Abstract
Close associations between adult males and lactating females and their dependent infants are not commonly described in non-monogamous mammals. However, such associations [sometimes called “friendships” (Smuts 1985)] are regularly observed in several primate species in which females mate with multiple males during the fertile period. The absence of mating exclusivity among “friends” suggests that males should invest little in infant care, raising questions about the adaptive significance of friendship bonds. Using data from genetic paternity analyses, patterns of behavior, and long-term demographic and reproductive records, we evaluated the extent to which friendships in four multi-male, multi-female yellow baboon (Papio cynocephalus) groups in Amboseli, Kenya represent joint parental care of offspring or male mating effort. We found evidence that mothers and infants benefited directly from friendships; friendships provided mother–infant dyads protection from harassment from other adult and immature females. In addition, nearly half of all male friends were the genetic fathers of offspring and had been observed mating with mothers during the days of most likely conception for those offspring. In contrast, nearly all friends who were not fathers were also not observed to consort with the mother during the days of most likely conception, suggesting that friendships between mothers and non-fathers did not result from paternity confusion. Finally, we found no evidence that prior friendship increased a male’s chances of mating with a female in future reproductive cycles. Our results suggest that, for many male–female pairs at Amboseli, friendships represented a form of biparental care of offspring. Males in the remaining friendship dyads may be trading protection of infants in exchange for some resources or services not yet identified. Our study is the first to find evidence that female primates gain social benefits from their early associations with adult males.
Keywords: Male–female associations, Parental care, Mating effort, Infant harassment
Introduction
Year-round associations between adult males and females are typical of most birds (Lack 1968) but are generally rare in mammals (Clutton-Brock 1989), except in primates where over two thirds of genera exhibit year-round male–female associations (Wilson 1975; van Schaik and Kappeler 1997). Year round associations between male and female animals may serve multiple functions, including joint parental care (Clutton-Brock 1991), joint resource defense (Armstrong 1991), or mating effort by males (Seyfarth 1978b), in which males provide care to infants in exchange for future mating opportunities with the infant’s mother. In birds (e.g., Morris 1967; Lack 1968; Wittenberger and Tilson 1980; Gubernick 1994) and some rodents (e.g., Wolff and Cicirello 1991) and insects (e.g., Eggert and Muller 1997) male–female associations are typically found in socially monogamous species exhibiting biparental care of offspring (reviewed in Palombit 2000). In contrast, in primates, year-round male–female associations are regularly observed even in non-monogamous species. In addition, highly specific individual male–female bonds or “friendships” (consisting of close spatial proximity and frequent affiliative behavior; Smuts 1985) between adult males and non-fertile or lactating females and their dependent offspring are regularly observed in a number of cercopithecine primates living in multi-male, multi-female social groups in which females mate with multiple males during the fertile period [Papio anubis (Ransom and Ransom 1971; Smuts 1985; Bercovitch 1991; Lemasson et al. 2008); P. cynocephalus (Altmann 1980); P. ursinus (Seyfarth 1978b; Palombit et al. 1997); Macaca fuscata (Takahata 1982); M. mulatta (Chapais 1986a; Mason 1994); M. sylvanus (Paul et al. 1996; Menard et al. 2001)]. When females mate with multiple males, males may not be able to distinguish their own offspring from unrelated offspring and are thus expected to invest little in infant care. The prevalence of friendships in many multi-male primate species raises questions about the adaptive significance of these relationships (Smuts 1985).
Friendship with a male may provide protection for a female’s infant against various sources of injury or mortality (Hrdy 1979; Altmann 1980). Mothers and infants are particularly vulnerable to social aggression (Nicolson 1987), a pervasive feature of life in primate social groups (Aureli and de Waal 2000). By associating with an adult male, a mother may gain the male’s protection for her offspring from social aggression committed by members of her own or nearby groups, including immature and adult females (the “female harassment” hypothesis, Altmann 1980) and adult males (the “anti-infanticide” hypothesis, Hrdy 1979).
In many group-living primates, females other than the mother are often highly motivated to interact with infants, but the reluctance of mothers to let other group members handle their newborn infants suggests some forms of handling may be harmful to infants (Nicolson 1987). Persistent aggressive infant handling or harassment and kidnapping are associated with higher risk of infant injury or mortality in some populations (Silk 1980; Kleindorfer and Wasser 2004) and may represent a form of reproductive competition among females (Silk 1980; Wasser and Barash 1981). Mothers with young infants may reduce their risk of harassment by associating with male friends who intervene on their behalf in agonistic interactions (Manson 1994) or who, by their mere presence, could protect the dyad from intrusive infant handling and other aggressive acts by immature and adult females (Altmann 1980).
In addition to protection from female harassment, male friends may offer mothers and infants protection from infanticide by other adult males (Hrdy 1979). Infanticide is regularly observed in mammals ranging from lions [Panthera leo (Pusey and Packer 1994)] to rodents (reviewed in Blumstein 2000) and is widely considered to be a sexually selected alternative male reproductive strategy (van Schaik and Janson 2000). By killing unrelated infants and effectively terminating the suppressive effects of suckling on postpartum fertility, infanticidal males can (a) accelerate the return to fertility of lactating females, thereby increasing the number of fertile females with whom they can potentially mate and (b) remove potential competitors for their future offspring (van Schaik and Janson 2000).
Evidence that male–female bonds protect infants from infanticide is available from several animal populations where infanticide is a significant source of infant mortality (reviewed in Palombit 2000). Comparable data are scarce on the adaptive benefits of male–female bonds in populations where infanticide is rare (Lemasson et al. 2008). Although infanticide has rarely been observed in most primate societies in which friendships have been described (Maestripieri 2000), this lack of infanticide may be attributable to the widespread success of primate friendships in deterring infant killing. If male–female bonds evolved as a counter-strategy to infanticide, these bonds are expected to be responsive to variation in infanticide risk. Infanticide is commonly associated with changes in male breeding status (Hausfater and Hrdy 1984; Parmigiani et al. 1994; van Schaik and Janson 2000). Infanticide risk is therefore expected to be highest during periods of turnover in the alpha position in the male dominance hierarchy and after the immigration of an adult male into a new social group (Alberts et al. 1992; Sapolsky 1993; Beehner et al. 2005). During these periods of heightened infanticide risk, mothers with young infants are expected to seek protection from male friends against potentially infanticidal males.
Although mothers may gain protection for their offspring from a range of possible sources of injury or mortality, the benefits that males gain by associating with particular mother–infant pairs is less clear. For males, friendships may represent a form of paternal care or male mating effort or both. The “paternal investment” hypothesis proposes that by associating preferentially with his own offspring, a male friend will help to improve the fitness of the offspring he has sired with the female (Bales 1980; Kleiman and Malcolm 1981; Smuts 1985; Taub 1990; Taub and Mehlman 1991).
Given that female baboons mate with multiple males during the fertile period (Hausfater 1975; Seyfarth 1978a; Smuts 1985), how can male friends distinguish their own neonates from unrelated infants? Emerging evidence suggests that males in some species can and do recognize paternal kin (e.g., Alberts 1999; Borries et al. 1999a, b; Soltis et al. 2000; Widdig et al. 2001; Buchan et al. 2003; Smith et al. 2003; Charpentier et al. 2008). The behavior of males in several non-primate and primate species demonstrates that males distinguish their own young from unrelated ones [birds (Moller and Birkhead 1993; Dixon et al. 1994; Weatherhead et al. 1994; Chuang-Dobbs et al. 2001), fish (Rios-Cardenas and Webster 2005), hyenas (Van Horn et al. 2004), and primates (Borries et al. 1999b; Soltis et al. 2000; Buchan et al. 2003)]. However, even if a male distinguishes his own from unrelated infants, competing forces may influence his behavior toward any one infant, as the time and energy a male devotes to any one offspring are time and energy he cannot devote to other activities or offspring (Clutton-Brock 1991). Therefore, a male’s participation in a friendship bond with a particular mother–infant dyad could depend not only on the male’s genetic relationship to the infant but also on the number of other related infants in the group or on the physical or social threats facing a particular infant.
Friendships could instead (or also) represent a form of male mating effort; that is, male care of infants (Price 1990; Buss 1994; Freeman-Gallant 1997) or friendship with mothers (Smuts 1985; Smuts and Gubernick 1992; Menard et al. 2001) may function to enhance a male’s attractiveness as a future mating partner. This idea, called the “mating effort” hypothesis, proposes that by demonstrating their willingness to invest in an infant, male friends may enhance their chances of being chosen by the female (or by other females) as a mating partner in future reproductive cycles (Seyfarth 1978b; Smuts 1985).
Through friendships, fathers can potentially achieve mating effort as well as paternal care of genetic offspring. In addition, the importance of mating effort and paternal care are expected to vary with male age and experience (Smuts 1985), making the task of identifying the adaptive value of friendships to males a difficult one. Nonetheless, data on the benefits of friendships to males are needed to provide insight into the evolution of male–female bonds.
