Summary
In both eukaryotes and prokaryotes, chromosomal DNA undergoes replication, condensation-decondensation and segregation, sequentially, in some fixed order. Other conditions, like sister-chromatid cohesion (SCC), may span several chromosomal events. One set of these chromosomal transactions within a single cell cycle constitutes the “chromosome cycle”. For many years it was generally assumed that the prokaryotic chromosome cycle follows major phases of the eukaryotic one: -replication-condensation-segregation-(cell division)-decondensation-, with SCC of unspecified length. Eventually it became evident that, in contrast to the strictly consecutive chromosome cycle of eukaryotes, all stages of the prokaryotic chromosome cycle run concurrently. Thus, prokaryotes practice “progressive” chromosome segregation separated from replication by a brief SCC, and all three transactions move along the chromosome at the same fast rate. In other words, in addition to replication forks, there are “segregation forks” in prokaryotic chromosomes. Moreover, the bulk of prokaryotic DNA outside the replication-segregation transition stays compacted. I consider possible origins of this concurrent replication-segregation and outline the “nucleoid administration” system that organizes the dynamic part of the prokaryotic chromosome cycle.
Keywords: chromosome replication, chromosome segregation, sister-chromatid cohesion, chromosome condensation, nucleoid administration, chromosome organization
Definition of the chromosome cycle
Cells grow and divide, producing daughter cells that can do the same. The overt cellular events between two consecutive cell divisions include G1 phase of “rest” after the previous cell division, S phase of the DNA content doubling, G2 phase of preparation for cell division and M (mitosis) phase of cell division itself. The letters are different in prokaryotes: G1 = I (or B), S = C (“chromosome”), while G2 + M = D (“division”) (Helmstetter, 1987). All cells go through the same four events every generation, - G1 - S - G2 - M - (in prokaryotes, - I - C - D -), in this particular order, constituting the cell cycle (Cooper, 2000). The main difference between prokaryotic and eukaryotic cells (besides the smaller size of the former) is that bacterial chromosomal DNA is organized as a nucleoid, an amorphous mass without defined borders floating in the cytoplasm (Pettijohn, 1996, Woldringh & Nanninga, 1985), whereas in eukaryotes the chromosomal DNA is contained within the nucleus, an organelle with its own double membrane (Cooper, 2000).
The cell cycle in its linear presentation with the DNA content as a read-out reveals no difference between mammalian cells (higher eukaryote) and slowly-growing E. coli (prokaryote) (Fig. 1A) (Baserga & Wiebel, 1969, Bates et al., 2005, Boye et al., 1996), indicating that the DNA replication-cell division relationship is the same across kingdoms. However, due to the apparent differences between eukaryotes and prokaryotes in genetic material organization and dynamics (de la Cueva-Mendez & Labib, 2008), this does not have to be true for other chromosomal transactions. Chromosomes undergo four major transactions: replication, segregation, condensation and decondensation (Hirano, 2000) (Fig. 1D–F). These transactions are performed in every cell, in a fixed order one after another, forming their own cycle within the cell cycle. Therefore, one set of these transactions within the cell cycle forms the chromosome cycle. The term “chromosome cycle” has been used lately to emphasize chromosome cycling between replication and segregation (Stillman, 2005, Blow & Tanaka, 2005, de la Cueva-Mendez & Labib, 2008). I will use “chromosome cycle” to mean a specific order of the chromosome transactions (replication, segregation, condensation and decondensation) within the cell cycle. This definition provides a framework to discuss differences and similarities between the chromosome cycles of eukaryotes and prokaryotes.
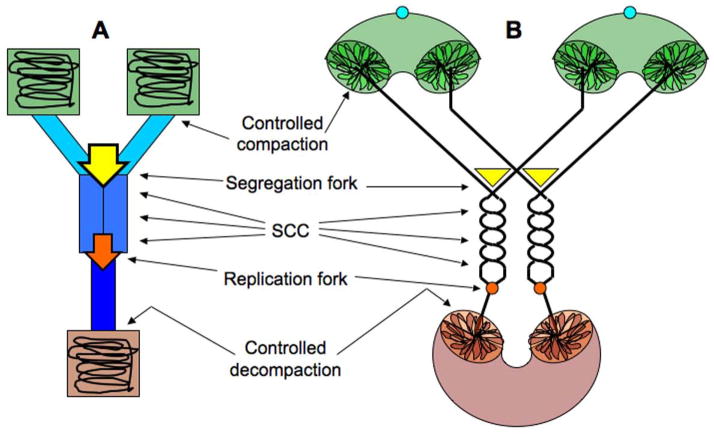
Fig. 1. The eukaryotic chromosome cycle.
A–C: Timing of events of the eukaryotic chromosomal cycle in the overall cell cycle. The cell cycle is presented in the linear form and is plotted along the X axis. A. Chromosomal DNA replication. B. Condensation-decondensation. C. Sisiter-chromatid cohesion (SCC) and chromosome segregation. In “B” and “C”, the plot of chromosomal DNA replication is shown on the background.
D–F: The three major chromosomal transactions. These three panels represent mechanisms behind the read-outs in panels A–C. In this and subsequent diagrams, DNA duplex is shown as a single line. D. Replication and sister-chromatid cohesion. There are two types of SCC: the physical cohesion due to sister chromatid catenation on the left and the “biochemical” cohesion due to encircling by cohesin protein on the right. E. Condensation-decondensation. F. Segregation.
G. The eukaryotic chromosome cycle. The colored triangle covering the top right part of the diagram shows duration of sister-chromatid cohesion. It also coincides with the area of the cell cycle under the control of CDK.
The eukaryotic chromosome cycle
The chromosome cycle of eukaryotes (—> replicate —> condense in preparation for mitosis —> segregate —(cell division)—> decondense —>) (Losada & Hirano, 2001, Mazia, 1987) (Fig. 1G) is an unsung textbook fixture. Sister-chromatid cohesion (SCC) is an important chromosome condition in eukaryotes that spans several individual transactions of the chromosome cycle and responds directly to the cell-cycle engine. SCC ensures that the two sister duplexes stay together (Fig. 1D) during the colored part of the chromosome cycle (Hirano, 2000, Nasmyth et al., 2000) (Fig. 1G), which coincides with the part of the cell cycle regulated by cycline-dependent kinases (Blow & Tanaka, 2005, Stillman, 2005). The protein bonds holding sisters chromatids together are completely broken only after condensation, in preparation for chromosome segregation (Hirano, 2000, Nasmyth et al., 2000).
The linear presentation of the eukaryotic cell cycle taking chromosome condensation-decondensation as the read-out (Fig. 1B) starts with condensed chromosomes at the beginning of G1 which rapidly decondense and stay decondensed through most of G1, S and all G2 phases, only to be recondensed at the beginning of M phase and to stay condensed throughout M. When the read-out is the number of chromosomes (the segregation status) (Fig. 1C), the action initiates with the beginning of M phase, coincident with the metaphase separation of the sister chromatids due to the cessation of SCC, resulting in the doubling of the chromosome number, followed by their immediate segregation, with the numbers again becoming one half of the maximal at cell division. Below I will review the evidence for or against these phases in the context of the prokaryotic chromosome cycle, as being recently described in C. cresentus, V. cholera, B. subtilis and P. aeruginosa and of course, E. coli. In fact, because of the dominance of E. coli’s research on the subject, discussion unavoidably becomes E. coli-centric, but the finding in other bacteria supports this overall picture of the prokaryotic chromosome cycle.
A snapshot of the prokaryotic chromosome cycle
A typical bacterial chromosome is circular (Fig. 2A) and compactly organized in an oval-shaped nucleoid, perhaps as a stack of rosette-like layers (Kavenoff & Bowen, 1976), that condenses into a toroid if translation is blocked (Fig. 2B) (Zimmerman, 2006). The chromosome is replicated by a single replication bubble (“unibubble” replication), — reflected in the familiar Cairns structure of theta-replication (Fig. 2C) (Cairns, 1963, Wake, 1973), sometimes drawn as “butterfly” to emphasize segregation (Fig. 2D). However, it is not a simple DNA circle that is replicated, but a single higher-order structure, a DNA molecule packed into a nucleoid, is converted into two such structures (Fig. 2E). When the period of SCC following replication is also shown, a scheme of the replicating nucleoid depicting all the major events of the bacterial chromosome cycle becomes elaborate (Fig. 2F). Similar schemes, starting with Dingman’s replication factory (Dingman, 1974), are now ubiquitous, instantly recognizable and broadly useful for discussion of prokaryotic nucleoid behavior (Deng et al., 2002, Draper & Gober, 2002, Gordon & Wright, 2000, Holmes & Cozzarelli, 2000, Lemon & Grossman, 1998, Lemon & Grossman, 2001, Løbner-Olesen & Kuempel, 1992, Reyes-Lamothe et al., 2008b, Sawitzke & Austin, 2001, Toro & Shapiro, 2010, Wang et al., 2013). These schemes make it obvious that the bacterial chromosome cycle is distinct from the eukaryotic chromosome cycle, in that events that are sequential in eukaryotic chromosomes and affect the whole set of chromosomes at once, appear contemporaneous in the prokaryotic chromosome, affecting only a small portion of it in a moving window. In particular, this snapshot of prokaryotic chromosome cycle stresses the limited amount of DNA being both replicated and segregated outside of the otherwise compacted nucleoids, that pack the bulk of prokaryotic DNA during the chromosome cycle (Fig. 2F). In an effort to place the events of the prokaryotic chromosome cycle within the established framework of the eukaryotic chromosome cycle, or to reveal the differences between the two cycles, I will start with the most conserved phase, which is DNA replication.
