Abstract
Objectives
Antineutrophil cytoplasmic antibody small-vessel vasculitis (ANCA-SVV) is an autoimmune systemic process increasingly recognised since the advent of antibody testing for the disease. Prompt diagnosis and institution of immunosuppressive therapy has been shown to improve patient outcome. The goal of this study was to better understand how patients navigate the health care system from symptom presentation to biopsy diagnosis, and to study the effects of prompt versus delayed diagnosis.
Methods
Disease symptoms and number of physicians seen prior to renal biopsy were assessed for 127 ANCA-SVV patients. Direct, delayed, and quest pathways to diagnosis and treatment of vasculitis were defined for both patients and providers. Kruskal-Wallis and Fisher exact tests were used to evaluate continual measures and compare categorical variables across pathways.
Results
Among patients who sought direct care, physician delay in referral to a nephrologist was common (49/127, 71%, p=0.0023). Patients who delayed seeking care also experienced a delayed diagnosis 57% of the time (p=0.0023). Patients presenting with prodromal flu or upper respiratory involvement were more likely to have a delay/quest patient pathway (56% and 55%, respectively) than a direct patient pathway (44%, p=0.033 and 45%, p=0.019, respectively). There was a trend for patients with more severe loss of renal function to have a more direct referral to a nephrologist.
Conclusion
Delay in diagnosis of ANCA SVV may be due to lack of or non-specific symptoms, especially in patients who present with non-renal manifestations of disease. Better algorithms are needed to identify extra-renal manifestations, expedite diagnosis and improve patient outcomes.
Keywords: vasculitis, renal biopsy, provider education, diagnostic tools, symptomology
Introduction
Small-vessel inflammation and necrosis associated with antineutrophil cytoplasmic antibodies (ANCA) leads to significant morbidity and mortality in affected patients. Approximately 90% of patients with pauci-immune small-vessel vasculitis have circulating ANCA which play an active role in its pathogenesis (1, 2). ANCA small-vessel vasculitis (ANCA-SVV) can present with a constellation of symptoms and the clinical course can be progressive and potentially fatal, primarily due to pulmonary haemorrhage (3-5). Immunosuppressive therapy leads to remission of ANCA-SVV in 80% of patients, but some may suffer substantial sequelae from irreversible organ injury due to vasculitis or adverse effects of treatment (5-7). Conversely, a rapid initiation of therapy significantly improves morbidity and mortality from vasculitis (6-10).
ANCA-SVV may present with protean manifestations, often affecting more than one organ, making a definitive and timely diagnosis difficult. Pauci-imune necrotising and crescentic glomerulonephritis affects 75 to 90% of patients with ANCA-SVV and may present in a spectrum ranging from asymptomatic microscopic haematuria to rapidly deteriorating renal function (11). Unless there is overt haematuria (which is uncommon), the disease usually goes unnoticed by patients until there is severe chronic damage. More clinically overt disease manifestations involve the lungs, upper respiratory tract, peripheral nerves, skin and joints (12-16). Commonly associated constitutional symptoms such as weight loss, fatigue, migratory joint pains and flu-like illness broaden the differential diagnosis (17). Prompt institution of immunosuppressive therapy with cyclophosphamide and corticosteroids improves long term renal outcome and patient survival (18, 19). Approximately 20% of patients are resistant to treatment and progress to end stage kidney disease (ESKD) within two months (8, 20). Treatment resistance is primarily associated with a presentation of severely depressed renal function and the presence of severe glomerular and interstitial scarring on renal biopsy.
The rapidity with which a patient presents to medical attention and proper treatment is commenced have a critical impact on ultimate organ damage and overall patient outcome. Understanding how patients come to medical attention and how the diagnosis is established once in the medical system are critical in terms of minimising chronic damage, especially the irreversible loss of kidney function. The purpose of this study was to evaluate pathways from onset of symptoms to diagnosis of ANCA-SVV, considering disease awareness and barriers to care by both patients and physicians. We hypothesised that the presence of more severe and clinically apparent symptoms (e.g. haemoptysis) lead patients to seek medical attention more promptly, and result in a quicker diagnosis compared to more subtle or indolent disease manifestations (e.g. asymptomatic microscopic haematuria). We also postulated that a delay in diagnosis would result in more frequent progression to ESKD.
