Abstract
Purpose
Temporal lobe epilepsy is associated with the inflammatory process related to the basic mechanisms that lead to seizure susceptibility and brain damage. Platelet-activating factor (PAF), a potent, short-lived phospholipid mediator of inflammation participates in physiological signaling in the brain. However, after seizures PAF accumulates in the brain and activates intracellular signaling related with inflammation-mediated excitotoxicity and hippocampal hyperexcitability. The objective of this study is to evaluate the effect of PAF antagonism on hippocampal hyperexcitability, seizure susceptibility and neuroprotection using the kindling paradigm and pilocarpine-induced seizure damage models.
Methods
The PAF antagonist, LAU-0901 (60 mg/kg, i.p.), or vehicle was administrated each day of kindling or daily during the four weeks after status epilepticus (SE). We analyzed seizure severity, electrical activity, cellular damage and inflammation in the hippocampi of both treated groups.
Results
LAU-0901 limits the progression of kindling and attenuates seizure susceptibility one week after the kindling procedure. Also, under the seizure-damage conditions studied here, we observed that LAU-0901 induces hippocampal neuroprotection and limits somatostatin interneuronal cell loss and inflammation.
Discussion
Our results indicate that modulation of PAF over-activity attenuates seizure susceptibility, hippocampal hyperexcitability and neuroinflammation.
Keywords: Kindling, epileptogenesis, platelet-activating factor, neuroinflammation, seizures, neuroprotection, somatostatin
Introduction
A sustained inflammatory process is involved in temporal lobe epilepsy (TLE) (Aronica et al., 2010; Crespel et al., 2002; Ravizza et al., 2008). Seizures induced by experimental epilepsy activate glia and microglia (Avignone et al., 2008; Khurgel and Ivy, 1996) that up-regulate transcriptional factors and increase the production of cytokines (Vezzani, 2008) and lipid mediators (Bazan, 2006, 2007). These mediators orchestrate a prominent inflammatory response that initiates and propagates an epileptic response (Plata-Salamán et al., 2000; Vezzani and Granata, 2005), which contributes to hippocampal hyperexcitability (Rodgers et al., 2009) and seizure-related brain damage such as neuronal death, gliosis and aberrant plasticity (Cavalheiro et al., 2006).
PAF is a potent, short-lived, phospholipid mediator of inflammation that accumulates in the synaptic cleft (Marcheselli et al., 1990), potentiates glutamate action, participates in long-term potentiation (LTP) and hippocampal plasticity, and enhances inflammatory signaling (Koltai et al., 1991). PAF exerts its actions through a seven-transmembrane G-protein linked receptor (PAF-r) (Izumi et al., 1995), which activates intracellular calcium changes and increases early gene expression and neuronal cell death (Bazan, 2006; Kato et al., 1994).
Therefore, we speculated that over-activated PAF-r (through PAF accumulation) was involved in TLE pathogenesis. We show here that limiting PAF-r activation attenuated progression from partial to generalized seizures, which resulted in modulation of hippocampal hyperexcitabilty through high-frequency oscillatory activity and promotion of neuroprotection.
Methods
Animals
Studies were performed on C57BL/6 adult male mice and adult Male Wistar rats (20-25 g and 175-200 g, respectively; Charles River Labs, Wilmington, MA). Animals were housed individually within a temperature- and light-controlled vivarium (12-h light/dark cycle) and given access to food and water ad libitum according to National Institutes of Health guidelines. The Institutional Animal Care and Use Committee at the Louisiana State University Health Sciences Center, New Orleans, approved all protocols for the animals used in these studies.
Kindling procedure in mice and hippocampal electrographic analysis
Bipolar electrode units for electrical stimulation and hippocampal electrographic (HEG) recordings (Plastic One Inc., Roanoke, VA) were implanted in the dorsal right hippocampus (coordinates: 2.3 mm caudal to bregma; 1.75 mm lateral to midline; 2.00 mm ventral to dura), and a ground wire was placed on the occipital bone. The electrodes and ground wire were fixed to the skull with acrylic cement guided by stereotaxis. For the implantation procedure, animals were kept under anesthesia induced by intraperitoneal (i.p.) injection of a mixture of ketamine (200 mg/kg) and xylazine (10 mg/kg) (Vedco Inc., Saint Joseph, MO).
Seven days after surgery, kindling acquisition was achieved by stimulating the dorsal hippocampus with sub-convulsive electrical stimulations (10-s train containing 50-Hz biphasic pulses of 100-μA amplitude) six times daily for four days at 30-min intervals. The setup for this stimulation was based on previous results obtained by progressive increases in intensity to acquire an afterdischarge (AD) in adult C57BL/6 mice. All electrical stimulations were performed sequentially with a 10-second delay in groups of up to eight animals placed in individual Plexiglas boxes. Animals that displayed signs of electrode movement during stimulations, such as bleeding around the acrylic cement or excessive noise from the signal, were excluded from the study.
As shown in previous studies, animals that reach stage 3-5 (Racine’s Score) and obtain an AD duration of more than 25 seconds maintain a similar score and duration at least one week after subconvulsive hippocampal stimulation (Tu and Bazan, 2003; Musto et al., 2009). Therefore, in our studies, groups treated with the PAF antagonist, LAU-0901 (2,4,6-Trimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid ester), or vehicle (2-hydroxypropyl-β-cyclodextrin,1.4mg/kg, ip, Sigma H107) received sub-convulsive electrical stimulations one week after kindling. The behavioral responses were scored by an investigator blinded to the number of stimulations according to a modified Racine’s Score (Racine, 1972) (1 = standing still or wet dog shaking; 2 = head nodding or jerking; 3 = forelimb myoclonus; 4 = rearing; 5 = falling; 6 = burst of rapid locomotion with or without jumping; 7 = prolonged violent locomotion with repeated jumping). HEG recordings were made during each AD throughout the kindling procedure and one week after using Enhanced Graphics Acquisition for Analysis (Version 3.63, RS Electronics Inc., Santa Barbara, CA). The signal was amplified 1000 times, filtered from 0.5 to 40 Hz (3dB/octave) (Med Associates, St. Albans, VT), and digitized at a sampling rate of 200 Hz. AD duration and different morphologic components of each AD, such as spikes, sharp waves, and poly-spike waves, were analyzed using Neuroexplorer software (Next Technology, Littleton, MA). The number of spikes was analyzed as follows: 1) the raw signal was visualized offline in a time/μV window using Sort Client program (Rasputin software, Plexon Inc., Dallas TX); 2) before stimulation, a line was set up above the signals as a baseline threshold of amplitude; and 3) the number of spikes above the threshold were automatically quantified, selecting for peak and timestamp function.
Also band frequencies for theta (4-8 Hz), beta (13-20 Hz) and gamma (21-40 Hz) were analyzed. Signal values were multiplied by the coefficients of the Hann window, and discrete fast Fourier transformation of the results were calculated using previously defined formulas (Press et al., 1992). Power spectrum was normalized from raw power spectral densities and represented the sum of all the spectrum values equal to the mean squared of the signal (Neuroexplorer, Next Technology, Littleton, MA).
