Abstract
Background
Individualizing arterial blood pressure (ABP) targets during cardiopulmonary bypass (CPB) based on cerebral blood flow (CBF) autoregulation monitoring may provide a more effective means for preventing cerebral hypoperfusion than the current standard of care. Autoregulation can be monitored in real-time with transcranial Doppler (TCD). We have previously demonstrated that near infrared spectroscopy (NIRS) derived regional cerebral oxygen saturation (rScO2) provides a clinically suitable surrogate of CBF for autoregulation monitoring. The purpose of this study was to determine the accuracy of a stand-alone “plug-and-play” investigational system for autoregulation monitoring that uses a commercially available NIRS monitor with TCD methods.
Methods
TCD monitoring of middle cerebral artery CBF velocity and NIRS monitoring was performed in 70 patients during CPB. Indices of autoregulation were computed by both a personal computer-based system and an investigational prototype NIRS-based monitor. A moving linear correlation coefficient between slow waves of ABP and CBF velocity (mean velocity index, M×) and between ABP and rScO2 (cerebral oximetry index, CO×) were calculated. When CBF is autoregulated, there is no correlation between CBF and ABP; when CBF is dysregulated, M× and CO× approach 1 (i.e., CBF and ABP are correlated). Linear regression and bias analysis was performed between time-averaged values of M× and CO× derived from the personal computer-based system and from CO× measured with the prototype monitor. Values for M× and CO× were categorized in 5 mmHg bins of ABP for each patient. The lower limit of CBF autoregulation) was defined as the ABP where M× incrementally increased to ≥ 0.4.
Results
There was correlation and good agreement between CO× derived from the prototype monitor and M× (r=0.510, 95% confidence interval [CI], 0.414 to 0.595, p<0.001; bias -0.07 ± 0.19). The correlation and bias between the personal computer-based CO× and CO× from the prototype NIRS monitor were r=0.957, 95% CI, 0.945 to 0.966, p<0.001 and 0.06±0.06, respectively. The average ABP at the lower limit of autoregulation was 63 ± 11 mmHg (95% prediction interval, 52 to 74 mmHg mmHg). While the mean ABP at the CO×-determined lower limit of autoregulation determined with the prototype monitor was statistically different from that determined by M× (59 ± 9 mmHg, 95% prediction interval, 50 to 68 mmHg, p=0.026), the difference is not likely clinically meaningful.
Conclusions
Monitoring CBF autoregulation with an investigational stand-alone NIRS monitor is correlated and in good agreement with TCD based methods. Availability of such a device would allow wide-spread autoregulation monitoring as a means of individualizing ABP targets during CPB.
Monitoring of cerebral blood flow (CBF) autoregulation with a moving linear regression correlation coefficient between blood pressure and middle cerebral artery transcranial Doppler (TCD) measured blood flow velocity has been validated in volunteers and in patients with head trauma, carotid artery stenosis, acute ischemic stroke, subarachnoid hemorrhage, and those undergoing cardiac surgery.1-5 By allowing clinicians to individualize arterial blood pressure (ABP) targets, monitoring of autoregulation may have benefits for improving neurological outcomes for patients undergoing cardiac surgery with cardiopulmonary bypass (CPB) whom have a high prevalence of cerebral vascular disease.
There is no “gold standard” for measuring CBF to determine autoregulation.6 Monitors of brain oxygenation, such as direct tissue O2 tension and jugular bulb O2 saturation measurements have been used as surrogates of CBF for monitoring autoregulation.6,7 Near-infrared spectroscopy (NIRS) is increasingly used during cardiac surgery to monitor regional cerebral oxygenation (rScO2). Since these measurements are weighted toward venous blood, regional cerebral O2 saturation is an indicator of the adequacy of cerebral O2 supply versus demand. We have found that rScO2 provides a clinically acceptable surrogate of CBF for experimental and clinical autoregulation monitoring.5,8,9 Unlike transcranial Doppler methods, the use of rScO2 as a proxy for CBF does not require a trained technician, is non-invasive and continuous and, thus, could be widely applied in a broad range of clinical settings. Our previously validated methods, though, require complex signal processing and analysis using specialized software and a personal computer that limits its applications mostly to research. The availability of a stand-alone, “plug-and-play” system for monitoring cerebral autoregulation would provide clinicians with a method for optimizing ABP during CPB and in other clinical areas.
