Abstract
Oxidative stress has been implicated in the pathogenesis of cardiovascular diseases, including myocardial hypertrophy and infarction. Although impairment of antioxidant defense mechanisms has been thought to provoke oxidative stress-induced myocardial dysfunction, it has been difficult to clearly demonstrate. Nuclear erythroid 2 p45-related factor 2 (Nrf2) is a redox-sensitive, basic leucine zipper protein that regulates the transcription of several antioxidant genes. We previously reported that sustained activation of Nrf2 upregulates transcription of a number of endogenous antioxidants in the heart. Here, we show that acute exercise stress (AES) results in activation of Nrf2/ARE (antioxidant response element) signaling and subsequent enhancement of antioxidant defense pathways in wild-type (WT) mouse hearts, while oxidative stress, along with blunted defense mechanisms, was observed in Nrf2−/− mice. We also find that AES is associated with increased trans-activation of ARE-containing genes in exercised animals when compared to age-matched sedentary WT mice. However, enhanced oxidative stress in response to AES was observed in Nrf2−/− mice due to lower basal expression and marked attenuation of the transcriptional induction of several antioxidant genes. Thus, AES induces ROS and promotes Nrf2 function, but disruption of Nrf2 increases susceptibility of the myocardium to oxidative stress. Our findings suggest the basis for a nonpharmacological approach to activate Nrf2/ARE signaling, which might be a potential therapeutic target to protect the heart from oxidative stress-induced cardiovascular complications.
Keywords: Nrf2, Keap1, Exercise, Oxidative stress, ROS, AES
Introduction
From a public health perspective, the increase in the aged population is a major concern in the United States [1–3]. Age associated decline in antioxidant potential and accumulation of reactive oxygen/nitrogen species (ROS/RNS) are a primary cause for multiple health problems, such as cardiovascular diseases, diabetes, cancer, and neurodegenerative diseases (Alzheimer’s, Parkinson’s, and Huntington), etc. [4–8]. Major cardiovascular diseases, such as atherosclerosis, hypertension, myocardial infarction, cardiac hypertrophy, and cardiomyopathy, are ascribed to increased oxidative stress [9–15]; however, the underlying molecular mechanisms remain poorly defined.
Changes in the transcriptional regulation of antioxidant genes have been recently reported in humans and mouse models of disease [16–18]. Evidence indicates that Nrf2 is the primary transcriptional regulator of a majority of the antioxidants including NAD(P)H-quinone oxydase-1 (NQO1), hemoxygenase-1 (HO1), γ-glutamyl cysteine ligase-catalytic (GCLC), γ-glutamyl cysteine ligase-modulatory (GCLM), glucose-6-phosphate dehydrogenase (G6PD), glutathione peroxydase-1 (GPX1), glutathione peroxydase-2 (GPX2), glutathione reductase (GSR), and catalase [19]. A recent study has reported that a significant decrease of Nrf2 nuclear expression in aged rats is associated with hepatic glutathione depletion [20], which is strongly coupled with significant down-regulation of transcription and translation products for GCLC and GCLM (rate-limiting enzyme for biosynthesis of GSH) [20]. The similarities with older wild-type and Nrf2−/− mice [21] suggest that impairment of Nrf2-ARE signaling may occur in the course of aging. Davies and co-workers have demonstrated that exercise can reduce oxidative stress [22–24]. Recent studies have observed that chronic exercise results in activation of Nrf2 in human skeletal muscle and rat kidney [25–27]. However, the mechanisms of Nrf2 activation and its downstream transcriptional regulation of antioxidants have not been clearly elucidated. Further, the role of exercise on Nrf2/ARE signaling has not been investigated in the context of the heart.
In this study, we investigate the role of acute exercise stress (AES) on Nrf2 and myocardial antioxidant defense mechanisms. We hypothesize that Nrf2 deficiency will lead to deregulation of myocardial cytoprotective mechanisms on AES. More importantly, our goal is to investigate whether exercise can induce ROS and activate the Nrf2-Keap1 pathway, an essential component of myocardial redox homeostasis, and also to assess whether disruption of Nrf2/ARE signaling is detrimental to the heart. Age-matched (8–10 weeks old) WT and Nrf2−/− mice were subjected to AES; we investigated the cytoprotective response in the myocardium.
Materials and methods
Animals
Breeding pairs of Nrf2−/−male and female mice were generously provided by Dr. Li Wang, University of Utah. Originally, the Nrf2-null mice were generated in Dr. Yamamoto’s laboratory as previously described [28]. The mice were bred and genotyped for Nrf2 expression by PCR amplification of genomic DNA using the following primers (Fig. 1A):
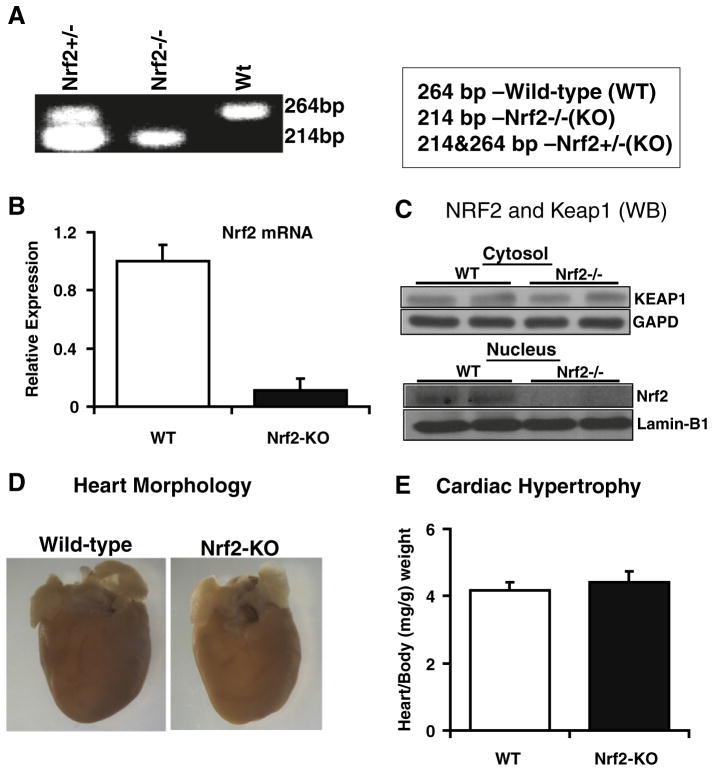
Fig. 1.
Nrf2−/− mouse model: Disruption of Nrf2 has no effect on heart structure and function. (A) PCR and agarose electrophoresis of tail DNA showing WT (264 bp), Nrf2+/− (214 and 264 bp), and Nrf2−/− (214 bp) genotypes. (B) Nrf2 mRNA expression in the heart. Real-time qPCR analysis showing basal or no expression of Nrf2 gene in the Nrf2−/− mouse myocardium (n=5). (C) Western blotting analysis for Nrf2 and Keap1 protein expression. Anti-Nrf2-ab reaction showing Nrf2 protein expression in WT, but not in Nrf2−/−mouse hearts. (Upper panel) WB confirmed the Keap1 protein expression in WT and Nrf2−/− mouse myocardium. (D) Heart morphology. Heart tissues from WT and Nrf2−/−mouse appear normal and no evidence for cardiac abnormalities including hypertrophy. (E) Organ-to-body (heart/body) weight ratios on autopsy confirm no hypertrophy either in WT or in Nrf2−/− mice.
Nrf2 forward: 5-GCCTGAGAGCTGTAGGCCC-3
Nrf2 reverse: 5-GGAATGGAAAATAGCTCCTGCC-3
Nrf2 mutant: 5-GGGTTTTCCCAGTCACGAC-3.
Mice were housed under conditions controlled for temperature and humidity, using a 12 h light/dark cycle. They were fed a standard rodent diet and water ad libitum. Age-matched WT and Nrf2−/− male mice were subjected to acute exercise as described below. All experimental protocols conducted on the mice were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Utah in accordance with the standards established by the US Animal Welfare Act.
