Abstract
Amyloid precursor protein (APP) and its secreted form, sAPP, contribute to the development of neurons in hippocampus, a brain region critical for learning and memory. Full-length APP binds the low-density lipoprotein receptor-related protein (LRP), which stimulates APP endocytosis. LRP also contributes to neurite growth. Furthermore, the receptor associated protein (RAP) binds LRP in a manner that blocks APP-LRP interactions. To elucidate APP contributions to neurite growth for full-length APP and sAPP, we cultured wild type (WT) and APP knockout (KO) neurons in sAPPα and/or RAP and measured neurite outgrowth at 1 day in vitro. Our data reveal that WT neurons had less axonal outgrowth including less axon branching. RAP treatment potentiated the inhibitory effects of APP. KO neurons had significantly more outgrowth and branching, especially in response to RAP, effects which were also associated with ERK2 activation. Our results affirm a major inhibitory role by full-length APP on all aspects of axonal and dendritic outgrowth, and show that RAP-LRP binding stimulated axon growth independently of APP. These findings support a major role for APP as an inhibitor of neurite growth and reveal novel signaling functions for LRP that may be disrupted by Alzheimer’s pathology or therapies aimed at APP processing.
Keywords: Axon growth, axon branching, dendritic growth, dendritic branching, low-density lipoprotein receptor-related protein
Introduction
During development, axonal outgrowth connects various brain regions by forming synapses between neurons in order to create normal patterning and connectivity in the brain (Kimmel, 1993). In neurodegeneration, synapse loss leads to widespread disconnection of the neuronal circuits, leading to a coordinate loss of normal brain function. This type of synaptic loss is widespread in Alzheimer’s disease (AD) (DeKosky and Scheff, 1990), a major brain disorder associated with severe cognitive loss, in which individuals lose memory, reasoning, language, and emotional control (Selkoe, 2012).
The pathology in AD brain is characterized by neurofibrillary tangles and amyloid plaques. AD plaques contain high levels of the amyloid peptide, Aβ, a 4 kDa fragment of the amyloid precursor protein (APP) (Glenner and Wong, 1984). Amyloid peptide is secreted following cleavage of APP by two secretase, β-secretase, which releases the long N-terminal fragment sAPPβ, and γ-secretase, which releases Aβ. Non-amyloidogenic APP processing occurs by α-secretase, which releases sAPPα after which γ-secretase cleavage yields p3 (Selkoe et al., 1996). Abundant full-length APP resides at the cell surface, with α-secretase cleavage occurring mainly in this pool (De Strooper and Annaert, 2000, Hui and Koo, 2011), while β-secretase acts primarily on an endocytic pool of APP (Perez et al., 1999, Thinakaran and Koo, 2008).
APP is a type 1 integral transmembrane protein (De Strooper and Annaert, 2000, Hui and Koo, 2011) that is highly expressed on neuronal precursors (Young-Pearse et al., 2007) and on cortical and hippocampal neurons (Löffler and Huber, 1992, Gralle and Ferreira, 2007). Although widely implicated in neurodegeneration, APP normally contributes to cell signaling, adhesion, axon branching and synaptic plasticity (Mattson, 1997, Perez et al., 1997, Wallace et al., 1997, Hui and Koo, 2006, Ikin et al., 2007, Thinakaran and Koo, 2008). In fact, APP knockout (KO) mice display brain gliosis (Zheng et al., 1995) and impairment of both hippocampal neuroplasticity and long term potentiation, both of which are associated with memory (Dawson et al., 1999, Seabrook et al., 1999). Neuronal cultures from APP KO mice form abnormal axons and dendrites (Perez et al., 1997). Remarkably, the deficits found in APP KO mice can be rescued by knocking-in APP expression (Ring et al., 2007), supporting an important normal role for APP in brain.
Compared to full-length APP, unique roles have been proposed for the long N-terminal secreted fragments sAPPα and sAPPβ. Data from cells expressing wild type APP or Swedish mutant APP showed that sAPPα has neurotrophic effects while sAPPβ does not, although the high levels of Aβ secreted by mutant cells may have contributed to the sAPPβ effects (Li et al., 1997). Our earlier work revealed that both sAPP and cell surface APP contribute to neurite growth (Perez et al., 1997). Newer data demonstrate that sAPPα and sAPPβ both contribute to axon growth (Chasseigneaux et al., 2011). Others have shown that sAPPα modulates axon growth, but only in the presence of full-length APP, occurring by cell surface APP and sAPPα competing for Integrin β1 binding (Young-Pearse et al., 2008).
In addition to APP, another type 1 transmembrane protein that is highly expressed on hippocampal and cortical neurons is the low-density lipoprotein receptor-related protein (LRP). LRP, sometimes called LRP1, a member of the low density lipoprotein (LDL) receptor family (Jordan et al., 1998, Van Uden et al., 2000, Lillis et al., 2008). LRP binds lipoproteins such as apoE and α2m to regulate their uptake by endocytosis. Curiously, adding α2m and/or apoE to neurons in culture raises LRP protein levels and redistributes LRP to neurites (Qiu et al., 2004). In addition to lipoproteins, LRP also binds to cell surface APP751 through the KPI domain, which stimulates APP endocytosis (Kounnas et al., 1995). The receptor-associated protein (RAP) normally assists in the intracellular transport of endocytic receptors such as LRP (Willnow, 1998), but when added extracellularly, RAP binds LRP in a manner to block the uptake of all known LRP ligands (Bu and Rennke, 1996). Cultured cortical neurons treated with RAP redistribute LRP out of the neurites and back into the soma (Qiu et al., 2004), however, RAP effects on hippocampal neurons have never been studied. It is noteworthy that hippocampal neurons express similar levels of both APP695, which lacks the KPI domain, and APP751 (Johnson et al., 1990).
