Abstract
NSAIDs display promising antineoplastic activity for colorectal and other cancers, but toxicity from cyclooxygenase (COX) inhibition limits their long-term use for chemoprevention. Previous studies have concluded that the basis for their tumor cell growth inhibitory activity does not required COX inhibition, although the underlying mechanism is poorly understood. Here we report that the NSAID, sulindac sulfide (SS) inhibits cyclic guanosine monophosphate phosphodiesterase (cGMP PDE) activity to increase intracellular cGMP levels and activate cGMP dependent protein kinase (PKG) at concentrations that inhibit proliferation and induce apoptosis of colon tumor cells. SS did not activate the cGMP/PKG pathway, nor affect proliferation or apoptosis in normal colonocytes. Knockdown of the cGMP-specific PDE5 isozyme by siRNA and PDE5-specific inhibitors, tadalafil and sildenafil, also selectively inhibited the growth of colon tumor cells that expressed high levels of PDE5 compared with colonocytes. The mechanism by which SS and the cGMP/PKG pathway inhibits colon tumor cell growth appears to involve the transcriptional suppression of β-catenin to inhibit Wnt/β-catenin TCF transcriptional activity, leading to down-regulation of cyclin D1 and survivin. These observations suggest that safer and more efficacious sulindac derivatives can be developed for colorectal cancer chemoprevention by targeting PDE5 and possibly other cGMP degrading isozymes.
Keywords: colorectal cancer, chemoprevention, sulindac, phosphodiesterase, β-catenin
Introduction
Colorectal cancer (CRC) is the 3rd most common malignant disease in the western world (1). In the United States, CRC accounts for 12% of all newly diagnosed cancers (2) in which approximately 6% of Americans will die each year from this form of cancer (3). Despite the benefits of endoscopic screening to reduce the risk of developing CRC, there is an unmet medical need to develop safe and effective drugs for CRC chemoprevention.
Epidemiologic studies have reported that the long-term use of NSAIDs is associated with a significant reduction in the incidence of CRC and associated mortality (4, 5). Certain prescription strength NSAIDs such as sulindac also cause the regression of precancerous adenomas in patients with familial or sporadic adenomatous polyposis (6–10). These observations are consistent with preclinical studies that have shown the ability of sulindac and other NSAIDs to inhibit tumorigenesis in various animal models of CRC (11–13). While the basis for the anticancer activity of NSAIDs merits further study, their cyclooxygenase (COX) inhibitory activity is associated with potentially fatal gastrointestinal, renal, and cardiovascular toxicities that preclude their long-term use for cancer chemoprevention. Another limitation is that NSAIDs do not completely protect from disease progression in all individuals. Case reports, for example, have described individuals with FAP who developed CRC despite long-term treatment with sulindac (14).
Although COX-2 is well established to play an important role in tumorigenesis, multiple investigators have concluded that the biochemical basis for the antineoplastic activity of NSAIDs is unrelated to their COX-1 or COX-2 inhibitory activity (15, 16). It may therefore be feasible to develop safer drugs for CRC chemoprevention by designing derivatives that do not disrupt prostaglandin biosynthesis, but retain or have improved anticancer activity. In support of this possibility, the non-COX inhibitory sulfone metabolite of sulindac was shown to inhibit tumor formation in the azoxymethane-induced rat model of colon tumorigenesisis without inhibiting COX-1 or COX-2 and affecting prostaglandin levels in the colonic mucosa (13). Sulindac sulfone (exisulind) was also reported to be effective in clinical trials involving individuals with familial or sporadic adenomatous polyposis (17, 18), but did not receive FDA approval because of hepatotoxicity. The mechanism for the antineoplastic activity of sulindac sulfone was previously described to involve the inhibition of cyclic guanosine monophosphate phosphodiesterase (cGMP PDE), which can increase intracellular cGMP levels to activate cGMP dependent protein kinase (PKG), leading to the suppression of tumor cell growth and the induction of apoptosis (19, 20). More recently, we and others have shown that the COX inhibitory metabolite of sulindac, sulindac sulfide (SS), as well as other NSAIDs from various chemical families, including indomethacin, meclofenamic acid, and celecoxib, also inhibit cGMP PDE and that this activity is closely associated with their tumor cell growth inhibitory activity (21, 22).
Cyclic nucleotide phosphodiesterases are a superfamily of enzymes that hydrolyze the 3′, 5′-phosphodiester bond in cGMP and/or cAMP. There are 11 PDE isozymes having different substrate specificity, regulatory properties, tissue localization, and inhibitor sensitivity (23). PDE1, 2, 3, 10 and 11 are dual substrate-degrading isozymes, while PDE5, 6, 9 are selective for cGMP, and PDE4, 7 and 8 are cAMP selective. PDE inhibitors have the potential to cause a transient or sustained increase in intracellular levels of cAMP and/or cGMP to activate cAMP-dependent protein kinase (PKA) and/or cGMP-dependent protein kinase (PKG), as well as other cyclic nucleotide regulated processes. While several PDE inhibitors have been developed for various disease indications, there has been relatively little attention given to cancer, despite the well-known regulation of cell growth by cyclic nucleotides (24).
Although SS can inhibit several cGMP degrading isozymes, including PDE2, 3, 5, and 10, PDE5 appears to be a critical target for SS as previously reported (21, 25, 26). For example, SS can inhibit recombinant PDE5 within a concentration range that can suppress tumor cell growth and induce apoptosis in vitro. PDE5 is also widely expressed in colon tumor cell lines and levels of this enzyme are elevated in colon adenocarcinomas and adenomas compared with normal colonic mucosa (21). In addition, we recently described a novel non-COX inhibitory derivative of sulindac that selectively inhibits PDE5 and potently inhibits colon tumor cell growth (27). Here we provide additional evidence that the antitumor activity of SS involves PDE5 inhibition and that this isozyme plays an important role in colon tumor cell proliferation and survival. We also describe a novel regulatory influence of the cGMP/PKG pathway over Wnt/β-catenin-TCF transcriptional activity that appears to fully or partially account for the antiproliferative and pro-apoptotic properties of sulindac.
Materials and Methods
Drugs and Reagents
Sulindac sulfide (SS) was purchased from Sigma-Aldrich. 8-bromo-cGMP was purchased from Biolog. Tadalafil and sildenafil were extracted from CIALIS® and VIAGRA® tablets, respectively. All antibodies were purchased from Cell Signaling Technologies. DMSO was used as vehicle for all compounds unless otherwise noted.
