Abstract
Recent evidence suggests those with autism may be generally impaired in visual motion perception. To examine this, we investigated both coherent and biological motion processing in adolescents with autism employing both psychophysical and fMRI methods. Those with autism performed as well as matched controls during coherent motion perception but had significantly higher thresholds for biological motion perception. The autism group showed reduced posterior Superior Temporal Sulcus (pSTS), parietal and frontal activity during a biological motion task while showing similar levels of activity in MT+/V5 during both coherent and biological motion trials. Activity in MT+/V5 was predictive of individual coherent motion thresholds in both groups. Activity in dorsolateral prefrontal cortex (DLPFC) and pSTS was predictive of biological motion thresholds in control participants but not in those with autism. Notably, however, activity in DLPFC was negatively related to autism symptom severity. These results suggest that impairments in higher-order social or attentional networks may underlie visual motion deficits observed in autism.
Introduction
Recent papers exploring visual motion perception in people with autism spectrum disorders (ASDs) have re-energized the debate about possible visual processing impairments in those with autism (for reviews, see Dakin & Frith, 2005; Kaiser & Shiffrar, 2009; Simmons, Robertson, McKay, Toal, McAleer & Pollick, 2009). Autism itself is a neurodevelopmental disorder characterized by deficits in social understanding and behavior, delayed and/or impoverished verbal and non-verbal language skills as well as restricted and stereotyped interests and repetitive actions. Studies of visual motion perception in autism span different ages and different levels of the visual system. Flicker contrast sensitivity (Pellicano, Gibson, Maybery, Durkin & Badcock, 2005; Pellicano & Gibson, 2008; Bertone, Mottron, Jelenic & Faubert, 2003) and first-order motion perception (Bertone et al., 2003) have been reported as unimpaired while those with autism have shown deficits in second-order motion perception (Bertone et al., 2003). Reports on coherent motion perception, a task requiring spatial integration across time, have been mixed. Coherent motion (or ‘global’ motion) is the perception of average motion direction in a group of objects moving in varying directions – like snow flakes blowing in the wind or flocks of birds wheeling in the sky. Sensitivity to such global motion develops quite early, with children as young as 4 years of age showing motion coherence thresholds similar to those of adults (Parrish, Giaschi, Boden & Dougherty, 2005). While several groups have reported impairments for both adults and children with autism assessed on a global dot motion task (Pellicano et al., 2005; Milne, White, Campbell, Swettenham, Hansen & Ramus, 2006; Milne, Swettenham, Hansen, Campbell, Jeffries & Plaisted, 2002; Spencer, O’Brien, Riggs, Braddick, Atkinson & Wattam-Bell, 2000; Tsermentseli, O’Brien & Spencer, 2008), other groups have found no such impairment (White, Frith, Milne, Stuart, Swettenham & Ramus, 2006; Del Viva, Igliozzi, Tancredi & Brizzolara, 2006). In addition, two groups using quite different tests of coherent motion (plaid motion and motion signal detection in Gaussian noise) have also found no impairments in autism (Vandenbroucke, Scholte, van Engeland, Lamme & Kemner, 2008; Sanchez-Marin & Padilla-Medina, 2008).
Biological motion perception has also often been reported as impaired in people with autism (though see Murphy, Brady, Fitzgerald & Troje, 2009). Biological motion consists of characteristic human and animal body movements. While it is most intuitive to think of biological motion in terms of whole-body movement, it also includes facial affect changes, eye-gaze shifts, hand motions and speech movements and is thus one important source of information for understanding others in social settings (Zilbovicius, Meresse, Chabane, Brunelle, Samson & Boddaert, 2006). Biological motion is investigated using many different stimuli, from full-body, full-light movies, to individual body part movement, to still pictures that merely imply body movement. Johansson (1973) was the first to demonstrate sensitivity to the motion of human movement alone by showing that when only the joints of a walking human are illuminated, the movement of those dots of light is immediately recognizable as human. When static, such dots convey little information about figure shape; it is the characteristic motions of the dots that, when combined, prompt the visual system to group them as a single figure. Biological motion perception is considered a fundamental skill developed early in life. Babies as young as 3 months of age can distinguish between biological and non-biological motion shown in point-light displays (Fox & McDaniel, 1982) while toddlers as young as 3 years can recognize and identify different animals and movements from point-light-displays (Pavlova, Krageloh-Mann, Sokolov & Birbaumer, 2001). Sensitivity to biological motion in noise shows a more protracted development, with accuracy not reaching adult levels until after the age of 9 (Freire, Lewis, Maurer & Blake, 2006).
People with autism are typically unimpaired at simple point-light biological motion identification (Moore, Hobson & Lee, 1997) but are less accurate than controls when discriminating between biological and scrambled motion (Blake, Turner, Smoski, Pozdol & Stone, 2003; Freitag, Konrad, Häberlen, Kleser, von Gontard, Reith, Troje & Krick, 2008; Kaiser, Delmolino, Tanaka & Shiffrar, 2010). Even in studies where biological motion recognition was largely unimpaired, both children and adults with autism were less sensitive to more subtle/complex information in these displays, such as emotional content (Parron, Da Fonséca, Santos, Moore, Monfardini & Deruelle, 2008; Hubert, Wicker, Moore, Monfardini, Duverger, Da Fonséca & Deruelle, 2007; Atkinson, 2009). That biological motion perception impairments could contribute to the development of abnormal social cognition is supported by recent studies showing deficits in biological motion perception in children with autism as young as 15 months (Klin, Lin, Gorrindo, Ramsay & Jones, 2009; Klin & Jones, 2008). In addition, autism severity is correlated with deficits in biological motion perception (Blake et al., 2003). Brain activity differences have also been demonstrated in those with autism in response to both non point-light biological motion tasks (Pelphrey, Morris & McCarthy, 2005a; Pinkham, Hopfinger, Pelphrey, Piven & Penn, 2008; Castelli, Frith, Happé & Frith, 2002; Gervais, Belin, Boddaert, Leboyer, Coez, Sfaello, Barthélémy, Brunelle, Samson & Zilbovicius, 2004; Redcay, 2008) and point-light tasks (Herrington, Baron-Cohen, Wheelwright, Singh, Bullmore, Brammer & Williams, 2007; Freitag et al., 2008).
Several groups have proposed that a specific visual motion perception deficit in those with autism, reflected in both coherent and biological motion perception impairments, could indicate disruptions in brain regions in the dorsal stream of the visual system (Milne, Swettenham & Campbell, 2005; Spencer et al., 2000; Pellicano et al., 2005). Certainly, deficits in coherent motion processing could support a putative dorsal stream deficit, especially if functional differences were found in ‘dorsal’ area MT+/V5. As perception of coherent motion appears to be driven primarily by activity in MT+/V5 (Britten, Shadlen, Newsome & Movshon, 1992; Aspell, Tanskanen & Hurlbert, 2005; Culham, He, Dukelow & Verstraten, 2001), reductions in MT+/V5 activity would be expected to accompany coherent motion perception deficits. Biological motion perception is a ‘form-from-motion’ task and recruits both dorsal and ventral stream regions in fMRI experiments (Cowey & Vaina, 2000; Vaina, Solomon, Chowdhury, Sinha & Belliveau, 2001; Grossman & Blake, 2002). Deficits in biological motion perception could support a theory of dorsal stream dysfunction if MT+/V5 reductions co-occurred with activity reductions in right-hemisphere pSTS, an area particularly sensitive to biological motion (Pelphrey, Morris, Michelich, Allison & McCarthy, 2005b; Thompson, Clarke, Stewart & Puce, 2005; Grossman & Blake, 2002)
If dorsal stream dysfunction is a major source of visual motion perception deficits in autism, we expect to find impairments in both coherent and biological motion in our autism group and reductions in the function of brain areas along the dorsal pathway, most specifically in both area MT+/V5 and pSTS. Some initial support for this idea comes from previous fMRI studies investigating brain response in those with autism during both coherent motion perception (Brieber, Herpertz-Dahlmann, Fink, Kamp-Becker, Remschmidt & Konrad, 2010) and point-light biological motion perception (Freitag et al., 2008; Herrington et al., 2007) tasks. Brieber and colleagues found that adolescents with autism, in contrast to control participants, did not recruit area MT+/V5 more strongly for coherent motion than for incoherent motion. Activity in area MT+/V5 and bilateral pSTS (Herrington et al., 2007; Freitag et al., 2008) was reduced in those with autism in response to point-light biological motion stimuli. Our intention in the current study was to investigate global motion and biological motion tasks in the same cohort of participants using the same noise manipulation and utilizing both psychophysics and functional imaging (fMRI). Doing so allowed us to more fully characterize possible visual motion perception impairments and their neural correlates in those with autism. Further, we were able to assess if our findings were consistent with the theory of dorsal stream dysfunction in autism.
