Abstract
Epidemiological studies consistently find correlations between nicotine and alcohol use, yet the neural mechanisms underlying their interaction remain largely unknown. Nicotine and alcohol (i.e., ethanol) share many common molecular and cellular targets that provide potential substrates for nicotine-alcohol interactions. These targets for interaction often converge upon the mesocorticolimbic dopamine system, where the link to drug self-administration and reinforcement is well documented. Both nicotine and alcohol activate the mesocorticolimbic dopamine system, producing downstream dopamine signals that promote the drug reinforcement process. While nicotine primarily acts via nicotinic acetylcholine receptors, alcohol acts upon a wider range of receptors and molecular substrates. The complex pharmacological profile of these two drugs generates overlapping responses that ultimately intersect within the mesocorticolimbic dopamine system to promote drug use. Here we will examine overlapping targets between nicotine and alcohol and provide evidence for their interaction. Based on the existing literature, we will also propose some potential targets that have yet to be directly tested. Mechanistic studies that examine nicotine-alcohol interactions would ultimately improve our understanding of the factors that contribute to the associations between nicotine and alcohol use.
Keywords: Nicotine, Alcohol, Dopamine, drug abuse, stress
1. Introduction
Tobacco and alcohol use is a worldwide health problem, accounting for more than 7 million deaths per year combined [1, 2]. Epidemiological studies demonstrate a shared vulnerability to tobacco and alcohol abuse, in which one substance influences the use of the other [3–6]. Estimates indicate that over 83% of alcoholics also smoke, with alcoholism approximately 10 times more prevalent in smokers than in non-smokers [7–9]. Adolescent and college-age smoking increases the risk of developing alcohol use disorders [10, 11]. Similarly, binge patterns of alcohol drinking occur more often in regular or occasional smokers compared to non-smokers [4, 6].
Rodent models often show that exposure to nicotine can increase alcohol self-administration [12–14]. Rats exposed to nicotine during adolescence show significantly higher alcohol intake as adults upon re-exposure to nicotine [15]. Some studies have reported other effects of nicotine on alcohol intake [16], which possibly arise from methodological differences in the self-administration procedures. There is also evidence for the converse relationship (i.e., the influence of alcohol on nicotine self-administration). Alcohol consumption potently increases ad-lib smoking behavior in humans [17, 18]. Alcohol also enhances the craving to smoke and the positive feelings associated with smoking [19–21].
Thus, there is a potent and complex interaction between tobacco and alcohol reinforcement. Many factors contribute to this interaction, including psychosocial and genetic influences, as well as the widespread availability of tobacco and alcohol [22, 23]. Accumulating evidence suggests that nicotine and alcohol also interact through neurobiological mechanisms [24]. In rodents and humans, chronic nicotine administration is associated with a reduced sensitivity to the pharmacological effects of ethanol, a phenomenon known as cross-tolerance [25, 26]. Behavioral studies that have examined nicotine and alcohol interactions suggest the involvement of the cerebellum, hippocampus, hypothalamus, and striatum [27–29]. Although the mesocorticolimbic dopamine (DA) system has a central role in the development of drug reinforcement and addiction [30], there is little mechanistic information on how nicotine and alcohol interact within this system. Other brain systems contribute significantly to drug-seeking behavior, but we have limited the scope of our discussion mainly to nicotine and alcohol interactions that implicate the DA system and its afferent connections. To identify common targets of action, we will describe the separate actions of nicotine and alcohol within the context of the DA system. The review will highlight relevant functional interactions when appropriate and will examine potential mechanistic interactions that largely await future investigations.
2. The Mesocorticolimbic DA System
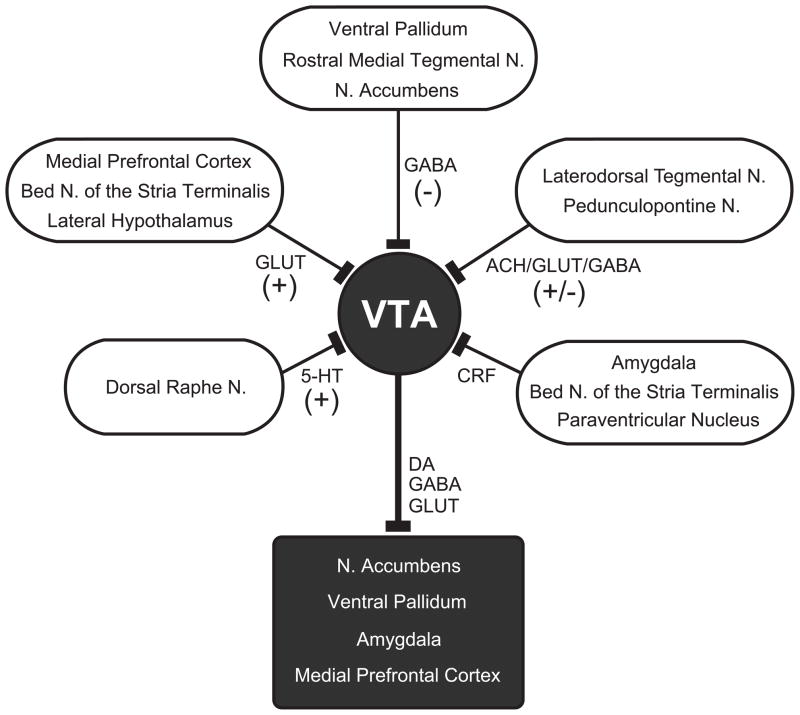
The mesocorticolimbic DA system regulates mood, emotional responses, and incentive-based behavior [31, 32], and dysregulation of this system is a hallmark of the drug-addicted state [30, 33, 34]. Neurons in the ventral tegmental area (VTA) are the primary source of the mesocorticolimbic DA system. Those neurons project to many cortical and forebrain limbic structures, including the nucleus accumbens (NAc), ventral pallidum, amygdala, and the medial prefrontal cortex (mPFC). A simplified summary of the mesocorticolimbic pathway is shown in Fig. 1 (darkened symbols). DA neurons represent an abundant cell type in the VTA, but the exact percentage of DA neurons varies between subregions [35–37]. The VTA also contains glutamate neurons and GABA neurons that project to the forebrain and can form local synapses with other VTA neurons [37–41]. A fourth cell type synthesizes both DA and glutamate, and there are potentially other mixed transmitter neurons in this area [42, 43].
Figure 1.
A simplified schematic of the mesocorticolimbic DA system (darkened symbols) and its afferent inputs. The ventral tegmental area (VTA) is the primary source of the mesocorticolimbic DA system, which projects to several limbic and cortical structures. Numerous excitatory (+) and inhibitory (−) inputs, as well as neuromodulatory signals (e.g., CRF), regulate the output of the VTA. N. = nucleus, GLUT = glutamate, ACH = acetylcholine.
Numerous afferent inputs regulate the activity of DA and non-DA neurons in the VTA. Major excitatory inputs arise from the laterodorsal and pedunculopontine tegmentum, the lateral hypothalamus, the bed nucleus of the stria terminalis, and the prefrontal cortex [44–47]. The major GABAergic inhibitory inputs to the VTA arise from the rostromedial tegmental nucleus, the ventral pallidum, the laterodorsal tegmentum, and the nucleus accumbens (NAc) [48–50]. The serotonergic (5-HT) neurons of the dorsal raphe nucleus constitute another important afferent projection, which transmits mainly excitatory signals to the VTA [51]. Fig. 1 summarizes these different afferent inputs to the VTA, including their relevant neurotransmitters. Some inputs target specific subsets of VTA neurons. The excitatory inputs from the laterodorsal tegmentum, for example, mainly target VTA DA neurons that project to the nucleus accumbens [44], whereas the inhibitory inputs from the nucleus accumbens were shown to target non-dopaminergic VTA neurons [49].
2.1. VTA Neuron Properties
DA neurons are the most well studied class of cells in the VTA. The classic DA neurons (found mainly in the lateral VTA and in the substantia nigra compacta) display various modes of firing activity in vivo, including tonic and phasic activation [52, 53]. Tonic activity involves slow irregular patterns of single action potentials, whereas phasic activity involves short-latency bursts of action potentials (typically 2–4 action potentials, each separated by less than 80 ms). DA neurons in brain slices do not display normal burst activity [54], indicating that burst firing depends on afferent synaptic input, which is altered and disrupted in brain slices. The tonic firing frequency of DA neurons in freely moving animals is approximately 2–10 Hz. However, within a single burst of action potentials the average firing frequency increases to approximately 15–28 Hz [53, 55]. The transition from tonic to phasic firing activity is hypothesized to be a key mechanism for transmitting behaviorally relevant information [31]. Molecular substrates, including the DA transporters and DA autoreceptors, further filter the information encoded by DA neurons to regulate DA release. Owing to regional differences in these molecular substrates, phasic DA signals lead to greater extracellular DA levels in the ventral striatum compared to the dorsal striatum [56–58].
In addition to DA neurons, VTA GABAergic and glutamatergic neurons provide input to the mesocorticolimbic target areas (Fig. 1). A high firing frequency (typically above 20 Hz) and relatively short action potential duration characterize a well-studied subset of GABAergic neurons [39, 55, 59]. Recent studies indicate that VTA GABA neurons make selective synaptic connections with cholinergic interneurons in the NAc that influence local DA transmission and contribute to associative learning [60, 61]. Glutamatergic VTA neurons also innervate the nucleus accumbens and other structures, and their properties and their regulation continue to be studied [37, 42, 43, 62].
3. Nicotine and Alcohol Regulate DA Function
Rodents will learn to press a lever to obtain nicotine or alcohol, suggesting that nicotine and alcohol are reinforcing. The development of nicotine and alcohol reinforcement involves the DA system [33, 63]. In contrast to the many and diverse molecular targets of alcohol, nicotine initially acts upon the DA system via nicotinic acetylcholine receptors (nAChRs). Targeted manipulation of nAChRs in the VTA has determined a critical role for these receptors in mediating the reinforcing properties of nicotine [64–68]. Blockade of nAChRs in the VTA or upon afferents to the VTA consistently reduces operant self-administration of nicotine.