Cells and Cell Culture
Human colon tumor cell lines HCT116, HT-29, SW480 and Caco-2 were obtained from the American Type Culture Collection (ATCC) (Manassas, VI) and grown under standard cell culture conditions in RPMI 1640 medium containing 5% serum at 37 °C in a humidified atmosphere with 5% CO2. The human colonocyte line, NCM460, that is derived from normal human colon mucosa (28) was obtained from INCELL (San Antonio, TX) and grown in INCELL’s enriched M3:10 medium with 10% serum as recommended by supplier. All cell lines were expanded upon delivery, and numerous aliquots of low passage cells were preserved in liquid N2. Cells were passaged no longer than 2 months. Tumor cell lines obtained from ATCC were characterized by STR profiling as performed by ATCC. The NCM460 line was characterized by INCELL as described previously (e.g. tumorigenicity testing) (28). No additional re-authentication of the cell lines was performed except for experimental reasons (e.g. confirmation of cell doubling time, morphology, sensitivity to SS, PDE5 expression levels, etc).
siRNA Transfection
Two different sequences of siRNA targeting human PDE5 and scrambled control siRNA were purchased from Qiagen. The siRNA target sequences were as follows: siPDE5-1, 5′-GCCATCTGCTTGCAACTGTAT-3′; siPDE5-2, 5′-CCAGCTTTACTGCCATTCAAT-3′. The scrambled control siRNA, containing nonspecific sequences do not have homology in the human genome. siRNA duplexes were transfected into NCM460 and HCT116 cells using Hiperfect transfection reagent (Qiagen) and HT29 cells using RNAiMAX transfection reagent (Invitrogen) according to manufacturer’s specifications and incubated at 37°C for 72 hours.
Cell Viability Assay
Cells were plated in 96-well microtiter plates at a density of 5,000 cells per well. For drug treatment, cells were treated with compound or vehicle, and incubated at 37°C for 72 hours. For siRNA assays, cells were transfected with siRNA under the same condition described above. All viability assays were performed in 5% serum using either tumor cells or normal colonocytes, with the exception of those involving sildenafil and tadalafil that were performed in 1.5% serum to reduce binding to albumin and increase levels of free drugs. The effect of treatment on cell viability was measured using the Cell Titer Glo Assay as specified by the manufacturer (Promega).
Apoptosis Assay
Cells were plated in 96-well microtiter plates at a density of 10,000 cells per well, and allowed to attach overnight. Cells were treated with compounds or vehicle, and incubated at 37°C for 6 hours. For siRNA assays, cells were plated in a 96-well plate (10,000 cells per well) and transfected with siRNA under the same conditions above. All apoptosis assays were done in 5% serum using either tumor cells or normal colonocytes. The induction of apoptosis caused by treatment was determined using Caspase 3/7 Glo Assay (Promega).
EdU Cell Proliferation Assay
The antiproliferative activity of SS and PDE5 siRNA was determined by measuring EdU (5-ethynyl-2′-deoxyuridine) incorporation during DNA synthesis. Cells were plated at a density of 1.5×106 cells per 10cm tissue culture dish and incubated overnight at 37°C. After growing the cells in serum-free media overnight, the cells were treated with SS or vehicle in media with 10% serum for 24 hours. A final concentration of 10 μmol/L EdU was added to each dish after 6 hours treatment. For siRNA assays, cells were plated at a density of 2×105 cells per well in 6-well tissue culture plates, transfected with siRNA, and incubated at 37° C for 54 hours prior to the addition of EdU. After another 18 hours of incubation with EdU, cells were harvested and analyzed using the Click-iT EdU Alexa Fluor 488 Proliferation Assay (Invitrogen) according to the manufacturer’s specifications. The percentage of proliferating cells was quantified using a Guava EasyCyte Plus flow cytometer.
PDE Assay
PDE activity in cell lysates was measured using the IMAP fluorescence polarization PDE assay (Molecular Devices) as described previously (26). For experiments involving siRNA, cells were plated at a density of 2×105 cells per well in 6-well tissue culture plates and transfected with siRNA for 72 hours prior to cell lysis.
cGMP Assay
Cells were plated at a density of 1×106 cells per 10cm tissue culture dish, incubated for 48 hours, and treated with SS or vehicle control. After 45 min of treatment, cells were lysed and assayed for cGMP content using the cGMP Direct Biotrak EIA kit (GE Healthcare Life Sciences). The assay was performed according to the manufacturer’s specifications.
Cell Lysis
Cells were lysed and protein concentrations were determined as described previously (26).
Western Blotting
Western blotting was performed as described previously (26). The band intensities in the images were quantified by ImageJ software.
Luciferase Reporter Assay
Cells were plated at a density of 5×104 cells per well in 24-well tissue culture plates. After 24 hours of incubation, cells were transiently transfected with 0.1 μg TOP-FLASH/FOP-FLASH constructs (Millipore) or CTNNB1 construct (generously provided by Dr. Darren Browning from Georgia Regents University Cancer Center) and 0.1 μg β-galactosidase-expressing vector (Promega). The CTNNB1 reporter construct was previously shown to subclone the essential promoter region (−2760 to +27) of β-catenin gene into the luciferase reporter vector pGL3Basic (29). After 42 hours of transfection, cells were treated with compound or vehicle for 6 hours. For siRNA assays, cells were cotransfected with reporter constructs and β-galactosidase-expressing vector 24 hours after an initial siRNA transfection and incubated for another 48 hours. At the end of treatment, cells were lysed and both luciferase and β-galactosidase activities were measured using kits from Promega. All luciferase activity was normalized to β-galactosidase activity. For TCF activity, the normalized FOP-FLASH values were further subtracted from the normalized TOP-FLASH values.
Semi-quantitative RT-PCR
HCT116 cells were plated at a density of 1.5×106 cells per 10 cm tissue culture dish and allowed to adhere overnight. After 24 hours, growth media was replaced with serum-free media. After 18 hours of serum starvation, cells were treated with compound or vehicle for 6 hours. At the end of incubation period, cells were lysed and RNA was extracted using the RNAqueous-4PCR Kit (Ambion) according to manufacturer’s instructions. Semi-quantitative RT-PCR was performed using BluePrint RT-PCR Kit (Takara) according to manufacturer’s instructions with 23 cycles at an anneal temperature of 60° C. The primers (Invitrogen) were as follows: β-catenin forward, 5′-ATCCCACTGGCCTCTGATAAA-3′ and reverse, 5′-CAATAGCTTCTGCAGCTTCCT-3′; GAPDH forward, 5′-TGATGACATCAAGAAGGTGGTGAAG-3′ and reverse, 5′-TCCTTGGAGGCCATGTGGGCCAT-3′. The band intensities were quantified by ImageJ software.
Experimental Design and Data Analysis
Drug effects on cell growth and IC50 values were determined as described previously (26). Experiments were performed with a minimum of 3 replicates per data point. Each experiment was performed a minimum of three times to verify reproducibility. All error bars represent standard error of the mean (SEM). Calculation of p values was done by comparing the specified treatment group with vehicle-treated controls using a Student’s t test. A P value of <0.05 was considered statistically significant.