The purpose of this study was to evaluate the accuracy of an investigational prototype NIRS cerebral autoregulation monitor with specialized hardware and software compared with transcranial Doppler methods. We hypothesize that the average autoregulation indices obtained during CPB obtained using an investigational prototype NIRS monitor will be correlated and have good agreement with those calculated using standard Doppler methods. We further hypothesize that the lower limit of CBF autoregulation determined using the investigational prototype NIRS autoregulation monitor will be equivalent with that obtained using transcranial Doppler autoregulation methods. A secondary aim of the study was to compare NIRS autoregulation indices from the prototype monitor with our standard personal computer-based methods.
Methods
Using a protocol approved by the Johns Hopkins Medical Institutes research review board, and after receiving written informed consent, 70 patients undergoing cardiac surgery at The Johns Hopkins Hospital with CPB between July 30, 2010 and July 29, 2011 were enrolled in this study. Patient care during surgery including management during CPB were similar to our prior reports.5,9,10 Briefly, the patients received midazolam, fentanyl, pancuronium, and isoflurane for anesthesia and muscle relaxation. The patient's blood pressure was monitored with a direct radial artery catheter. Non-pulsatile CPB was with a non-occlusive roller pump with flows of 2.0 and 2.4 L/min/m2 and a membrane oxygenator. Alpha-stat pH management was used and the patients were monitored with continuous in-line arterial blood gas monitoring calibrated hourly with arterial blood gas measurements. Blood pressure during CPB was based on usual institutional practice. Clinicians caring for the patients were blinded to the autoregulation monitoring data.
Autoregulation Monitoring
Autoregulation measurements were observed during spontaneous fluctuations in blood pressure that occur during the conduct of cardiac surgery. No medications or maneuvers were performed to manipulate blood pressure for measuring autoregulation. Bilateral middle cerebral artery CBF velocity was monitored with transcranial Doppler (Doppler Box, DWL, Compumedics, Charlotte, NC) using two 2.5-MHz transducers held in place with brackets fitted on a headband. Depth of insonation was varied between 35 and 52 mm until representative spectral middle cerebral artery flow was identified and the probes slightly manipulated to obtain the maximal flow signal. The TCD signals were monitored throughout the procedure to ensure that the probes remained appropriately positioned. Bilateral rScO2 was monitored by means of a NIRS monitor (Somanetics INVOS, Covidien, Boulder, CO) using sensors placed on the right and left forehead. Baseline calibration was performed while the patients were breathing room air. The algorithm for derivation of rScO2 have been described.11 Arterial blood pressure was obtained from the operating room hemodynamic monitor (GE Medical, Milwaukee, WI). The ABP and TCD signals were processed by using a personal computer-based system using ICM+ software (University of Cambridge, Cambridge, UK) to compute reference indices of autoregulation as previously described.5,9,10 The ABP and rScO2 signals from the same Invos™ were also processed by an investigational prototype NIRS-based monitor (Covidien, Boulder, CO) with customized software running on a multi-parameter monitoring system (VitalSync, Covidien, Boulder, CO), which computed a second index of autoregulation (CO×) for evaluation. Specifically, the analog ABP signal from the operating room monitor and TCD signals were connected to an analog-to-digital convertor directly connected to the personal computer-based system. Using a customized cable the ABP signal from the hemodynamic monitor was simultaneously connected directly to the prototype NIRS-based monitor containing an internal analog-to-digital convertor. The digital output of the latter was then processed by the prototype autoregulation monitor. A schemata of the signal acquisition system is shown in Figure 1.
Figure 1.