As for nicotine and other addictive drugs, alcohol self-administration coincides with an increase in DA levels in the NAc [69–71], and with learning, this DA signal may arise from the initial sensory cues of the alcohol solution [72, 73]. Local infusion of DA receptor antagonists into the VTA and the NAc reduces subsequent operant responses for alcohol [74–76], and neurotoxic lesions of the DA system decrease alcohol intake [77, 78]. The lesions only appear to alter alcohol intake when applied prior to the acquisition of drinking behavior (i.e., prior to training), and not after self-administration behavior becomes established, suggesting that DA signaling is particularly critical during the acquisition and development of alcohol reinforcement. Many of these features of alcohol self-administration are shared by other addictive drugs.
3.1. Nicotine and Alcohol Increase Dopamine Excitability
The behavioral responses to nicotine and alcohol arise from their cellular and molecular actions in the brain, including those acting directly and indirectly upon the mesocorticolimbic DA system. Although alcohol targets a wider range of molecular targets and neural substrates than nicotine, both drugs potently act and converge upon the mesocorticolimbic DA system to increase DA transmission. In vivo administration of either nicotine or alcohol increases the action potential firing rate of DA neurons and the phasic burst activity of DA neurons [79–82]. Concomitant with this activation, nicotine increases the correlated firing activity among subsets of VTA DA neurons [55], which would amplify and synchronize the output of the DA signal to the target areas. Nicotine enhances the firing rate and burst activity of DA neurons, in part, by activating cholingeric and glutamatergic inputs from the laterodorsal and pedunculopontine tegmentum [44, 83–85], and via a direct activation of nAChRs within the VTA [79, 86]. NMDA glutamate receptors also participate in nicotine-induced burst firing of DA neurons [87]. A recent study provides evidence that nicotine increases burst firing through the co-activation of VTA GABA neurons [68]. Alcohol-induced activation of the DA system may also involve cholingeric and glutamatergic inputs from the laterodorsal and pedunculopontine tegmentum, but support for this hypothesis is limited at this point [88–90].
3.2. Nicotine and Alcohol Increase DA Release
The DA neuron activity induced by nicotine and alcohol correlates with increased DA release at target areas, including the NAc, ventral pallidum, and mPFC [91–98]. In target areas in the dorsal and ventral striatum, nAChRs located on DA terminals (and/or axons), as well as on cholinergic interneurons, regulate DA release [58, 86, 99, 100]. Different regulatory mechanisms involving nAChRs allow initial nicotine administrations to increase extracellular DA levels largely in the ventral striatum compared to the dorsal striatum [101]. Nicotine also increases the DA input to the hippocampus to modulate perforant pathway long-term potentiation and conditioned behavior [102, 103]. Nicotine and alcohol administered simultaneously produce an additive increase in DA release in the NAc compared to each drug alone [104]. However, pretreatment with nicotine has been shown to decrease subsequent alcohol-induced DA release [105, 106]. Therefore, the DA system represents a prime target for functional interactions between nicotine and alcohol.
4. Cellular and Molecular Actions of Nicotine
The following sections summarize some of the cellular and molecular actions of nicotine associated with the DA system, but we do not mean to imply that the DA system is the primary system through which nicotine acts. Nicotinic AChRs mediate the effects of nicotine throughout the brain, and non-dopaminergic effects are essential to nicotine reinforcement [65]. We simply wish to convey how nicotine targets specific substrates within the DA system that are also critical for the responses to alcohol.
The nAChR is a pentameric ligand-gated ion channel formed by different combinations of subunits (α2-α10 and β2-β4) [107, 108]. Much of the work with nAChR subunit combinations has been done in rodents. The most common nAChRs in the rodent VTA are the high affinity β2-containing subtype (often in combination with α4 and/or α6) and the lower affinity α7-containing subtype [109–111]. DA and GABA VTA neurons, as well as the afferent GABAergic inputs to VTA neurons, express the high affinity β2-containing nAChR [109, 110, 112]. The α7-containing nAChR is located predominately on presynaptic glutamatergic inputs and to a lesser extent on VTA GABA neurons [109, 111].
4.1. Acute Nicotine Action in the VTA
The low brain concentrations of nicotine obtained from tobacco (~20–100 nM) activate the β2-containing nAChRs [113–115]. Within minutes of exposure, nicotine begins to desensitize mainly the high affinity nAChRs [110, 114]. This process leads to a decrease particularly in GABA inhibition of VTA DA neurons [112, 116]. Due to differences in agonist affinity, these low concentrations of nicotine do not readily desensitize α7-containing nAChRs [110, 116]. This distinction may allow α7-containing nAChRs to exert a prolonged excitatory effect over glutamatergic afferents onto DA neurons, favoring the induction of long-term synaptic potentiation [112, 116]. However, the β2-containing, and not the α7-containing, nAChRs are of greater importance during the initiation of nicotine self-administration [115, 117]. During those initial exposures, a single administration of nicotine is sufficient to induce long-term synaptic potentiation of glutamatergic afferents onto DA neurons, as indicated by an increase in the AMPA/NMDA receptor ratio and an increase in the probability of glutamate release [89, 118, 119]. These kinds of synaptic changes underlie learning and memory. Working throughout the brain, this “drug associated learning” may link the drug use to environmental cues [33].
4.2. Chronic Nicotine Action in the VTA
In addition to any acute effects, chronic repeated exposure to nicotine causes a persistent upregulation of some nAChRs subtypes, meaning that nicotine increases the number of nAChRs available for binding [120, 121]. Upregulation of nAChRs varies between nAChR subtypes, between brain regions, and even between local cell types [120, 122–124]. Various cellular mechanisms may account for upregulation, including increased receptor assembly and trafficking to the plasma membrane, increased nAChR function, and decreased rate of nAChR turnover [125–129]. Behavioral studies also indicate that nicotine self-administration upregulates AMPA receptors in the VTA [130]. These neuroadaptations and others could contribute to changes in the long-term sensitivity to nicotine and alcohol [10, 131, 132].
Lester and colleagues showed that chronic nicotine exposure tends to upregulate high affinity nAChRs on VTA GABA neurons, which was related to an increase in GABAergic inhibition of DA neurons [124]. Increased GABAergic inhibition could contribute to withdrawal by causing a reduction in tonic DA levels in the nucleus accumbens [133, 134]. A stronger inhibition of the tonic DA response after nicotine withdrawal increases the ratio between tonic and phasic DA signaling [134]. Therefore, during the withdrawal period, re-exposure to nicotine or alcohol may induce DA signals that increase the vulnerability to the drug abuse. Relapse to smoking often occurs within two weeks of abstinence, suggesting that early withdrawal from nicotine is a critical period for relapse [135].
Lastly, evidence also suggests that some actions of nicotine within the DA system occur through non-nicotinic receptor mechanisms. Prolonged exposure to nicotine can inhibit ubiquitin-related proteasomal activity, which was shown to regulate α7-containing nAChRs as well as several ionotropic and metabotropic glutamate receptors [136]. These responses were only partially blocked by a nAChR antagonist, indicating that nicotine activation of nAChRs does not completely account for the results. Therefore, nicotine influences a diverse array of molecular substrates and systems (including the cholinergic, glutamatergic, and GABAergic systems), and these substrates represent known targets of alcohol.
5. Alcohol Regulation of Dopamine and Behavior
Alcohol is an allosteric modulator of many ligand-gated ion channels, giving alcohol broad access to the DA system and to the brain as a whole. Alcohol potentiates the function of GABAA, nACh, and 5-HT3 receptors, and can inhibit the function of glutamatergic receptors [137]. Although not comprehensive, the following section will review evidence of how alcohol can modulate these and other neurotransmitter systems, including upstream and downstream effects, to influence the DA system and behavior. This will provide a basis for understanding potential interactions between nicotine and alcohol.
5.1. Direct Action on Dopamine Neurons
At concentrations achieved during intoxication (20–100 mM), alcohol moderately excites VTA DA neurons in brain slices, as well as in acutely dissociated DA neurons lacking synaptic inputs, indicating a direct alcohol action on DA neurons [138–140]. Alcohol reduces the amplitude of the after-hyperpolarization phase of the DA action potential and influences other ionic conductances and metabotropic signals, which may increase DA neuron excitability [140–144]. In addition to these direct actions, the in vivo effects of alcohol on DA neurons arise from a complex interplay between alcohol and many neurotransmitter systems that converge on the local circuits of the VTA.
5.2. Alcohol and GABA
Substantial evidence indicates a role of GABA signaling in alcohol self-administration [145, 146]. Local blockade of GABAA receptors in the VTA and the ventral pallidum and knockout of GABAA receptors in the NAc decreases alcohol self-administration [147–149]. At the molecular level, alcohol binds to specific amino acid residues on the GABAA receptor, which enhances inward chloride conductance and inhibition [150–152]. Alcohol also modulates GABAA receptors indirectly via the activation of neuroactive steroids [153], which we discuss further below.
Application of alcohol to VTA brain slices increases presynaptic GABA release onto DA neurons [154, 155], and evidence suggests that in vivo alcohol exposure induces a long lasting (at least 24 h to 1 week) potentiation of GABA inhibition in the VTA [156]. The precise mechanism by which ethanol enhances presynaptic GABA release is not understood, but could involve 5-HT transmission and stress signaling pathways [155, 157]. Evidence from the hippocampus also suggests that nicotinic receptors mediate varying degrees of GABAergic inhibition in target cells [158]. If these mechanisms extend to the VTA, local circuit interactions involving alcohol-induced activation of the cholinergic system (see section 5.4) could potentially influence GABAergic activity in the VTA. In vivo recordings from putative VTA GABA neurons indicate that GABAergic responses to alcohol can vary substantially between cells, and single cells can even show transient excitation and inhibition during a single recording [159, 160]. The dose of ethanol and the timing between injections are factors that likely contribute to different GABAergic responses [159, 161].
