Abstract
The synthesis and operation of a light-operated nanovalve that controls the pore openings of mesoporous silica nanoparticles containing gold cores nanoparticles is described. The nanoparticles, consisting of 20 nm gold cores inside ~150 nm mesoporous silica spheres, were synthesized using a unique one-pot method. The nanovalves are comprised of cucurbit[6]uril rings encircling stalks that are attached to the ~2 nm pore openings. Plasmonic heating of the gold core raises the local temperature and decreases the ring-stalk binding constant, thereby unblocking the pore and releasing the cargo molecules that were preloaded inside. Bulk heating of the suspended particles to 60 °C is required to release the cargo, but no bulk temperature change was observed in the plasmonic heating release experiment. High intensity irradiation caused thermal damage to the silica particles, but low intensity illumination caused a sufficient local temperature increase to operate the valves without damaging the nanoparticles containers. These light-stimulated, thermally activated mechanized nanoparticles demonstrate a new system with potential utility for on-command drug release.
Multifunctional drug delivery systems are currently being studied intensively because of their potential to combine multiple essential properties in a single nanovehicle.1-4 The ability to control the location, time, and amount of drug released are important in nanomedicine.5,6 In the specific case of photothermal control of the release, multifunctional nanoparticles combining the photothermal heating of metal particles that have plasmonic properties with core or shell nanoparticles that have drug-carrying capability as specific remote triggered-release have been exploited with bare gold nanoparticles,7,8 core@shellAu@Liposome,9,10 Au@poly-electrolytes-multilayers@Lipid,11 polymer@Au,12 and Silica nanorattle@Mesoporoussilica@Au.3 None of these systems are robust nanocarriers that prevent premature release because of drug leakage through phospholipid membranes, polymer irregularities, and shell imperfections respectively. Very recently, gold nanorods were coated with mesoporous silica to photothermally release doxorubicin electrostatically trapped in the pores, but premature leakage from the uncapped pores before irradiation was severe.13
Mesoporous silica nanoparticles (MSNs) have been shown to be non-toxic,14,15 are taken up (endocytosed) by cells,16-18 and are able to transport various drugs.19-21 Many gate-keeping mechanisms have been developed and attached to MSN pore openings to trap the drug payload.4,22 As a result, a remarkable variety of mesoporous silica nanocarriers have been designed with both autonomous activation (pH- or redox opening of nano- valves 23-25 and external (light or magnetic field) control.26-28 Note that in most of these on-command release systems, precise spatial control cannot be achieved.
In this communication we report the synthesis and successful operation of nanovalves on mesoporous silica nanoparticles that are remotely controlled by light by using a photo-thermal mechanism involving plasmonic properties of a gold nanoparticle core. During the course of this study we discovered a facile one-pot synthesis of gold nanoparticles embedded in the mesoporous silica matrix, Au@MSN, through the autoreduction of tetrachloroaurate ions in the presence of the CTAB that also induces the template directed assembly. This one-pot synthesis is a faster and greener preparation of such nanovehicles than the multistep methods previously reported.29 Irradiation of the Au@MSNs that are mechanized with nanovalves at wavelengths corresponding to the plasmon resonance of the gold core causes internal heating and subsequent opening of the nanovalve that allow the contents of the pore to escape. Investigation of the state of the particles after release showed that they remain intact at moderate light intensities but that some degradation occurs at high intensity.
The nearly monodisperse Au@MSN nanoparticles (Figures 1A-D) were obtained through the condensation of tetraethoxysilane on freshly prepared gold Nanoparticles (21 ± 4 nm) in a basic water/ethanol mixture. The formation of the gold nanoparticles was surprising because no additional reducing agent was provided. The reduction of the gold precursor was sensitively dependent on the precise experimental conditions (basic pH, order of introduction of the reactants, and temperature, see table SI). Interestingly, the injection of sodium hydroxide was found to be necessary to obtain the gold nanoparticles. Previously studies reported the reduction of tetrachloroaurate ions via quaternary ammonium ions under γ-irradiation,30 and the encapsulation of gold nanoparticles via a similar autoreduction in the presence of a cross-linked tertiary amine polymer.31
Figure 1.