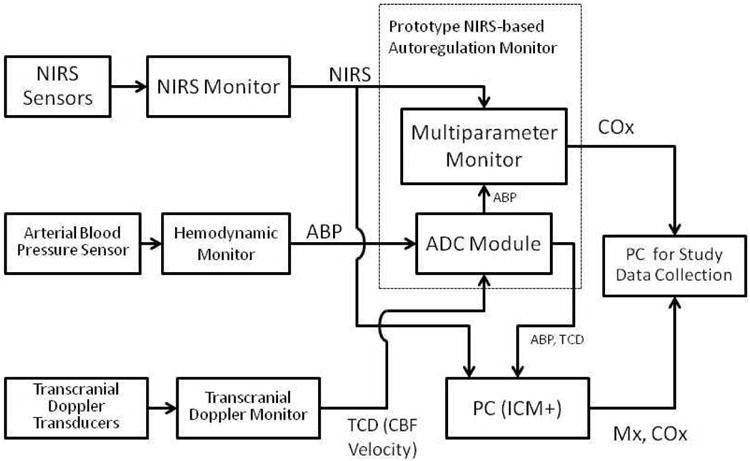
A schematic diagram of the prototype near infrared spectroscopy (NIRS) based autoregulation monitor and the additional equipment used in the study. Digital signals from the same standard Invos™ 5100 monitor (Covidien, Boulder, CO) were simultaneously sampled by the personal computer (pc) based system and the prototype monitor. Arterial blood pressure (ABP) signals were digitized with an analog-to-digital convertor (ADC) that was internal for prototype monitor. Mean velocity index (M×) and cerebral oximetry index (CO×) cerebral oximetry index were then calculated as the Pearson correlation coefficient between blood pressure and transcranial Doppler (TCD) cerebral blood flow velocity or cerebral oximetry signals, respectively (see text for details). Note: ICM+ (University of Cambridge, Cambridge, UK) software was used for the pc-based autoregulation monitoring.
The personal computer-based system sampled the ABP, TCD, and NIRS signals at 60 Hz and time-integrated them as non-overlapping 10-second mean values, which is equivalent to applying a moving average filter with a 10-second time window and resampling at 0.1 Hz.5,9,10 This approach eliminates high frequency noise from the respiratory and pulse frequencies, while allowing detection of oscillations and transients occurring below 0.05 Hz. A continuous, moving Pearson correlation coefficient was performed between the ABP and TCD signals, rendering mean velocity index (M×). The same calculation was performed using the ABP and rScO2 signals rendering cerebral oximetry index (CO×). For each consecutive 10-second time period, averaged paired values of 300-seconds duration were used for analysis, incorporating 30 data points for each index.
The prototype NIRS autoregulation monitor used a proprietary algorithm, resulting in a simultaneously determined, independent autoregulation index CO× which could be compared to both metrics computed by the personal computer-based system (M× and CO×). These methods included similar sampling frequencies and filtering processes as the personal computer based system. Cerebral oximetry index was computed as a Pearson correlation coefficient between ABP and rScO2 signals using a similar time period of sampling and data averaging. Intact CBF autoregulation is indicated by values of M× and CO× that approach 0 or that are negative, since CBF and ABP are not correlated. When ABP is below the autoregulation limit, M× and CO× approach 1 indicating that CBF is pressure passive.
Sample Size Estimates
The sample size estimates of the study were based on the correlation and agreement between the measures of M× and CO× during CPB. This estimate was based on our prior experiences with similar monitoring of 227 adult patients undergoing CPB where the M× value (mean±SD) during CPB was 0.23±0.17. We randomly sampled data from this population comparing the simultaneous M× and CO× measurements. This preliminary analysis demonstrated that 50 patients would provide correlation between M× and CO× with p-value=0.0385 and bias of -0.10±0.21. A final sample of 70 patients was chosen to allow for incomplete data collection due to unanticipated technical difficulties.
Data Analysis
Time-averaged values for M× and for CO× obtained with the NIRS autoregulation prototype monitor recorded during CPB were compared with linear regression and Pearson correlation. Bland-Altman analysis was used to compare the differences in M× and CO× versus the average of these values.12 This analysis was repeated for CO× obtained from the personal computer-based system and CO× obtained from the prototype monitor. Values of M× and CO× were further categorized into 5 mmHg bins of ABP for each patient. The M× cut-off indicating the lower limit of autoregulation is not clearly known but it is likely to be between 0.3 and 0.5 as previously noted.1,5,8-10 The lower limit of autoregulation was defined in this study as the ABP where M× incrementally increased to ≥ 0.4. When M× was ≥ 0.4 at all ABP during CPB, the autoregulation threshold was defined as that ABP where M× had the lowest value. The average CO× value at the ABP associated with the lower limit of autoregulation was determined. This value was then applied to the data as the CO× lower limit of autoregulation. Arterial blood pressure at the lower limit of autoregulation determined with CO× was compared with that determined by M× with Wilcoxon Signed Rank test. Associations between indices were assessed with Pearson correlation, using the Fisher Transformation to calculate the 95% confidence intervals. Analysis was performed with GraphPad Prism software (GraphPad Software, Inc, La Jolla, CA), Stata software (Version 9.0; Stata Corp, College Station, TX) and SPSS (SPSS version 17, IBM Statistics, Armonk NY).