Studies of LRP and APP often focus on Aβ production in search of novel targets for AD therapy. However, the normal physiology of APP and LRP might be inadvertently dysregulated by therapies that target amyloid production. Thus, in the current study we explored the contributions of full-length APP, sAPPα, and LRP on axonal and dendritic growth from hippocampal neurons from APP WT and APP KO mice. The neurons were grown in varying concentrations of sAPPα and in the presence or absence of RAP, to block sAPP binding and uptake via LRP (Kounnas et al., 1995). The extracellular regulated kinase (ERK) contributes to axon elongation in both chick retina and mouse hippocampus (Perron and Bixby, 1999, Wang et al., 2011) and ERK2 can be activated by LRP ligands that regulate neurite growth (Qiu et al., 2004), therefore, we also measured ERK2 activation in our hippocampal cultures.
The use of hippocampal neurons for our studies is important because hippocampus (1) regulates short term memory (Suzuki, 2007), (2) undergoes continued stem cell renewal (Eriksson et al., 1998, von Bohlen und Halbach, 2011), and (3) is a brain area that undergoes dynamic synaptic plasticity (Ho et al., 2011), processes that are all severely impaired in AD. Our findings confirm an inhibitory role on axonal growth for full-length APP; a novel role for full-length LRP in signal transduction that may be co-regulated by APP, and novel effects on minor process (dendritic) growth in response to APP, LRP, and sAPPα interactions. These data provide evidence that neurons with too little full-length APP could form axons that are overly long and may bypass normal postsynaptic targets. The data further suggest that hippocampal neurons with too much full-length APP could produce short axons with underdeveloped terminal arbors, again producing synaptic disconnection. Changes resulting in abnormal APP expression may ultimately lead to impaired plasticity and further affect memory formation. Cumulatively, these findings suggest that ideal AD therapies should act to preserve normal APP levels in order to sustain the optimal synaptic contacts that subserve normal memory function.
Methods
APP Knockout mice
Mice lacking APP (APP KO) (Zheng et al. 1995) were rederived on a C57BL6 background by Taconic Farms (Germantown, NY) and wild type C57BL6 mice (APP WT) served as controls. Timed breeding was done at Taconic and animals were handled consistent with USPH Policy in Humane Care and Use of Laboratory Animals and NIH guidelines on protocols approved by the Institutional Animal Care and Use Committee at both Allegheny General Hospital and the University of Pittsburgh. To minimize pain or discomfort mice were euthanized by CO2 inhalation followed by decapitation. Experiments were performed according to the guidelines of Animal Research: Reporting of In Vivo Experiments (ARRIVE).
ERK Activation
Triplicate neuron cultures were plated 160,000 cells/well in 24 well plates pretreated with poly-L-lysine. Media were gently changed 4 hr before subsequent treatments. Cells were given vehicle alone, 150 nM RAP alone, or U0126 (U120, Sigma/Aldrich) for 15 min. Controls were pretreated with U0126 1 hr prior to RAP. Media were aspirated and lysates prepared by application of boiling 2X Laemmli buffer. Proteins were separated by SDS-PAGE and ERK activation was determined from Western blots reacted with antibodies specific for phosphorylated ERK1/2 (Cell Signaling, cat # 9101, Danvers, MA) and total ERK1/2 (BD-Pharmingen, cat # 554095). Densitometry was performed as before (Perez et al., 1997).
Primary hippocampal cultures and sAPP and RAP treatments
Hippocampi dissected from embryonic day 16 mice were prepared as described (Goslin and Banker, 1991). Briefly, hippocampi were suspended in trypsin-EDTA 15 min, 37°C, washed with calcium-and magnesium-free Hanks’ buffered saline solution, and triturated with a fire polished pipet to dissociate. Cells were plated onto 12-mm round glass coverslips (Carolina Biological Supply, Burlington, NC) pretreated with poly-L-lysine hydrobromide (1 mg/ml, Sigma, St. Louis) in 0.1 M borate buffer (pH 8.5). Cells attached for 3–5 hr in MEM/10% horse serum/0.6% glucose (Plating-MEM), at 37°C 5% CO2 and then transferred to APP KO glial conditioned N2.1 medium. Freshly resuspended sAPPα was added to media at 0 nM – 20 nM. In pilot studies 75–600 nM RAP (recombinant RAP, gift of Dr. Rebeck, Georgetown University) was applied to APP WT and APP KO cultures with optimal viability noted in 150 nM RAP, the concentration selected for our experiments. Cells were fixed in 4% paraformaldehyde/4% sucrose phosphate buffered saline (PBS) 24 hr after treatments.
Primary Astrocyte Cultures
Glial cultures enriched in type 1 astrocytes (>95%) were prepared using cortical tissue from postnatal day 1 mice, as described (Goslin and Banker, 1991). Briefly, APP KO cortex was dissected free of choroid and meninges and dissociated using trypsin and trituration. Astrocytes were grown in 75-mm tissue culture flasks with minimal essential medium (MEM), 10% horse serum, 0.6% glucose (Glial-MEM), 37°C, 5% CO2 until confluent. For astrocyte-conditioned medium, cultures were rinsed 1x with unsupplemented MEM and changed to N2.1 supplemented MEM and allowed to condition the medium with secreted factors for 6–7 days. Media were collected, cells eliminated by centrifugation, and aliquots stored at −80°C.
Immunocytochemistry
For morphometric analyses neurons were labeled with anti-α-tubulin (DM1A antibody, Sigm/Aldrich, St. Louis) and anti-mouse IgG-HRP (Amersham, Arlington Heights, IL) reacted with diaminobenzidine (DAB) (Sigma, St. Louis, MO) for bright field microscopy as before (Perez et al., 1997).