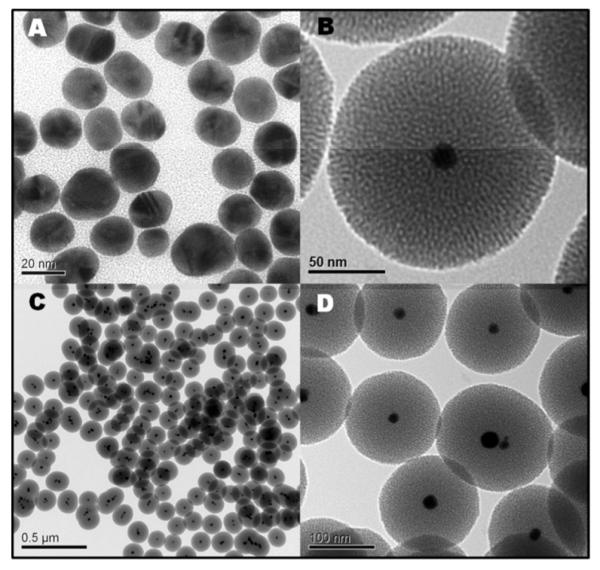
Transmission electron microscopy images of the gold Nanoparticles prepared by the CTAB mediated autoreduction of tetrachloroaurate ions (A), and of the final gold@mesoporous silica Nanoparticles from the one-pot synthesis (B-D).
The mechanism of operation of the molecular machine involves temperature-dependent non-covalent interactions between the stalk and the cucurbit[6]uril ring (Figure 2). The two ammonium groups of the stalk interact with the carbonyl groups of the cucurbituril via hydrogen bonds, while the alkyl chain of the thread interacts with the hydrophobic cucurbit[6]uril core through London forces. The stalk-ring binding constant decreases exponentially with the temperature, so that at 25°C the cucurbit[6]uril rings dwell on the threads, but at 60°C these rings slip off and open the pores (Figure 2A).
Figure 2.
(A) External heating of a suspension of God@MSN@Valve to 60 °C causes dissociation of the cucubituril caps from the stalks and release of the cargo molecules from the pores. (B) The release profile caused by bulk thermal activation of the nanomachines.
The molecular mechanization was performed by condensing N-(6-N-Aminohexyl)aminomethyltriethoxysilane stalks on the silica porous surface in dried toluene (see SSNMR 13C and 29Si figure S5, SI).28 Then the rhodamine B cargo was loaded by soaking the CTAB-extracted Gold@ValveMSN-thread nanoparticles in a concentrated aqueous solution. Finally the pores were closed by complexing cucurbit[6]uril on the stalks (Fig. S1, SI). This step was performed by adding cucurbit-6-uril (and sodium chloride to increase its solubility) to the previous rhodamine loading solution in order to avoid the loss of cargo molecules during the pore capping process.
The thermal operation of the machine was assessed in a control experiment (without light) by heating the solution. The dye loaded Gold@MSN@valve nanomachines were placed in the bottom of a glass cuvette filled with water and heated on a hot-plate. The release of cargo molecules was monitored by a probe diode laser (448 nm, 18 mW) irradiating the upper part of the cuvette and a CCD detector to measure the fluorescence of the dye that escaped from the pores. This experiment showed that a temperature of 60°C or higher was required to induce the release by disrupting the supramolecular temperature-dependent thread-ring interactions (Figure 2A-B). At room temperature the release profile exhibits a flat baseline characteristic of a non-leaky carrier, which validates its usefulness as a robust drug delivery system without premature leakage of the cargo.
Operation of the system by photo-induced internal heating was studied in a similar manner except that no external heating of the solution occurred. Figure 3A illustrates schematically the surface plasmon effect induced by an appropriate laser irradiation on the Gold@MSN@Valve, which produces a photo-thermal conversion of the laser energy. The mechanism involves the internal temperature increase inside the particle that is produced by the photo-thermal effect in order to disrupt the thread-ring interactions and release the cargo. The dye loaded particles were placed in a corner of a glass cuvette as was done in the bulk external heating control experiments, but the sample was irradiated at 514 nm (100 mW) to excite the gold cores at their plasmon band maximum at 530 nm (measured by the extinction spectrum Figure S3C in the SI). The release of cargo molecules was monitored by a probe diode laser (448 nm, 18 mW) irradiating the upper part of the cuvette, and a CCD detector to measure the dye fluorescent emission. The release profile displays the laser triggered instantaneous release of rhodamine B under irradiation, thus demonstrating the control of a temporal remote-photothermal release of cargo molecules encapsulated in Gold@MSN nanocarriers.
Figure 3.

Photothermal-induced internal heating of Gold@MSN@Valve through the surface plasmon effect of the gold cores releases dye molecules under power-dependant laser actuation. The release profiles were normalized to the plateaus representing the maximum amount of released dye. The maximum (2.5 %wt) varied according to the geometry of the experiments and the laser power. The rate of release increased with laser power.