Status epilepticus induction, behavioral and electrographic monitoring of spontaneous seizure occurrence
One week prior to status epilepticus (SE) induction, rats were anesthetized with ketamine hydrochloride and xylazine (50-80 mg/kg + 5-10 mg/kg, i.p.). Bipolar electrode units were placed into the right dorsal hippocampus (AP: −3 mm, L: 3 mm, DV: 3 mm below dura) and a ground wire was attached to screws placed into the occipital bone for detecting HEG activity. The rats were allowed to recover for 7 days before SE induction. Protocol for SE induction was adapted from the pilocarpine model of temporal lobe epilepsy (André et al., 2001; Dinocourt et al., 2003; Mello and Covolan, 1996). Thirty min prior to SE induction, methyl scopolamine nitrate (1 mg/kg; i.p.) and saline (5 ml; i.p.) were administered to prevent the peripheral muscarinic and dehydrating effects of pilocarpine, respectively. An initial loading dose of pilocarpine (280 mg/kg; i.p.) was administered and behaviors were closely monitored using a modified Racine’s Score (Racine, 1972). An animal was considered to be in SE once it had continuous seizure activity of stage 3 or higher. Additional doses of pilocarpine were given to animals that did not display continuous convulsive seizure activity within 40 min of the initial loading dose of pilocarpine. The total amount of pilocarpine administered did not exceed 560 mg/kg.
Once sustained convulsive-status epilepticus began, seizure activity was continuously monitored for 150 min and then stopped with a single dose of diazepam (10 mg/kg). Although the diazepam did not stop SE completely, it did facilitate the recovery period, improve survival rate, and reduce systemic stress. After SE, animal behavior in the LAU-0901- and vehicle-treated groups was monitored daily by continuous video recordings between 8:00 a.m. and 5:00 p.m. using a digital camera (JVC, HD Everio). Groups of LAU-0901- and vehicle-treated rats were monitored simultaneously. Racine’s Score (Racine, 1972) was used for rating the severity of spontaneous seizures observed in the recorded video sessions. The modal value (the seizure severity stage that occurred most often) was used for each rat (Polascheck et al., 2010). The total number of spontaneous seizures (stage 4-5) per day for each treated rat was determined, and the animals that presented two or more unprovoked spontaneous seizures 24 h apart were considered epileptic (Brooks-Kayal et al., 1998). Latency of spontaneous seizures was represented in days, beginning from day 1 after SE induction to the day of occurrence of the first spontaneous seizure (stage 4-5). The numbers were calculated using product-limit, survival-fit, log-rank Wilcoxon tests and plotted using a logarithmic scale (JMP, Version 8). At 35-36 days after SE, both treated groups were randomly selected for HEG analysis. A total of 1.6 h of HEG signals was recorded. The number of spikes was analyzed with the same data acquisition and signal analysis equipment used for the kindling experiments.
Novel PAF-receptor antagonist LAU-0901 administration
Animals were randomly assigned for treatment with the PAF antagonist, LAU-0901, or vehicle. Dosage response studies (data not shown) indicated that 60 mg/kg (i.p.) of LAU-0901 attenuates kindling. Also, previous experiments in rodents demonstrated that LAU-0901 crosses the blood brain barrier, has a physiological effect in the brain up to 6 h after injection (i.p.), and has no apparent adverse effects (Belayev et al., 2008). LAU-0901 (60 mg/kg, i.p.) or vehicle was given immediately before the first stimulation on each day of kindling acquisition or daily for four weeks after status epilepticus.
Histology
Five weeks post-SE, animals were deeply anesthetized with ketamine hydrochloride and xylazine (50-80 mg/kg + 5-10 mg/kg; i.p.) and transcardially perfused with 4% paraformaldehyde. Samples were cryoprotected thereafter in 30% sucrose. For sectioning, brains were treated overnight with 20% glycerol and 2% dimethylsulfooxide to prevent freeze-artifacts. Multiple brains (n=8) were embedded in a gelatin matrix using MultiBrain™ Technology (Neuroscience Associates, Knoxville, TN). Therefore, since all histology techniques were conducted in a like manner, this resulted in all brains having the same level of coronal sectioning, thickness, interval sectioning, and a consequent uniform distribution of staining solutions. This procedure reduced variability in total profile estimation (see Imaging Analysis below) in order to attribute an equal opportunity rule for each sample and reduce unbiased estimation (Coggeshall and Lekan, 1996). After curing, brain blocks were rapidly frozen by immersion in isopentane, chilled up to −70°C with crushed dry ice, and mounted on a freezing stage of a microtome. The MultiBrain block was sectioned coronally (40 μm), and all sections were collected sequentially into 4×6 containers with Antigen Preserve solution (50% PBS pH 7.0, 49% ethylene glycol, 1% polyvinyl pyrrolidone). Sections were stored at −20°C until staining. For Nissl staining, sections were dehydrated using alcohol prior to defatting in a chloroform/ether/alcohol solution. The slides were then rehydrated and stained in 0.05% thionine/0.08M acetate buffer (pH 4.5). Following deionized water rinses, the slides were differentiated in 95% alcohol/acetic acid and dehydrated in a standard alcohol series, cleared in xylene, and then coverslipped. Sections for Fluoro-Jade C (FJC) staining were mounted onto gelatinized slides and air dried. After pre-wetting with 95% EtOH, slides underwent the following immersions/rinses: 1% NaOH in 50% EtOH (5 min), 70% EtOH (3 min), deionized water (2 min), 0.06% potassium permanganate (10 min), deionized water (2 min), FJC (0.0001%; 10 min), three brief rinses with deionized water, and then were cleared in xylene. Immunohistochemistry staining was completed in free-floating sections. Following the blocking serum, Tris buffered saline (TBS) with Triton X100 (TX) was added to all incubation solutions and rinses were performed with TBS. After hydrogen peroxide treatment and serum blocking, sections were immunostained overnight at room temperature with the appropriate primary antibody: somatostatin (SOM, 1:15,000; Peninsula Labs), and Iba-1 (1;15000, Wako). Following rinses, the appropriate biotinylated secondary antibody was applied. To visualize the location of the primary antibody binding site, an avidin-biotin-HRP complex was applied (Vectastain Elite ABC kit; Vector, Burlingame, CA). After rinses, the sections were treated with diaminobenzidine (DAB) and hydrogen peroxide to create a visible reaction product. The sections were then mounted, air-dried, dehydrated in alcohol, cleared in xylene, and coverslipped.