Withdrawal from chronic alcohol exposure is associated with decreases in GABAA function [162]. Prolonged exposure to alcohol can decrease GABAA synaptic transmission and decrease GABAA receptor levels in the VTA and elsewhere [163, 164]. During abstinence, alcoholics show a reduced sensitivity to GABAA receptor ligands, such as benzodiazepines [165], which is consistent with blunted GABAA receptor function. Interestingly, nicotine and alcohol interactions during withdrawal may involve changes in GABAA responses [166]. Smoking appears to attenuate some of the deficits in GABAA function that occur during withdrawal [167].
5.3. Alcohol and Glutamate
In contrast to its effects on other ligand-gated receptors, alcohol primarily inhibits the function of NMDA-type glutamate receptors [168, 169]. However, prior activation of DA receptors (and DARPP-32 signaling) was shown to attenuate alcohol-induced inhibition of NMDA receptors in the NAc [170], suggesting that DA signaling may circumvent this inhibition. In addition, alcohol can inhibit non-NMDA (AMPA/kainate) receptors [169, 171, 172], particularly in the amygdala, but results can vary depending on the brain region and the age of the subject [168, 173]. The inhibition of the metabotropic glutamate receptor (mGluR) by alcohol [174] is of additional interest due to the influence of these receptors on alcohol self-administration. Several studies demonstrate that inhibitors of the mGluR5 subtype decrease alcohol self-administration and reinforcement [175–177], which is intriguing because mGluR5 inhibitors were also shown to decrease nicotine self-administration and reinstatement [178, 179].
Regarding its presynaptic action, acute application of alcohol to VTA brain slices enhances glutamatergic transmission onto DA neurons [180], but glutamatergic transmission can differ in other brain regions [169, 172, 173]. Increased extracellular glutamate levels have also been reported in the NAc as measured by in vivo microdialysis [181]. Lastly, long-term exposure to alcohol upregulates glutamatergic receptors in the VTA, amygdala, and elsewhere [182–185]. There is little information on functional interactions between alcohol and nicotine involving glutamatergic transmission. However, one study has shown that co-abuse of alcohol and nicotine regulates the expression of the vesicular glutamate transporter differently from nicotine abuse alone [186].
5.4. Alcohol and ACh
There is a direct interaction between alcohol and the nicotinic cholinergic system via the nAChRs. Low concentrations of alcohol increase the affinity of acetylcholine for neuronal nAChRs and potentiate the nicotinic currents produced by acetylcholine [187, 188]. Heterologously expressed recombinant nAChRs that contain the α2 or α4 subunit are particularly sensitive to activation by alcohol [189, 190]. Evidence indicates that alcohol potentiates receptor function by stabilizing the open state of the α4-containing nAChR [191], but prolonged exposure to alcohol may increase nAChR desensitization [192]. In contrast, alcohol seems to inhibit the nicotinic responses of some α7-containing nAChRs, but the results can vary depending on the expression system or the duration of the bath alcohol application [189, 193]. Interestingly, chronic exposure to alcohol was shown to upregulate high affinity nAChRs in the thalamus and hypothalamus [194], similar to chronic nicotine exposure. Whether alcohol induces similar nAChR upregulation within the DA system remains to be tested.
In addition to any direct actions on nAChR function, alcohol may influence the DA system via activation of afferent cholinergic inputs. Alcohol self-administration elevates extracellular acetylcholine levels in the VTA [88], and a single dose of alcohol activates cholinergic interneurons in the nucleus accumbens, as measured by Fos expression [195]. Substantial evidence indicates an involvement of distinct nAChRs in alcohol-induced DA release and self-administration. Intra-VTA or systemic blockade of nAChRs antagonists with mecamylamine (a general nAChR antagonist) reduces alcohol consumption and alcohol-induced DA release in the NAc [12, 196, 197]. Mecamylamine was also shown to reduce the subjective rewarding effects of alcohol in humans [198]. One caveat is that mecamylamine acts as an antagonist at the NMDA glutamate receptor [199], which raises the issue of specificity.
Using antagonists that are more selective than mecamylamine, several studies have indicated a contribution of different nAChR subtypes to alcohol responses. Blocking α3β2-containing nAChRs (and/or β3* nAChRs) with α-conotoxin MII prevents alcohol-induced DA release and alcohol self-administration [200–202]. Pharmacological studies have not provided reliable evidence for the involvement of other high affinity nAChRs, nor for α7-containing nAChRs, in these responses [201–203]. However, using a novel nAChR ligand, one study has suggested that α4β2-containing nAChRs participate in self-administration of both nicotine and alcohol [204]. Likewise, recent work using α4 nAChR knockout models demonstrates that alcohol-induced DA responses and alcohol conditioned place preference require α4-containing nAChRs [205]. Knockout studies also suggest a role for the α7-containing nAChRs in alcohol consumption [206]. Other alcohol-induced behaviors, such as sedation, anxiolytic responses, and motor activity/coordination involve high affinity nAChRs and the lower affinity α7-containing nAChRs [207–209]. Interestingly, varenicline, an anti-smoking agent and a partial agonist at several nAChR subtypes, effectively reduces alcohol consumption in rodents and in humans [210, 211]. Varenicline administration also appears to decrease DA release induced by alcohol [212]. Although the role of specific nAChR subtypes remains unclear, the facts suggest that nAChRs mediate many actions of alcohol.
5.5. Alcohol and 5-HT
Another mechanism by which alcohol influences the DA system is through 5-HT signaling. Alcohol concentrations achieved during intoxication potentiate the function of the 5-HT3 ligand-gated receptor [213, 214]. Similar to its action on nAChRs, alcohol appears to stabilize the open state of the 5-HT3 receptor and may slow the rate of receptor desensitization [215]. Substantial evidence indicates that 5-HT receptors contribute to the many systemic and behavioral effects of alcohol. Alcohol administration increases serotonin release in the VTA and the NAc, and blockade of 5-HT3 receptors prevents this increase in serotonin [216–218].
Activation of 5-HT3 receptors also contributes to alcohol reinforcement. Systemic or intra-VTA administration of 5-HT3 receptor antagonists attenuates operant alcohol self-administration [219, 220]. Moreover, intra-VTA self-administration of alcohol is prevented by locally blocking 5-HT3 receptors during training [221]. There are many metabotropic 5-HT receptors (5-HT1A–F, 5-HT2A–C, 5-HT4, 5-HT5, 5-HT6, and 5-HT7), but alcohol does not appear to interact with them directly. Instead, these receptors appear to modulate alcohol-induced responses. Blockade of 5-HT2A receptors, for example, decreases ethanol drinking in several rodent lines [222]. The 5-HT2C receptor modulates specific actions of alcohol within the VTA. Blockade of 5-HT2C receptors blocks alcohol-induced GABAergic inhibition onto DA neurons, suggesting that alcohol induces GABA release via 5-HT transmission [155]. 5-HT2C receptors have also been shown to modulate nicotine reinforcement [223]. Furthermore, recent studies show that a 5-HT6 receptor antagonist was effective at reducing both nicotine and ethanol reinstatement [224].
5.6. Alcohol and Endocannabinoids
Although endocannabinoid (eCB) receptors are not direct targets of alcohol and nicotine, substantial evidence suggests that certain aspects of alcohol and nicotine reinforcement involve eCB signaling [225]. Endogenous cannabinoid pathways modify the central nervous system and immune responses through CB1 and CB2 receptors, respectively [226]. DA neurons appear to release eCBs, which act in a retrograde manner to bind CB1 receptors on glutamatergic and GABAergic inputs [227]. CB1 antagonists reduce alcohol self-administration and alcohol conditioned place preference [228–230]. CB1 receptor antagonists also reduce nicotine self-administration as well as nicotine-induced DA release in the NAc [231, 232]. Local blockade of CB1 receptors in the VTA and the NAc reduces alcohol consumption, suggesting a critical role of eCB signaling in the mesoaccumbens pathway [233]. Pretreatment with a CB1 receptor agonist or with nicotine blocked alcohol-induced DA release, suggesting that these agents may interact with alcohol through a common mechanism [105]. In addition, alcohol increases conditioned place preference induced by nicotine, and this effect is prevented by blocking eCB receptors [234]. Repeated nicotine or alcohol exposure correlates with an increase in eCB levels across different brain regions [235]. Furthermore, clinical trials evaluating CB1 antagonist rimonabant as an aid for smoking cessation report a doubling in abstinence rates [236]. Rimonabant may therefore be useful in the treatment of alcohol use disorders.
6. Potential Interactions via Neuroendocrine Pathways
In addition to the acute effects of nicotine and alcohol, nicotine influences the long-term use of alcohol, suggesting that neuroadaptative mechanisms involving gene transcription and protein synthesis contribute to this interaction. One way that nicotine and alcohol could interact is through neuroendocrine pathways associated with the hypothalamic-pituitary-adrenal (HPA) axis. Stressful or arousing events activate the HPA axis to induce long-term adaptive changes in the brain and in behavior [237]; and drugs of abuse activate these pathways [238]. The stress response is governed by neurons in the paraventricular nucleus (PVN) of the hypothalamus that release corticotropin-releasing factor (CRF) into the hypophyseal portal system. CRF then activates corticotrophs in the pituitary that release adrenocorticotropic hormone (ACTH) into the bloodstream. ACTH stimulates the secretion of stress hormones, such as glucocorticoids, from the adrenal cortex [239], which influence neuronal activity via both genomic and non-genomic mechanisms [240]. Other HPA-related hormones, such as progesterone and its metabolites, also participate in stress responses [241, 242].