To verify that the local temperature increase in the nanoparticles (rather than an increase of the temperature of the bulk solvent) is responsible for the cargo release, the solvent temperature was monitored during the photothermal-induced release experiment. The solution temperature remained unchanged within experimental error during experimental runs as long as ten hours. These results show that the heat necessary for uncapping the pore and releasing the cargo was provided by the very local hearing of the nanoparticles by photo-thermal conversion of the laser electromagnetic energy. Local temperatures in the vicinity of the stalk and cap must reach at least 60 °C in Au@MSN. Such a local temperature increase should be very useful to apply these nanomachines for spatially controlled dual therapy: by delivering the cargo to cells, and by necrosis through hyperthermia. This dual use of plasmonic heating distinguishes this light sensitive nanomachine from others based on chromophores alone and may be advantageous for increasing killing efficiency.32, 26-27
An alternative mechanism for releasing the cargo could be thermal damage to the silica shell itself. The nanocarriers were analyzed after photo-irradiation and cargo release by transmission electron microscopy (Figure 4 A-B). Under the highest irradiation intensity (100 mW), some of the particles were severely degraded. Thus some of the cargo release could have been produced by cracking the silica rather than by the opening of the nanovalves. As a control, bare MSN nanoparticles (no gold core) were irradiated at 514 nm (100 mW for 14h), and no silica damage was observed, confirming that only the gold embedded nanoparticles were light-sensitive (Fig S4, SI). Note that the addition of sodium hydroxide aliquots at the end of this laser irradiation both confirmed that the nanoparticles were loaded and capped with Stalk-CB[6] nanomachines. Release experiments were carried out at lower power (15 mW), with equal irradiation times, and the release profiles were characterized similarly (Figure 3B). The release was slower at lower power as expected. The particles were undamaged (Figure 4B). Thus it is clear that plasmonic heating at high light intensities produces enough heat or a rapid enough temperature change to degrade the silica, but that lower powers do not damage the silica but do cause enough of a local temperature change to open the valves and release the contents.
Figure 4.

TEM images of Nanoparticles irradiated with 100 mW (A), and 15 mW (B).
In summary, we have demonstrated that novel gold-core mesoporous silica nanoparticles are effective in actuating a thermosensitive nanovalve under exposure to laser irradiation. The release mechanism was demonstrated to be caused by local internal heat produced by the photo-thermal conversion of the light energy, and not a bulk temperature increase. A novel one-pot synthesis of the Au@MSN Nanoparticles was presented. The mechanized Au@MSN Nanoparticles enabled remotely controlled triggered release of the cargo molecules “on command” via a robust matrix without premature leaking. This novel nanocarrier fulfills the strict criterion of controlled release of cargo molecules at a desired time in a specified spatial location that is a significant advancement for nanomedicine. It is envisioned that Au@MSN nanomachines could efficiently kill cancer cells through hyperthermia, as well as synergistically enhancing the cytotoxicity of drugs such as docetaxel.33
Supplementary Material
ACKNOWLEDGMENT
The research was supported by the US National Institute of Health grant NIH R01-133697, the French American Cultural Exchange-Partner University Fund grant FACE-PUF 20091853 and the Agence Nationale de la Recherche (ANR-2010-NANO-022-01). The authors thank Min Xue for assistance in obtaining and interpreting the solid state NMR spectrum, and Lorraine Raboin for helpful discussions.
Footnotes
Supporting Information. Experimental details, UV-VISIBLE spectra of Gold@MSN compounds. Solid state NMR of 13C and 29Si of Gold@MSN@Thread compounds. Transmission electron microscopy images of MSN irradiated under 200 mW, and the MSN@Valve control laser experiment.
REFERENCES
- (1).Torchilin VP. Advanced Drug Delivery Reviews. 2006;58:1532. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- (2).Sanvicens N, Marco MP. Trends in Biotechnology. 2008;26:425. doi: 10.1016/j.tibtech.2008.04.005. [DOI] [PubMed] [Google Scholar]