Results
Clinical data from the 70 patients included in the study are listed in Table 1. Average M× for the cohort was 0.27 ± 0.16 and average CO× derived from the prototype monitor 0.34 ± 0.21. There was significant correlation between M× and CO× derived from the prototype NIRS autoregulation monitor (r=0.510, 95% confidence interval, 0.414 to 0.595, p<0.001) and good agreement between the methods (bias -0.07 ± 0.19) as shown in Figures 2 and 3, respectively. Comparison was made between CO× determined with the prototype monitor and that determined with our personal computer-based method that has been previously validated.5,8 There was strong correlation (r=0.957, 95% confidence interval, 0.945 to 0.966, p<0.001) and good agreement between CO× determined with both methods (0.06 ± 0.06).
Table 1.
Patient medical and operative data.
| Parameter | Patient Data (n=70) |
|---|---|
| Age (mean ± SD) | 61±12 |
| Males | 49 (70%) |
| Females | 21 (30%) |
| Prior CVA | 4 (6%) |
| Hypertension | 50 (71%) |
| Diabetes | 22 (31%) |
| COPD | 7 (10%) |
| Current tobacco abuse | 12 (17%) |
| Peripheral vascular disease | 6 (9%) |
| Congestive heart failure | 16 (23%) |
| Left ventricular ejection fraction < 30% | 11 (16%) |
| Prior myocardial infarction | 11 (16%) |
| Prior cardiac surgery | 16 (23%) |
| Left carotid artery stenosis | |
| <50% | 68 (97%) |
| 50-69% | 2 (3%) |
| Right carotid artery stenosis | |
| <50% | 67 (96%) |
| 50-69% | 2 (3%) |
| 70-99% | 1 (1%) |
| Medications | |
| Aspirin | 42 (60%) |
| Beta blockers | 40 (57%) |
| Statin drugs | 41 (59%) |
| Calcium channel blockers | 17 (24%) |
| ACE inhibitors | 21 (30%) |
| Type of surgery | |
| CABG | 33 (47%) |
| CABG/AVR | 6 (9%) |
| CABG/MVR or Repair | 13 (19%) |
| AVR | 7 (10%) |
| MVR or repair | 2 (3%) |
| Ascending aorta replacement | 2 (3%) |
| Valve-sparing aortic root replacement | 6 (9%) |
| Cardiopulmonary bypass duration (min) | 107±49 |
| Aortic cross-clamp duration (min) | 59±33 |
Figure 2.
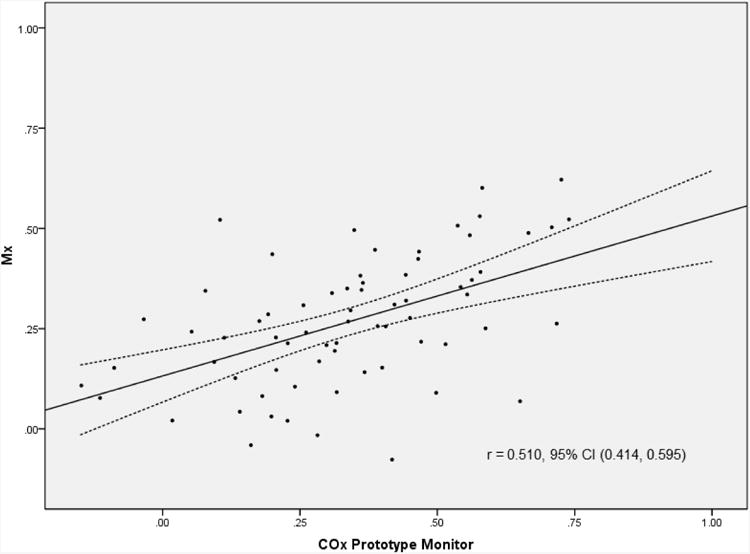
Correlation and 95% confidence intervals between mean velocity index (M×) and cerebral oximetry index (CO×). M× was determined with a personal computer based system as the correlation coefficient between transcranial Doppler measured cerebral blood flow velocity and mean arterial pressure. CO× is the correlation between near infrared spectroscopy-measured cerebral oximetry and mean arterial pressure.