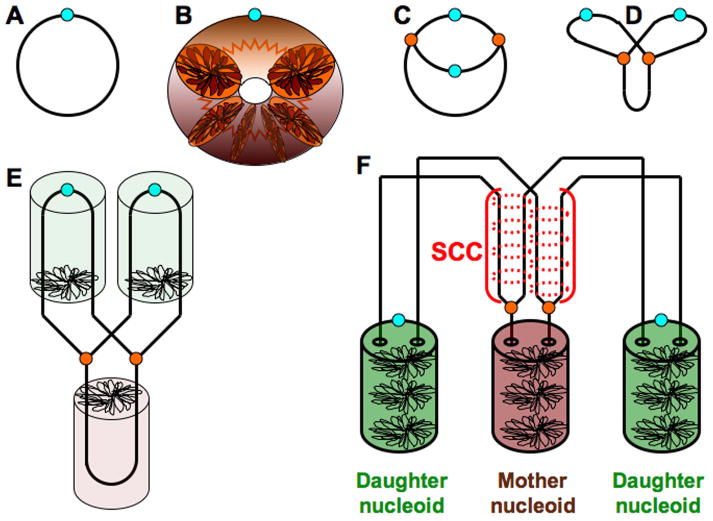
Fig. 2. The prokaryotic nucleoid.
In this and subsequent diagrams, the cylinders represent condensed chromosomal DNA, small light blue circles represent the replication origins, and small orange circles represent replication forks. A. A scheme of a circular prokaryotic chromosome with a unique replication origin. B. In fact, the chromosomal DNA is an extremely long molecule, roughly 1,000 times longer than the nucleoid structure in which it is packed. A chloramphenicol-condensed nucleoid of a non-replicating monomer chromosome is a toroid with a rosette-like DNA packing at the cross-section (several of these rosettes are shown). C. A scheme of bacterial chromosome undergoing theta-replication (the Cairns structure). D. The same theta-structure represented in its segregation-oriented “butterfly” form (Peter et al., 1998). E. Replication of the packed chromosome followed by immediate segregation leads to the formation of two packed daughter nucleoids. In this and some subsequent diagrams, replicating nucleoids are shown as cylinders, instead of partial toroids, for simplicity. F. Same as in “E”, but the period of sister-chromatid cohesion (SCC) is shown right behind the replication forks.
Replication
Enzymatically, the replication forks are organized similarly in both eukaryotes and prokaryotes (Marians, 1992, Masai et al., 2010). The main difference in the overall format of chromosomal replication is the number of replication bubbles. While there are several bubbles per chromosome in lower eukaryotes and hundreds of bubbles per chromosome in higher eukaryotes (Fig. 3A, the “multibubble single round” replication) (Masai et al., 2010), in prokaryotes there is typically a unique replication origin initiating a single bubble which is responsible for replication of the entire chromosome (Sernova & Gelfand, 2008) (Fig. 3B, the “unibubble single round” replication). Apparently, the replication fork progress in bacteria is fast enough for the entire chromosome to be replicated by a single bubble. However, in the best growth conditions, E. coli cells can divide every 20 minutes, while their shortest chromosome replication time is ~40 minutes (Bremer & Dennis, 1987). In a second difference from eukaryotes, instead of initiating additional replication bubbles from secondary origins to accelerate replication rate within the tardy round, as eukaryotes would do (Masai et al., 2010), E. coli initiates additional replication rounds to run in parallel in the same cell (Bremer & Dennis, 1987) (Fig. 3C, the “unibubble multiround” replication). Other bacteria that can divide faster than it takes to replicate their genome, like B. subtilis (Quinn & Sueoka, 1970) and V. cholera (Stokke et al., 2011) are also capable of unibubble multiround replication. Thus, the format of chromosomal replication in prokaryotes is dramatically different from the one in eukaryotes, both in the number and position of replication bubbles per chromosome and the possible number of replication rounds per cell.
Fig. 3. Chromosome replication and segregation in prokaryotes.
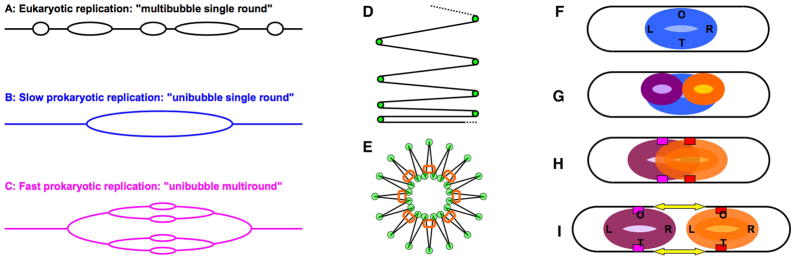
A–C: General formats of eukaryotic versus prokaryotic replication. A. Multi-bubble single round replication of eukaryotes. B. Unibubble single round replication of slowly-growing prokaryotes. C. Unibubble multiround replication of fast-growing prokaryotes.
D and E: Bacterial DNA compaction. D. The general pattern of DNA compaction: “continuous-feed printer paper”. Green circles here and in “E”, DNA-compacting proteins. E. The radial loop model of a compacted bacterial chromosome. Orange ovals, condensins MukBEF.
F–I: The first model of chromosomal segregation in prokaryotes by Jacob, Brenner and Cuzin (1963). Letters in the nucleoid: O, origin; T, terminus; L, the left replichore; R, the right replichore. F. An unreplicated nucleoid. G. A nucleoid in the process of replication. H. Fully replicated but unsegregated nucleoid. Small red or magenta rectangles designate nucleoid attachment to the cell envelope. I. Preferential growth of the envelope between the sites of attachment of the sister nucleoids not only elongates the cell, but also moves the sister nucleoids apart, effecting segregation.
Sister-chromatid cohesion
In eukaryotes, the newly-replicated sister chromatids are held together as a pair, encircled by “cohesins”, in a phenomenon called “sister-chromatid cohesion” (Fig. 1D) (Hirano, 2000, Nasmyth et al., 2000). In its basics, the eukaryotic paradigm demands that sister-chromatid cohesion — the physical linking of two newly-replicated DNA duplexes and eventually of the whole daughter chromosomes — should last much longer than the chromosome replication period (S), because cohesins are loaded before replication and removed during segregation (Uhlmann, 2009), making SCC encompass not only S, but also the whole of G2 and even part of M, lasting exactly half of the eukaryotic chromosome cycle (Fig. 1G) (time-wise, this may correspond to 80% of the cell cycle in some organisms). SCC is eroded gradually in chromosomal arms, allowing their condensation. When it disappears by the end of metaphase by removal of the centromere cohesins, all chromosomes became free from their sisters simultaneously, as a result of the dedicated cell cycle signaling cascade (Hirano, 2000, Nasmyth et al., 2000).
In typical laboratory growth conditions, sister-chromatid cohesion in E. coli lasts 15–20 minutes at the replication origin and longer at the replication terminus (Bates & Kleckner, 2005, Lesterlin et al., 2012, Nielsen et al., 2006, Nielsen et al., 2007, Wang et al., 2008), although substantial variations in distinct growth conditions were reported (Adachi et al., 2008). In slowly-growing cells, SCC has a similar duration (15 minutes) for loci in the rest of the chromosome (Nielsen et al., 2006, Nielsen et al., 2007), but in fast-growing cells, SCC in the bulk of the chromosome drops below 6 minutes (Nielsen et al., 2007), roughly 15% of the fastest C period in E. coli (~40 minutes). In an unrelated and indirect observation, nicks in the newly-synthesized DNA in fast-growing E. coli are converted into double-strand breaks five minutes after replication fork passage (Kouzminova & Kuzminov, 2012), perhaps reflecting a qualitative transition in the nascent DNA, most likely from SCC to segregation and/or condensation. In Caulobacter, SCC must be also short, as segregation of the chromosomal loci quickly follows their replication (Viollier et al., 2004). The segregation times of chromosomal loci in Pseudomonas are also consistent with a short SCC period (Vallet-Gely & Boccard, 2013). Because SCC lasts only a fraction of the S-period in these bacteria, it cannot be released at the same time for all chromosomal loci, so it is both established and released progressively (by analogy with “progressive segregation” (Nielsen et al., 2006, Nielsen et al., 2007) below), squeezed between replication and segregation.
Finally, in contrast to eukaryotes, where their long SCC is mostly “biochemical” in nature, promoted by special proteins called “cohesins” that actively encircle the two sisters (Fig. 1D) (Díaz-Martínez et al., 2008, Uhlmann, 2009), the progressive SCC in E. coli appears to be mostly physical in nature, caused by DNA precatenanes, which are coils of one sister duplex around the other (Fig. 1D), formed as a result of incomplete removal of torsional stress in front of the replication fork (Peter et al., 1998). The model of precatenane-based SCC in E. coli is based on the inverse correlation of SCC length with Topo IV activity in the cell (Lesterlin et al., 2012, Wang et al., 2008); Topo IV is the topoisomerase responsible for removal of precatenanes (reviewed in (Espeli & Marians, 2004)). Sister chromosome catenation is also known in eukaryotes, first described in replicating SV40 (Sundin & Varshavsky, 1980), but it is a minor part of SCC in eukaryotic chromosomes (Díaz-Martínez et al., 2008, Farcas et al., 2011). As an exception to the rule, the longer SCC in the terminus macrodomain of the E. coli chromosome does depend on the sequence-specific DNA-binding protein MatP (Espéli et al., 2012, Mercier et al., 2008), that can be considered a chromosome domain-specific bacterial cohesin. Nevertheless, in contrast to the eukaryotic SCC, which is a CDK-regulated chromosome condition spanning several phases of the eukaryotic chromosome cycle (Fig. 1G), progressive prokaryotic SCC constitutes a distinct phase of the prokaryotic chromosome cycle, sandwiched between the replication and segregation phases.