In this study, we evaluate the adaptive value and determinants of “friendships” between adult males and mothers with newborn infants in a wild baboon (P. cynocephalus) population in Amboseli, Kenya. Using data from genetic paternity analyses, patterns of behavior among parents, offspring, and other group members and the long-term (>30 years) demographic and reproductive records of the Amboseli baboon population, we examine the patterning of male–female bonds during the early postnatal period and evaluate the nature and extent to which friendships represent joint parental care of offspring or male mating effort in this study population.
Materials and methods
Subjects and study population
Baboons are non-seasonal polygynandrous breeders and are among the largest, most sexually dimorphic, omnivorous, and terrestrial of the monkeys (Estes 1992; Kingdon 2003). Males typically disperse from their natal groups as they near adult size, and many continue to migrate between groups throughout their lives (Pusey and Packer 1987). Females, in contrast, remain in their natal group their entire life (Pusey and Packer 1987). Most matings occur within the context of mate-guarding episodes, called sexual consortships, during the follicular phase of the sexual cycle (Seyfarth 1978a; Smuts 1985; Bercovitch 1987; Alberts et al. 2003). During this phase, females have prominent, swollen sex skins that increase in size until around the time of ovulation (Shaikh et al. 1982).
The subjects for this study are part of a larger population of wild baboons inhabiting the Amboseli basin, a semi-arid, short grass savannah ecosystem situated at the base of Mt. Kilimanjaro in East Africa. The population has been under continuous observation since 1971, and data on life history are known since birth from near-daily records of demographic events and reproductive cycles for several hundred animals (Altmann 1980, 1998; Alberts and Altmann 2003; Altmann and Alberts 2003, see http://www.princeton.edu/~baboon for a complete bibliography and the Baboon Project Monitoring Guide, which outlines data collection protocols for this population). Twenty-nine mother–infant pairs and their associated male friends, members of four distinct multi-male groups, were observed from July 2002 to November 2003 by NN.
Behavioral data collection
Observations began on pregnant females 2 months before parturition and continued for 2 months after birth. On average, pregnant females were observed in 31.3 ± 2.5SD focal animal samples of 20-min duration (Altmann 1974), yielding an average of 10.9 ± 0.9SD in-sight hours of observation per female. In addition, an average of 11.6 ± 0.8SD in-sight hours of observation was collected for each mother–infant dyad after birth. Particular effort was made to ensure that observations of each pregnant female or mother–infant dyad were evenly distributed between morning and afternoon samples and across weeks. Samples of mothers also served as samples on infants since infant baboons spent almost all of their time in bodily contact or in close proximity to mothers during the first 2 months of life (Altmann 1980). Data were collected on a Psion Workabout™ handheld computer using a custom program.
Within the focal samples, embedded point samples (Altmann 1974) were collected every 5 min on the activity of the focal female and on the identities of the female’s (1) nearest neighbor, (2) nearest adult neighbor, and (3) nearest adult male neighbor within 5 m. During each focal sample, continuous data were collected on all occurrences of affiliative and agonistic interactions between focal females and infants (if present) and other group members, including actor and recipient identities and the time of onset and termination for each grooming bout.
Following birth, continuous records were also kept on all bouts of infant distress and all occurrences of infant handling during focal samples. A bout of infant distress was defined as beginning with the infant’s first distress vocalization and ending when the infant no longer gave distress cries in relation to the original cause of distress. We limit our analysis to those distress bouts that were due to the behavior or proximity of other group members or external stimuli or that were otherwise clearly not arising from mother-infant interactions. We recorded three different infant handling behaviors. A touch was recorded when one animal made manual contact with the infant in a non-aggressive manner. A pull was recorded when an animal tugged at the infant while it was in contact with the mother, and either the infant or the mother counteracted the force of the pull by holding the other member of the dyad tighter. Lastly, an act of contact breaking was recorded when an animal succeeded in pulling the infant away from the mother’s body.
Behavioral measures
Measures of grooming and proximity between male and female pairs were used to differentiate pairs with particularly strong and persistent bonds from all other pairs. Grooming plays a key role in the formation and maintenance of social bonds in primates (Seyfarth 1983), and grooming intensity can be used as an indicator of the strength of affiliation between grooming partners. In addition, spatial arrangements within groups provide the broad outlines for social opportunities among group members, and consistent spatial patterning can provide an indirect measure of the “value” of a relationship to an animal (Rowell and Olson 1983). Indeed, spatial proximity is often used as an indicator of partner preference in mate choice tests, and females in some species have enhanced reproductive success when paired with their “preferred” male (e.g., Drickamer et al. 2000, Sandvik et al. 2000, Wong 2004).
For each female, we calculated the proportion of total point samples spent in close proximity (i.e., within 5 m) to each adult male in her group and determined her most frequent male nearest neighbor. We used data on frequency and lengths of grooming bouts to calculate the proportion of each female’s total time spent grooming all males and the proportion of each female’s total time grooming and total time being groomed that was accounted for by individual males. From these data, we also determined each female’s most frequent male grooming and being-groomed partner. Data were divided into pre- and postnatal phases, the former coinciding with the last 8 weeks of pregnancy and the latter with the first 8 weeks of infancy.
The social dangers facing infants during the study period were inferred from patterns of infant handling and rates of infant distress. We calculated the hourly rate at which infants received each type of handling (touch, pull, contact breaking) by dividing the totals for each type received by each infant by the total number of hours the mother–infant dyad was observed. We then calculated the rate at which infants received each type of handling per potential handler by dividing the hourly rate of each handling type by the number of potential partners of each age/sex category present in the group during the 2 months of infancy. Because pulling and contact breaking were both disruptive acts, we combined these two behaviors into a single measure, “rough handling.” Finally, we calculated the average number of distress bouts given by each infant for each hour the dyad was observed.
Demographic and dominance records
For each mother–infant dyad, we determined (1) the mother’s parity (the number of prior pregnancies, including the current infant), (2) the number of months since an unfamiliar adult male had emigrated into the group, and (3) the number of months since the alpha male during the infant’s second month of life had achieved his top ranking position. Unfamiliar adult males were defined as any immigrant male who had not previously resided in the group. Dominance ranks were tabulated once each month for all adult members of the study population based on the pattern of wins and losses in agonistic interactions (Hausfater et al. 1982; Alberts et al. 2003) recorded as part of regular monitoring of groups in the study population.
“Behavioral” fathers and future mating success with the mother
The identity of known male and female participants in mating events and mate-guarding episodes were recorded as part of regular monitoring of study groups. We used these data to identify a set of “behavioral” fathers for each infant [i.e., all adult males who were observed consorting with the mother during the 5 days of most likely conception of the infant during the cycle in which she conceived the infant (Buchan et al. 2003; Alberts et al. 2006)]. Because insufficient sampling may cause bias in estimates of mating success, we restricted our analyses to include only those cycles in which females were observed consorting for a minimum of 5 h (consort times are available in ESM Table S4). Furthermore, as in previous studies (Buchan et al. 2003; Alberts et al. 2006), for each conception period, we restricted our analyses to those consortships that occurred during the 5 days before the onset of deturgescence of the highly visible sexual skin swelling (i.e., the days of most likely conception of the infant) because this is the period in which ovulation and conception are most likely to occur (Hendrickx and Kraemer 1969; Wildt et al. 1977; Shaikh et al. 1982). We then determined the amount of the mother’s total consort time achieved by each behavioral father during the days of most likely conception of the infant. Finally, to determine subsequent mating success with the mother, we compared each male friend’s consorting activity with his female friend (once she resumed cycling) with his average overall consorting activity with each other adult female in the group (see below).
Genetic paternity analyses
Paternity was known for 340 individuals in our long-term study population, including 23 infants included in this study. We assigned paternity by genotyping 504 individuals in the study population at multiple microsatellite loci (13.81 ± 0.78 SD loci per individual), genotyping most animals at 14 loci (459 of 504, 91%) as described in Buchan et al. (2003) and Alberts et al. (2006). All loci were in Hardy–Weinberg equilibrium, with a mean of 11.07 ± 2.62 SD alleles per locus. Mean observed heterozygosity across loci was 0.813 ± 0.039. Males were considered potential fathers if they were present in the population and had achieved testes enlargement when the offspring was conceived (see Alberts et al. 2006). Males were assigned as fathers if they met one of two criteria (see Alberts et al. 2006): (1) they were the only male with no exclusions for a given offspring, considering maternal genotypes, or (2) they had a single genetic mismatch with the offspring, considering maternal genotypes, and the paternity assignment was supported at the 95% confidence level by the maximum-likelihood method employed in Cervus 2.0 (Marshall et al. 1998). To generate confidence levels with Cervus, 10,000 simulations were run with the following parameters: 70% of candidate parents (62) sampled, 98% of loci typed, and a typing error rate of 0.01, set as the rate of mismatches between mother and offspring as in Buchan et al. 2003.