Antibodies and reagents
The following antibodies and reagents were used: Nrf2-ab (SC-722, Santa Cruz Bio, SC, USA), Keap-1 (10503-2-AP, Proteantech, Chicago, USA), HO-1 (ab13248, Abcam Inc., SF, USA), NQO-1 (ab34173), GAPDH (ab9485), SOD1 (ab13498), SOD2 (ab13534), lamin-B1 (ab16048), catalase (219010, Calbiochem, Merck kGaA, Germany), γ-GCS (RB-1697-P1, Labvision/Neomarkers, CA, USA), Nox2 (sc5827), and Nox4 (sc21860). Secondary antibodies conjugated with horseradish peroxidase IgG (Rabbit and Mouse/PI-1000 and PI-2000, Vector Labs, Burlingame, USA) were used. Radical detecting EPR probes, CMH (NOX-2.1), TEMPOL (705748, Sigma-Aldrich, St. Louis, MO, USA), EPR grade water (NOX-7.7.1), Krebs-HEPES buffer (NOX-7.6.1), DETC (NOX-10.1), DF (NOX-9.1), glass capillary tubes (NOX-G.3.1), and critoseal (NOX-A. 3.1-VP), were purchased from Noxygen Diagnostics, Germany. Bio-Rad Protein Assay (500–0006, Bio-Rad, Hercules, CA) was used to determine protein levels in heart tissue extracts. All reagents and primers for RNA extraction and real-time RT-PCR quantification were purchased from Qiagen Inc. (Valencia, CA).
Acute exercise stress
Wild-type (WT) and Nrf2−/− mice at 2 months of age (n=6–8/ group/experiment) were subjected to exercise on a treadmill for 2 consecutive days [60 min per day; 14 m/min; 10% grade]. Immediately after exercise on the second day, mice were sacrificed and the hearts excised and processed/frozen for subsequent analysis [29,30]. EPR analysis was performed in fresh heart ventricles obtained immediately after the exercise. For GSH redox determination, fresh tissues were homogenized with MES buffer and precipitated proteins with 10% meta-phosphoric acid (MPA) and the supernatants were collected after centrifugation. The MPA supernatants were flash-frozen in liquid nitrogen and stored in −80 °C.
Myocardial glutathione (GSH) redox state
Glutathione redox (GSH/GSSG) state was determined in heart tissue extracts from WT and Nrf2−/− mice at 2 months of age. In brief, heart tissue extracts were prepared in MES buffer and centrifuged at 5000 rpm for 5 min at 4 °C. A small aliquot of the supernatants was used for protein determination and the remaining samples were mixed with equal volumes of 10% MPA (meta-phosphoric acid, Cat. No. 239275, Sigma) to precipitate proteins. A known volume of the MPA extracts was treated with TEAM (triethanolamine) reagent and 2-vinyl pyridine (only for GSSG analysis), and appropriate GSH and GSSG standards were treated similarly to prepare a standard graph. A kinetic GSH-reductase recycling assay was performed following the manufacturer’s instructions (703002, Cayman Chemicals, Ann Arbor, MI, USA) using a plate reader (Bio-Tek, FLx-800) [31–33].
Reactive oxygen species using electron paramagnetic resonance (EPR) spectroscopy
To determine the ROS load in the WT and Nrf2−/− myocardial tissue, we measured the tissue redox stress/ROS formation and assessed the temporal effect of Nrf2 deficiency under basal and AES conditions. Briefiy, mice (control or exercised) were injected (ip) with heparin (4 U/g bw) and sacrificed by CO2 inhalation. Hearts were perfused in situ with HEPES buffer (20 μM, pH 7.4) to remove residual blood. A portion of the ventricle was cut into small pieces, placed in a clean 24-well tissue culture plate containing 500 μl HEPES, washed twice, and immersed in 300 μl of the same buffer. About 10 mg of processed ventricular tissues was incubated with 150 μl of 500 μM CMH (1-hydroxy-3-methoxy-carbonyl-2,2,5,5- tet-ramethyl pyrrolidine), at 37 °C for 30 min. ROS released by tissues react with the CMH to form a stable nitroxide radical that can be measured using EPR. Aliquots (50 μl) of the incubated probe media were taken in glass capillary tubes and analyzed using an EMX-ESR spectrometer (Bruker Instruments, Germany) as reported previously [33,34].
Myocardial tissue homogenization and immunoblotting
Harvested hearts from WT and Nrf2−/− animals were initially flash frozen in liquid nitrogen [33]. Cytosolic lysates from the tissues were prepared by homogenizing the tissue using cytosolic/homogenizing buffer (10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.5 mM MgCl2, with freshly prepared 1 mM dithiothreitol and 0.1 mM phenyl methyl-sulfonyl fluoride (PMSF) and 1% Triton X-100, pH 7.9), followed by centrifugation at 5200 rpm for 5–6 min. The nuclear pellet was washed with 4 vol of homogenizing buffer to remove cytosolic contaminants. Nuclear fractions were prepared in complete lysis buffer (20 mM HEPES, 420 mM NaCl, 0.1 mM EDTA, 1.5 mM MgCl2, 25% glycerol and 1 mM dithiotheitol, 0.5 mM PMSF, pH 7.9). Samples were incubated on ice with mild shaking and centrifuged at 8200 rpm for 10 min. Later, proteins were determined using Biorad Bradford reagent and the samples for Western blots were prepared in 0.25 vol of Laemmli buffer with 5% freshly added β-mercapthoethanol and boiled for 5 min. About 30 μg of both nuclear and cytosolic proteins was resolved separately on 10–12% SDS-PAGE. The blots were probed using antibodies against Nrf2, Keap1, catalase, SOD, GPX1, GSR, G6PD, HO-1, NQO-1, γ-GCS, GAPDH, and lamin-B1. Secondary antibodies conjugated with horseradish peroxidase IgG (rabbit and mouse) were used for chemiluminescence detection.
Determination of Nrf2 nuclear translocation by immunoblotting
Nuclear proteins (30–40 μg) from WT and Nrf2−/− mice heart were resolved separately on 10% SDS-PAGE. The blots were probed using anti-Nrf2 antibody, and the same blots were stripped and reprobed with lamin-B1 or β-actin antibodies.
RNA isolation, reverse transcription, and gene expression using qPCR analysis
Myocardial tissues were harvested after in situ perfusion with 10 ml of RNase-free PBS and 10 ml of RNA later reagent. To extract RNA, ~30 mg of ventricular tissue was processed from WT and Nrf2−/− mice (n=5) using RNA extraction kits (Qiagen No. 74106) following the suppliers instructions. RNA samples were then quantified by measuring the absorbance at 260 nm. The reverse transcription reaction was performed on 2.5 μg RNA using a Qiagen Reverse Transcription Kit (Cat. No. 205311) as per the manufacturers instructions to synthesize cDNA using oligo(dT). For quantitative real-time RT-PCR analysis, 100 ng of cDNA template, 10 μl of SYBR green master mix (Qiagen No. 204054), and respective Qiagen primer sets for Ho-1 (QT00095270), Nqo-1 (QT00094367), catalase (QT01058106), G6pd (QT00120750), Gpx1 (QT01195936), Gsr (QT01758232), Gclc (QT QT00130543), Gclm (QT00174300), Nrf2 (QT00095270), and Keap-1 (QT00147371) were used and analyzed in a Light Cycler real-time thermocycler (Roche Bio). Copy numbers of cDNA targets were quantified using Ct values, and the mRNA expression levels for all samples were normalized to the level of the housekeeping gene Arbp1 (QT00249375) or GAPDH (QT01658692).
Analysis of Nrf2 binding with ARE by trans-activation assay
Nrf2 activation and antioxidant response element (ARE) binding efficacy under basal and AES conditions were evaluated in WT and Nrf2−/− mouse myocardial nuclear extracts using a Trans AM Nrf2 Kit (50296, Active Motif, Carlsbad, CA). A 10-μg aliquot of nuclear protein was incubated with immobilized oligonucleotides containing the ARE consensus binding site (5′-GTCACAGTACTCAGCAGAATCTG-3′) and the active form of Nrf2 that bound to the oligo was detected using anti-Nrf2 primary antibody after treating with HRP-conjugated secondary antibody. The chromogen formed as a result of specific activity of the transcription factor in the nuclear extracts was determined using a plate reader at 450 nm, and absorbance was expressed as the direct activity of Nrf2.