Imaging analysis
For quantification of FJC and SOM cell profiles, approximately 324 coded, non-overlapping images [6 sections × 2 dorsal hippocampus × 3 region (CA1, CA3 and hilus) × 4 rats × 4 treatments (naïve, vehicle and LAU-0901)] were captured using bright microscopy or a fluorescent filter by trained personnel blinded to the treatments. Images were recorded using an Axioplan 2 microscope coupled with an Axiocam and Axiovision software (Carl Zeiss Inc., Thornwood, NY). A pilot study using immunostained samples from naïve rats contributed to effective verification of the incorporation of each hippocampal region, thus increasing the probability of identifying interneuronal soma (Dinocourt et al., 2003; Petilla Interneuron Nomenclature Group et al., 2008). Sections where electrodes were displaced were not included in the data analysis. Cell body profiles and immunopositive or immunoflourescent positive stains in each picture were tagged and counted by personnel blinded to the staining and treatment. The total profile number was Σ for each hippocampal region from all observed sections. Semi-quantitative measurement of microglia reactivity was conducted according to established procedures (Blackbeard et al., 2007; Colburn et al., 1997). The scores were characterized as follows: Score 1 – isolated microglia cell bodies containing many small, thin ramifications are spread throughout; Score 2 – some microglia cell bodies overlap, and cell bodies and ramifications are wider than those of Score 1; Score 3 – extensive ramifications and overlap among microglia cell bodies are observed, and cell bodies and ramifications are wider than those of Scores 2 and 1.
Estimation of hippocampal damage was conducted using a semi quantitative grading system modified from Chen et al. (2004). The system scores were characterized as follows: Score 1 - normal pyramidal morphology and cellular arrangement in parallel lines; Score 2 - shrinkage of some pyramidal cells, few separation between cellular lines; Score 3 - shrinkage of pyramidal cells in 50% of the area, increased separation among lines of cells, and partial disruption of linear formation; Score 4 - extensive shrinkage of pyramidal cells, separation of lines of cells in 70% of the area, and total disruption of linear formation; Score 5 - loss of pyramidal cells, shrinkage of pyramidal cells, and complete extensive separation of cells. Trained laboratory personnel conducted the quantification in a blind fashion.
Pictures were taken (40X magnification field) of CA1, CA3 and hilus samples from dorsal hippocampi of randomly treated rats. The total hippocampal score was determined from the Σ of each hippocampal region score from the hippocampus of each animal.
Prostaglandin E2 and PAF measurements
To rapidly inactivate brain enzymes, head-focused, high-powered microwave irradiation (10 KW, 400 V, 750 msec) was applied after anesthesia induced by isofluorane, followed by rapid immersion of the mouse head in ice water. The brain regions were dissected and homogenized in 5 volumes of cold chloroform:methanol (1:1). Protein precipitates were separated by centrifugation at 3000 rpm for 5 min at 4°C. Solvent extracts were collected and kept under nitrogen at −80°C until purification for solid-phase extraction. Extracts were pre-equilibrated at pH 3.0 in 10% methanol/water, loaded to 500 mg C18 columns (Varian), and then eluted with 1% methanol/ethyl acetate. Eluates were concentrated on an N2 stream evaporator. Samples were loaded to a liquid chromatograph-tandem mass spectrometer (LC-MS-MS; LC-TSQ Quantum, Thermo-Finnigan) installed with a Biobasic-AX column (Thermo-Hypersil-Keystone) (100 mm × 2.1 mm, 5-μm particle sizes). Samples were eluted in a linear gradient [100% solution A (40:60:0.01 methanol/water/acetic acid pH 4.5) to 100% solution B (99.99:0.01 methanol/acetic acid)] at a flow rate of 300 μl/min for 30 min. LC effluents were diverted to an electrospray-ionization probe (ESI) on a TSQ Quantum (Thermo-Finnigan) triple quadrupole mass spectrometer (Marcheselli et al., 2003); lipid standards (Cayman Chem., Ann Arbor, MI) were used for tuning, optimization, and calibration curves. The instruments were set on full-scale mode to detect parent ions and also were set to selected-reaction mode (SRM) for quantitative analysis to detect product ions simultaneously. The selected parent/product ions (m/z) and collision energy (v) obtained on negative ion detection mode were 351.1/333.1/16 for prostaglandin E2 (PGE2) and 370.0/171.3/2 for PAF.
Statistical analysis
Data were averaged and expressed as means ± S.E.M. and accompanied by the number of observations. Data comparison between LAU-0901- and vehicle-treated groups was performed using the Student’s t test using SAS system programs and procedures (Statistical Analysis System, SAS Institute, Cary NC). The probability level interpreted as statistically significant was P<0.05.
Results
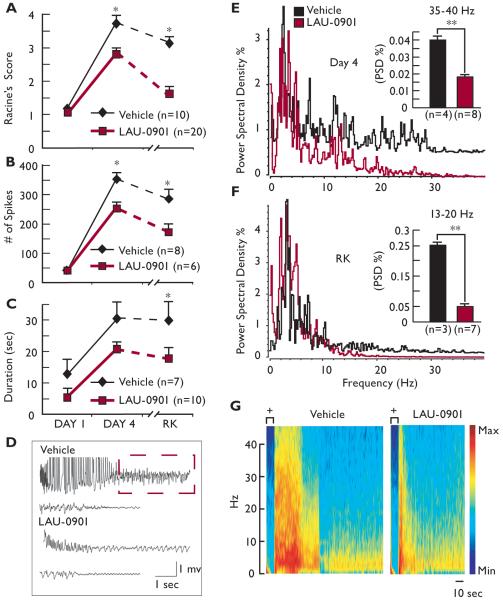
When administered systemically to mice during kindling acquisition, the novel potent PAF antagonist, LAU-0901, limited progression of locomotor seizures and reduced evoked hippocampal electrical activity. The number of spikes was reduced by day 4 of kindling, and attenuation of the evoked seizure responses occurred one week after kindling acquisition (Fig. 1A and B). Although a trend of reduced AD duration was observed in LAU-0901-treated mice during kindling, significant differences were noted one week after kindling acquisition (rekindling, RK) (Fig. 1C). The AD morphology of LAU-0901-treated mice was associated with a reduced number of spikes as well as suppression of the initial high amplitude for spikes and subsequent synchronized poly-spike complex (Fig. 1D). Previous studies indicate that different frequency bands participate in kindling (Musto et al., 2009). Here, we observed that LAU-0901-treated mice experienced a significant reduction in gamma and beta bands within each AD at day 4 and in the gamma band one week after kindling acquisition (Fig. 1E-G).
Figure 1. LAU-0901 limits kindling epileptogenesis.
(A) LAU-0901 attenuates evoked seizure responses induced by kindling procedure compared with vehicle-treated mice (Racine’s Score). This difference is accentuated when mice are stimulated one week after (rekindling, RK). (B) Number of evoked spikes from each afterdischarge (AD) during kindling and one week after. LAU-0901-treated mice show less evoked spikes associated with short AD duration (C) than vehicle-treated mice at day 4 and RK. (D) Representative hippocampal AD from vehicle and LAU-0901-treated mice at day 4 of kindling. Note the initial high amplitude spikes, observed in vehicle, and the poly-spike profile (dashed box) are abolished and reduced respectively in LAU-0901-treated mice. (E, F) Representative power spectral densities (PSD) in the frequency domain, measured in Hz, at day 4 and RK from treated groups, respectively. LAU-0901 attenuates gamma and delta band frequencies in mice at day 4 and at RK, especially in the specific bands indicated in the inset graphics. (G) Representative spectrogram of AD at day 4 of kindling procedure from LAU-0901- and vehicle-treated mice; + = stimulation. Note that LAU-0901 reduces duration and frequencies above 35 Hz. Values represent averages and ± S.E.M; * = p< 0.0001; ** = p<0.05.