DNA compaction and chromosome condensation
Since the linear size of genomic DNA is many times longer than the size of the compartment that houses it, chromosomal DNA in the prokaryotic cells is compacted by a factor of 1,000 (Holmes & Cozzarelli, 2000, Trun & Marko, 1998), while in eukaryotic interphase nuclei this factor is about 100 (Guacci et al., 1994, Trask et al., 1989). The “chromatin architecture” proteins from eukaryotes and prokaryotes have three similar modes of action (Luijsterburg et al., 2008), suggesting that principles of DNA compaction are preserved across kingdoms. A growing E. coli cell has tens of thousands of copies of small nucleoid-associated proteins, like HU, IHF, H-NS and Fis, that act to compact bacterial DNA by bending it at angles sometimes approaching 180°. Bent in such a way at regular intervals, DNA collapses on itself like a long strip of continuous-feed printer paper, neatly packed in a box 1,000 times shorter than paper’s length, with 180° bends separating one “page” from the next one (Fig. 3D). The distinct packing format of eukaryotic DNA includes compaction by histones and coiling into 30 nm fibers (Grigoryev & Woodcock, 2012).
After DNA replication and the visually uneventful G2 period, eukaryotic chromosomes undergo additional condensation in prophase to prepare for alignment in metaphase and segregation in anaphase, before cell division (Fig. 1) (Cooper, 2000). This additional mitotic condensation is only ~2X for the diminutive chromosomes of S. cerevisiae (Loidl, 2003), but is an order of magnitude higher in human chromosomes (Trask et al., 1993) and reaches an astonishing 1,000X in mitotic chromosomes of barley (Wanner & Formanek, 2000), effected by a protein complex called “condensin” that organizes DNA in loops (Losada & Hirano, 2001). The varying degree of mitotic condensation reflects the length of the DNA in the chromosome vis-à-vis the size of the mitotic spindle in that particular organism: the longer the chromosomes and the shorter the spindle, the greater the required degree of mitotic condensation. On a more general level, mitotic chromosome condensation reflects the fact that entire chromosomes are segregated as one unit during mitotic anaphase.
In another significant departure from the eukaryotic chromosome cycle, no additional chromosome condensation, similar to the one that occurs during eukaryotic prophase, is observed prior to cell division in prokaryotes (Errington et al., 2005, Nordstrom & Dasgupta, 2001, Woldringh, 1976, Zusman et al., 1973). In fact, no additional chromosomal DNA condensation is observed in prokaryotes even leading up to chromosome segregation proper; the reason for this will become evident in the next section. Due to this lack of apparent “process of condensation” or the period of obvious tightening of the entire nucleoid in prokaryotes, there is even an argument that the prokaryotic chromosome does not have to be condensed in any organized way, because of the “mandatory condensation” in prokaryotic cells due to molecular crowding (de Vries, 2010, Zimmerman & Murphy, 1996). One reason prokaryotic nucleoids do not globally change their compaction state, except for the apparently required decompaction in the parts that are undergoing duplication (casually mentioned in (Holmes & Cozzarelli, 2000); for the eukaryotic chromosomes, this pre-replicative de-compaction is reviewed in (Takahashi, 1989)), may be because the prokaryotic genome is transcribed continuously. In E. coli, transcription of some genes does show short dips around the time of replication fork passage, while transcription of other genes is unaffected (Zhou et al., 1997).
With the concurrent obligatory translation of the still growing transcripts, this continuous transcription opens up the nucleoid structure, through the transient attachment of its parts to the inner membrane, via the synthesis of membrane proteins (Woldringh, 2002). This continuous transcription also makes nucleoids fluid, by constantly bringing the currently expressed genes to the surface of the nucleoid, because ribosomes are excluded from compact DNA mass (Bakshi et al., 2012). If translation is blocked with chloramphenicol or several other agents, bacterial nucleoids become highly condensed and toroidal in shape (Bakshi et al., 2012, Zimmerman, 2006, Zusman et al., 1973) (Fig. 2B). Why do bacteria need to transcribe their genes continuously and therefore have to avoid tight DNA packing that would be perfect for eukaryotic-like mitosis will be explored later. The absence of mitotic-like nucleoid condensation suggests that the bacterial chromosome does not segregate as one unit.
Segregation
Eukaryotic chromosomes are segregated by the concerted action of mitotic spindle, the structure at least an order of magnitude larger than the biggest chromosome in a karyotype, that makes all chromosomes and all individual loci on these chromosomes segregate simultaneously and relatively quickly (Cooper, 2000). Before segregation, sister chromatids are held together by SCC through S- and G2-phases, as well as through condensation during mitotic prophase (Hirano, 2000, Nasmyth et al., 2000). Since there is no obvious suprachromosomal system to forcefully pull entire chromosomes within prokaryotic cells, the first idea of prokaryotic chromosome segregation was that the two complete sisters would be attached to the cell wall, and the preferential growth of the cell wall in between the attachment sites would push the condensed sister chromatids apart (Fig. 3H—>I) (Jacob et al., 1963). Again, all the genes on these chromatids would segregate together, even though relatively slowly. In elaboration of this scheme, the replication origin was proposed to function as a transient centromere, when oriC was found to bind the cell membrane in the hemimethylated state that lasts 10 minutes after initiation of chromosomal replication (Ogden et al., 1988).
However, experimental characterization gradually painted a different picture. First, segregation of the bacterial nucleoid was found to be continuous and concurrent with DNA replication, rather than a separate event after replication (Løbner-Olesen & Kuempel, 1992, van Helvoort & Woldringh, 1994, Woldringh, 1976). Since no segregation functions were identified by mutations, it was naturally proposed that this piece-meal segregation of the bacterial nucleoid is driven by entropy (Jun & Mulder, 2006, Pelletier et al., 2012). However, tracing the relative positions of pairs of individual chromosomal loci showed that bacterial nucleoid segregation, even though looking slow and primitive, is in fact a fast and highly controlled event (Niki et al., 2000). First, even though the nucleoid appears to be lacking specific organization, its four quarters in E. coli (that can be called “origin”, “terminus”, “left “ and “right”) are never mixed and occupy their own fixed places in the cell, suggesting a defined structure (Wang et al., 2006b) (Fig. 3F). Exquisite control of this structure is revealed by the striking translational symmetry of the two daughter nucleoids (Fig. 3I) (Wang et al., 2006b), that can be achieved only if segregation specifically sends the leading strands of the replication bubble to the poles of the cells, while the lagging strands to the center of the cell (White et al., 2008). Moreover, careful measurements in individual Caulobacter and E. coli cells showed that segregation of various loci along the chromosome is strictly sequential, rather than simultaneous, and for any given locus shortly follows replication of this locus, after a brief SCC (Nielsen et al., 2006, Nielsen et al., 2007, Viollier et al., 2004). Also, even though reaching home positions by the sister loci does take time (Espeli et al., 2008), statistically-significant segregation of individual loci is achieved within less than 10 minutes (Espeli et al., 2008, Nielsen et al., 2006, Viollier et al., 2004) suggesting that it operates on limited regions of unconstrained (naked?) DNA, rather than on condensed chromosomes or even sub-chromosomal domains. This type of continuous segregation, concurrent with DNA replication, was termed “progressive” (Nielsen et al., 2006, Nielsen et al., 2007). The same type of progressive segregation of individual chromosomal loci was recently described in Pseudomonas (Vallet-Gely & Boccard, 2013). There are also studies that are consistent with sequential, rather than continuous, segregation of the origin and terminus chromosomal macrodomains in E. coli (Bates & Kleckner, 2005, Espeli et al., 2008, Fisher et al., 2013, Joshi et al., 2011, Lesterlin et al., 2012). Although sequential segregation appears different from the progressive one, the overall conclusion is still that segregation of bacterial nucleoid is concurrent with replication.
The concept of concurrent replication/segregation brings up an important point. In principle, one could argue that bacteria keep their chromosome formally decondensed during segregation because it is so small in size and simple in organization that it can be segregated by entropy. On the other hand, blocking translation does condense bacterial chromosomes dramatically and could be used by the cell just before segregation, to facilitate movement of compact nucleoids. The bacterial cell does not use either option because, apparently, it needs to segregate with speed and precision and at the same time cannot stop gene expression, even temporarily. Therefore, it segregates the newly-replicated daughter DNA duplexes either in a linear sliding zipper-like fashion (Nielsen et al., 2007) (Fig. 4), or bundling them in chromosomal macrodomains first and segregating these subdomains sequentially ((Espeli et al., 2008) and as will be reviewed elsewhere). In either case, the overall rate of segregation matches the rate of replication, while the segregated DNA is immediately compacted into the daughter nucleoids. That is, in another dramatic departure from the eukaryotic paradigm, segregation of bacterial chromosome operates either with a naked DNA or with a minimally bundled one, the substrate opposite to the highly-condensed entire eukaryotic chromosomes segregated by the mitotic spindle.