Data analysis
For each mother–infant dyad, we identified a single male “friend” and a set of “non-friends” (i.e., all other adult males present in the group during the infant’s first 2 months of life). We then evaluated factors that might contribute to variation in male friendships by comparing friends to non-friends in (1) age, (2) dominance rank, (3) number of other immature offspring sired by the male that were present in the group during the focal infant’s first 2 months of life, and (4) proportion of the female’s consort time achieved by each male on the days of most likely conception of the infant, using independent samples t tests. Male age at infant birth was calculated from known or estimated male birth-dates. Male dominance rank was calculated as the average of the male’s ranks during the month the infant was born and during each of the next 2 months, with rank one being the highest. The number of immature offspring sired by each male was drawn from the genetic records for the population. Immature offspring were all offspring, not including the current infant, who had been sired by the male and who had not attained sexual maturity [i.e., for females, before the age of menarche; for males, before the age of testicular enlargement (Alberts and Altmann 1995)] by the time the infant turned 2 months old.
To evaluate whether friendships with males provided infants with protection from harassment by other females, we treated the rate at which infants were handled roughly as the response variable in a linear regression on the amount of time mothers spent in proximity to their male friend. We also compared the rate of rough handling received by infants whose mothers spent the most amount of time with male friends (mothers who ranked in the top quartile, i.e., those that spent at least 28% of time in proximity to her male friend) to infants whose mothers spent less time with male friends. To evaluate whether friendships with males were in response to infants needing protection from potentially infanticidal males, we treated the amount of time females spent in proximity to male friends as the response variable in a multiple regression on two measures of instability in male social relations: tenure of the highest ranking male and the months since an adult male had emigrated into the group. In a recent survey of reported cases of infanticide in wild primate populations, van Schaik (2000) found that 85% of reported cases of infanticide occur within the context of (1) an adult male rising in rank within a group or (2) the immigration of an adult male into a new social group. Although infanticide has rarely been observed in the baboons at Amboseli, even during periods of immigration or instability in the male dominance rank hierarchy (Altmann and Alberts, unpublished data), in light of van Schaik (2000)’s findings, we defined infanticide risk in terms of instability in male social relations.
Next, we sought to evaluate whether friendships represented a form of paternal care of offspring or male mating effort. First, among the genetic fathers who were present in their infant’s group during its first 2 months of life, we identified those who were also male friends. Then, we examined whether prior mating success with mothers differed between fathers who were friends and fathers who were not, using independent samples t tests. Next, we evaluated whether an individual father’s decision to form a friendship alliance with one mother–infant dyad but not others might reflect (a) tradeoffs between time invested in that offspring and time that could be invested in other offspring or (b) the infant’s risk of injury or mortality. Because our sample size was not adequate for multivariate analyses, to evaluate these potential tradeoffs, we used bivariate tests (independent samples t tests) to compare dyads in which fathers were friends to dyads in which fathers were not friends with respect to (1) the father’s number of other immature offspring in the group, (2) the infant’s risk of infanticide by males (based on the number of months since an adult male had immigrated into the group), and (3) the mother–infant dyad’s risk of social aggression from females (based on maternal dominance rank).
Because matings achieved through prior affiliation and female choice may be of only secondary importance, compared with other mating tactics arising from direct male–male contest competition (Anderson 1983; Berenstain and Wade 1983; Noe and Sluijter 1990; Bercovitch 1991; Bulger 1993; Alberts et al. 2003), males that form friendships are not expected to have greater mating success overall compared to non-friends. Instead, the “mating effort” hypothesis predicts that males will have greater mating success with former friends (once they resume cycling) than expected relative to the males’ overall consorting activity (i.e., their share of all available female consort time; Smuts 1985).
Therefore, to evaluate the mating effort hypothesis, we compared each male’s consorting activity with his female friend (once she resumed cycling) with what was expected relative to his overall consorting activity with all adult females in his group (adapted from Smuts 1985: 166). The “male’s share of the female’s consort time” was defined as the male’s achieved proportion of the total consort hours that the female gave to all males on all her fertile days (from the day she resumed cycling to the day she conceived her next infant). The “male’s expected share of consort time” was defined as the proportion of the total consort hours available in the group that the male achieved over the same time period. Each dyad in which the male’s individual share of a female’s consort time exceeded his expected share of consort time was assigned a plus, while dyads in which the male’s individual share was the same as his expected share was assigned an equal, and all other dyads were assigned minuses. Pluses were interpreted to mean that the male consorted with the female on a greater percent of her consort hours than he did, on average, with other females. In contrast, minuses were interpreted to mean that the male consorted with the female on a smaller percent of her consort hours than he did, on average, with all females in his group. We then examined whether former affiliation increased a male’s chances of consorting with a female more than expected given his consorting activity overall by comparing the performance of all friend dyads with all non-friend dyads using Fisher’s exact test. We consider consort time a reasonable measure of mating success because a recent study of our population found that observed mating success was a good predictor of paternity success (Alberts et al. 2006).
All statistical analyses were conducted using SPSS 13.0 (SPSS Inc., 2003). Relationships between explanatory variables were explored using Pearson’s correlation coefficients. Values reported are means±SE unless otherwise noted. All analyses were two-tailed with α=0.05.
Results
Identifying friendships from spatial proximity and grooming patterns
Females spent significantly more time in close proximity to all adult males after birth (43±2% of total time budget) than before (21±2%; paired samples t test, p<0.0005), and the greatest increase was seen in the time spent in proximity to the most frequent male nearest neighbor. Time in proximity to females’ most frequent male neighbor nearly tripled from late pregnancy (8±1%) to the postnatal period (21±2%; paired samples t test, p<0.0005). Many females exhibited consistency in their choice of male neighbors across the peripartum period; in more than half of all cases (15 of 29), the female’s most frequent male neighbor was the same individual before as after birth (ESM Table S1).
Females spent significantly less time grooming after birth than before (3.7±0.5 vs. 6.9±0.6% of total time budget; paired samples t test, p<0.0005), although time spent grooming adult males did not change after birth (1.3± 0.3 vs. 1.2±0.3; paired samples t test, p=0.93). Consequently, the percent of females’ total grooming time accounted for by adult males increased significantly after parturition (paired samples t test, p<0.0005), and most of the increase was due to females’ grooming one adult male. During late pregnancy, females’ most frequent male grooming partner accounted for 14±3% of females’ total grooming time, and this value nearly doubled after infant birth (to 29±5%; paired samples t test, p=0.001). In contrast, all other adult males combined accounted for less than 6% of females’ total grooming time before and after birth. In one third of all cases (eight of 25), females’ most frequent male grooming partner was the same individual before and after birth (ESM Table S1).
Females spent significantly more time being groomed by other group members after the birth of their infants (8.5± 0.6% of total time budget) than before (3.6±0.4%; paired samples t test, p<0.0005), although grooming by adult males accounted for only a fraction of one percent of females’ time before birth (0.3±0.1%), and this value did not change after infant birth.
Females exhibited consistency in their choice of male social partners. Twenty-five mothers had both a most frequent male nearest neighbor and a most frequent male grooming partner; for just under 80% (19 of 25) of these mothers, the two frequent partners were the same (ESM Table S1). Because of the concordance between grooming and proximity partners and because four mothers were not seen grooming with any adult male, we henceforth consider each mother’s most frequent male nearest neighbor as her male “friend” in all subsequent analyses. We found that females spent significantly more time in proximity to their most frequent male nearest neighbor (i.e., their male friend) than to the male with whom they spent the second most amount of time in proximity (21±2 vs. 10±1%; paired samples t test; p<0.0005). For this reason, we chose to assign only one male friend to each mother–infant dyad. We use the amount of time a female spent in close proximity to her male friend as a measure of the strength of their friendship.
The characteristics of friendship bonds
The 29 mothers in our sample included females of all dominance ranks and parities (ESM Table S2). The amount of time a mother spent in proximity to her male friend was not predictable from the sex of her infant or maternal rank or parity [general linear model (GLM), , p=0.445 for the entire model]. Thirty-four adult males were resident in the four groups during the study period, and of these, 16 males were friends with at least one female, giving a total of 29 friend dyads and 172 non-friend dyads. On average, friends were similar in age (12.6±0.5 years) to non-friends (12.4±0.2), but the ranks of male friends (3.5± 0.4) were significantly higher during the period of friendship (i.e., the infants’ first two months of life) than those of non-friends (4.9±0.02; t test, p<0.01). In addition, friends had more immature offspring in the group than non-friends (3.0±0.4 vs. 2.2±0.2; t test, p=0.06), though this difference was not significant. Finally, friends obtained, though not significantly, a higher proportion of the mother’s total consort time during the cycle in which she conceived the infant (0.3±0.1; n=22 dyads) than did non-friends (0.1±0.0; n=122 dyads; t test, p=0.09).