Results
Growth and cGMP PDE inhibitory activity of SS
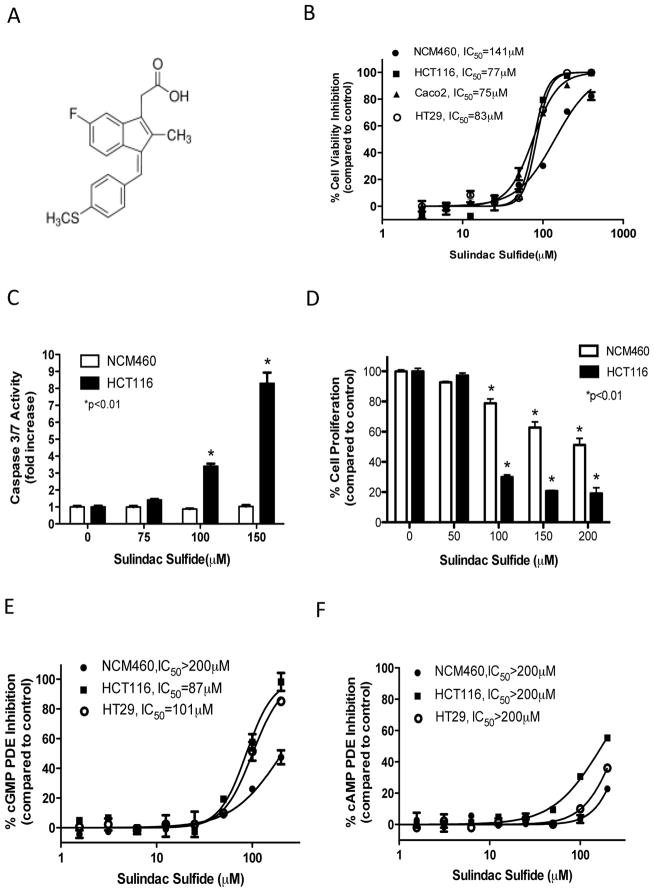
Sulindac is a non-steroidal anti-inflammatory drug from the arylalkanoic acid class in which the sulfide metabolite as shown in Figure 1A is responsible for its antineoplastic activity. Initial experiments were conducted to quantify the inhibitory effect of SS on the viability of colon cells derived from either malignant or normal tissues. As shown in Figure 1B, SS inhibited the viability of human HCT116, HT29, and Caco2 colon tumor cell lines with IC50 values ranging from 75–83 μmol/L following 72 hours of treatment. By comparison, human NCM460 normal colonocytes were less sensitive to SS with an IC50 value of 141 μmol/L. SS also induced apoptosis of HCT116 tumor cells as evident by an increase in caspase activity that occurred within 6 hours of treatment, but did not induce caspase activity in colonocytes (Figure 1C). In addition, SS inhibited the proliferation of HCT116 tumor cells as measured by EdU incorporation following 24 hours of treatment (Figure 1D). Colonocytes were again less sensitive in which 100 μmol/L SS inhibited proliferation by only 20% compared with 75% inhibition of colon tumor cell proliferation.
Figure 1.
Colon tumor cell growth and cGMP PDE inhibitory activity of SS. A, chemical structure of sulindac sulfide. B, dose-dependent decrease of cell viability following 72 hours of SS treatment. C, apoptosis induction of HCT116 cells by SS after 6 hours of treatment. D, inhibition of proliferation by SS treatment after 24 hours in NCM460 colonocytes and HCT116 colon tumor cells. E and F, dose-dependent inhibition of cGMP (E) and cAMP (F) PDE activity in whole cell lysates from NCM460 colonocytes and HCT116, and HT29 colon tumor cells.
A fluorescence polarization assay that can simultaneously measure cGMP and cAMP hydrolysis was used to determine if SS can inhibit PDE activity in whole cell lysates. As shown in Figure 1E, SS inhibited cGMP PDE activity in colon tumor cell lysates with IC50 values equivalent to those required for inhibition of viability, but did not significantly affect cAMP hydrolysis (Figure 1F). SS also did not inhibit cGMP or cAMP hydrolysis in lysates from colonocytes, which paralleled their reduced sensitivity to the effects of SS on viability, apoptosis and proliferation.
SS activates cGMP/PKG signaling in colon tumor cells
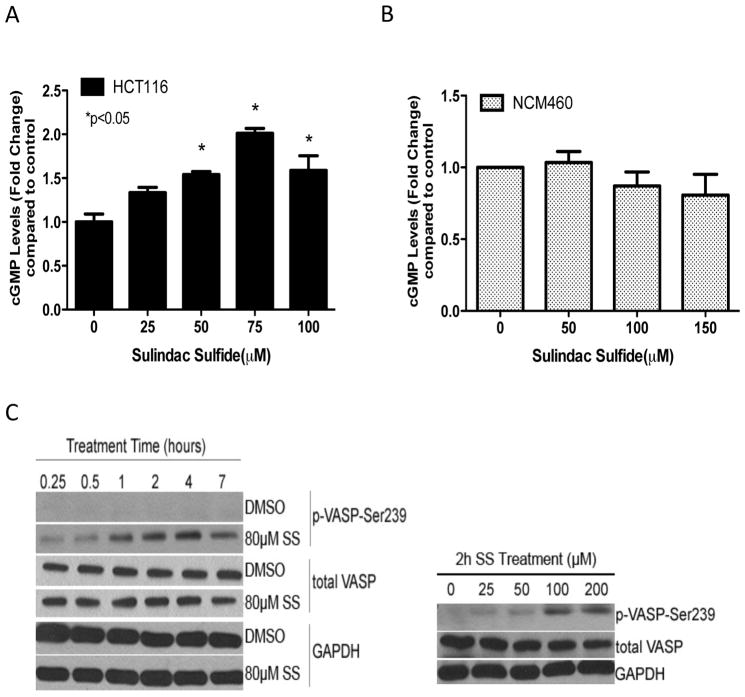
Intracellular cGMP levels were next measured to determine if SS can inhibit cGMP PDE activity in intact cells. As shown in Figure 2A, SS treatment of HCT116 colon tumor cells for 45 minutes increased intracellular cGMP levels within the same concentration range that inhibited cGMP PDE activity in cell lysates. In accord with its lack of cGMP PDE inhibitory activity in lysates from colonocytes, SS did not affect intracellular cGMP levels in colonocytes (Figure 2B).
Figure 2.
SS activation of cGMP signaling in HCT116 human colon tumor cells. A and B, dose-dependent increase in intracellular cGMP levels after 45 min of SS treatment in HCT116 colon tumor cells (A), but not NCM460 colonocytes (B). C, time-dependent (left) and dose-dependent (right) increase in VASP phosphorylation at Ser239 after SS treatment in HCT116 cells.