6.1. Complexity of the Stress Pathways
Although stress is a known risk factor for nicotine and alcohol abuse, the mechanisms by which HPA axis contributes to drug interactions have only been hypothesized. The complexity of the HPA system has limited our ability to identify stress-mediated adaptations that promote subsequent nicotine or alcohol use. First, glucocorticoids and other stress hormones act throughout the brain and can serve different functions [243]. Within the PVN of the hypothalamus, for example, glucocorticoids act as a negative feedback signal to inhibit CRF activity [244]. However, in extra-hypothalamic regions, such as the central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST), glucocorticoids deliver a positive feedback signal to potentiate CRF activity [245]. CRF activity in extra-hypothalamic regions, including the DA system, contributes to different aspects of drug abuse [243]. Second, stress hormones can induce fast, non-genomic interactions at the cell membrane (seconds to minutes) as well as slow, transcription-dependent effects via intracellular receptors (15 minutes to hours or days) [240, 246]. Finally, stress hormones can serve as precursors for the production of neuroactive steroids, which exert rapid effects on neural transmission and regulate neuroendocrine responses [247, 248]. More specifically, progesterone and other structurally related molecules can act as potent allosteric modulators of nACh, GABAA, and NMDA receptors [249–252]. Therefore, stress hormones and neuroactive steroids may act in concert through multiple mechanisms to influence the responses to drugs of abuse.
6.2. Stress Pathways and Drug Abuse
Several studies support the hypothesis that activation of the HPA axis contributes to the motivational properties of nicotine and alcohol. Animals will learn to self-administer glucocorticoids intravenously, suggesting that glucocorticoids are reinforcing [253]. Nicotine and alcohol separately activate stress pathways to elicit glucocorticoid release [254, 255]. Glucocorticoids and other stress-related hormones act directly on the DA system to modulate DA transmission and behavior [256, 257]. Adrenalectomized rodents show reduced dopamine responses to nicotine and reduced alcohol self-administration [258, 259]. Conversely, local activation of glucocorticoid receptors in the ventral striatum has been shown to increase alcohol intake [260], and the development of alcohol dependence may involve the activation of glucocorticoid receptors in the DA system [261]. Consistent with these findings, studies in humans suggest that low glucocorticoid levels among alcoholic patients are a risk factor for early relapse [262]. In terms of interactions between nicotine and alcohol, it is essential to determine how glucocorticoids acting during nicotine exposure influence the responses to alcohol and vice versa.
The tolerance associated with repeated exposure to nicotine or alcohol involves stress hormones and their neuroactive metabolites [263–265]. Whether neuroendocrine systems undergo cross-tolerance to the effects of nicotine and alcohol is an intriguing question but remains to be determined. Inhibition of nAChRs by stress hormones may contribute to nicotine tolerance [266]. Because alcohol also targets nAChRs, stress-mediated alterations in nAChR function induced by nicotine could potentially influence subsequent sensitivity to alcohol.
Chronic drug exposure and persistent HPA activation may sensitize extra-hypothalamic CRF systems during drug withdrawal [245, 267]. The abrupt cessation of nicotine or alcohol intake after chronic exposure initiates a withdrawal syndrome characterized by negative affect, deficits in reward function, and a propensity for relapse [268, 269]. Elevated CRF signaling in the extended amygdala is hypothesized to mediate these symptoms, as CRF antagonists block their expression [270–272]. Even during protracted abstinence when withdrawal symptoms have subsided, the CRF circuitry remains sensitized to stress responses and increases the vulnerability to relapse [273].
6.3. Neuroactive Steroids
Another possible mechanism that could contribute to drug sensitivity is the modulation of GABAA receptors by neuroactive steroids. Initially, nicotine or alcohol exposure increases the levels of the allopregnanolone [255, 274], a progesterone-derived steroid that acts as a positive allosteric modulator of GABAA receptors. Like glucocorticoids, allopregnanolone has reinforcing properties, causes dopamine release, and induces conditioned place preference [275–277]. In addition, allopregnanolone contributes to the pharmacological action of alcohol [278, 279]. Evidence suggests that allopregnanolone-sensitive receptors mediate the discriminative stimulus effects of alcohol [280], promote alcohol consumption [281, 282], and contribute to alcohol tolerance [283]. Allopregnanolone also acts within the VTA, influencing alcohol-induced withdrawal behaviors [284]. Interestingly, nicotine dose-dependently increases allopregnanolone levels [255, 285], suggesting that nicotine could alter the responses to alcohol via allopregnanolone signaling.
In summary, stress hormones may act through multiple mechanisms to promote drug reinforcement, tolerance, and withdrawal. Because stress hormones and drugs of abuse target similar brain regions, adaptations mediated by neuroendocrine pathways are centrally linked to the development of nicotine and alcohol abuse. Therefore, detailed studies of the mechanisms by which stress hormones influence nicotine and alcohol interactions would advance this field.
7. Potential Interactions via Neuroimmune Pathways
Nicotine and alcohol could also interact through neuroimmune signaling pathways. The neuroimmune system consists of neurons that regulate immune responses, as well as the inflammatory signals that feedback to modulate neural function. During an immune response, foreign stimuli activate macrophages (peripheral) and microglia (central) of the innate immune system to elicit the release of inflammatory cytokines [286]. Importantly, the activation of immune signals is implicated in the actions of drugs of abuse [287].
Immune responses could mediate nicotine-alcohol interactions through direct and indirect mechanisms. In terms of a direct mechanism, α7 nAChRs are expressed on macrophages and microglia of the innate immune system [288]. These receptors mediate cholinergic anti-inflammatory pathways within the immune system [289]. Interestingly, alcohol can inhibit the function of α7 nAChRs [290], a receptor that mediates the responses to both nicotine and alcohol. To our knowledge, no experiments have examined the contribution of α7 nAChRs to alcohol’s inflammatory profile, but such work would be relevant to the neuroimmune hypothesis of alcohol abuse [291].
Repeated drug exposure potentially leads to complex interactions between nicotine, alcohol, and the immune system. During chronic nicotine exposure, for example, the activation of some proinflammatory pathways, such as IL-1β-mediated signaling, are required for nicotine’s immunosuppressive effects [292]. These same proinflammatory pathways can directly modulate alcohol intake [293, 294]. In fact, evidence now suggests that immune signaling in the VTA and amygdala is a factor in alcohol abuse [295, 296]. Stress hormones could mediate additional indirect interactions between nicotine, alcohol, and the immune system. Glucocorticoids potently interact with the inflammatory pathways [297–299] and modify the immune responses to nicotine and alcohol [297, 300]. Taken together, these findings warrant further research to explore the contribution of immune signaling to the development of nicotine and alcohol co-abuse.
8. Summary and Conclusions
Nicotine can influence the use of alcohol and vice versa. This multifaceted interaction arises from a combination of genetic, psychosocial, and neurobiological factors. Because of its central role in drug reinforcement, the mesolimbic DA system may represent an important locus for mechanistic interactions between nicotine and alcohol. Nicotine and alcohol share many common targets that directly and indirectly impinge on the mesolimbic DA system. Nicotine and alcohol influence GABAergic, glutamatergic, cholinergic, serotonergic, endocannabinoid, stress, and immune signaling pathways that regulate the DA system. These common pathways provide neural targets for nicotine and alcohol interactions. The complexity of the responses arises from the diverse actions of these drugs, which can differ between cell types and brain regions. Alcohol and nicotine modulate the function of numerous membrane-bound proteins, as well as neuroendocrine effectors, to influence inhibitory and excitatory transmission. Because of this inherent complexity, multiple mechanisms likely contribute to nicotine-alcohol interactions within and outside of the DA system.
The concurrent use of nicotine and alcohol may involve the modulation of nAChR transmission in concert with other acute mechanisms. Concurrent administration of nicotine and alcohol produces an additive increase in DA release in the NAc compared to each drug alone, suggesting that nicotine and alcohol in combination are more reinforcing. However, the fact that early age smoking is a risk factor for future alcohol abuse suggests that the interaction is multidimensional and involves long-term adaptive processes. Long-term adaptations in the DA and neuroendocrine systems may represent one important process. These systems could undergo a cross-tolerance to the effects of nicotine and alcohol, in which one drug alters the sensitivity to the other [25]. However, the cellular and system level events contributing to cross-tolerance remain unclear. To advance our understanding of these different interactions, future studies involving nicotine and alcohol should examine the functional relationships between these many signaling pathways.