The value of friendships for mothers and infants
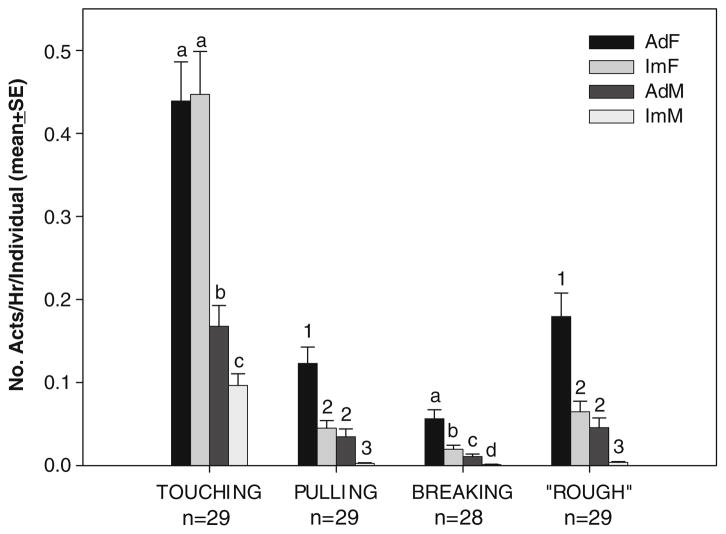
Adult and immature females were responsible for the vast majority of acts of infant handling (Fig. 1). On average, infants were handled most often by adult females, followed by immature females and then adult males and, finally, least often by immature males (Fig. 1). Comparisons of the number of handling acts performed per hour per potential partner of each age/sex class showed that (1) females of all ages were more active than males in handling infants, and (2) disparities in handling frequencies by the different age/sex classes were consistent across handling behaviors (Fig. 1).
Fig. 1.
Mean (±SE) hourly rate of infant handling interactions per potential partner. This value was obtained by dividing the hourly rate of interactions of each type by the number of potential partners of each age/sex category present in the group. The rate of “rough” handling represents the sum of the rate of pulling and contact breaking. Letters and numbers indicate where values differ significantly among age sex classes (when symbols are not shared among classes) and where they do not (when symbols are shared) for a given handling behavior, based on repeated applications of paired-samples t tests. Numbers below the interactions represent the sample size of infants
Touching was the most common form of infant handling. Pulling was second most common, and contact-breaking was least common. The rate at which individual infants were handled roughly (i.e., were pulled or had contact with their mothers broken) by all group members was not predictable from variation among mother–infant dyads in maternal rank, parity, or infant sex (GLM, , p=0.69 for the entire model). However, the rate at which infants were handled roughly predicted the rate at which infants gave distress vocalizations (linear regression, R2= 0.386, p<0.0005), a result that suggests that the more intrusive forms of handling were distressing to infants.
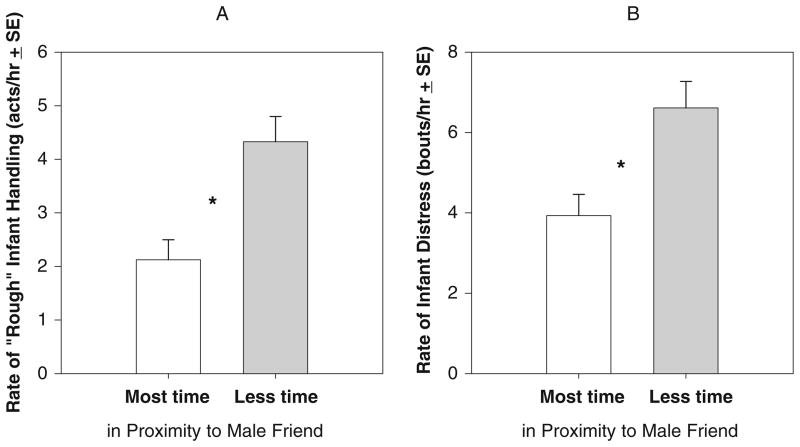
In general, infants whose mother spent more time with male friends received less rough handling and cried less often. The amount of time dyads spent in proximity to male friends was inversely related to (a) the rate at which infants received rough handling (Pearson correlation, R2=0.112, p=0.072) and (b) the rate at which infants gave distress cries (R2=0.110, p=0.078), though these differences were not significant. In addition, mother–infant dyads who ranked in the top quartile of time in proximity to male friends experienced significantly lower rates of rough infant handling than those dyads who were not (Fig. 2a; t test, p= 0.011). Moreover, infants whose mothers spent the most time with male friends exhibited significantly lower rates of distress vocalizations than those infants whose mothers did not (Fig. 2b; t test, p=0.025).
Fig. 2.
Mean (±SE) rates of rough infant handling and distress cries in dyads that spent the most and those that spent less time in proximity to male friends. Infants whose mothers ranked in the top quartile (i.e., spent at least 28%) of time in proximity to male friends a experienced significantly lower rates of rough infant handling, and b gave significantly lower rates of distress vocalizations than infants whose mothers spent less time in proximity to male friends. Asterisk=difference is statistically significant; α =0.05
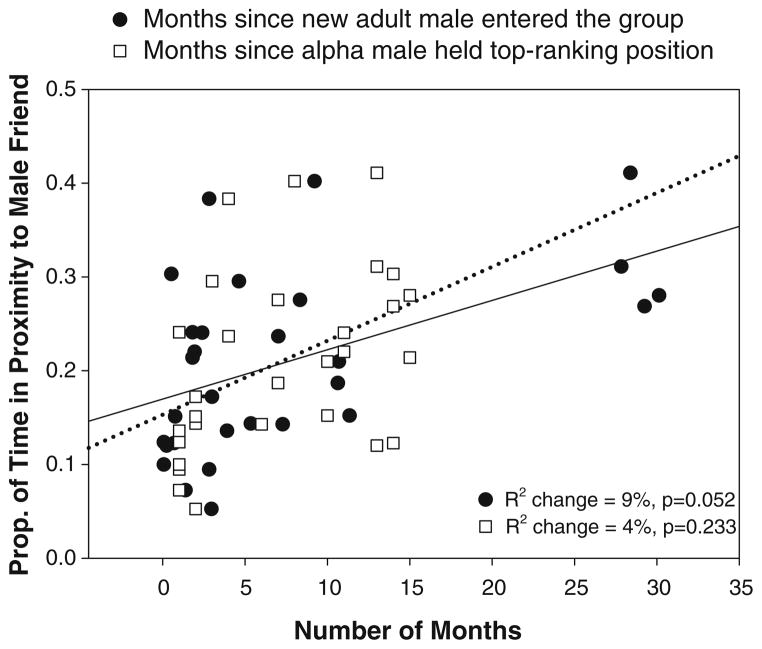
The two measures of instability in male social relations—the number of months since an unfamiliar adult male had entered the group and the tenure length of the alpha male at rank one—were correlated (Pearson correlation, R2=0.26, p= 0.005) but at a sufficiently low value to allow both to be used as predictor variables in the same GLM. The amount of time a mother spent in proximity to her male friend was predicted by the number of months since an unfamiliar adult male had entered the group but not by variance in the tenure of the highest-ranking male (GLM, , p=0.010 for the whole model, p=0.052 for the partial regression coefficient of the number of months since an adult male had immigrated into the group; Fig. 3). However, the effect of instability in male social relations on male–female associations was opposite to the one predicted; mothers with young infants spent less time in proximity to male friends the more recently an unfamiliar adult male had emigrated into the group.
Fig. 3.
The effect of instability in male social relations on the strength of male-female bonds. Mothers with young infants spent less time in proximity to male friends the more unstable male social relationships were within the group, i.e., the fewer the number of months since an unfamiliar adult male had emigrated into the group and the fewer the number of months since the alpha male achieved his top ranking position (adjusted R2=0.242, df=28, p=0.010). Of these two variables, months since an unfamiliar adult male entered the group made a larger unique contribution to the prediction of time spent in proximity to male friends (9%) than did number of months since the alpha male has retained his position (4%)
The value of friendships to males
Paternity was known for 78% (183 of 235) of all individuals present in the four groups during the study period, including 80% (91 of 114) of immature individuals, 23 of whom were infants included in this study whose fathers were present in their group during their first 2 months of life. Fathers were friends with mothers for nearly half (11 of 23) of these study infants, and adult males that were not fathers were friends with mothers for the other half (12 of 23; ESM Table S3). Friendships with fathers did not differ in intensity (i.e., in the amount of time dyads spent in proximity to male friends) from friendships with non-fathers (25.0±3.3% vs. 18.8±2.3% of the total time budgets of mothers, respectively; t test, p=0.14).