Morphometry and Statistical Analyses
An imaging system equipped with AIS software (MCID, UK) was used to measure neurite growth. Briefly, microscopic images of isolated neurons were displayed on a video monitor and captured to a computer. Outgrowth was traced using a mouse and AIS software. Neurons were evaluated for axon growth, minor process growth, axon and minor process branching, minor process numbers, minor process length, average axon branch length, total neurite growth and main axonal process growth. The main axonal process refers to the length of the single longest neurite extending from the neuronal cell body. Axon outgrowth refers to the length of the single longest neurite from the neuronal cell body plus the sum of all processes emanating from the longest process. All neurons analyzed had a single axon. At 1 day in vitro, dendritic-like growth is referred to as a minor process. Minor process growth consists of the sum of the lengths of all non-axonal processes emanating from cell body including the sum of the lengths of side processes arising from minor processes. Total outgrowth is the sum of all axonal and minor process growth. Branches refer to the small processes extending laterally from an axon or minor process. Minor process number refers to the total number of processes extending directly from the neuronal cell body, not including the main axonal process. Data from 10 independent experiments measured outgrowth from a minimum of 37 neurons per treatment condition, and most experiments analyzed 50 neurons per condition. Data collected as Excel files were analyzed by one way ANOVA (Instat3, GraphPad, San Diego, CA), with significance set to p < 0.05 or better, and data given as mean ± the standard error of the mean (SEM).
Purification of sAPPα
We previously showed that CHO cells transfected with APP751 lacking the last 43 amino acids secrete sAPPα (Perez et al., 1999). Cells were grown to 90% confluence in DMEM, 10 % FBS, G418 then changed to serum-free defined media for 48 hr. Conditioned media were collected, supplemented with protease inhibitors, cooled and spun at 2000 × g at 4°C, 30 min to eliminate cells. sAPPα was purified by Affigel-Heparin followed by Mono-Q FPLC as previously described (Kametani et al., 1993). Purity of sAPP was confirmed by silver staining and immunoblotting with antibody 8E5, which recognizes residues 444–592 of human APP. Purified sAPPα fractions were pooled, protein concentrations determined, and aliquots lyophilized and stored at −80°C.
Results
Full-length APP decreases total axon outgrowth
Hippocampi were dissected from APP WT and APP KO E16 embryos, and dissociated neurons were cultured in APP KO glial conditioned medium for 1 day in 0 nM, 10 nM, or 20 nM sAPPα bearing a KPI domain, in the absence (0 nM) or presence of RAP (150 nM). We then evaluated hippocampal neurons for differences in total axon outgrowth and noted that sAPPα alone had limited effects (Fig. 1A). Bigger effects were noted for full-length APP. In all conditions APP KO neurons had significantly more total axonal outgrowth compared to APP WT neurons (Fig. 1). Remarkably, addition of 150 nM RAP to APP KO neurons grown in 0 nM and 10 nM sAPPα induced a further increase in already robust axon outgrowth, while outgrowth was similar for APP KO neurons in both 20 nM sAPPα and 20 nM sAPPα + RAP (APP KO; Fig. 1A). With regard to APP WT neurons, there was a slight tendency for sAPPα at 10 nM, with or without RAP, to decrease total axon outgrowth although not significantly (Fig. 1A). These data suggest that full-length APP plays an inhibitory role on axon outgrowth. They further suggest that RAP-LRP interactions, in the absence of full-length APP, have outgrowth inducing effects that are independent of full-length APP.
Figure 1. Full-length APP diminishes total axon outgrowth and RAP further enhances axon growth of neurons lacking APP (APP KO).
Hippocampal neurons were grown for 1 day in APP KO glial-conditioned medium without, or in the presence of sAPPα ± RAP. A. In the absence of RAP, axon growth was significantly greater for APP KO neurons (gray bars) compared to APP WT neurons (white bars). In the presence of RAP, axon growth was even more stimulated for APP KO neurons (black bars) compared to APP WT neurons (crosshatched bars), as also seen for APP KO neurons by comparing gray to black bars in 0 and 10 nM sAPPα. * p < 0.05; ** p <.0.01; *** p <.0.001. Note: Y axis begins at 100 μm to reduce the height of the graph. B. Representative images of hippocampal neurons grown in 0 nM sAPPα without or with 150 nM RAP. Top left, APP WT, 0 RAP; Top right, APP WT, 150 nM RAP; Bottom left, APP KO, 0 RAP; Bottom right, APP KO, 150 nM RAP. Scale bar = 25 μm for all images.
Full-length APP decreases the number of axon branches
Next we sought to determine the effects of full-length APP, sAPPα and RAP on axonal branching. Branching was similar for APP WT and APP KO neurons grown without sAPPα or RAP (Fig. 2). However, APP WT and APP KO neurons grown without RAP but in the presence of sAPPα exhibited significant differences in axonal branching (Fig. 2, 10 nM and 20 nM sAPPα, compare white to gray bars). APP KO neurons cultured in 150 nM RAP, with or without sAPPα, also had significantly more axon branches compared to APP WT neurons in the same conditions (Fig. 2, compare crosshatched to black bars). Indeed, for APP WT neurons sAPPα had no significant effect on axonal branching in the absence of RAP, while in 150 nM RAP with 0 nM or 10 nM sAPPα, APP WT neurons had significantly less axon branching (Fig. 2, compare white bars to cross hatched bars). Much like total axonal outgrowth, these findings again support a major inhibitory role for full-length APP on axonal branching.
Figure 2. The number of axon branches is diminished by full-length APP and further still by addition of RAP to WT neurons grown in 0 nM or 10 nM sAPPα; while branching is significantly enhanced by sAPPα for APP KO neurons lacking APP.
Left side - minus RAP: Axon branching was similar for APP WT and KO neurons grown without sAPPα (0 nM), but significantly increased for APP KO neurons grown with sAPPα (compare white to gray bars, 10 nM, 20 nM). Right side - plus RAP: Addition of RAP in the absence of sAPPα (0 nM sAPPα, 150 nM RAP) further inhibited branching of APP WT axons (compare white to crosshatched bars, 0 nM sAPPα), but resulted in significantly more branching for APP KO axons (compare crosshatched to black bars, 0 nM sAPPα). RAP + 10 nM sAPPα also significantly inhibited APP WT axon branching (compare white to crosshatched bars, 10 nM). * p < 0.05; *** (p < 0.001).