- (3).Liu H, Chen D, Li L, Liu T, Tan L, Wu X, Tang F. Angew. Chem. Int. Ed. 2011;50:891. doi: 10.1002/anie.201002820. [DOI] [PubMed] [Google Scholar]
- (4).Li Z, Barnes JC, Boscoy A, Stoddart JF, Zink JI. Chem. Soc. Rev. 2012;41:2590–2605. doi: 10.1039/c1cs15246g. [DOI] [PubMed] [Google Scholar]
- (5).Liu Y, Miyoshi H, Nakamura M. Int. J. Cancer. 2007;120:2527. doi: 10.1002/ijc.22709. [DOI] [PubMed] [Google Scholar]
- (6).De Jong WH, Borm PJA. Inter. J. Nano. 2008;2:133. [Google Scholar]
- (7).Jones MR, Millstone JE, Giljohann DA, Seferos DS, Young KL, Mirkin CA. ChemPhyChem. 2009;10:1461. doi: 10.1002/cphc.200900269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Poon L, Zandberg W, Hsiao D, Erno Z, Sen D, Gates B, D., Branda N,R. ACS Nano. 2010;4:6395. doi: 10.1021/nn1016346. [DOI] [PubMed] [Google Scholar]
- (9).Anderson LJE, Hansen E, Lukianova-Hleb YE, Hafner JH, Lapotko OD. J. Controlled Release. 2010;144:151. doi: 10.1016/j.jconrel.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Paasonen L, Laaksonen L, Johans C, Yliperttula M, Kontturi K, Urtti A. J. Controlled Release. 2007;122:86. doi: 10.1016/j.jconrel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- (11).Angelatos SA, Radt B, Caruso F. J. Phys. Chem. B. 2005;109:3071. doi: 10.1021/jp045070x. [DOI] [PubMed] [Google Scholar]
- (12).Park H, Yang J, Seo S, Kim K, Suh J, Kim D, Haam S, Yoo K-H. Small. 2008;4:192. doi: 10.1002/smll.200700807. [DOI] [PubMed] [Google Scholar]
- (13).Zhang Z, Wang L, Wang J, Jiang X, Li X, Hu Z, Ji Y, Wu X, Chen C. Adv. Mater. 2012;24:1418–1423. doi: 10.1002/adma.201104714. [DOI] [PubMed] [Google Scholar]
- (14).Lin Y-S, Wu S-H, Hung Y, Chou Y-H, Chang C, Lin M-L, Tsai C-P, Mou C-Y. Chem. Mater. 2006;18:5170. [Google Scholar]
- (15).Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. ACS Nano. 2008;2:889. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Slowing I, Trewyn BG, Lin VS-Y. J. Am. Chem. Soc. 2006;128:14792. doi: 10.1021/ja0645943. [DOI] [PubMed] [Google Scholar]
- (17).Lu J, Liong M, Zink JI, Tamanoi F. Small. 2007;3:1341. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- (18).Trewyn BG, Slowing II, Giri S, Chen HT, Lin VSY. Acc. Chem. Res. 2007;40:846–853. doi: 10.1021/ar600032u. [DOI] [PubMed] [Google Scholar]
- (19).Slowing II, Trewyn BG, Giri S, Lin VS-Y. Adv. Funct. Mater. 2007;17:1225–1236. [Google Scholar]
- (20).Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE. ACS Nano. 2010;4:4539. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Vallet-Regí M, Francisco B, Daniel A. Angew. Chem. Int. Ed. Engl. 2007;46:7548–7558. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- (22).Ambrogio MW, Thomas CR, Zhao YL, Zink JI, Stoddart JF. Accounts Of Chemical Research. 2011;44:903. doi: 10.1021/ar200018x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Angelos S, Khashab NM, Yang Y-W, Trabolsi A, Khatib HA, Stoddart JF, Zink JI. J. Am. Chem. Soc. 2009;131:12912. doi: 10.1021/ja9010157. [DOI] [PubMed] [Google Scholar]
- (24).Meng H, Xue M, Xia T, Zhao T, Tamanoi T, Stoddart JF, Zink JI, Nel AE. J. Am. Chem. Soc. 2010;132:12690. doi: 10.1021/ja104501a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Liu R, Zhao X, Wu T, Feng P. J. Am. Chem. Soc. 2008;130:14418–14419. doi: 10.1021/ja8060886. [DOI] [PubMed] [Google Scholar]
- (26).Lu J, Choi E, Tamanoi F, Zink JI. Small. 2008;4:421. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Angelos S, Choi E, Vogtle F, DeCola L, Zink JI. J. Phys. Chem. C. 2007;111:6587. [Google Scholar]
- (28).Thomas CR, Ferris DP, Lee J-H, Choi E, Cho MH, Kim ES, Stoddart JF, Shin JS, Cheon J, Zink JI. J. Am. Chem. Soc. 2010;132:10623. doi: 10.1021/ja1022267. [DOI] [PubMed] [Google Scholar]
- (29).Liu S, Han M-Y. Chem. Asian J. 2010;5:36. doi: 10.1002/asia.200900228. [DOI] [PubMed] [Google Scholar]
- (30).Chen S, Liu Y, Wu G. Nanotechnology. 2005;16:2360. doi: 10.1088/0957-4484/16/10/061. [DOI] [PubMed] [Google Scholar]
- (31).Oishi M, Hayashi H, Uno T, Ishii T, Iijima M, Nagasaki Y. Macromol. Chem. Phys. 2007;208:1176. [Google Scholar]
- (32).Mal NK, Fujiwara M, Tanaka Y. Nature. 2003;421:350–353. doi: 10.1038/nature01362. [DOI] [PubMed] [Google Scholar]
- (33).Mohamed F, Marchettini P, Stuart A, Urano M, Sugarbaker PH. Ann. Surg. Oncol. 2003;10:463. doi: 10.1245/aso.2003.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



