Abstract
Neurologic impairments associated with human immunodeficiency virus (HIV) infection in pediatric patients may affect quality of life, and can develop despite antiretroviral therapy (ART). Behavioral changes observed in clinical studies of HIV-infected children suggest alterations in dopaminergic neurotransmission. Findings from our model of choice, the HIV-1 transgenic rat, reveal a significant increase in phosphorylated tyrosine hydroxylase protein expression and a decrease in dopamine transporter mRNA, without changes in tyrosine hydroxylase (TH) or dopamine transporter (DAT) protein or in more general markers of protein and gene expression levels in the HIV-1 transgenic rat midbrain. Thus these findings suggest selective vulnerability of the dopamine system in developing brains to HIV-1 infection.
Keywords: dopamine, HIV-1, pediatric, transgenic
Introduction
Of the estimated 40 million global human immunodeficiency virus (HIV) infections, over 2 million are infections in children under the age of 15 (UNAIDS, 2008). Symptoms of HIV in pediatric patients may include a multitude of infections affecting the skin, liver, spleen, lymph nodes, and respiratory system; further complicating the health of HIV-infected children are deficits in neurologic functions, collectively referred to as pediatric encephalopathy. Pediatric HIV-1 infections may manifest as motor dysfunctions (Mintz, 1996), expressive language disorders (Wolters et al, 1997), and attention deficits (Havens et al, 1994; Mintz, 2005), all of which may have a substantial negative impact on the functioning of HIV-positive children.
Both dopamine (DA) pathology and dysfunction have been established in adult HIV tissues (Kieburtz et al, 1991; Lopez et al, 1999; Silvers et al, 2006) as well as in animal models of adult neuroAIDS (Gelman et al, 2006; Silvers et al, 2007; Ferris et al, 2009a, 2009b; Zhu et al, 2009). Studies of adult human tissues have revealed a significant reduction in tyrosine hydroxylase (TH) concentration in the substantia nigra of HIV-infected adult tissues (Silvers et al, 2006). Similar results from another study of human postmortem tissues describe a decrease in TH as well as phosphorylated TH (pTH), in addition to an increase in dopamine transporter (DAT) expression as measured by Western blot in the striatum, suggesting a compensatory mechanism for an overall decrease in DA synthesis (Gelman et al, 2006). Other studies have described decreases in DAT in the putamen and ventral striatum of infected patients with HIV-associated dementia, as measured by positronemission tomography (Wang et al, 2004). Collectively these findings indicate that there can be pronounced DAergic changes in HIV-infected adults (for review see Ferris et al, 2008).
As attention, motor abilities, and expressive language are modulated to a substantial degree by dopamine (DA) neurotransmission, it has been suggested that dopamine-based functional and molecular changes may exist in HIV-infected children as well (for review see Webb et al, 2009). Despite the clinical observations of HIV-infected children and the functional and pathologic findings in adults, the effects of HIV on the pathology and function of developing DA systems have yet to be assessed.
Our model of choice is the HIV-1 transgenic rat (Reid et al, 2001); these animals contain a viral plasmid from which the genes Gag and Pol have been removed, but genes encoding toxic viral proteins such as Tat have been unchanged, rendering the animals noninfectious. In this study we utilized a developmental model of HIV neurologic disorders to examine the effects of HIV on the expression of the biomarkers essential to DA neurotransmission.
Results
Data from Western blotting (Figure 1) indicated a significant increase in pTH (P < .05; 215% ± 45% increase in transgenic [Tg] animals relative to control animals). No significant changes in the expression of TH (P = .12) (Figure 2A and B), DAT (P = .84) (Figure 3A and B), or neuron-specific enolase (NSE) (P = .67) (data not shown) were found between control and HIV-1 Tg animals. All Westerns were verified by observing that immunoreactivity for each antibody is proportional to the amount of protein loaded (Figures 1C, 2C, and 3C). Optical densities for pTH, TH, and DAT were normalized to NSE optical densities.