Materials and methods
Study participants
Data was collected through telephone interviews as part of a population-based case-control study to evaluate the association of environmental factors, drugs and co-existing diseases with the onset of ANCA-SVV (21, 22). Details of the case-control study have previously been described (21), with only information on case participants used for this analysis. In brief, eligible patients were between 18 and 84 years old and had a diagnosis of ANCA-SVV with renal biopsy proof of pauci-immune necrotising glomerulonephritis. Nephropathologists throughout the region identified patients with an initial renal biopsy between October 1997 and October 2003. This allowed comprehensive ascertainment of patients with newly diagnosed ANCA-SVV with glomerulonephritis, although an unknown number may progress to ESKD without undergoing a renal biopsy. ANCA positivity, determined by indirect immunofluorescence microscopy and/or antigen-specific ELISA, was classified as either cytoplasmic and/or proteinase 3-ANCA (referred to as C/PR3-ANCA) or perinuclear and/or myeloperoxidase-ANCA (referred to as P/MPO-ANCA) (23-25). P-ANCA alone required a negative antinuclear antibody test.
Of 498 potential cases identified, 214 cases met the entry criteria and 128 (60%) completed the full interview and had complete information on their diagnosis history. Patients who participated in the telephone interview were similar to the overall population of patients identified during the study period with respect to age, sex and race (21).
Survey instrument
Trained interviewers from Battelle Centers for Public Health Research and Evaluation conducted the structured, computer-assisted telephone interviews. All study materials were approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Information on a variety of topics was collected during the interview, including symptoms at disease presentation, occupational history, smoking history, and medical and medication history. Participants were asked to identify the time of onset and the type of first symptoms of vasculitis or kidney disease. Patients were also asked the number and type of physicians they had seen, whether or not they were hospitalised for their symptoms and/or whether they were diagnosed with vasculitis at the time of their hospitalisation. A list of presenting symptoms typically associated with vasculitis or kidney disease was used for the survey, followed by an open-ended question about any symptoms not listed. These symptoms were then categorised into groups: prodromal syndrome (flu and/or night sweats), weight loss, joints, lung (coughing up blood, trouble breathing), upper respiratory (recurrent or persistent sinus problems/nose bleeds, ear infections), eyes (red/painful eyes), skin, neurological, and renal (haematuria).
Conceptualisation and operationalisation of diagnostic pathways
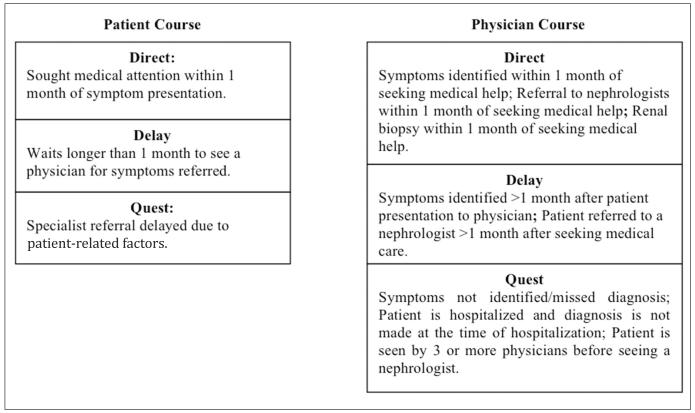
Definitions of pathways to diagnosis were adapted from those used in a study of the journey to diagnosis for paediatric patients with chronic illness (26). Responses were coded into pathway categories defined as Direct, Delay, and Quest, separately for the patient and their physician(s). In general, pathway categories were defined by the time it took for the patient to seek care and/or get referred to a nephrologist by their primary doctor, number of doctors seen prior to their renal biopsy, and symptoms that were missed or mis-identified (Fig. 1).
Fig. 1.
Individual pathway definitions*.
*For overall/combined pathway, the worst (longest) pathway was chosen of the two.