Statistical analysis
Data are expressed as mean±SD. Analysis of variance (ANOVA) followed by the Newman-Kuels multiple comparison test was used to determine significant differences between the groups. P values less than 0.05 were considered statistically significant.
Results
Nrf2 and Keap1 proteins are expressed in the mouse myocardium
The Nrf2-Keap1 pathway is critical for maintaining intracellular redox homeostasis in multiple cells and organs [21,35]. We performed Western blots to test whether Nrf2 and Keap1 proteins are expressed in the mouse myocardium. As shown in our recent report [33], we confirmed that the Nrf2 and Keap1 proteins are present in the WT mouse hearts (Fig. 1C). We observed comparable expression of Keap1 in the Nrf2−/− mouse myocardium, suggesting that abrogation of Nrf2 did not influence Keap1 protein expression (Fig. 1C). The protein (WB) and RNA expression (qPCR) analysis confirmed disruption of Nrf2 in the KO mouse heart (Figs. 1B and C).
Under basal physiological setting, loss of Nrf2 has no effect on heart morphology and function
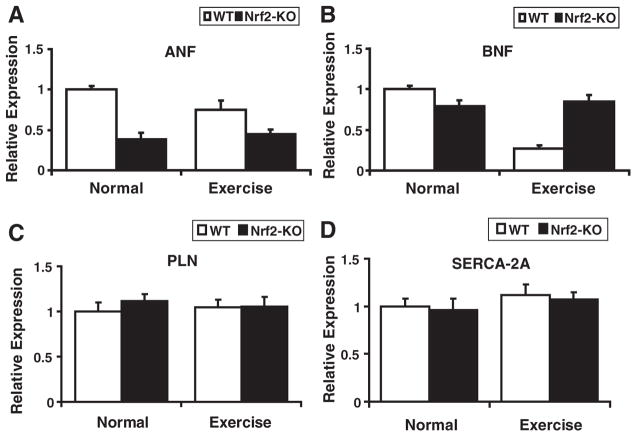
To investigate whether loss of Nrf2 can induce cardiac hypertrophy, we determined heart/body weight ratio and morphology (Fig. 1). As shown in Fig. 1D, morphology of WT and Nrf2−/− hearts was similar and no signs of hypertrophy were evident. Heart-to-body weight ratios were comparable among the WT and Nrf2−/− mice (Fig. 1E). Further, qPCR analyses for cardiac hypertrophy markers, atrial natriuretic factor (ANF) and brain natriuretic factor (BNF), did not increase after AES in these mice (Figs. 7A and B). Next, we performed echocardiograph analysis of WT and Nrf2−/− mice to investigate Nrf2′s physiological function in the heart (data not shown). These results indicate that there is no evidence for cardiac dysfunction due to disruption of Nrf2. Thus, loss of Nrf2 is not deleterious to the heart under basal-physiological or unstressed states.
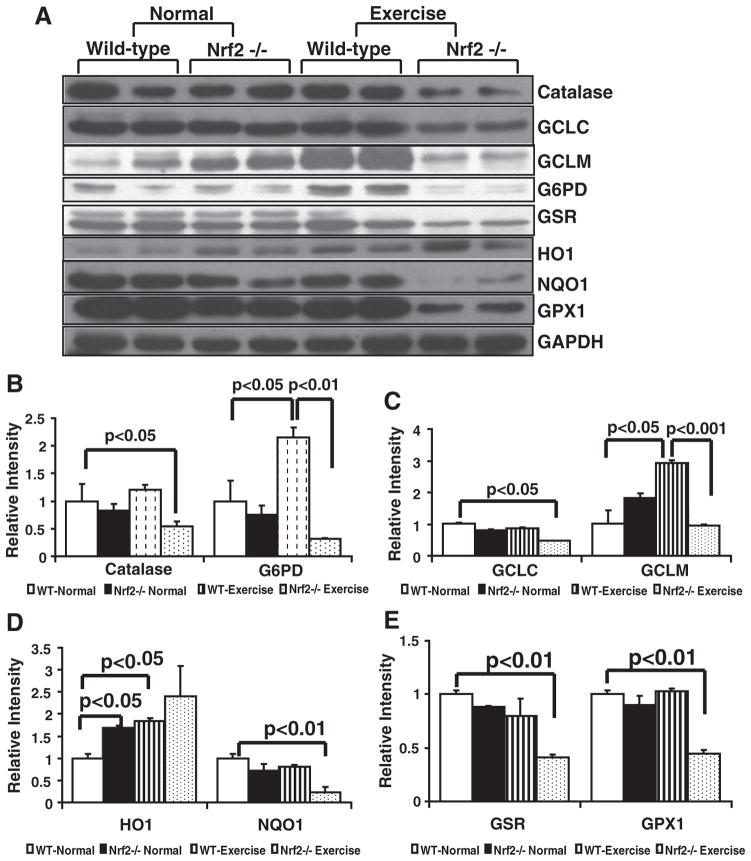
Fig. 7.
Decreased protein expression of Nrf2-dependent antioxidants in the myocardium of Nrf2−/− mice on AES. (A) Representative immunoblot experiments of cytosolic extracts from WT and Nrf2−/− mice at basal and after AES conditions. Blots with proteins were probed with anti-catalase, G6PD, GSR, GCLC, GCLM, HO1, NQO1, and GAPDH. Individual lanes indicate each animal (n=6). (B, C, D, and E) Densitometry analysis of respective protein signals were performed using Image-J and expressed as relative intensity units calculated as mean values of WT basal/young. While significant decreases in catalase, GCLC, GCLM, G6PD, NQO1, GSR, and GPX1 proteins were observed in Nrf2−/− mice after AES, some antioxidants (GCLM, G6PD, catalase) were significantly increased in WT on AES, indicating activation of Nrf2-dependent cytoprotective pathways.
Induction of ROS in the myocardium of WT and Nrf2−/− mice on AES
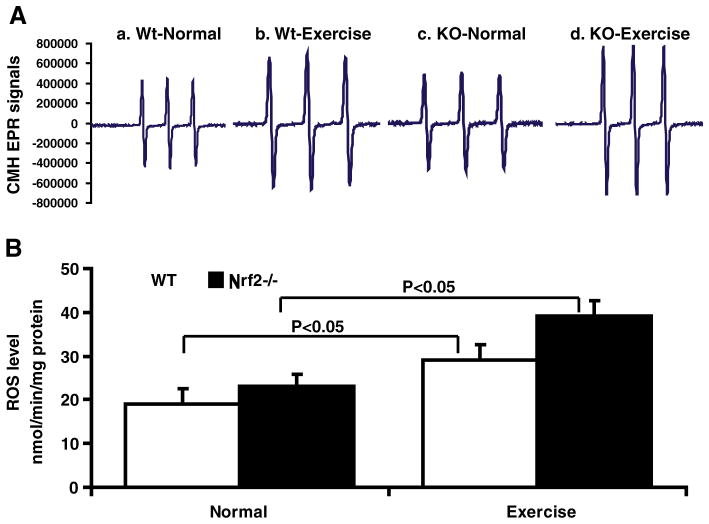
We hypothesized that acute exercise induces ROS, which might then dissociate the Nrf2/Keap1 complex and activate ARE-dependent antioxidants in the heart. Davies and co-workers have previously reported that oxygen- and nitrogen-centered free radicals and reactive oxygen and nitrogen (ROS/RNS) species are generated during exercise [24]. The EPR signal for CMH (superoxide+hydroxyl) were determined under basal and AES conditions. On AES, Nrf2−/− mouse had increased ROS accumulation (~2- and >1.5-fold; Figs. 2A and B) when compared with sedentary WT and Nrf2−/−, respectively, suggesting that Nrf2 deficiency is coupled to impaired antioxidant capacity. Notably, under basal conditions there were no distinguishable changes in the CMH signals observed between WT and Nrf2−/− mice (19.81±1.82 vs 21.9±1.54 nmol/min/mg protein).