RAP and full-length APP modulate axon growth in multiple ways
Side branching off the main axonal process as measured by total axon outgrowth less the longest axonal process
As we found the most robust changes in axon growth associated with the loss of full-length APP and in response to RAP, we sought to further scrutinize the contributions of APP and LRP by measuring subtler aspects of axon outgrowth. We first looked at total axon outgrowth less the longest axonal process, to determine if APP and RAP may have affected axon branch outgrowth independently of effects on the main axonal process. This type of outgrowth was significantly shorter in APP WT neurons compared to APP KO neurons grown without RAP (Fig. 3A, 0 nM RAP). RAP significantly increased branching off the main axonal process for APP KO neurons, but not for APP WT neurons (Fig. 3A, 150 nM RAP). These RAP results revealed that axon branching (not including the longest axonal process) significantly increased for cells lacking APP, suggesting that RAP binding to LRP alone can transduce a signal into neurons to stimulate axonal branching.
Figure 3. Subtle measures of full-length APP effects on axons.

A. Axon growth, less the main axon process is diminished by full-length APP, while RAP further stimulates axon growth of APP KO neurons. Branches were significantly more abundant for APP KO neurons compared to APP WT neurons grown without RAP. Addition of RAP further increased branching off the main axonal process for KO neurons. ** p < 0.01, *** p < 0.001. B. Full-length APP, in the presence of RAP, decreases the length of the longest axonal process. The length of the main axon process was similar for APP WT and APP KO neurons grown in 0 nM RAP. Addition of 150 nM RAP significantly decreased the longest axonal process for APP WT neurons (compare white bars), which resulted in significantly longer main axonal processes for APP KO neurons grown in RAP compared to APP WT neurons (compare white to gray bars in 150 nM RAP). ** p < 0.01, *** p < 0.001. C. Full-length APP increases the length of axon branches for APP WT neurons treated with RAP. Axon branch length was significantly increased for APP WT neurons in the presence of RAP. APP KO neurons had no change in axon branch length in response to RAP. * p < 0.05, ** p <.0.01. D. The ratio of total axon outgrowth to longest axonal process is reduced by full-length APP, but increases in response to RAP for APP WT neurons. APP KO neurons had a significantly higher ratio of total axon outgrowth to the longest axon process compared to APP WT neurons grown in 0 nM RAP (compare white to gray bars). The ratio was significantly increased for APP WT neurons grown in RAP (compare white bars). *** p < 0.001.
Length of the main axonal process
A measure of the longest axon process showed that APP WT and APP KO neurons were similar when grown in medium without sAPPα or RAP (Fig. 3B, left side). In 150 nM RAP, APP WT neurons main axon processes were significantly shorter compared to parallel cultures grown without RAP (Fig. 3B, compare white bars), while APP KO neurons had similar main axonal length when grown in the presence or absence of RAP (Fig. 3B, gray bars). Because RAP treatment significantly reduced the length of the main axonal process of APP WT neurons, that reduction produced a significant difference in the length of the main axonal process between APP WT and APP KO neurons grown in the presence of RAP (Fig. 3B, compare white to gray bars in 150 nM RAP).
Length of the axonal side branches
To further scrutinize the source of differences in outgrowth, we measured the length of each axonal side branch. We found that RAP significantly increased axon branch lengths of APP WT neurons compared to parallel WT cultures grown without RAP (Fig. 3C, white bars). RAP had no effect on APP KO axon branch lengths (Fig. 3C, gray bars). From these results we conclude that the more robust total axon outgrowth noted for APP KO neurons (Fig. 1) was likely due to increased numbers of axon branches (Fig. 2), not increased axon branch lengths.
Ratio of total axon outgrowth to main axon process length
As a measure of full-length APP effects on axon growth, we also calculated the ratio of total axon outgrowth compared to the length of the main axon process for APP WT and APP KO neurons grown without RAP. This ratio was significantly greater for APP KO compared to APP WT neurons (Fig. 3D, 0 RAP). We also calculated the ratio of total axonal outgrowth compared to growth of the main axonal process in response to 150 nM RAP. RAP tended to increase this ratio for all neurons, but only APP WT neurons had a significant increase (Fig. 3D, white bars), a result that might have been expected since RAP significantly decreased the length of the main axon process (Fig. 3B) while RAP significantly increased axon branch lengths for APP WT neurons (Fig. 3C). From these results we infer that full-length APP primarily affects axon growth by modulating branch numbers, not the length of each axon branch. This suggests that RAP-LRP interactions that block the binding of LRP to full-length APP, act to inhibit the growth of the main axon process (Fig. 3B), whilst RAP in the absence of full-length APP, increases the total number of axonal branches (Fig. 1, 2).
APP inhibits minor process outgrowth
In addition to measuring all aspects of axonal outgrowth we also measured the effects of full-length APP, sAPPα and RAP on minor processes (presumptive dendrites). As shown in Table 1 (upper three rows), neurons which lack APP (APP KO) had significantly more minor processes, increased minor process branching, and increased minor process outgrowth compared to APP WT neurons. We observed similar effects on minor processes in the presence or absence of sAPPα. As might be expected, the enhanced minor process growth noted for APP KO neurons, when summed with axonal outgrowth, accounted for significantly greater total neurite outgrowth for APP KO neurons compared to APP WT neurons (Table 1, bottom row). RAP treatment decreased total neurite outgrowth for APP WT neurons but increased total neurite outgrowth for APP KO neurons. These results imply that full-length APP inhibits minor process growth and that RAP blockade of an APP-LRP binding interaction further accentuates the inhibitory effects of APP, while RAP treatment of neurons lacking APP enhances their already robust minor process growth; raising the possibility that a growth-associated-signal transduced by RAP was different in neurons that express APP compared those from APP KO mice, which lack APP. ERK2 has been implicated in neurite outgrowth (Perron and Bixby, 1999, Wang et al., 2011), thus, we did experiments to evaluate RAP effects on ERK2 as follows.