Almost all friends who were genetic fathers had achieved at least one third of the mother’s available consort time when she was most likely to have conceived the infant (seven of eight cases in which we had at least 5 h of mating data for the female). In contrast, nearly all friends who were not genetic fathers were not observed consorting with the mother when she conceived the infant (eight of nine cases in which we had at least 5 h of mating data for the female; Fisher’s exact test comparing these two proportions, p= 0.003; ESM Table S3). Furthermore, friends who were fathers were significantly more likely than friends who were not fathers to be present in the group during the mother’s future reproductive cycles (82% of 11 cases vs. 25% of 12 cases; Fisher’s exact test, p=0.012).
Of the 13 adult males identified as fathers of the 23 infants for whom paternity could be assigned (ESM Table S4), five sired multiple infants in this study. Because fathers were not consistent in their behavior toward their own infants, we examined the determinants of affiliation for fathers in bivariate analyses (because our sample size prohibited multivariate analyses), by analyzing whether characteristics of the father or the mother–infant dyad predicted whether fathers formed friendships with particular dyads. We found that fathers who were friends did not differ significantly from fathers who were not friends in age, dominance rank, or the number of other immature offspring present in the group (ESM Table S5). Moreover, a dyad’s presumed risk of social aggression from males (i.e., months since the immigration of a new adult male) and females (i.e., maternal dominance rank), mothers’ prior infant care experience (i.e., parity number) and infant sex did not predict whether fathers formed friendships with dyads (ESM Table S5). Finally, although father–friends had achieved a higher proportion of the mother’s consort time at conception compared to father–non-friends (0.71±0.15 vs. 0.43 ± 0.15), this difference was not statistically significant (t test, p=0.20; ESM Table S5). These results suggest that either an individual father’s decision to befriend particular mother–infant dyads was not predicted by his current status, past mating success with the mother, or by maternal or infant characteristics, or it suggests that the basis of male decisions are complex and can only be revealed with larger sample sizes that accommodate multivariate analyses.
Did male friends (regardless of whether they were fathers) achieve more mating success with female friends (once those females resumed cycling) than what was expected given their average consort activity with all other adult females in the group? To address this question, we first restricted our analyses to those dyads in which both the male and female were present in the group when the female partner resumed cycling, and a minimum of 5 h total consort observations were available for her before she conceived her next offspring (see “Materials and methods”). For one of 21 dyads that met these criteria, an expected value of consortship with the mother could not be calculated because the male did not consort with any female during the period in question. On average, mothers resumed cycling 9.6±2.5 SD months after infant birth and cycled for 4.8±3.0 SD months before conceiving the next infant (n= 29). Among the 20 friendship dyads in which data were available, male friends consorted with the female more often, as often, or less than expected in 20% (n=4), 30% (n=6), and 50% (n=10), respectively, of all cases (ESM Table S6). Next, we further restricted our analyses to only those friendship dyads in which both the male and female were present in the group on all days up to and including the day the female conceived her next offspring (n=15). In four of these 15 dyads consisting of former friends (27%), former friends consorted with the female more than expected (given the male’s overall consorting activity that period); this is not a significantly different proportion of greater than expected consorting compared to dyads consisting of non-friends (21% of 72; Fisher’s exact test, p=0.732). In this study, friendship in the past did not predict whether a male would consort with a female in the future.
Discussion
Definitions and characteristics of friendships at Amboseli
In the large majority of dyads, the female’s most frequent male proximity partner was also her most frequent male grooming partner, and this provided a basis for differentiating male–female pairs with particularly strong bonds (i.e., friendships) from all other pairs. Our criteria for defining friendships differed somewhat from those in previous studies in which data on the identity of the individuals responsible for the onset and termination of proximity bouts were used to calculate composite scores with which to identify friendship bonds (see Smuts 1985; Palombit et al. 1997). These data are labor intensive to collect and do not always provide an objective means of differentiating friend from non-friend dyads. For example, although two thirds of the friend dyads in Smuts’s (1985) study were identified using the dyads’ composite scores, one third of the friend dyads were identified subjectively, based on prior anticipation of them qualifying as friend dyads even though their scores did not meet the author’s objective criteria for friendship.
Despite differences in the criteria used to identify friendships between our study and those in previous studies, friendships at Amboseli did not differ qualitatively from those at other sites. Females at Amboseli spent more time grooming males than being groomed by them before and after birth. Females also devoted a greater share of their grooming time to adult males after the birth of their infants than before. These patterns are consistent with those described in three well-studied primate systems where stable male–female bonds have been described. Female olive baboons at Gilgil (Smuts 1985), chacma baboons in the Okavango Delta (Palombit et al. 1997), and mountain gorillas at Karisoke (Harcourt 1979; Sicotte 1994; Watts 1996) all contribute more than their male partners to the formation and maintenance of friendship bonds.
Friendships provide protection to females’ infants
Our study is the first to find evidence consistent with the hypothesis that, by forming friendships with males, mothers gain direct benefits for their offspring in the form of protection against harassment by other females. As in other primates (Hiraiwa 1981; Nicolson 1987; Silk 1999), female baboons at Amboseli (of all ages) handled infants more roughly and at higher rates than did males. Infants who were handled roughly at higher rates gave more distress cries, indicating that rough forms of handling was distressing and may be harmful to infants. However, infants whose mothers spent the most time with male friends received less rough handling and cried less often than infants whose mothers spent less time in proximity to friends. Aggressive handling of infants is associated with lower survival in two other primate populations (Silk 1980; Kleindorfer and Wasser 2004), and kidnapping has resulted in infant death at this site in the past (Shopland and Altmann 1987). Friendship with males may therefore help to reduce an infant’s risk of injury or mortality. Mortality at Amboseli (Altmann and Alberts 2003) and other long-term study populations (Gerald 1995; Hill et al. 2001; Cheney et al. 2004) is highest during infancy. Perhaps mothers in these other species and other baboon populations benefit, as those in Amboseli do, from male friends’ protection against female harassment of offspring, a potentially underappreciated source of danger to primate infants. Finally, our finding that variation in the rate at which infants were handled was not influenced by infant sex, maternal parity, or dominance rank suggests that infants of all backgrounds may benefit from male protection.
Infanticide avoidance has been suggested to be the adaptive reason for year-round male–female bonds in social animals (Hrdy 1979; Palombit 2000). Some prior studies have found data consistent with this hypothesis (Palombit et al. 1997; Borries et al. 1999b), and others have produced more equivocal results (Wright 1995; Overdorff 1998). Baboon mothers at Amboseli did not increase their association with male friends the more recently (a) an adult male had immigrated into the group or (b) there had been a change in the topmost position in the male dominance hierarchy. Instead, mothers appeared to be avoiding male friends during these times of greater presumed infanticide risk, possibly as a defense against becoming a focus of aggression during periods of enhanced male–male conflict (Smuts 1985).
One possibility is that infanticide may have been the initial selective force for the evolution of year-round male–female bonds in primates, but in those populations where infanticide risk is low, friendships may be maintained by the secondary benefits gained through protection from other dangers. At Amboseli, infants whose mothers maintained particularly intense friendships with males suffered from less social harassment by conspecific females and cried less often. Our results, in conjunction with evidence from other studies suggesting that year-round male–female associations may protect infants from infanticide (e.g., Palombit et al. 1997; Borries et al. 1999b), indicate that friendships in primates may function as female counter-strategies against a variety of social threats to infant safety.
Friendships may represent joint parental care
Because young mammals depend for their nutrition solely on unique maternal physiological adaptations (mammary glands and milk production), opportunities for paternal care are more limited in mammals than in those species in which males can support nutritionally dependent young (Clutton-Brock 1991). Indeed, the prevalence of male–infant interactions in primates has commonly been attributed to male mating effort, rather than to paternal care (reviewed in van Schaik and Paul 1996). In contrast to this interpretation, our results suggest that prior friendship with a particular female did not predict whether a male would consort with that female in the future. Nearly half of all male friends emigrated from the female’s social group before she conceived her next offspring, and those friends who continued to reside in the female’s social group did not have greater consortship success with her when she resumed cycling than they would have achieved otherwise. Prior friendship has been found to lead to enhanced male mating success with former friends in two primate populations (Smuts 1985; Menard et al. 2001). However, in the majority of studies, male friends have not had greater mating success with females once these females resumed cycling (Chapais 1983a, b; Chapais 1986b; Hill 1990; Manson 1994; Paul 1999).