Figure 3.
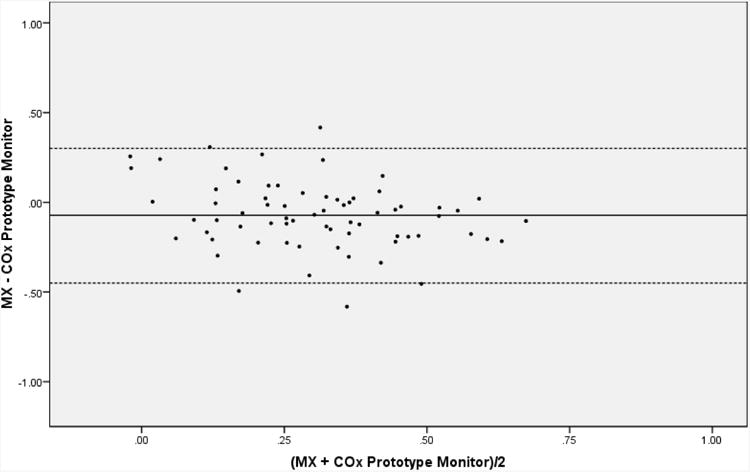
Bias and 95% confidence intervals between mean velocity index (M×) and cerebral oximetry index (CO×).
A lower limit of autoregulation was observed in all patients with M× and CO×. The ABP at the lower limit of autoregulation based on M× monitoring was 63±11 mmHg (95% prediction interval, 52 to 74 mmHg). The average CO× at this ABP was 0.38 ± 0.26 for the personal computer-based method and 0.44 ± 0.26 for the prototype monitor. Based on the CO× determined by the prototype monitor, the ABP at the lower limit of autoregulation was 59 ± 9 mmHg (95% prediction interval, 50 to 68 mmHg, p=0.026 vs. M×).
Discussion
These results show that CO× determined with an investigational prototype NIRS autoregulation monitor is correlated and in good agreement with previously validated TCD methods for autoregulation monitoring. The ABP at the lower limit of autoregulation was similar between the two methods suggesting that CO× using this monitor maybe an acceptable substitute for M× monitoring during CPB.
During cardiopulmonary bypass (CPB) systemic blood flow is calculated based on body surface area and temperature and then adjusted, depending on indicators of adequate global tissue perfusion (mixed venous O2 saturation, pH, etc.). Cerebral blood flow is assumed to be sufficient based on the fact that CBF–arterial pressure autoregulation remains intact with CPB flows between 1.6 and 2.4 L/min/m2 when α-stat pH management is utilized.13,14 Supported by the latter data, an ABP of 50 to 60 mmHg is widely considered to be the minimal acceptable blood pressure during CPB. This practice fails to consider that CBF–blood pressure autoregulation has wide individual variation, maybe altered in many common conditions (e.g., hypertension, diabetes, stroke), and is derived using statistical methods that have been questioned (i.e., based on limited data from individuals).15-22 Importantly, the current arbitrary standard of care for managing ABP during CPB may predispose the increasing number of surgical patients with cerebral vascular disease to cerebral hypoperfusion and ischemic brain injury.23,24 In fact, a high proportion of strokes after cardiac surgery are hypoperfusion-type watershed strokes that have been shown to be related to decreases in ABP during CPB.24
Prior laboratory and clinical studies have validated CO× as a reliable monitor of CBF autoregulation. In piglets made hypotensive by inflation of a balloon in the inferior vena cava CO× was correlated (r=0.67) and had good agreement (bias, 0.03) with Doppler flux monitoring of the frontal-partial cortex.8 A CO× value of >0.36 had 92% sensitivity (73% to 99%) and 63% specificity (48% to 76%) for identifying the autoregulation threshold. In a study of 60 adult patients we found significant correlation (r=0.55, P<0.0001) and good agreement (bias, 0.08±0.18) between M× and CO× during CPB.5 In patients undergoing CPB the average lower limit of autoregulation was found to be 66 mmHg, but this value ranged between 40mmHg to 90 mmHg.5,25 The range of ABPs at the lower limit of autoregulation in this study (33 to 83 mmHg) is similar to our prior studies. The data from this study corroborates other investigations during CPB and in non-cardiac surgery settings showing that CO× monitoring is a clinically reliable method for autoregulation monitoring.5,8,26-28