Methods
Participants
Participants included 16 typically developing (TD) adolescents (two female) and 16 adolescents (two female) with ASD (three participants had Asperger syndrome diagnoses, the remaining 13 had Autism diagnoses). All participants also participated in a previously reported psychophysical study (Koldewyn, Whitney & Rivera, 2010). Autism diagnosis was confirmed using the Autism Diagnostic Observation Schedule (ADOS; Lord, Risi, Lambrecht, Cook, Leventhal, DiLavore, Pickles & Rutter, 2000) for all participants with autism. The three participants with Asperger syndrome met full autism diagnosis criteria on the social subscale but not the communication subscale of the ADOS; all other participants with autism met full autism criteria. Six additional participants with autism diagnoses were initially recruited but later excluded because they either were unable to tolerate the scanning environment (three), or their in-scanner movement exceeded 3.4mm (three). Two additional TD adolescents completed the protocol but were later excluded because of excessive in-scanner movement. The two groups were matched on age, gender and non-verbal IQ as measured by the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) (see Table 1). All participants had normal or corrected-to-normal vision. Every participant signed an assent form and a parent or guardian signed an informed consent approved by the University of California at Davis Institutional Review Board.
Table 1.
Participant Information
| Measure | Control (n = 16)
|
Autism (n = 16)
|
T | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |||
| Performance IQ (WASI) | 112.6 | 11.35 | 99–140 | 106.7 | 12.04 | 82–128 | 1.43 | 0.164 |
| Verbal IQ* (WASI) | 120.3 | 11.26 | 103–141 | 112.3 | 21.5 | 77–143 | 1.32 | 0.146 |
| Full-Score IQ* (WASI) | 118.6 | 10.88 | 105–144 | 110.6 | 13.94 | 82–133 | 1.81 | 0.080 |
| Age | 15.6 | 2.32 | 11.90–19.72 | 15.4 | 2.81 | 11.41–19.53 | .220 | 0.828 |
| SCQ | 3.42 | 2.67 | 0–8 | 22.07 | 7.21 | 14–30 | −9.7 | <.001 |
| ADOS (social) | – | – | – | 8.63 | 2.72 | 5–14 | – | – |
| ADOS (communication) | – | – | – | 5.07 | 1.83 | 2–8 | – | – |
| ADOS (total) | – | – | – | 13.23 | 3.91 | 8–21 | – | – |
Missing data on two participants with autism and one control participant.
Apparatus and stimuli
Out of scanner psychophysics
All stimuli were presented on a 17-inch screen with a resolution of 1280 × 1024 pixels and a 50 Hz refresh rate. Presentation™ was used to present stimuli and collect participant responses. Biological and coherent motion stimuli were presented as 2-second video clips with a frame rate of 30 Hz. Participants were seated 60 cm from the screen and asked to keep their heads still but were given no explicit instructions on where to fixate. All psychophysical testing was completed in a single session (approximately 1.5 hours of testing), with breaks taken as needed. Each task was broken into two blocks of 120 trials for a total of 240 trials in each condition. Block order varied between individuals and was counterbalanced both within and between participant groups. Participants also completed one additional behavioral task whose data are included in a previous publication but will not be discussed here (Koldewyn et al., 2010).
Coherent motion stimuli
Coherent motion sensitivity was assessed through a Global Dot Motion task (Newsome & Pare, 1988) with 200 black dots (.1° visual angle in diameter) presented on a white screen in a rectangle (10.67° × 8.5° visual angle) centered on the screen. Display coherence was manipulated using a standard ‘random walk’ paradigm (example: Williams & Sekuler, 1984). Every dot in the display was given the same ‘intended’ direction (left or right). Between frames, each dot was independently given a new direction from a uniform probability distribution centered around the intended direction. We manipulated the probability of perceiving coherent motion by varying the range of the distribution of direction vectors. These ranges are most easily expressed in degrees. At 360°, no coherent direction can be perceived; dot movement is essentially Brownian. At 0°, no variance is allowed in any direction and all dots move in a straight line. All dots were assigned movement directions from the same distribution and no single dot gave more directional information than any other. To facilitate the use of the coherent motion display as a mask in the biological motion task, dots varied in speed (between 4.5° and 9° visual angle/sec) and the length of time between direction changes. Global (or mean) direction was leftward on 50% of trials and rightward on 50%. On each trial, participants were asked to indicate the direction of global motion and could answer at any time. Forty trials at each of the same six coherence levels (0°, 252°, 288°, 324°, 342°, 360°) were presented to all participants. Coherence levels between 252° and 360° were chosen to assess sensitivity in participants’ dynamic range while the 0° condition allowed for direct measurement of lapse rates (the rate of random errors made by participants – measured at the tail-end of the psychometric function). Participants completed 20 practice trials during which they were provided with feedback before the first experimental block. No feedback was provided during experimental trials.
Biological motion stimuli
Thresholds for biological motion perception were assessed by introducing noise into a standard point-light biological motion display of a walking human (Johansson, 1973). Each walker consisted of 13 points (black dots on a white background) placed on the major joints and head of a walking figure shown in profile. Original stimuli obtained through Vanrie and Verfaillie (2004) were manipulated for our use within Matlab. The ‘walker’ stayed in one location as though walking on a treadmill. Noise was introduced by superimposing the same stimuli utilized in the coherent motion task, described above, on the point-light displays. The walker remained constant across trials, except for direction of movement, while the coherence of the noise mask was manipulated at six difference levels (0°, 72°, 144°, 216°, 288°, 360°). The walker was presented in different positions on each trial within the middle 1/16th of the stimulus rectangle to reduce the likelihood of participants forming a location-specific ‘template’. In addition, each trial started at a random frame within the walk cycle. Each stimulus was presented for 2 seconds and participants reported the facing direction of the figure. Participants completed 40 trials in each of the six coherence levels. Prior to experimental blocks, participants were presented with 10 trials without noise to familiarize them with the walking figure. They then also completed 10 practice trials at the lowest noise level (360°), during which feedback was provided. During experimental trials, no feedback was given.
Threshold estimation
To obtain 75% thresholds for each task after data collection, a logistic function was fit to each individual participant’s data using the psignifit toolbox (version 2.5.6) for Matlab which utilizes the maximum-likelihood procedure described by Wichmann and Hill (Wichmann & Hill, 2001a; Wichmann & Hill, 2001b). To obtain the best fit for most participants on all tasks, lambda (1-lambda = ceiling performance) was allowed to vary but constrained to values between 0 and 0.05 and delta (chance performance) was set at 0.5. To estimate threshold, a bootstrapping technique was used which included 5000 replications for each fitted function (Wichmann & Hill, 2001b).