Could friendships at Amboseli represent a form of paternal investment in offspring? Recent studies of the baboon population at Amboseli provide compelling evidence that males in this society engage in true paternal care. First, male baboons at Amboseli were found to selectively support their own juvenile offspring in agonistic disputes (Buchan et al. 2003). Second, the father’s presence during the offspring’s immature period was found to accelerate offspring maturation (Charpentier et al. 2008). The results of the present study, conducted on the same population, suggest that some friendships represent a form of paternal investment in offspring and also provide intriguing evidence that paternal kin recognition and investment in offspring may begin immediately after birth. Nearly one half of all friendship dyads consisted of a mother–father pair. In addition, friends who were fathers were more likely than friends who were not fathers to be present in the group during the mother’s future reproductive cycles.
Our results suggest that, at Amboseli, an individual father’s decision to form a friendship alliance with a particular mother–infant dyad was not predicted in any simple way by (1) the father’s current status (age, dominance, or other offspring in the group), (2) his past mating success with the mother, (3) or by maternal or infant characteristics. The factors prompting some fathers to enter into friendships with mother–infant dyads thus remain unclear and represent a critical avenue for future research at Amboseli and other sites where male–female friendships occur.
Another puzzle remains: If prior friendship with a female does not increase a male’s chances of mating with her in future reproductive cycles, what other benefits can be obtained by a male friend who is not the infant’s father? It is possible that, by associating with a new mother, a male may gain additional matings (either presently or in the future) with other females who observe his behavior (van Schaik and Paul 1996). Support for this idea comes from studies that have shown that (1) males are more attractive as mates when caring for offspring (Price 1990), including offspring sired by other males (Gori et al. 1996), or that (2) males are more affiliative toward infants when the mother was in sight than when she was not (Keddy-Hector et al. 1989). Males may also benefit from the grooming they receive from female friends. Because grooming confers both health and social benefits (Keverne et al. 1989; Dunbar 1991; Tanaka and Takefushi 1993), it could be traded [as a commodity in biological markets (Barrett et al. 1999)] in exchange for itself or for other services. Female friends at Amboseli groomed male partners more often than they were groomed by them and devoted a greater share of their grooming time to these males after birth than before birth. It is possible that male friends who were not fathers may trade protection of unrelated infants in exchange for grooming, for the chance to increase their attractiveness as mates to other females or for some services or benefits not yet identified.
Intriguingly, nearly all friends who were not genetic fathers were also not ‘behavioral’ fathers (i.e., males who had consorted with the mother when she was likely to have conceived the infant), a finding that indicates that friendships between non-fathers and mothers in this population were not simply the result of paternity confusion. That non-father friends were not misled by past mating behavior into forming friendships raises the question of what actually led them to do so. Additional information on the benefits of friendships for non-fathers from this and other populations are needed before a full understanding of the adaptive value of friendships for all male and female participants is possible.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of a National Science Foundation (NSF) Dissertation Improvement Grant to NN and JA as well as support to NN from the LSB Leakey Foundation and Princeton University. During this study, the Amboseli Baboon Project was supported by NSF grants IOB-0322613, IOB-0322781, BCS-0323553, and BCS-0323596 to JA and SCA. We thank the Republic of Kenya and the Kenya Wildlife Service for permission to work in Amboseli, the Institute of Primate Research for local sponsorship, and the Wardens and staff of Amboseli National Park and the pastoralist communities of Amboseli and Longido for continuous cooperation. R. Zimmerman designed the software program used to collect the behavioral data; Amboseli field workers R. S. Mututua, S. N. Sayialel, and J. K. Warutere provided field assistance and C. Markham and L. Gerber provided assistance with the Amboseli database. The manuscript benefited greatly from discussions with or comments by P. Fashing, M. Hau, D. Rubenstein, and two anonymous reviewers. All procedures were noninvasive and comply with relevant regulations in Kenya (Kenya Research Permit MOEST 13/001/C351 Vol. II) and the USA (IACUC 1456, renewed 12 November 2002).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00265-009-0786-6) contains supplementary material, which is available to authorized users.
Contributor Information
Nga Nguyen, Email: ntn@clevelandmetroparks.com, Department of Conservation & Science, Cleveland Metroparks Zoo, Cleveland, OH 44109, USA. Department of Biology, Case Western Reserve University, Cleveland, OH 44106, USA. Department of Anthropology, California State University Fullerton, Fullerton, CA 92834, USA.
Russell C. Van Horn, Conservation & Research for Endangered Species, Zoological Society of San Diego, San Diego, CA 92112, USA
Susan C. Alberts, Department of Biology, Duke University, Durham, NC 27708, USA. Institute for Primate Research, National Museums of Kenya, Nairobi, Kenya
Jeanne Altmann, Institute for Primate Research, National Museums of Kenya, Nairobi, Kenya. Department of Ecology & Evolutionary Biology, Princeton University, Princeton, NJ 08544, USA. Department of Animal Physiology and Veterinary Medicine, University of Nairobi, Nairobi, Kenya.
References
- Alberts SC. Paternal kin discrimination in wild baboons. Proc R Soc Lond B Biol Sci. 1999;266:1501–1506. doi: 10.1098/rspb.1999.0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts SC, Altmann J. Preparation and activation—determinants of age at reproductive maturity in male baboons. Behav Ecol Sociobiol. 1995;36:397–406. [Google Scholar]
- Alberts SC, Altmann J. Matrix models for primate life history analysis. In: Kappeler P, Pereira ME, editors. Primate life history and socioecology. University of Chicago Press; Chicago: 2003. pp. 66–102. [Google Scholar]
- Alberts SC, Buchan JC, Altmann J. Sexual selection in wild baboons: from mating opportunities to paternity success. Anim Behav. 2006;72:1177–1196. [Google Scholar]
- Alberts SC, Sapolsky RM, Altmann J. Behavioral, endocrine, and immunological correlates of immigration by an aggressive male into a natural primate group. Horm Behav. 1992;26:167–178. doi: 10.1016/0018-506x(92)90040-3. [DOI] [PubMed] [Google Scholar]
- Alberts SC, Watts HE, Altmann J. Queuing and queue-jumping: long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Anim Behav. 2003;65:821–840. [Google Scholar]
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Altmann J. Baboon mothers and infants. Harvard University Press; Cambridge: 1980. [Google Scholar]
- Altmann J, Alberts SC. Intraspecific variability in fertility and offspring survival in a nonhuman primate: behavioral control of ecological and social sources. In: Wachter KW, Bulatao RA, editors. Offspring: the biodemography of fertility and family behavior. National Academy Press; Washington, DC: 2003. pp. 140–169. [Google Scholar]
- Altmann SA. Foraging for survival: yearling baboons in Africa. University of Chicago Press; Chicago: 1998. [Google Scholar]
- Anderson CM. Levels of social organization and male female bonding in the genus Papio. Am J Phys Anthropol. 1983;60:15–22. doi: 10.1002/ajpa.1330600104. [DOI] [PubMed] [Google Scholar]
- Armstrong DP. Aggressive of breeding territorial honeyeaters corresponds to seasonal changes in nectar availability. Behav Ecol Sociobiol. 1991;29:103–111. [Google Scholar]
- Aureli F, de Waal FBM. Why natural conflict resolution? In: Aureli F, de Waal FBM, editors. Natural conflict resolution. University of California Press; Berkeley: 2000. pp. 3–10. [Google Scholar]
- Bales KB. Cumulative scaling of paternalistic behavior in primates. Am Nat. 1980;116:454–461. [Google Scholar]
- Barrett L, Henzi SP, Weingrill T, Lycett JE, Hill RA. Market forces predict grooming reciprocity in female baboons. Proc R Soc Lond B Biol Sci. 1999;266:665–670. [Google Scholar]
- Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL. The effect of new alpha males on female stress in free-ranging baboons. Anim Behav. 2005;69:1211–1221. [Google Scholar]