A detailed log for each patient was constructed from the telephone interview responses by a social worker familiar with symptoms and patient diagnosis issues related to ANCA-SVV (CJP). The detailed log documented the date of first symptoms, date of first renal biopsy diagnosis of ANCA glomerulonephritis, a list of symptoms at or before the renal biopsy, which symptoms prompted the patient to seek medical attention or required hospitalisation, number and types of doctors seen, and information on if and when the patient reached ESKD. These logs were then used to apply a “patient pathway category” and a “MD pathway category”. A written explanation for categorisation was included for each patient for further clarification. A second reviewer (SLH) examined each patient log and pathway category. Discrepancies in pathway categories between the two reviewers were discussed until an agreement was reached for a final pathway categorisation for the patient and the physician. Patient and physician categories were also synthesised to create an “overall” pathway category. The worse (longest) pathway of the two was selected as the overall category.
Estimated glomerular filtration rate (eGFR) was used to determine the level of kidney function and was calculated using the abbreviated Modification in Diet and Renal Disease (MDRD) Study formula based on serum creatinine, age, sex and race (27, 28). Acute dialysis was defined as a period of dialysis lasting three months or less, followed by recovery and discontinuation of dialysis. ESKD was defined as the need for chronic dialysis or a kidney transplant.
Statistical methods
Descriptive statistics in this study are presented as number with percent, mean with standard deviation, or median with interquartile range. Kruskal-Wallis tests were used to evaluate for differences in continuous measures between the three pathway categories - Direct, Delay, and Quest. Fisher’s exact tests were used to compare categorical variables across pathway and disease categories. Exact p-values are reported, with an alpha level of 0.05 considered statistically significant. Statistical calculations were performed using SAS software, version 9.1 (Cary, NC).
Results
Patient characteristics
The 128 patients included in the study had a mean age of 62 (± 14) years and 84% were Caucasian. Forty-four percent of patients were MPO-ANCA positive, 34% PR3-ANCA positive and 22% had a positive ANCA test by IIFM, but no information on antigen specificity. Details on antigen specificity as well as on diagnosis category was missing in 25 patients. The characteristics of this cohort were representative of the entire ANCA registry and of ANCA SVV in the general population (3, 8, 29, 30). Chart review was available in 103 patients (80%), among whom 22% were categorised as having granulomatosis with polyangiitis (GPA, formerly Wegeners Granulomatosis (30)), 44% with microscopic polyangiitis (MPA) and 34% with ANCA glomerulonephritis without systemic manifestations of the disease (renal limited).
Individual patient and physician pathways
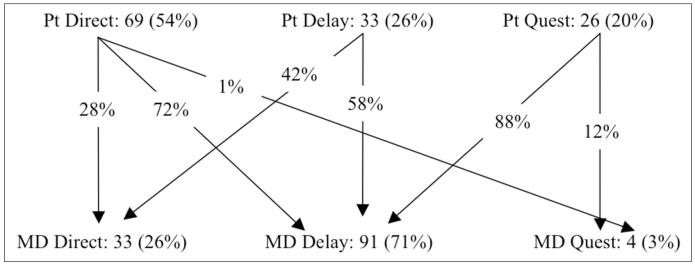
With respect to patient pathways to diagnosis (Fig. 2), 54% of patients sought direct care (n=69), 26% (n=33) had a delayed course due to patient-related factors and 20% (n=26) had a quest course. Patients with skin involvement (n=31) were more likely to have a direct (74%) than a delay/quest patient pathway (26%, p=0.015); whereas patients with prodromal flu-like (n=64) or upper respiratory tract symptoms (n=76) were more likely than expected to have a delay/quest patient pathway (56% and 55%, respectively) than a direct patient pathway (44%, p=0.033 and 45%, p=0.019, respectively). Demographic characteristics, health insurance or symptoms associated with other organ-system involvement did not influence the patient pathway.
Fig. 2.
Schematic representation of patient pathways (n=128).
With respect to physician pathways (Fig. 2), direct referrals to nephrology occurred in only 26% of cases (n=33) whereas it was delayed in 71% (n=91), and 3% (n=4) had a quest course (p=0.0001). There was a trend for upper respiratory involvement to also lead to more frequent delay/quest physician pathway (80%) than a direct physician pathway (20%, p=0.07). Patients who received a direct referral to nephrology had a lower eGFR (median 14.5 ml/min/1.73m2, IQR 11.1) than those who had a delay/quest physician pathway (median eGFR 20.6 ml/min/1.73m2, IQR 25.4; p=0.028). Similarly, the need for acute dialysis was associated with a higher than expected direct physician pathway (50%) than delay/quest physician pathway (50%, p=0.08). Demographics, health insurance and other organ system involvement did not influence the physician pathway.