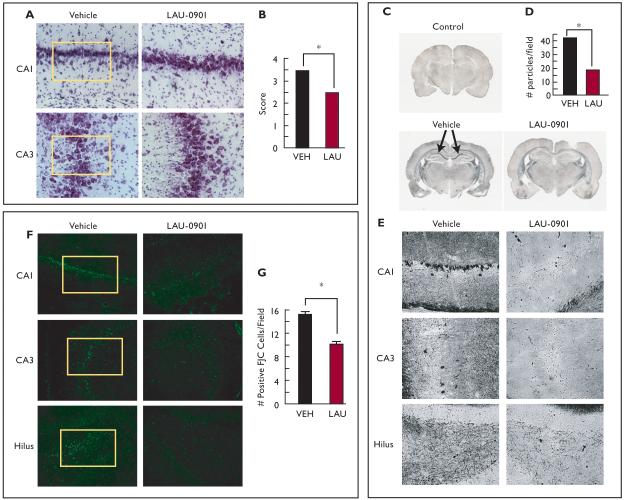
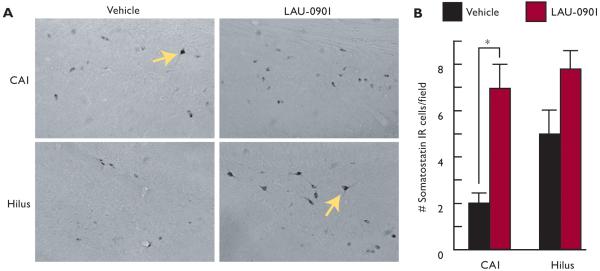
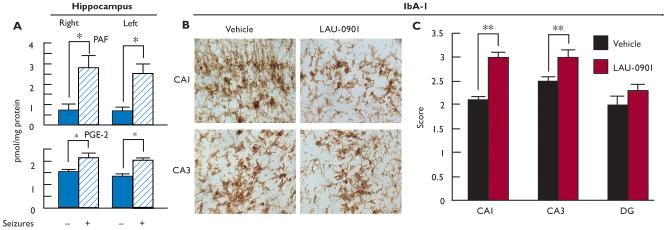
Some features associated with temporal lobe epilepsy are present following pilocarpine-induced status epilepticus, including neuronal loss in the hippocampal CA1 and hilus regions (Cavalherio, 2006, Turski et al., 1989). Neuronal and interneuronal damage have been detected as early as 24 h after SE and were still observed up to 3 months later (André et al., 2001; Dinocourt et al., 2003). Here, hippocampal damage was attenuated when LAU-0901 was injected (60 mg/kg, i.p.) daily for 4 weeks after SE, as indicated by Nissl (Fig. 2A and B) and silver staining (Fig. 2C, D, and G). The CA1 and CA3 regions from LAU-0901-treated rats presented less picnotic cells and more lines of cellular organization, as compared with the vehicle-treated group. Hippocampal sections from LAU-0901-treated rats showed lower numbers of positive FJC cells in the CA1, CA3 and hilus regions compared to sections from vehicle-treated rats (Fig. 2F and G, box). Also, LAU-0901 rats contained lower silver granule profiles in the CA1, CA3 and hilus regions (Fig. 2D and E). Moreover, the loss of SOM interneuronal cells in the SE-induced rats (Dinocourt et al., 2003) was lower in the LAU-0901-treated rats (Fig. 3A and B). It has been shown that seizures induced by kindling trigger inflammatory responses involving cyclooxygenase (COX)-2 up-regulation in neuronal cells (Tu and Bazan, 2003). Also, the presence of FJC-positive degenerating neurons is associated with interleukin (IL)-1β, nuclear factor kappa B (NF-κB), and COX-2 protein expression during status epilepticus (Voutsinos-Porche et al., 2004). Our results also show that kindling induced PAF and PGE2 accumulation in the hippocampus (Fig. 4A). In addition, our studies show that inflammatory responses resulting from pilocarpine-induced SE were attenuated by PAF antagonism. Animals treated with LAU-0901 displayed a marked reduction of activated microglia cells in the CA1 and CA3 hippocampal regions (Fig. 4B and C).
Figure 2. LAU-0901 limits hippocampal cell damage resulting from status epilepticus.
(A) Representative coronal sections of CA1 and CA3 hippocampal regions (Nissl staining, 40X) showing that LAU-0901 attenuates loss of structural organization in CA1 and CA3 observed vehicle-treated animals (box). (B) LAU-0901 reduces hippocampal damage compared with vehicle. (C) Coronal section of brain from representative control (naïve rat), vehicle, and LAU-0901-treated rats. LAU-0901 attenuates the degenerative process in hippocampi, compared with the strong staining (arrows) observed in hippocampi from vehicle-treated rats. (D, E) Representative hippocampal region of LAU-0901-treated rats showing lower dark neurons and granule density (D) in all hippocampal regions compared with vehicle-treated rats (20X). (F) Representative coronal section of CA1, CA3 and DG region of hippocampus from vehicle- and LAU-0901-treated rats using Fluoro-Jade C (FJC). Note that LAU-0901 reduces positive cells in hippocampal regions. (G) LAU-0901 limits the degeneration of neurons in the hippocampus after status epilepticus (SE) compared with vehicle (20X). Values represent averages and ± S.E.M., * = p<0.05.
Figure 3. LAU-0901 limits somatostatin interneuronal loss in the hippocampus after status epilepticus.
(A) Representative coronal sections of CA1 and hilus showing somatostatin (SOM) IR cell profiles (arrows) from vehicle- and LAU-0901-treated rats under high magnification (40X). Note that LAU-0901 presents more IR cell profiles than vehicle. (B) Quantification of IR cell profiles showing that LAU-0901 limits SOM IR cell loss as a consequence of SE as compared to vehicle. Note that LAU-0901 significantly limits SOM cell profile loss in CA1, which reveals a trend in the hilus. Values represent averages and ± S.E.M., *=p<0.05.
Figure 4. LAU-0901 attenuates hippocampal cellular inflammation resulting from status epilepticus.
(A) PAF and PGE2 increase in hippocampi after seizures (stage 5) evoked by kindling, compared with mice with an electrode implanted in hippocampus that was not stimulated. (B) Representative coronal section of CA1 and CA3 from vehicle- and LAU-0901-treated rats after SE. Note that LAU-0901 reduces microglia ramification and number and cells in both regions. (C) Microglia activation (IbA-1) is attenuated in CA1 and CA3 regions in LAU-0901-treated rats five weeks after SE. Values represent averages and ± S.E.M, * = p<0.001, ** = p< 0.05.
