Preface
The availability of neuroimaging technology has spurred a marked increase in the human cognitive neuroscience literature, including the study of cognitive aging. Although there is a growing consensus that the aging brain retains considerable plasticity of function, currently measured primarily by means of functional magnetic resonance imaging, it is less clear how age differences in brain activity relate to cognitive performance. The field also is hampered by the complexity of the aging process itself and the large number of factors that are influenced by age. In this review, current trends and unresolved issues in the cognitive neuroscience of aging are discussed.
Introduction
Age differences in cognitive function have been studied for many years, and it is well-established that older adults have particular difficulty with episodic memory, defined as the conscious recollection of events1. In the laboratory, these age differences in episodic memory are manifested by a reduced ability to learn and retrieve both non-verbal and verbal material, such as a list of words2. Substantial age-related differences also are seen in tasks involving working memory3,4, attention5–7, and task switching8–10, all of which can be considered as types of high level “executive” functions. Older adults also are more susceptible to the effects of distracting interference during cognitive tasks11,12 and have generally slower processing speed13. Nevertheless, some aspects of cognition are maintained with age, such as semantic memory, or the accumulation of knowledge about the world14,15, and emotional regulation16,17. In addition, age differences in cognition are not immutable; for example, the experimental conditions under which memory is studied in older adults can be modified so that age differences are reduced or eliminated18. A challenge in this field has been to understand the brain mechanisms that might underlie better or worse performance in old adults.
It is on this challenge that functional and structural neuroimaging studies of aging have focused, and in the past decade functional magnetic resonance imaging (fMRI) studies have provided ample evidence of age differences in task-related brain activity19,20. However, the interpretation of these differences is difficult, as sometimes brain activity is reduced in older relative to younger adults, and sometimes it is increased. Decreased brain activity has typically been interpreted as a reflection of cognitive deficits in older adults21, and increased activity has often been interpreted as compensatory22. However, other mechanisms may also explain age-related increases of brain activity, including a lack of efficiency in the utilization of neural resources, or a reduction in the selectivity of responses, known as dedifferentiation23.
Another issue is how brain activity is related to other aspects of brain aging, such as changes in structure (volumes or white matter myelination, for example), or neurotransmitters. There also is the question of how age differences in brain function might be affected by undetected neuropathological changes due to dementing illnesses. That is, some otherwise healthy older adults might eventually be diagnosed with Alzheimer’s disease (AD), and the “silent” pathological processes in their brains might account for some of the age differences reported in the literature. The purpose of this review is to cover some recent developments in the field that address these longstanding issues and to discuss some interesting new trends in this area of research.
Before reviewing this work on cognitive aging and the brain, it is important to note that there is general consensus that the blood oxygen level dependent (BOLD) signal obtained from fMRI is a reasonable, although indirect, index of neural activity, especially the synaptic activity reflected in local field potentials24,25. In regard to the use of fMRI to study aging, peak stimulus-related BOLD responses are similar in young and older adults,26–28 although some work has shown that the magnitude of the BOLD response can be reduced in older adults, at least in some brain regions29. In addition, it is important to keep in mind that there are alterations in the cerebral vasculature with age, and these have the potential to influence the BOLD signal in as yet unknown ways30,31. Alhough much remains to be done to understand the impact of aging on the physiology underlying the BOLD signal, the relatively small age differences noted in the properties of these signals and recent work suggesting a small vascular contribution to BOLD signals in older adults during cognitive tasks32 encourage the continued use of this technique to study cognitive aging.
Compensation in the Older Brain
An early idea in the literature was that older adults (i.e., those above the age of 65) might be able to engage some brain areas above the level seen in younger adults (in their 20’s), particularly the frontal lobes, to compensate for impaired function elsewhere in the brain 22. In these early studies, older adults were noted to have more activity in prefrontal cortex (PFC) during memory tasks relative to younger adults33–35, which was thought to compensate for reduced activity in visual processing regions22,36 (a phenomenon recently termed the posterior-anterior shift with aging, or PASA36). This PFC activity was often bilateral in the older adults on tasks for which younger adults typically showed unilateral PFC activity, leading to the idea that the increased bilaterality of PFC activation in older adults reflects a compensatory mechanism that can aid cognitive performance37.
A compensatory interpretation is often invoked when older adults show more activity in a brain region than younger adults whilst they perform a task at the same level as younger adults38, or when increased activity is positively correlated with performance in older adults, but not younger adults35,36,39–41. Several researchers have suggested that compensatory mechanisms might still play a role even if performance in older adults is impaired42. For example, increased activity in an older adult might not be associated with preserved performance on a given task to the level seen in a young adult, but this performance might be even worse without the over-recruitment. Thus, despite the continued attention that this idea has received, it is still not clear exactly which regions might act in a compensatory manner or under which conditions this might occur43,44.
Several recent papers have provided further evidence in favor of the compensatory hypothesis. One examined age differences in inhibition using a series of tasks that assessed the ability to inhibit prepotent responses45. Older adults displayed more activity in a set of dorsal PFC and parietal regions, sometimes called the dorsal attention network46,47, compared with younger adults. Importantly, activity in these attention-related regions correlated with better inhibition only in older adults. This result is consistent with the idea of a compensatory mechanism whereby additional activity in task-relevant regions increases the ability of older adults to carry out the task. Similarly, another experiment examined face perception48 and found a set of regions in right PFC and occipital cortex where increased activity was associated with better face recognition in older adults, but not a younger group. Furthermore, in a task requiring attention to right and left visual fields49, only old adults showed increased activity in bilateral PFC that was positively correlated with better performance. Interestingly, studies using transcranial magnetic stimulation (TMS) also have provided some support for the idea that increased bilateral PFC activation is beneficial for performance in older adults. Two studies have shown that using TMS to reduce activity in either the left PFC during encoding or the right PFC during retrieval reduces memory performance in younger adults, but has less effect in older adults, presumably because the unstimulated hemisphere can support the function when the other is inactivated 50,51. Therefore, these studies all suggest that older adults can recruit higher levels of brain activity than young adults, often in the PFC, and that this additional activity can aid performance of the old adults who are best able to engage it.
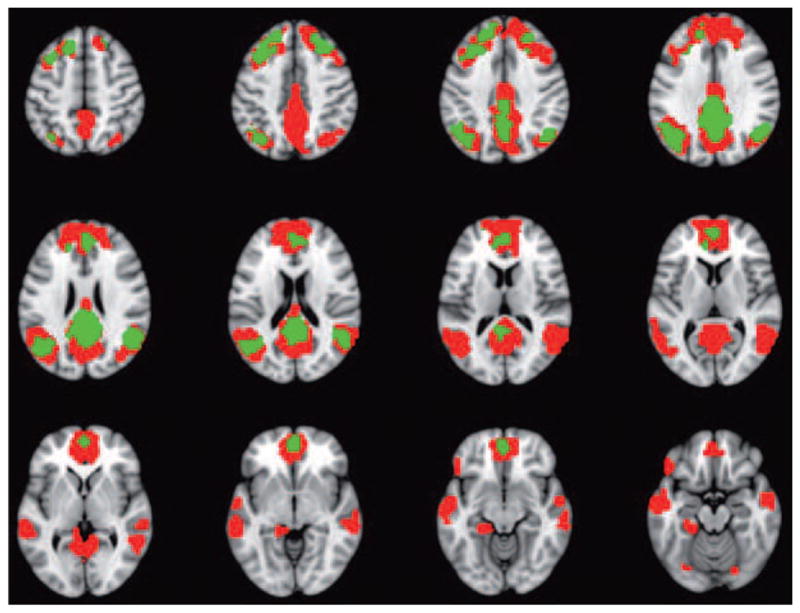
By contrast, other work has provided evidence that over-recruitment of brain activity does not necessarily lead to better task performance. For example, some researchers42,52,53 have suggested that when performance is matched between age groups, over-recruitment reflects less efficient use of neural resources in the older group, not compensation. In addition, more activation in old adults can sometimes be associated with poorer, not better, performance54. Recent studies have reported greater activity in the PFC during memory encoding55 or retrieval56 in older adults, both of which were correlated with poorer memory. Similarly, higher activity in a distributed set of regions, including PFC and parietal cortex, in old adults compared to young adults57,58 was found to be correlated with slower and more variable reaction times on a set of visual tasks. To complicate matters further, some of these regions associated with slower responses in older adults are very similar to the fronto-parietal regions reported to support better inhibitory function in older adults45 (see above), suggesting that the association between activity in a given brain region and performance in older adults is task specific, response specific (e.g., accuracy vs. response time), or both (Figure 1).
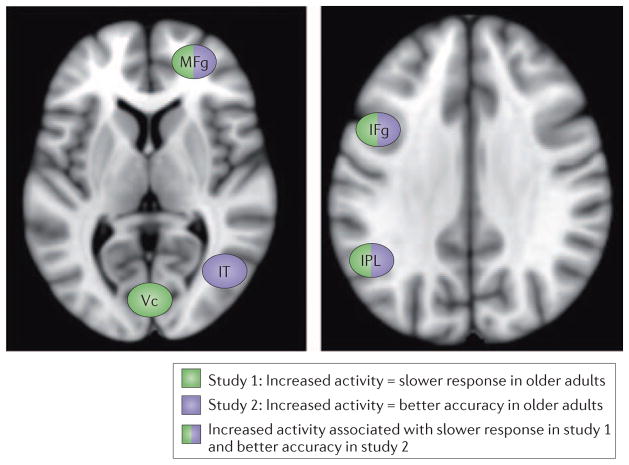
Figure 1. Increased brain activity in older adults may be associated with better or worse task performance.
This figure summarizes the results of two studies that differ in how increased brain activity in older adults was associated with task performance. In one of these studies, several brain regions (indicated in blue) showed a correlation between more activity and more accurate performance on an task requiring inhibition of responses in a go/no-go task45. In the other study the regions shown in green showed a correlation between more activity and slower reaction times on perceptual and working memory tasks57. Note that some regions (colored blue and green) showed an association with better performance in Study 1 and the opposite effect in Study 2. This discrepancy highlights the complexity of trying to relate brain activity in older adults to their behaviour, and indicates that the specific relationships between regional brain activity and task performance in older adults depend on the task demands or on the behavioral measure that is assessed (or both). Abbreviations: MFg, middle frontal gyrus; IFg, inferior frontal gyrus; IPL, inferior parietal lobe; IT, inferior temporal cortex; Vc, visual cortex.