Figure 1.
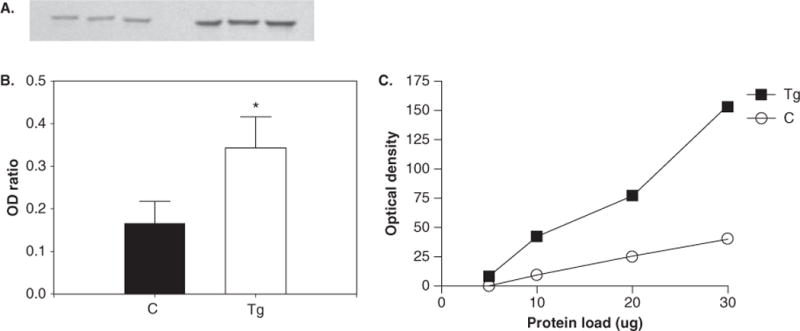
Western blotting results for phosphorylated tyrosine hydroxylase. Blotting membrane (A) shows the intensity of band staining between control (left) and Tg (right) animals, and the ratio of band optical density (OD) of pTH to NSE (B). *Significant increase in the expression of pTH in Tg animals (P < .05). (C) Protocol optimization indicates that immunoreactivity is linear over a range of protein loading.
Figure 2.
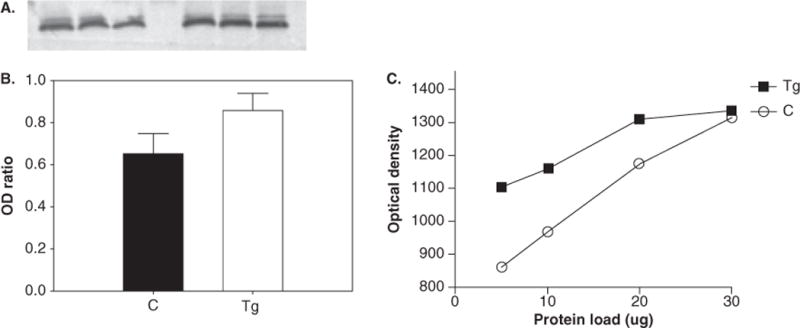
Western blotting results for tyrosine hydroxylase (TH). Blotting membrane (A) shows the intensity of band staining between control (left) and Tg (right) animals, and the ratio of band OD of TH to NSE (B). There were no significant changes in protein of TH between Tg and control animals. (C) Protocol optimization indicates that immunoreactivity is linear over a range of protein loading.
Figure 3.
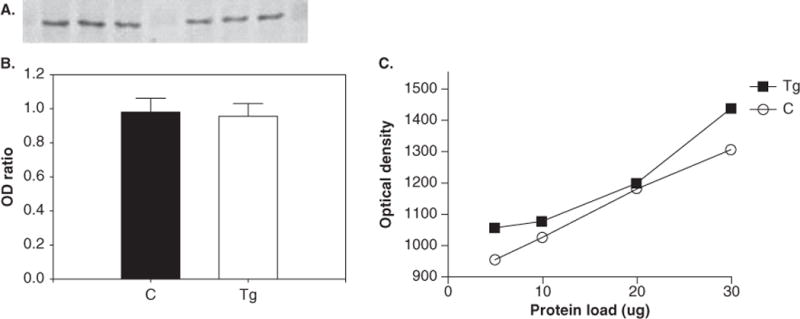
Western blotting results for dopamine transporter (DAT). Blotting membrane (A) shows the intensity of band staining between control (left) and Tg (right) animals, and the ratio of band OD of DAT to NSE (B). (C) Protocol optimization indicates that immunoreactivity is linear over a range of protein loading.
Quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) data demonstrated a significant decrease in levels of DAT mRNA (P < .05; 6.4-fold decrease) (Table 1), but no reduction in TH mRNA levels (P > .1) in HIV-1 Tg animals relative to control.