- Bercovitch FB. Reproductive success in male savanna baboons. Behav Ecol Sociobiol. 1987;21:163–72. [Google Scholar]
- Bercovitch FB. Mate selection, consortship formation, and reproductive tactics in adult female savanna baboons. Primates. 1991;32:437–452. [Google Scholar]
- Berenstain L, Wade TD. Intrasexual selection and male mating strategies in baboons and macaques. Int J Primatol. 1983;4:201–235. [Google Scholar]
- Blumstein DT. The evolution of infanticide in rodents: A comparative analysis. In: van Schaik CP, Janson C, editors. Infanticide by males and its implications. Cambridge University Press; Cambridge: 2000. pp. 178–197. [Google Scholar]
- Borries C, Launhardt K, Epplen C, Epplen JT, Winkler P. DNA analyses support the hypothesis that infanticide is adaptive in langur monkeys. Proc R Soc Lond B Biol Sci. 1999a;266:901–904. doi: 10.1098/rspb.1999.0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borries C, Launhardt K, Epplen C, Epplen JT, Winkler P. Males as infant protectors in Hanuman langurs (Presbytis entellus) living in multimale groups - defence pattern, paternity and sexual behaviour. Behav Ecol Sociobiol. 1999b;46:350–356. [Google Scholar]
- Buchan JC, Alberts SC, Silk JB, Altmann J. True paternal care in a multi-male primate society. Nature. 2003;425:179–181. doi: 10.1038/nature01866. [DOI] [PubMed] [Google Scholar]
- Bulger JB. Dominance rank and access to estrous females in male savanna baboons. Behaviour. 1993;127:67–103. [Google Scholar]
- Buss DM. The evolution of desire. Harper Collins; New York: 1994. [Google Scholar]
- Chapais B. Reproductive activity in relation to male dominance and the likelihood of ovulation in rhesus monkeys. Behav Ecol Sociobiol. 1983a;12:215–228. [Google Scholar]
- Chapais B. Structure of the birth season relationship among adult male and female rhesus monkeys. In: Hinde RA, editor. Primate social relationships. Blackwell Scientific; Oxford: 1983b. pp. 200–208. [Google Scholar]
- Chapais B. Why do adult male and female rhesus monkeys affiliate during the birth season? In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago macaques: history, behavior, and biology. State University of New York Press; Albany: 1986a. pp. 173–200. [Google Scholar]
- Chapais B. Why do adult male and female rhesus monkeys affiliate during the birth season? In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago macaques. SUNY Press; Albany: 1986b. pp. 173–200. [Google Scholar]
- Charpentier MJE, Van Horn RC, Altmann J, Alberts SC. Paternal effects on offspring fitness in a multimale primate society. Proc Natl Acad Sci U S A. 2008;105:1988–1992. doi: 10.1073/pnas.0711219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM, Fischer J, Beehner J, Bergman T, Johnson SE, Kitchen DM, Palombit RA, Rendall D, Silk JB. Factors affecting reproduction and mortality among baboons in the Okavango Delta, Botswana. Int J Primatol. 2004;25:401–428. [Google Scholar]
- Chuang-Dobbs HC, Webster MS, Holmes RT. Paternity and parental care in the black-throated blue warbler, Dendroica caerulescens. Anim Behav. 2001;62:83–92. [Google Scholar]
- Clutton-Brock TH. Mammalian mating systems. Proc R Soc Lond B Biol Sci. 1989;236:339–372. doi: 10.1098/rspb.1989.0027. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. The evolution of parental care. Princeton University Press; Princeton: 1991. [Google Scholar]
- Dixon A, Ross D, Omalley SLC, Burke T. Paternal investment inversely related to degree of extra pair paternity in the reed bunting. Nature. 1994;371:698–700. [Google Scholar]
- Drickamer LC, Gowaty PA, Holmes CM. Free female mate choice in house mice affects reproductive success and offspring viability and performance. Anim Behav. 2000;59:371–378. doi: 10.1006/anbe.1999.1316. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. Functional significance of social grooming in primates. Folia Primatol. 1991;57:121–131. [Google Scholar]
- Estes RD. The behavior guide to african mammals: including hoofed mammals, carnivores. University of California Press; Berkeley, Primates: 1992. [Google Scholar]
- Eggert AK, Muller JK. Biparental care and social evolution in burying beetles: lessons from the larder. In: Choe JC, Crespi BJ, editors. The evolution of social behavior in insects and arachnids. Cambridge University Press; Cambridge: 1997. pp. 216–236. [Google Scholar]
- Freeman-Gallant CR. Parentage and paternal care: consequences of intersexual selection in Savannah sparrows? Behav Ecol Sociobiol. 1997;40:395–400. [Google Scholar]
- Gerald CN. MSc thesis. Princeton University; Princeton: 1995. Demography of the Virunga mountain gorilla (Gorilla gorilla beringei) [Google Scholar]
- Gori DF, Rohwer S, Caselle J. Accepting unrelated broods helps replacement male yellow-headed blackbirds attract mates. Behav Ecol. 1996;7:49–54. [Google Scholar]
- Gubernick DJ. Biparental care and male-female relations in mammals. In: Parmigiani S, von Saal FS, editors. Infanticide and parental care. Harwood; Chur, Switzerland: 1994. pp. 427–463. [Google Scholar]
- Harcourt AH. Social relationships between adult male and female mountain gorillas in the wild. Anim Behav. 1979;27:325–342. [Google Scholar]
- Hausfater G. Dominance and reproduction in baboons (Papio cynocephalus) Karger; Basel: 1975. [PubMed] [Google Scholar]
- Hausfater G, Altmann J, Altmann S. Long-term consistency of dominance relations among female baboons (Papio cynocephalus) Science. 1982;217:752–755. doi: 10.1126/science.217.4561.752. [DOI] [PubMed] [Google Scholar]
- Hausfater G, Hrdy SB. Infanticide: comparative and evolutionary perspectives. Aldine de Gruyter; New York: 1984. [Google Scholar]
- Hendrickx AG, Kraemer DC. Observations on the menstrual cycle, optimal mating time and pre-implantation embryos in the baboon, Papio anubis and Papio cynocephalus. J Reprod Fertil Supp. 1969;6:119–128. [Google Scholar]
- Hill DA. Social relationships between adult male and female rhesus macaques.2. Nonsexual affiliative behavior. Primates. 1990;31:33–50. [Google Scholar]
- Hill K, Boesch C, Goodall J, Pusey A, Williams J, Wrangham R. Mortality rates among wild chimpanzees. J Hum Evol. 2001;40:437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
- Hiraiwa M. Maternal and alloparental care in a troop of free-ranging Japanese monkeys. Primates. 1981;22:309–329. [Google Scholar]
- Hrdy SB. Infanticide among animals - Review, classification, and examination of the implications for the reproductive strategies of females. Ethol Sociobiol. 1979;1:13–40. [Google Scholar]
- Keddy-Hector AC, Seyfarth RM, Raleigh MJ. Male parental care, female choice and the effect of an audience in vervet monkeys. Anim Behav. 1989;38:262–271. [Google Scholar]
- Keverne EB, Martensz ND, Tuite B. Beta endorphin concentrations in cerebrospinal-fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology. 1989;14:155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- Kingdon J. The Kingdon field guide to african mammals. Black; London: 2003. [Google Scholar]
- Kleiman DG, Malcolm JR. The evolution of male parental investment in mammals. In: Gubernick DJ, Klopfer PH, editors. Parental care in mammals. Plenum; New York: 1981. pp. 347–387. [Google Scholar]
- Kleindorfer S, Wasser SK. Infant handling and mortality in yellow baboons (Papio cynocephalus): evidence for female reproductive competition? Behav Ecol Sociobiol. 2004;56:328–337. [Google Scholar]
- Lack DL. Ecological adaptations for breeding in birds. Methuen; London: 1968. [Google Scholar]
- Lemasson A, Palombit RA, Jubin R. Friendships between males and lactating females in a free-ranging group of olive baboons (Papio hamadryas anubis): evidence from playback experiments. Behav Ecol Sociobiol. 2008;62:1027–1035. [Google Scholar]
- Maestripieri D. Determinants of affiliative interactions between adult males and lactating females in pigtail macaques (Macaca nemestrina nemestrina) Ethology. 2000;106:425–439. [Google Scholar]
- Manson JH. Mating patterns, mate choice, and birth season heterosexual relationships in free-ranging rhesus macaques. Primates. 1994;35:417–433. [Google Scholar]
- Marshall TC, Slate J, Kruuk LEB, Pemberton JM. Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- Mason JH. Mating patterns, mate choice, and birth season heterosexual relationships in free-ranging rhesus macaques. Primates. 1994;35:417–433. [Google Scholar]
- Menard N, von Segesser F, Scheffrahn W, Pastorini J, Vallet D, Gaci B, Martin RD, Gautier-Hion A. Is male-infant caretaking related to paternity and/or mating activities in wild Barbary macaques (Macaca sylvanus)? C R Acad Sci SeÂr 3 Sci Vie. 2001;324:601–610. doi: 10.1016/s0764-4469(01)01339-7. [DOI] [PubMed] [Google Scholar]