Fig. 2.
Reactive oxygen species (ROS) generation is enhanced in the heart of AES mice. (A) Electron paramagnetic resonance (EPR) spectroscopy signals for CMH in the WT and Nrf2−/−mouse hearts at 2 months. (A) Representative EPR signals for CMH indicate significant differences between WT and Nrf2−/− hearts with increased ROS generation after AES. (B) ROS levels determined by EPR in the heart of WT and Nrf2−/− mice with or without AES (n=5).
Source for ROS generation on AES
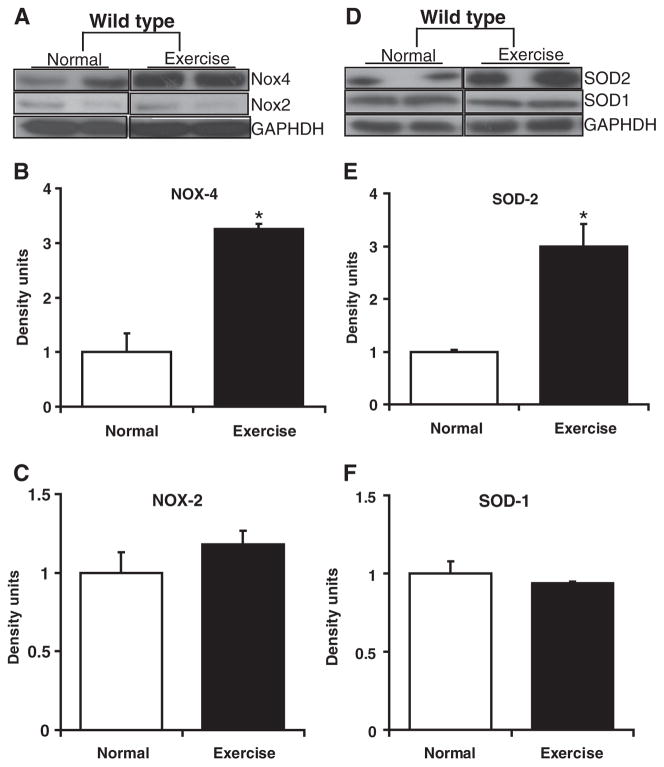
A variety of mechanisms have been reported to generate ROS in the cardiovascular system, which includes mitochondria (ETC), NADPH-oxidases (NOX), nitric oxide synthase (NOS), xanthine oxidase, etc. [24,36,37]. We have examined the levels of SOD1, SOD2, Nox2, and Nox4 in WT mouse myocardium under basal/AES conditions (Fig. 3). Interestingly, Nox4 and SOD2 were significantly (P<0.01) upregulated in AES when compared to sedentary WT myocardium (Figs. 3A–B and 3D–E) while the SOD1 and Nox2 levels were unaltered. AES-mediated activation of Nox4 is expected to generate ROS in mitochondria, which might have resulted in acute induction of SOD2. These results indicate that mitochondria could be one of the major sources for ROS generation on AES.
Fig. 3.
Source for reactive oxygen species (ROS) generation in the heart of AES mice. Western blots showing SOD and NOX isoforms in the sedentary and AES WT mice (n=4) myocardium. Cytosolic SOD1 (D, F) and Nox2 (A, C) levels were unaltered, while the mitochondrial Nox4 (A, B) and SOD2 (D, E) were significantly (*P<0.01) increased in AES when compared to sedentary WT myocardium.
Disruption of Nrf2/ARE signaling causes myocardial glutathione (GSH) depletion
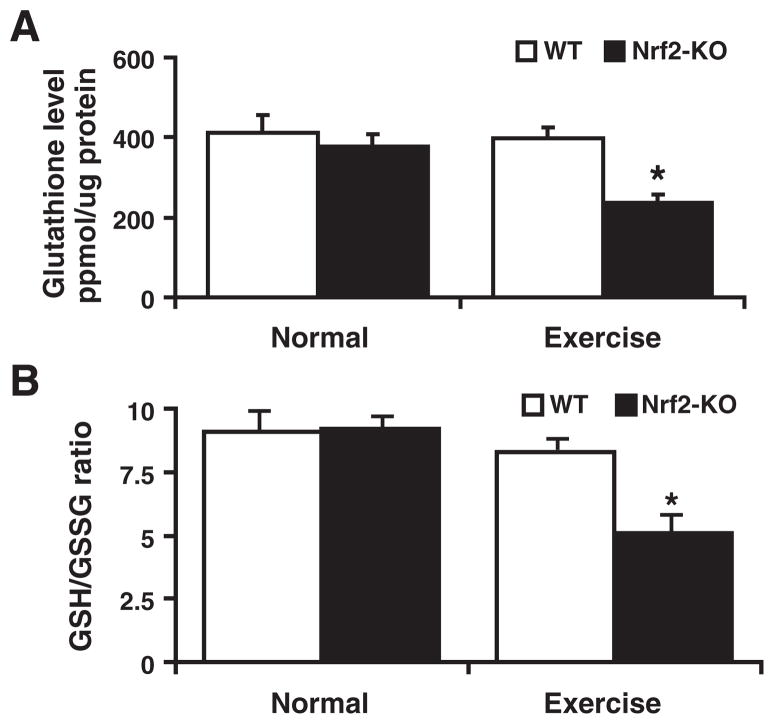
The heart constitutively generates ROS through mitochondria and NADPH oxidase(s) [38–41]. To limit accumulation of these reactive species, the myocardium has an inducible antioxidant system that includes glutathione and thioredoxins [42–44]. GSH is a tripeptide, nonprotein thiol antioxidant that is ubiquitously present in the majority of cells/tissues [45,46]. De novo biosynthesis pathways for GSH are largely conserved in various tissues, which facilitates local GSH production through GCL and GSH-synthase on stress and other demands that occur due to chronic hepatic dysfunction [47–50]. We assessed whether the Nrf2-deficiency state influenced GSH levels in the myocardium. Under basal conditions we observed a trend toward GSH depletion in Nrf2−/− when compared with WT mice, but these changes were not significant (Figs. 4A and B). However, on AES dramatic depletion of GSH (1.0±0.2 vs 0.57±0.04; WT vs Nrf2−/−) and decreased GSH/GSSG ratio (10:1 vs 5:1; WT vs Nrf2−/−) were observed in Nrf2−/− when compared to WT mice myocardium. Thus, in a stressed state the loss of Nrf2 impairs cellular antioxidant defenses.
Fig. 4.
Glutathione redox (GSH/GSSG) state of the myocardium in the WT and Nrf2−/− mice: Determined the redox state of myocardial (ventricular) glutathione in WT and Nrf2−/− mice at 2 months of age (n=6) under basal and after AES conditions. (A) Under basal conditions, no statistically significant change in GSH was measured in WT and Nrf2−/− groups. Significant decrease in GSH was evident in the Nrf2−/− mice after AES, indicating onset of oxidative stress (*P<0.01). (B) Glutathione redox ratio (GSH/GSSG) was comparable under the basal state, but it was tremendously decreased in Nrf2−/− when compared to WT on AES, suggesting oxidative stress in the Nrf2−/− mice. Values are mean±SD for 6 or more animals in each group.