Table 1.
qRT-PCR primer sequences and resultant fold changes in for TH and DAT gene expression
| Gene | Primer sequence | Fold change |
|---|---|---|
| TH | Forward 5′-TCTCAGAGCAGGATGCCAAGCA-3′ | 2.09 |
| Reverse 5′-GATGCTGTCCTCTCGGTAGCCA-3′ | ||
| DAT | Forward 5′-GTCACCAACGGTGGCATCTA-3′ | 6.44* |
| Reverse 5′-GCTGACCAGCTTCCAGCA-3′ |
Note. There was a significant decrease in DAT mRNA expression in Tg animals
P < .05.
There was no difference in the expression of the β-actin gene between groups (P = .30) (data not shown). All statistical analyses were conducted using GraphPad software and graphs were generated in SigmaPlot 8.0 (La Jolla, CA).
Discussion
Given the DA-based alterations that have been found in adult tissues and the suggestive DAergic nature of the neurologic deficits in children with HIV infections (see Webb et al, 2009, for review), an assessment of the nature of potential DAergic changes in a developmental model was warranted. We utilized postnatal day 24 HIV-1 transgenic rats for this study, which provide a developmental model of HIV, as the brain of a 24-day-old rat is roughly equivalent to a periadolescent child (Spear, 2007). Examination of the midbrain DAergic system was critical, as this brain region contains nuclei that give rise to DA-synthesizing neurons and DA afferent fibers that are important in motor and cognitive functions modulated by dopamine (Tzschentke, 2001).
Our findings indicate a significant increase in pTH protein expression, as well as a significant decrease in DAT mRNA in HIV-1 Tg animals, but no significant changes in the expression of DAT or TH protein or in TH mRNA. The stability of the levels of reference protein expression, NSE, and the β-actin reference gene suggests that the potential DAergic differences we observed are attributable to a decrease in specific biomarkers for DA-producing neurons, rather than a generalized degradation of tissues.
The increase in the protein expression of pTH, a more catalytically active enzyme than non-phosphorylated TH (Albert et al, 1984; Campbell et al, 1986; Daubner et al, 1992), may indicate a potential decrease in DA production, as TH is the rate-limiting step in DA synthesis and therefore the presynaptic driving force for its production and concentration (Nagatsu et al, 1964). However, whether the increase in pTH protein expression begets a functional change needs to be the subject of further studies that examine whether increases in DA content or alterations in the enzymatic activity of pTH exist.
Previous studies have demonstrated that exposure to Tat in vitro and in vivo causes a significant reduction in TH mRNA and protein expression (Zauli et al, 2000). As our data reflect no significant changes in either mRNA or protein expression of TH, it may be that the effects of Tat alone and in adult animals is differentially neurotoxic to DA neurons in comparison to Tat and the other HIV genes included in the transgene of the still-developing animals utilized in our study.
Earlier findings from our laboratory have also suggested that HIV neurotoxicity may have some selectivity for DA systems. In vivo microdialysis studies have shown that intrastriatal HIV Tat protein injection results in a decrease in DAT function that occurs early after Tat exposure and in the absence of a significant loss of DAergic terminals (Ferris et al, 2009a, 2009b). Furthermore, assessments of DAT function in Tat-treated synaptosomal preparations have demonstrated a time- and dose-dependent reduction of DAT function (Zhu et al, 2009). In the present study, the absence of a change in DAT immunoreactivity confirms previous data from a 2006 study of the neurotoxic effects of Tat on primary midbrain cultures, which demonstrated neuron death in the absence of changes in DAT immunoreactivity in response to Tat exposure (Aksenova et al, 2006). Our present findings reveal that although there are no detectable changes in DAT protein expression, there is a 6.5-fold decrease in DAT mRNA, which may suggest that there is an alteration in transporter recycling. If as in other models of HIV-induced DAergic impairment, DAT function is in some way impaired in our animal model, a decrease in transporter turnover may indicate a compensatory mechanism for a decrease in transporter function. Further studies are warranted to determine whether there may be an alteration in transporter recycling.