Although a decrease in blood pressure is a common consequence of general and regional anesthesia, there is currently no universally accepted definition of intraoperative hypotension. In fact, in a systematic review Bijker et al29 identified 130 articles in the anesthesiology literature that referred to 140 different definitions of intraoperative hypotension. An absolute threshold or relative change in systolic blood pressure or ABP from baseline or the requirement of a clinical intervention for treatment were most often applied. Monitoring of CBF autoregulation may provide a more clinically precise method for individualizing a blood pressure threshold that might compromise organ perfusion. In fact, our current data and prior reports suggest a wide variability in the lower limit of autoregulation making a priori definition of intraoperative hypotension nearly impossible.5,9,10,25 Further, in our prior investigation we found that monitoring the patients with CO× was more accurate than clinical history and preoperative blood pressure in identifying the ABP at the lower limit of autoregulation.25 While there may be varying organ tolerance to low blood pressure depending on disease state, in patients undergoing CPB, blood pressure management aimed at assessments of cerebral perfusion with NIRS was found to lower the frequency of major organ morbidity and mortality.30
The prototype monitor used in this study requires simple connection of the blood pressure output signal from the OR hemodynamic monitor to a modified NIRS monitor now currently available. Further refinement of the methods could further enhance the clinical application of the monitor to any clinical situation where NIRS and invasive blood pressure monitoring are currently performed. Future development of interfaces with non-invasive arterial blood pressure monitoring systems could extend this use to other operative and critical areas for more physiologic targeting of blood pressure.31
As mentioned, our use of a M× ≥ 0.4 as indicating the lower limit of autoregulation, while supported by experimental studies, is admittedly arbitrary.1,5,8-10 Clinically, rather than attempting to determine an exact autoregulation threshold, clinicians may rather target a blood pressure associated with the lowest M× or the blood pressure with optimal autoregulation. Indeed, optimizing cerebral perfusion pressure within the autoregulation range is associated with improved outcomes in patients with traumatic brain injury.32,33 In prior studies we have noted that some patients have an impaired autoregulation pattern during CPB based on an average M× ≥ 0.4 or an M× ≥ 0.4 at all blood pressures.10,25,34 In these situations, though, an autoregulation “curve” is still often present albeit with a limited plateau. The latter might allow for targeting an ABP in an optimal autoregulatory range by choosing that ABP associated with the lowest M×. In this study we denoted the MAP at the lower limit of autoregulation when when M× was ≥ 0.4 at all ABP during CPB. This approach is only is relevant for our comparison of the ABP at the limit of autoregulation between our standard methods of autoregulation testing and the NIRS-based autoregulation monitor. Our approach does not affect the primary analysis where we compare the average M× with CO× with correlation and bias analyses.
In conclusion, monitoring CBF autoregulation with a modified, stand-alone NIRS monitor is correlated and in good agreement with TCD based methods. Availability of such a device would allow wide-spread autoregulation monitoring as a means of individualizing ABP targets during CPB.
Acknowledgments
Funding: Funded in part by Grant-In-Aid Number 103363 from the Mid-Atlantic Affiliate of the American Heart Association; Grant R01HL092259 from the National Institutes of Health (to Dr. Hogue); and a direct research grant from Somanetics, Corp/Covidien PLC, Boulder, CO. Clinical Trials Registration NCT00769691at www.clinicaltrials.gov. Covidien has licensed aspects of the investigational software from The Johns Hopkins University.