Scanning methods
Brain image acquisition
Images were acquired on a 3.0 Tesla Siemens Trio scanner using a standard Siemens whole-head coil. fMRI was performed using a gradient echoplanar imaging (EPI) sequence with TR 2000 ms, TE 25 ms, Flip angle 90 degrees, FOV 22 cm, 3.4 mm slice thickness, 64 × 64 matrix, and 34 axial slices resulting in a voxel size of 3.44 × 3.44 × 3.44 mm. A T1-weighted MPRAGE 3D MRI sequence was acquired in the same scan session. The functional task was programmed in Presentation™, projected to a screen at the participant’s feet and viewed with a head-coil mounted mirror.
Image preprocessing
Images were corrected for movement using least square minimization without higher-order corrections for spin history, then normalized to stereotaxic MNI (Montreal Neurological Institute) coordinates and re-sampled every 2 mm. Normalized images were smoothed using a 6 mm Gaussian kernel. All participants included in the data analysis moved less than 3.4 mm and the two groups did not differ in average displacement (t(31) = −1.27, p = .214) nor showed significant regions of differential residuals.
fMRI task design
Localization paradigms
To identify regions of interest (ROIs), two localizer scans were run. The first, designed to localize area MT+/V5, consisting of a 7.2-minute scan with 12 18-second blocks of coherently moving dots alternating with 12 blocks of static dots. Dots were .1° of visual angle in diameter and, when moving, had a constant speed of 8° visual angle/second. During motion blocks, dots changed direction every 2 seconds. To discourage eye-movements, participants performed a simple task at fixation detecting rare, subtle color changes in the fixation cross (black to dark gray). The second ‘localizer’ was generally designed to locate areas in the brain responsive to biological motion including right pSTS, right IPS, a region in right inferior frontal gyrus and a region in bilateral IT cortex. This 5.3 minute scan consisted of 10 20-sec blocks showing unobscured point-light walkers alternating with 10 blocks of simple fixation. During biological motion blocks, participants indicated the walker’s facing direction. During fixation blocks, participants indicated which bar of the fixation cross was darker in color (left or right) for each 2-second trial. It is important to note that the contrast between point-light biological motion and simple fixation is not as selective as the more standard contrast between point-light biological movement and scrambled point-light displays used in many previous studies (Grossman & Blake, 2001, 2002; Peuskens, Vanrie, Verfaillie & Orban, 2005; Saygin, Wilson, Hagler, Bates & Sereno, 2004). As previous papers (Herrington et al., 2007; Freitag et al., 2008) failed to find significant pSTS and DLPFC activity in response to point-light displays contrasted with scrambled motion in participants with autism, we chose this much more inclusive ‘localizer’ to ensure that we could locate cortex sensitive to biological motion if not selective for biological motion in every participant. A region that is selective must also be sensitive while the reverse is not necessarily true; our localizer is more inclusive and therefore less likely to exclude regions that are genuinely important for biological motion perception in those with autism (i.e. less likely to suffer from a type 1 error). As a result, our biological motion ROIs cannot and are not intended to be considered as specific only to biological motion perception, though voxels selectively responsive to coherent motion in the MT+/V5 localizer scan were excluded from biological motion sensitive ROIs.
Experimental paradigm
The in-scan task was very similar to that performed during the out-of-scanner psychophysics session. In the scanner, both coherent motion and biological motion trials were presented in a single run. To assess how response in our ROIs changed as motion coherence changed for each task, scanning runs were blocked by both motion coherence level and task. The stimuli used were the same as those presented outside the scanner, but were presented for only 1.5 seconds to allow brief breaks between trials. Blocks were 20 seconds long and consisted of 10 trials. Participants could indicate their answer at any time during a trial or between trials. Data were collected in three 6.4-minute runs. Each run contained two blocks of each task and coherence level, two blocks of a static dot field and two blocks of simple fixation. Four different coherence levels were presented for coherent motion trials (0°, 108°, 324° and 360°) and three different coherence levels were presented for biological motion trials (0°, 324°, 360°). The number of coherence levels was restricted in this way to allow a parametric design while still collecting adequate data in each condition. Three levels in the coherent motion tasks were matched to the coherence level of the noise mask in biological motion trials. As MT+/V5 response might already be saturated at 324°, we included the fourth coherence level (108°) to be within what we anticipated would be MT+/V5’s dynamic range. Participants received brief one-word instructions (‘person’, ‘dots’, ‘rest’) indicating which task to perform before the initiation of each block. They indicated the direction of either dot or figure motion with a button press. During simple fixation and static dot field blocks, participants were instructed to simply ‘rest’ and keep their eyes focused on the center of the screen. All participants completed the psychophysics portion of the study before being scanned and also completed a brief practice session to become familiar with the task timing and instructions. The practice session included two blocks each of the coherent and biological motion tasks at the easiest level. All participants could also practice lying still using a mock-scanner that simulated both the sounds and feeling of being inside a real MRI scanner. When utilized, mock-scanner practice sessions usually lasted approximately 30 minutes.
fMRI analysis
Statistical analysis was performed on both individual and group data using the modified General Linear Model and the theory of Gaussian random fields as implemented in SPM5 (Friston, Holmes, Worsley, Poline, Frith & Frackowiak, 1995) with one predictor (convolved with a standard canonical hemodynamic response function) for each condition. All effects of interest were modeled using a standard within-subjects procedure for each participant by contrasting blocks of one condition vs. another (e.g. coherent motion vs. still frames). Regressors were also included to account for differences in global signal across scanning runs. Within-group analyses were performed to identify voxels and brain regions showing similar response modulation across participants in each group for a given contrast (e.g. biological motion trials–coherent motion trials). In addition, between-group analyses were performed to determine how the two groups differed in their average activation in response to each contrast of interest. We identified brain activations showing significant contrasts of parameter estimates with a voxel-wise (t = 2.95, p < .005, uncorrected) and cluster-wise (p < .05, corrected) significance threshold. All reported activations survived Bonferroni correction for multiple comparisons at the cluster level. Once subjected to threshold analysis, the activation was superimposed on a group-average MPRAGE image and localized manually (Duvernoy & Bourgouin, 1999).
>Region of interest definition
ROIs were defined separately for each individual using unsmoothed, unnormalized functional images. We used unsmoothed images to minimize overlap between proximal ROIs and unnormalized functional images to minimize the possibility that distortions inherently induced by standard normalization procedures would differentially affect our two groups.
MT+/V5 was defined functionally as described above and chosen as a strongly activated cluster near the temporal-occipital junction (typically in the ascending limb of the inferior temporal sulcus). Right pSTS, right IPS, right DLPFC and bilateral IT regions were defined using a contrast between point-light biological motion and simple fixation. All ROIs defined using this contrast were additionally constrained to exclude voxels that were significantly active in the motion–static contrast of the MT+/V5 localizer, although this constraint was primarily relevant only to the rIPS and rpSTS regions. Right pSTS was defined as the cluster of highest activation within anatomical STS, rIPS was defined as the cluster of highest activation within or on the bank of the IPS, right DLPFC was defined as the highest cluster of activity on or proximal to the inferior frontal gyrus. Activity was cluster-corrected for multiple comparisons at p < .01 and a height-threshold of at least 20 voxels. For most regions in most participants, ROI choice for each of these areas was unambiguous. While different ROIs were of varying sizes, all defined ROIs were at least 50 voxels in size. These clusters of significant activation were then combined with 10 mm radius sphere centered on the most active voxel in the functional cluster, thus limiting the final ROIs to significantly activated voxels within that sphere. If ROIs were close to each other, any overlapping voxels were assigned to one ROI or another based on their relative proximity.