In addition to assessing DA alterations in adult models, we have previously utilized an injection-based model of pediatric infection in which neurotoxic HIV proteins are directly injected into the central nervous system (CNS) in early postnatal life. Measuring prepulse inhibition of the auditory startle reflex, a dopamine-driven sensorimotor gating ability, the deficits induced by Tat observable at 30 and 60 days of age suggest that dopamine-based deficits first appearing during development may last into adulthood (Fitting et al, 2006). Complementing these developmental findings are studies utilizing adult HIV-1 transgenic rats that indicate that long-term behavioral changes may exist in this particular animal model (Lashomb et al, 2009). Further studies examining the longevity of the DAergic alterations we have here described may provide additional insights into DAergic vulnerabilities associated with HIV infection.
These early findings describe biochemical DAergic changes in a developmental model of HIV-infection, the HIV-1 transgenic rat. When considered alongside previous findings from our laboratory (Fitting et al, 2006; Silvers et al, 2007; Ferris et al, 2009a, 2009b; Zhu et al, 2009), the data we present here suggest that observable changes in dopamine-driven behaviors may be accompanied by significant developmental changes in DA biomarkers, indicating a functional and chemical vulnerability of DA systems that may persist into adulthood and suggests that there may be dopamine-specific pathology in infected pediatric patients.
Furthermore, understanding DAergic alterations occurring in an animal model that lacks viral replication, such as the HIV-1 transgenic rat, can provide insight into the nature of deficits when replication is controlled as in most developed nations (Biadgilign et al, 2008). In summary, we found a significant vulnerability of the DA system during early development to the neurotoxic effects of HIV, as evident by a significant increase in the expression of phosphorylated tyrosine hydroxylase and a significant decrease in DAT mRNA expression in HIV-1 transgenic rats. These data are likely highly clinically relevant, as understanding the HIV neuropathology of DA systems helps establish a positive precedent for the development of treatment strategies aimed at correcting the clinically observed DAergic manifestations of pediatric HIV infection, in addition to providing the basic groundwork for determining the potential mechanisms of DA-specific neurologic damage associated with developmental HIV infections.
Methods
Animals
Male Sprague-Dawley HIV-1 transgenic rats (Hsd: HIV-1 SD [Tg]; n = 3) and age-matched normal Sprague-Dawley animals (controls [C]; n = 3) were purchased from Harlan Laboratories (Hsd: SD); Indianapolis, IN) and were delivered on postnatal day 21. Transgenic animals were readily identified by their cataract phenotype (Reid et al, 2001). Animals were group housed under isolator lids to prevent exposure to pathogens. Dietary needs were met with standard rodent chow (Pro-Lab Rat, Mouse and Hamster Chow no. 3000) and water available ad libitum. The vivarium was kept on a 12-h light/dark cycle with lights on at 0700 h, and animals were maintained according to the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care–accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of South Carolina, Columbia.