Fig. 4. “Everything progressive”: SCC, segregation and controlled compaction/decompaction.
A. The “sliding zipper” segregation scheme. Brown box, mother nucleoid; green boxes, daughter nucleoids. Dark blue, parental DNA duplex; medium blue, unsegregated daughter duplexes; light blue, segregated daughter duplexes. Orange arrow, the replisome; yellow arrow (the slider of the zipper), the “segresome”.
B. The main events of the prokaryotic chromosomal cycle: mother nucleoid decompaction, replication of the decompacted DNA, SCC due to precatenanes, segregation of uncompacted DNA, daughter nucleoid organization (DNA recompaction). Yellow triangles designate “segresomes”. Rudner and colleagues (Wang et al., 2013) present essentially the same collection and order of events in their Fig. 2C.
Because of the short period of SCC that keeps sister duplexes together, similarly to the replication fork as a point of duplication of DNA sequences, we can now define “segregation fork” as the point of observable chromosomal foci doubling. This point of overt chromosome duplication moves along the already replicated portion of the chromosome, still held together by pre-catenanes, unzipping it with its own “slider”, that, by analogy with the replisome can be called the “segresome” (Fig. 4). Moreover, by analogy with “replication fork collapse” at nicks in template DNA (Kuzminov, 1995), there should be also “segregation fork collapse” at double-strand breaks formed at nicks behind the replication forks (Kouzminova & Kuzminov, 2012), the point that will be explored elsewhere.
Chromosome decondensation versus DNA recompaction
After segregation and cell division, eukaryotic chromosomes decondense and become available for transcription and replication (Cooper, 2000). In contrast to the metaphase chromosomes, which are organized around a fixed scaffold (Laemmli, 1978), the interphase eukaryotic chromosomes show no specific organization pattern (Bekers et al., 1986), even though evidence of chromosome territories (Cremer & Cremer, 2010) and of distinct interaction domains within individual chromosomes (Bickmore & van Steensel, 2013) argues for local as well as long-range arrangements similar to nucleoid macrodomains in E. coli (Espeli et al., 2008, Valens et al., 2004).
In contrast, nothing special, like loosening of the chromatin, happens in prokaryotes after segregation of the entire daughter nucleoids is complete. As mentioned above, prokaryotic DNA stays transcriptionally active throughout the entire cell cycle, with a small dip coinciding with the passage of replication forks. In fact, a transaction opposite to decondensation happens right after segregation of a particular DNA region, because the segregated DNA has to be organized into the new nucleoid (Fig. 4) (Wang et al., 2013). This organization restores regular compaction on bacterial DNA (see “DNA compaction and chromosomal condensation” section above). The compact structure of the E. coli chromosome is secured by the MukBEF protein complex (Wang et al., 2006a), a classic eukaryotic-like condensin (Chen et al., 2008, Uhlmann, 2009), so the overall format of local bacterial DNA packing is likely based on radial loops (Fig. 3E). Thus, during the post-segregation phase we again find no events compatible with the overall narrative of the eukaryotic chromosome cycle and, in fact, something opposite.
Reflective of organized compaction, if released from cells gently, nucleoids hold their overall shape (Foley et al., 2010, Murphy & Zimmerman, 1997). Electron microscopy of isolated nucleoids shows a rosette-like structure around a proteinaceous scaffold, with uniformly-sized independently-supercoiled loops (Kavenoff & Bowen, 1976), strikingly resembling the radial structure of histone-depleted human metaphase chromosomes (Laemmli, 1978). If the compact nucleoids are treated with agents that disrupt protein-DNA contacts (like SDS), they spread several-fold (Foley et al., 2010, Kavenoff & Bowen, 1976, Murphy & Zimmerman, 1997, Stonington & Pettijohn, 1971, Worcel & Burgi, 1972), confirming that they were compacted, even though still actively transcribed. Thus, another big difference from eukaryotes is that the prokaryotic chromosome, although accessible to transcription and translation, always stays maximally compacted, the overall format of this compaction being analogous to the radial loop structure of condensed human metaphase chromosomes (Marsden & Laemmli, 1979).
The prokaryotic chromosome cycle
The prokaryotic chromosome cycle has always appeared distinct from the eukaryotic one (de la Cueva-Mendez & Labib, 2008, Nordstrom & Dasgupta, 2001, Stillman, 2005). In fact, we see dramatic differences at every phase: 1) prokaryotes have a single replication bubble in their chromosome, in contrast to the multiple ones in eukaryotes; 2) prokaryotes have a brief and progressive sister-chromatid cohesion that affects only the newly-replicated DNA and is mostly controlled by topoisomerases, in contrast to the very long, genome-wide and cell cycle-controlled cohesion in eukaryotes; 3) prokaryotes lack post-replicational chromosome condensation in preparation for segregation that characterizes eukaryotic prophase of mitosis; 4) prokaryotes practice progressive segregation following DNA replication and SCC in real time and perhaps even operating at the level of naked DNA, in contrast to the “ensemble” segregation of the entire highly-condensed chromosomes in eukaryotes during anaphase of mitosis; 5) prokaryotes recompact their new DNA after segregation into daughter nucleoids, organizing them similarly to eukaryotic metaphase chromosomes, which is the exact opposite of eukaryotic post-segregational chromosome decondensation in G1; 6) overall, the prokaryotic chromosome stays compacted and organized at least 85% of the cell cycle and is only released from this compaction, in a sliding window, to replicate and segregate.
As mentioned in the Introduction, with the genome content as a read-out, the prokaryotic cell cycle looks no different than the eukaryotic one (cf. Fig. 1A and 5A). However, when the read-out is changed to the degree of condensation/compaction or the number of chromosomes per cell (segregation), there are clear differences between the two chromosome cycles. Eukaryotic chromosomes are decondensed during most of the cell cycle (G1, S and G2 phases) and are condensed only during mitosis (Fig. 1B). In contrast, prokaryotic chromosomes are compacted most of the cell cycle (D, I and most of C), but their individual regions become decompacted during the C-period, to be replicated, repaired if needed and segregated, only to be recompacted again (Fig. 5B). The timing of this short decompaction-replication-SCC-segregation-recompaction act is different for various chromosomal loci: those around the replication origin are first to undergo the process, while those around the replication terminus must wait until some 40 minutes later (in rapidly growing E. coli).
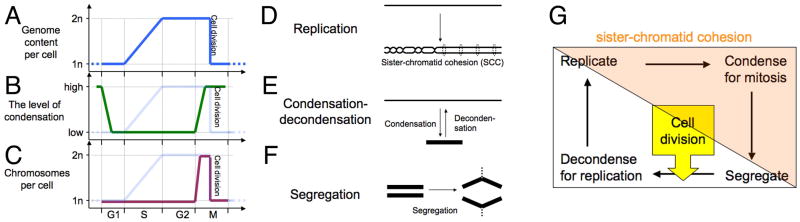
Fig. 5. The prokaryotic chromosome cycle.
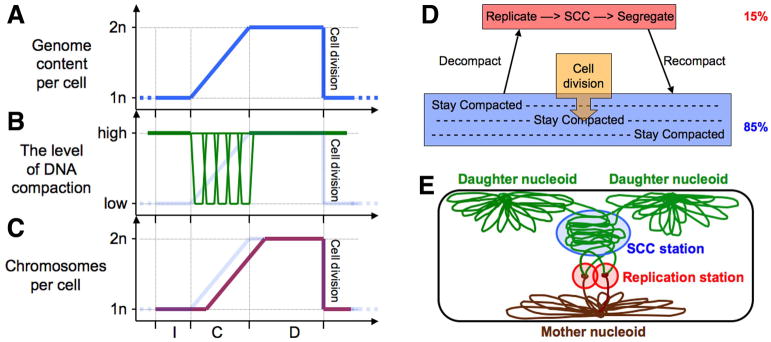
A–C: Timing of events of the prokaryotic chromosomal cycle in the overall cell cycle. See the legend of Fig. 1A–C for description.
D. A scheme of the prokaryotic chromosomal cycle. The percentages on the right show approximately how much of the total chromosomal DNA falls into these categories.
E. A snapshot of the prokaryotic chromosomal cycle within the cell. The “replication station” shows the two replisomes together, like in Bacillus subtilis (Lemon & Grossman, 1998), but they may also be physically unlinked, as in Escherichia coli (Reyes-Lamothe et al., 2008a).
In the eukaryotic chromosome cycle, because of the lengthy SCC, the number of visible chromosomes doubles just before their segregation and cell division, forming a short spike (Fig. 1C). In contrast, in the prokaryotic chromosome cycle, the doubling of the number of nucleoids per cell is a gradual process that shadows DNA replication, separated from it by a brief precatenane-based SCC (Fig. 5C). After segregation, the number of nucleoids stays doubled until cell division.
