitology. 1993 Jul;107(Pt 1):107–117. doi: 10.1017/s0031182000079464. [DOI] [PubMed] [Google Scholar]
- Proudfoot L., Kusel J. R., Smith H. V., Kennedy M. W. External stimuli and intracellular signalling in the modification of the nematode surface during transition to the mammalian host environment. Parasitology. 1993 Dec;107(Pt 5):559–566. doi: 10.1017/s0031182000068141. [DOI] [PubMed] [Google Scholar]
- Riddle D. L., Swanson M. M., Albert P. S. Interacting genes in nematode dauer larva formation. Nature. 1981 Apr 23;290(5808):668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- Sigurdson D. C., Spanier G. J., Herman R. K. Caenorhabditis elegans deficiency mapping. Genetics. 1984 Oct;108(2):331–345. doi: 10.1093/genetics/108.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27(1-2):57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Brenner S. The DNA of Caenorhabditis elegans. Genetics. 1974 May;77(1):95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. M., Riddle D. L. Critical periods in the development of the Caenorhabditis elegans dauer larva. Dev Biol. 1981 May;84(1):27–40. doi: 10.1016/0012-1606(81)90367-5. [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Birnby D. A., Vowels J. J. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics. 1993 Aug;134(4):1105–1117. doi: 10.1093/genetics/134.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent C., Tsuing N., Horvitz H. R. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983 Aug;104(4):619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowels J. J., Thomas J. H. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics. 1992 Jan;130(1):105–123. doi: 10.1093/genetics/130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]