Together, these results suggest that increased activity in older relative to younger adults can be associated with better performance on some tasks, but that this additional activity is not always compensatory (in the sense that it is directly related to better task performance). In some cases, over-recruitment of brain areas may reflect a greater demand on neural resources or less efficient use of them, and may or may not be related specifically to individual differences in behavior. One explanation for this is the ‘partial compensation hypothesis’55, whereby over-recruitment of the right PFC during memory encoding may aid old adults in carrying out the encoding task because of less effective use of the left PFC, which would normally carry out this task 21,59,60. However, this additional right PFC activity during encoding cannot compensate for a reduction in encoding effectiveness of the left PFC, and so does not provide a benefit for subsequent memory of the encoded items. This is similar to the idea that over-recruitment might help cognition in a general way, but may not be related to performance on a specific task42. Regardless, the papers cited in this section indicate that one should be careful about interpreting age increases in brain activity as compensatory without sufficient evidence from behavior to support such an interpretation.
Potential explanations for compensatory activity
One idea is that older adults shift from proactive strategies early in a decision process to reactive strategies that occur later. Support for this idea was reported in an experiment61 that found PFC activity in young adults during the early phase of memory retrieval trials, and PFC activity in older adults that occurred later in time. Another experiment examining task switching found that younger adults showed sustained PFC activity throughout the period in which they had to switch between tasks, whereas older adults showed transient increases to cues indicating that a switch was required62. This pattern suggests that cognitive control is engaged differently with aging, and also supports the notion of a shift from proactive control to a more reactive strategy that occurs in response to task demands. A similar proactive/reactive age difference was reported for the medial temporal lobes (MTL) in older adults during a memory task63. Younger adults had more activity in these regions during preparation for memory retrieval, whereas older adults showed more activity during retrieval. These studies suggest that a shift in the timing of resource engagement is required to deal with the influence of age on proactive strategies that make these less effective or accessible, and that compensation in aging may have a temporal component to it.
Another idea to explain compensatory activity is the ‘Compensation-Related Utilization of Neural Circuits hypothesis’, or CRUNCH64. The idea of CRUNCH is that more neural resources are recruited by older adults at low levels of cognitive load, i.e., when tasks are easier, than in younger adults, who don’t need them53. At higher levels of load, this compensatory mechanism is no longer effective, leading to equivalent or less activation in older adults relative to young. Data consistent with this idea have been reported in PFC65, and in both PFC and parietal cortex66,67 during working memory tasks that varied in the number of items that had to be kept in mind. In these studies, older adults had more activation at low levels of working memory load, where performance was equivalent to that of younger adults, but less activity and lower accuracy at higher loads. This kind of result also has been found during episodic memory tasks68; younger adults showed recruitment of bilateral PFC during a difficult version of the memory task, whereas older adults showed activation of these areas for both easy and difficult versions of the task. All of these studies are consistent with CRUNCH (Figure 2), which suggests that the relationship between brain activity and cognitive load is “S” shaped and plateaus at higher levels of load regardless of age. The older adult curve would be shifted to the left relative to younger adults, such that older adults would have greater brain activity at lower levels of load, and reach their plateau at levels where younger adults are still able to increase their brain activity. According to this hypothesis, old adults engage neural resources, such as the PFC, at lower task loads to compensate for less effective use of these resources, or perhaps because of degraded input to the PFC64, thus shifting the curve leftward. Although this idea has considerable appeal, and may be able to account for both the age-related increases and decreases of brain activity described in the literature, it is not clear if one would need to see recruitment of a unique region in older adults in order to interpret this activity as compensatory. That is, the engagement at lower load of the same region active in younger adults at higher loads might reflect an increase in the “normal” inter-individual variability in the brain/load function that must exist even in young adults, rather than compensation per se.
Figure 2. ‘Compensation-Related Utilization of Neural Circuits hypothesis’.
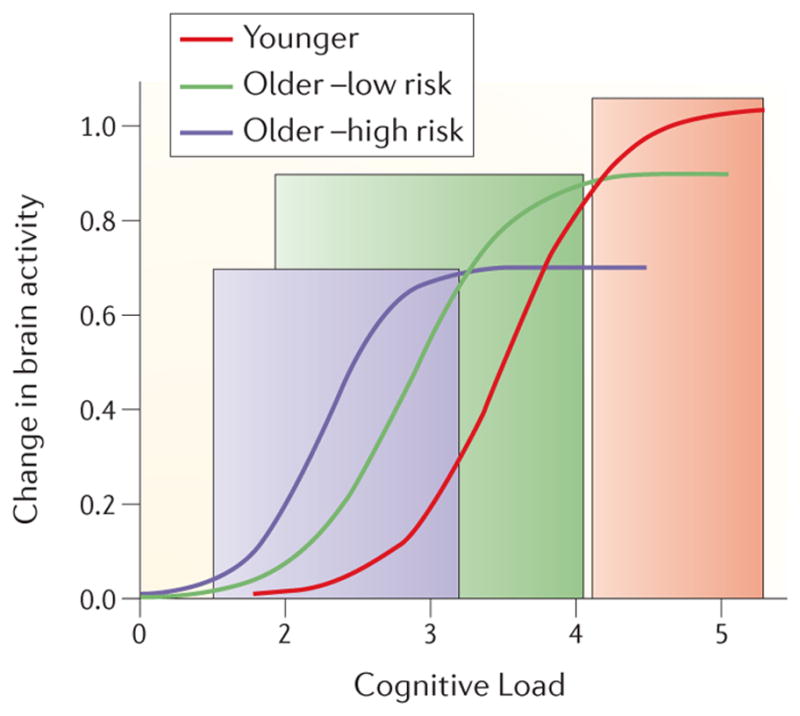
The function relating the change in brain activity (measured by fMRI during a task of interest) to levels of cognitive load is shown for young adults, old adults with a low risk of developing AD and old adults with high risk of developing dementia. The function in low risk older adults would be shifted to the left relative to that seen in younger adults. At relatively low levels of cognitive load this shift would result in higher activity in older relative to younger adults (green shaded area). However, activity in older adults would reach its peak and level off while younger adults’ activity is still increasing, so that at higher load levels there would be no age difference in activity or younger adults would have higher activity (gray shaded area). A similar effect would be seen when high-risk older adults are compared to low-risk older adults – higher activity in high risk groups relative to low risk at low levels of cognitive load (blue shaded area), with the reverse seen at higher levels of load. This hypothesized set of load-dependent functions could explain why studies have reported both under- and over-recruitment in older adults compared to young adults, and in high-risk older adults compared to low- risk older adults.
Dedifferentiation
The concept of dedifferentiation was originally proposed to explain the increased correlations among behavioral measures in older adults69, but was adopted by neuroimagers because it also seemed to characterize brain activity in older adults. Early examples included bilateral prefrontal activity associated with abilities that typically yield lateralized activity in younger adults37,70, more diffuse activation patterns71, and less selective activity in task-relevant regions across a variety of tasks22,72,73. Like the idea of compensation, dedifferentiation continues to be a viable explanation for some age differences in brain activity.
One way to investigate dedifferentiation is to compare the patterns of activity across tasks to see if they are more similar, i.e., less selective, in older adults. This kind of result was found in an experiment contrasting implicit memory for a sequence of repeated visual stimuli to explicit memory for a list of words74. Young adults showed more activity in the hippocampus for explicit learning, and more activity in the striatum for implicit learning. Older adults showed equivalent activation in these regions during the two tasks. Another experiment75 also found that implicit memory in younger adults was accompanied by increased activity in striatum and decreased activity in the hippocampus, whereas older adults showed increases in both. Simlarly, older adults are reported to have less distinctive activity in the visual cortex during perception and working memory tasks76,77. In both kinds of task, old adults had less distinctive patterns of activity in occipital cortex than young adults, consistent with dedifferentiation. Interestingly, distinctiveness in PFC and parietal regions was higher in old adults compared to young adults, which was interpreted as compensation. In another study 78, young adults were found to have unique patterns of activity during retrieval of three different kinds of memory content: autobiographical (personally relevant), episodic (not personally relevant but related to stimuli seen during the experiment) and semantic memory (world knowledge). These included activity in the MTL for autobiographical retrieval, dorsolateral PFC and parietal regions during episodic retrieval, and left temporal cortex during retrieval of semantic memories. These patterns of activity were also seen in old adults, but were less distinct for the autobiographical and episodic conditions, consistent with reported age differences in autobiographical and episodic memory but maintained, or even increased, semantic memory with age. Finally, less selective responses to specific categories of visual stimuli also have been reported79, and are associated with measures of task switching and working memory in old adults80. Thus, all of these studies indicate that young adults have activation patterns that are typically quite selective for the particular stimulus features or task demands involved, whereas in older adults activation can be much less distinct, consistent with the idea of dedifferentiation across cognitive processes. These studies further suggest that the loss of selective brain responses may be a marker of a more general cognitive disruption.
Another way to assess dedifferentiation is to use adaptation, which is a reduction in the response of a given brain region, or regions, when a stimulus is presented repeatedly, relative to the first presentation81. Several recent studies have used this method to look at selectivity of brain responses in aging. One assessed activity in the region of the brain that is most responsive to faces, the Fusiform Face Area, or FFA82 to faces that were the same, that had been morphed by varying amounts (similar), or that were different83. Young adults showed more FFA activity during presentation of morphed faces than for the same face shown repeatedly, indicating that the FFA treated morphed faces as “different” even though they were relatively similar to each other. In contrast, the older adults showed equivalent activity for same and morphed faces. Moreover, discrimination thresholds for distinguishing same from different faces were correlated with the degree of adaptation in the FFA across younger and older adults, indicating that this adaptation was important for behavior. A similar study48 assessed adaptation in the FFA during presentation of faces that also varied in viewpoint (right/left orientation). Young adults showed the least activity when the same face was seen in the same viewpoint, more activity when the face or the viewpoint changed, and the most activity when both the face and viewpoint changed. Older adults showed no differences in activity in FFA across the conditions, and performed worse than young adults on a face-matching task involving changes in viewpoint. Adaptation in the auditory domain also has been examined84, and, like visual adaptation, is seen more prominently in younger than in older adults. These experiments show that when adaptation is used to examine differentiation of responses in regions of cortex that respond to specific features of stimuli, regardless of modality, older age is associated with dedifferentiation of responses that are relatively selective in younger adults. Furthermore, this loss of selectivity may be associated with decrements in the ability to discriminate similarities and/or differences among these stimuli.