The overall scheme of the prokaryotic chromosome cycle (Fig. 5D) reflects these distinctions from the eukaryotic chromosome cycle (compare to Fig. 1G). The overall difference is that, while the eukaryotic chromosome cycle operates consecutively, by defined stages affecting simultaneously the entire chromosome set, the prokaryotic chromosome cycle operates concurrently, by the multi-stage “activity” (decondense-replicate-SCC-segregate-recondense) progressively affecting successive parts of the chromosome. This is why, in contrast to the eukaryotic chromosome cycle, it is possible to represent all phases of the prokaryotic chromosome cycle on a single scheme (Fig. 5E). It is worth repeating that the concurrent nature of bacterial chromosomal transactions was postulated a long time ago (Løbner-Olesen & Kuempel, 1992), and the clear difference from the sequential eukaryotic chromosomal transactions has been pointed out on several occasions (Dasgupta et al., 2000, de la Cueva-Mendez & Labib, 2008, Nordstrom & Dasgupta, 2001, Stillman, 2005).
What could be the selection behind the concurrent replication-segregation?
It is obvious that for stable reproduction, the chromosome cycle has to be linked with the cell cycle. This basic rule has no exceptions — otherwise, there will be cells with too many or too few chromosomes in the population, reducing the overall productivity of the latter. However, does it mean that only one chromosome cycles can be run in the same cell? The logic of the eukaryotic chromosome cycle, with its “ensemble” segregation temporally disconnected from replication and instead linked to cell division, indeed allows only one chromosome cycle per cell. The same is true for bacteria like Caulobacter, in which chromosome segregation is also linked to cell division, supporting the primordial logic of “one chromosome cycle per cell”. Yet, the typical prokaryotic chromosome cycle, with its progressive segregation mechanistically linked to DNA replication instead of cell division, offers the option of multiple chromosome cycles in the same cell.
The feasibility of having several chromosome cycles in the same cell is illustrated by the “unibubble-multiround” replication format of the rapidly-growing E. coli cells (Fig. 3C), commonly referred to as “multifork replication” (Lemon & Grossman, 2001, Quinn & Sueoka, 1970). Indeed, if the sister chromatids are not only replicated, but also completely segregated and compacted in the nucleoids of their own, there is nothing mechanistic standing in the way of new replication initiations in the daughter chromosomes, even though they are themselves still in the process of being created. The directional nature of this prokaryotic replication-segregation wave makes the incomplete daughter nucleoids initiation-competent immediately following the end of the eclipse period after the previous initiation (Olsson et al., 2002, von Freiesleben et al., 2000). In fact, the fastest-growing E. coli cells have either eight or even 16 replication origins in one cell (Morigen et al., 2009), translating into up to four overlapping chromosome cycles proceeding in parallel in the same cell and producing massive quantities of DNA at extremely high rates.
However, it is unlikely that this ability to increase DNA production exponentially was the driving selection for the development of the prokaryotic concurrent replication-segregation chromosome cycle. Bacteria are capable of incredible growth rates in the laboratory, but they never grow at these fast rates in their natural habitats (Savageau, 1983), and the first primordial prokaryotes were unlikely to enjoy conditions supporting fast growth either. One possible selection for multiple chromosome cycles per cell could have been the ability to temporarily disconnect the chromosome cycle from the cell cycle. That is, if the cell division is blocked for whatever reason, with such separation available the bacterial cells are still able to grow in size (in length), having regular chromosome cycles filling the filamentous cells with complete nucleoids, as observed in E. coli growing at low temperatures (Visvalingam et al., 2012) or upon encounter with sublethal concentrations of cell envelope-targeting antibiotics (Tanaka et al., 1976), excreted by competing microorganisms. Perhaps the major practitioners of multiple chromosome cycles without cell division are Actinobacteria, like Streptomyces (Jakimowicz & van Wezel, 2012).
The opposite situation in which multiround replication/segregation again becomes beneficial is during rapid vegetative growth in combination with bacteriostatic DNA damage (Suzuki et al., 1967), for example again due to inter-species competition, or upon general inhibition of DNA replication (Heinonen et al., 1976). Overlapping chromosome cycles under these conditions ensure that the rapidly growing cells initiate sufficient number of replication/segregation rounds to generate enough finished chromosomes by the time of cell division. Finally, the decision to divide may be delayed by the cell itself, in order to “outgrow” predation (Ammendola et al., 1998) or to facilitate nutrient acquisition and substrate exploration (Steinberger et al., 2002), as is the rationale in Streptomyces. This is somewhat analogous to the formation of syncytium tissues in multicelullar eukaryotic organisms, with the distinction that while nuclei in syncytial tissues are independent entities following their own individual cell cycles, the individual nucleoids in multinucleoid bacterial cells are still governed by the shared cytoplasmic signals, such as the concentration of DnaA initiator protein, allowing synchronous initiation at the multiple replication origins (Skarstad et al., 1986, Skarstad et al., 1988). Once cell division is unblocked, or becomes beneficial, the long multinucleoid cells would split into individual cells with single nucleoids, restoring the crucial link between the chromosome cycle and the cell cycle.
Concurrent replication-segregation in eukaryotes?
The concurrent replication-segregation was likely the original format of the chromosome cycle in the primitive eukaryotes. In fact, chromosomes of modern eukaryotes may still continuously segregate during the S-phase, as was repeatedly proposed (Dingman, 1974, Pflumm, 2002, Stack & Anderson, 2001), but this segregation is so short-distanced due to cohesin-imposed SCC, that its reality was never tested experimentally. According to this logic, the massive mitotic condensation and ensemble segregation, separate from replication but linked to cell division, evolved later, in response to the distinct requirements of meiosis (Bruce Stillman, personal communication). Eukaryotic chromosomes, in which autonomously-replicating sequences (ARSes or replication origins) coincide with centromeres, like in the yeast Yarrowia lipolytica (Vernis et al., 2001) or in the holocentric chromosomes of Caenorhabditis elegans (Felsenstein & Emmons, 1988), are organized similarly to prokaryotic chromosomes. DNA replication in these eukaryotic chromosomes could be directly followed by local condensation and segregation, that would pre-position centromeres for subsequent global segregation by the spindle (Dingman, 1974, Pflumm, 2002, Stack & Anderson, 2001). In fact, the phenomenon of non-random sister-chromatid segregation, indicative of such pre-positioning, is observed in both bacteria and eukaryotes (Falconer et al., 2010, Lark, 2012, White et al., 2008), suggesting that the primary segregation in eukaryotic chromosomes is concurrent with replication, just like in prokaryotes, so that it can pre-segregate sister chromosomes and their centromeres in such a way than the results of subsequent ensemble centromere-based segregation during mitosis become non-random.
Conclusion: nucleoid administration as a system to run the prokaryotic chromosome cycle
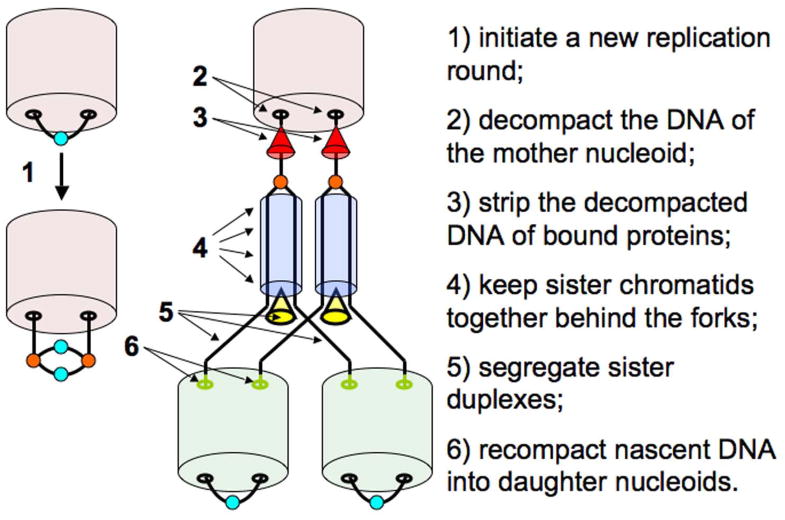
Since the prokaryotic chromosome cycle combines all its transitions (decompaction, replication, SCC, segregation and recompaction) together in a single event, this hyper-transition is performed in the same cellular location and, most likely, by physically-interacting activities. In fact, it has been proposed that the key events of the E. coli chromosome cycle are performed by a dedicated supramolecular structure around the SeqA protein (Norris et al., 2000). Sawitzke and Austin (Sawitzke & Austin, 2001) postulated five specific actions that are essential for the correct replication and segregation of the bacterial nucleoid. We would like to add replication initiation to this list, to make a total of six, and to call the overall system that runs the prokaryotic chromosome cycle “nucleoid administration”.
The six actions of nucleoid administration are (Fig. 6): 1) fire the replication origin on schedule to initiate a new replication round; 2) decompact the mother nucleoid in the chromosomal regions to be immediately replicated; 3) strip the decompacted DNA of bound proteins and enzymes, such as transcription regulators and RNA polymerases (the next action of feeding the naked mother DNA into the replisomes, and feeding daughter DNAs out is likely the function of replisomes themselves); 4) keep the nascent duplexes together for a period of sister-chromatid cohesion, while introducing regular helicity and removing pre-catenanes between them; 5) separate the two sister duplexes, channeling them towards the places of formation of the daughter nucleoids; 6) recompact the newly-replicated DNA into daughter nucleoids. How these several postulated activities of nucleoid administration are spatially and temporarily organized and controlled is currently unknown, with the exception of the regulation of replication initiation (Skarstad & Katayama, 2013), an exciting topic outside of the scope of this minireview.
Fig. 6. The proposed steps of nucleoid administration.
Red cones: activities that clean the template DNAs entering the replisomes of the bound proteins; yellow cones, the DNA segregation activities.