Region of interest analyses
Region of interest analyses were carried out on individuals’ unnormalized functional images using Marsbar (Brett, Anton, Valabregue & Pauline, 2002). Contrasts were first defined during separate localizer scans as described above and then analyzed only in voxels that fell within a defined ROI. All ROI analyses carried out on data from the main experiment were done so only in ROIs defined in separate localizer scans and thus are based solely on independent data (Vul 2010). A t-statistic term was calculated for each ROI as the mean of all voxel t-values within the defined ROI for each contrast and then used in between-group and correlational analyses.
Results
Psychophysical experiment outside the scanner
There was no significant difference between groups’ mean 75% threshold (see Methods) for coherent motion perception either for sessions inside the scanner (control M = 264.71°, autism M = 247.46°; t(30) = .967, p = .17) or outside (control M = 305.75°, autism M = 279.94°; t(30) = 1.693, p = .10). Mean 75% thresholds for biological motion, however, were different between groups both during sessions inside the scanner (control M = 175.32°, autism M = 97.92°; t(30) = 2.944, p = .006) and outside (control M = 168.19°, autism M = 92.48°; t(30) = 2.244, p = .032). Although the two groups did not differ significantly in IQ, the IQ range of the autism group was lower than that of the control group. To ensure that IQ range differences were not affecting our results, an AN-COVA was performed with 75% threshold entered as the dependent measure, group as a fixed factor and IQ as a covariate. Group did not emerge as a significant factor (F[1, 26] = .130, p = .721) for 75% thresholds in the coherent motion task and remained a significant factor for the biological motion task (F[1, 26] = 5.019, p = .034). Inter-subject variability was high within the autism group for the coherent motion task and in both groups for the biological motion task (Figure 1). Performance on the coherent motion task was correlated with performance on the biological motion task in the autism group (r = .797, p = .001) but not the control group (r = .350, p = .201). This relationship remains unchanged when IQ is accounted for in both autism (r = .703, p = .011) and control (r = .396, p = .202) groups. This autism-specific relationship between dynamic tasks echoes, unsurprisingly, the same relationship previously reported in a larger group of which the current sample is a subset (Koldewyn et al., 2010). As out-of-scanner sessions gathered data at more motion coherence levels, providing more data on which to fit a psychometric function, the threshold estimate from the out-of-scanner data is likely more accurate. For that reason, out-of-scanner thresholds were used for all additional analyses involving threshold. The in- and out-of-scanner threshold values were strongly correlated with each other both across and within groups. Across the whole group (n = 32) the correlation between coherent motion thresholds in and outside of the scanner was highly significant (r = .647, p < .001) and that for biological motion was even stronger (r = .858, p < .001).
Figure 1.
75% thresholds (from out-of-scanner psychophysics) for coherent motion (a) and biological motion (b) perception showing individual subject performance. Higher 75% thresholds indicate better performance (individuals need less coherent stimuli to perform at 75%).
Neuroimaging results
Between-group fMRI analysis
Across the whole brain, the two groups showed highly similar brain activity in response to all coherent motion perception trials compared to fields of static dots. Indeed, there were no areas showing significant difference between groups. This remained true when the same contrast was computed for each coherence level separately.
When assessing whole-brain function during all biological motion perception trials compared to brain activity during coherent motion perception trials (where the coherence of the noise-mask in biological motion trials matched the motion coherence during coherent motion trials), the control group showed greater activity than those with autism in several regions. The most notable of these areas included: a large area in bilateral parietal cortex, primarily along the IPS; right DLPFC centered in the inferior frontal gyrus; a cluster in anterior cingulate and a region in rpSTS (see Table 2, Figure 2a). The autism group, by contrast, showed only a single area of greater activity when compared to the control group: an area in bilateral inferior temporal (IT) cortex, including cortex in both lateral occipital gyrus and fusiform gyrus (Table 2, Figure 2b). When this pattern was assessed qualitatively at each coherence level, the same pattern was present at each level but was strongest when the noise mask was least coherent, becoming slightly weaker as noise-mask coherence increased.
Table 2.
Stereotaxic locations and Z-scores of activation peaks in the between-group maps. All activity reported was significant at the p < .05 level, corrected for multiple comparisons at the cluster level
| Biological–Coherent (all coherence levels combined) | ||||||
|---|---|---|---|---|---|---|
| Group contrast | Areas | # of voxels in cluster | Z Max | Peak coordinates | ||
| Control>Autism | R Insula, Bilateral Caudate, Bilateral Pulvinar | 5495 | 4.65 | 30 | 18 | 0 |
| R Intraparietal Sulcus, R Angular Gyrus, R Superior Temporal Sulcus | 2042 | 4.39 | 14 | −62 | 50 | |
| R Inf Frontal Sulcus, R Middle frontal gyrus, R Inf Frontal Gyrus | 2287 | 4.11 | 30 | 34 | 30 | |
| L Intraparietal Sulcus, L Angular Gyrus, L Superior Temporal Sulcus | 785 | 3.87 | −40 | −58 | 46 | |
| Anterior Cingulate Sulcus & Gyrus | 1208 | 3.67 | 4 | 38 | 16 | |
| Autism>Control | R Inf Temporal Gyrus, R Inf Occipital Gyrus | 2110 | 4.26 | 42 | −76 | −4 |
| R Inf Temporal Gyrus R Inf Occipital Gyrus | 3215 | 4.17 | −40 | −78 | −4 | |
Figure 2.
(a) Regions of greater activation in control participants than those with autism in response to biological motion trials when compared to coherent trials rendered on the surface of a MNI-normalized template image. Activation here is significant at the p < .01 level corrected for multiple comparisons at the cluster level (cluster threshold = 500 voxels).(b) The same contrast, here showing areas more active in those with autism than controls rendered on a single axial slice of a normalized, averaged group brain. Activation is significant at the p < .05 level corrected for multiple comparisons at the cluster level (cluster threshold = 200 voxels).
ROI analyses
MT+/V5 results
A repeated measures ANOVA with coherence level (0°, 108°, 324°, 360°) as a within subjects factor revealed that activity in bilateral MT+/V5 during coherent motion trials decreased significantly across coherence level for both the control (F(3, 45) = 11.477, p < .001) and autism (F(3, 45) = 8.678, p < .001) groups. When data from the two groups were combined and the same repeated measures ANOVA was run with group (ASD, TD) as a between subjects factor, no mean effect of group was observed. This suggests that the two groups did not differ in the degree of change in MT+/V5 activity in response to changes in motion coherence. Not surprisingly, the degree to which MT+ response was modulated by variations in motion coherence was significantly correlated with participant performance (75% threshold for coherent motion discrimination) in both the control (roh = −.650, p = .009) and autism groups (roh = −.643, p = .018) and remained significantly correlated when possible effects of IQ were partialed out in both control (r = .635, p = .027) and autism groups (r = .671, p = .017). In other words, how responsive MT+/V5 was to changes in motion coherence was directly related to how well individual participants were able to perform in both groups. MT+ function in response to coherent motion trials was virtually identical between groups, both in response ‘level’ and how MT+ response related to both changes in stimulus motion coherence and participant performance.