Acknowledgments
The authors are supported by grants from the National Institutes of Health, NIDA DA09411 and NINDS NS21229, and the Cancer Prevention and Research Institute of Texas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- 1.WHO. WHO Report on the Global Tobacco Epidemic. World Health Organization; 2008. pp. 1–342. [Google Scholar]
- 2.WHO. Global Status Report on Alcohol and Health. World Health Organization; 2011. pp. 1–286. [Google Scholar]
- 3.Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Harrison EL, Desai RA, McKee SA. Nondaily smoking and alcohol use, hazardous drinking, and alcohol diagnoses among young adults: findings from the NESARC. Alcohol Clin Exp Res. 2008;32:2081–7. doi: 10.1111/j.1530-0277.2008.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG. Smoking status as a clinical indicator for alcohol misuse in US adults. Arch Intern Med. 2007;167:716–21. doi: 10.1001/archinte.167.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weitzman ER, Chen YY. The co-occurrence of smoking and drinking among young adults in college: national survey results from the United States. Drug Alcohol Depend. 2005;80:377–86. doi: 10.1016/j.drugalcdep.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Burling TA, Ziff DC. Tobacco smoking: a comparison between alcohol and drug abuse inpatients. Addict Behav. 1988;13:185–90. doi: 10.1016/0306-4603(88)90010-x. [DOI] [PubMed] [Google Scholar]
- 8.Batel P, Pessione F, Maitre C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977–80. doi: 10.1046/j.1360-0443.1995.90797711.x. [DOI] [PubMed] [Google Scholar]
- 9.DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–5. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- 10.Grant BF. Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:59–73. doi: 10.1016/s0899-3289(99)80141-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Unger JB, Palmer P, Weiner MD, Johnson CA, Wong MM, et al. Prior cigarette smoking initiation predicting current alcohol use: evidence for a gateway drug effect among California adolescents from eleven ethnic groups. Addict Behav. 2002;27:799–817. doi: 10.1016/s0306-4603(01)00211-8. [DOI] [PubMed] [Google Scholar]
- 12.Smith BR, Horan JT, Gaskin S, Amit Z. Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology (Berl) 1999;142:408–12. doi: 10.1007/s002130050906. [DOI] [PubMed] [Google Scholar]
- 13.Le AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl) 2003;168:216–21. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Moreno JA, Trigo-Diaz JM, Rodriguez de Fonseca F, Gonzalez Cuevas G, Gomez de Heras R, Crespo Galan I, et al. Nicotine in alcohol deprivation increases alcohol operant self-administration during reinstatement. Neuropharmacology. 2004;47:1036–44. doi: 10.1016/j.neuropharm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Kemppainen H, Hyytia P, Kiianmaa K. Behavioral consequences of repeated nicotine during adolescence in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin Exp Res. 2009;33:340–9. doi: 10.1111/j.1530-0277.2008.00838.x. [DOI] [PubMed] [Google Scholar]
- 16.Sharpe AL, Samson HH. Repeated nicotine injections decrease operant ethanol self-administration. Alcohol. 2002;28:1–7. doi: 10.1016/s0741-8329(02)00238-0. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell SH, de Wit H, Zacny JP. Effects of varying ethanol dose on cigarette consumption in healthy normal volunteers. Behav Pharmacol. 1995;6:359–65. [PubMed] [Google Scholar]
- 18.Glautier S, Clements K, White JA, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behav Pharmacol. 1996;7:144–54. [PubMed] [Google Scholar]
- 19.Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine Tob Res. 2004;6:133–44. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- 20.King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcohol Clin Exp Res. 2005;29:547–52. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- 21.Harrison EL, Hinson RE, McKee SA. Experimenting and daily smokers: episodic patterns of alcohol and cigarette use. Addict Behav. 2009;34:484–6. doi: 10.1016/j.addbeh.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobo JK, Husten C. Sociocultural influences on smoking and drinking. Alcohol Res Health. 2000;24:225–32. [PMC free article] [PubMed] [Google Scholar]
- 23.Schlaepfer IR, Hoft NR, Ehringer MA. The genetic components of alcohol and nicotine co-addiction: from genes to behavior. Curr Drug Abuse Rev. 2008;1:124–34. doi: 10.2174/1874473710801020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res Health. 2006;29:186–92. [PMC free article] [PubMed] [Google Scholar]
- 25.Collins AC, Wilkins LH, Slobe BS, Cao JZ, Bullock AE. Long-term ethanol and nicotine treatment elicit tolerance to ethanol. Alcohol Clin Exp Res. 1996;20:990–9. doi: 10.1111/j.1530-0277.1996.tb01936.x. [DOI] [PubMed] [Google Scholar]
- 26.Madden PA, Bucholz KK, Martin NG, Heath AC. Smoking and the genetic contribution to alcohol-dependence risk. Alcohol Res Health. 2000;24:209–14. [PMC free article] [PubMed] [Google Scholar]
- 27.Tracy HA, Jr, Wayner MJ, Armstrong DL. Nicotine blocks ethanol and diazepam impairment of air righting and ethanol impairment of maze performance. Alcohol. 1999;18:123–30. doi: 10.1016/s0741-8329(98)00074-3. [DOI] [PubMed] [Google Scholar]
- 28.Al-Rejaie S, Dar MS. Behavioral interaction between nicotine and ethanol: possible modulation by mouse cerebellar glutamate. Alcohol Clin Exp Res. 2006;30:1223–33. doi: 10.1111/j.1530-0277.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 29.Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology (Berl) 2008;196:483–95. doi: 10.1007/s00213-007-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–7. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–88. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 33.De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 2011;34:105–30. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 36.Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–31. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–90. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995;682:215–21. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- 39.Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–15. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–29. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, et al. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–56. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–33. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–35. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- 45.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–73. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–23. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 47.Massi L, Elezgarai I, Puente N, Reguero L, Grandes P, Manzoni OJ, et al. Cannabinoid receptors in the bed nucleus of the stria terminalis control cortical excitation of midbrain dopamine cells in vivo. J Neurosci. 2008;28:10496–508. doi: 10.1523/JNEUROSCI.2291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. The Journal of comparative neurology. 2005;490:270–94. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- 49.Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci. 2011;31:7811–6. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herve D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435:71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- 52.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–90. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–92. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 54.Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–81. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W, Doyon WM, Dani JA. Acute in vivo nicotine administration enhances synchrony among dopamine neurons. Biochem Pharmacol. 2011;82:977–83. doi: 10.1016/j.bcp.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- 57.Chergui K, Suaud-Chagny MF, Gonon F. Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 1994;62:641–5. doi: 10.1016/0306-4522(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Doyon WM, Clark JJ, Phillips PE, Dani JA. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 2009;76:396–404. doi: 10.1124/mol.109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo AH, Georges FE, Aston-Jones GS. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. Eur J Neurosci. 2008;27:408–22. doi: 10.1111/j.1460-9568.2007.05985.x. [DOI] [PubMed] [Google Scholar]
- 60.Brown MT, Tan KR, O’Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–6. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- 61.van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–94. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chuhma N, Choi WY, Mingote S, Rayport S. Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience. 2009;164:1068–83. doi: 10.1016/j.neuroscience.2009.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–46. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–84. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 65.Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–30. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–7. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- 67.Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–27. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tolu S, Eddine R, Marti F, David V, Graupner M, Pons S, et al. Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol Psychiatry. 2012;18:382–93. doi: 10.1038/mp.2012.83. [DOI] [PubMed] [Google Scholar]
- 69.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–8. [PubMed] [Google Scholar]
- 70.Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26:318–25. [PubMed] [Google Scholar]
- 71.Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–31. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- 72.Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93:1469–81. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- 73.Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, et al. Beer Flavor Provokes Striatal Dopamine Release in Male Drinkers: Mediation by Family History of Alcoholism. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109:92–8. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- 75.Samson HH, Hodge CW, Tolliver GA, Haraguchi M. Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: the involvement of the nucleus accumbens. Brain Res Bull. 1993;30:133–41. doi: 10.1016/0361-9230(93)90049-h. [DOI] [PubMed] [Google Scholar]
- 76.Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21:1083–91. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ikemoto S, McBride WJ, Murphy JM, Lumeng L, Li TK. 6-OHDA-lesions of the nucleus accumbens disrupt the acquisition but not the maintenance of ethanol consumption in the alcohol-preferring P line of rats. Alcohol Clin Exp Res. 1997;21:1042–6. [PubMed] [Google Scholar]
- 78.Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- 79.Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–21. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 80.Schilstrom B, Rawal N, Mameli-Engvall M, Nomikos GG, Svensson TH. Dual effects of nicotine on dopamine neurons mediated by different nicotinic receptor subtypes. Int J Neuropsychopharmacol. 2003;6:1–11. doi: 10.1017/S1461145702003188. [DOI] [PubMed] [Google Scholar]
- 81.Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–3. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- 82.Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–6. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- 83.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 84.Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167–72. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol. 2008;153 (Suppl 1):S438–45. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, et al. Distinct contributions of nicotinic acetylcholine receptor subunit {alpha}4 and subunit {alpha}6 to the reinforcing effects of nicotine. Proc Natl Acad Sci U S A. 2011;108:7577–82. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schilstrom B, Nomikos GG, Nisell M, Hertel P, Svensson TH. N-methyl-D-aspartate receptor antagonism in the ventral tegmental area diminishes the systemic nicotine-induced dopamine release in the nucleus accumbens. Neuroscience. 1998;82:781–9. doi: 10.1016/s0306-4522(97)00243-1. [DOI] [PubMed] [Google Scholar]