PKG is an important downstream mediator of cGMP signaling, which we predicted would be activated in colon tumor cells treated with SS. PKG activity was measured by the phosphorylation level of vasodilator-stimulated phosphoprotein (VASP) that is preferentially phosphorylated at the serine 239 residue by PKG (30). As shown in Figure 2C, SS caused a time-and dose-dependent increase in phospho-VASP levels in HCT116 colon tumor cells without affecting total VASP levels.
A role of the cGMP-specific PDE5 isozyme
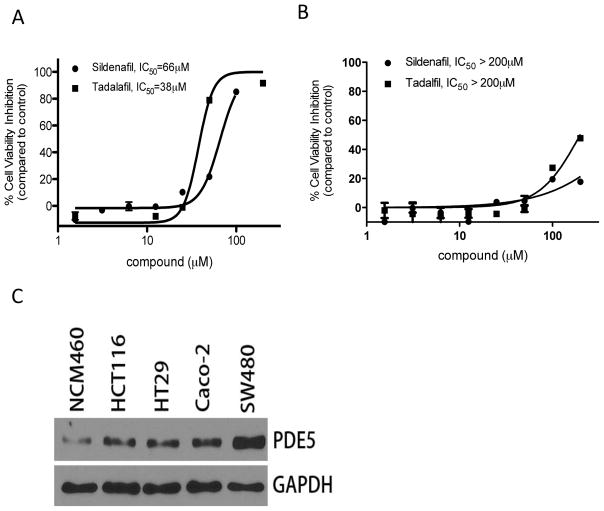
PDE5 appears to be an important target for the anticancer properties of SS based on several lines of evidence as previously reported (21, 25, 26). The requirement of PDE5 for colon tumor cell proliferation and/or survival is evident by the ability of highly specific PDE5 inhibitors, tadalafil and sildenafil, to selectively inhibit colon tumor cell viability without affecting the viability of colonocytes (Figures 3A and B). We next determined if the difference in sensitivity between colon tumor cells and colonocytes to SS was associated with differences in PDE5 expression levels. As shown in Figure 3C by Western blotting, PDE5 levels were appreciably higher in four colon tumor cell lines compared with colonocytes. These results are consistent with previously published immunohistochemistry studies using patient specimens in which PDE5 was found to be expressed in colon adenomas and adenocarcinomas at higher levels compared with normal colonic mucosa (21).
Figure 3.
Sensitivity to PDE5 inhibitors and expression of PDE5 in colon tumor cells and colonocytes. A and B, inhibitory effect of sildenafil and tadalafil on viability of HCT116 colon tumor cells (A), but not NCM460 colonocytes (B) after 72h treatment. C, expression of PDE5 in normal colonocytes and colon tumor cell lines.
PDE5 siRNA selectively inhibits colon tumor cell growth
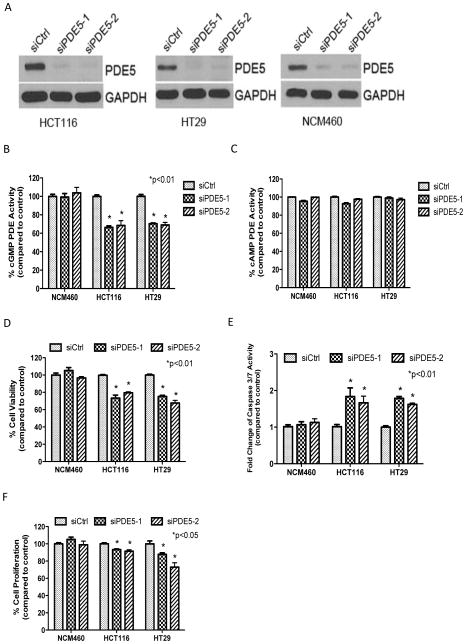
To further study the requirement of PDE5 for tumor cell proliferation and survival, siRNA knockdown studies were performed in colonocytes and two colon tumor cell lines, HCT116 and HT29. Two sequences of PDE5 siRNA, designated as siPDE5-1 and siPDE5-2, were used to avoid potential off-target effects of siRNA. Western blotting showed that transient transfection with both sequences of PDE5 siRNA suppressed PDE5 expression by approximately 90% in all three cell lines compared with control transfection using a scrambled sequence (Figure 4A). Both siRNA sequences of PDE5 resulted in 40% reduction in total cGMP hydrolysis in tumor cells, while there was no effect of cGMP hydrolysis in colonocyte lysates (Figure 4B). The tumor selectivity of the siRNA knockdown is consistent with differences in PDE5 expression levels between colon tumor cells and colonocytes as described above and may be attributed to the presence of other cGMP PDE isozymes in colonocytes that can compensate for PDE5 knockdown. As an additional control for assessing the PDE isozyme specificity of the siRNA knockdown, PDE5 siRNA was confirmed to have no effect on cAMP hydrolysis in all three cell lines (Figure 4C), which is to be expected based on the known substrate specificity of PDE5 for cGMP.
Figure 4.
Suppression of PDE5 with siRNA is sufficient to selectively inhibit colon tumor cell growth. A, siRNA knockdown of PDE5 protein expression in HCT116 and HT29 colon tumor cells and NCM460 colonocytes (Western Blotting). B, selective suppression of cGMP hydrolysis by PDE5 siRNA in HCT116 and HT29 colon tumor cells, but not NCM460 colonocytes. C, no effect of PDE5 siRNA on cAMP hydrolysis in HCT116, HT29 and NCM460 cells. D, selective inhibition of tumor cell viability by PDE5 siRNA. E, tumor cell specific induction of apoptosis by PDE5 siRNA. F, selective inhibition of tumor cell proliferation by PDE5 siRNA. All treatment effects (A–F) were measured 72 hours post transfection.
Significant suppression of cell viability was observed in HCT116 and HT29 colon tumor cells transfected with PDE5 siRNA compared with the control scrambled siRNA. In contrast, PDE5 siRNA knockdown did not affect the viability of colonocytes (Figure 4D). PDE5 siRNA knockdown also increased caspase activity in HCT116 and HT29 tumor cells, but did not affect caspase activity in colonocytes (Figure 4E). We also observed a significant inhibition of cell proliferation in tumor cells following PDE5 knockdown, while there was no effect in colonocytes (Figure 4F). Thus, PDE5 knockdown by siRNA closely mirrored the tumor cell selective effects of SS on viability, apoptosis and proliferation.