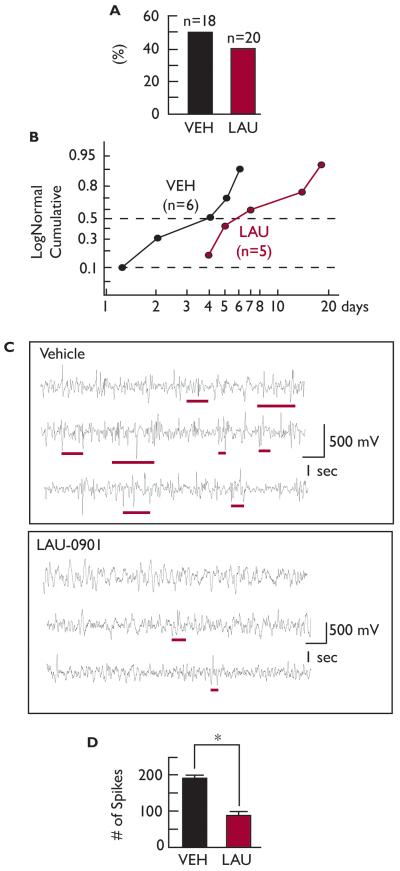
Spontaneous seizures were observed in LAU-0901- and vehicle-treated rats, respectively. LAU-0901 treatment did not reduce seizure frequency, but there was a trend toward increased latency (Fig. 5A and B). Since the CA1 hippocampal region presents hyperexcitability after pilocarpine-induced SE (El-Hassar et al., 2007), we decided to explore whether or not PAF antagonism following SE would have an effect on spontaneous electrical activity in the CA1 region after five weeks. Hippocampal electrographical activity was monitored in vivo for LAU-0901- and vehicle-treated rats. During the recording session, no spontaneous seizures (stage 4-5) were observed in either of the treated groups. LAU-0901-treated rats presented lower hippocampal epileptiform electrical activity (as represented by the number of spikes) compared to vehicle-treated rats (Fig. 5C).
Figure 5. LAU-0901 attenuates hippocampal epileptiform electrical activity after SE.
(A) LAU-0901 shows no statistical difference for the percentage of spontaneous epileptic events in rats during the first three weeks after SE and (B) an increase in spontaneous seizures (stage 4-5) after SE (p > 0.07). (C) Representative hippocampal spontaneous electrical activity five weeks after SE from vehicle- and LAU-0901-treated rats. Epileptiform activity, represented by repetitive spikes or burst of spikes with high frequency components (underlines), are increased in vehicle-treated rats compared with LAU-0901. (D) LAU-0901-treated rats present fewer spontaneous numbers of spikes in the hippocampus after five weeks of SE, compared with vehicle-treated rats.
Discussion
The rapid kindling model represents an experimental approach for understanding the basic mechanism/s that contribute to the evolution of partial complex to generalized seizures from a single epileptic focus in the hippocampus (Morimoto et al., 2004; Musto et al., 2009). Like other experimental in vivo models of epilepsy (Morimoto et al., 2004), this model can also determine the progression of the electrical discharge associated with the cellular reorganization and signal transduction pathways that promote a permanent state of seizure susceptibility.
Kindling is a complex phenomenon where repeated induction of focal seizure discharges produce a progressive and permanent increase in epileptic activity in response to the inducing agent (which is usually an electrical stimulation targeting a specific area of the brain) (Goddard et al., 1969). This often results in neuronal damage and spontaneous seizures, depending of the number of stimulations (Morimoto et al., 2004). In our experiments, we used a rapid kindling protocol (Lothman et al., 1985; Sankar et al., 2010) that permits the study of LAU-0901’s effect more rapidly than traditional kindling models. This protocol also provided information that correlates with studies related with early molecular and cellular changes in the long-term circuitry rearrangement seen in chronic TLE. Therefore, PAF-receptor signaling activation may participate in early gene activation (Akjama et al., 2008; Cole et al., 2006; Christensen et al., 2010; Tu and Bazan, 2003). Some secondary messengers display similar up-regulation after pilocarpine-induced SE (Okamoto et al., 2010). Although the half-life of LAU-0901 has not yet been determined, we estimate that its bioactivity period is more than 2 h, according to previous observations in experimental stroke (Belayev et al., 2009). Therefore, the actions of LAU-0901 in this protocol suggest that this drug interferes with establishment of the kindling state and the anti-epileptogenetic effects of LAU-0901 would require further experimental studies.
Using LAU-0901 during kindling acquisition, we showed that PAF antagonism limits progression from partial complex to generalized seizures and limits the permanent state of hippocampal seizure susceptibility in mice (Musto et al., 2009). Although LAU-0901 affects AD duration, initial responses after stimulations performed on days 1 (Fig. 1A-C), 2 and 3 (data not shown) showed no statistical differences. PAF is virtually undetectable in the brain, but it accumulates there in slightly greater amounts after seizures induced by kindling (Fig. 3A). These observations suggest that PAF activation might participate in a mechanism required for the hippocampal threshold of seizure susceptibility or that PAF activation may mediate the seizure propagation mechanism. PAF’s action in the brain is mediated through its interaction with distinct binding sites present in presynaptic and intracellular membranes (Marcheselli et al., 1990), suggesting that its role could mediate synaptic activity (Chen et al., 2001) through COX-2/PGE2 pathways (Oliveira et al., 2008; Takemiya et al., 2003; Tu and Bazan, 2003). Also, we observed that LAU-0901 attenuates spontaneous spikes in the CA1 region following SE (Fig. 5C). We propose that a permanent state of PAF signaling activation might promote initiation of epileptiform activity; however, the mechanism behind this is not clear yet. Damage to the CA1 region after epileptic seizures is associated with decreased inhibitory input to the CA1 region, which contributes to excitability in vivo due to selective loss of GABA-ergic interneurons and decreased expression of postsynaptic GABA (A) receptor function (Gibbs et al., 1997; Kapur and Lothman, 1989; Kapur and Coulter, 1995; Titulaer et al., 1995). This could lead to the development of a recurrent state of hyperexcitability after SE (Wu and Leung., 2003). Moreover, bursting neurons drive the entire CA1 network into interictal-like population bursts (Sanabria et al., 2001). Therefore, it is likely that the early emergence of intrinsic bursters, perhaps in conjunction with reduced GABA-ergic inhibition (Dudek et al., 2002), fosters the appearance of interictal HEG recorded spikes during the early stages of epileptogenesis (Stewart and Leung, 2003). These spikes could be correlated with spontaneous seizures (Racine’s Score 1-2) within the week following SE (Goffin et al., 2007). Since PAF inhibits inotropic GABA receptor activity in neurons, LAU-0901 could prevent the imbalance of inotropic GABA after SE. Another explanation is that an inhibitory cellular mechanism might participate in epileptiform activity. The SE-induced loss of SOM and other types of interneurons in the hilar region of the hippocampus leads to the loss of 1) dendritic inhibition, and 2) control of the action-potential generation of pyramidal neurons (Dinocourt et al., 2003). We observed that LAU-0901 limits cell loss of SOM-positive cells in the CA1 region (Fig. 3). Taken together, this indicates that these events may be directly related to hyperexcitability. However, these events may not be necessary for spontaneous seizure and therefore may not be directly involved in epileptogenesis (Polascheck et al., 2010).