References
- 1.Czonsnyka M, Brady K, Reinhard M, Smielewski P, Steiner L. Monitoring of cerebrovascular autoregulation: Facts, Myths, and Missing Links. Neurocrit Care. 2009;10:373–86. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- 2.Lang EW, Mehdorn HM, Dorsch NW, Czosnyka M. Continuous monitoring of cerebrovascular autoregulation: a validation study. J Neurol Neurosurg Psychiatry. 2002;72:583–6. doi: 10.1136/jnnp.72.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinhard M, Roth M. Effect of carotid endarterectomy or stenting on impairment of dynamic cerebral autoregulation. Stroke. 2004;35:1381–7. doi: 10.1161/01.STR.0000127533.46914.31. [DOI] [PubMed] [Google Scholar]
- 4.Minhas PS, Smielewski P, Kirkpatrick PJ, Pickard JD, Czosnyka M. Pressure autoregulation and positron emission tomography-derived cerebral blood flow acetazolamide reactivity in patients with carotid artery stenosis. Neurosurg. 2004;55:63–8. doi: 10.1227/01.neu.0000126876.10254.05. [DOI] [PubMed] [Google Scholar]
- 5.Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley B, Hogue J CW. Real time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41:1951–6. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panerai R. Assessment of cerebral pressure autoregulation in humans-a review of measurement methods. Physiol Meas. 1998;19:305–38. doi: 10.1088/0967-3334/19/3/001. [DOI] [PubMed] [Google Scholar]
- 7.Olsen K, Svendsen L, Larsen F. Validation of transcranial near-infrared spectroscopy for evaluation of cerebral blood flow autoregulation. J Neurosurg Anesthesiol. 1996;8:280–5. doi: 10.1097/00008506-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Brady K, Lee J, Kibler K, Smielewski P, Czosnyka M, Easley R, Koehler R, Shaffner D. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38:2818–25. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barodka V, Joshi B, Berkowitz D, Hogue J CW, Nyhan D. Implications of vascular aging. Anesth Analg. 2011;112:1048–60. doi: 10.1213/ANE.0b013e3182147e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi B, Brady K, Lee J, Easley B, Panigrahi R, Smielewski P, Czosnyka M, Hogue J CW. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010;110:321–8. doi: 10.1213/ANE.0b013e3181c6fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MB, Ward DS, Cartwright CR, Kolano J, Chlebowski S, Henson LC. Estimation of jugular venous O2 saturation from cerebral oximetry or arterial O2 saturation during isocapnic hypoxia. J Clin Monit Comput. 2000;16:191–9. doi: 10.1023/a:1009940031063. [DOI] [PubMed] [Google Scholar]
- 12.Bland J, Altman D. Statistical methods fo rassessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 13.Schell R, Kern F, Greeley W, Schulman S, Frasco P, Croughwell N, Newman M, Reves J. Cerebral blood flow and metabolism during cardiopulmonary bypass. Anesth Analg. 1993;76:849–65. doi: 10.1213/00000539-199304000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Taylor K. The hemodynamics of cardiopulmonary bypass. Sem Thorac Cardiovasc Surg. 1990;2:300–12. [PubMed] [Google Scholar]
- 15.Newman M, Croughwell N, White W, Lowry E, Baldwin B, Clements F, Davis R, Jr, Jones R, Amory D, Reves J. Effect of perfusion pressure on cerebral blood flow during normothermic cardiopulmonary bypass. Circulation. 1996;94(Suppl II):II-353–II-7. [PubMed] [Google Scholar]
- 16.Mutch W, Sutton I, Teskey J, Cheang M, Thomson I. Cerebral pressure-flow relationship during cardiopulmonary bypass in the dog at normothermia and moderate hypothermia. J Cereb Blood Flow Metab. 1994;14:510–8. doi: 10.1038/jcbfm.1994.63. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz A, Sandhu A, Kaplon R, Young W, Jonassen A, Adams D, Edwards N, Sistino J, Kwiatkowski P, Michler R. Cerebral blood flow is determined by arterial pressure and not cardiopulmonary bypass flow rate. Ann Thorac Surg. 1995;60:165–9. [PubMed] [Google Scholar]