AES-induced ROS generation results in enhanced nuclear translocation of Nrf2 and its binding to ARE in WT mice
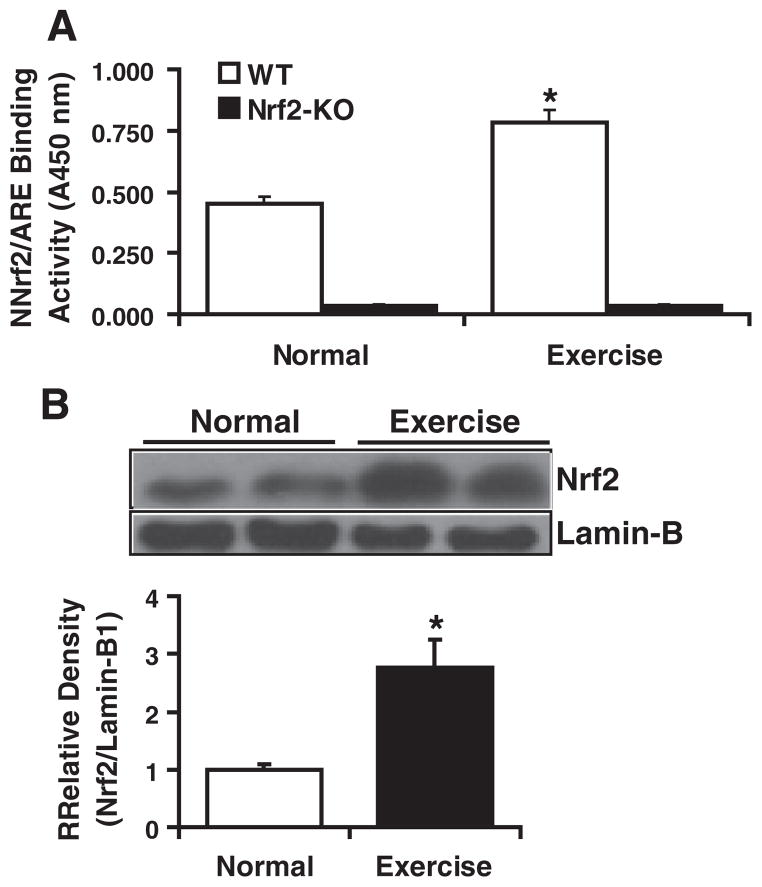
Next, to investigate the AES-induced activation of Nrf2 in the myocardium, we determined the Nrf2 activity using an ARE-oligonucleotide-based trans-activation assay in the nuclear extracts of WT mouse hearts at ~2 months of age. On AES, the myocardial Nrf2 activity was significantly higher (~2.0-fold) in exercised when compared with sedentary WT mice, suggesting its induction/stabilization and nuclear translocation due to AES (Fig. 5A). As predicted, basal (background) signals were detected for the nuclear extracts from Nrf2−/− mice (Fig. 5A). Further, immunoblot analysis showed that acute exercise stress (AES) mediated the increase of Nrf2 nuclear translocation (Fig. 5B).
Fig. 5.
Increased Nrf2/ARE binding activity in the WT mouse myocardium after AES. (A) TransAM-Nrf2 activity assay: Ten micrograms of nuclear proteins from WT and Nrf2−/− mice (n=6) was incubated with the oligonucleotides (precoated on 96-well plate/strips) for ARE (antioxidant response element). Using HRP-conjugated anti-Nrf2-ab binding activity for Nrf2 in the nuclear extracts was determined in a plate reader. Final values were expressed as mean A540 nm±standard deviation for four hearts three from each group. Significant increase of Nrf2 binding was noted in WT mice after AES when compared with basal conditions. Also detected were background values (ranging from 0.020 to 0.047 OD) in Nrf2−/− mice after blanking the wells without protein samples. (B) Increased nuclear translocation of Nrf2 is evident in the WT mice after AES. Representative immunoblot (IB) of nuclear proteins collected from WT mice under basal and AES conditions. Each lane represents an individual animal/heart. Lamin-B1 served as loading control and to normalize the density of the IB signals. Density analysis reveals >2.5-fold increase in the WT mice after AES compared to their respective basal nuclear levels for Nrf2. Diminished Nrf2-binding (Fig. 5A) is due to decreased nuclear Nrf2 in sedentary WT when compared to AES mice (Fig. 5B).
This increase in Nrf2 nuclear translocation is associated with elevated ROS and subsequent up-regulation of ARE-dependent trans-activation of antioxidants in WT mice. Though WT exhibited compensatory trans-activation of ARE-containing antioxidant genes, the Nrf2−/− mice were susceptible to AES-induced ROS generation. Absence of ARE binding in the nuclear extracts of Nrf2−/− myocardium might be associated with blunted transcription of antioxidants and accumulation of ROS, which result in chronic oxidative stress.
Abrogation of Nrf2 down-regulates transcription of ARE containing antioxidant genes on AES
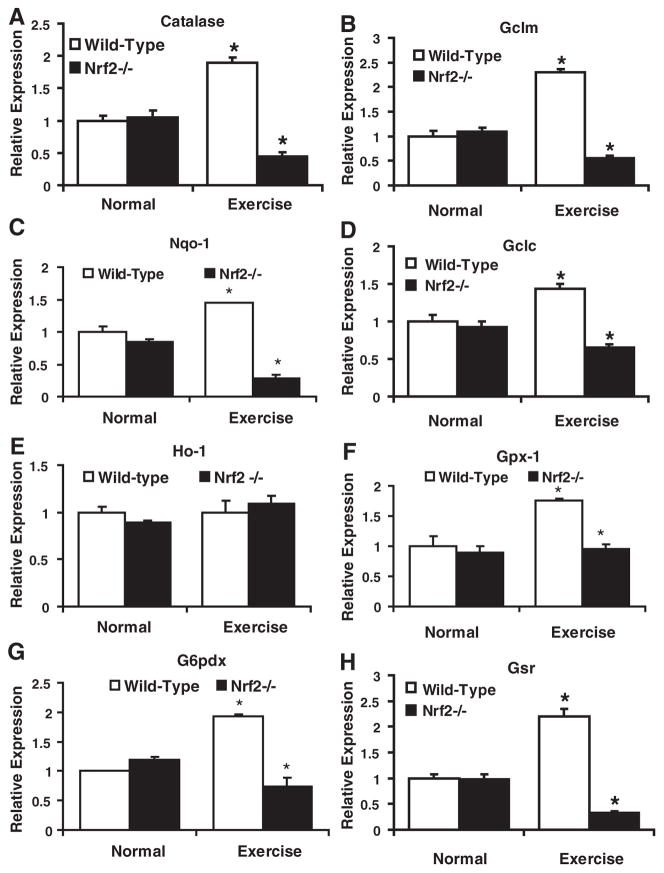
We hypothesized that abrogation of Nrf2 might inhibit transcriptional activation of downstream targets including critical antioxidant pathways associated with glutathione homeostasis in the myocardium. To test this hypothesis, we performed real-time RT-PCR using RNA isolated from sedentary/AES WT and Nrf2−/− mouse hearts. We selected major antioxidant genes that are regulated by NRF2/ ARE. Expression of catalase, G6pdx, Gclm, Gclc, Gsr, and Nqo1 (Figs. 6A–H) was significantly lower in the Nrf2−/− compared with WT mouse hearts, suggesting a lack of compensation against AES-induced ROS. However, under basal conditions, the WT and Nrf2−/− mice had similar transcript expression. Interestingly, transcript levels for most of the antioxidant genes (Cat, Gclm, Gclc, Nqo1, Gsr, G6pdx, and Gpx1) were significantly (P<0.05) increased in WT mice on exercise (Fig. 6) while the Nrf2−/− mice exhibited poor compensatory response to AES.
Fig. 6.
Disruption of NRF2 down regulates transcription of ARE-dependent antioxidants in the myocardium of AES mice (A–H). Real-time qPCR analyses of major antioxidants regulated by Nrf2/ARE signaling in WT and Nrf2−/− mice heart at 2 months (n=6) were performed using appropriate Qiagen primer sets. Data were primarily normalized to Arbp1 and then to the corresponding gene expression in the WT-normal group. Under basal conditions, gene expression for most of the antioxidants was comparable between WT and Nrf2−/− mice. But after AES, there was significant down regulation of most of the genes (catalase, Gclm, Nqo1, Gclc, Gpx1, G6pdx, and Gsr) in Nrf2−/− when compared to the corresponding WT mice. Interestingly, WT mice had significant upregulation of all these genes [Catalase (~2.0-fold), Gclm (>2.0-fold), Gsr (>2.0-fold), Gpx1 (>1.5-fold), Gsr (>2.0-fold), and G6pdx (~2-fold)] on AES, suggesting potential activation of Nrf2 and subsequent compensatory mechanisms at 4 transcriptional levels (*P<0.05; **P<0.01 vs WT-normal). No significant changes were observed in Ho1 mRNA expression (E).