In conclusion, I revisited the accumulating body of evidence that the concurrent and progressive decompaction-replication-SCC-segregation-recompaction set of transactions in prokaryotes makes their chromosome cycle dramatically different from the eukaryotic one. I suggest that the prokaryotic chromosome cycle was developed to enable transient decoupling of the chromosome cycle from the cell cycle, as it allows a single cell to run several chromosome cycles in parallel during either inhibited DNA replication or postponed cell divisions. This transient decoupling temporarily removes limitations on the rate of cell mass growth from either the rate of DNA replication or from the rate of cell division. The idea of the distinct prokaryotic chromosome cycle offers an intellectual framework for characterization of the proposed system of nucleoid administration that executes the major chromosomal transactions in bacteria.
Acknowledgments
I would like to thank Bruce Stillman for encouragement and for the eukaryotic insights and Glen Cronan for helpful suggestions, hard-to-find references and general editing of the manuscript. Two anonymous referees helped a great deal to balance and clarify the presentation. Experimental work in this laboratory is supported by grant # GM 073115 from the National Institutes of Health.
Footnotes
The author declares no conflict of interest.
References
- Adachi S, Fukushima T, Hiraga S. Dynamic events of sister chromosomes in the cell cycle of Escherichia coli. Genes Cells. 2008;13:181–197. doi: 10.1111/j.1365-2443.2007.01157.x. [DOI] [PubMed] [Google Scholar]
- Ammendola A, Geisenberger O, Andersen JB, Givskov M, Schleifer KH, Eberl L. Serratia liquefaciens swarm cells exhibit enhanced resistance to predation by Tetrahymena sp. FEMS Microbiol Lett. 1998;164:69–75. doi: 10.1111/j.1574-6968.1998.tb13069.x. [DOI] [PubMed] [Google Scholar]
- Bakshi S, Siryaporn A, Goulian M, Weisshaar JC. Superresolution imaging of ribosomes and RNA polymerase in live Escherichia coli cells. Mol Microbiol. 2012;85:21–38. doi: 10.1111/j.1365-2958.2012.08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga R, Wiebel F. The cell cycle of mammalian cells. Int Rev Exp Pathol. 1969;7:1–30. [PubMed] [Google Scholar]
- Bates D, Epstein J, Boye E, Fahrner K, Berg H, Kleckner N. The Escherichia coli baby cell column: a novel cell synchronization method provides new insight into the bacterial cell cycle. Mol Microbiol. 2005;2005:380–391. doi: 10.1111/j.1365-2958.2005.04693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Kleckner N. Chromosome and replisome dynamics in E. coli: loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell. 2005;121:899–911. doi: 10.1016/j.cell.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekers AG, Pieck AC, Rijken AA, Wanka F. Evidence for the persistence of a decondensed chromosome scaffold in the interphase nucleus. J Cell Sci. 1986;86:155–171. doi: 10.1242/jcs.86.1.155. [DOI] [PubMed] [Google Scholar]
- Bickmore WA, van Steensel B. Genome architecture: domain organization of interphase chromosomes. Cell. 2013;152:1270–1284. doi: 10.1016/j.cell.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Blow JJ, Tanaka TU. The chromosome cycle: coordinating replication and segregation. EMBO Rep. 2005;6:1028–1034. doi: 10.1038/sj.embor.7400557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye E, Stokke T, Kleckner N, Skarstad K. Coordinating DNA replication initiation with cell growth: differential roles for DnaA and SeqA proteins. Proc Natl Acad Sci USA. 1996;93:12206–12211. doi: 10.1073/pnas.93.22.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H, Dennis PP. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt FC, editor. Escherichia coli and Salmonella typhimurium. Cellular and Molecular Biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1527–1542. [Google Scholar]
- Cairns J. The chromosome of Escherichia coli. Cold Spring Harbor Symp Quant Biol. 1963;28:43–46. doi: 10.1101/sqb.1974.038.01.007. [DOI] [PubMed] [Google Scholar]
- Chen N, Zinchenko AA, Yoshikawa Y, Araki S, Adachi S, Yamazoe M, Hiraga S, Yoshikawa K. ATP-induced shrinkage of DNA with MukB protein and the MukBEF complex of Escherichia coli. J Bacteriol. 2008;190:3731–3737. doi: 10.1128/JB.01863-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GM. The Cell: A Molecular Approach. Sinauer Associates; Sunderland (MA): 2000. p. 689. [Google Scholar]
- Cremer T, Cremer M. Chromosome territories. Cold Spring Harb Perspect Biol. 2010;2:a003889. doi: 10.1101/cshperspect.a003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Maisnier-Patin S, Nordström K. New genes with old modus operandi. The connection between supercoiling and partitioning of DNA in Escherichia coli. EMBO Rep. 2000;1:323–327. doi: 10.1093/embo-reports/kvd077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cueva-Mendez G, Labib K. New insights into the chromosome cycle. Conference on the Replication & Segregation of Chromosomes. EMBO Rep. 2008;9:1177–1181. doi: 10.1038/embor.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries R. DNA condensation in bacteria: Interplay between macromolecular crowding and nucleoid proteins. Biochimie. 2010;92:1715–1721. doi: 10.1016/j.biochi.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Deng W, Burland V, Plunkett Gr, Boutin A, Mayhew GF, Liss P, Perna NT, Rose DJ, Mau B, Zhou S, Schwartz DC, Fetherston JD, Lindler LE, Brubaker RR, Plano GV, Straley SC, McDonough KA, Nilles ML, Matson JS, Blattner FR, Perry RD. Genome sequence of Yersinia pestis KIM. J Bacteriol. 2002;184:4601–4611. doi: 10.1128/JB.184.16.4601-4611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Martínez LA, Giménez-Abián JF, Clarke DJ. Chromosome cohesion - rings, knots, orcs and fellowship. J Cell Sci. 2008;121:2107–2114. doi: 10.1242/jcs.029132. [DOI] [PubMed] [Google Scholar]
- Dingman CW. Bidirectional chromosome replication: some topological considerations. J Theor Biol. 1974;43:187–195. doi: 10.1016/s0022-5193(74)80052-4. [DOI] [PubMed] [Google Scholar]
- Draper GC, Gober JW. Bacterial chromosome segregation. Annu Rev Microbiol. 2002;56:567–597. doi: 10.1146/annurev.micro.56.012302.160729. [DOI] [PubMed] [Google Scholar]
- Errington J, Murray H, Wu LJ. Diversity and redundancy in bacterial chromosome segregation mechanisms. Philos Trans R Soc Lond B Biol Sci. 2005;360:497–505. doi: 10.1098/rstb.2004.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espéli O, Borne R, Dupaigne P, Thiel A, Gigant E, Mercier R, Boccard F. A MatP-divisome interaction coordinates chromosome segregation with cell division in E. coli. EMBO J. 2012;31:3198–3211. doi: 10.1038/emboj.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeli O, Marians KJ. Untangling intracellular DNA topology. Mol Microbiol. 2004;52:925–931. doi: 10.1111/j.1365-2958.2004.04047.x. [DOI] [PubMed] [Google Scholar]
- Espeli O, Mercier R, Boccard F. DNA dynamics vary according to macrodomain topography in the E. coli chromosome. Mol Microbiol. 2008;68:1418–1427. doi: 10.1111/j.1365-2958.2008.06239.x. [DOI] [PubMed] [Google Scholar]
- Falconer E, Chavez EA, Henderson A, Poon SS, McKinney S, Brown L, Huntsman DG, Lansdorp PM. Identification of sister chromatids by DNA template strand sequences. Nature. 2010;463:93–97. doi: 10.1038/nature08644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farcas AM, Uluocak P, Helmhart W, Nasmyth K. Cohesin’s concatenation of sister DNAs maintains their intertwining. Mol Cell. 2011;44:97–107. doi: 10.1016/j.molcel.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein KM, Emmons SW. Nematode repetitive DNA with ARS and segregation function in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:875–883. doi: 10.1128/mcb.8.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JK, Bourniquel A, Witz G, Weiner B, Prentiss M, Kleckner N. Four-dimensional imaging of E. coli nucleoid organization and dynamics in living cells. Cell. 2013;153:882–895. doi: 10.1016/j.cell.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley PL, Wilson DB, Shuler ML. Macromolecular crowding can account for RNase-sensitive constraint of bacterial nucleoid structure. Biochem Biophys Res Commun. 2010;395:42–47. doi: 10.1016/j.bbrc.2010.03.128. [DOI] [PubMed] [Google Scholar]
- Gordon GS, Wright A. DNA segregation in bacteria. Annu Rev Microbiol. 2000;54:681–708. doi: 10.1146/annurev.micro.54.1.681. [DOI] [PubMed] [Google Scholar]
- Grigoryev SA, Woodcock CL. Chromatin organization - the 30 nm fiber. Exp Cell Res. 2012;318:1448–1455. doi: 10.1016/j.yexcr.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Guacci V, Hogan E, Koshland D. Chromosome condensation and sister chromatid pairing in budding yeast. J Cell Biol. 1994;125:517–530. doi: 10.1083/jcb.125.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen J, Joronen I, Tuokko H. Adaptation of the cells of Escherichia coli to the presence of hydroxyurea increases the level of inorganic pyrophosphatase acttivity. Chem Biol Interact. 1976;12:91–98. doi: 10.1016/0009-2797(76)90070-3. [DOI] [PubMed] [Google Scholar]