Table 1. Minor process outgrowth and total neurite outgrowth for APP WT and APP KO hippocampal neurons grown without (w/o RAP, 0 nM) or with RAP (w/RAP, 150 nM).
At the developmental stage evaluated, presumptive dendrites are called minor processes. APP KO neurite growth was significantly greater than APP WT growth in all conditions.
| Between Groups Measures | WT w/o RAP | KO w/o RAP | P Value | WT w/RAP | KO w/RAP | P Value |
|---|---|---|---|---|---|---|
| Minor Process Number | 5.200 ± 0.3 | 7.516 ± 0.5 | p < 0.001 | 3.925 ± 0.2 | 7.211 ± 0.4 | p < 0.001 |
| Minor Process Branches | 6.373 ± 0.6 | 18.122 ± 1.8 | p < 0.001 | 4.050 ± 2.9 | 18.688 ± 1.4 | p < 0.001 |
| Minor Process Outgrowth (μm) | 218.16 ± 8.8 | 312.11 ± 13.8 | p < 0.001 | 184.22 ± 11.4 | 329.63 ± 16.0 | p < 0.001 |
| Total neurite outgrowth (μm) | 411.79 ± 14.0 | 553.82 ± 17.4 | p < 0.001 | 282.11 ± 18.7 | 640.18 ± 20.2 | p < 0.001 |
| Within Groups Measures | WT w/o RAP | WT w/RAP | P Value | KO w/o RAP | KO w/RAP | P Value |
| Total neurite outgrowth (μm) | 411.79 ± 14.0 | 282.11 ± 18.7 | p < 0.001 | 553.82 ± 17.4 | 640.18 ± 20.2 | p < 0.01 |
Note: In the presence of RAP, total neurite outgrowth was even more pronounced for WT and KO neurons, however, in opposite the direction.
ERK2 response to RAP is different in APP WT and APP KO neurons
We evaluated signaling effects of RAP associated with blockade of APP-LRP binding (APP WT) or associated with RAP-LRP binding in the absence of APP (APP KO). Neurons were grown 1 day without or with RAP after which ERK2 activation was measured (Fig. 4). Controls included vehicle alone for baseline ERK2 activity; and in response to U0126, an inhibitor of MAP kinase kinase 2 (MEK2), to block ERK2 activity. Immunoblots were probed for total ERK2 and phosphorylated ERK2 and quantified using established methods (Ugarte et al., 2003, Lin et al., 2008). In four independent experiments, we noted a trend toward lower baseline ERK2 activation in neurons without APP (APP KO) compared to APP WT neurons, though the difference was not significant (Fig. 4, white bars). As expected, U0126 treatment reduced ERK2 activity (Fig. 4, black bars) as also noted when U0126 was given prior to RAP treatment (not shown). Opposite effects of RAP on ERK2 were noted in APP WT and APP KO neurons; whereby RAP diminished ERK2 activity in APP WT neurons, presumably by blocking the interaction between APP and LRP; but enhanced ERK2 activity in APP KO neurons that lack full-length APP (Fig. 4, shaded bars). These findings provide the first evidence that binding of RAP directly to LRP in the absence of APP activates ERK2 in an APP-independent manner.
Figure 4. Full-length APP diminishes ERK2 activation in response to RAP.
Baseline ERK2 activity tended to be higher in APP WT neurons compared to APP KO neurons (white bars), though the difference was not significant. Pretreatment with a MEK inhibitor, U0126, significantly diminished ERK2 activation in all cultures, demonstrating down regulation of ERK2 activity in the presence of MEK2 inhibitor. RAP stimulated ERK2 activity significantly only in APP KO neurons. * p < .05, *** p < 0.001.
Discussion
Many studies have evaluated APP effects on neuron development in both the central nervous system (CNS) and peripheral nervous system (PNS). Some of the most intriguing findings have come from neuronal cultures using APP KO mice. In our current study, we evaluated low density hippocampal cultures from APP WT and APP KO mice to further explore the impact of full-length APP and sAPPα on neurite growth as a model of hippocampal plasticity, controlling for sAPP effects using purified sAPPα and RAP. It is known that hippocampal neurons express similar levels of APP751, which contains the KPI domain, and APP695 (Johnson et al., 1990). Extracellular RAP, which blocks sAPPα uptake into neurons via LRP, was used as a control to help elucidate our earlier findings (Perez et al., 1997). We noted a significant increase in axon branching for APP KO neurons exposed to sAPPα (Fig. 2), however, there were very limited sAPPα effects overall, even in APP WT cultures, in which small amounts of endogenous sAPPα, sAPPβ, or Aβ may have been released by the neurons during the time after plating. Thus, we focused the remainder of our studies on the effects of full-length APP and on novel findings pertaining to RAP in both APP WT and APP KO neurons.
Abundantly apparent in our results is the finding that the absence of full-length APP on KO hippocampal neurons led to increased axon outgrowth, axon branching, minor process outgrowth, and total neurite growth, supporting a major inhibitory role by full-length cell surface APP on neurite outgrowth. Remarkably, RAP potentiated already robust total axon outgrowth for APP KO neurons, suggesting that in cells lacking APP, the binding of RAP to LRP transduces a signal that enhances neurite growth. Moreover, for APP WT neurons, RAP reduced axon branching even further as described below, consistent with the findings of Qiu et al (2004) using cortical neurons.