Overall MT+ response to biological motion trials (contrasted with static dots) did not differ between groups. In addition, MT+ response did not change as noise-mask coherence increased. This pattern of response (while participants attended the biological motion figure) was in marked contrast to that seen during coherent motion trials (when participants attended the whole field of dots). A repeated measures ANOVA with coherence level as a within-subjects factor showed that MT+/V5 activity did not change significantly across noise-mask coherence levels within the control group (F(2, 30) = 1.369, p = .537) and increased only slightly, though consistently across subjects, within the autism group (F(2, 30) = 8.947, p = .001). This increase was minimal and thus may not reflect anything of true neural meaning. Alternatively, it could reflect incomplete ‘filtering’ of the coherent motion ‘noise’ by those with autism. Despite this possible between-groups difference, change in MT function in response to motion coherence changes during biological motion trials did not predict subject performance in either the control (rho = .112, p = .68; controlling for IQ: r = .172, p = .575) or autism groups (roh = −.291, p = .274; controlling for IQ: r = −.206, p = .462). While MT+ function at least partially drives participant performance during motion coherence trials it does not appear to directly influence performance during biological motion trials (Figure 3).
Figure 3.
(a) Correlation between the change in MT response across different coherence levels (slope) and participant performance (measured as 75% threshold of motion detection) during coherent motion perception. To calculate the change of MT response changes across coherence, we fitted a line to the mean MT+/V5 response at each coherence level and used the slope of this line as our measure of change in MT+ response across coherence level. (b) The same correlation shown for data collected in response to biological motion trials.
Parietal results
Parietal response did not differ between groups during coherent motion trials and was not related to coherent motion perception in either group. While Parietal ROI analysis confirmed that TD individuals showed greater activity within parietal cortex than those with autism in response to combined biological motion perception trials (t(30) = 2.36, p = .02), activity in parietal cortex was not correlated with participant performance for either the control (rho = .3, p = .277) or the autism (rho = .075, p = .791) group and differences in parietal recruitment were not predictive of autism symptomatology (with ADOS total score: rho = .117, p = .679). Although parietal activity differed between groups during biological motion trials, that difference is not clearly related to either between-group performance differences or autism symptomatology.
pSTS results
Response in pSTS did not differ between groups during coherent motion trials and was not related to coherent motion perception in either group. Contrary to our expectations, pSTS activity did not increase as coherence of the mask increased and the walker was more readily seen. Instead, the pSTS maintained a significant but steady level of activity at every coherence level. STS averaged across all trials when the biological motion figure was present predicted participant performance in the control group (roh = .665, p = .005; controlling for IQ: r = .669, p = .012) but did not do so in the autism group (roh = .232, p = .387; controlling for IQ: r = .115, p = .683) (see Figure 4a). Control participants who recruited this biological motion sensitive region more strongly had correspondingly lower biological motion perception thresholds, but activity in the pSTS in those with autism did not show an obvious relationship with biological motion perception ability.
Figure 4.
Activity averaged across all biological motion trials compared to coherent motion trials (a) in the pSTS and (b) in the DLPFC correlated with participant performance as measured by 75% threshold on the biological motion perception task. Activity is measured as the mean t-value of all voxels in a particular ROI in response to the contrast of interest.
DLPFC results
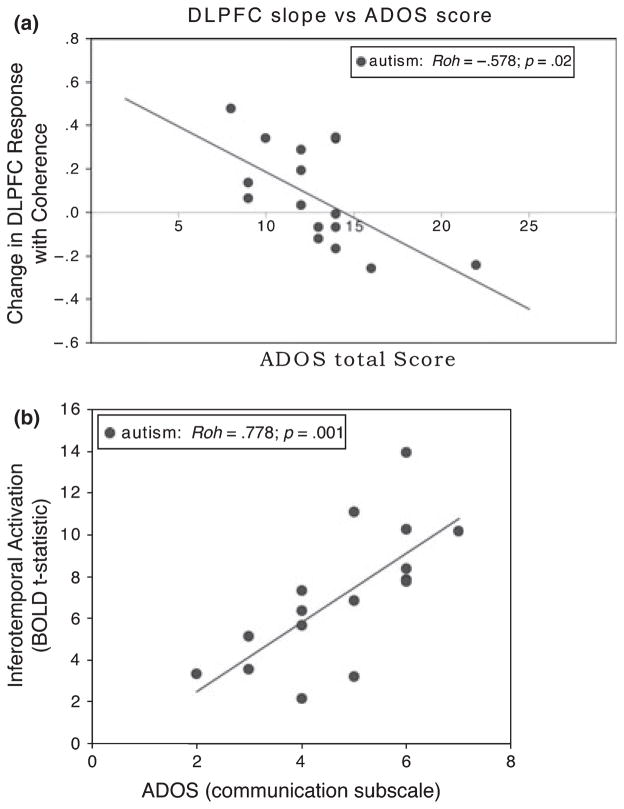
ROIs defined within DLPFC were primarily within inferior frontal gyrus. The exceptions were two participants with autism and one typical control whose DLPFC ROIs were centered in the superior frontal gyrus. As DLPFC regions were not typically recruited during coherent motion trials, DLPFC ROIs were only assessed during biological motion trials. Activity in DLPFC ROIs increased with increasing noise-mask for many participants in both groups. This change across coherence levels was significant for the control group (F(2, 30) = 3.763, p = .035) but not for the autism group (F(2, 30) = 1.475, p = .245). Despite this, the sensitivity of DLPFC cortex to changes in motion coherence (the degree to which DLPFC response changed with changes in motion coherence and/or visibility of the biological motion figure) was correlated with autism symptomatology as measured by the ADOS (rho = −.578, p = .02; controlling for IQ: r = −.583, p = .023) (Figure 5a). The more DLPFC activity increased with motion coherence increases, the lower the score on the ADOS. Additionally, DLPFC activity averaged across all biological motion trials was predictive of performance for the control group (rho = .683, p = .004; controlling for IQ: r = .574, p = .040) but, much like the relationship seen in pSTS, not for the autism group (rho = .309, p = .244; r = .293, p = .288) (Figure 4b). DLPFC activity thus appears to be related both to biological motion perception in typical individuals and to autism symptomatology within the autism group.
Figure 5.
(a) Correlation between change in DLPFC recruitment across coherence levels and ADOS total score. Change in DLPFC recruitment was assessed by fitting a line to the mean DLPFC response at each coherence level and using the slope of that line as our measure of change. (b) Activity in IT (mean t-statistic within the defined ROI) averaged across all biological motion trials correlated with the communication subscale of the ADOS.
IT results
During biological motion trials, activity in IT generally increased with noise-mask coherence as the figure became more distinguishable. This change across coherence level was significant for both control (F(2, 30) = 4.647, p = .017) and autism (F(2, 30) = 6.557, p = .004) groups. Neither change across coherence level nor average activity (across all biological motion trials) correlated with subject performance for either group. Intriguingly, despite the lack of correlation between activity and perceptual ability, there was a positive correlation between autism symptom severity and average activity in this region. This relationship is significant for the ADOS total score (rho = .564, p = .023; controlling for IQ: r = .564, p = .029) but is particularly strong for the communication subscale (rho = .778, p < .001; controlling for IQ: r = .803, p < .001) (Figure 5b).
Discussion
We approached the question of visual motion deficits in autism from a systems neuroscience perspective, hypothesizing that we would see activity differences in areas along the dorsal stream. Although there was a non-significant trend toward lower performance on the coherent motion task for the autism group, we did not replicate some earlier reports of a coherent motion processing deficit in those with autism (Pellicano et al., 2005; Pellicano & Gibson, 2008; Spencer et al., 2000; Tsermentseli et al., 2008). Instead, our results are more consistent with reports suggesting that coherent motion perception is not strongly impaired in those with autism (White et al., 2006; Del Viva et al., 2006; Vandenbroucke et al., 2008; Sanchez-Marin & Padilla-Medina, 2008). The large inter-subject variability in coherent motion thresholds seen in the autism group has also been observed in previous studies (Pellicano & Gibson, 2008; Milne et al., 2005) and could indicate that either only a subset of those with autism show a deficit in visual motion or that a higher-level process is the main deficit. In a larger sample of participants, of which those in this study are a subset, only 34% of participants performed at worse than one standard deviation from the control mean on the coherent motion tasks while 56% did so on the biological motion task (Koldewyn et al., 2010). Such observations underscore the need to approach studies in autism with an eye towards individual differences and potential sub-typing. In contrast to the coherent motion results, clear performance deficits on biological motion perception were seen in participants with autism.