The neuroprotective mechanism provided by PAF antagonism after brain injury is currently unknown (Belayev et al., 2009). However, there is evidence that PAF activates intracellular signaling related to neuronal damage: for example, 1) PAF accumulation activates the fos/jun/AP-1 transcriptional signaling system (Squinto et al., 1989), suggesting a role for PAF in the genomic response activated in seizures; and 2) PAF is activated during c-Jun N-terminal kinase (JNK) and p38 activity (Mukherjee et al., 1999). The effects of PAF have been successfully blocked by different antagonists (Marcheselli et al., 1990). We suggest that PAF antagonism mediates neuroprotective mechanism/s on this specific interneuronal cell. PAF-r abundance in damage-prone cells (like interneurons) will need to be explored in future studies.
Our studies point to activated microglia or gliosis as the source of inflammatory molecules in experimental epilepsy (Ravizza et al., 2008; Vezzani et al., 2002) including PAF (Teather and Wurtman, 2003). Lipopolysaccharide (LPS)-induced inflammatory processes stimulate PGE2 production, which enhances seizure severity (Sayyah et al., 2003). Also, COX-2, mRNA, and protein levels increase during seizures (Tu and Bazan, 2003). Neuroinflammation increases spike-wave discharges, which are blocked by indomethacin (a prostaglandin synthesis inhibitor), suggesting that spike-wave discharges involve COX-2 induction and subsequent synthesis and action of PGE2 (Kovacs et al., 2006). In a previous study, animals that underwent kindling-induced seizures exhibited a significant up-regulation of IL-1β, IL-1RI, tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β1 mRNAs in the amygdala, hippocampus, and all three cortical brain regions two hours after seizure induction; however, this up-regulation was not observed three weeks after seizures occurred (Plata-Salaman et al., 2000). This suggests that cytokines might play a role in seizure induction or susceptibility. Cytokine administration induces prolonged epileptiform discharges, which display a decrease in power in the delta band, an increase in theta and alpha activity, and a marked increase in power in the beta and gamma bands (Shandra et al., 2002). Increased cytokine production has a negative correlation with SOM interneuronal survival (Gavilan et al., 2007). However intra-amygdaloid administration of PGD2, PGE2 and PGF2a has no effect on seizures (Croucher et al., 1991), suggesting that other factors may participate in seizure and brain hyperexcitability. Also, our studies suggest that LAU-0901 may protect the integrity of the blood brain barrier after SE (Marcon et al., 2009) or attenuate peripheral inflammation (Marchi et al., 2009).
In summary, we report here that PAF-r antagonism attenuates neuronal damage, hyperexcitability, neuroinflammation, and epileptiform activity mechanism/s in an animal model of seizures. We also show here that PAF-r antagonism modulates the neuronal network, thus making it less susceptible to seizure initiation and propagation (Fig. 6). Overall, our studies show that the neuroprotective effect of LAU-0901 is related with mechanisms independent to those that mediate spontaneous seizures.
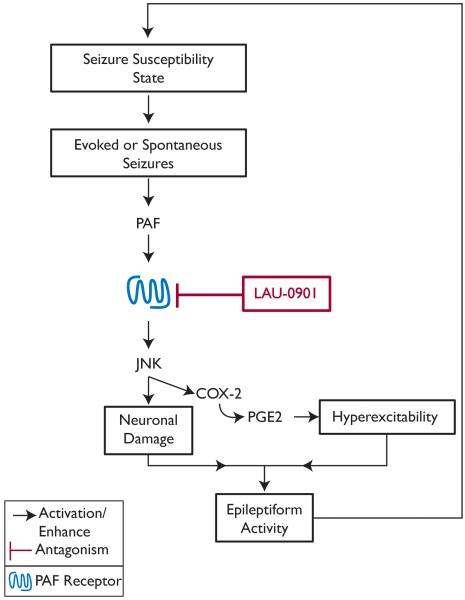
Figure 6. Hypothetical mechanism of PAF in experimental epilepsy.
Hyperexcitability state facilitates evoked or spontaneous seizures that release platelet-activating factor (PAF). PAF induces molecular signaling, c-jun N-terminal kinase (JNK) and cyclooxygenase (COX)-2 activation through its receptor. This signaling cascade promotes neuronal damage and neuronal hyperexcitability, which contributes to epileptiform activity. LAU-0901, a PAF antagonist, attenuates PAF receptor signaling and therefore limits seizure susceptibility and hippocampal hyperexcitability.
Acknowledgements
We would like to thank Dr. Nicolas Bazan for providing LAU-0901 and critical discussion of the results; Justin Hayes and Lori Hutcherson for their technical assistant in the kindling and pilocarpine model; Dr. Hilary Thompson for his statistical advise; and Fannie Jackson for LAU-0901 preparation. This work was supported by United States Public Health Service grant P20 RR016816 from the National Institutes of Health, National Center for Research Resources.