Our most robust findings revealed that full-length APP strongly inhibited axonal growth, much like results from other laboratories (Ikin et al., 2007, Young-Pearse et al., 2008). In the current studies we measured all aspects of outgrowth including the main processes and all side branches of both axons and minor processes. We found that neurons expressing full-length APP (APP WT) had significantly less axon growth including fewer branches off the main axonal process (Fig. 2), which resulted in less total axon outgrowth (Fig. 1A). When APP WT neurons were exposed to RAP we noted a further decrease in axon branch number per cell (Fig. 2) suggesting that RAP blockade of APP-LRP interactions further contributed to inhibition of already limited axon branching of APP WT neurons. A review of the literature allows us to propose the following explanation for these finding. Ikin et al. (2007) found that full-length APP binds to the cytoskeletal regulatory protein Mena via interactions with the adaptor molecule, Fe65, to form a complex with APP that inhibits branching of axons. Young-Pearse et al. (2008) found that full-length APP inhibits the growth of the main axonal process by interacting with Integrin β1. In addition, Integrin β1 can modulate ERK2 activation (Reyes-Reyes et al., 2002). Our results suggest that APP-LRP binding, when blocked by RAP, acts to limit growth associated ERK2 signaling.
Based on the above-mentioned studies and our results we propose a model whereby full-length APP and LRP both modulate axon growth (Fig. 5). With regard to full-length APP, interactions between APP and Integrin β1 at the cell surface combined with intracellular interactions between Fe65, Mena, and APP serve to limit axon growth and branching (Fig. 5A). The addition of RAP to APP WT neurons decreased the length of the longest axonal process (Fig. 3B) as well as axonal branches (Fig. 2). This may be because RAP bound to LRP and prevented LRP binding to full-length APP, diminishing APP endocytosis and increasing cell-surface APP levels (Ulery et al., 2000, Conboy et al., 2005), thus amplifying APP inhibitory effects on neurite growth (Fig. 5B). Lack of full-length APP on APP KO neurons changed outgrowth in a manner that also supports a role for APP as an outgrowth inhibitory molecule. APP KO neurons had more robust outgrowth that was primarily associated with increased axon branching, likely attributable to the absence of full-length APP (Fig. 5C). No significant differences were seen for APP WT and APP KO neurons with regard to axonal branch lengths or the length of the main axonal process.
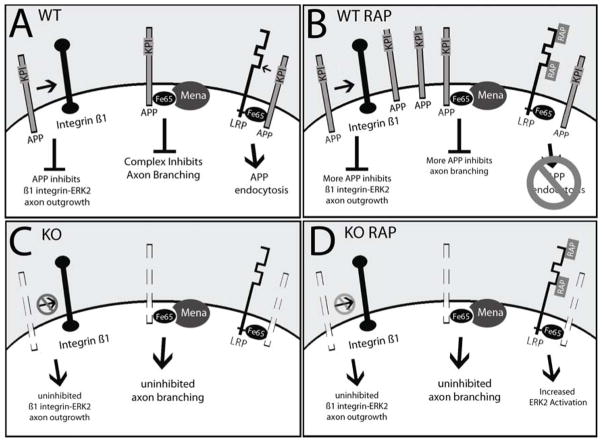
Figure 5. Hypothetical model for the effects of cell-surface full-length APP on axonal growth; RAP accentuation of APP inhibitory effects; and RAP-associated stimulation of APP KO axon growth in an APP-independent manner.
A. WT neurons without RAP. In APP WT neurons (WT), APP interacts with Integrin β1 at the cell surface, combined with intracellular interactions between Fe65, Mena, and APP, which together act to limit axonal growth and branching. Binding of APP to LRP stimulates endocytosis. B. WT neurons treated with RAP. RAP binds LRP preventing the APP-KPI domain from binding to LRP at the cell surface, which diminishes endocytosis, leaving cell surface APP to further amplify its inhibitory effects on axonal processes and axonal branches. Increased levels of full-length APP at the cell surface also allow more interaction with Integrin β1, and between Fe65, Mena, which further inhibit axon growth and branching. C. KO neurons without RAP. Absence of full-length APP allowed robust axon outgrowth that was primarily associated with increased axonal branching. D. KO neurons with RAP. When RAP binds LRP on neurons without APP, axonal branching is potentiated. No significant difference between APP WT and APP KO neurons was noted with regard to lengths of either the main axonal process or axonal branches. The dashed outlines in C and D represent the absence of APP in KO neurons.
We initially thought that administration of RAP to APP KO neurons might have no effect since there is no APP-LRP interaction to be blocked. Instead, we found that the administration of RAP to APP KO neurons resulted in increased axon outgrowth (Fig. 1), a tendency to increase axon branch numbers (Fig. 2) and an increase in branching off the main axonal process (Fig. 3A). Considering that APP is not present on APP KO neurons to interact with LRP, in that situation we might assume that an interaction between RAP and LRP initiated a signaling cascade that accentuated the growth of branches emanating from the main axonal process (Fig. 5D). In support of this, ERK2 is implicated in neurite outgrowth (Perron and Bixby, 1999, Wang et al., 2011) and we anticipated that higher levels of activated ERK2 may be present in APP KO neurons, which also had the most total outgrowth of axons and dendritic minor processes (Table 1). In fact, APP KO neurons exposed to RAP had significantly elevated ERK2 activity (Fig. 4), suggesting that a RAP-LRP interaction itself transduces ERK2 signaling in hippocampal neurons when full-length APP is missing (Fig. 5D). LRP is associated with signaling (Herz, 2001), and a study by Qiu et al. (2004) noted that RAP alone added to mouse cortical neurons stimulates ERK2, though their cells expressed APP and no increase in outgrowth occurred. Interestingly, we find that hippocampal and cortical neurons from APP KO mice display unique neurite outgrowth patterns (unpublished).
In conclusion, our data reveal that because APP plays such a major inhibitory role on neurite outgrowth, changes associated with AD pathology or therapies directed at APP, may tip the balance of APP in a manner to impair synaptic plasticity in hippocampus. It has long been appreciated that there is aberrant neurite growth in AD brain (Cotman et al., 1990, Masliah et al., 1991). Furthermore, levels of LRP decrease with age and a polymorphism in the LRP gene is linked to late onset AD (Kang et al., 1997). Additionally, as LRP and its ligands modulate Aβ synthesis and degradation, LRP is becoming an increasingly popular target for AD therapy (Vana et al., 2009, Zlokovic et al., 2010). Thus, obtaining a better understanding of normal APP and LRP functions in hippocampal neurite growth offers an opportunity for translating findings from the bench into new AD therapies.