The fMRI results from the coherent motion perception task support our psychophysical findings as the two groups showed no significant differences in neural recruitment in the whole-brain analysis. Both groups recruited area MT+/V5 during the task but did not differ significantly in MT+/V5 activity at any coherence level. Moreover, activity change across coherence level in area MT+/V5 was predictive of coherent motion perception performance for participants in both groups. These findings contrast somewhat with both a study of point-light biological motion perception in autism (Herrington et al., 2007) and a recent report on coherent motion perception in autism (Brieber et al., 2010). Herrington and colleagues reported between-group differences in a large cluster which included both putative MT+ (near previously reported coordinates) and pSTS during biological motion compared with fixation but did not document group differences when assessing activity directly comparing point-light figures with ‘randomized walkers’. It is difficult to assess differences between this study and the current findings both because there are substantial task differences and because, in contrast to the current paper, Herrington et al. did not take an ROI approach in assessing either MT+/V5 or STS activity. In addition, both MT+ and STS differences were part of two large clusters of activity spanning regions from the cerebellum to parietal cortex bilaterally, making it difficult to make region-specific conclusions. While Brieber and colleagues, much like the current study, did not document direct differences between groups in MT+/V5 activity in a whole-brain analysis, in an ROI analysis they found that those with autism did not activate MT+/V5 more during perception of coherent motion compared to incoherent motion. While there are also stimulus and task differences between the two studies, the choice of MT+/V5 definition is the most likely source of difference between Brieber et al.’s findings and the current study. Brieber and colleagues used an anatomically defined probability map of MT+/V5 (from an SPM toolbox) in normalized group space, while the current study used individual-specific ROIs that were functionally defined in a separate run in unnormalized native space. It was therefore more likely that the current study had already limited the between-group ROI analysis to coherent-motion sensitive cortex. Results from the current study suggest that MT+ function is unaffected in at least high-functioning adolescents with autism but such between-study differences may indicate that more in-depth study of MT+/V5 function in those with autism may be warranted.
In contrast to the results from the coherent motion task, areas of the brain sensitive to biological motion showed significant neural recruitment differences between groups during biological motion trials. The TD group showed much more activity in cortex along the IPS, DLPFC and pSTS than did the autism group, while the autism group showed greater activity in IT cortex. In agreement with numerous other studies, however, both groups activated a network of areas including IPS, inferior frontal gyrus, pSTS and anterior cingulate in response to biological motion trials (Grossman, Donnelly, Price, Pickens, Morgan, Neighbor & Blake, 2000; Grossman & Blake, 2002; Grossman, Blake & Kim, 2004; Vaina et al., 2001). Thus, while activity in all these areas was significantly lower in the autism group as a whole, they appeared to utilize the same basic network of areas to process biological motion as TD individuals.
Activity in both DLPFC and the pSTS was predictive of individual participant performance during biological motion perception in the control group but not the autism group. This finding supports previous work documenting not only that DLPFC and pSTS are activated by point-light displays (Saygin et al., 2004) but also that both areas are necessary for intact biological motion perception (Saygin, 2007). As the current findings do not include a causal network analysis, they cannot directly address the question of whether deficits in pSTS or DLPFC function might be most related to biological motion perception deficits in the autism group. Consistent with the idea that functional differences in the pSTS region could be primary to biological motion and social cognition impairments in people with autism, earlier neuroimaging studies have shown hypoactivation in the pSTS in response to a variety of tasks, including point-light displays (Pelphrey et al., 2005a; Pinkham et al., 2008; Herrington et al., 2007; Castelli et al., 2002; Gervais et al., 2004; Redcay, 2008; Freitag et al., 2008). Consistent with the idea that deficits in DLPFC function could be driving biological motion perception deficits in the autism group, DLPFC function correlates negatively with ADOS score. Alternatively, the deficit could lie in the connectivity between these two areas, similar to previously reported reductions in connectivity between prefrontal and sensory areas (Just, Cherkassky, Keller, Kana & Minshew, 2007; Cherkassky, Kana, Keller & Just, 2006). Unless particularly severe, such a failure of communication might only mildly affect less ‘complex’ tasks, like coherent motion perception, but would show greater effects on tasks requiring top-down attentional and/or cognitive control.
The autism group showed greater activity than the control group in IT cortex. Despite this, IT activity did not predict individual participant performance in either group, yet activity in the IT ROI was positively correlated with autism symptom severity within the autism group. The absence of a relationship between IT recruitment and perceptual ability in either group makes it difficult to assess the implications of this correlation. While it is possible that increased IT recruitment within the autism group reflects a higher reliance on object perception areas in ventral visual cortex, it is equally possible that the IT findings may not be task specific but might be seen across tasks that recruit this region. If so, assessing IT function will be important to pursue in future studies of visual processing in those with autism.
One complicating aspect of this study is that our biological motion task involved more than simple biological motion discrimination. Unlike the coherent motion task, changes in coherence during biological motion trials were not changes in the signal on which participants based their response. Instead, coherence changes affected the masking noise obscuring the biological motion signal. Our task thus required not only integration of the walker ‘signal’ dots into a coherent moving form, but also the segmentation of that form from dynamic noise dots. Deficits on this task could reflect the autism group struggling with segmentation rather than biological motion perception. As such, although the current data are suggestive of a biological motion deficit, they cannot rule out that similar deficits might be observed in other dynamic tasks that require segmentation. One recent study made exactly this comparison, using both point-light walkers and an articulated but non-biological point-light object (a tractor) (Kaiser et al., 2010). Although this paper did not directly compare groups, subjects with autism did not show the typical advantage for biological stimuli but instead showed similar performance on both biological and non-biological tasks. In addition, though assessed only in a small group of those with ASD, their results suggest that noise-masking may not affect the performance of those with autism in the same way as it affects TD children. If this holds true, their data would support the idea that our findings reflect a deficit in biological motion perception itself rather than a more general deficit in segmentation ability.
The biological motion task in the current study also conflated the facing direction of the point-light figure with walking direction. There is some evidence that spatial configuration form cues are sufficient to determine facing direction while biological motion direction (e.g. actual direction of walking motion) requires the fully intact spatio-temporal pattern (Lange & Lappe, 2007; Vangeneugden, Vancleef, Jaeggli, VanGool & Vogels, 2010). In addition, it is known that facing direction can influence judgments of actual biological motion direction (Pavlova, Krägeloh-Mann, Birbaumer & Sokolov, 2002; Verfaillie, 2000). The task used in the current study does not allow us to separately assess the influence of form and dynamic cues on biological motion perception in those with autism nor assess whether facing direction influences the perception of motion direction to the same extent in those with autism as it does TD individuals. While general form perception has been reported to be relatively intact in those with autism (Spencer et al., 2000; Milne et al., 2002; Milne et al., 2006; Blake et al., 2003; Koldewyn et al., 2010; Tsermentseli et al., 2008), it may prove to be important to assess these two types of cues separately to better understand the source of biological motion perception deficits in those with autism.