- Helmstetter CE. Timing of synthetic activities in the cell cycle. In: Neidhardt FC, editor. Escherichia coli and Salmonella typhimurium. Cellular and Molecular Biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1594–1605. [Google Scholar]
- Hirano T. Chromosome cohesion, condensation, and separation. Annu Rev Biochem. 2000;69:115–144. doi: 10.1146/annurev.biochem.69.1.115. [DOI] [PubMed] [Google Scholar]
- Holmes VF, Cozzarelli NR. Closing the ring: links between SMC proteins and chromosome partitioning, condensation, and supercoiling. Proceedings of the National Academy of Sciences, United States of America. 2000;97:1322–1324. doi: 10.1073/pnas.040576797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F, Brenner S, Cuzin F. On the Regulation of DNA Replication in Bacteria. Cold Spring Harbor Symp Quant Biol. 1963;28:329–348. [Google Scholar]
- Jakimowicz D, van Wezel GP. Cell division and DNA segregation in Streptomyces: how to build a septum in the middle of nowhere? Mol Microbiol. 2012;85:393–404. doi: 10.1111/j.1365-2958.2012.08107.x. [DOI] [PubMed] [Google Scholar]
- Joshi MC, Bourniquel A, Fisher J, Ho BT, Magnan D, Kleckner N, Bates D. Escherichia coli sister chromosome separation includes an abrupt global transition with concomitant release of late-splitting intersister snaps. Proceedings of the National Academy of Sciences, United States of America. 2011;108:2765–2770. doi: 10.1073/pnas.1019593108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun S, Mulder B. Entropy-driven spatial organization of highly confined polymers: lessons for the bacterial chromosome. Proc Natl Acad Sci U S A. 2006;103:12388–12393. doi: 10.1073/pnas.0605305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavenoff R, Bowen BC. Electron microscopy of membrane-free folded chromosomes from Escherichia coli. Chromosoma. 1976;59:89–101. doi: 10.1007/BF00328479. [DOI] [PubMed] [Google Scholar]
- Kouzminova EA, Kuzminov A. Chromosome demise in the wake of ligase-deficient replication. Mol Micorbiol. 2012;84:1079–1096. doi: 10.1111/j.1365-2958.2012.08076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzminov A. Collapse and repair of replication forks in Escherichia coli. Mol Microbiol. 1995;16:373–384. doi: 10.1111/j.1365-2958.1995.tb02403.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Levels of organization of the DNA in eucaryotic chromosomes. Pharmacol Rev. 1978;30:469–476. [PubMed] [Google Scholar]
- Lark KG. Discovering non-random segregation of sister chromatids: the naïve treatment of a premature discovery. Front Oncol. 2012;2:211. doi: 10.3389/fonc.2012.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon KP, Grossman AD. Localization of bacterial DNA polymerase: evidence for a factory model of replication. Science. 1998;282:1516–1519. doi: 10.1126/science.282.5393.1516. [DOI] [PubMed] [Google Scholar]
- Lemon KP, Grossman AD. The extrusion-capture model for chromosome partitioning in bacteria. Genes Dev. 2001;15:2031–2041. doi: 10.1101/gad.913301. [DOI] [PubMed] [Google Scholar]
- Lesterlin C, Gigant E, Boccard F, Espéli O. Sister chromatid interactions in bacteria revealed by a site-specific recombination assay. EMBO J. 2012;31:3468–3479. doi: 10.1038/emboj.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løbner-Olesen A, Kuempel PL. Chromosome partitioning in Escherichia coli. J Bacteriol. 1992;174:7883–7889. doi: 10.1128/jb.174.24.7883-7889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl J. Chromosomes of the budding yeast Saccharomyces cerevisiae. Int Rev Cytol. 2003;222:141–196. doi: 10.1016/s0074-7696(02)22014-8. [DOI] [PubMed] [Google Scholar]
- Losada A, Hirano T. Shaping the metaphase chromosome: coordination of cohesion and condensation. Bio Essays. 2001;23:924–935. doi: 10.1002/bies.1133. [DOI] [PubMed] [Google Scholar]
- Luijsterburg MS, White MF, van Driel R, Dame RT. The major architects of chromatin: architectural proteins in bacteria, archaea and eukaryotes. Crit Rev Biochem Mol Biol. 2008;43:393–418. doi: 10.1080/10409230802528488. [DOI] [PubMed] [Google Scholar]
- Marians KJ. Prokaryotic DNA replication. Annu Rev Biochem. 1992;61:673–719. doi: 10.1146/annurev.bi.61.070192.003325. [DOI] [PubMed] [Google Scholar]
- Marsden MP, Laemmli UK. Metaphase chromosome structure: evidence for a radial loop model. Cell. 1979;17:849–858. doi: 10.1016/0092-8674(79)90325-8. [DOI] [PubMed] [Google Scholar]
- Masai H, Matsumoto S, You Z, Yoshizawa-Sugata N, Oda M. Eukaryotic chromosome DNA replication: where, when, and how? Annu Rev Biochem. 2010;79:89–130. doi: 10.1146/annurev.biochem.052308.103205. [DOI] [PubMed] [Google Scholar]
- Mazia D. The Chromosome Cycle and the Centrosome Cycle in the Mitotic Cycle. Int Rev Cytol. 1987;100:49–92. doi: 10.1016/s0074-7696(08)61698-8. [DOI] [PubMed] [Google Scholar]
- Mercier R, Petit MA, Schbath S, Robin S, El Karoui M, Boccard F, Espéli O. The MatP/matS site-specific system organizes the terminus region of the E. coli chromosome into a macrodomain. Cell. 2008;135:475–485. doi: 10.1016/j.cell.2008.08.031. [DOI] [PubMed] [Google Scholar]
- Morigen, Odsbu I, Skarstad K. Growth rate dependent numbers of SeqA structures organize the multiple replication forks in rapidly growing Escherichia coli. Genes Cells. 2009;14:643–657. doi: 10.1111/j.1365-2443.2009.01298.x. [DOI] [PubMed] [Google Scholar]
- Murphy LD, Zimmerman SB. Isolation and characterization of spermidine nucleoids from Escherichia coli. J Struct Biol. 1997;119:321–335. doi: 10.1006/jsbi.1997.3883. [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Peters JM, Uhlmann F. Splitting the chromosome: cutting the ties that bind sister chromatids. Science. 2000;288:1379–1385. doi: 10.1126/science.288.5470.1379. [DOI] [PubMed] [Google Scholar]
- Nielsen HJ, Li Y, Youngren B, Hansen FG, Austin S. Progressive segregation of the Escherichia coli chromosome. Mol Microbiol. 2006;61:383–393. doi: 10.1111/j.1365-2958.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- Nielsen HJ, Youngren B, Hansen FG, Austin S. Dynamics of Escherichia coli chromosome segregation during multifork replication. J Bacteriol. 2007;189:8660–8666. doi: 10.1128/JB.01212-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki H, Yamaichi Y, Hiraga S. Dynamic organization of chromosomal DNA in Escherichia coli. Genes Dev. 2000;14:212–223. [PMC free article] [PubMed] [Google Scholar]
- Nordstrom K, Dasgupta S. Partitioning of the Escherichia coli chromosome: superhelicity and condensation. Biochimie. 2001;83:41–48. doi: 10.1016/s0300-9084(00)01204-9. [DOI] [PubMed] [Google Scholar]
- Norris V, Fralick J, Danchin A. A SeqA hyperstructure and its interactions direct the replication and sequestration of DNA. Mol Microbiol. 2000;37:696–702. doi: 10.1046/j.1365-2958.2000.02019.x. [DOI] [PubMed] [Google Scholar]
- Ogden GB, Pratt MJ, Schaechter M. The replicative origin of the E. coli chromosome binds to cell membranes only when hemimethylated. Cell. 1988;54:127–135. doi: 10.1016/0092-8674(88)90186-9. [DOI] [PubMed] [Google Scholar]
- Olsson J, Dasgupta S, Berg OG, Nordström K. Eclipse period without sequestration in Escherichia coli. Mol Microbiol. 2002;44:1429–1440. doi: 10.1046/j.1365-2958.2002.02954.x. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Halvorsen K, Ha BY, Paparcone R, Sandler SJ, Woldringh CL, Wong WP, Jun S. Physical manipulation of the Escherichia coli chromosome reveals its soft nature. Proc Natl Acad Sci U S A. 2012;109:E2649–E2656. doi: 10.1073/pnas.1208689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BJ, Ullsperger C, Hiasa H, Marians KJ, Cozzarelli NR. The structure of supercoiled intermediates in DNA replication. Cell. 1998;94:819–827. doi: 10.1016/s0092-8674(00)81740-7. [DOI] [PubMed] [Google Scholar]
- Pettijohn DE. The Nucleoid. In: Neidhardt FC, editor. Escherichia coli and Salmonella. Washington, D.C: ASM Press; 1996. pp. 158–166. [Google Scholar]
- Pflumm MF. The role of DNA replication in chromosome condensation. Bio Essays. 2002;24:411–418. doi: 10.1002/bies.10092. [DOI] [PubMed] [Google Scholar]
- Quinn WG, Sueoka N. Symmetric replication of the Bacillus subtilis chromosome 1970. 1970;67:717–723. doi: 10.1073/pnas.67.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Lamothe R, Possoz C, Danilova O, Sherratt DJ. Independent positioning and action of Escherichia coli replisomes in live cells. Cell. 2008a;133:90–102. doi: 10.1016/j.cell.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Lamothe R, Wang X, Sherratt D. Escherichia coli and its chromosome. Trends Microbiol. 2008b;16:238–245. doi: 10.1016/j.tim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Savageau MA. Escherichia coli habitats, cell types, and molecular mechanisms of gene control. Amer Nat. 1983;122:732–744. [Google Scholar]