Brain Networks and Functional Connectivity
Cognitive neuroscientists are becoming increasingly interested in assessing the integrated activity among groups of brain regions as a way of defining brain networks (Box 1). One way of doing this is to measure the functional connectivity of a given brain region or set of regions85,86. Several recent studies have looked at specific functional connections during a task, and how these are affected by age. One study examined changes in brain activity during a working memory task with varying degrees of difficulty87 and found that young adults had load-dependent increases of activity in PFC. Older adults showed relatively high levels of PFC activity across all load levels (consistent with CRUNCH, see above), and weaker functional connectivity between the premotor cortex and a left dorsolateral PFC region. Another study addressed age differences in the resolution of interference during working memory for scenes using a delayed match-to-sample task88. Interference was introduced by presenting a face during the delay period and asking participants to make a gender and age judgment about it. Connectivity was measured between a brain region that responds preferentially to scenes, (the parahippocampal place area, or PPA89) and a region of PFC thought to be important for resolving interference. In young adults, the correlation between activity in the PPA and PFC was disrupted when the face was presented, but returned to pre-interruption levels after the face was removed, suggesting that the resumption of PPA-PFC functional connectivity resolved the interference effect. Older adults showed a similar disruption of PPA-PFC functional connectivity, but the effect persisted after the face was removed, suggesting a deficient ability to dynamically modulate network connectivity, consistent with the poorer performance of the older adults on the task in the presence of the interfering face. Both of these studies highlight the importance of functional connectivity between task-relevant regions and the influence of age on these connections, which in turn might affect behavior.
Box 1. Measuring Activity in Brain Networks.
There is currently much interest in using neuroimaging to assess brain networks, and a number of methods have emerged in recent years for identifying such networks and measuring individual or group differences in network integration and activity. Most of these methods are based on correlations or covariance between regional measures of activity obtained with fMRI (or some other neuroimaging technique), and range from relatively simple assessments of correlations between the time courses of two or more brain regions105, to more complex multivariate approaches that assess brain-wide patterns of connectivity, such as independent component analysis116, or partial least squares198,199. Another approach is that of graph theory200, which uses the number of correlations that characterize various regions to identify areas with large numbers of connections (hubs) and to cluster together subgroups of regions with strong connectivity inside larger collections of areas. Some have attempted to design methods that can assign temporal or functional causality, such as dynamic causal modeling201 and Granger causality analysis202, but these have been somewhat controversial203.
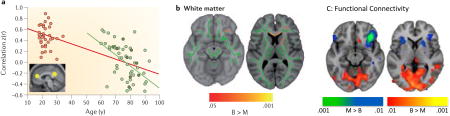
An example of the pair-wise correlation approach is shown in the Figure (panel A)105. In this study, the time course of resting activity in the posterior cingulate cortex, a main node of the default network (DN), was correlated with the time course of activity in the other primary network node, medial PFC (these regions are shown as yellow circles on the brain image in panel A). The correlation values are shown (panel A) for a group of older adults (green dots) and younger adults (black dots). Not only are the correlations lower in older adults, relative to the young, but are reduced with age even in the older group (note regression line shown in green). Studies assessing whole brain functional connectivity have shown age differences in global DN functional connectivity58 (see also Figure 3), suggesting weaker network integration overall in older adults. Functional connectivity in the DN (and other networks) is weakened further in older adults with dementia180,204. However, recent work has shown that some imaging artifacts that are more common in older adults, such as the influence of motion in the scanner205, can weaken functional connectivity, so issues such as this need to be examined further.
Structural connections appear to be important for at least some of the functional connectivity seen in brain networks206. For example, the posterior cingulate is a hub for structural connections207, as well as functional connections in the DN117, and DN regions are strongly connected structurally208. In terms of aging, older adults with better maintained white matter show stronger functional connectivity49. In addition, life experience can influence this relationship between structure and function in older adults. Recently it was shown that bilingualism was associated with better white matter integrity as well as more distributed patterns of functional connectivity in older adults209. Bilingual older adults had better white matter integrity in a number of tracts, including the corpus callosum, as measured with DTI (see panel B in the Figure, which shows white matter tracts in green on two representative structural images, and the areas with greater integrity in bilinguals [Bi] than monolinguals [Mono] in red and yellow). The bilingual older adults also had stronger resting functional connectivity between a region of inferior PFC (circled in panel B) and posterior brain regions (red areas in panel C, where brain images correspond to the slices seen in panel B) compared to age and education-matched monolinguals, whereas monolinguals had stronger functional connectivity within PFC areas (blue regions, panel C). Since older bilinguals typically show better cognitive control than monolinguals210, this finding suggests that better maintained white matter structure and more distributed functional connectivity support maintained cognitive function in older age. Panel A in the Figure is reproduced with permission from REF. 105; Panels B and C in the Figure are adapted from REF. 209 and reproduced with permission.
Age differences in functional connectivity during episodic memory tasks have been studied using a verbal recognition task90. Old adults had reduced functional connectivity within a hippocampal-parietotemporal network relative to young adults, but increased connectivity within a parahippocampal-frontal network. This result was interpreted as evidence that older adults compensate for hippocampal deficits by relying more on the parahippocampal cortex. Similar results have been reported in studies that measured brain activity during successful encoding of words91, scenes92 or objects93 by comparing encoding activity for subsequently remembered versus forgotten stimuli. In these studies, functional connectivity during successful encoding between MTL regions and posterior regions, such as occipital cortex, was weaker in old adults, but connections between the MTL and the PFC were stronger in the older adults compared to a younger group. The results of these studies are reminiscent of the PASA effect involving more PFC activity in conjunction with less occipital activity36, and suggest that successful memory encoding in older adults might be mediated by similar posterior-to-anterior shifts in the functional connectivity of memory-related regions in the MTL.
Functional connectivity has also been studied using attentional tasks. One recent study94 showed that attention to specific task-related cues was associated with activation of the dorsal PFC and parietal attention-related regions in both younger and older adults, but functional connectivity of these regions was higher in young adults than for older adults. Interestingly, increasing cue-related functional connectivity was associated with more efficient performance on the task. In another study95 attention and expectancy were manipulated by predictively cueing which type of stimulus would be presented in a working memory task. When the cue indicated that a picture of a face would be presented, young adults showed greater functional connectivity between the FFA and dorsal attention regions compared to older adults, consistent with their greater memory for predictively cued faces. Both of these studies suggest that weakened functional connectivity between PFC and parietal regions may explain the reduced ability of older adults to attend to and make use of stimuli in the environment. In general, the studies in this section suggest that task-relevant functional connections between specific brain regions can be disrupted with age, and that these disruptions have a negative impact on task performance.
Another recent trend in the neuroimaging literature is to examine functional connectivity within particular large-scale brain networks, such as the default network (DN), which is active when people are resting and engaged in spontaneous thought96–101. In young adults, DN regions maintain strong functional interconnections during tasks requiring self-reference or theory of mind101,102, and also during the resting state103,104. Several studies have found that the reduction of DN activity during externally-driven cognitive tasks is less pronounced in old adults, relative to young adults58,105–112. Functional connectivity of the DN also is reduced with age during working memory tasks113 and during periods of rest105,114–116. These reductions in task-related deactivation and functional connectivity are seen in the two regions thought to be the major nodes of the DN, the posterior cingulate cortex (PCC) and ventromedial PFC117, as well as in other DN regions, such as the MTL and inferior parietal lobes (see Figure 3). Interestingly, intrinsic connectivity during the resting state among nodes of the DN is related to the performance of older individuals on a variety of cognitive tasks105,106,111,118. Given that DN modulation is associated with the degree of task difficulty and performance119,120, a deficit in the ability to modulate DN activity and functional connectivity with advancing age may be a mechanism for deficient resource allocation to the task at hand, accounting for some age differences in cognitive performance108.
Figure 3. The default network in young and older adults.
The regions making up the default network (DN) are shown in this figure (brain areas shown in red). The DN was defined using a multivariate, whole-brain approach198 that identified regions where activity at rest was correlated with activity in the posterior cingulate cortex (PCC, a major node of the DN). The DN includes lateral inferior parietal regions (IPL), as well as ventromedial prefrontal cortex (vmPFC), superior frontal gyrus (SFG), and the medial temporal lobe (MTL). The green regions represent a subset of DN areas, both medial and lateral, with weaker resting functional connectivity in older compared to young adults (also see Box 1).
Factors Influencing Age Differences in Cognition and Brain Activity
An important issue in understanding age differences in brain activity is how these are related to other factors that are affected by aging and that influence brain function, such as brain structure. It has been known for some time that gray matter structures in the brain undergo changes with aging, such as reduced volume and thinning of the cortex, particularly in the frontal lobes121,122. Integrity of white matter, which is typically assessed with diffusion tensor imaging (DTI)123, also is reduced in old compared to young adults124–126. In addition to structural changes, age reductions in neurotransmitter binding potential and receptor density have been found for both dopamine127–129 and serotonin130,131. Finally, the incidence of dementing illnesses such as AD increases with age132, making the impact of risk factors for AD in healthy older adults an area of interest.
Brain Structure
There is a fairly extensive literature on age differences and decline in gray and white matter structures in the brain, especially in the frontal lobes133,134. A longitudinal study135 showed that both hippocampal volume and integrity of white matter in the corpus callosum were reduced in older adults and correlated with declining memory performance. Some studies49,55 have found that age differences in activation within PFC were mediated by white matter integrity, such that more intact white matter was related to more activation, but others have failed to find this effect94,136. Despite this inconsistency in results, the use of DTI to assess white matter integrity holds considerable promise for the study of cognitive aging, particularly as the integrity of specific tracts has been shown to be related to speed of performance in older adults137 or to accuracy of performance 138–140. Of particular interest will be studies examining the relations among white matter integrity underlying specific functional networks, functional connectivity in those networks, and how these measures are related to behaviour in older adults. For example, it has been shown that stronger functional connectivity in a network involving inferior PFC was associated both with better integrity of the corpus callosum and faster response times in older adults141 (see also Box 1).