Our results are not supportive of general dorsal stream impairment in those with autism which would predict significant performance deficits in both coherent and biological motion perception tasks and marked between-group activity difference in MT+/V5 – neither of which are supported by our data. Our findings could support the theory primarily put forward by Just and colleagues of autism as an ‘underconnectivity’ syndrome (Just et al., 2007) as the DLPFC, IPS and STS finding could be consistent with such a theory. Our experimental design, however, did not allow for a network analysis and we could not directly test connectivity differences between groups. Alternatively, the social nature of biological motion perception could mean that those with autism are impaired on this task principally because of their well-documented deficits in many aspects of social cognition. Even perception of simple point-light walkers in a noise mask is, after all, a social perception task. Such an interpretation of the data would support ‘social brain’ theories of autism (Schultz, Gauthier, Klin, Fulbright, Anderson, Volkmar, Skudlarski, Lacadie, Cohen & Gore, 2000) and predict that visual perception differences in those with autism are inextricably linked to their deficits in social cognition. Autism-specific correlations between coherent motion and biological motion perception reported previously both in a larger group that participated only in psychophysical testing (Koldewyn et al., 2010) and in an experiment where the biological motion task involved emotional-attribution (Atkinson, 2009) suggest that social cognition deficits cannot be a complete explanation. Such correlations suggest that the dynamic nature of the biological motion stimuli may also be a contributing factor.
One dynamic factor that could help to explain our findings is the possibility that those with autism could have a deficit in dynamic attention. Dynamic attention gates the perception of certain types of visual motion and form-from-motion information, including motion in noise and motion in ambiguous displays. It is not the same as generalized attention or synonymous with static spatial attention. Simple perception of a biological motion figure requires allocation of dynamic attentional resources (Battelli, Cavanagh & Thornton, 2003; Cavanagh, Labianca & Thornton, 2001). Our task, which in addition requires the segmentation of a moving form from moving noise, would require still more (Thornton, Rensink & Shiffrar, 2002). A dynamic attention deficit would be consistent with the confusing mix of findings in various motion perception tasks reported in those with autism. That a dynamic attention deficit would only mildly affect coherent motion perception might also help to explain the contradictory results reported in the literature; differences in subject cohort, group size and task difficulty could easily push results into or out of significance when a deficit only mildly affects task performance. Although our data are consistent with this idea, the current study was not designed to test it and our data cannot substantiate it. Further research that manipulates dynamic attentional resources during biological motion perception and tasks that more directly measure dynamic attention in those with autism would help to clarify these issues.
The results presented here provide direct evidence that activity in early dorsal stream areas cannot explain either coherent motion or biological motion perception deficits in those with autism. Our findings instead implicate a network of areas including DLPFC, IPS and pSTS. Our data generally support the literature on biological motion deficits within those with autism and raises the possibility that, rather than being indicative of a dorsal stream deficit, visual motion processing deficits in those with autism may be the result of higher-order deficits in social cognition and dynamic attention.
Acknowledgments
We are grateful to the research participants and their families; to Ken Britten for his constructive criticism and assistance with stimulus creation, to Chris Bishop and Lee Miller for the use of their MT localizer script and to Tom Kiely for his help with participant recruitment. Funding from Autism Speaks through a Pre-doctoral Mentored Research Award (SMR) supported this work.
References
- Aspell JE, Tanskanen T, Hurlbert AC. Neuromagnetic correlates of visual motion coherence. European Journal of Neuroscience. 2005;22 (11):2937–2945. doi: 10.1111/j.1460-9568.2005.04473.x. [DOI] [PubMed] [Google Scholar]
- Atkinson AP. Impaired recognition of emotions from body movements is associated with elevated motion coherence thresholds in autism spectrum disorders. Neuropsychologia. 2009;47 (13):3023–3029. doi: 10.1016/j.neuropsychologia.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Thornton IM. Perception of biological motion in parietal patients. Neuropsychologia. 2003;41(13):1808–1816. doi: 10.1016/s0028-3932(03)00182-9. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: a ‘complex’ issue. Journal of Cognitive Neuroscience. 2003;15 (2):218–225. doi: 10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychological Science. 2003;14 (2):151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Pauline JB. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16 (2):S497. [Google Scholar]
- Brieber S, Herpertz-Dahlmann B, Fink GR, Kamp-Becker I, Remschmidt H, Konrad K. Coherent motion processing in autism spectrum disorder (ASD): an fMRI study. Neuropsychologia. 2010;48 (6):1644–1651. doi: 10.1016/j.neuropsychologia.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. Journal of Neuroscience. 1992;12 (12):4745–4767. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125 (Pt 8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Labianca AT, Thornton IM. Attention-based visual routines: sprites. Cognition. 2001;80 (1–2):47–60. doi: 10.1016/s0010-0277(00)00153-0. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. NeuroReport. 2006;17 (16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Cowey A, Vaina LM. Blindness to form from motion despite intact static form perception and motion detection. Neuropsychologia. 2000;38 (5):566–578. doi: 10.1016/s0028-3932(99)00117-7. [DOI] [PubMed] [Google Scholar]
- Culham J, He S, Dukelow S, Verstraten FA. Visual motion and the human brain: what has neuroimaging told us? Acta Psychologica. 2001;107 (1–3):69–94. doi: 10.1016/s0001-6918(01)00022-1. [DOI] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48 (3):497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Del Viva MM, Igliozzi R, Tancredi R, Brizzolara D. Spatial and motion integration in children with autism. Vision Research. 2006;46 (8–9):1242–1252. doi: 10.1016/j.visres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Bourgouin P. The human brain: Surface, three-dimensional sectional anatomy with MRI, and blood supply. New York: Springer; 1999. [Google Scholar]
- Fox R, McDaniel C. The perception of biological motion by human infants. Science. 1982;218 (4571):486–487. doi: 10.1126/science.7123249. [DOI] [PubMed] [Google Scholar]
- Freire A, Lewis TL, Maurer D, Blake R. The development of sensitivity to biological motion in noise. Perception. 2006;35 (5):647–657. doi: 10.1068/p5403. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Konrad C, Häberlen M, Kleser C, von Gontard A, Reith W, Troje NF, Krick C. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46 (5):1480–1494. doi: 10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley JP, Poline CD, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gervais H, Belin P, Boddaert N, Leboyer M, Coez A, Sfaello I, Barthélémy C, Brunelle F, Samson Y, Zilbovicius M. Abnormal cortical voice processing in autism. Nature Neuroscience. 2004;7 (8):801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain activity evoked by inverted and imagined biological motion. Vision Research. 2001;41 (10–11):1475–1482. doi: 10.1016/s0042-6989(00)00317-5. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain areas active during visual perception of biological motion. Neuron. 2002;35 (6):1167–1175. doi: 10.1016/s0896-6273(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R, Kim CY. Learning to see biological motion: brain activity parallels behavior. Journal of Cognitive Neuroscience. 2004;16 (9):1669–1679. doi: 10.1162/0898929042568569. [DOI] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, Blake R. Brain areas involved in perception of biological motion. Journal of Cognitive Neuroscience. 2000;12 (5):711–720. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Baron-Cohen S, Wheelwright SJ, Singh KD, Bullmore ET, Brammer M, Williams SCR. The role of MT+/V5 during biological motion perception in Asperger Syndrome: an fMRI study. Research in Autism Spectrum Disorders. 2007;1 (1):14–27. [Google Scholar]