Of the patients who sought direct care, 28% (n=19) received direct referral from the primary care physician to a nephrologist, representing the most expeditious combined pathway. Seventy-two percent of patients who sought direct care received a delayed referral to a nephrologist (n=49 delayed and n=1 quest by the physician). For patients in this group disease-related symptoms placed them on a direct pathway toward treatment but provider delays lead to delayed diagnosis. The cause(s) of this delay are not apparent from our analysis. There were no statistical differences in patient demographics, health insurance, or organ involvement comparing patient-direct to physican-direct pathway, or comparing patient-delay/quest to physician- delay/quest pathway. Among patients who were in the delay (n=33) and quest (n=26) categories by patient related factors, 24% received a direct referral to a nephrologist from the primary care physician. This group of patients was given the most expeditious diagnosis by the medical community despite patient related delays. Seventy-six percent of patients had a delay from patient related factors and were further delayed by the provider (p=0.62).
Combined/overall pathways
The combined/overall pathway categorisation resulted in a majority of participants (79/128 or 62%) with a delayed diagnosis, either by the physician or the patient’s not seeking care. Only fifteen percent of patients (19/127) were categorised as having a direct pathway and 23% (29/127) fell into the quest category. There were no significant differences between pathway categories by age, race, sex, insurance status, or education (Table I). There was a trend of increasing predominance of PR3-ANCA positivity along the continuum of the direct, delay and quest categories respectively (27%, 41% and 56%, respectively, p=0.06) (Table I). Disease diagnosis (GPA, MPA, Renal Limited) was not different between groups (Table I).
Table I.
Demographic and disease characteristics by overall pathway category.
| Mean ± standard deviation or n. (%) |
Direct n=19 (15%) |
Delay n=79 (62%) |
Quest n=30 (23%) |
p-value† |
|---|---|---|---|---|
| Mean age (years) | 63.8 ± 11.8 | 62.2 ± 14.4 | 58.0 ± 14.7 | 0.27 |
| Sex: | 0.47 | |||
| Male | 11 (58%) | 50 (63%) | 15 (50%) | |
| Race | 0.83 | |||
| White | 17 (89%) | 65 (82%) | 26 (87%) | |
| High school education or less | 11 (58%) | 38 (48%) | 16 (53%) | 0.72 |
| Any form of health insurance | 17 (89%) | 65 (82%) | 25 (83%) | 0.89 |
| Diagnosis category†† | ||||
| MPA* | 5 (36%) | 34 (52%) | 8 (33%) | 0.46 |
| Renal limited | 6 (43%) | 18 (28%) | 9 (38%) | |
| GPA | 3 (21%) | 13 (20%) | 7 (29%) | |
| Measures used to define pathway categories: | ||||
| Number of Physicians seen (median months and IQR¶) |
1 (0) | 1 (1) | 4 (2) | <0.0001 |
| Hospitalisation without a diagnosis of vasculitis |
4 (21%) | 15 (19%) | 14 (47%) | 0.0165 |
| Time from first symptom to renal biopsy (median months and IQR¶) |
1.0 (2) | 4.0 (10) | 8.5 (50) | <0.0001 |
Comparison of measures across the 3 overall pathway groups.
Disease diagnosis only available for n=103 patients.
2 Churg-Strauss were included as having MPA.
IQR: Inter-quartile range.
Patients with upper respiratory symptoms represented 60% of those in the delay category and 77% in the quest category in contrast with 32% in the direct category; p=0.0078 (Table II). Lung involvement was also more common among those categorised in the quest pathway compared to those categorised as direct or delay, (57% vs. 26% and 32%, respectively, p=0.04). There was less frequent history of joint symptoms and a trend for less prodromal flu-like symptoms among those in the direct category compared to those in the delay and quest categories (Table II).
Table II.