Reduced functional activation also has been associated with age differences in gray matter volumes142. One recent study143 assessed the relationship between age reductions in gray matter volume of a region in the right middle frontal gyrus (MFG) and brain activity. In young adults, larger right MFG volume was positively correlated with greater activity in bilateral dorsolateral PFC and inferior parietal cortex, both of which have been implicated in memory retrieval144,145. In older adults right MFG volume was not positively correlated with activity in any regions that showed correlations in young adults, but was negatively correlated with activity in several regions, including parahippocampal cortex. Less activity in these regions predicted better memory in older adults, suggesting that older adults with larger right MFG volume may be better able to compensate for the effects of age on this region by modifying activity in other brain regions to help memory retrieval. Interestingly, in this case, the compensation, if that is what it is, appears to take the form of decreased activity in some regions, which may indicate suppression of processes that would conflict with memory retrieval.
Another study146 assessed the relation between brain activity and gray matter volume in younger and older adults across the whole brain. There was under-recruitment of occipital cortex during encoding of face-name pairs in old, compared to young adults, which was mostly accounted for by atrophy in these regions. At retrieval, older adults over-recruited a number of regions including dorsolateral PFC and parietal cortex. This over-recruitment was eliminated after accounting for volume loss in the PFC, but age differences remained in parietal cortex after accounting for the effect of age differences in volume. These results suggest that structural age changes may account for some, but not all, of the differences in brain activity between older and younger adults. Perhaps more important is the evidence that age differences in brain structure can influence the relationship between activity in task-related brain regions and behavior, indicating a complex interplay between structure and function.
Dopamine
One of the most studied aspects of dopamine is its role in reward. Current conceptions of how reward is processed in the brain propose that a circuit of regions, including the ventral striatum and dopaminergic cells in the ventral tegmental area, is necessary for learning about and using rewards to guide behavior147. Several studies have shown that there are age reductions in striatal responses to learned reward148, and reward anticipation149. Only one study has directly examined the relation between functional activation during reward tasks and dopamine binding levels150. It showed that old adults not only have less activity in the ventral striatum during reward anticipation, they also show a weaker relationship between this activity and dopamine levels in the midbrain, relative to younger adults, suggesting that age-related dysfunction in this neurotransmitter system could impact multiple everyday decisions that rely on reward processing.
The role of dopamine in non-reward tasks also has been examined. One study151 assessed brain activity during a low-level working memory task and the influence of a common polymorphism in the gene for COMT in young and old adults. COMT is an enzyme that is thought to regulate dopamine levels in the PFC152, and the Val(158)Met polymorphism results in differing levels of available dopamine in the brain. The Met variant is associated with lower dopamine-degrading activity relative to the Val variant, leading to greater dopamine levels. Individuals who were Met carriers showed no age difference in brain activity, whereas those with the Val allele showed a robust age difference in left PFC activity. Older adults with the Val allele, presumably those with lower dopamine levels, had higher activity than their younger counterparts. These findings suggest that the Val(158)Met polymorphism influences the activity of brain regions within working memory networks and that over-recruitment of PFC activity in older adults can be linked to specific gene effects.
Another recent study153 measured the binding potential of dopamine, as an index of receptor density, and related it to brain activity during working memory. Young adults had increased activity in frontal and parietal regions in a high load memory condition relative to low load conditions, and these load-dependent increases were greater in younger than in older adults. Older adults showed reductions of dopamine binding potential in the caudate nucleus and dorsolateral PFC, and when the contribution of these differences in dopamine binding was accounted for, the age effects on frontal and parietal activity were eliminated or greatly reduced. These findings suggest that some of the age-related differences seen in brain activity during varying cognitive loads (see Figure 2) may be due to alterations in dopaminergic neurotransmission.
Unlike binding potential, dopamine synthesis capacity can be increased in old adults relative to younger adults154, which could reflect an attempt to compensate for the reduced receptor density. Greater synthesis capacity in the caudate nucleus correlated with better verbal working memory performance and more PFC activity during the task in old adults155. However, the relationship between dopamine synthesis capacity and task-related modulation of activity in the PCC (a default network region), is disrupted in old adults156. These studies suggest that age differences in dopamine synthesis capacity, as with binding potential, influence functional activity in multiple brain circuits that are relevant for working memory performance, but whether these differences have a causal role in the reduced working memory performance in older adults is still unknown.
Two studies have manipulated dopamine levels directly to assess the relationship between dopamine, aging and cognition. Dopamine depletion in young adults (by blocking dopamine D1 receptors) resulted in reduced activation in frontal and parietal regions during a high-load working memory task to levels similar to those seen in older adults157. Performance also was lower in young adults after D1 blockade, although still better than that seen in older adults. However, when a dopamine agonist was administered to old adults, to test the idea that boosting dopamine function would induce similar brain activity to that observed in young adults when carrying out episodic memory tasks, an enhancement, rather than a reduction, in age differences was seen158. Clearly much more research is required before any strong statements about the interactions among dopamine alterations and brain activity in aging can be made.
Risk Factors for Alzheimer’s Disease: APOE
There is evidence that memory reductions can be seen at least 6 years prior to a diagnosis of AD159, suggesting that pathology in memory-related regions is well advanced prior to diagnosis160. Therefore, it is important to assess the potential influence of AD risk on studies of “normal” aging. The impact of the different alleles of the APOE gene have been examined in this context, as the presence of one or two ε4 alleles is a known risk factor for AD161. Some studies have reported greater activation in memory-related areas, notably the hippocampus, in healthy old adults who were ε4 carriers compared with non-carriers of the ε4 allele162–164, and even in young ε4 carriers relative to non-carriers165, suggesting an increase in demand on these regions prior to the appearance of any symptoms of memory loss. However, a couple of studies166,167 have found evidence of lower brain activity in the hippocampus of aged ε4 allele carriers during memory tasks. These inconsistent findings regarding brain activity in high-risk individuals compared to their low-risk counterparts could be due to differences in specific task demands, the influence of any number of life-style or health factors, or where in the trajectory of longitudinal change one happens to measure brain activity and cognition. For example, if a participant is on a trajectory towards eventual dementia, measuring brain activity early in this trajectory might reveal an over-recruitment of activity in a given region, whereas a later measurement might show under-recruitment. It also is possible that differential responses to cognitive load could account for over- or under-recruitment in older individuals with either high or low risk for AD (see Figure 2). It nevertheless seems clear that APOE genotype influences age-related changes in brain function, and that the altered task-related brain activity in ε4 carriers may reflect the increased vulnerability of these individuals to AD pathology and cognitive decline.
Finally, it was recently shown that over-recruitment of brain activity in older ε4 carriers is enhanced in those with greater physical activity168. Older adults with the ε4 allele who engaged in more physical activity had greater memory-related activation in posterior temporal and parietal regions than non ε4-carriers or those with lower physical activity. This result is particularly interesting as these areas are some of the first regions of cortex to show metabolic deficits in early AD169–171. This work shows interesting influences of both APOE genotype and physical activity on memory-related brain activation in cognitively intact but genetically at-risk older adults, but it is not clear if this increase is compensatory or protective against future cognitive decline.
Risk Factors for Alzheimer’s Disease: Mild Cognitive Impairment
Mild cognitive impairment (MCI) in older adults is another risk factor for AD, as a relatively high proportion of older adults with MCI, particularly those with amnestic symptoms, will progress to clinical dementia172. There is an extensive literature on functional and structural brain changes in MCI, much of which has shown that individuals with MCI have greater activation in the MTL during memory tasks relative to healthy older controls173,174. Recent research has focused on understanding what might underlie this over-activity. For example, one recent study175 examined subregions of the hippocampus using high resolution fMRI to explore the CA3 region, thought to be involved in pattern separation during memory. Participants with MCI showed over-recruitment of the CA3 region, but not other regions, relative to controls, as well as impaired pattern separation ability, consistent with the idea of a dysfunctional encoding mechanism due to early neuropathological changes in this hippocampal region. Interestingly, healthy older adults also show memory-related deficits in CA3 function relative to younger adults176. Another study177 found that over-recruitment of the hippocampus in MCI was related to cognitive load, such that it was only seen at lower levels of memory load during a paired-associates task. At higher loads, activity in the hippocampus was lower in the MCI group relative to controls, consistent with the CRUNCH hypothesis (similar results have also been reported in MCI for other brain regions178). These studies point both toward specific processing deficits as well an impairment in the ability to respond to increases in cognitive demand as potential explanations for MTL over-recruitment in MCI. This work also highlights the similarities between age differences in healthy older vs younger adults and differences between MCI and healthy older individuals (e.g., both can be characterized by CRUNCH and involve over-recruitment of brain activity). This similarity suggests a continuum of effects due to age and neuropathological brain changes, perhaps because both aging and risk for dementia can impact cognition in a general way that impairs the ability to respond to increasing cognitive demand.
Other recent work has emphasized how MCI affects larger scale brain networks. One such study showed that healthy older adults utilize a network of regions, including the MTL, for successful encoding179. Although participants with MCI showed engagement of this network, activity in it was not associated with memory performance; instead activity in a network involving anterior temporal regions thought to be involved in semantic retrieval was correlated with memory in MCI. This shift was interpreted as a compensatory response to dysfunction in the MTL. The DN also has been studied in older adults with MCI, who show weaker functional connectivity in this network compared to healty elderly, consistent with studies showing that AD patients have less deactivation of and weaker functional connectivity in the DN109,180. These effects of MCI have been found in the PCC181, and in its connections to other regions182. In addition, DN functional connectivity is more affected in those MCI individuals who later progress to dementia than in those who remain stable over time183. Indeed, the weaker functional connectivity of the DN in MCI and AD, in conjunction with the finding of amyloid deposition and other neuropathological changes in DN regions, including the MTL160, has led to the suggestion that the DN is intimately involved in the neuropathology of AD184. Again, there is a similarity in the vulnerability of the DN in those with risk factors for AD (APOE, MCI) and the vulnerability seen in healthy older adults relative to young adults, suggesting that DN activity and functional connectivity in older samples might be a useful marker for predicting cognitive decline.