- 88.Larsson A, Edstrom L, Svensson L, Soderpalm B, Engel JA. Voluntary ethanol intake increases extracellular acetylcholine levels in the ventral tegmental area in the rat. Alcohol Alcohol. 2005;40:349–58. doi: 10.1093/alcalc/agh180. [DOI] [PubMed] [Google Scholar]
- 89.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–82. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 90.Bernier BE, Whitaker LR, Morikawa H. Previous ethanol experience enhances synaptic plasticity of NMDA receptors in the ventral tegmental area. J Neurosci. 2011;31:5205–12. doi: 10.1523/JNEUROSCI.5282-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bradberry CW. Dose-dependent effect of ethanol on extracellular dopamine in mesolimbic striatum of awake rhesus monkeys: comparison with cocaine across individuals. Psychopharmacology (Berl) 2002;165:67–76. doi: 10.1007/s00213-002-1233-9. [DOI] [PubMed] [Google Scholar]
- 92.Melendez RI, Rodd-Henricks ZA, McBride WJ, Murphy JM. Alcohol stimulates the release of dopamine in the ventral pallidum but not in the globus pallidus: a dual-probe microdialysis study. Neuropsychopharmacology. 2003;28:939–46. doi: 10.1038/sj.npp.1300081. [DOI] [PubMed] [Google Scholar]
- 93.Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. Disparity Between Tonic and Phasic Ethanol-Induced Dopamine Increases in the Nucleus Accumbens of Rats. Alcohol Clin Exp Res. 2009 doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Domino EF, Evans CL, Ni L, Guthrie SK, Koeppe RA, Zubieta JK. Tobacco smoking produces greater striatal dopamine release in G-allele carriers with mu opioid receptor A118G polymorphism. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:236–40. doi: 10.1016/j.pnpbp.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 96.Lecca D, Cacciapaglia F, Valentini V, Gronli J, Spiga S, Di Chiara G. Preferential increase of extracellular dopamine in the rat nucleus accumbens shell as compared to that in the core during acquisition and maintenance of intravenous nicotine self-administration. Psychopharmacology (Berl) 2006;184:435–46. doi: 10.1007/s00213-005-0280-4. [DOI] [PubMed] [Google Scholar]
- 97.Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–25. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schier CJ, Dilly GA, Gonzales RA. Intravenous ethanol increases extracellular dopamine in the medial prefrontal cortex of the long-evans rat. Alcohol Clin Exp Res. 2013;37:740–7. doi: 10.1111/acer.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–9. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 100.Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29:4035–43. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang J, Dani JA. Dopamine enables in vivo synaptic plasticity associated with the addictive drug nicotine. Neuron. 2009;63:673–82. doi: 10.1016/j.neuron.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–13. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 104.Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–9. [PubMed] [Google Scholar]
- 105.Lopez-Moreno JA, Scherma M, Rodriguez de Fonseca F, Gonzalez-Cuevas G, Fratta W, Navarro M. Changed accumbal responsiveness to alcohol in rats pre-treated with nicotine or the cannabinoid receptor agonist WIN 55,212-2. Neurosci Lett. 2008;433:1–5. doi: 10.1016/j.neulet.2007.11.074. [DOI] [PubMed] [Google Scholar]
- 106.Doyon WM, Zhang L, Zhang T, Dong Y, Placzek AN, Dani JA. Long lasting attenuation of ethanol-induced dopamine signals after in vivo nicotine exposure. Journal of Neurochemistry, Abstract 22nd Biennial Meeting of the International Society for Neurochemistry. 2009;110:1. [Google Scholar]
- 107.McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–46. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 108.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 109.Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–63. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–85. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–52. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–19. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 113.Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol. 1989;98:135–40. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–4. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 115.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–7. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 116.Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, et al. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–9. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:328–38. doi: 10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- 118.Gao M, Jin Y, Yang K, Zhang D, Lukas RJ, Wu J. Mechanisms involved in systemic nicotine-induced glutamatergic synaptic plasticity on dopamine neurons in the ventral tegmental area. J Neurosci. 2010;30:13814–25. doi: 10.1523/JNEUROSCI.1943-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mao D, Gallagher K, McGehee DS. Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J Neurosci. 2011;31:6710–20. doi: 10.1523/JNEUROSCI.5671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–25. [PubMed] [Google Scholar]
- 121.Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci. 1999;19:4804–14. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Walsh H, Govind AP, Mastro R, Hoda JC, Bertrand D, Vallejo Y, et al. Up-regulation of nicotinic receptors by nicotine varies with receptor subtype. J Biol Chem. 2008;283:6022–32. doi: 10.1074/jbc.M703432200. [DOI] [PubMed] [Google Scholar]
- 123.Alkondon M, Albuquerque EX. Nicotinic receptor subtypes in rat hippocampal slices are differentially sensitive to desensitization and early in vivo functional up-regulation by nicotine and to block by bupropion. J Pharmacol Exp Ther. 2005;313:740–50. doi: 10.1124/jpet.104.081232. [DOI] [PubMed] [Google Scholar]
- 124.Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, et al. Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–18. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–72. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23:130–6. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- 127.Darsow T, Booker TK, Pina-Crespo JC, Heinemann SF. Exocytic trafficking is required for nicotine-induced up-regulation of alpha 4 beta 2 nicotinic acetylcholine receptors. J Biol Chem. 2005;280:18311–20. doi: 10.1074/jbc.M501157200. [DOI] [PubMed] [Google Scholar]
- 128.Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, et al. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 129.Govind AP, Walsh H, Green WN. Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J Neurosci. 2012;32:2227–38. doi: 10.1523/JNEUROSCI.5438-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang F, Chen H, Steketee JD, Sharp BM. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacology. 2007;32:103–9. doi: 10.1038/sj.npp.1301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, et al. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–6. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jackson KM, Sher KJ, Cooper ML, Wood PK. Adolescent alcohol and tobacco use: onset, persistence and trajectories of use across two samples. Addiction. 2002;97:517–31. doi: 10.1046/j.1360-0443.2002.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rahman S, Zhang J, Engleman EA, Corrigall WA. Neuroadaptive changes in the mesoaccumbens dopamine system after chronic nicotine self-administration: a microdialysis study. Neuroscience. 2004;129:415–24. doi: 10.1016/j.neuroscience.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 134.Zhang L, Dong Y, Doyon WM, Dani JA. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol Psychiatry. 2012;71:184–91. doi: 10.1016/j.biopsych.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shiffman S. Reflections on smoking relapse research. Drug Alcohol Rev. 2006;25:15–20. doi: 10.1080/09595230500459479. [DOI] [PubMed] [Google Scholar]
- 136.Rezvani K, Teng Y, Shim D, De Biasi M. Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. J Neurosci. 2007;27:10508–19. doi: 10.1523/JNEUROSCI.3353-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dopico AM, Lovinger DM. Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev. 2009;61:98–114. doi: 10.1124/pr.108.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–9. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- 139.Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–52. [PubMed] [Google Scholar]
- 140.Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–26. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brodie MS, Appel SB. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin Exp Res. 1998;22:236–44. [PubMed] [Google Scholar]
- 142.Appel SB, Liu Z, McElvain MA, Brodie MS. Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J Pharmacol Exp Ther. 2003;306:437–46. doi: 10.1124/jpet.103.050963. [DOI] [PubMed] [Google Scholar]
- 143.Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci. 1999;2:1084–90. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- 144.Mulholland PJ, Becker HC, Woodward JJ, Chandler LJ. Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol Psychiatry. 2011;69:625–32. doi: 10.1016/j.biopsych.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boyle AE, Segal R, Smith BR, Amit Z. Bidirectional effects of GABAergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1993;46:179–82. doi: 10.1016/0091-3057(93)90338-t. [DOI] [PubMed] [Google Scholar]
- 146.Chester JA, Cunningham CL. GABA(A) receptor modulation of the rewarding and aversive effects of ethanol. Alcohol. 2002;26:131–43. doi: 10.1016/s0741-8329(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 147.Nowak KL, McBride WJ, Lumeng L, Li TK, Murphy JM. Blocking GABA(A) receptors in the anterior ventral tegmental area attenuates ethanol intake of the alcohol-preferring P rat. Psychopharmacology (Berl) 1998;139:108–16. doi: 10.1007/s002130050695. [DOI] [PubMed] [Google Scholar]
- 148.Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic delta-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci U S A. 2011;108:4459–64. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, et al. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–37. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- 150.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–9. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 151.Harris RA. Ethanol actions on multiple ion channels: which are important? Alcohol Clin Exp Res. 1999;23:1563–70. [PubMed] [Google Scholar]
- 152.Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–8. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- 153.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 154.Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol Clin Exp Res. 2008;32:1040–8. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 2009;329:625–33. doi: 10.1124/jpet.108.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–82. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–4. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- 158.Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–55. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- 159.Gallegos RA, Lee RS, Criado JR, Henriksen SJ, Steffensen SC. Adaptive responses of gamma-aminobutyric acid neurons in the ventral tegmental area to chronic ethanol. J Pharmacol Exp Ther. 1999;291:1045–53. [PubMed] [Google Scholar]
- 160.Doyon WM, Li W, Dani JA. Discrete excitation and inhibition of VTA neurons by ethanol in freely moving rats. San Diego, CA: Society for Neuroscience; 2010. p. Abstract 47316. [Google Scholar]
- 161.Steffensen SC, Walton CH, Hansen DM, Yorgason JT, Gallegos RA, Criado JR. Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacol Biochem Behav. 2009;92:68–75. doi: 10.1016/j.pbb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, et al. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–64. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Charlton ME, Sweetnam PM, Fitzgerald LW, Terwilliger RZ, Nestler EJ, Duman RS. Chronic ethanol administration regulates the expression of GABAA receptor alpha 1 and alpha 5 subunits in the ventral tegmental area and hippocampus. J Neurochem. 1997;68:121–7. doi: 10.1046/j.1471-4159.1997.68010121.x. [DOI] [PubMed] [Google Scholar]