Telephone survey and clinical results by overall pathway category.
| Median (IQR) or n. (%) total n=128 |
Direct n=19 (15%) |
Delay n=79 (62%) |
Quest n=30 (23%) |
p-value† |
|---|---|---|---|---|
| Diagnosed by | 0.0120 | |||
| Specialist | 4 (21%) | 27 (34%) | 18 (60%) | |
| Usual MD | 15 (79%) | 52 (66%) | 12 (40%) | |
| Onset symptoms: | ||||
| Prodromal flu | 5 (26%) | 41 (52%) | 18 (60%) | 0.0624 |
| Lung | 5 (26%) | 25 (32%) | 17 (57%) | 0.04 |
| Upper respiratory | 6 (32%) | 47 (60%) | 23 (77%) | 0.0078 |
| Joints | 4 (21%) | 41 (52%) | 18 (60%) | 0.0204 |
| Skin | 5 (26%) | 22 (28%) | 4 (13%) | 0.25 |
| Brown urine | 4 (21%) | 21 (27%) | 12 (40%) | 0.29 |
| eGFR; median (IQR) | n=17 | n=71 | n=28 | 0.13 |
| (Missing12) | 13.4 (11.0) | 20.0 (26.3) | 16.6 (24.9) | |
| Acute dialysis* | 3 (16%) | 7 (9%) | 2 (7%) | 0.56 |
| ESKD | 6 (32%) | 20 (25%) | 10 (33%) | 0.64 |
| Time to ESKD | n=6 | n=18 | n=10 | 0.53 |
| (median months and IQR¶) | 12.7 (29.5) | 1.1 (8.0) | 4.0 (19) | |
| Death | 4 (21%) | 16 (20%) | 2 (7%) | 0.21 |
| Time to death | n=4 | n=16 | n=2 | 0.10 |
| (median months and IQR¶) | 88.7 (31.8) | 48.8 (28.1) | 92.0 (79.3) | |
IQR: Inter-quartile range.
Comparison of measures across the 3 overall pathway groups.
Acute dialysis: patients who presented with need for renal replacement therapy but recovered renal function with treatment and did not require permanent dialysis.
The need for acute dialysis at or before renal biopsy, eGFR, and progression to ESKD or death were not statistically different across overall pathway groups. Patients diagnosed with renal limited disease had a lower median eGFR at diagnosis compared to those with extra-renal involvement (15.82 vs. 27.9 ml/min/1.73m2; p=0.0084), with 50% versus 27%, respectively, having an eGFR ≤15 ml/min/1.73m2 (p=0.038).
Discussion
It has previously been suggested that significant delays in diagnosis of ANCA vasculitis are common and have a negative impact on the long term outcomes of patients. Our study documents and confirms these frequent delays in diagnosis. These delays are attributable to patient delays in seeking care, as well as delays by their treating physicians in establishing the diagnosis. Our study was not designed to critically establish the causes of patient delays. These are conceivably attributable to a number of possible clinical, socioeconomic or personal causes.
The specific manifestation of symptoms and the concern a patient feels about any given symptoms may help to explain whether a patient presents to a physician expeditiously or with a delay. The presence of non-specific systemic symptoms alone, such as flu-like or upper-respiratory tract symptoms, appears to be associated with a delay in seeking medical attention. Interestingly, there is a strong association between skin manifestations and a direct patient pathway to care. Unfortunately, our data were not detailed enough to evaluate the kind of skin findings that prompted such a strong patient response.
Although not definitive, our results provide an interesting window into patients’ interpretation and response to their symptoms, and may help improve patient education in the future. Previous studies suggested that disease onset with systemic features lead to patients seeking more prompt medical care (32-36). Our study documents delays in diagnosis even after patients have sought medical attention. In this setting, we hypothesised that the presence of overt extra-renal manifestation of disease would lead to a more rapid diagnosis because the glomerulonephritis associated with ANCA-SVV may be associated with relatively few symptoms until far advanced. Our data however refute this hypothesis. In this study, the presence of extra-renal manifestations were all more frequent than expected in the MD delay/quest group than the direct group, although these differences reached statistical significance only for musculoskeletal symptoms. Instead of acting as clues for the presence of systemic vasculitis, the extra-renal manisfestations appear to lead the treating physician to focus on these, and their common causes (e.g. pneumonia, sinusitis). In such situations, the diagnosis of systemic vasculitis is typically entertained only after the patient fails to improve, or presents with recurrent signs and symptoms despite common therapy. In the absence of multi-organ involvement, and when symptoms are non-specific, systemic vasculitis is typically not entertained in the differential diagnosis.