Activation of cGMP pathway inhibits β-catenin signaling
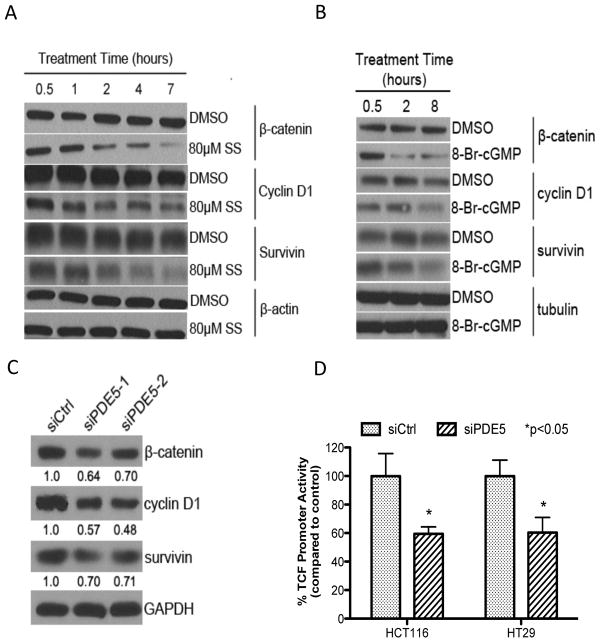
Numerous investigators have reported that sulindac and other NSAIDs can inhibit Wnt/β-catenin signaling and have concluded that this effect is important for their cancer chemopreventive activity (25, 31–34). We therefore conducted a series of experiments to determine if PDE5 inhibition and activation of the cGMP/PKG pathway can mediate the inhibitory effect of SS on Wnt/β-catenin signaling. Within the same time period as required for PKG activation (1–2 hours), SS reduced β-catenin protein levels, as well as the expression of cyclin D1 and survivin that are known to be regulated by Wnt/β-catenin-dependent TCF transcriptional activity (Figure 5A). In addition, 8-bromo-cGMP, a PKG activator, reduced levels of β-catenin, along with cyclin D1 and survivin in colon tumor cells (Figure 5B). Similar to the effects caused by SS and 8-bromo-cGMP, PDE5 siRNA also reduced β-catenin, cyclin D1 and survivin levels in HCT116 cells (Figure 5C). While we have previously reported that SS and other PDE5 inhibitors (e.g. MY5445) can inhibit TCF transcriptional activity (21), PDE5 siRNA was found to suppress TCF transcriptional activity as measured by the TOPFLASH luciferase reporter construct following transient transfection of HCT116 and HT29 colon tumor cells (Figure 5D). These results provide evidence for a novel role of the cGMP/PKG pathway in regulating oncogenic β-catenin signaling.
Figure 5.
Activation of cGMP signaling correlates with β-catenin signaling. A, time-dependent decrease in the expression of β-catenin, cyclin D1 and survivin with 80 μmol/L SS treatment in HCT116 cells. B, time-dependent decrease in the expression of β-catenin, cyclin D1 and survivin with 1 mmol/L 8-bromo-cGMP in HCT116 cells. C, suppression of β-catenin, cyclin D1 and survivin with PDE5 siRNA after 72 hours transfection in HCT116 cells. The numbers indicated below the blots derive from band quantification normalized to GAPDH and are expressed as ratio of scrambled control siRNA treatment. D, inhibition of TCF transcriptional activity after 72 hours transfection of PDE5 siRNA in HCT116 and HT29 colon tumor cells. Data obtained were normalized for β-galactosidase activity, and normalized FOP-FLASH values were then subtracted from the normalized TOP-FLASH values.
PDE5 suppression inhibits β-catenin synthesis
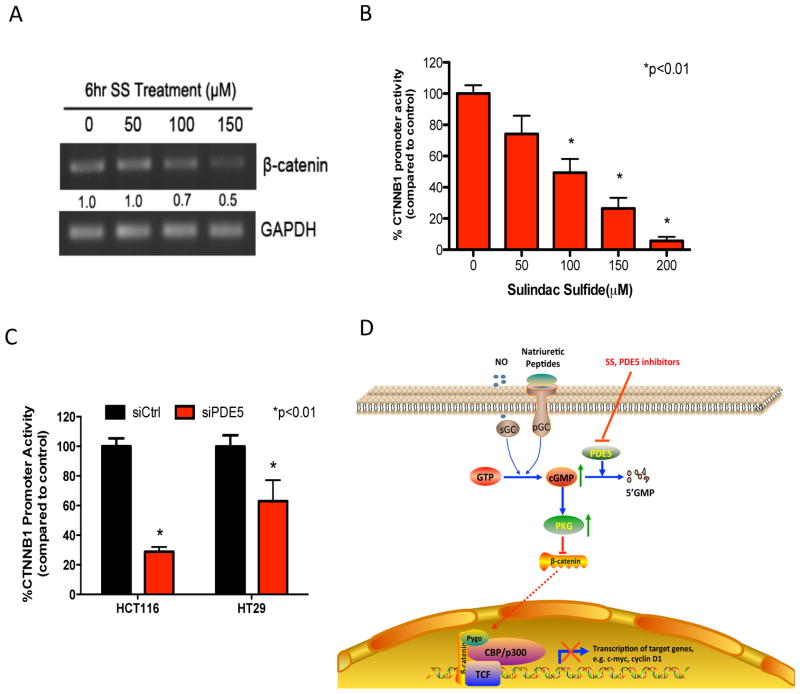
Previous studies have reported that PKG can directly suppress the transcription of β-catenin (35), although other studies have suggested a mechanism involving the phosphorylation of β-catenin to induce ubiquitin-mediated proteosome degradation (20, 36). To further study the mechanism by which SS attenuates Wnt/β-catenin signaling, we measured the effect of SS treatment on steady-state levels of β-catenin mRNA in HCT116 colon tumor cells by RT-PCR. As shown in Figure 6A, SS treatment reduced β-catenin mRNA levels in a dose-dependent manner at concentrations that can inhibit the growth of colon tumor cells. A reporter construct that encodes for the promoter regions of β-catenin gene (CTNNB1) was used to confirm that reduced levels of β-catenin mRNA were due to transcriptional repression. Figure 6B showed that SS treatment of HCT116 tumor cells caused a dose-dependent suppression of CTNNB1 activity. PDE5 siRNA also significantly inhibited CTNNB1 activity in HCT116 and HT29 colon tumor cells (Figure 6C). We also measured levels of phosphorylated β-catenin by Western blotting using a phospho-specific antibody with specificity for Ser33/37/Thr41 residues that are known to target β-catenin for degradation, but did not observe an increase in β-catenin phosphorylation in response to SS treatment (data not shown). Thus, the suppression of PDE5 by either SS or siRNA can inhibit the transcription of β-catenin, which appears to explain their inhibitory effects on TCF-dependent transcriptional activity as well as their effects on viability, proliferation, and apoptosis.
Figure 6.