- 164.Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 165.Lingford-Hughes AR, Wilson SJ, Cunningham VJ, Feeney A, Stevenson B, Brooks DJ, et al. GABA-benzodiazepine receptor function in alcohol dependence: a combined 11C-flumazenil PET and pharmacodynamic study. Psychopharmacology (Berl) 2005;180:595–606. doi: 10.1007/s00213-005-2271-x. [DOI] [PubMed] [Google Scholar]
- 166.Cosgrove KP, Esterlis I, Mason GF, Bois F, O’Malley SS, Krystal JH. Neuroimaging insights into the role of cortical GABA systems and the influence of nicotine on the recovery from alcohol dependence. Neuropharmacology. 2011;60:1318–25. doi: 10.1016/j.neuropharm.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Staley JK, Gottschalk C, Petrakis IL, Gueorguieva R, O’Malley S, Baldwin R, et al. Cortical gamma-aminobutyric acid type A-benzodiazepine receptors in recovery from alcohol dependence: relationship to features of alcohol dependence and cigarette smoking. Arch Gen Psychiatry. 2005;62:877–88. doi: 10.1001/archpsyc.62.8.877. [DOI] [PubMed] [Google Scholar]
- 168.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–4. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 169.Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Maldve RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippmann MJ, Snyder GL, et al. DARPP-32 and regulation of the ethanol sensitivity of NMDA receptors in the nucleus accumbens. Nat Neurosci. 2002;5:641–8. doi: 10.1038/nn877. [DOI] [PubMed] [Google Scholar]
- 171.Moykkynen T, Korpi ER, Lovinger DM. Ethanol inhibits alpha-amino-3-hydyroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function in central nervous system neurons by stabilizing desensitization. J Pharmacol Exp Ther. 2003;306:546–55. doi: 10.1124/jpet.103.050666. [DOI] [PubMed] [Google Scholar]
- 172.Zhu W, Bie B, Pan ZZ. Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci. 2007;27:289–98. doi: 10.1523/JNEUROSCI.3912-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mameli M, Zamudio PA, Carta M, Valenzuela CF. Developmentally regulated actions of alcohol on hippocampal glutamatergic transmission. J Neurosci. 2005;25:8027–36. doi: 10.1523/JNEUROSCI.2434-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Minami K, Gereau RWt, Minami M, Heinemann SF, Harris RA. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;53:148–56. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- 175.Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–70. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cowen MS, Djouma E, Lawrence AJ. The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005;315:590–600. doi: 10.1124/jpet.105.090449. [DOI] [PubMed] [Google Scholar]
- 177.Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–8. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- 178.Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–64. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- 179.Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, et al. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49 (Suppl 1):167–78. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 180.Xiao C, Shao XM, Olive MF, Griffin WC, 3rd, Li KY, Krnjevic K, et al. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2009;34:307–18. doi: 10.1038/npp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lallemand F, Ward RJ, De Witte P, Verbanck P. Binge drinking +/− chronic nicotine administration alters extracellular glutamate and arginine levels in the nucleus accumbens of adult male and female Wistar rats. Alcohol Alcohol. 2011;46:373–82. doi: 10.1093/alcalc/agr031. [DOI] [PubMed] [Google Scholar]
- 182.Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, et al. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–98. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- 183.Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology. 2006;31:988–96. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- 184.Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307:1020–9. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- 185.Trevisan L, Fitzgerald LW, Brose N, Gasic GP, Heinemann SF, Duman RS, et al. Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J Neurochem. 1994;62:1635–8. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- 186.Flatscher-Bader T, Zuvela N, Landis N, Wilce PA. Smoking and alcoholism target genes associated with plasticity and glutamate transmission in the human ventral tegmental area. Hum Mol Genet. 2008;17:38–51. doi: 10.1093/hmg/ddm283. [DOI] [PubMed] [Google Scholar]
- 187.Aistrup GL, Marszalec W, Narahashi T. Ethanol modulation of nicotinic acetylcholine receptor currents in cultured cortical neurons. Mol Pharmacol. 1999;55:39–49. doi: 10.1124/mol.55.1.39. [DOI] [PubMed] [Google Scholar]
- 188.Narahashi T, Aistrup GL, Marszalec W, Nagata K. Neuronal nicotinic acetylcholine receptors: a new target site of ethanol. Neurochem Int. 1999;35:131–41. doi: 10.1016/s0197-0186(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 189.Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–80. [PubMed] [Google Scholar]
- 190.Borghese CM, Henderson LA, Bleck V, Trudell JR, Harris RA. Sites of excitatory and inhibitory actions of alcohols on neuronal alpha2beta4 nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;307:42–52. doi: 10.1124/jpet.102.053710. [DOI] [PubMed] [Google Scholar]
- 191.Zuo Y, Nagata K, Yeh JZ, Narahashi T. Single-channel analyses of ethanol modulation of neuronal nicotinic acetylcholine receptors. Alcohol Clin Exp Res. 2004;28:688–96. doi: 10.1097/01.alc.0000125349.99823.8a. [DOI] [PubMed] [Google Scholar]
- 192.Nagata K, Aistrup GL, Huang CS, Marszalec W, Song JH, Yeh JZ, et al. Potent modulation of neuronal nicotinic acetylcholine receptor-channel by ethanol. Neurosci Lett. 1996;217:189–93. [PubMed] [Google Scholar]
- 193.Covernton PJ, Connolly JG. Differential modulation of rat neuronal nicotinic receptor subtypes by acute application of ethanol. Br J Pharmacol. 1997;122:1661–8. doi: 10.1038/sj.bjp.0701568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yoshida K, Engel J, Liljequist S. The effect of chronic ethanol administration of high affinity 3H-nicotinic binding in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1982;321:74–6. doi: 10.1007/BF00586353. [DOI] [PubMed] [Google Scholar]
- 195.Herring BE, Mayfield RD, Camp MC, Alcantara AA. Ethanol-induced Fos immunoreactivity in the extended amygdala and hypothalamus of the rat brain: focus on cholinergic interneurons of the nucleus accumbens. Alcohol Clin Exp Res. 2004;28:588–97. doi: 10.1097/01.alc.0000122765.58324.6d. [DOI] [PubMed] [Google Scholar]
- 196.Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–96. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- 197.Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–63. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- 198.Blomqvist O, Hernandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol Clin Exp Res. 2002;26:326–31. [PubMed] [Google Scholar]
- 199.O’Dell TJ, Christensen BN. Mecamylamine is a selective non-competitive antagonist of N-methyl-D-aspartate- and aspartate-induced currents in horizontal cells dissociated from the catfish retina. Neurosci Lett. 1988;94:93–8. doi: 10.1016/0304-3940(88)90276-5. [DOI] [PubMed] [Google Scholar]
- 200.Larsson A, Jerlhag E, Svensson L, Soderpalm B, Engel JA. Is an alpha-conotoxin MII-sensitive mechanism involved in the neurochemical, stimulatory, and rewarding effects of ethanol? Alcohol. 2004;34:239–50. doi: 10.1016/j.alcohol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 201.Jerlhag E, Grotli M, Luthman K, Svensson L, Engel JA. Role of the subunit composition of central nicotinic acetylcholine receptors for the stimulatory and dopamine-enhancing effects of ethanol. Alcohol Alcohol. 2006;41:486–93. doi: 10.1093/alcalc/agl049. [DOI] [PubMed] [Google Scholar]
- 202.Kuzmin A, Jerlhag E, Liljequist S, Engel J. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology (Berl) 2009;203:99–108. doi: 10.1007/s00213-008-1375-5. [DOI] [PubMed] [Google Scholar]
- 203.Hendrickson LM, Zhao-Shea R, Tapper AR. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology (Berl) 2009;204:563–72. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rezvani AH, Slade S, Wells C, Petro A, Lumeng L, Li TK, et al. Effects of sazetidine-A, a selective alpha4beta2 nicotinic acetylcholine receptor desensitizing agent on alcohol and nicotine self-administration in selectively bred alcohol-preferring (P) rats. Psychopharmacology (Berl) 2010;211:161–74. doi: 10.1007/s00213-010-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu L, Hendrickson LM, Guildford MJ, Zhao-Shea R, Gardner PD, Tapper AR. Nicotinic acetylcholine receptors containing the alpha4 subunit modulate alcohol reward. Biol Psychiatry. 2013;73:738–46. doi: 10.1016/j.biopsych.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 2010;208:613–26. doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kamens HM, Hoft NR, Cox RJ, Miyamoto JH, Ehringer MA. The alpha6 nicotinic acetylcholine receptor subunit influences ethanol-induced sedation. Alcohol. 2012;46:463–71. doi: 10.1016/j.alcohol.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dawson A, Miles MF, Damaj MI. The beta2 nicotinic acetylcholine receptor subunit differentially influences ethanol behavioral effects in the mouse. Alcohol. 2013;47:85–94. doi: 10.1016/j.alcohol.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM. Deletion of the alpha7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcohol Clin Exp Res. 2005;29:295–302. doi: 10.1097/01.alc.0000156116.40817.a2. [DOI] [PubMed] [Google Scholar]
- 210.Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–23. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, et al. Varenicline Reduces Alcohol Self-Administration in Heavy-Drinking Smokers. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ericson M, Lof E, Stomberg R, Soderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009;329:225–30. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- 213.Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–70. [PubMed] [Google Scholar]
- 214.Machu TK, Harris RA. Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther. 1994;271:898–905. [PubMed] [Google Scholar]
- 215.Zhou Q, Verdoorn TA, Lovinger DM. Alcohols potentiate the function of 5-HT3 receptor-channels on NCB-20 neuroblastoma cells by favouring and stabilizing the open channel state. J Physiol. 1998;507 (Pt 2):335–52. doi: 10.1111/j.1469-7793.1998.335bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wozniak KM, Pert A, Linnoila M. Antagonism of 5-HT3 receptors attenuates the effects of ethanol on extracellular dopamine. Eur J Pharmacol. 1990;187:287–9. doi: 10.1016/0014-2999(90)90015-x. [DOI] [PubMed] [Google Scholar]
- 217.Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol. 1996;13:569–74. doi: 10.1016/s0741-8329(96)00069-9. [DOI] [PubMed] [Google Scholar]
- 218.Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- 219.McKinzie DL, McBride WJ, Murphy JM, Lumeng L, Li TK. Effects of MDL 72222, a serotonin3 antagonist, on operant responding for ethanol by alcohol-preferring P rats. Alcohol Clin Exp Res. 2000;24:1500–4. [PubMed] [Google Scholar]
- 220.Rodd ZA, Bell RL, Oster SM, Toalston JE, Pommer TJ, McBride WJ, et al. Serotonin-3 receptors in the posterior ventral tegmental area regulate ethanol self-administration of alcohol-preferring (P) rats. Alcohol. 2010;44:245–55. doi: 10.1016/j.alcohol.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. Effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol within the ventral tegmental area of Wistar rats. Psychopharmacology (Berl) 2003;165:252–9. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- 222.Overstreet DH, McArthur RA, Rezvani AH, Post C. Selective inhibition of alcohol intake in diverse alcohol-preferring rat strains by the 5-HT2A antagonists amperozide and FG 5974. Alcohol Clin Exp Res. 1997;21:1448–54. [PubMed] [Google Scholar]