Research done with primary care physicians found that diagnosis and disease management are negatively impacted by the lack of availability of diagnostic tests, symptoms not typically associated with a certain disease, and physician reluctance to committing to an intensive course of action (37, 38). Moreover co-morbidities related to age and the increased risk of toxicities from immunosuppressive treatments might negatively influence the workup for vasculitis. This phenomenon has also been recognised in other autoimmune diseases with a wide spectrum of clinical manifestations such as systemic lupus erythematosus and rheumatoid arthritis (39, 40). Our findings imply that more education may be needed to provide physicians with tools and algorithms to raise the suspicion for and more expeditiously diagnose systemic vasculitis. Whether a particular constellation of findings can point the primary care physician towards a diagnosis of vasculitis requires further research.
It is intriguing that patients in the delay/quest MD pathway had a statistically significantly higher eGFR than those in the direct MD pathway (median 20.6 ml/min/1.73m2, IQR 25.4; vs. 14.5 ml/min/1.73m2, IQR 11.1; p=0.03), although the median eGFR was low in both groups. These results suggest that patients are referred to a nephrologist only when very severe renal failure is detected, and the need for renal replacement therapy is imminent. Such delays are expected to be associated with a decreased likelihood of response to therapy from the renal point of view, as the GFR at the time of treatment initiation has been demonstrated to be a strong predictor of outcome (3).
There are a number of limitations to this study. Many confounding variables were not explored in this paper that may affect the pathways to diagnosis, including access to health care, educational level, and type of specialist seen, due to the nature of the survey questions asked. There was also the risk of recall bias, as this was a telephone interview asking for past medical history solely from a patient’s perspective. However, all patients were interviewed within 5 years of their diagnosis thus limiting recall of events in the distant past.
In conclusion, ANCA-SVV is a multi-systemic disease with a variety of presenting symptoms that can baffle both the affected person and the attending physician. Better algorithms are needed to help physicians consider a vasculitic or autoimmune process with or without renal involvement in their differential diagnosis. Further study is needed, using patient and provider input, as well as structured medical chart review, to better understand and expedite a patient’s pathway to ANCA-SVV diagnosis.
Acknowledgements
We acknowledge the time and effort required by the subjects in this study and by the nephropathologists and study staff who helped in participant identification and recruitment.
Funding: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases under the Program Project “ANCA Glomerulonephritis from Molecules to Man” [grant number P01-DK58335].
Footnotes
Competing interests: none declared.
References
- 1.FALK RJ, JENNETTE JC. ANCA are pathogenic – oh yes they are! J Am Soc Nephrol. 2002;13:1977–9. doi: 10.1681/ASN.V1371977. [DOI] [PubMed] [Google Scholar]
- 2.JENNETTE JC, FALK RJ. New insight into the pathogenesis of vasculitis associated with antineutrophil cytoplasmic autoantibodies. Curr Opin Rheumatol. 2008;20:55–60. doi: 10.1097/BOR.0b013e3282f16c0a. [DOI] [PubMed] [Google Scholar]
- 3.HOGAN SL, NACHMAN PH, WILKMAN AS, JENNETTE JC, FALK RJ. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:23–32. doi: 10.1681/ASN.V7123. [DOI] [PubMed] [Google Scholar]
- 4.FAUCI AS, DOPPMAN JL, WOLFF SM. Cyclophosphamide-induced remissions in advanced polyarteritis nodosa. Am J Med. 1978;64:890–4. doi: 10.1016/0002-9343(78)90533-8. [DOI] [PubMed] [Google Scholar]