Footnotes
Disclosure of Conflicts of Interest
The authors have no conflicts of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- André V, Ferrandon A, Marescaux C, Nehlig A. Vigabatrin protects against hippocampal damage but is not antiepileptogenic in the lithium-pilocarpine model of temporal lobe epilepsy. Epilepsy Res. 2001;47:99–117. doi: 10.1016/s0920-1211(01)00299-6. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Ishikawa M, Saito A. mRNA expression of activity-regulated cytoskeleton-associated protein (arc) in the amygdala-kindled rats. Brain Res. 2008;1189:236–246. doi: 10.1016/j.brainres.2007.10.102. [DOI] [PubMed] [Google Scholar]
- Aronica E, Fluiter K, Iyer A, Zurolo E, Vreijling J, van Vliet EA, Baayen JC, Gorter JA. Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur J Neurosci. 2010;31:1100–1107. doi: 10.1111/j.1460-9568.2010.07122.x. [DOI] [PubMed] [Google Scholar]
- Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E. Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci. 2008;28:9133–9144. doi: 10.1523/JNEUROSCI.1820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Siegel G. Eicosanoids, docosanoids, platelet-activating factor and inflammation. In: Albers RW, Brady S, Price D, editors. Basic Neurochemistry. 7th ed. Elsevier; London, England: 2006. pp. 575–591. [Google Scholar]
- Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- Behr J, Heinemann U, Mody I. Glutamate receptor activation in the kindled dentate gyrus. Epilepsia. 2000;6:S100–S103. doi: 10.1111/j.1528-1157.2000.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Atkins K, Gordon WC, Alvarez-Builla J, Bazan NG. LAU-0901, a novel platelet-activating factor antagonist, is highly neuroprotective in cerebral ischemia. Exp Neurol. 2008;214:253–258. doi: 10.1016/j.expneurol.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40:3121–3126. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackbeard J, O’Dea KP, Wallace VC, Segerdahl A, Pheby T, Takata M, Field MJ, Rice AS. Quantification of the rat spinal microglial response to peripheral nerve injury as revealed by immunohistochemical image analysis and flow cytometry. J Neurosci Methods. 2007;164:207–217. doi: 10.1016/j.jneumeth.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin DE, Sanderson JL, Peterson S, Connor JA, Müller WS. Development of epileptiform excitability in the deep enthorinal cortex after status epilepticus. Eur J Neurosci. 2009;30:611–624. doi: 10.1111/j.1460-9568.2009.06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Mello ML, Leite JP. The pilocarpine model of seizures. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. Elsevier; Burlington, MA: 2006. pp. 433–448. [Google Scholar]
- Chen C, Magee JC, Marcheselli V, Hardy M, Bazan NG. Attenuated LTP in hippocampal dentate gyrus neurons of mice deficient in the PAF receptor. J Neurophysiol. 2001;85:384–390. doi: 10.1152/jn.2001.85.1.384. [DOI] [PubMed] [Google Scholar]
- Chen Z, Duan RS, Q HC, Wu Q, Mix E, Winblad B, Ljunggren HG, Zhu J. IL-12p35 deficiency alleviates kainic acid-induced hippocampal neurodegeneration in C57BL/6 mice. Neurobiol Dis. 2004;17:171–178. doi: 10.1016/j.nbd.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Christensen KV, Leffers H, Watson WP, Sánchez C, Kallunki P, Egebjerg J. Levetiracetam attenuates hippocampal expression of synaptic plasticity-related immediate early and late response genes in amygdala-kindled rats. BMC Neurosci. 2010;27:11–19. doi: 10.1186/1471-2202-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Cole-Edwards KK, Musto AE, Bazan NG. c-Jun N-terminal kinase activation responses induced by hippocampal kindling are mediated by reactive astrocytes. J Neurosci. 2006;26:8295–8304. doi: 10.1523/JNEUROSCI.1986-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespel A, Coubes P, Rousset MC, Brana C, Rougier A, Rondouin G, Bockaert J, Baldy-Moulinier M, Lerner-Natoli M. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 2002;952:159–169. doi: 10.1016/s0006-8993(02)03050-0. [DOI] [PubMed] [Google Scholar]
- Croucher MJ, Marriott DR, Bradford HF, Wilkin GP. Lack of effect of focally administered prostaglandins on electrically kindled seizure activity. Prostaglandins. 1991;42:29–38. doi: 10.1016/0090-6980(91)90091-s. [DOI] [PubMed] [Google Scholar]
- Dinocourt C, Petanjek Z, Freund TF, Ben-Ari Y, Esclapez M. Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J Comp Neurol. 2003;459:407–425. doi: 10.1002/cne.10622. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Staley KJ, Sutula TP. The search for animal models of epileptogenesis and pharmacoresistance: are there biologic barriers to simple validation strategies? Epilepsia. 2002;43:1275–1277. doi: 10.1046/j.1528-1157.2002.35102.x. [DOI] [PubMed] [Google Scholar]
- El-Hassar L, Esclapez M, Bernard C. Hyperexcitability of the CA1 hippocampal region during epileptogenesis. Epilepsia. 2007;48:131–139. doi: 10.1111/j.1528-1167.2007.01301.x. [DOI] [PubMed] [Google Scholar]
- Gavilán MP, Revilla E, Pintado C, Castaño A, Vizuete ML, Moreno-González I, Baglietto-Vargas D, Sánchez-Varo R, Vitorica J, Gutiérrez A, Ruano D. Molecular and cellular characterization of the age-related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J Neurochem. 2007;103:984–996. doi: 10.1111/j.1471-4159.2007.04787.x. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Sombati S, DeLorenzo RJ, Coulter DA. Physiological and pharmacological alterations in postsynaptic GABA(A) receptor function in a hippocampal culture model of chronic spontaneous seizures. J Neurophysiol. 1997;77:2139–2152. doi: 10.1152/jn.1997.77.4.2139. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Goffin K, Nissinen J, Van Laere K, Pitkänen A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp Neurol. 2007;205:501–505. doi: 10.1016/j.expneurol.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Henshall DC, Murphy BM. Modulators of neuronal cell death in epilepsy. Curr Opin Pharmacol. 2008;8:75–78. doi: 10.1016/j.coph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Izumi T, Honda Z, Mutoh H, Kume K, Shimizu T. Regulation and signal transduction of PAF receptor. Adv Prostaglandin Thromboxane Leukot Res. 1995;23:461–466. [PubMed] [Google Scholar]
- Kapur J, Lothman EW. Loss of inhibition precedes delayed spontaneous seizures in the hippocampus after tetanic electrical stimulation. J Neurophysiol. 1989;61:427–434. doi: 10.1152/jn.1989.61.2.427. [DOI] [PubMed] [Google Scholar]
- Kapur J, Coulter DA. Experimental status epilepticus alters gamma-aminobutyric acid type A receptor function in CA1 pyramidal neurons. Ann Neurol. 1995;38:893–900. doi: 10.1002/ana.410380609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Clark GD, Bazan NG, Zorumski CF. Platelet-activating factor as a potential retrograde messenger in CA1 hippocampal long-term potentiation. Nature. 1994;367:175–179. doi: 10.1038/367175a0. [DOI] [PubMed] [Google Scholar]
- Khurgel M, Ivy GO. Astrocytes in kindling: relevance to epileptogenesis. Epilepsy Res. 1996;26:163–175. doi: 10.1016/s0920-1211(96)00051-4. [DOI] [PubMed] [Google Scholar]
- Koltai M, Hosford D, Guinot P, Esanu A, Braquet P. PAF. A review of its effects, antagonists and possible future clinical implications (Part II) Drugs. 1991;42:174–204. doi: 10.2165/00003495-199142020-00002. [DOI] [PubMed] [Google Scholar]