- 18.Drummond J. The lower limit of autoregulation: Time to revise our thinking? Anesthesiology. 1997;86:1431–3. doi: 10.1097/00000542-199706000-00034. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Meyer J, Sakai F, Yamaguchi F. Aging and cerebral vasodilator responses to hypercarbia. Responses in normal aging and in persons with risk factors for stroke. Arch Neurol. 1980;37:489–96. doi: 10.1001/archneur.1980.00500570037005. [DOI] [PubMed] [Google Scholar]
- 20.Davis S, Ackerman R, Correia J, Alpert N, Chang J, Buonanno F, Kelley R, Rosner B, Taveras J. Cerebral blood flow and cerebrovascular CO2 reactivity in stroke-age normal controls. Neurology. 1983;33:391–9. doi: 10.1212/wnl.33.4.391. [DOI] [PubMed] [Google Scholar]
- 21.Muhonen M, Sawin P, Loftus C, Heistad D. Pressure-flow relations in canine collateral-dependent cerebrum. Stroke. 1992;23:988–94. doi: 10.1161/01.str.23.7.988. [DOI] [PubMed] [Google Scholar]
- 22.Strandgaard S, Olesen J, Skinhoj E, Lassen N. Autoregulation of brain circulation in severe arterial hypertension. Br Med J. 1973;1:507–10. doi: 10.1136/bmj.1.5852.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moraca R, Lin E, Holmes J, IV, Fordyce D, Campbell W, Ditkoff M, Hill M, Gutyon S, Paull D, Hall R. Impaired baseline regional cerebral perfusion in patients referred for coronary artery bypass. J Thorac Cardiovasc Surg. 2006;131:540–6. doi: 10.1016/j.jtcvs.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman R, Sherman P, Grega M, Yousem D, Borowicz LJ, Selnes O, Baumgartner W, McKhann G. Watershed strokes after cardiac surgery: diagnosis, etiology, and outcome. Stroke. 2006;37:2306–11. doi: 10.1161/01.STR.0000236024.68020.3a. [DOI] [PubMed] [Google Scholar]
- 25.Joshi B, Ono M, Brown C, Brady K, Easley R, Yenokyan G, Gottesman R, Hogue J CW. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg. 2012;114:503–10. doi: 10.1213/ANE.0b013e31823d292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Kibler K, Benni P, Easley R, Czosnyka M, Smielewski P, Koehler R, Shaffner D, Brady K. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009;40:1820–6. doi: 10.1161/STROKEAHA.108.536094. [DOI] [PubMed] [Google Scholar]
- 27.Zweifel C, Castellani G, Czosnyka M, Carrera E, Brady K, Kirkpatrick P, Pickard J, Smielewski P. Continuous assessment of cerebral autoregulation with near-Infrared spectroscopy in adults after subarachnoid hemorrhage. Stroke. 2010;41:1963–68. doi: 10.1161/STROKEAHA.109.577320. [DOI] [PubMed] [Google Scholar]
- 28.Zweifel C, Castellani G, Czosnyka M, Helmy A, Anne M, Carrera E, Brady K, Hutchinson P, Menon D, Pickard J, Smieleweski P. Non-invasive monitoring of cerebrovascular reactivity with near infrared spectroscopy in head injured patients. J Neurotrauma. 2007 2010 Sep 2; doi: 10.1089/neu.2010.1388. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Bijker J, van Klei W, Kappen T, van Wolfswinkel L, Moons K, Kalkman C. Incidence of intraoperative hypotension as a function of the chosen definition. Anesthesiology. 2007:213–20. doi: 10.1097/01.anes.0000270724.40897.8e. [DOI] [PubMed] [Google Scholar]
- 30.Murkin J, Adams SJ, Novick RJ, al e. Monitoring brain oxygen saturation during coronary artery bypass surgery: A randomized, prospective study. Anesth Analg. 2007;104:51–8. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 31.Lavinio A, Schmidt E, Haubrich C, Smielewski P, Pickard JD, Czosnyka M. Noninvasive evaluation of dynamic cerebrovascular autoregulation using Finapres plethysmograph and transcranial Doppler. Stroke. 2007;38:402–4. doi: 10.1161/01.STR.0000254551.92209.5c. [DOI] [PubMed] [Google Scholar]
- 32.Steiner L, Czosnyka M, Piechnik S, Smielewski P, Chatfield D, Menon D, Pickard J. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733–8. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Aries M, Czosnyka M, Budohoski K, Steiner L, Lavinio A, Kolias A, Hutchinson P, Brady K, Menon D, Pickard J, Smielewski P. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012 May 22; doi: 10.1097/CCM.0b013e3182514eb6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Ono M, Joshi B, Brady K, Easley R, Zheng Y, Brown C, Baumgartner W, Hogue C. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth. 2012 Jun 1; doi: 10.1093/bja/aes148. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