- Hubert B, Wicker B, Moore DG, Monfardini E, Duverger H, Da Fonséca D, Deruelle C. Brief report: recognition of emotional and non-emotional biological motion in individuals with autistic spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37 (7):1386–1392. doi: 10.1007/s10803-006-0275-y. [DOI] [PubMed] [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Perception and Psychophysics. 1973;14:201–211. [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17 (4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MD, Delmolino L, Tanaka JW, Shiffrar M. Comparison of visual sensitivity to human and object motion in autism spectrum disorder. Autism Research. 2010;3 (4):191–195. doi: 10.1002/aur.137. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Shiffrar M. The visual perception of motion by observers with autism spectrum disorders: a review and synthesis. Psychonomic Bulletin & Review. 2009;16 (5):761–777. doi: 10.3758/PBR.16.5.761. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W. Altered face scanning and impaired recognition of biological motion in a 15-month-old infant with autism. Developmental Science. 2008;11 (1):40–46. doi: 10.1111/j.1467-7687.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459 (7244):257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Whitney D, Rivera SM. The psychophysics of visual motion and global form processing in autism. Brain. 2010;133(Pt. 2):599–610. doi: 10.1093/brain/awp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange J, Lappe M. The role of spatial and temporal information in biological motion perception. Advances in Cognitive Psychology. 2007;3 (4):419–428. doi: 10.2478/v10053-008-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EHJ, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30 (3):205–223. [PubMed] [Google Scholar]
- Milne E, Swettenham J, Campbell R. Motion perception and autistic spectrum disorder: a review. Cahiers de Psychologie Cognitive/Current Psychology of Cognition. 2005;23 (1):3–36. [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2002;43 (2):255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Milne E, White S, Campbell R, Swettenham J, Hansen P, Ramus F. Motion and form coherence detection in autistic spectrum disorder: relationship to motor control and 2:4 digit ratio. Journal of Autism and Developmental Disorders. 2006;36 (2):225–237. doi: 10.1007/s10803-005-0052-3. [DOI] [PubMed] [Google Scholar]
- Moore DG, Hobson RP, Lee A. Components of person perception: an investigation with autistic, non-autistic retarded and typically developing children and adolescents. British Journal of Developmental Psychology. 1997;15:401–423. [Google Scholar]
- Murphy P, Brady N, Fitzgerald M, Troje NF. No evidence for impaired perception of biological motion in adults with autistic spectrum disorders. Neuropsychologia. 2009;47 (14):3225–3235. doi: 10.1016/j.neuropsychologia.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) Journal of Neuroscience. 1988;8 (6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish EE, Giaschi DE, Boden C, Dougherty R. The maturation of form and motion perception in school age children. Vision Research. 2005;45 (7):827–837. doi: 10.1016/j.visres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Parron C, Da Fonséca D, Santos A, Moore DG, Monfardini E, Deruelle C. Recognition of biological motion in children with autistic spectrum disorders. Autism. 2008;12(3):261–274. doi: 10.1177/1362361307089520. [DOI] [PubMed] [Google Scholar]
- Pavlova M, Krägeloh-Mann I, Birbaumer N, Sokolov A. Biological motion shown backwards: the apparent-facing effect. Perception. 2002;31 (4):435–443. doi: 10.1068/p3262. [DOI] [PubMed] [Google Scholar]
- Pavlova M, Krageloh-Mann I, Sokolov A, Birbaumer N. Recognition of point-light biological motion displays by young children. Perception. 2001;30 (8):925–933. doi: 10.1068/p3157. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Gibson LY. Investigating the functional integrity of the dorsal visual pathway in autism and dyslexia. Neuropsychologia. 2008;46 (10):2593–2596. doi: 10.1016/j.neuropsychologia.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: a possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43 (7):1044–1053. doi: 10.1016/j.neuropsychologia.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005a;128(Pt. 5):1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G. Functional anatomy of biological motion perception in posterior temporal cortex: an fMRI study of eye, mouth and hand movements. Cerebral Cortex. 2005b;15(12):1866–1876. doi: 10.1093/cercor/bhi064. [DOI] [PubMed] [Google Scholar]
- Peuskens H, Vanrie J, Verfaillie K, Orban GA. Specificity of regions processing biological motion. European Journal of Neuroscience. 2005;21 (10):2864–2875. doi: 10.1111/j.1460-9568.2005.04106.x. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophrenia Research. 2008;99 (1–3):164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E. The superior temporal sulcus performs a common function for social and speech perception: implications for the emergence of autism. Neuroscience and Bio-behavioral Reviews. 2008;32 (1):123–142. doi: 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Sanchez-Marin FJ, Padilla-Medina JA. A psychophysical test of the visual pathway of children with autism. Journal of Autism and Developmental Disorders. 2008;38 (7):1270–1277. doi: 10.1007/s10803-007-0507-9. [DOI] [PubMed] [Google Scholar]
- Saygin AP. Superior temporal and premotor brain areas necessary for biological motion perception. Brain. 2007;130(Pt. 9):2452–2461. doi: 10.1093/brain/awm162. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Wilson SM, Hagler DJ, Bates E, Sereno MI. Point-light biological motion perception activates human premotor cortex. Journal of Neuroscience. 2004;24 (27):6181–6188. doi: 10.1523/JNEUROSCI.0504-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57 (4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Research. 2009;49 (22):2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Spencer J, O’Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency. NeuroReport. 2000;11 (12):2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- Thompson JC, Clarke M, Stewart T, Puce A. Configural processing of biological motion in human superior temporal sulcus. Journal of Neuroscience. 2005;25 (39):9059–9066. doi: 10.1523/JNEUROSCI.2129-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton IM, Rensink RA, Shiffrar M. Active versus passive processing of biological motion. Perception. 2002;31 (7):837–853. doi: 10.1068/p3072. [DOI] [PubMed] [Google Scholar]
- Tsermentseli S, O’Brien JM, Spencer JV. Comparison of form and motion coherence processing in autistic spectrum disorders and dyslexia. Journal of Autism and Developmental Disorders. 2008;38 (7):1201–1210. doi: 10.1007/s10803-007-0500-3. [DOI] [PubMed] [Google Scholar]
- Vaina LM, Solomon J, Chowdhury S, Sinha P, Belliveau JW. Functional neuroanatomy of biological motion perception in humans. Proceedings of the National Academy of Sciences of the United States of America. 2001;98 (20):11656–11661. doi: 10.1073/pnas.191374198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke MW, Scholte HS, van Engeland H, Lamme VA, Kemner C. A neural substrate for atypical low-level visual processing in autism spectrum disorder. Brain. 2008;131(Pt. 4):1013–1024. doi: 10.1093/brain/awm321. [DOI] [PubMed] [Google Scholar]
- Vangeneugden J, Vancleef K, Jaeggli T, VanGool L, Vogels R. Discrimination of locomotion direction in impoverished displays of walkers by macaque monkeys. Journal of Vision. 2010;10 (4):22. doi: 10.1167/10.4.22. [DOI] [PubMed] [Google Scholar]
- Vanrie J, Verfaillie K. Perception of biological motion: a stimulus set of human point-light actions. Behavior Research Methods, Instruments, & Computers. 2004;36 (4):625–629. doi: 10.3758/bf03206542. [DOI] [PubMed] [Google Scholar]
- Verfaillie K. Perceiving human locomotion: priming effects in direction discrimination. Brain and Cognition. 2000;44 (2):192–213. doi: 10.1006/brcg.2000.1228. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- White S, Frith U, Milne E, Stuart R, Swettenham J, Ramus F. A double dissociation between sensorimotor impairments and reading disability: a comparison of autistic and dyslexic children. Cognitive Neuropsychology. 2006;23:748–761. doi: 10.1080/02643290500438607. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Perception and Psychophysics. 2001a;63 (8):1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Perception and Psychophysics. 2001b;63 (8):1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]
- Williams DW, Sekuler R. Coherent global motion percepts from stochastic local motions. Vision Research. 1984;24 (1):55–62. doi: 10.1016/0042-6989(84)90144-5. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. Autism, the superior temporal sulcus and social perception. Trends in Neurosciences. 2006;29(7):359–366. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]