Depletion of major antioxidant enzymes on AES, but not under basal conditions, in the myocardium of Nrf2-deficient mice
To characterize whether Nrf2 deficiency, in part, is responsible for the decreased GSH levels on AES in the mouse heart, we measured protein expression for major targets of Nrf2 and the enzymes that are involved in GSH metabolism/redox homeostasis using immuno-blotting. Under basal conditions, though there appeared to be decreases in some of the antioxidant enzymes in Nrf2−/− mice (Figs. 7A–E), most of these changes were not statistically significant when compared with levels in the WT myocardium. Surprisingly, while protein expression for GCLC was comparable, GCLM was higher in Nrf2−/− when compared to WT. However, on AES, many of the antioxidants including GCLC (1.0 vs 0.61), GCLM (1.0 vs 0.55), catalase (1.0 vs 0.48), NQO1 (1.0 vs 0.25), G6PD (1.0 vs 0.28), GPX1 (1.0 vs 0.48), and GSR (1.0 vs 0.45) were significantly (P<0.05 – 0.001 as indicated) decreased (Figs. 7A–E) while there was profound increase in HO-1 levels (Fig. 7A) in the Nrf2−/− when compared to WT mice. Independent of Nrf2, the HO1 is either tightly regulated by or responding to redox changes. These results indicate that Nrf2−/− mice were highly susceptible to AES-induced ROS accumulation. Overall, Western blot analysis revealed significant depletion of major antioxidants in Nrf2−/− mice, while WT mice exhibited compensatory antioxidant responses to the AES.
Analysis of cardiac hypertrophy in Nrf2−/− mice
Based on the evidence indicating an impaired antioxidant mechanism and increased ROS in the Nrf2−/− mouse, next we analyzed the cardiac function and molecular markers of cardiac hypertrophy. Increased ROS has been tightly correlated with cardiac hypertrophy in mouse models and human patients. M-mode echocardiograph analysis revealed no evidence for cardiac dysfunction in Nrf2−/− mice in either basal or AES states (data not shown). Further, gene expressions for the major hypertrophy markers (ANF, BNF, PLN, and SERCA2A) were not increased in either WT or Nrf2−/− on AES (Fig. 8). These results indicate that short-term acute exercise has very minimal physiological effects on myocardial function despite impaired antioxidant mechanisms in Nrf2−/− mice.
Fig. 8.
Disruption of Nrf2 and AES has no effect on cardiac hypertrophy. (A–D) Expression for cardiac hypertrophy markers; atrial natriuretic factor (ANF), brain natriuretic factor (BNF), phospholamban (PLN), and SERCA2A in WT and Nrf2−/− mice under basal and AES conditions were determined by qPCR analysis (n=3). Comparisons between WT and Nrf2−/− revealed no indication of cardiac hypertrophy under either basal or AES conditions.
Discussion
Exercise is the natural mode of improving physiological outcomes by reducing morbidity/mortality and preventing the progression of chronic pathological conditions including CVD [51–54]. Through augmenting the function of Nrf2, the master transcriptional regulator of antioxidant genes, it is possible to impact the antioxidant mechanisms and protect the myocardium from well-defined oxidative stress-related pathological processes. Recently, we have reported an Nrf2-dependent increase of major antioxidants in the mouse myocardium [33]. Experimental and clinical studies using antioxidant supplements against oxidative stress disorders report nonspecific and deleterious side effects. Our investigation has identified Nrf2 as a critical factor that determines the fate of myocardial cytoprotective mechanisms under basal and stressed states. The role of oxido-reductive stress and associated pathogenic processes in human cardiovascular diseases has received considerable attention from both basic and translational researchers. However, the role of Nrf2-Keap1 signaling in myocardium has not been previously investigated in the context of acute exercise stress. Our current findings reveal that (i) under unstressed conditions, Nrf2 has a minimal role on cardiac antioxidant defenses, (ii) AES promotes Nrf2 and up-regulates ARE-dependent antioxidant transcription and (iii) acute exercise represents a nonpharmacological inducer of Nrf2/ARE signaling. Precise mechanisms for Nrf2 signaling under various stress conditions are poorly elucidated. In this study, we used genetic depletion of Nrf2 to explicitly explore the role of antioxidant responses to AES. Our results demonstrate that Nrf2-dependent ARE signaling pathways are significantly activated in the AES mouse hearts.
Disruption of Nrf2 has a minimal role in myocardial antioxidant system under basal conditions
A recently elucidated leucine zipper Cap-‘n’-collar transcription factor, Nrf2, is believed to play a major role in protecting a range of tissues (multiorgan protector) from a variety of stressful and pathogenic insults [21,55,56]. Under unstressed physiological conditions, Nrf2 is attracted by its negative regulator, Keap1, in the cytoplasm and ubiquitinated [57–59]. In response to chemical, electrophilic, physical (UV-radiation), or xenobiotic stress, Nrf2 is released from Keap1 and translocates to the nucleus to bind with ARE of target genes [60–62]. As briefly reported previously, loss of Nrf2 has minimal or no effect on its target genes basal expression, suggesting the existence of mechanisms controlling expression apart from Nrf2. Nevertheless, transcriptional down-regulation of some Nrf2/ARE-dependent genes along with an insignificant decrease of their protein expression in the Nrf2−/− mice indicates that the magnitude of regulation is based on the number and proximity of ARE sites that interact with Nrf2. Thus Nrf2 is not required until a stress state exists. Investigating mechanisms for activation/stabilization of Nrf2 is crucial for preventing, as well as treating, various oxidative stress diseases.
Abrogation of Nrf2 leads to deregulation of cardiac antioxidant system under stress conditions
To our knowledge, Nrf2-ARE redox signaling has not been characterized in heart tissue in the context of exercise-mediated redox stress. Recent studies document that Nrf2 enhances oxidative stress tolerance/life span of Drosophila and intestinal stem cell proliferation by promoting redox homeostasis [63–65]. Previous reports have documented that the loss of Nrf2 is strongly coupled with dysregulation of antioxidant pathways and progression of disease [16–18]. A question addressed in this study is whether AES could trigger ROS generation in the myocardium. Earlier investigations reported evidence that acute exercise can induce ROS generation through a variety of mechanisms [66]. Here we demonstrate that ROS were generated in WT and Nrf2−/− mouse hearts on AES. The magnitude of ROS accumulation was significantly higher in Nrf2−/− mice, suggesting insufficient activation of compensatory cytoprotective measures at transcriptional and translational levels. The striking decline in several antioxidant proteins (catalase, NQO1, -GCS, GSR, GPX-1, G6PD, etc.) on AES in Nrf2−/− mice might be attributed to an increased utilization of these enzymes and restricted protein synthesis due to lack of Nrf2/ARE-dependent transcription. Further, gene expression (real-time qPCR) analysis revealed subsequent down-regulation of major antioxidant genes in Nrf2−/− mice after AES, indicating a principal role for Nrf2 in myocardial antioxidant defenses. Thus, our findings support the notion that Nrf2 is critical for protecting the heart from such acute stress conditions as previously demonstrated in exercised rat kidney [25,27] and human skeletal muscle [26].