Influence of Training on the Aging Brain
The influence of expertise on the adult brain has been demonstrated185,186 (see Box 1 for an example of how a lifelong experience can influence brain structure and function), but less is known about how short-term behavioral training can affect brain activity in older adults. This question is important because it has implications for rehabilitating cognitive decline in older people. A few neuroimaging studies have looked at this issue and their results are intriguing. One study provided training to older adults on a divided attention task in five one-hour sessions over a two-week interval and found improved performance and reduced age differences in brain activity that were apparent prior to training187. PFC activity that was greater in older adults prior to training was reduced to the level seen in younger adults after training, presumably because the practice on the two tasks had reduced the effort required to carry them out simultaneously, reducing the need for PFC mediated cognitive control. Similarly, a reduction in the amplitude of an electrophysiological evoked response during a working memory task was reported in older adults after 10 hours of perceptual discrimination training, and this reduction predicted the increase in accuracy on the working memory task that was achieved after training188.
Increased activity in older adults after episodic memory training has been reported in a study189 that scanned young and old adults during encoding of words to assess baseline age differences. The older adults then underwent two training sessions (for a total of 2.5 hours), in which they were trained on the use of three different learning strategies and then allowed to use the strategy of their choice to learn lists of words. A post training fMRI session was then carried out in the older group. Older adults’ reported use of the strategies during the encoding condition at the second fMRI session coincided with an elimination of the pre-training age differences in word memory. Training also increased older adults’ brain activity in the left frontal and temporal regions that have been previously associated with verbal processing and successful encoding70,190,191. These increases of brain activity were correlated with the degree of improvement in memory after training, suggesting a direct role for training in influencing both brain function and behavior. These training studies suggest that increased brain activity after even limited training could be due to the adoption of a different strategy, whereas decreased brain activity after training is more likely due to a practice-related increase of efficiency on a task187.
Conclusions
There are a number of issues in the aging literature that have yet to be resolved satisfactorily, including that of compensatory brain activity. For example, over-recruitment of brain activity in older adults has been interpreted as compensation both when there was a positive correlation between activity and behavior39, and when this correlation was negative146. Although it seems unlikely that both positive and negative correlations could be compensatory, perhaps a more careful and consistent definition of what is ‘compensatory’ is needed. One model192 defines three different types of compensation. When there is a mismatch between available cognitive resources and current task demands, this leads to the recruitment of additional neural resources, reflected in increased brain activity. At this point, without a link to behavior, the over-recruitment is called “attempted compensation”. The increase in brain activity may be associated with better task performance, in which case it is defined as “successful compensation”, or not (“unsuccessful compensation”). In this model, determining the relationship between the engagement of additional neural resources and task performance is critical for determining whether the compensation has been successful or not. Adopting terms such as unsuccessful or partial compensation, to make a clear distinction between these phenomena and successful compensation, where over-recruitment is clearly linked to better performance, may help to remove discrepancies in the literature and clarify the compensatory role of various regions. In addition, others have suggested23 that the term “compensation” should be used only for those instances in which old adults recruit brain activity that is not seen in younger adults, and the engagement of this area or areas is directly correlated with better performance only in the older adults and not in the young (i.e., there is a unique pattern of neural activity that supports task performance in an age-specific manner).
Another initiative that would be welcome in this field is the use of lifespan studies to identify the changes that occur, both in cognitive processes and the brain mechanisms underlying them, from childhood to old age. Such developmental changes could take a variety of forms, including both linear and non-linear changes. There is recent evidence suggesting that some behavioral and brain trends in development might take different forms (see Box 2), indicating that much could be learned about the links between brain and behavior using a comprehensive lifespan approach. In addition, longitudinal studies will be important for understanding brain aging. Although cross-sectional studies are easier to carry out, and have contributed most of what we know to date about aging of the brain, they are vulnerable to cohort effects, and longitudinal studies are necessary for identifying the effects of aging within individuals. There have been a few longitudinal studies of brain function in older adults, which have shown decreased task-related activity over time193, both decreases and increases, depending on the specific brain region and cognitive demands194,195, and a greater decline of activity in older individuals with risk factors for AD196. With so few data points it is difficult to come to any strong conclusions about change over time, highlighting the need for these kinds of studies.
Box 2. Assessing Behavioral and Brain Trajectories over the lifespan.
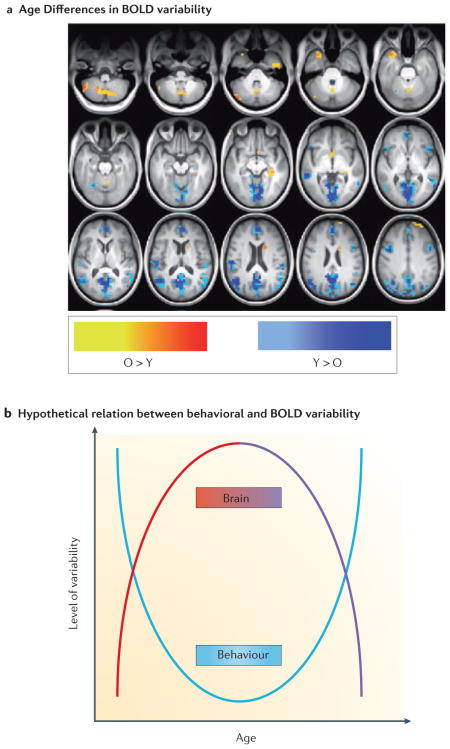
An example of how lifespan studies could add to our knowledge of brain-behavior interactions can be found in the study of variability. It is well known that measures of behavioral performance, such as response times, are variable both between and within individuals120,211,212 and that behavioral variability is higher in children and older adults relative to younger adults213–216. In aging, behavioral variability also can serve as a marker for cognitive decline217,218, and increases prior to death219. However, the relation between behavioral variability and variability in brain activity has not been extensively examined, although evidence indicates that variability in ongoing activity is important for the expression of evoked patterns of activity220. The use of fMRI to study brain function has relied primarily on assessing average brain activation patterns. Nevertheless, brain activity is inherently variable, and several lines of research have shown that our ability to understand important aspects of brain function is enhanced by considering the variability of brain signals221–223. In particular, networks that are more variable may be more robust to disruption and may explore more neural states, thus enhancing learning and promoting optimal performance221,222,224,225.
Recent studies have shown that there are developmental increases in variability and complexity of brain activity, from childhood to the young adult ages, along with increased accuracy and stability of task performance226. In addition, a recent study assessed variability of the BOLD signal with age, using the standard deviation of activity in all brain voxels, and found that the majority of regions with age differences had less variability in the older group227 (see Figure, blue regions in panel A). In addition, lower BOLD variability in these regions was associated with slower and more variable response times on cognitive tasks57. Thus, this accumulating evidence suggests that behavioural variability has a U-shaped function over the lifespan216, with larger variability in children and older adults compared to young adults (see Figure, panel B), whereas variability of brain activity shows the opposite trend (inverted U shape). Lifespan studies examining this kind of question using the same behavioral and imaging paradigms from children up to older adults would shed much light on how developmental changes in brain function can impact behavior. Panel A in the Figure is adapted from REF. 227, and reproduced with permission.
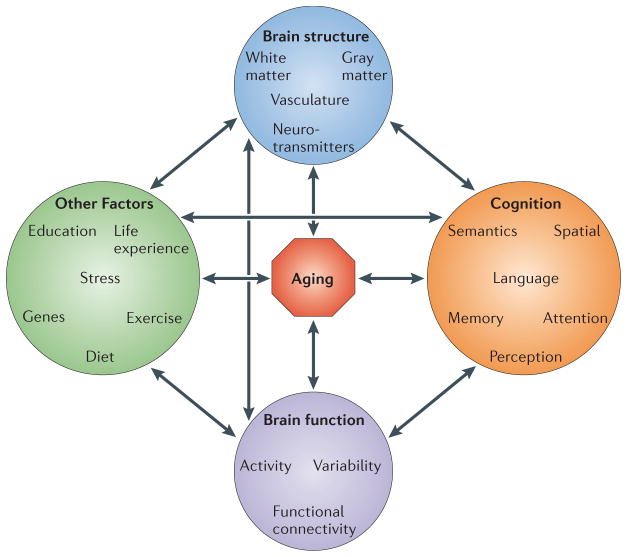
Finally, it is clear that aging is influenced by a large number of factors that vary from individual to individual, including genetics and life experiences (Figure 4). Although it is probably impossible to account for all of these factors in a single study, the current trend is to include an assessment of multiple influencing factors and multiple measures of brain structure and function, as the experiments reviewed here can attest. Publicly accessible databases, such as the Alzheimer Disease Neuroimaging Initiative (ADNI)197, that contain information on a large number of individuals collected across multiple laboratories will aid greatly in this effort. Sharing of data and meta-analyses will allow for larger scale examinations of aging effects than is possible with data from a single laboratory, and ultimately add to our knowledge in a substantial way.
Figure 4. A hypothetical model of the various dimensions that can interact with aging.
The model is intended to show the interplay among a wide array of physical and behavioral aspects (some of which are discussed in this review) and the aging process. The arrows are bidirectional to indicate that the influence can potentially arise from these factors on the aging process, or vice versa. For example, genetic factors could influence how an individual ages, and aging can enhance the effects of genes on specific behaviors. There are other factors that could be included here, such as risk factors for vascular disease or dementia, but this incomplete list gives a sense of how complex the study of aging is, and how difficult it would be to comprehensively assess these variables in a single experiment.
Online Summary.
The main challenge in the field of neurocognitive aging is to understand the brain mechanisms that might underlie age differences in cognitive performance or why some functions are maintained into older age.
A number of ideas have been suggested to explain age differences in brain activity during cognitive tasks, including compensation, dedifferentiation, and less efficient use of neural resources. Although there is evidence to support all of these theories, there also is evidence to the contrary, and it is not yet clear if one is more characteristic of aging than the others.
Recently there has been increasing interest in examining the effects of age on large-scale brain networks. One of these in particular, the default network, appears to be especially vulnerable to the effects of age.
There is evidence that age differences in brain structure can influence the relationship between activity in task-related brain regions and behaviour, indicating a complex interplay between structure and function.