Downregulation of β-catenin by PKG activation is associated with inhibition of β-catenin transcription. A, dose-dependent decrease in β-catenin mRNA levels after 6 hours of treatment with SS in HCT116 cells as measured by semi-quantitative RT-PCR. The band intensities were quantified by ImageJ software and the β-catenin mRNA values were normalized against GAPDH levels. Fold change values under each lane indicate the normalized β-catenin intensities as compared to vehicle control. B, dose-dependent inhibition of CTNNB1 transcriptional activity after 6 hours of SS treatment in HCT116 cells. C, suppression of CTNNB1 transcriptional activity by PDE5 siRNA after 72 hours transfection in HCT116 and HT29 colon tumor cells. D, proposed model showing the COX-independent anticancer mechanism of SS involving activation of cGMP/PKG pathway and suppression of β-catenin signaling in colon cancer cells. Inhibition of PDE5 by SS/PDE5 inhibitors results in accumulation of cGMP, activation of PKG, and suppression of β-catenin mediated gene transcription.
Discussion
Numerous epidemiological studies have reported that NSAIDs can significantly reduce the incidence and mortality from CRC and other cancers, but toxicities associated with COX-1 and COX-2 inhibition and the suppression of physiologically important prostaglandins limit their long-term use for cancer chemoprevention. However, a few investigators have concluded that the basis for the antineoplastic activity of NSAIDs does not require COX inhibition, which suggest that it may be feasible to develop safer and more efficacious derivatives for CRC chemoprevention by targeting the underlying mechanism. Studies described here establish strong evidence that the cGMP-specific PDE5 isozyme is a target for sulindac and provide insight as to how the cGMP/PKG pathway can regulate tumor cell proliferation and survival.
Our findings show that SS can selectively inhibit cGMP hydrolysis in lysates from colon tumor cells without affecting cAMP hydrolysis. The biological relevance of this observation was confirmed by experiments demonstrating the ability of SS treatment to induce cGMP elevation and PKG activation in intact colon tumor cells. These experiments also demonstrate that concentration range of SS required to inhibit tumor cell growth matched the concentration range required to inhibit cGMP PDE activity and induce cGMP/PKG signaling. Specifically, the 50–100 μmol/L concentration range of SS that was required to activate PKG in colon tumor cells was the same range as for cGMP PDE inhibition in tumor cell lysates as well as for the elevation of intracellular cGMP levels. It is notable that all three measurements involved different assay formats. Moreover, the IC50 values of SS to inhibit purified cGMP PDE isozymes as described previously (27) were also within the same concentration range to inhibit cGMP PDE activity in whole cell lysates. It is also remarkable that the time required to activate PKG (1–4 hours) followed a relatively short period of time (45 minutes) when a maximal increase in intracellular cGMP levels was observed, which proceeded a longer duration of time (6 hours) required to induce apoptosis as detected biochemically by an increase in caspase activity.
A unique characteristic of sulindac and likely other NSAIDs that may be highly pertinent to their safety and efficacy for cancer chemoprevention is their ability to selectively inhibit proliferation and induce apoptosis of cancer cells without toxicity to normal proliferating tissues as is the case for conventional chemotherapy. The COX-independent nature by which sulindac can induce apoptosis is suggest by in a clinical trial involving FAP patients in which sulindac sulfone treatment was found to be associated with an increase in apoptosis indices in adenomas without affecting apoptosis rates in adjacent normal mucosa (17). To model the tumor selectivity in vitro, we compared the effects of SS on colon tumor cells with cells derived from normal colonic mucosa. We showed that colonocytes were appreciably less sensitive to SS, especially by using specific assays measuring proliferation and apoptosis. The reduced sensitivity of colonocytes to SS was paralleled by the inability of SS to inhibit cGMP PDE activity in lysates from colonocytes or to induce cGMP signaling in colonocytes.
The ability of SS to selectively inhibit colon tumor cell proliferation and induce apoptosis was associated with increased PDE5 expression levels compared with colonocytes. The role of the PDE5 isozyme is supported by the ability of highly specific PDE5 inhibitors used for the treatment of erectile dysfunction to selectively suppress colon tumor cell growth as well as by a siRNA knockdown approach in which suppression of PDE5 mimicked the tumor cell growth inhibitory activity and selectivity of SS. The high levels of PDE5 as measured in cultured colon tumor cells compared with colonocytes is consistent with studies involving human clinical specimens that have reported higher PDE5 levels in colorectal, bladder, lung and breast carcinomas compared with normal epithelium from these tumor types (19, 21, 37, 38). PDE5 expression may therefore be essential for colon tumor cell growth or survival, although further studies are needed to fully define its role in tumor initiation and progression.
We have previously reported that SS can inhibit recombinant PDE5 with an IC50 value of 38 μmol/L, while slightly higher concentrations in the 50–100 μmol/L range are required to activate cGMP/PKG signaling in intact cells. Although these potency values matched those required for growth inhibition, they are appreciably higher than those required to inhibit COX-1 or -2 in which IC50 values in the 10−6 mol/L range or lower have been described previously (39). Nonetheless, dosages of sulindac (150 mg bid) that have been reported to be effective for the treatment of adenomas in FAP patients (7) can achieve blood levels in the 10−5 mol/L range that are sufficient to inhibit PDE5 in vivo (40). Higher dosages of sulindac could be more effective but would be associated with a higher risk of COX-dependent toxicities. Alternatively, it may be feasible to design derivatives that lack COX inhibitory activity and hold the potential to be safer and more efficacious for CRC chemoprevention. The possibility of uncoupling COX and PDE5 inhibitory activity from sulindac was recently demonstrated by an amine derivative of sulindac that was found to be PDE5 selective, but did not inhibit COX-1 or COX-2, yet potently inhibited colon tumor cell growth and induced apoptosis (27).
An important question that remains from these studies is whether targeting PDE5 alone is ideal or if there are advantages in targeting additional cGMP PDE isozymes. On one hand, we previously reported that SS can inhibit several cGMP PDE isozymes (e.g. PDE2, 3, 5, and 10), but not others such as PDE1, 6, 9, or 11 (27). On the other hand, we show here that highly selective PDE5 inhibitors (e.g. sildenafil and tadalafil) and PDE5 siRNA can inhibit colon tumor cell growth, which implies that the inhibition of PDE5 is sufficient. However, high concentrations of PDE5 inhibitors in the micromolar range were required to suppress tumor cell growth compared with nanomolar concentrations required to inhibit PDE5 in isolated enzyme assays. In addition, the suppression of colon tumor cell growth by PDE5 siRNA was limited to a 30–40% reduction of viable cell number compared with control cultures, which is in contrast to SS that causes >95% reduction of viable cell number. Further research is therefore necessary to study the involvement of additional cGMP PDE isozymes.