- Sawitzke J, Austin S. An analysis of the factory model for chromosome replication and segregation in bacteria. Mol Microbiol. 2001;40:786–794. doi: 10.1046/j.1365-2958.2001.02350.x. [DOI] [PubMed] [Google Scholar]
- Sernova NV, Gelfand MS. Identification of replication origins in prokaryotic genomes. Brief Bioinform. 2008;9:376–391. doi: 10.1093/bib/bbn031. [DOI] [PubMed] [Google Scholar]
- Skarstad K, Boye E, Steen HB. Timing of initiation of chromosome replication in individual Escherichia coli cells. EMBO J. 1986;5:1711–1717. doi: 10.1002/j.1460-2075.1986.tb04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K, Katayama T. Regulating DNA Replication in Bacteria. Cold Spring Harb Perspect Biol. 2013 doi: 10.1101/cshperspect.a012922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K, von Meyenburg K, Hansen FG, Boye E. Coordination of chromosome replication initiation in Escherichia coli: effects of different dnaA alleles. J Bacteriol. 1988;170:852–858. doi: 10.1128/jb.170.2.852-858.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack SM, Anderson LK. A model for chromosome structure during the mitotic and meiotic cell cycles. Chromosome Res. 2001;9:175–198. doi: 10.1023/a:1016690802570. [DOI] [PubMed] [Google Scholar]
- Steinberger RE, Allen AR, Hansa HG, Holden PA. Elongation correlates with nutrient deprivation in Pseudomonas aeruginosa unsaturated biofilms. Microb Ecol. 2002;43:416–423. doi: 10.1007/s00248-001-1063-z. [DOI] [PubMed] [Google Scholar]
- Stillman B. Origin recognition and the chromosome cycle. FEBS Lett. 2005;579:877–884. doi: 10.1016/j.febslet.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Stokke C, Waldminghaus T, Skarstad K. Replication patterns and organization of replication forks in Vibrio cholerae. Microbiology. 2011;157:695–708. doi: 10.1099/mic.0.045112-0. [DOI] [PubMed] [Google Scholar]
- Stonington OG, Pettijohn DE. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci USA. 1971;68:6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin O, Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980;21:103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Pangborn J, Kilgore WW. Filamentous cells of Escherichia coli formed in the presence of mitomycin. J Bacteriol. 1967;93:683–688. doi: 10.1128/jb.93.2.683-688.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M. A model of chromatin-dependent DNA replication sequences based on the decondensation units hypothesis. J Theor Biol. 1989;136:427–465. doi: 10.1016/s0022-5193(89)80157-2. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Iseki M, Miyoshi T, Aoki H, Imanaka H. Mechanism of action of bicyclomycin. J Antibiot (Tokyo) 1976;29:155–168. doi: 10.7164/antibiotics.29.155. [DOI] [PubMed] [Google Scholar]
- Toro E, Shapiro L. Bacterial chromosome organization and segregation. Cold Spring Harb Perspect Biol. 2010;2:a000349. doi: 10.1101/cshperspect.a000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask B, Pinkel D, van den Engh G. The proximity of DNA sequences in interphase cell nuclei is correlated to genomic distance and permits ordering of cosmids spanning 250 kilobase pairs. Genomics. 1989;5:710–717. doi: 10.1016/0888-7543(89)90112-2. [DOI] [PubMed] [Google Scholar]
- Trask BJ, Allen S, Massa H, Fertitta A, Sachs R, van den Engh G, Wu M. Studies of metaphase and interphase chromosomes using fluorescence in situ hybridization. Cold Spring Harb Symp Quant Biol. 1993;58:767–775. doi: 10.1101/sqb.1993.058.01.084. [DOI] [PubMed] [Google Scholar]
- Trun NJ, Marko JF. Architecture of a bacterial chromosome. ASM News. 1998;64:276–283. [Google Scholar]
- Uhlmann F. A matter of choice: the establishment of sister chromatid cohesion. EMBO Rep. 2009;10:1095–1102. doi: 10.1038/embor.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valens M, Penaud S, Rossignol M, Cornet F, Boccard F. Macrodomain organization of the Escherichia coli chromosome. EMBO J. 2004;23:4330–4341. doi: 10.1038/sj.emboj.7600434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet-Gely I, Boccard F. Chromosomal organization and segregation in Pseudomonas aeruginosa. PLoS Genet. 2013;9:e1003492. doi: 10.1371/journal.pgen.1003492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helvoort JM, Woldringh CL. Nucleoid partitioning in Escherichia coli during steady-state growth and upon recovery from chloramphenicol treatment. Mol Microbiol. 1994;13:577–583. doi: 10.1111/j.1365-2958.1994.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Vernis L, Poljak L, Chasles M, Uchida K, Casarégola S, Käs E, Matsuoka M, Gaillardin C, Fournier P. Only centromeres can supply the partition system required for ARS function in the yeast Yarrowia lipolytica. J Mol Biol. 2001;305:203–217. doi: 10.1006/jmbi.2000.4300. [DOI] [PubMed] [Google Scholar]
- Viollier PH, Thanbichler M, McGrath PT, West L, Meewan M, McAdams HH, Shapiro L. Rapid and sequential movement of individual chromosomal loci to specific subcellular locations during bacterial DNA replication. Proc Natl Acad Sci U S A. 2004;101:9257–9262. doi: 10.1073/pnas.0402606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvalingam J, Gill CO, Holley RA. The viabilities of cells in cultures of Escherichia coli growing with formation of filaments at 6 °C. Int J Food Microbiol. 2012;153:129–134. doi: 10.1016/j.ijfoodmicro.2011.10.027. [DOI] [PubMed] [Google Scholar]
- von Freiesleben U, Krekling MA, Hansen FG, Løbner-Olesen A. The eclipse period of Escherichia coli. EMBO J. 2000;19:6240–6248. doi: 10.1093/emboj/19.22.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake RG. Circularity of the Bacillus subtilis chromosome and further studies on its bidirectional replication. J Mol Biol. 1973;77:569–572. doi: 10.1016/0022-2836(73)90223-4. [DOI] [PubMed] [Google Scholar]
- Wang Q, Mordukhova EA, Edwards AL, Rybenkov VV. Chromosome condensation in the absence of the non-SMC subunits of MukBEF. J Bacteriol. 2006a;188:4431–4441. doi: 10.1128/JB.00313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu X, Possoz C, Sherratt DJ. The two Escherichia coli chromosome arms locate to separate cell halves. Genes Dev. 2006b;20:1727–1731. doi: 10.1101/gad.388406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Llopis PM, Rudner DZ. Organization and segregation of bacterial chromosomes. Nat Rev Genet. 2013 doi: 10.1038/nrg3375. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Reyes-Lamothe R, Sherratt DJ. Modulation of Escherichia coli sister chromosome cohesion by topoisomerase IV. Genes Dev. 2008;22:2426–2433. doi: 10.1101/gad.487508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner G, Formanek H. A new chromosome model. J Struct Biol. 2000;132:147–161. doi: 10.1006/jsbi.2000.4310. [DOI] [PubMed] [Google Scholar]
- White MA, Eykelenboom JK, Lopez-Vernaza MA, Wilson E, Leach DR. Non-random segregation of sister chromosomes in Escherichia coli. Nature. 2008;455:1248–1250. doi: 10.1038/nature07282. [DOI] [PubMed] [Google Scholar]
- Woldringh CL. Morphological analysis of nuclear separation and cell division during the life cycle of Escherichia coli. J Bacteriol. 1976;125:248–257. doi: 10.1128/jb.125.1.248-257.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh CL. The role of co-transcriptional translation and protein translocation (transertion) in bacterial chromosome segregation. Mol Micorbiol. 2002;45:17–29. doi: 10.1046/j.1365-2958.2002.02993.x. [DOI] [PubMed] [Google Scholar]
- Woldringh CL, Nanninga N. Structure of nucleoid and cytoplasm in the intact cell. In: Nanninga N, editor. Molecular Cytology of Escherichia coli. London: Academic Press; 1985. pp. 161–197. [Google Scholar]
- Worcel A, Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972;71:127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Zhou P, Bogan JA, Welch K, Pickett SR, Wang HJ, Zaritsky A, Helmstetter CE. Gene transcription and chromosome replication in Escherichia coli. J Bacteriol. 1997;179:163–169. doi: 10.1128/jb.179.1.163-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SB. Shape and compaction of Escherichia coli nucleoids. J Struct Biol. 2006;156:255–261. doi: 10.1016/j.jsb.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Zimmerman SB, Murphy LD. Macromolecular crowding and the mandatory condensation of DNA in bacteria. FEBS Lett. 1996;390:245–258. doi: 10.1016/0014-5793(96)00725-9. [DOI] [PubMed] [Google Scholar]
- Zusman DR, Carbonell A, Haga JY. Nucleoid condensation and cell division in Escherichia coli MX74T2 ts52 after inhibition of protein synthesis. J Bacteriol. 1973;115:1167–1178. doi: 10.1128/jb.115.3.1167-1178.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]