There is a growing literature on how various risk factors for Alzheimer disease, such as the APOE gene and mild cognitive impairment, impact task-related brain activity in older adults. This work also highlights the similarities between age differences in healthy older vs younger adults and differences between MCI and healthy older individuals, suggesting a continuum of effects due to age and neuropathological brain changes.
Future work should aim to more clearly define compensatory brain activity, make more use of lifespan and longitudinal approaches, and attempt to account for the large number of factors influence the aging process and that vary from individual to individual, including genetics and life experiences.
Biography
Dr. Cheryl Grady is a senior scientist at the Rotman Research Institute at Baycrest, and a professor in the departments of Psychiatry and Psychology at the University of Toronto. She obtained her PhD in experimental psychology from Boston University, and her main research interest is in the cognitive neuroscience of aging, with a focus on fMRI studies of brain networks during memory and perception. She holds the Canada Research Chair in Neurocognitive Aging.
References
- 1.Tulving E. Elements of Episodic Memory. Oxford University Press; 1983. [Google Scholar]
- 2.Craik FIM, Bosman EA. In: Gerontechnology: Proceedings of the First International Conference on Technology and Aging. Bouma H, Graafmans J, editors. IOS Press; 1992. pp. 79–92. [Google Scholar]
- 3.Balota DA, Dolan PO, Duchek JM. In: The Oxford Handbook of Memory. Tulving E, Craik F, editors. Oxford University Press; 2000. pp. 395–410. [Google Scholar]
- 4.Zacks RT, Hasher L, Li KZH. In: The Handbook of Aging and Cognition. Craik FIM, Salthouse TA, editors. Erlbaum; 2000. pp. 200–230. [Google Scholar]
- 5.Connelly SL, Hasher L, Zacks RT. Age and reading: the impact of distraction. Psychol Aging. 1991;6:533–541. doi: 10.1037//0882-7974.6.4.533. [DOI] [PubMed] [Google Scholar]
- 6.Allen PA, Makken DJ, Groth KE, Crozier LC. Impact of age, redundancy, and perceptual noise on visual search. J Gerontol. 1992;47:P69–P74. doi: 10.1093/geronj/47.2.p69. [DOI] [PubMed] [Google Scholar]
- 7.Madden DJ. Adult age differences in attentional selectivity and capacity. Eur J Cogn Psychol. 1990;2:229–252. [Google Scholar]
- 8.Anderson ND, Craik FIM, Naveh-Benjamin M. The attentional demands of encoding and retrieval in younger and older adults: I. Evidence from divided attention costs. Psychol Aging. 1998;13:405–423. doi: 10.1037//0882-7974.13.3.405. [DOI] [PubMed] [Google Scholar]
- 9.Kramer AF, Hahn S, Gopher D. Task coordination and aging: explorations of executive control processes in the task switching paradigm. Acta Psychol (Amst) 1999;101:339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 10.Cepeda NJ, Kramer AF, Gonzalez de Sather JC. Changes in executive control across the life span: examination of task-switching performance. Dev Psychol. 2001;37:715–730. [PubMed] [Google Scholar]
- 11.Hasher L, Zacks RT. In: The psychology of learning and motivation. Bower GH, editor. Vol. 22. Academic Press; 1988. pp. 193–225. [Google Scholar]
- 12.Healey MK, Campbell KL, Hasher L. Progress in Brain Research. In: Sossin W, Lacaille JC, Castellucci VF, Belleville S, editors. The Essence of Memory. Vol. 169. Elsevier; 2008. This review describes work characterizing age differences in inhibition. When younger and older adults are presented with a task in the presence of distraction (and told to ignore the distracting information), older adults have better memory for the distracting material when tested subsequently. This effect is thought to be due to an age-related reduction in inhibitory effectiveness. [DOI] [PubMed] [Google Scholar]
- 13.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 14.Craik FIM, Jennings JM. In: The Handbook of Aging and Cognition. Craik FIM, Salthouse TA, editors. Lawrence Erlbaum; 1992. pp. 51–110. [Google Scholar]
- 15.Laver GD. Adult aging effects on semantic and episodic priming in word recognition. Psychol Aging. 2009;24:28–39. doi: 10.1037/a0014642. [DOI] [PubMed] [Google Scholar]
- 16.Carstensen LL, Fung HF, Charles ST. Socioemotional selectivity theory and the regulation of emotion in the second half of life. Motiv Emot. 2003;27:103–123. [Google Scholar]
- 17.Carstensen LL, et al. Emotional experience improves with age: evidence based on over 10 years of experience sampling. Psychol Aging. 2011;26:21–33. doi: 10.1037/a0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahhal TA, May CP, Hasher L. Truth and character: sources that older adults can remember. Psychol Sci. 2002;13:101–105. doi: 10.1111/1467-9280.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Eyler LT, Sherzai A, Kaup AR, Jeste DV. A review of functional brain imaging correlates of successful cognitive aging. Biological Psychiatry. 2011;70:115–122. doi: 10.1016/j.biopsych.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grady CL, et al. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- 22.Grady CL, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grady CL. Cognitive neuroscience of aging. Ann N Y Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- 24.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 25.Mukamel R, et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 26.Aizenstein HJ, et al. The BOLD hemodynamic response in healthy aging. J Cogn Neurosci. 2004;16:786–793. doi: 10.1162/089892904970681. [DOI] [PubMed] [Google Scholar]
- 27.Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage. 2001;13:161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- 28.D’Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the BOLD hemodynamic response. Neuroimage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- 29.Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;12:24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- 30.Hillary FG, Biswal B. The influence of neuropathology on the FMRI signal: a measurement of brain or vein? Clin Neuropsychol. 2007;21:58–72. doi: 10.1080/13854040601064542. [DOI] [PubMed] [Google Scholar]
- 31.D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 32.Kannurpatti SS, Motes MA, Rypma B, Biswal BB. Neural and vascular variability and the fMRI-BOLD response in normal aging. Magn Reson Imaging. 2010;28:466–476. doi: 10.1016/j.mri.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabeza R, et al. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madden DJ, et al. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Map. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reuter-Lorenz PA, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 36.Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. This paper presents a model to explain over-recruitment of bilateral prefrontal cortex in older adults and argues that this over-recruitment is compensatory. This idea of bilaterality in aging is still being assessed in the literature. [DOI] [PubMed] [Google Scholar]
- 38.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 39.McIntosh AR, et al. Recruitment of unique neural systems to support visual memory in normal aging. Cur Biol. 1999;9:1275–1278. doi: 10.1016/s0960-9822(99)80512-0. [DOI] [PubMed] [Google Scholar]
- 40.Della-Maggiore V, et al. Corticolimbic interactions associated with performance on a short-term memory task are modified by age. J Neurosci. 2000;20:8410–8416. doi: 10.1523/JNEUROSCI.20-22-08410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grady CL, McIntosh AR, Craik F. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging. 2007;28:784–798. doi: 10.1016/j.neurobiolaging.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- 44.Greenwood PM. Functional plasticity in cognitive aging: Review and hypothesis. Neuropsychology. 2007;21:657–673. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- 45.Vallesi A, McIntosh AR, Stuss DT. Overrecruitment in the aging brain as a function of task demands: evidence for a compensatory view. J Cogn Neurosci. 2011;23:801–815. doi: 10.1162/jocn.2010.21490. [DOI] [PubMed] [Google Scholar]
- 46.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee Y, Grady CL, Habak C, Wilson HR, Moscovitch M. Face Processing Changes in Normal Aging Revealed by fMRI Adaptation. J Cogn Neurosci. 2011;23:3433–3447. doi: 10.1162/jocn_a_00026. [DOI] [PubMed] [Google Scholar]
- 49.Davis SW, Kragel JE, Madden DJ, Cabeza R. The architecture of cross-hemispheric communication in the aging brain: Linking behavior to functional and structural connectivity. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossi S, et al. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. J Neurosci. 2004;24:7939–7944. doi: 10.1523/JNEUROSCI.0703-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manenti R, Cotelli M, Miniussi C. Successful physiological aging and episodic memory: a brain stimulation study. Behav Brain Res. 2011;216:153–158. doi: 10.1016/j.bbr.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 52.Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cereb Cortex. 2007;17:2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- 53.Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation- performance relations in delayed-response tasks: a multiple component analysis. Cortex. 2007;43:65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- 54.Stevens WD, Hasher L, Chiew K, Grady CL. A neural mechanism underlying memory failure in older adults. J Neurosci. 2008;28:12820–12824. doi: 10.1523/JNEUROSCI.2622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Chastelaine M, Wang TH, Minton B, Muftuler LT, Rugg MD. The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cereb Cortex. 2011;21:2166–2176. doi: 10.1093/cercor/bhq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Persson J, Kalpouzos G, Nilsson LG, Ryberg M, Nyberg L. Preserved hippocampus activation in normal aging as revealed by fMRI. Hippocampus. 2011;21:753–766. doi: 10.1002/hipo.20794. [DOI] [PubMed] [Google Scholar]
- 57.Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The importance of being variable. J Neurosci. 2011;31:4496–4503. doi: 10.1523/JNEUROSCI.5641-10.2011. This is one of a series of papers showing that the variability of the fMRI signal is less in older adults, compared to younger adults, and that less variable brain signals are associated with greater behavioral variability on cognitive tasks. This is a novel approach to the study of brain function with fMRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grady CL, et al. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Otten LJ, Rugg MD. Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb Cortex. 2001;11:1150–1160. doi: 10.1093/cercor/11.12.1150. [DOI] [PubMed] [Google Scholar]
- 60.Wagner AD, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 61.Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cereb Cortex. 2007;17:1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- 62.Jimura K, Braver TS. Age-related shifts in brain activity dynamics during task switching. Cereb Cortex. 2010;20:1420–1431. doi: 10.1093/cercor/bhp206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dew IT, Buchler N, Dobbins IG, Cabeza R. Where Is ELSA? The Early to Late Shift in Aging. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008:177–182. This paper presents the CRUNCH model of brain function and aging, and suggests a mechanism to explain both under and over-recruitment of brain activity in older adults. [Google Scholar]
- 65.Mattay VS, et al. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 66.Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schneider-Garces NJ, et al. Span, CRUNCH, and beyond: working memory capacity and the aging brain. J Cogn Neurosci. 2010;22:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spaniol J, Grady C. Aging and the neural correlates of source memory: over-recruitment and functional reorganization. Neurobiol Aging. 2012;33:425.e423–425.e418. doi: 10.1016/j.neurobiolaging.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 70.Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- 71.Madden DJ, et al. Aging and recognition memory: changes in regional cerebral blood flow associated with components of reaction time distributions. J Cogn Neurosci. 1999;11:511–520. doi: 10.1162/089892999563571. [DOI] [PubMed] [Google Scholar]