Given that the canonical Wnt/β-catenin signaling pathway plays an important role in CRC progression (41–43) and the ability of sulindac to suppress this pathway, we investigated the potential for crosstalk with the cGMP/PKG pathway. We report here for the first time that SS and siRNA knockdown of PDE5 can reduce β-catenin protein expression and transcriptional activity of TCF leading to the suppression of important tumor cell cycle and survival regulatory proteins, such as cyclin D1 and survivin. Moreover, the ability of 8-bromo-cGMP, a highly specific PKG activator, to attenuate β-catenin signaling confirmed a role of PKG. These observations are consistent with reports from other investigators showing that sulindac metabolites or activators of the cGMP/PKG pathway can suppress the oncogenic activity of β-catenin (20, 21, 33, 35, 36, 44, 45).
The mechanism by which SS suppresses the oncogenic activity of β-catenin appears to be at the transcriptional level given that β-catenin mRNA levels were reduced in colon tumor cells following treatment with SS at concentrations that reduced β-catenin protein expression. PDE5 knockdown by siRNA also resulted in a significant suppression of β-catenin transcription, which suggest that the inhibitory effect of SS on PDE5 and activation of the cGMP/PKG signaling involves the repression of β-catenin transcription. However, the underlying mechanism by which PKG inhibits β-catenin transcription requires further study. A previous report has shown binding sites of numerous transcription factors within CTNNB1 promoter, including AP-1, TCF/LEF, E2F1, NF-κB, MEF1, etc. (29). Moreover, another report demonstrated that PKGII inhibits the transcriptional activity of AP-1, and expression level of components of AP-1, c-Jun and c-Fos, thereby suppressing the proliferation of gastric cancer cells (46). It is possible that PKG may inhibit transcription of β-catenin through negative regulation of these transcription factors (e.g. AP-1), although further studies are necessary to test this possibility. Other investigators have reported that PKG activation can also down-regulate β-catenin expression by promoting its degradation (20, 36). Although SS did not increase the level of phosphorylated β-catenin in our experiments, it is possible that SS stimulated the phosphorylation of β-catenin, but may not have been detectable due to its rapid degradation.
As depicted in Figure 6D, we conclude that SS can selectively inhibit colon tumor cell proliferation and induce apoptosis by inhibiting PDE5 to increase intracellular cGMP levels and activate PKG signaling. The relevance of this pathway to tumorigenesis was demonstrated by its ability to inhibit Wnt/β-catenin-dependent TCF transcriptional activity, which leads to the suppression of critical proteins that regulate colon tumor cell proliferation and apoptosis. These data suggest PDE5 is an important target of SS that is partially or fully responsible for its antineoplastic activity, and which can be targeted to develop safer and more efficacious drugs for CRC chemoprevention.
Acknowledgments
Grant Support: This work was supported by National Institutes of Health Grants, 1R01CA131378, 1R01CA148817, 1R01CA155638, and 1R21CA160280.
We thank Dr. Darren Browning from Georgia Regents University Cancer Center for providing the CTNNB1 luciferase reporter construct.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- COX
cyclooxygenase
- CRC
colorectal cancer
- FP
fluorescence polarization
- NSAID
nonsteroidal anti-inflammatory drug
- PDE
phosphodiesterase
- PKA
cAMP dependent protein kinase
- PKG
cGMP dependent protein kinase
- SS
sulindac sulfide
- VASP
vasodilator-stimulated phosphoprotein
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Author Contributions: Conception and design: N.L., G.A.P.; Development of methodology: N.L, H.N.T., B.D.G., A.B.K., Y.L.; Acquisition of data: N.L., H.N.T., Y.X., B.Z., E.G., W.C.; Analysis and interpretation of data: N.L., Y.X., W.E.G., M.L.C., G.A.P.; Writing, review, and/or revision of the manuscript: N.L., G.A.P.; Administrative, technical, or material support: X.C., A.H.A., M.P.M.; Study supervision: G.A.P.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Wingo PA, Ries LA, Parker SL, Heath CW., Jr Long-term cancer patient survival in the United States. Cancer Epidemiol Biomarkers Prev. 1998;7:271–82. [PubMed] [Google Scholar]
- 3.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Chan TA. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol. 2002;3:166–74. doi: 10.1016/s1470-2045(02)00680-0. [DOI] [PubMed] [Google Scholar]
- 5.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–66. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 6.Rigau J, Pique JM, Rubio E, Planas R, Tarrech JM, Bordas JM. Effects of long-term sulindac therapy on colonic polyposis. Ann Intern Med. 1991;115:952–4. doi: 10.7326/0003-4819-115-12-952. [DOI] [PubMed] [Google Scholar]
- 7.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–6. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 8.Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–9. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 9.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 10.Matsuhashi N, Nakajima A, Fukushima Y, Yazaki Y, Oka T. Effects of sulindac on sporadic colorectal adenomatous polyps. Gut. 1997;40:344–9. doi: 10.1136/gut.40.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, Vogelstein B, et al. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis. 1996;17:1757–60. doi: 10.1093/carcin/17.8.1757. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoud NN, Boolbol SK, Dannenberg AJ, Mestre JR, Bilinski RT, Martucci C, et al. The sulfide metabolite of sulindac prevents tumors and restores enterocyte apoptosis in a murine model of familial adenomatous polyposis. Carcinogenesis. 1998;19:87–91. doi: 10.1093/carcin/19.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Li H, Finn T, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer research. 1997;57:2909–15. [PubMed] [Google Scholar]
- 14.Niv Y, Fraser GM. Adenocarcinoma in the rectal segment in familial polyposis coli is not prevented by sulindac therapy. Gastroenterology. 1994;107:854–7. doi: 10.1016/0016-5085(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 15.Alberts DS, Hixson L, Ahnen D, Bogert C, Einspahr J, Paranka N, et al. Do NSAIDs exert their colon cancer chemoprevention activities through the inhibition of mucosal prostaglandin synthetase? J Cell Biochem Suppl. 1995;22:18–23. doi: 10.1002/jcb.240590804. [DOI] [PubMed] [Google Scholar]
- 16.Rigas B, Kashfi K. Cancer prevention: a new era beyond cyclooxygenase-2. J Pharmacol Exp Ther. 2005;314:1–8. doi: 10.1124/jpet.104.080564. [DOI] [PubMed] [Google Scholar]