- Kovács Z, Kékesi KA, Szilágyi N, Abrahám I, Székács D, Király N, Papp E, Császár I, Szego E, Barabás K, Péterfy H, Erdei A, Bártfai T, Juhász G. Facilitation of spike-wave discharge activity by lipopolysaccharides in Wistar Albino Glaxo/Rijswijk rats. Neuroscience. 2006;140:731–742. doi: 10.1016/j.neuroscience.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Hatlelid JM, Zorumski CF, Conry JA, Moon PF, Perlin JB. Kindling with rapidly recurring hippocampal seizures. Brain Res. 1985;360:83–91. doi: 10.1016/0006-8993(85)91223-5. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Rossowska MJ, Domingo MT, Braquet P, Bazan NG. Distinct platelet-activating factor binding sites in synaptic endings and in intracellular membranes of rat cerebral cortex. J Biol Chem. 1990;265:9140–9145. [PubMed] [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, Batra A, Carlton E, Najm I, Granata T, Janigro D. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33:171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcon J, Gagliardi B, Balosso S, Maroso M, Noé F, Morin M, Lerner-Natoli M, Vezzani A, Ravizza T. Age-dependent vascular changes induced by status epilepticus in rat forebrain: implications for epileptogenesis. Neurobiol Dis. 2009;34:121–132. doi: 10.1016/j.nbd.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Mello LE, Covolan L. Spontaneous seizures preferentially injure interneurons in the pilocarpine model of chronic spontaneous seizures. Epilepsy Res. 1996;26:123–129. doi: 10.1016/s0920-1211(96)00048-4. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, DeCoster MA, Campbell FZ, Davis RJ, Bazan NG. Glutamate receptor signaling interplay modulates stress-sensitive mitogen-activated protein kinases and neuronal cell death. J Biol Chem. 1999;274:6493–6498. doi: 10.1074/jbc.274.10.6493. [DOI] [PubMed] [Google Scholar]
- Musto AE, Samii MS, Hayes JF. Different phases of afterdischarge during rapid kindling procedure in mice. Epilepsy Res. 2009;85:199–205. doi: 10.1016/j.eplepsyres.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MS, Furian AF, Royes LF, Fighera MR, Fiorenza NG, Castelli M, Machado P, Bohrer D, Veiga M, Ferreira J, Cavalheiro EA, Mello CF. Cyclooxygenase-2/PGE2 pathway facilitates pentylenetetrazol-induced seizures. Epilepsy Res. 2008;79:14–21. doi: 10.1016/j.eplepsyres.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Okamoto OK, Janjoppi L, Bonone FM, Pansani AP, da Silva AV, Scorza FA, Cavalheiro EA. Whole transcriptome analysis of the hippocampus: toward a molecular portrait of epileptogenesis. BMC Genomics. 2010;11:230. doi: 10.1186/1471-2164-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petilla Interneuron Nomenclature Group. Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsáki G, Cauli B, Defelipe J, Fairén A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvárday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Muñoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata-Salamán CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Romanovitch AE, Kelly ME, Bureau Y, Anisman H, McIntyre DC. Kindling modulates the IL-1beta system, TNF-alpha, TGF-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Mol Brain Res. 2000;75:248–258. doi: 10.1016/s0169-328x(99)00306-x. [DOI] [PubMed] [Google Scholar]
- Polascheck N, Bankstahl M, Löscher W. The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp Neurol. 2010;224:219–233. doi: 10.1016/j.expneurol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Press WB, Flannery SA, Teukolsky WT, Vetterling . Numerical recipes in C. the art of scientific computing. 2nd edition Cambridge University Press; United Kingdom: 1992. [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Noe F, Zardoni D, Vaghi V, Sifringer M, Vezzani A. Interleukin Converting enzyme inhition impairs kindling epilpetogenesis in rats by blocking astrocytic IL-beta production. Neurobiol Dis. 2008;31:327–33. doi: 10.1016/j.nbd.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Rodgers KM, Hutchinson MR, Northcutt A, Maier SF, Watkins LR, Barth DS. The cortical innate immune response increases local neuronal excitability leading to seizures. Brain. 2009;132:2478–2486. doi: 10.1093/brain/awp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria ER, Su H, Yaari Y. Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J Physiol. 2001;532:205–216. doi: 10.1111/j.1469-7793.2001.0205g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar R, Auvin S, Kwon YS, Pineda E, Shin D, Mazarati A. Evaluation of development-specific targets for antiepileptogenic therapy using rapid kindling. Epilepsia. 2010;3:39–42. doi: 10.1111/j.1528-1167.2010.02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyah M, Najafabadi IT, Beheshti S, Majzoob S. Lipopolysaccharide retards development of amygdala kindling but does not affect fully-kindled seizures in rats. Epilepsy Res. 2003;57:175–180. doi: 10.1016/j.eplepsyres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Shandra AA, Godlevsky LS, Vastyanov RS, Oleinik AA, Konovalenko VL, Rapoport EN, Korobka NN. The role of TNF-alpha in amygdala kindled rats. Neurosci Res. 2002;42:147–153. doi: 10.1016/s0168-0102(01)00309-1. [DOI] [PubMed] [Google Scholar]
- Squinto SP, Block AL, Braquet P, Bazan NG. Platelet-activating factor stimulates a fos/jun/AP-1 transcriptional signaling system in human neuroblastoma cells. J Neurosci Res. 1989;24:558–566. doi: 10.1002/jnr.490240414. [DOI] [PubMed] [Google Scholar]
- Stewart LS, Leung LS. Temporal lobe seizures alter the amplitude and timing of rat behavioral rhythms. Epilepsy Behav. 2003;4:153–160. doi: 10.1016/s1525-5050(03)00006-4. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Suzuki K, Sugiura H, Yasuda S, Yamagata K, Kawakami Y, Maru E. Inducible brain COX-2 facilitates the recurrence of hippocampal seizures in mouse rapid kindling. Prostaglandins Other Lipid Mediat. 2003;71:205–216. doi: 10.1016/s1098-8823(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Teather LA, Wurtman RJ. Cyclooxygenase-2 mediates platelet-activating factor-induced prostaglandin E2 release from rat primary astrocytes. Neurosci Lett. 2003;340:177–180. doi: 10.1016/s0304-3940(03)00129-0. [DOI] [PubMed] [Google Scholar]
- Titulaer MN, Kamphuis W, Lopes da Silva FH. Autoradiographic analysis of [35S]t-butylbicyclophosphorothionate binding in kindled rat hippocampus shows different changes in CA1 area and fascia dentata. Neuroscience. 1995;66:547–54. doi: 10.1016/0306-4522(94)00570-u. [DOI] [PubMed] [Google Scholar]
- Tu B, Bazan NG. Hippocampal kindling epileptogenesis upregulates neuronal cyclooxygenase-2 expression in neocortex. Exp Neurol. 2003;179:167–175. doi: 10.1016/s0014-4886(02)00019-5. [DOI] [PubMed] [Google Scholar]
- Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA. Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154–171. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T, Perego C, De Simoni MG. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43(Suppl 5):30–35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Koning E, Kaplan H, Ferrandon A, Guenounou M, Nehlig A, Motte J. Temporal patterns of the cerebral inflammatory response in the rat lithium-pilocarpine model of temporal lobe epilepsy. Neurobiol Dis. 2004;17:385–402. doi: 10.1016/j.nbd.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Wu K, Leung LS. Increased dendritic excitability in hippocampal ca1 in vivo in the kainic acid model of temporal lobe epilepsy: a study using current source density analysis. Neuroscience. 2003;116:599–616. doi: 10.1016/s0306-4522(02)00567-5. [DOI] [PubMed] [Google Scholar]