Western blotting
At 24 days of age, animals were deeply anesthetized with sevoflurane (Abbott Laboratories, North Chicago, IL) and sacrificed by rapid decapitation. Brains were removed from the cranial vault for dissection of the midbrain, inclusive of striatum, substantia nigra, and basal ganglia. Tissues were maintained at −80°C; unilateral midbrains were pooled and homogenized in tissue lysis buffer to which protease and phosphatase inhibitor cocktails were added (Sigma, St. Louis, MO), and then centrifuged at 4°C and 14,000 × g. Following protein concentration measurement by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL), 20 μg of tissue lysates were combined with an equal volume of 2 × Laemmli buffer (Sigma), boiled for 5 min, and separated at 200 V for 50 min on 10% Tris–HCl gels, then semidry-transferred to nitrocellulose membranes at 15 V for 1 h (Bio-Rad, Hercules, CA). The nitrocellulose membranes were blocked in Tris-buffered saline (TBS) containing 3% bovine serum albumin (BSA) for 1 h at room temperature on a rocking platform. Membranes were incubated with primary antibody diluted in fresh block solution overnight at 4°C and were then washed three times at room temperature for 5 min each with TTBS (0.05% Tween-20 in 1 × TBS) on a rocking platform. Membranes were then incubated with secondary antibodies diluted in blocking wash for 1 h at room temperature on a rocking platform. The antibodies used are as follows: DAT 1:250 (Sigma), TH 1:500 (Aves, Tigard, OR), THphosphorylated at Ser401:400 (Sigma), andneuron-specific enolase (NSE) 1:20,000 (Aves), which served as a reference protein. Secondary antibodies used were bovine anti-rabbit alkaline phosphatase–conjugated immunoglobulin G (IgG) (Santa Cruz, Santa Cruz, CA; 1:2500) and goat anti-chicken alkaline phosphatase–conjugated IgY (Abcam, Cambridge, MA; 1:2500). After incubation in secondary antibody, membranes were washed three times, and then developed with BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/nitro blue ztetrazolium) (Sigma). Membranes were imaged and analyzed on a ChemiDoc XRS Molecular Imager (Bio-Rad). Optical density values were normalized to control and analyzed with Prism4 software (GraphPad, La Jolla, CA).
cDNA Preparation and qRT-PCR
Unilateral midbrain tissues taken from each animal were prepared for RNA isolation using the RNAqueous-4PCR kit per manufacturer’s instructions (Ambion, Austin, TX). RNA purity and concentration were measured by ultraviolet (UV) spectroscopy on a GeneQuant RNA/DNA Calculator (Pharmacia/GE Healthcare, Piscataway, NJ) and cDNAs were generated from 5 μg of isolated RNA using random hexamers provided in the reverse transcription kit (Cloned AMV First-Strand Synthesis kit; Invitrogen, Carlsbad, CA). cDNA samples were run in a 40 cycle protocol of 95°C melting, 50°C annealing, and 60°C elongation using the Platinum SYBR Green qPCR SuperMix-UDG system (Invitrogen). Glutaraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin served as reference genes and were purchased from R&D Systems (Minneapolis, MN) and used according to manufacturer’s instructions. cDNAs were amplified as triplicates for each reference gene and gene of interest.
Primer sequences were designed with the National Institute of Health Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast) tool from mRNA RefSeqs NM_012740 and NM_012694 for TH and DAT respectively, and custom oligonucleotides were synthesized through Invitrogen (Table 1).
Statistical analysis
Western blot optical densities were measured and normalized to NSE and expression ratios were compared by two-tailed t test. For qRT-PCR, C(t) values were normalized to β-actin averaged for each sample and analyzed by two-tailed t test. All results were considered significant at P < .05.
Acknowledgments
This research was supported by NIH grants HD043680 (C.F.M.) and DA013137 (R.M.B.)