AES promotes Nrf2 signaling through ROS generation in WT, but induces oxidative stress in Nrf2−/− mice
To determine the mechanisms for AES-induced Nrf2 signaling, we examined the levels of ROS in WT and Nrf2−/− mice myocardium. We found that AES induces ROS generation along with increased nuclear translocation of Nrf2 compared to unstressed controls. Our findings also indicate that mitochondria might be one of the major sources for ROS generation during AES. Recent findings revealed localization of Nox4 in the mitochondria of myocardium [67,68], which contributes to ROS production despite the mitochondrial electron transport chain (ETC) [24,69,70]. Increased Nox4, but not Nox2, suggests potential activation of NADPH-oxidase system in the mitochondria on AES. Accordingly, increased SOD2 is attributed to stress-induced upregulation (feedback induction) of this enzyme to combat excessively produced ROS on AES. Consistent with previous reports that ROS and electrophilic stress can be the primary trigger for the dissociation of the Nrf2-Keap1 complex [60,61], AES-mediated ROS resulted in Nrf2 activation and subsequent transcription of target antioxidant genes. However, Nrf2 deficiency resulted in poor compensatory response to AES and exhibited oxidative stress as indicated by decreased GSH and GSH/GSSG ratio in Nrf2−/− mice. A recent investigation documented that disruption of Nrf2 is associated with enhanced susceptibility to pressure overload hypertrophy, indicating a critical pathophysiological role for Nrf2 in the heart [71]. Hence, it will be important in future studies to determine whether or not stress conditioning of aged myocardium leads to protection against myocardial infarction, ischemia/reperfusion, or drug-induced cardiac remodeling.
Physical activity and adaptive exercise: A drug-free approach to potentiate defense mechanisms
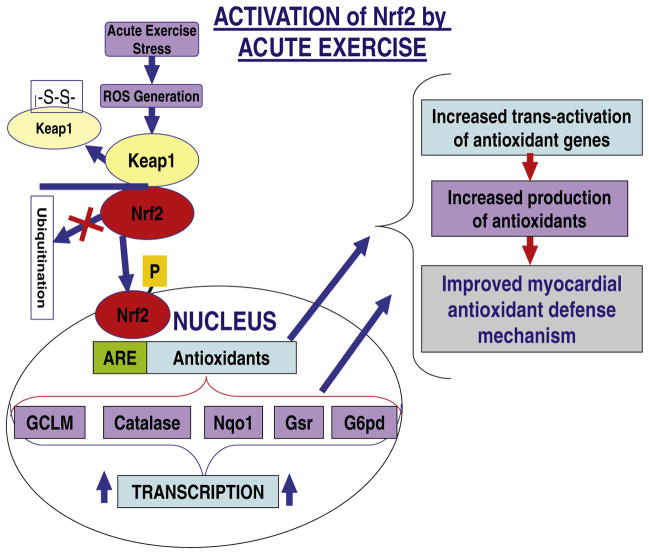
To date, the Nrf2-Keap1 pathway has been shown to be activated as an adaptive response to various stresses including oxidative stress caused by toxic chemicals, electrophilic stress, and UV radiation [60,61]. Recently, several other small molecules, anticancer agents, and plant-derived chemical species have been reported to regulate Nrf2/Keap1 signaling [26]. The molecular mechanisms to explain how the Nrf2-Keap1 pathway is modulated by any of these factors in a dose/time-dependent manner remain largely unknown. Our findings provide evidence, for the first time, that exercise stress activates this important pathway that regulates transcription of an array of cytoprotective/antioxidant genes (Fig. 9). A continuous nonpharmacological mode of Nrf2 activation might be efficacious in maintaining the steady-state function of Nrf2/ARE signaling and to prevent multiple cardiovascular complications (Fig. 9).
Fig. 9.
Proposed model for exercise-induced Nrf2 activation (a drug-free approach) to maintain redox homeostasis and protection against oxidative stress-mediated cardiovascular diseases.
Conclusions
Acute exercise induces ROS and activates Nrf2 in the myocardial tissue. However, disruption of Nrf2 increases the susceptibility of myocardial tissue to oxidative stress-induced potential damage, and Nrf2 signaling might be a potential therapeutic target to protect the heart tissue from ROS-dependent ischemia/reperfusion (I/R) injury and myocardial infarction (MI). Our findings show that induction of a battery of major antioxidant genes at transcriptional and subsequent translational capacity is feasible through acute exercise (Fig. 9). Future investigations addressing the effect of chronic regimented and spontaneous exercise (to increase adaptive response) on maintaining a steady-state function of Nrf2 and thereby achieving potential intracellular cytoprotective defense mechanisms and redox homeostasis are warranted.
Acknowledgments
This work is supported by the Center on Aging (CoA), Utah-Pilot Grant Program Award, Beginning Grant-In Aid from the AHA (086501F), and the University of Utah Interdisciplinary Seed Grant to Namakkal Soorappan Rajasekaran. Nrf2−/− mice, protocol, and primers for genotyping were kindly provided by Dr. Li Wang. We are grateful to Dr. Mark A. Supiano (Director of Center on Aging) for his support and valuable comments on this project. Thanks to Dr. John Michael, Dr. Robert Paine III and the Division of Cardiology for seed funding. We appreciate and thank Dr. Goutam Karan for his expert help in performing whole-heart images/microscopy. The authors thank Corey J. Miller for editorial assistance.
References
- 1.Kulminski AM, Ukraintseva SV, Arbeev KG, Manton KG, Oshima J, Martin GM, Il’yasova D, Yashin AI. Health-protective and adverse effects of the apolipo-protein E epsilon2 allele in older men. J Am Geriatr Soc. 2008;56:478–483. doi: 10.1111/j.1532-5415.2007.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manton KG. Recent declines in chronic disability in the elderly U.S. population: risk factors and future dynamics. Annu Rev Public Health. 2008;29:91–113. doi: 10.1146/annurev.publhealth.29.020907.090812. [DOI] [PubMed] [Google Scholar]
- 3.Manton KG, Gu X, Lowrimore GR. Cohort changes in active life expectancy in the U.S. elderly population: experience from the 1982–2004 National Long-Term Care Survey. J Gerontol B Psychol Sci Soc Sci. 2008;63:S269–S281. doi: 10.1093/geronb/63.5.s269. [DOI] [PubMed] [Google Scholar]
- 4.Jacob MH, da Janner DR, Araujo AS, Jahn MP, Kucharski LC, Moraes TB, Dutra Filho CS, Ribeiro MF, Bello-Klein A. Redox imbalance influence in the myocardial Akt activation in aged rats treated with DHEA. Exp Gerontol. 2010;45:957–963. doi: 10.1016/j.exger.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Fukagawa NK. Age-related changes in redox signaling and VSMC function. Antioxid Redox Signal. 2010;12:641–655. doi: 10.1089/ars.2009.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104: e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 8.Du Y, Wooten MC, Gearing M, Wooten MW. Age-associated oxidative damage to the p62 promoter: implications for Alzheimer disease. Free Radic Biol Med. 2009;46:492–501. doi: 10.1016/j.freeradbiomed.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capell BC, Collins FS, Nabel EG. Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ Res. 2007;101:13–26. doi: 10.1161/CIRCRESAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- 10.Hazzard WR, Ettinger WH., Jr Aging and atherosclerosis: changing considerations in cardiovascular disease prevention as the barrier to immortality is approached in old age. Am J Geriatr Cardiol. 1995;4:16–36. [PubMed] [Google Scholar]
- 11.Jennings JR, Kamarck T, Manuck S, Everson SA, Kaplan G, Salonen JT. Aging or disease? Cardiovascular reactivity in Finnish men over the middle years. Psychol Aging. 1997;12:225–238. doi: 10.1037//0882-7974.12.2.225. [DOI] [PubMed] [Google Scholar]
- 12.Rhoades DA, Welty TK, Wang W, Yeh F, Devereux RB, Fabsitz RR, Lee ET, Howard BV. Aging and the prevalence of cardiovascular disease risk factors in older American Indians: the Strong Heart Study. J Am Geriatr Soc. 2007;55:87–94. doi: 10.1111/j.1532-5415.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 13.Segal BL, Tecce MA, Sherman FT. Cardiovascular disease and the aging U.S. population. Geriatrics. 2003;58:43. [PubMed] [Google Scholar]
- 14.Waller BF, Bloch T, Barker BG, Roe SJ, Hawley DA, Pless JC, Eble JN. The old-age heart: aging changes of the normal elderly heart and cardiovascular disease in 12 necropsy patients aged 90 to 101 years. Cardiol Clin. 1984;2:753–779. [PubMed] [Google Scholar]
- 15.Wissler RW, Robert L. Aging and cardiovascular disease: a summary of the Eighth Munster International Arteriosclerosis Symposium. Circulation. 1996;93:1608–1612. doi: 10.1161/01.cir.93.9.1608. [DOI] [PubMed] [Google Scholar]