- 223.Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, et al. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology. 2012;37:1177–91. doi: 10.1038/npp.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.de Bruin NM, McCreary AC, van Loevezijn A, de Vries TJ, Venhorst J, van Drimmelen M, et al. A novel highly selective 5-HT6 receptor antagonist attenuates ethanol and nicotine seeking but does not affect inhibitory response control in Wistar rats. Behav Brain Res. 2013;236:157–65. doi: 10.1016/j.bbr.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 225.Parolaro D, Vigano D, Realini N, Rubino T. Role of endocannabinoids in regulating drug dependence. Neuropsychiatr Dis Treat. 2007;3:711–21. doi: 10.2147/ndt.s976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 227.Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105–16. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 228.Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164:206–13. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 229.Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277–82. [PubMed] [Google Scholar]
- 230.Gessa GL, Serra S, Vacca G, Carai MA, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcohol. 2005;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- 231.Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–63. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 232.Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–84. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 233.Malinen H, Hyytia P. Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2008;32:1976–83. doi: 10.1111/j.1530-0277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 234.Biala G, Budzynska B. Rimonabant attenuates sensitization, cross-sensitization and cross-reinstatement of place preference induced by nicotine and ethanol. Pharmacol Rep. 2010;62:797–807. doi: 10.1016/s1734-1140(10)70340-6. [DOI] [PubMed] [Google Scholar]
- 235.Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- 236.Steinberg MB, Foulds J. Rimonabant for treating tobacco dependence. Vasc Health Risk Manag. 2007;3:307–11. [PMC free article] [PubMed] [Google Scholar]
- 237.Selye H. Stress and the general adaptation syndrome. Br Med J. 1950;1:1383–92. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Armario A. Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: different pathways, common outcome. Trends Pharmacol Sci. 2010;31:318–25. doi: 10.1016/j.tips.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 239.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–71. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 240.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4:525–33. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 241.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–7. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wirth MM. Beyond the HPA Axis: Progesterone-Derived Neuroactive Steroids in Human Stress and Emotion. Front Endocrinol (Lausanne) 2011;2:19. doi: 10.3389/fendo.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 244.Albeck DS, Hastings NB, McEwen BS. Effects of adrenalectomy and type I or type II glucocorticoid receptor activation on AVP and CRH mRNA in the rat hypothalamus. Brain Res Mol Brain Res. 1994;26:129–34. doi: 10.1016/0169-328x(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 245.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–95. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- 246.Mani SK, Oyola MG. Progesterone signaling mechanisms in brain and behavior. Front Endocrinol (Lausanne) 2012;3:7. doi: 10.3389/fendo.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dubrovsky BO. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:169–92. doi: 10.1016/j.pnpbp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 248.Morrow AL, Devaud LL, Purdy RH, Paul SM. Neuroactive steroid modulators of the stress response. Ann N Y Acad Sci. 1995;771:257–72. doi: 10.1111/j.1749-6632.1995.tb44687.x. [DOI] [PubMed] [Google Scholar]
- 249.Bullock AE, Clark AL, Grady SR, Robinson SF, Slobe BS, Marks MJ, et al. Neurosteroids modulate nicotinic receptor function in mouse striatal and thalamic synaptosomes. J Neurochem. 1997;68:2412–23. doi: 10.1046/j.1471-4159.1997.68062412.x. [DOI] [PubMed] [Google Scholar]
- 250.Ke L, Lukas RJ. Effects of steroid exposure on ligand binding and functional activities of diverse nicotinic acetylcholine receptor subtypes. J Neurochem. 1996;67:1100–12. doi: 10.1046/j.1471-4159.1996.67031100.x. [DOI] [PubMed] [Google Scholar]
- 251.Majewska MD, Mienville JM, Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988;90:279–84. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- 252.Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991;40:333–6. [PubMed] [Google Scholar]
- 253.Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A. 1993;90:11738–42. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ellis FW. Effect of ethanol on plasma corticosterone levels. J Pharmacol Exp Ther. 1966;153:121–7. [PubMed] [Google Scholar]
- 255.Porcu P, Sogliano C, Cinus M, Purdy RH, Biggio G, Concas A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol Biochem Behav. 2003;74:683–90. doi: 10.1016/s0091-3057(02)01065-1. [DOI] [PubMed] [Google Scholar]
- 256.Wanat MJ, Bonci A, Phillips PE. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat Neurosci. 2013;16:383–5. doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc Natl Acad Sci U S A. 2011;108:18459–64. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Shoaib M, Shippenberg TS. Adrenalectomy attenuates nicotine-induced dopamine release and locomotor activity in rats. Psychopharmacology (Berl) 1996;128:343–50. doi: 10.1007/s002130050143. [DOI] [PubMed] [Google Scholar]
- 259.Fahlke C, Engel JA, Eriksson CJ, Hard E, Soderpalm B. Involvement of corticosterone in the modulation of ethanol consumption in the rat. Alcohol. 1994;11:195–202. doi: 10.1016/0741-8329(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 260.Fahlke C, Hansen S. Effect of local intracerebral corticosterone implants on alcohol intake in the rat. Alcohol Alcohol. 1999;34:851–61. doi: 10.1093/alcalc/34.6.851. [DOI] [PubMed] [Google Scholar]
- 261.Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–71. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, et al. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–93. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- 263.Caggiula AR, Epstein LH, Antelman SM, Saylor SS, Perkins KA, Knopf S, et al. Conditioned tolerance to the anorectic and corticosterone-elevating effects of nicotine. Pharmacol Biochem Behav. 1991;40:53–9. doi: 10.1016/0091-3057(91)90319-w. [DOI] [PubMed] [Google Scholar]
- 264.Cam GR, Bassett JR. Effect of prolonged exposure to nicotine and stress on the pituitary-adrenocortical response; the possibility of cross-adaptation. Pharmacol Biochem Behav. 1984;20:221–6. doi: 10.1016/0091-3057(84)90246-6. [DOI] [PubMed] [Google Scholar]
- 265.Boyd KN, Kumar S, O’Buckley TK, Morrow AL. Chronic ethanol exposure produces tolerance to elevations in neuroactive steroids: mechanisms and reversal by exogenous ACTH. J Neurochem. 2010;115:142–52. doi: 10.1111/j.1471-4159.2010.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Caggiula AR, Epstein LH, Antelman SM, Saylor S, Knopf S, Perkins KA, et al. Acute stress or corticosterone administration reduces responsiveness to nicotine: implications for a mechanism of conditioned tolerance. Psychopharmacology (Berl) 1993;111:499–507. doi: 10.1007/BF02253543. [DOI] [PubMed] [Google Scholar]
- 267.Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–43. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 268.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–9. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 269.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 270.Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009;66:110–7. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- 272.Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–66. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 274.Boyd KN, Kumar S, O’Buckley TK, Porcu P, Morrow AL. Ethanol induction of steroidogenesis in rat adrenal and brain is dependent upon pituitary ACTH release and de novo adrenal StAR synthesis. J Neurochem. 2010;112:784–96. doi: 10.1111/j.1471-4159.2009.06509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Finn DA, Phillips TJ, Okorn DM, Chester JA, Cunningham CL. Rewarding effect of the neuroactive steroid 3 alpha-hydroxy-5 alpha-pregnan-20-one in mice. Pharmacol Biochem Behav. 1997;56:261–4. doi: 10.1016/s0091-3057(96)00218-3. [DOI] [PubMed] [Google Scholar]
- 276.Rouge-Pont F, Mayo W, Marinelli M, Gingras M, Le Moal M, Piazza PV. The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens. Eur J Neurosci. 2002;16:169–73. doi: 10.1046/j.1460-9568.2002.02084.x. [DOI] [PubMed] [Google Scholar]
- 277.Rowlett JK, Winger G, Carter RB, Wood PL, Woods JH, Woolverton WL. Reinforcing and discriminative stimulus effects of the neuroactive steroids pregnanolone and Co 8–7071 in rhesus monkeys. Psychopharmacology (Berl) 1999;145:205–12. doi: 10.1007/s002130051050. [DOI] [PubMed] [Google Scholar]
- 278.Biggio G, Concas A, Follesa P, Sanna E, Serra M. Stress, ethanol, and neuroactive steroids. Pharmacol Ther. 2007;116:140–71. doi: 10.1016/j.pharmthera.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, et al. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23:1933–40. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- 280.Grant KA, Helms CM, Rogers LS, Purdy RH. Neuroactive steroid stereospecificity of ethanol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2008;326:354–61. doi: 10.1124/jpet.108.137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav Brain Res. 2007;179:265–72. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–12. [PubMed] [Google Scholar]
- 283.Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22:2055–61. [PubMed] [Google Scholar]
- 284.Tanchuck MA, Cozzoli DK, He I, Kaufman KR, Snelling C, Crabbe JC, et al. Local changes in neurosteroid levels in the substantia nigra reticulata and the ventral tegmental area alter chronic ethanol withdrawal severity in male withdrawal seizure-prone mice. Alcohol Clin Exp Res. 2013;37:784–93. doi: 10.1111/acer.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Marx CE, Trost WT, Shampine L, Behm FM, Giordano LA, Massing MW, et al. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology (Berl) 2006;186:462–72. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]
- 286.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17:6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther. 2012;134:219–45. doi: 10.1016/j.pharmthera.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 288.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–43. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 289.Cui WY, Li MD. Nicotinic modulation of innate immune pathways via alpha7 nicotinic acetylcholine receptor. J Neuroimmune Pharmacol. 2010;5:479–88. doi: 10.1007/s11481-010-9210-2. [DOI] [PubMed] [Google Scholar]
- 290.Yu D, Zhang L, Eisele JL, Bertrand D, Changeux JP, Weight FF. Ethanol inhibition of nicotinic acetylcholine type alpha 7 receptors involves the amino-terminal domain of the receptor. Mol Pharmacol. 1996;50:1010–6. [PubMed] [Google Scholar]
- 291.Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: a key component of alcohol abuse. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Razani-Boroujerdi S, Langley RJ, Singh SP, Pena-Philippides JC, Rir-sima-ah J, Gundavarapu S, et al. The role of IL-1beta in nicotine-induced immunosuppression and neuroimmune communication. J Neuroimmune Pharmacol. 2010;6:585–96. doi: 10.1007/s11481-011-9284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2011;17:108–20. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25 (Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–58. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr, et al. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci U S A. 2004;108:4465–70. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Frank MG, Watkins LR, Maier SF. Stress- and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun. 2011;25 (Suppl 1):S21–8. doi: 10.1016/j.bbi.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 299.Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, et al. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res. 2000;859:193–201. doi: 10.1016/s0006-8993(99)02443-9. [DOI] [PubMed] [Google Scholar]
- 300.Glover M, Pruett SB. Role of corticosterone in immunosuppressive effects of acute ethanol exposure on Toll-like receptor mediated cytokine production. J Neuroimmune Pharmacol. 2006;1:435–42. doi: 10.1007/s11481-006-9037-z. [DOI] [PubMed] [Google Scholar]