Footnotes
Declaration of interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aksenova MV, Silvers JM, Akenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395:235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Albert KA, Helmer-Matyjek E, Nairn AC, Müller TH, Haycock JW, Greene LA, Goldstein M, Greengard P. Calcium/phospholipid-dependent protein kinase (protein kinase C) phosphorylates and activates tyrosine hydroxylase. Proc Natl Acad Sci USA. 1984;81:7713–7717. doi: 10.1073/pnas.81.24.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biadgilign S, Deribew A, Amberbir A, Deribe K. Adherence to highly active antiretroviral therapy and its correlates among HIV infected pediatric patients in Ethiopia. E-Pub BMC Pediatr. 2008;8:53. doi: 10.1186/1471-2431-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DG, Hardie DG, Vulliet PR. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J Biol Chem. 1986;261:10489–10492. [PubMed] [Google Scholar]
- Daubner SC, Lauriano C, Haycock JW, Fitzpatrick PF. Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. Effects of dopamine and cAMP dependent phosphorylation on enzyme activity. J Biol Chem. 1992;267:12639–12646. [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in neuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. The human immunodeficiency virus-1-associated protein, Tat1-86, impairs dopamine transporters and interacts with cocaine to reduce nerve terminal function: a no-net-flux microdialysis study. Neuroscience. 2009a;159:1292–1299. doi: 10.1016/j.neuroscience.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. In vivo microdialysis in awake, freely moving rats demonstrates HIV-1 Tat-induced alterations in dopamine transmission. Synapse. 2009b;63:181–185. doi: 10.1002/syn.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Spencer JA, Holzer CE, 3rd, Soukup VM. Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. J Neuroimmune Pharmacol. 2006;1:410–20. doi: 10.1007/s11481-006-9030-6. [DOI] [PubMed] [Google Scholar]
- Havens JF, Whitaker AH, Feldman JF, Ehrhardt AA. Psychiatric morbidity in school-age children with congenital human immunodeficiency virus infection: a pilot study. J Dev Behav Pediatr. 1994;15:S18–25. [PubMed] [Google Scholar]
- Kieburtz KD, Epstein LG, Gelbard HA, Greenamyre JT. Excitotoxicity and dopaminergic dysfunction in the acquired immunodeficiency syndrome dementia complex. Therapeutic implications. Arch Neurol. 1991;48:1281–4. doi: 10.1001/archneur.1991.00530240087028. [DOI] [PubMed] [Google Scholar]
- Lashomb A, Vigorito M, Chang SL. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J NeuroVirol. 2009;15:14–24. doi: 10.1080/13550280802232996. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Smith G, Meltzer CC, Becker JT. Dopamine systems in human immunodeficiency virus-associated dementia. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:184–92. [PubMed] [Google Scholar]
- Mintz M. Neurological and developmental problems in pediatric HIV infection. J Nutr. 1996;126:2663S–2673S. doi: 10.1093/jn/126.suppl_10.2663S. [DOI] [PubMed] [Google Scholar]
- Mintz M. Clinical features. In: Gendelman HE, Grant I, Everall IP, Lipton SA, Swindells S, editors. The neurology of AIDS. 2. New York: Oxford University Press; 2005. pp. 639–658. [Google Scholar]
- Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, HillM, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1transgenicrat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Aksenov MY, Aksenova MV, Beckley J, Olton P, Mactutus CF, Booze RM. Dopaminergic marker proteins in the substantia nigra of human immunodeficiency virus type 1-infected brains. J NeuroVirol. 2006;12:140–145. doi: 10.1080/13550280600724319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Neurotoxicity of HIV-1 Tat protein: involvement of D1 dopamine receptor. Neurotoxicology. 2007;28:1184–90. doi: 10.1016/j.neuro.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol Teratol. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol. 2001;63:241–320. doi: 10.1016/s0301-0082(00)00033-2. [DOI] [PubMed] [Google Scholar]
- UNAIDS. Report on the global AIDS epidemic. Joint United Nations Programme on HIV/AIDS. 2008 http://data.unaids.org/pub/GlobalReport/2008/JC1510_2008GlobalReport_en.zip.
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Webb KM, Mactutus CF, Booze RM. The ART of HIV therapies: dopaminergic deficits and future treatments for HIV pediatric encephalopathy. Expert Rev Anti Infect Ther. 2009;7:93–203. doi: 10.1586/14787210.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters PL, Brouwers P, Civitello L, Moss HA. Receptive and expressive language function of children with symptomatic HIV infection and relationship with disease parameters: a longitudinal 24-month follow-up study. AIDS. 1997;11:1135–1144. doi: 10.1097/00002030-199709000-00009. [DOI] [PubMed] [Google Scholar]
- Zauli G, Secchiero P, Rodella L, Gibellini D, Mirandola P, Mazzoni M, Milani D, Dowd DR, Capitani S, Vitale M. HIV-1 Tat-mediated inhibition of the tyrosine hydroxylase gene expression in dopaminergic neuronal cells. J Biol Chem. 2000;275:4159–4165. doi: 10.1074/jbc.275.6.4159. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]