- 5.KOUTANTJI M, PEARCE S, HARROLD E. Psychological aspects of vasculitis. Rheumatology (Oxford) 2000;39:1173–9. doi: 10.1093/rheumatology/39.11.1173. [DOI] [PubMed] [Google Scholar]
- 6.HOFFMAN GS. Therapeutic interventions for systemic vasculitis. JAMA. 2010;304:2413–4. doi: 10.1001/jama.2010.1676. [DOI] [PubMed] [Google Scholar]
- 7.TALARICO R, BALDINI C, DELLA ROSSA A, et al. Large- and small-vessel vasculitis: a critical digest of the 2010-2011 literature. Clin Exp Rheumatol. 2012;30(Suppl. 70):S130–8. [PubMed] [Google Scholar]
- 8.HOGAN SL, FALK RJ, CHIN H, et al. Predictors of relapse and treatment resistance in antineutrophil antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621–31. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- 9.KLEMMER PJ, CHALERMSKULRAT W, REIF MS, HOGAN SL, HENKE DC, FALK RJ. Plasmapheresis therapy for diffuse alveolar hemorrhage in patients with small-vessel vasculitis. Am J Kidney Dis. 2003;42:1149–53. doi: 10.1053/j.ajkd.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 10.JAYNE DR, GASKIN G, RASMUSSEN N, et al. EUROPEAN VASCULITIS STUDY GROUP: Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–8. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 11.FRANSSEN CF, STEGEMAN CA, KALLEN-BERG CG, et al. Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int. 2000;57:2195–206. doi: 10.1046/j.1523-1755.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 12.JENNETTE JC, FALK RJ, GREENBURG A. Kidney involvement in systemic vasculitis. In: CHEUNG AK, COFFMAN TM, FALK RJ, JENNETTE JC, editors. Primer on Kidney Diseases. Elsevier Saunders; Pennsylvania: 2005. pp. 226–233. [Google Scholar]
- 13.PESCI A, PAVONE L, BUZIO C, MANGANEL-LI P. Respiratory system involvement in ANCA-associated systemic vasculitides. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22:S40–8. Suppl. [PubMed] [Google Scholar]
- 14.PESCI A, MANGANELLI P. Respiratory system involvement in antineutrophil cytoplasmic-associated systemic vasculitides: clinical, pathological, radiological, and therapeutic considerations. Drugs R D. 2007;8:25–42. doi: 10.2165/00126839-200708010-00003. [DOI] [PubMed] [Google Scholar]
- 15.KAWAKAMI T, SOMA Y, SAITO C, et al. Cutaneous manifestations in patients with microscopic polyangiitis: two case reports and a minireview. Acta Derm Venereol. 2006;86:144–7. doi: 10.2340/00015555-0034. [DOI] [PubMed] [Google Scholar]
- 16.SEISHAMA M, OYANA Z, ODA M. Skin eruptions associated with microscopic polyangiitis. Eur J Dermatol. 2004;14:255–8. [PubMed] [Google Scholar]
- 17.JENNETTE JC, FALK RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–23. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 18.NACHMAN PH, HOGAN SL, JENNETTE JC, FALK RJ. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:33–9. doi: 10.1681/ASN.V7133. [DOI] [PubMed] [Google Scholar]
- 19.LITTLE MA, PUSEY CD. Glomerulonephritis due to antineutrophil cytoplasm antibody-associated vasculitis: an update on approaches to management. Nephrology (Carlton) 2005;10:368–76. doi: 10.1111/j.1440-1797.2005.00434.x. [DOI] [PubMed] [Google Scholar]
- 20.LIONAKI S, HOGAN SL, JENNETTE CE, et al. The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int. 2009;76:644–51. doi: 10.1038/ki.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HOGAN SL, COOPER GS, SAVITZ DA, et al. Association of silica exposure with anti-neutrophil cytoplasmic autoantibody small-vessel vasculitis: a population-based, case-control study. Clin J Am Soc Nephrol. 2007;2:290–9. doi: 10.2215/CJN.03501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LIONAKI S, HOGAN SL, FALK RJ, et al. Association between thyroid disease and its treatment with ANCA small-vessel vasculitis: a case-control study. Nephrol Dial Transplant. 2007;22:3508–15. doi: 10.1093/ndt/gfm493. [DOI] [PubMed] [Google Scholar]