- 17.Stoner GD, Budd GT, Ganapathi R, DeYoung B, Kresty LA, Nitert M, et al. Sulindac sulfone induced regression of rectal polyps in patients with familial adenomatous polyposis. Adv Exp Med Biol. 1999;470:45–53. doi: 10.1007/978-1-4615-4149-3_5. [DOI] [PubMed] [Google Scholar]
- 18.Arber N, Kuwada S, Leshno M, Sjodahl R, Hultcrantz R, Rex D, et al. Sporadic adenomatous polyp regression with exisulind is effective but toxic: a randomised, double blind, placebo controlled, dose-response study. Gut. 2006;55:367–73. doi: 10.1136/gut.2004.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piazza GA, Thompson WJ, Pamukcu R, Alila HW, Whitehead CM, Liu L, et al. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res. 2001;61:3961–8. [PubMed] [Google Scholar]
- 20.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, et al. Exisulind induction of apoptosis involves guanosine 3′,5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000;60:3338–42. [PubMed] [Google Scholar]
- 21.Tinsley HN, Gary BD, Thaiparambil J, Li N, Lu W, Li Y, et al. Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal anti-inflammatory drugs is associated with phosphodiesterase 5 inhibition. Cancer Prev Res (Phila) 2010;3:1303–13. doi: 10.1158/1940-6207.CAPR-10-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soh JW, Kazi JU, Li H, Thompson WJ, Weinstein IB. Celecoxib-induced growth inhibition in SW480 colon cancer cells is associated with activation of protein kinase G. Mol Carcinog. 2008;47:519–25. doi: 10.1002/mc.20409. [DOI] [PubMed] [Google Scholar]
- 23.Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651–90. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- 24.Pastan IH, Johnson GS, Anderson WB. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- 25.Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/beta-catenin-mediated transcription in human breast tumor cells. Cancer Prev Res (Phila) 2011;4:1275–84. doi: 10.1158/1940-6207.CAPR-11-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, et al. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8:3331–40. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitt JD, Li N, Tinsley HN, Chen X, Zhang W, Li Y, et al. A Novel Sulindac Derivative that Potently Suppresses Colon Tumor Cell Growth by Inhibiting cGMP Phosphodiesterase and beta-Catenin Transcriptional Activity. Cancer Prev Res (Phila) 2012;5:822–33. doi: 10.1158/1940-6207.CAPR-11-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyer MP, Manzano LA, Merriman RL, Stauffer JS, Tanzer LR. NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev Biol Anim. 1996;32:315–7. doi: 10.1007/BF02722955. [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Dashwood WM, Zhong X, Al-Fageeh M, Dashwood RH. Cloning of the rat beta-catenin gene (Ctnnb1) promoter and its functional analysis compared with the Catnb and CTNNB1 promoters. Genomics. 2004;83:231–42. doi: 10.1016/j.ygeno.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Deguchi A, Soh JW, Li H, Pamukcu R, Thompson WJ, Weinstein IB. Vasodilator-stimulated phosphoprotein (VASP) phosphorylation provides a biomarker for the action of exisulind and related agents that activate protein kinase G. Mol Cancer Ther. 2002;1:803–9. [PubMed] [Google Scholar]
- 31.Han A, Song Z, Tong C, Hu D, Bi X, Augenlicht LH, et al. Sulindac suppresses beta-catenin expression in human cancer cells. Eur J Pharmacol. 2008;583:26–31. doi: 10.1016/j.ejphar.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice PL, Kelloff J, Sullivan H, Driggers LJ, Beard KS, Kuwada S, et al. Sulindac metabolites induce caspase- and proteasome-dependent degradation of beta-catenin protein in human colon cancer cells. Mol Cancer Ther. 2003;2:885–92. [PubMed] [Google Scholar]
- 33.Chang WC, Everley LC, Pfeiffer GR, 2nd, Cooper HS, Barusevicius A, Clapper ML. Sulindac sulfone is most effective in modulating beta-catenin-mediated transcription in cells with mutant APC. Ann N Y Acad Sci. 2005;1059:41–55. doi: 10.1196/annals.1339.020. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Liu L, David ML, Whitehead CM, Chen M, Fetter JR, et al. Pro-apoptotic actions of exisulind and CP461 in SW480 colon tumor cells involve beta-catenin and cyclin D1 down-regulation. Biochem Pharmacol. 2002;64:1325–36. doi: 10.1016/s0006-2952(02)01345-x. [DOI] [PubMed] [Google Scholar]
- 35.Kwon IK, Wang R, Thangaraju M, Shuang H, Liu K, Dashwood R, et al. PKG inhibits TCF signaling in colon cancer cells by blocking beta-catenin expression and activating FOXO4. Oncogene. 2010;29:3423–34. doi: 10.1038/onc.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Li H, Underwood T, Lloyd M, David M, Sperl G, et al. Cyclic GMP-dependent protein kinase activation and induction by exisulind and CP461 in colon tumor cells. J Pharmacol Exp Ther. 2001;299:583–92. [PubMed] [Google Scholar]
- 37.Pusztai L, Zhen JH, Arun B, Rivera E, Whitehead C, Thompson WJ, et al. Phase I and II study of exisulind in combination with capecitabine in patients with metastatic breast cancer. J Clin Oncol. 2003;21:3454–61. doi: 10.1200/JCO.2003.02.114. [DOI] [PubMed] [Google Scholar]
- 38.Whitehead CM, Earle KA, Fetter J, Xu S, Hartman T, Chan DC, et al. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol Cancer Ther. 2003;2:479–88. [PubMed] [Google Scholar]
- 39.Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila) 2009;2:572–80. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies NM, Watson MS. Clinical pharmacokinetics of sulindac. A dynamic old drug. Clinical pharmacokinetics. 1997;32:437–59. doi: 10.2165/00003088-199732060-00002. [DOI] [PubMed] [Google Scholar]
- 41.Anderson CB, Neufeld KL, White RL. Subcellular distribution of Wnt pathway proteins in normal and neoplastic colon. Proc Natl Acad Sci U S A. 2002;99:8683–8. doi: 10.1073/pnas.122235399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 43.Shih IM, Yu J, He TC, Vogelstein B, Kinzler KW. The beta-catenin binding domain of adenomatous polyposis coli is sufficient for tumor suppression. Cancer Res. 2000;60:1671–6. [PubMed] [Google Scholar]
- 44.Lu W, Tinsley HN, Keeton A, Qu Z, Piazza GA, Li Y. Suppression of Wnt/beta-catenin signaling inhibits prostate cancer cell proliferation. Eur J Pharmacol. 2009;602:8–14. doi: 10.1016/j.ejphar.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clapper ML, Coudry J, Chang WC. beta-catenin-mediated signaling: a molecular target for early chemopreventive intervention. Mutat Res. 2004;555:97–105. doi: 10.1016/j.mrfmmm.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Sang J, Chen Y, Jiang L, Tao Y, Wu Y, Wang Y, et al. Type II cGMP-dependent protein kinase inhibits ERK/JNK-mediated activation of transcription factors in gastric cancer cells. Molecular medicine reports. 2012;6:1190–4. doi: 10.3892/mmr.2012.1050. [DOI] [PubMed] [Google Scholar]