Acknowledgments
We thank Bill Rebeck (Georgetown University) for RAP, Dominic Walsh (Harvard Medical School) for help purifying sAPPα, Merck Laboratories (Hui Zheng) for APP KO mice; Lupe Vidal, Ernesto Villanueva and Sesha Krishnamachari for critical reading of the manuscript. Funding was provided by the Alzheimer’s Association (to RGP) and the Alzheimer’s Disease Research Center at the University of Pittsburgh [NIH P50AG005133, Seed Award to RGP].
Abbreviations used
- Aβ
the 4 kDa peptide fragment of APP
- APP
Amyloid precursor protein
- sAPPα
secreted amyloid precursor protein cleaved by α-secretase
- sAPPβ
secreted amyloid precursor protein cleaved by β-secretase
- LRP
low density lipoprotein receptor related protein
- MEK2
map kinase kinase2
- RAP
Receptor associated protein
- apoE
apolipoprotein E
- α2m
α2macroglobulin
- ERK2
Extracellular regulated kinase 2
Footnotes
Author credits: RGP conceived of experiments and experimental design, prepared cultures, purified sAPPα, performed statistical analyses, supervised manuscript preparation, revised drafts for intellectual content, and had final approval of the submission. AJB performed literature searches, analyzed and interpreted data, prepared the figures, wrote the paper, and designed the hypothetical model. CY maintained cultures, performed immunostaining, did western blots, and worked on data acquisition; IB assisted with data acquisition.
The authors have no conflict of interest and declare that the funding agencies played no role in design, analysis, or interpretation of data.
References
- Bu G, Rennke S. Receptor-associated protein is a folding chaperone for low density lipoprotein receptor-related protein. J Biol Chem. 1996;271:22218–22224. doi: 10.1074/jbc.271.36.22218. [DOI] [PubMed] [Google Scholar]
- Chasseigneaux S, Dinc L, Rose C, Chabret C, Coulpier F, Topilko P, Mauger G, Allinquant B. Secreted amyloid precursor protein beta and secreted amyloid precursor protein alpha induce axon outgrowth in vitro through Egr1 signaling pathway. PLoS ONE. 2011;6:e16301. doi: 10.1371/journal.pone.0016301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy L, Murphy KJ, Regan CM. Amyloid precursor protein expression in the rat hippocampal dentate gyrus modulates during memory consolidation. J Neurochem. 2005;95:1677–1688. doi: 10.1111/j.1471-4159.2005.03484.x. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Geddes JW, Kahle JS. Axon sprouting in the rodent and Alzheimer’s disease brain: a reactivation of developmental mechanisms? Progress in brain research. 1990;83:427–434. doi: 10.1016/s0079-6123(08)61266-2. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O’Dowd G, Bowery BJ, Boyce S, Trumbauer ME, Chen HY, Van der Ploeg LH, Sirinathsinghji DJ. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. doi: 10.1016/s0306-4522(98)00410-2. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. Journal Of Cell Science. 2000;113 ( Pt 11):1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Annals of neurology. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G. Cellular and molecular neuroscience series. Cambridge, Mass: MIT Press; 1991. Culturing nerve cells/edited by Gary Banker and Kimberly Goslin; p. c1991. [Google Scholar]
- Gralle M, Ferreira ST. Structure and functions of the human amyloid precursor protein: the whole is more than the sum of its parts. Progress In Neurobiology. 2007;82:11–32. doi: 10.1016/j.pneurobio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Herz J. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001;29:571–581. doi: 10.1016/s0896-6273(01)00234-3. [DOI] [PubMed] [Google Scholar]
- Ho VM, Lee JA, Martin KC. The cell biology of synaptic plasticity. Science. 2011;334:623–628. doi: 10.1126/science.1209236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui Z, Koo EH. The amyloid precursor protein: beyond amyloid. Molecular Neurodegeneration. 2006;1:5–12. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui Z, Koo EH. Biology and pathophysiology of the amyloid precursor protein. Molecular Neurodegeneration. 2011;6:27–42. doi: 10.1186/1750-1326-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikin AF, Sabo SL, Lanier LM, Buxbaum JD. A macromolecular complex involving the amyloid precursor protein (APP) and the cytosolic adapter FE65 is a negative regulator of axon branching. Molecular and cellular neurosciences. 2007;35:57–63. doi: 10.1016/j.mcn.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, McNeill T, Cordell B, Finch CE. Relation of neuronal APP-751/APP-695 mRNA ratio and neuritic plaque density in Alzheimer’s disease. Science. 1990;248:854–857. doi: 10.1126/science.2111579. [DOI] [PubMed] [Google Scholar]
- Jordan J, Galindo MF, Miller RJ, Reardon CA, Getz GS, LaDu MJ. Isoform-specific effect of apolipoprotein E on cell survival and beta-amyloid-induced toxicity in rat hippocampal pyramidal neuronal cultures. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:195–204. doi: 10.1523/JNEUROSCI.18-01-00195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani F, Tanaka K, Ishii T, Ikeda S, Kennedy HE, Allsop D. Secretory form of Alzheimer amyloid precursor protein 695 in human brain lacks beta/A4 amyloid immunoreactivity. Biochemical And Biophysical Research Communications. 1993;191:392–398. doi: 10.1006/bbrc.1993.1230. [DOI] [PubMed] [Google Scholar]
- Kang DE, Saitoh T, Chen X, Xia Y, Masliah E, Hansen LA, Thomas RG, Thal LJ, Katzman R. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer’s disease. Neurology. 1997;49:56–61. doi: 10.1212/wnl.49.1.56. [DOI] [PubMed] [Google Scholar]
- Kimmel CB. Patterning the brain of the zebrafish embryo. Annual review of neuroscience. 1993;16:707–732. doi: 10.1146/annurev.ne.16.030193.003423. [DOI] [PubMed] [Google Scholar]
- Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, Tanzi RE, Hyman BT, Strickland DK. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- Li HL, Roch JM, Sundsmo M, Otero D, Sisodia S, Thomas R, Saitoh T. Defective neurite extension is caused by a mutation in amyloid beta/A4 (A beta) protein precursor found in familial Alzheimer’s disease. Journal of neurobiology. 1997;32:469–480. [PubMed] [Google Scholar]
- Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E, Cavanaugh JE, Leak RK, Perez RG, Zigmond MJ. Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells. J Neurosci Res. 2008;86:108–117. doi: 10.1002/jnr.21478. [DOI] [PubMed] [Google Scholar]
- Löffler J, Huber G. Beta-amyloid precursor protein isoforms in various rat brain regions and during brain development. Journal of neurochemistry. 1992;59:1316–1324. doi: 10.1111/j.1471-4159.1992.tb08443.x. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, Alford M, Albright T, DeTeresa R, Terry R, Baudier J, Saitoh T. Patterns of aberrant sprouting in Alzheimer’s disease. Neuron. 1991;6:729–739. doi: 10.1016/0896-6273(91)90170-5. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Cellular actions of...-Amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiological Reviews. 1997;77:1081. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ, Chen X, Stokin GB, Koo EH. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem. 1999;274:18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- Perez RG, Zheng H, Van der Ploeg LH, Koo EH. The beta-amyloid precursor protein of Alzheimer’s disease enhances neuron viability and modulates neuronal polarity. The Journal of neurosciences: the official journal of the Society for Neuroscience. 1997;17:9407–9414. doi: 10.1523/JNEUROSCI.17-24-09407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron JC, Bixby JL. Distinct neurite outgrowth signaling pathways converge on ERK activation. Molecular and cellular neurosciences. 1999;13:362–378. doi: 10.1006/mcne.1999.0753. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Hyman BT, Rebeck GW. Apolipoprotein E receptors mediate neurite outgrowth through activation of p44/42 mitogen-activated protein kinase in primary neurons. J Biol Chem. 2004;279:34948–34956. doi: 10.1074/jbc.M401055200. [DOI] [PubMed] [Google Scholar]
- Reyes-Reyes M, Mora N, Gonzalez G, Rosales C. Beta1 and beta2 integrins activate different signalling pathways in monocytes. The Biochemical journal. 2002;363:273–280. doi: 10.1042/0264-6021:3630273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, Herms J, Buchholz C, Eckman CB, Korte M, Wolfer DP, Müller UC. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. The Journal Of Neuroscience: The Official Journal Of The Society For Neuroscience. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, Morton RA, Zheng H, Dawson GR, Sirinathsinghji DJ, Davies CH, Collingridge GL, Hill RG. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology. 1999;38:349–359. doi: 10.1016/s0028-3908(98)00204-4. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Preventing Alzheimer’s Disease. Science. 2012;337:1488–1492. doi: 10.1126/science.1228541. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Yamazaki T, Citron M, Podlisny MB, Koo EH, Teplow DB, Haass C. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann N Y Acad Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. Making New Memories. Annals of the New York Academy of Sciences. 2007;1097:1–11. doi: 10.1196/annals.1379.007. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid Precursor Protein Trafficking, Processing, and Function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarte SD, Lin E, Klann E, Zigmond MJ, Perez RG. Effects of GDNF on 6-OHDA-induced death in a dopaminergic cell line: Modulation by inhibitors of PI3 kinase and MEK. Journal of neuroscience research. 2003;73:105–112. doi: 10.1002/jnr.10632. [DOI] [PubMed] [Google Scholar]
- Ulery PG, Beers J, Mikhailenko I, Tanzi RE, Rebeck GW, Hyman BT, Strickland DK. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer’s disease. J Biol Chem. 2000;275:7410–7415. doi: 10.1074/jbc.275.10.7410. [DOI] [PubMed] [Google Scholar]
- Van Uden E, Kang DE, Koo EH, Masliah E. LDL receptor-related protein (LRP) in Alzheimer’s disease: towards a unified theory of pathogenesis. Microscopy Research And Technique. 2000;50:268–272. doi: 10.1002/1097-0029(20000815)50:4<268::AID-JEMT3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Vana K, Zuber C, Pflanz H, Kolodziejczak D, Zemora G, Bergmann A-K, Weiss S. LRP/LR as an Alternative Promising Target in Therapy of Prion Diseases, Alzheimer’s Disease and Cancer. Infectious Disorders - Drug Targets. 2009;9:69–80. doi: 10.2174/1871526510909010069. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell and tissue research. 2011;345:1–19. doi: 10.1007/s00441-011-1196-4. [DOI] [PubMed] [Google Scholar]
- Wallace WC, Akar CA, Lyons WE. Amyloid precursor protein potentiates the neurotrophic activity of NGF. Brain Research Molecular Brain Research. 1997;52:201–212. doi: 10.1016/s0169-328x(97)00258-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang Z, Yao Y, Li J, Zhang X, Li C, Cheng Y, Ding G, Liu L, Ding Z. Essential role of ERK activation in neurite outgrowth induced by α-lipoic acid. BBA -Molecular Cell Research. 2011;1813:827–838. doi: 10.1016/j.bbamcr.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Willnow TE. Receptor-associated protein (RAP): a specialized chaperone for endocytic receptors. Biological chemistry. 1998;379:1025–1031. [PubMed] [Google Scholar]
- Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A Critical Function for beta-Amyloid Precursor Protein in Neuronal Migration Revealed by In Utero RNA Interference. J Neurosci. 2007;27:14459–14469. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Pearse TL, Chen AC, Chang R, Marquez C, Selkoe DJ. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural Dev. 2008;3:15. doi: 10.1186/1749-8104-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, Conner MW, Stevens KA, Slunt HH, Sisoda SS, Chen HY, Van der Ploeg LH. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain B. V. Zlokovic et al. LRP1: a key regulator of Aβ homeostasis. Journal of neurochemistry. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]