- 23.HAGEN EC, BALLIEUX BE, VAN ES LA, DAHA MR, VAN DER WOUDE FJ. Antineutrophil cytoplasmic autoantibodies: a review of the antigens involved, the assays, and the clinical and possible pathogenetic consequences. Blood. 1993;81:1996–2002. [PubMed] [Google Scholar]
- 24.HAGEN EC, DAHA MR, HERMANS J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int. 1998;53:743–53. doi: 10.1046/j.1523-1755.1998.00807.x. [DOI] [PubMed] [Google Scholar]
- 25.NIFLI AP, NOTAS G, MAMOULAKI M, et al. Comparison of a multiplex, bead-based fluorescent assay and immunofluorescence methods for the detection of ANA and ANCA autoantibodies in human serum. J Immunol Methods. 2006;311:189–97. doi: 10.1016/j.jim.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 26.KNAFL KA, ZOELLER LH, AYRES L, BREIT-MAYER BJ, GALLO AM. Learning from stories: parents’ accounts of the pathway to diagnosis. Pediatric Nurs. 2005;21:411–5. 1995. [PubMed] [Google Scholar]
- 27.LEVEY AS, BOSCH JP, LEWIS JB, GREENE T, ROGERS N, ROTH D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 28.LEVEY AS, GREEN T, KUSEK JW, BECK GJ, MDRD STUDY GROUP A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 29.ABDOU NI, KULLMAN GJ, HOFFMAN GS, et al. Wegener’s granulomatosis: survey of 701 patients in North America. Changes in outcome in the 1990s. J Rheumatol. 2002;29:309–16. [PubMed] [Google Scholar]
- 30.HOFFMAN GS, KERR GS, LEAVITT RY, et al. Wegener granulomatosis: an analysis of 158 patients. Arthritis Rheum. 1992;35:1322–9. doi: 10.1002/art.1780351113. [DOI] [PubMed] [Google Scholar]
- 31.FALK RJ, GROSS WL, GUILLEVIN L, et al. Granulomatosis with polyangiitis (Wegener’s): an alternative name for Wegener’s granulomatosis. J Am Soc Nephrol. 2011;22:587–8. doi: 10.1681/ASN.2011010081. [DOI] [PubMed] [Google Scholar]
- 32.FERRI E, ARMATO E, CAPUZZO P, CAVALERI S, IANNIELLO F. Early diagnosis of Wegener’s granulomatosis presenting with bilateral facial paralysis and bilateral serous otitis media. Auris Nasus Larynx. 2007;34:379–82. doi: 10.1016/j.anl.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 33.HERN JD, HOLLIS LJ, MOCHLOULIS G, MONTGOMERY PQ, TOLLEY NS. Early diagnosis of Wegener’s granulomatosis presenting with facial nerve palsy. J Laryngol Otol. 1996;110:459–61. doi: 10.1017/s0022215100133973. [DOI] [PubMed] [Google Scholar]
- 34.MACIAS JD, WACKYM PA, MCCABE BF. Early diagnosis of otologic Wegener’s granulomatosis using the serologic marker C-ANCA. Ann Otol Rhinol Laryngol. 1993;102:337–41. doi: 10.1177/000348949310200503. [DOI] [PubMed] [Google Scholar]
- 35.HELLMICH B, LAMPRECHT P, ARIES PM, GROSS WL. Early diagnosis of vasculitides. Z Rheumatol. 2005;64:538–46. doi: 10.1007/s00393-005-0794-5. [DOI] [PubMed] [Google Scholar]
- 36.CHEGAR BE, KELLEY RT. Wegener’s granulomatosis presenting as unilateral parotid enlargement. Laryngoscope. 2004;114:1730–3. doi: 10.1097/00005537-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 37.ANDERSON MS, TODD JK, GLODÉ MP. Delayed diagnosis of Kawasaki syndrome: an analysis of the problem. Pediatrics. 2005;115:e428–33. doi: 10.1542/peds.2004-1824. [DOI] [PubMed] [Google Scholar]
- 38.FUAT A, HUNGIN AP, MURPHY JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ. 2003;326:196. doi: 10.1136/bmj.326.7382.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LAZARO D. Elderly-onset systemic lupus erythematosus: prevalence, clinical course and treatment. Drugs Aging. 2007;24:701–15. doi: 10.2165/00002512-200724090-00001. [DOI] [PubMed] [Google Scholar]
- 40.TUTUNCU Z, REED G, KREMER J, KAVAN-AUGH A. Do patients with older-onset rheumatoid arthritis receive less aggressive treatment? Ann Rheum Dis. 2006;65:1226–9. doi: 10.1136/ard.2005.051144. [DOI] [PMC free article] [PubMed] [Google Scholar]