- 16.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202: 47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, Reddy SP. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol. 2009;182:7264–7271. doi: 10.4049/jimmunol.0804248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osburn WO, Kensler TW. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat Res. 2008;659:31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101: 3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47: 89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 22.Packer L, Cadenas E, Davies KJ. Free radicals and exercise: an introduction. Free Radic Biol Med. 2008;44:123–125. doi: 10.1016/j.freeradbiomed.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 23.Radak Z, Atalay M, Jakus J, Boldogh I, Davies K, Goto S. Exercise improves import of 8-oxoguanine DNA glycosylase into the mitochondrial matrix of skeletal muscle and enhances the relative activity. Free Radic Biol Med. 2009;46:238–243. doi: 10.1016/j.freeradbiomed.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachdev S, Davies KJ. Production, detection, and adaptive responses to free radicals in exercise. Free Radic Biol Med. 2008;44:215–223. doi: 10.1016/j.freeradbiomed.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Asghar M, George L, Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am J Physiol Renal Physiol. 2007;293:F914–F919. doi: 10.1152/ajprenal.00272.2007. [DOI] [PubMed] [Google Scholar]
- 26.Safdar A, deBeer J, Tarnopolsky MA. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic Biol Med. 2010;49:1487–1493. doi: 10.1016/j.freeradbiomed.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 27.George L, Lokhandwala MF, Asghar M. Exercise activates redox-sensitive transcription factors and restores renal D1 receptor function in old rats. Am J Physiol Renal Physiol. 2009;297:F1174–F1180. doi: 10.1152/ajprenal.00397.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 29.Hoene M, Lehmann R, Hennige AM, Pohl AK, Haring HU, Schleicher ED, Weigert C. Acute regulation of metabolic genes and insulin receptor substrates in the liver of mice by one single bout of treadmill exercise. J Physiol. 2009;587:241–252. doi: 10.1113/jphysiol.2008.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safdar A, Abadi A, Akhtar M, Hettinga BP, Tarnopolsky MA. miRNA in the regulation of skeletal muscle adaptation to acute endurance exercise in C57Bl/6J male mice. PLoS One. 2009;4:e5610. doi: 10.1371/journal.pone.0005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130: 427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajasekaran NS, Sathyanarayanan S, Devaraj NS, Devaraj H. Chronic depletion of glutathione (GSH) and minimal modification of LDL in vivo: its prevention by glutathione mono ester (GME) therapy. Biochim Biophys Acta. 2005;1741:103–112. doi: 10.1016/j.bbadis.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Rajasekaran NS, Varadharaj S, Khanderao GD, Davidson CJ, Kannan S, Firpo MA, Zweier JL, Benjamin IJ. Sustained activation of nuclear erythroid 2-related factor 2/ antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxid Redox Signal. 2011;14: 957–971. doi: 10.1089/ars.2010.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirayama A, Yoh K, Nagase S, Ueda A, Itoh K, Morito N, Hirayama K, Takahashi S, Yamamoto M, Koyama A. EPR imaging of reducing activity in Nrf2 transcriptional factor-deficient mice. Free Radic Biol Med. 2003;34:1236–1242. doi: 10.1016/s0891-5849(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 35.Lee SB, Kim CY, Lee HJ, Yun JH, Nho CW. Induction of the phase II detoxification enzyme NQO1 in hepatocarcinoma cells by lignans from the fruit of Schisandra chinensis through nuclear accumulation of Nrf2. Planta Med. 2009;75: 1314–1318. doi: 10.1055/s-0029-1185685. [DOI] [PubMed] [Google Scholar]
- 36.Powers SK, Ji LL, Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc. 1999;31: 987–997. doi: 10.1097/00005768-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Sugamura K, Keaney JF., Jr Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuroda J, Sadoshima J. NADPH oxidase and cardiac failure. J Cardiovasc Transl Res. 2010;3:314–320. doi: 10.1007/s12265-010-9184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rueckschloss U, Villmow M, Klockner U. NADPH oxidase-derived superoxide impairs calcium transients and contraction in aged murine ventricular myocytes. Exp Gerontol. 2010;45:788–796. doi: 10.1016/j.exger.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Sanz A, Caro P, Gomez J, Barja G. Testing the vicious cycle theory of mitochondrial ROS production: effects of H2O2 and cumene hydroperoxide treatment on heart mitochondria. J Bioenerg Biomembr. 2006;38:121–127. doi: 10.1007/s10863-006-9011-8. [DOI] [PubMed] [Google Scholar]
- 41.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart (Br Cardiac Soc) 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 43.Ago T, Sadoshima J. Thioredoxin1 as a negative regulator of cardiac hypertrophy. Antioxid Redox Signal. 2007;9:679–687. doi: 10.1089/ars.2007.1529. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meister A. Selective modification of glutathione metabolism. Science. 1983;220: 472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- 46.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 47.Griffith OW, Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979;76:5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meister A. Glutathione, metabolism and function via the gamma-glutamyl cycle. Life Sci. 1974;15:177–190. doi: 10.1016/0024-3205(74)90206-9. [DOI] [PubMed] [Google Scholar]
- 49.Meister A, Griffith OW, Novogrodsky A, Tate SS. New aspects of glutathione metabolism and translocation in mammals. Ciba Found Symp. 1979:135–161. doi: 10.1002/9780470720554.ch9. [DOI] [PubMed] [Google Scholar]
- 50.Meister A, Tate SS. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- 51.Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch PhysMed Rehabil. 2004;85:S31–S42. doi: 10.1016/j.apmr.2004.03.010. quiz S43–S34. [DOI] [PubMed] [Google Scholar]
- 52.Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- 53.Navarro-Arevalo A, Canavate C, Sanchez-del-Pino MJ. Myocardial and skeletal muscle aging and changes in oxidative stress in relationship to rigorous exercise training. Mech Ageing Dev. 1999;108:207–217. doi: 10.1016/s0047-6374(99)00013-5. [DOI] [PubMed] [Google Scholar]
- 54.Navarro-Arevalo A, Sanchez-del-Pino MJ. Age and exercise-related changes in lipid peroxidation and superoxide dismutase activity in liver and soleus muscle tissues of rats. Mech Ageing Dev. 1998;104:91–102. doi: 10.1016/s0047-6374(98)00061-x. [DOI] [PubMed] [Google Scholar]
- 55.Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Zhang C, Xing Y, Janicki JS, Yamamoto M, Wang XL, Tang DQ, Cui T. Up-regulation of p27kip1 contributes to Nrf2-mediated protection against angiotensin II-induced cardiac hypertrophy. Cardiovasc Res. 2011;90:315–324. doi: 10.1093/cvr/cvr010. [DOI] [PubMed] [Google Scholar]
- 57.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O’Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8:379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 58.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278: 21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 59.Zhang DD. The Nrf2-Keap1-ARE signaling pathway: the regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13:1623–1626. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]
- 60.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcino-genesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who’s listening? Antioxid Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2010;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sykiotis GP, Bohmann D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saborido A, Naudi A, Portero-Otin M, Pamplona R, Megias A. Stanozolol treatment decreases the mitochondrial ROS generation and oxidative stress induced by acute exercise in rat skeletal muscle. J Appl Physiol. 2010;110:661–669. doi: 10.1152/japplphysiol.00790.2010. [DOI] [PubMed] [Google Scholar]
- 67.Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging. 2010;2:1012–1016. doi: 10.18632/aging.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 70.Davies KJ, Hochstein P. Ubisemiquinone radicals in liver: implications for a mitochondrial Q cycle in vivo. Biochem Biophys Res Commun. 1982;107:1292–1299. doi: 10.1016/s0006-291x(82)80138-1. [DOI] [PubMed] [Google Scholar]
- 71.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, Cui T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]