- Moller AP, Birkhead TR. Certainty of paternity covaries with paternal care in birds. Behav Ecol Sociobiol. 1993;33:261–268. [Google Scholar]
- Morris D. The naked ape. Cape; London: 1967. [Google Scholar]
- Nicolson NA. Infants, mothers, and other females. In: Smuts B, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Unversity of Chicago Press; Chicago: 1987. pp. 330–342. [Google Scholar]
- Noe R, Sluijter AA. Reproductive tactics of male savanna baboons. Behaviour. 1990;113:117–170. [Google Scholar]
- Overdorff DJ. Are Eulemur species pair-bonded? Social organization and mating strategies in Eulemur fulvus rufus from 1988–1995 in southeast Madagascar. Am J Phys Anthropol. 1998;105:153–166. doi: 10.1002/(SICI)1096-8644(199802)105:2<153::AID-AJPA4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Palombit RA. Infanticide and the evolution of male–female bonds in animals. In: van Schaik CP, Janson C, editors. Infanticide by males and its implications. Cambridge University Press; Cambridge: 2000. pp. 239–268. [Google Scholar]
- Palombit RA, Seyfarth RM, Cheney DL. The adaptive value of ‘friendships’ to female baboons: experimental and observational evidence. Anim Behav. 1997;54:599–614. doi: 10.1006/anbe.1996.0457. [DOI] [PubMed] [Google Scholar]
- Parmigiani S, Palanza P, Mainardi D, Brain PF. Infanticide and protection of young in house mice (Mus domesticus): female and male strategies. In: Parmigiani S, von Saal FS, editors. Infanticide and parental care. Harwood; Chur, Switzerland: 1994. pp. 341–363. [Google Scholar]
- Paul A. The socioecology of infant handling in primates: is the current model convincing? Primates. 1999;40:33–46. doi: 10.1007/BF02557700. [DOI] [PubMed] [Google Scholar]
- Paul A, Kuester J, Arnemann J. The sociobiology of male-infant interactions in Barbary macaques, Macaca sylvanus. Anim Behav. 1996;51:155–170. [Google Scholar]
- Price EC. Infant carrying as a courtship strategy of breeding male cotton-top tamarins. Anim Behav. 1990;40:784–786. [Google Scholar]
- Pusey AE, Packer C. Dispersal and philopatry. In: Smuts B, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. University of Chicago Press; Chicago: 1987. pp. 250–266. [Google Scholar]
- Pusey AE, Packer C. Infanticide in lions: consequences and counterstrategies. In: Parmigiani S, von Saal FS, editors. Infanticide and parental care. Harwood; London: 1994. pp. 277–299. [Google Scholar]
- Ransom TW, Ransom BS. Adult male–infant relations among baboons (Papio anubis) Folia Primatol. 1971;16:179–195. doi: 10.1159/000155400. [DOI] [PubMed] [Google Scholar]
- Rios-Cardenas O, Webster AS. Paternity and paternal effort in the pumpkinseed sunfish. Behav Ecol. 2005;16:914–921. [Google Scholar]
- Rowell TE, Olson DK. Alternative mechanisms of social organization in monkeys. Behaviour. 1983;86:31–54. [Google Scholar]
- Sandvik M, Rosenqvist G, Berglund A. Male and female mate choice affects offspring quality in a sex-role-reversed pipefish. Proc R Soc Lond B Biol Sci. 2000;267:2151–2155. doi: 10.1098/rspb.2000.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Neuroendocrinology of the stress response. In: Becker JB, Breedlove SM, Crews D, editors. Behav Endocrinol. MIT Press; Cambridge: 1993. pp. 287–324. [Google Scholar]
- Seyfarth RM. Social relationships among adult male and female baboons. I. Behaviour during sexual consortship. Behaviour. 1978a;64:204–226. [Google Scholar]
- Seyfarth RM. Social relationships among adult male and female baboons. II. Behavior throughout the female reproductive cycle. Behaviour. 1978b;64:227–247. [Google Scholar]
- Seyfarth RM. Grooming and social competition in primates. In: Hinde RA, editor. Primate Social relationships: an integrated approach. Blackwell; London: 1983. pp. 182–189. [Google Scholar]
- Shaikh AA, Celaya CL, Gomez I, Shaikh SA. Temporal relationship of hormonal peaks to ovulation and sex skin deturgescence in the baboon. Primates. 1982;23:444–452. [Google Scholar]
- Shopland JM, Altmann J. Fatal intragroup kidnapping in yellow baboons. Am J Primatol. 1987;13:61–65. doi: 10.1002/ajp.1350130108. [DOI] [PubMed] [Google Scholar]
- Sicotte P. Effect of male competition on male female relationships in bi male groups of mountain gorillas. Ethology. 1994;97:47–64. [Google Scholar]
- Silk JB. Kidnapping and female competition in captive bonnet macaques. Primates. 1980;21:100–110. [Google Scholar]
- Silk JB. Why are infants so attractive to others? The form and function of infant handling in bonnet macaques. Anim Behav. 1999;57:1021–1032. doi: 10.1006/anbe.1998.1065. [DOI] [PubMed] [Google Scholar]
- Smith K, Alberts SC, Altmann J. Wild female baboons bias their social behaviour towards paternal half-sisters. Proc R Soc Lond B Biol Sci. 2003;270:503–510. doi: 10.1098/rspb.2002.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuts B. Sex and friendship in baboons. Cambridge University Press; Cambridge: 1985. [Google Scholar]
- Smuts B, Gubernick DJ. Male–infant relationships in nonhuman primates: paternal investment or mating effort? In: Hewlett BS, editor. Father–child relations. Aldine de Guyter; New York: 1992. pp. 1–30. [Google Scholar]
- Soltis J, Thomsen R, Matsubayashi K, Takenaka O. Infanticide by resident males and female counter-strategies in wild Japanese macaques (Macaca fuscata) Behav Ecol Sociobiol. 2000;48:195–202. [Google Scholar]
- Takahata Y. Social relations between adult males and females of Japanese monkeys in the Arashiyama B troop. Primates. 1982;23:1–23. doi: 10.1007/s10329-022-01024-x. [DOI] [PubMed] [Google Scholar]
- Tanaka I, Takefushi H. Elimination of external parasites (lice) is the primary function of grooming in free-ranging japanese macaques. Anthropol Sci. 1993;101:187–193. [Google Scholar]
- Taub DM. The functions of primate paternalism: a cross-species review. In: Feierman JR, editor. Pedophilia: biosocial dimensions. Springer; New York: 1990. pp. 338–377. [Google Scholar]
- Taub DM, Mehlman PT. Primate paternalistic investment: a cross-species view. In: Loy JD, Peters CB, editors. Understanding behavior: what primate studies tell us about human behavior. Oxford; New York: 1991. pp. 51–89. [Google Scholar]
- Van Horn RC, Wahaj SA, Holekamp KE. Role-reversed nepotism among cubs and sires in the spotted hyena (Crocuta crocuta) Ethology. 2004;110:413–426. [Google Scholar]
- van Schaik CP. Infanticide by male primates: The sexual selection hypothesis revisited. In: van Schaik CP, Janson CH, editors. Infanticide by males and its implications. Cambridge University Press; Cambridge: 2000. pp. 27–60. [Google Scholar]
- van Schaik CP, Janson CH. Infanticide by males and its implications. Cambridge University Press; Cambridge: 2000. [Google Scholar]
- van Schaik CP, Kappeler PM. Infanticide risk and the evolution of male-female association in primates. Proc R Soc Lond B Biolog Sci. 1997;264:1687–1694. doi: 10.1098/rspb.1997.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik CP, Paul A. Male care in primates: does it ever reflect paternity? Evol Anthropol. 1996;5:152–156. [Google Scholar]
- Wasser SK, Barash DP. The selfish allomother—a comment. Ethol Sociobiol. 1981;2:91–93. [Google Scholar]
- Watts DP. Comparative socio-ecology of gorillas. In: McGrew WC, Marchant LF, Nishida T, editors. Great ape societies. Cambridge University Press; Cambridge: 1996. pp. 16–28. [Google Scholar]
- Weatherhead PJ, Montgomerie R, Gibbs HL, Boag PT. The cost of extra-pair fertilizations to female red-winged blackbirds. Proc R Soc Lond B Biol Sci. 1994;258:315–320. [Google Scholar]
- Widdig A, Nurnberg P, Krawczak M, Streich WJ, Bercovitch FB. Paternal relatedness and age proximity regulate social relationships among adult female rhesus macaques. Proc Natl Acad Sci U S A. 2001;98:13769–13773. doi: 10.1073/pnas.241210198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt DE, Doyle LL, Stone SC, Harrison RM. Correlation of perineal swelling with serum ovarian hormone levels, vaginal cytology, and ovarian follicular development during the baboon reproductive cycle. Primates. 1977;18:261–270. [Google Scholar]
- Wilson EO. Sociobiology. Belknap; Cambridge: 1975. [Google Scholar]
- Wittenberger JF, Tilson RL. The evolution of monogamy—hypotheses and evidence. Annu Rev Ecol Syst. 1980;11:197–232. [Google Scholar]
- Wolff JO, Cicirello DM. Comparative paternal and infanticidal behavior of sympatric white-footed mice (Peromyscus leucopus noveboracensis) and deermice (P. maniculatus nubiterrae) Behav Ecol. 1991;2:38–45. [Google Scholar]
- Wong BBM. Superior fighters make mediocre fathers in the Pacific blue-eye fish. Anim Behav. 2004;67:583–590. [Google Scholar]
- Wright PC. Demography and life history of free-ranging Propithecus diadema edwardsi in Ranomafana National Park, Madagascar. Int J Primatol. 1995;16:835–854. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.