- 72.Grady CL. Age-related differences in face processing: A meta-analysis of three functional neuroimaging experiments. Canad J Exp Psychol. 2002;56:208–220. doi: 10.1037/h0087398. [DOI] [PubMed] [Google Scholar]
- 73.Townsend J, Adamo M, Haist F. Changing channels: an fMRI study of aging and cross-modal attention shifts. Neuroimage. 2006;31:1682–1692. doi: 10.1016/j.neuroimage.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 74.Dennis NA, Cabeza R. Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rieckmann A, Fischer H, Backman L. Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: relations to performance. Neuroimage. 2010;50:1303–1312. doi: 10.1016/j.neuroimage.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 76.Carp J, Gmeindl L, Reuter-Lorenz PA. Age differences in the neural representation of working memory revealed by multi-voxel pattern analysis. Frontiers in Human Neuroscience. 2010;4:217. doi: 10.3389/fnhum.2010.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carp J, Park J, Polk TA, Park DC. Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. Neuroimage. 2011;56:736–743. doi: 10.1016/j.neuroimage.2010.04.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.St-Laurent M, Abdi H, Burianov H, Grady CL. Influence of Aging on the Neural Correlates of Autobiographical, Episodic, and Semantic Memory Retrieval. J Cogn Neurosci. 2011;23:4150–4163. doi: 10.1162/jocn_a_00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park DC, et al. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Science U S A. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park J, Carp J, Hebrank A, Park DC, Polk TA. Neural specificity predicts fluid processing ability in older adults. J Neurosci. 2010;30:9253–9259. doi: 10.1523/JNEUROSCI.0853-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 82.Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goh JO, Suzuki A, Park DC. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. Neuroimage. 2010;51:336–344. doi: 10.1016/j.neuroimage.2010.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grady CL, Charlton R, He Y, Alain C. Age differences in FMRI adaptation for sound identity and location. Frontiers in Human Neuroscience. 2011;5:24. doi: 10.3389/fnhum.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McIntosh AR. Mapping cognition to the brain through neural interactions. Memory. 1999;7:523–548. doi: 10.1080/096582199387733. [DOI] [PubMed] [Google Scholar]
- 86.Horwitz B. In: Visuomotor Coordination. Ewert J-P, Arbib MA, editors. Plenum Press; 1989. pp. 873–892. [Google Scholar]
- 87.Nagel IE, et al. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J Cogn Neurosci. 2011;23:2030–2045. doi: 10.1162/jocn.2010.21560. [DOI] [PubMed] [Google Scholar]
- 88.Clapp WC, Rubens MT, Sabharwal J, Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7212–7217. doi: 10.1073/pnas.1015297108. This study suggests the intriguing possibility that older adults show a reduced ability to resolve interference (a type of inhibition deficit) because interfering stimuli disrupt functional connectivity in task-relevant brain networks, which does not return to normal as quickly as in young adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- 90.Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of Healthy Aging on Hippocampal and Rhinal Memory Functions: An Event-Related fMRI Study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dennis NA, et al. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.St Jacques P, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: A network analysis of fMRI data. Psychol Sci. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Addis DR, Leclerc CM, Muscatell KA, Kensinger EA. There are age-related changes in neural connectivity during the encoding of positive, but not negative, information. Cortex. 2010;46:425–433. doi: 10.1016/j.cortex.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Madden DJ, et al. Adult age differences in functional connectivity during executive control. Neuroimage. 2010;52:643–657. doi: 10.1016/j.neuroimage.2010.04.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bollinger J, Rubens MT, Masangkay E, Kalkstein J, Gazzaley A. An expectation- based memory deficit in aging. Neuropsychologia. 2011;49:1466–1475. doi: 10.1016/j.neuropsychologia.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Nat Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Raichle ME, et al. A default mode of brain function. Proceedings of the National Academy of Science U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shulman GL, et al. Common Blood Flow Changes Across Visual Tasks: Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 99.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 100.Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 101.Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection and theory-of-mind, and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- 102.Grigg O, Grady CL. The default network and processing of personally relevant information: Converging evidence from task-related modulations and functional connectivity. Neuropsychologia. 2010;48:3815–3823. doi: 10.1016/j.neuropsychologia.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. This review paper presents a comprehensive summary of the default network and the potential relevance of disrupted network function to dementia. [DOI] [PubMed] [Google Scholar]
- 104.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Science U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andrews-Hanna JR, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Damoiseaux JS, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 107.Esposito F, et al. Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI. Magn Reson Imaging. 2008;26:905–913. doi: 10.1016/j.mri.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 108.Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- 109.Lustig C, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Science U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller SL, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Science U S A. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- 112.Duzel E, Schutze H, Yonelinas AP, Heinze HJ. Functional phenotyping of successful aging in long-term memory: Preserved performance in the absence of neural compensation. Hippocampus. 2011;21:803–814. doi: 10.1002/hipo.20834. [DOI] [PubMed] [Google Scholar]
- 113.Sambataro F, et al. Age-related alterations in default mode network: Impact on working memory performance. Neurobiol Aging. 2010;31:839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park DC, Polk TA, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Frontiers in Human Neuroscience. 2010;3:article 75. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hedden T, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Allen EA, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang L, et al. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010;51:910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 120.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 121.Raz N, et al. Selective aging of human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 122.Fjell AM, et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johansen-Berg H, Behrens T. Academic Press; London: 2009. [Google Scholar]
- 124.Moseley M. Diffusion tensor imaging and aging - a review. NMR Biomed. 2002;15:553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- 125.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 126.Head D, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- 127.Kish SJ, Zhong XH, Hornykiewicz O, Haycock JW. Striatal 3,4-dihydroxyphenylalanine decarboxylase in aging: disparity between postmortem and positron emission tomography studies? Ann Neurol. 1995;38:260–264. doi: 10.1002/ana.410380220. [DOI] [PubMed] [Google Scholar]
- 128.Rinne JO, Lonnberg P, Marjamaki P. Age-dependent decline in human brain dopamine D1 and D2 receptors. Brain Research. 1990;508:349–352. doi: 10.1016/0006-8993(90)90423-9. [DOI] [PubMed] [Google Scholar]
- 129.Walker LC, et al. The neural basis of memory decline in aged monkeys. Neurobiol Aging. 1988;9:657–666. doi: 10.1016/s0197-4580(88)80130-1. [DOI] [PubMed] [Google Scholar]
- 130.Meltzer CC, et al. Reduced binding of [18F]altanserin to serotonin type 2A receptors in aging: persistence of effect after partial volume correction. Brain Research. 1998;813:167–171. doi: 10.1016/s0006-8993(98)00909-3. [DOI] [PubMed] [Google Scholar]
- 131.Moller M, Jakobsen S, Gjedde A. Parametric and regional maps of free serotonin 5HT1A receptor sites in human brain as function of age in healthy humans. Neuropsychopharmacology. 2007;32:1707–1714. doi: 10.1038/sj.npp.1301310. [DOI] [PubMed] [Google Scholar]
- 132.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Raz N. In: Handbook of Aging and Cognition - II. Craik FIM, Salthouse TA, editors. Lawrence Erlbaum; 2000. pp. 1–90. [Google Scholar]
- 134.Kaup AR, Mirzakhanian H, Jeste DV, Eyler LT. A review of the brain structure correlates of successful cognitive aging. J Neuropsychiatry Clin Neurosci. 2011;23:6–15. doi: 10.1176/appi.neuropsych.23.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Persson J, et al. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- 136.Madden DJ, et al. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Salami A, Eriksson J, Nilsson LG, Nyberg L. Age-related white matter microstructural differences partly mediate age-related decline in processing speed but not cognition. Biochim Biophys Acta. 2012;1822:408–415. doi: 10.1016/j.bbadis.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 138.Davis SW, et al. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lockhart S, et al. Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Frontiers in Human Neuroscience. 2012;6:article 56. doi: 10.3389/fnhum.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Charlton RA, Barrick TR, Lawes IN, Markus HS, Morris RG. White matter pathways associated with working memory in normal aging. Cortex. 2010;46:474–489. doi: 10.1016/j.cortex.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 141.Chen NK, Chou YH, Song AW, Madden DJ. Measurement of spontaneous signal fluctuations in fMRI: adult age differences in intrinsic functional connectivity. Brain Struct Funct. 2009;213:571–585. doi: 10.1007/s00429-009-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thomsen T, et al. Brain localization of attentional control in different age groups by combining functional and structural MRI. Neuroimage. 2004;22:912–919. doi: 10.1016/j.neuroimage.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 143.Rajah M, Languay R, Grady C. Age-related changes in right middle frontal gyrus volumes correlate with altered episodic retrieval activity. J Neurosci. 2011;31:17941–17954. doi: 10.1523/JNEUROSCI.1690-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Proc Natl Acad Sci USA. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kalpouzos G, Persson J, Nyberg L. Local brain atrophy accounts for functional activity differences in normal aging. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 147.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mell T, et al. Altered function of ventral striatum during reward-based decision making in old age. Frontiers in Human Neuroscience. 2009;3:article 34. doi: 10.3389/neuro.09.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Samanez Larkin GR, et al. Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proceedings of the National Academy of Science U S A. 2008;105:15106–15111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sambataro F, et al. Catechol-O-methyltransferase valine(158)methionine polymorphism modulates brain networks underlying working memory across adulthood. Biological Psychiatry. 2009;66:540–548. doi: 10.1016/j.biopsych.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Backman L, et al. Dopamine D(1) receptors and age differences in brain activation during working memory. Neurobiol Aging. 2011;32:1849–1856. doi: 10.1016/j.neurobiolaging.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 154.Braskie MN, et al. Relationship of striatal dopamine synthesis capacity to age and cognition. J Neurosci. 2008;28:14320–14328. doi: 10.1523/JNEUROSCI.3729-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Landau SM, Lal R, O’Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009;19:445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Braskie MN, et al. Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults. Human Brain Mapping. 2011;32:947–961. doi: 10.1002/hbm.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fischer H, et al. Simulating neurocognitive aging: effects of a dopaminergic antagonist on brain activity during working memory. Biological Psychiatry. 2010;67:575–580. doi: 10.1016/j.biopsych.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 158.Morcom AM, et al. Memory encoding and dopamine in the aging brain: a psychopharmacological neuroimaging study. Cereb Cortex. 2010;20:743–757. doi: 10.1093/cercor/bhp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Backman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain. 2001;124:96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- 160.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 161.Strittmatter WJ, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bookheimer SY, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dickerson BC, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Trivedi MA, et al. fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: risk factors for Alzheimer’s disease. Neuropsychologia. 2008;46:1667–1678. doi: 10.1016/j.neuropsychologia.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dennis NA, et al. Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimers Dement. 2010;6:303–311. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Trivedi MA, et al. Reduced hippocampal activation during episodic encoding in middle- aged individuals at genetic risk of Alzheimer’s disease: a cross-sectional study. BMC Med. 2006;4:1. doi: 10.1186/1741-7015-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Filippini N, et al. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage. 2011;54:602–610. doi: 10.1016/j.neuroimage.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 168.Smith JC, et al. Interactive effects of physical activity and APOE-epsilon4 on BOLD semantic memory activation in healthy elders. Neuroimage. 2011;54:635–644. doi: 10.1016/j.neuroimage.2010.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Grady CL, et al. Longitudinal study of the early neuropsychological and cerebral metabolic changes in dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1988;10:576–596. doi: 10.1080/01688638808402796. [DOI] [PubMed] [Google Scholar]
- 170.Duara R, et al. Positron emission tomography in Alzheimer’s disease. Neurology. 1986;36:879–887. doi: 10.1212/wnl.36.7.879. [DOI] [PubMed] [Google Scholar]
- 171.Frackowiak RSJ, et al. Regional cerebral oxygen supply and utilization in dementia. A clinical and physiological study with oxygen-15 and positron tomography. Brain. 1981;104:753–778. doi: 10.1093/brain/104.4.753. [DOI] [PubMed] [Google Scholar]
- 172.Petersen RC, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Filippi M, Agosta F. Structural and functional network connectivity breakdown in Alzheimer’s disease studied with magnetic resonance imaging techniques. Journal of Alzheimer’s disease. 2011;24:455–474. doi: 10.3233/JAD-2011-101854. [DOI] [PubMed] [Google Scholar]
- 174.Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: insights from functional MRI studies. Neuropsychologia. 2008;46:1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yassa MA, et al. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.de Rover M, et al. Hippocampal dysfunction in patients with mild cognitive impairment: a functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia. 2011;49:2060–2070. doi: 10.1016/j.neuropsychologia.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 178.Kochan NA, et al. Functional alterations in brain activation and deactivation in mild cognitive impairment in response to a graded working memory challenge. Dement Geriatr Cogn Disord. 2010;30:553–568. doi: 10.1159/000322112. [DOI] [PubMed] [Google Scholar]
- 179.Protzner AB, Mandzia JL, Black SE, McAndrews MP. Network interactions explain effective encoding in the context of medial temporal damage in MCI. Human Brain Mapping. 2011;32:1277–1289. doi: 10.1002/hbm.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Science U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sala-Llonch R, et al. Greater default-mode network abnormalities compared to high order visual processing systems in amnestic mild cognitive impairment: an integrated multi-modal MRI study. J Alzheimers Dis. 2010;22:523–539. doi: 10.3233/JAD-2010-101038. [DOI] [PubMed] [Google Scholar]
- 182.Han SD, et al. Functional connectivity variations in mild cognitive impairment: associations with cognitive function. Journal of the International Neuropsychological Society. 2012;18:39–48. doi: 10.1017/S1355617711001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology. 2011;76:511–517. doi: 10.1212/WNL.0b013e31820af94e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neuroscience. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neuroscience. 2000;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- 187.Erickson KI, et al. Training-induced plasticity in older adults: effects of training on hemispheric asymmetry. Neurobiol Aging. 2007;28:272–283. doi: 10.1016/j.neurobiolaging.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 188.Berry AS, et al. The influence of perceptual training on working memory in older adults. PLoS One. 2010;5:e11537. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kirchhoff BA, Anderson BA, Barch DM, Jacoby LL. Cognitive and Neural Effects of Semantic Encoding Strategy Training in Older Adults. Cereb Cortex. 2012;22:788–799. doi: 10.1093/cercor/bhr129. This is one of the few papers to address the effects on the brain of cognitive training in older adults and shows strong evidence that such changes are important for improved behavioral performance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- 191.Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring “how” from “where”. Neuropsychologia. 2003;41:280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- 192.Cabeza R, Dennis NA. In: Principles of Frontal Lobe Function. Stuss DT, Knight RT, editors. Oxford University Press; in press. [Google Scholar]
- 193.Nyberg L, et al. Longitudinal evidence for diminished frontal cortex function in aging. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22682–22686. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Beason-Held LL, Kraut MA, Resnick SMI. Longitudinal changes in aging brain function. Neurobiol Aging. 2008;29:483–496. doi: 10.1016/j.neurobiolaging.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Beason-Held LL, Kraut MA, Resnick SM. II. Temporal patterns of longitudinal change in aging brain function. Neurobiol Aging. 2008;29:497–513. doi: 10.1016/j.neurobiolaging.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Thambisetty M, Beason-Held L, An Y, Kraut MA, Resnick SM. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol. 2010;67:93–98. doi: 10.1001/archneurol.2009.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Weiner MW, et al. The Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Dement. 2011 [Google Scholar]
- 198.Grigg O, Grady CL. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS One. 2010;5:e13311. doi: 10.1371/journal.pone.0013311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56:455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 200.Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stephan KE, et al. Dynamic causal models of neural system dynamics:current state and future extensions. J Biosci. 2007;32:129–144. doi: 10.1007/s12038-007-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage. 2005;25:230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 203.Smith SM, et al. The danger of systematic bias in group-level FMRI-lag-based causality estimation. Neuroimage. 2012;59:1228–1229. doi: 10.1016/j.neuroimage.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 204.Sperling RA, et al. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Honey CJ, et al. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Science U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hagmann P, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Luk G, Bialystok E, Craik F, Grady C. Lifelong bilingualism maintains white matter integrity in older adults. J Neurosci. 2011;31:16808–16813. doi: 10.1523/JNEUROSCI.4563-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bialystok E, Craik F. Cognitive and linguistic processing in the bilingual mind. Current Directions in Psychological Science. 2010;19:19–23. [Google Scholar]
- 211.Mantyla T, Backman L. Encoding variability and age-related retrieval failures. Psychol Aging. 1990;5:545–550. doi: 10.1037//0882-7974.5.4.545. [DOI] [PubMed] [Google Scholar]
- 212.Morse CK. Does variability increase with age? An archival study of cognitive measures. Psychol Aging. 1993;8:156–164. doi: 10.1037//0882-7974.8.2.156. [DOI] [PubMed] [Google Scholar]
- 213.MacDonald SW, Nyberg L, Backman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 214.Hultsch DF, MacDonald SW, Dixon RA. Variability in reaction time performance of younger and older adults. Journal of Gerontology B Psychological Science and Social Science. 2002;57:P101–115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- 215.West R, Murphy KJ, Armilio ML, Craik FI, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain Cogn. 2002;49:402–419. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- 216.Williams BR, Hultsch DF, Strauss EH, Hunter MA, Tannock R. Inconsistency in reaction time across the life span. Neuropsychology. 2005;19:88–96. doi: 10.1037/0894-4105.19.1.88. [DOI] [PubMed] [Google Scholar]
- 217.Dixon RA, et al. Neurocognitive markers of cognitive impairment: exploring the roles of speed and inconsistency. Neuropsychology. 2007;21:381–399. doi: 10.1037/0894-4105.21.3.381. [DOI] [PubMed] [Google Scholar]
- 218.MacDonald SW, Hultsch DF, Dixon RA. Performance variability is related to change in cognition: evidence from the Victoria Longitudinal Study. Psychol Aging. 2003;18:510–523. doi: 10.1037/0882-7974.18.3.510. [DOI] [PubMed] [Google Scholar]
- 219.Macdonald SW, Hultsch DF, Dixon RA. Predicting impending death: inconsistency in speed is a selective and early marker. Psychol Aging. 2008;23:595–607. doi: 10.1037/0882-7974.23.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. An early landmark study addressing variability in brain activity by showing that intrinsic, ongoing brain activity influences the way in which stimulus-evoked activity is expressed. The authors describe the effect of such stimuli as “the additional ripples caused by tossing a stone into a wavy sea”. [DOI] [PubMed] [Google Scholar]
- 221.Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stein RB, Gossen ER, Jones KE. Neuronal variability: noise or part of the signal? Nat Rev Neurosci. 2005;6:389–397. doi: 10.1038/nrn1668. [DOI] [PubMed] [Google Scholar]
- 223.Ghosh A, Rho Y, McIntosh AR, Kotter R, Jirsa VK. Noise during rest enables the exploration of the brain’s dynamic repertoire. PLoS Comput Biol. 2008;4:e1000196. doi: 10.1371/journal.pcbi.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Basalyga G, Salinas E. When response variability increases neural network robustness to synaptic noise. Neural Comput. 2006;18:1349–1379. doi: 10.1162/neco.2006.18.6.1349. [DOI] [PubMed] [Google Scholar]
- 225.Ma WJ, Beck JM, Latham PE, Pouget A. Bayesian inference with probabilistic population codes. Nature Neuroscience. 2006;9:1432–1438. doi: 10.1038/nn1790. [DOI] [PubMed] [Google Scholar]
- 226.McIntosh AR, Kovacevic N, Itier RJ. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput Biol. 2008;4:e1000106. doi: 10.1371/journal.pcbi.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Garrett DD, Kovacevic N, McIntosh AR, Grady CL. Blood oxygen level-dependent signal variability is more than just noise. J Neurosci. 2010;30:4914–4921. doi: 10.1523/JNEUROSCI.5166-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]