Abstract
In an intriguing new study, Loffredo et al., report that joining the circulation of old mice with that of young mice reduces age-related cardiac hypertrophy. They also found that the growth factor GDF11 is a circulating negative regulator of cardiac hypertrophy which suggests that raising GDF11 levels may be useful to treat cardiac hypertrophy associated with aging.
As the human population ages, heart failure is increasing in prevalence.1 In heart failure, cardiac hypertrophy is associated with aging and is common in diastolic heart failure in which relaxation and filling is impaired.2–4 In a recent article in Cell, Amy Wagers, Richard Lee and colleagues demonstrate that age-related cardiac hypertrophy and increase in cardiomyocyte area in old mice can be reversed by surgically joining their circulatory system to that of young mice for four weeks.5 The expression of genes involved in hypertrophy in old mice were also altered by exposure to young blood. To identify candidate regulators of hypertrophy, the authors carried out proteomic analysis of plasma from young and old mice and found that the levels of 13 proteins differentiate old versus young plasma. They then show that one protein, growth/differentiation factor 11 (GDF11), a secreted growth factor and member of the transforming growth factor beta (TGF-beta) superfamily, declines with age in mice. Daily injection of GDF11 into old mice mimicked the effects of exposure to young serum on aged hearts. GDF11 had no effect, however, pressure overload-induced hypertrophy. These results suggest that increasing the circulating levels of GDF11 could potentially treat or prevent age-related cardiac hypertrophy.
The mechanisms by which cells age and lose their functional or regenerative capacity are of growing research interest spurred by the aging population. Some potential mechanisms of cellular aging include telomere shortening, oxidative damage, somatic mutations, and epigenetic changes. However, accumulating evidence suggests that the extracellular environment also changes with aging pointing to potentially new targets more amenable for therapeutics to improve or prevent age-related cellular decline.
In this study, an old technique is employed to address questions about the environment of young or old cells. Prompted by the realization that conjoined twin pairs could share circulation, an experimental technique called parabiosis was developed to surgically pair two animals by joining the skin from the left flank of one to the right flank of the other, sometimes together with abdominal wall musculature.6 In the first description of this technique by Paul Bert in 1862,6 fluids administered to one rat were detectable in the parabiont which demonstrated that the circulatory systems were shared. When healthy and affected animals are paired, the technique allows the researcher to test whether a circulatory factor from one animal can change some feature of a tissue of interest in the other. Parabiosis has been used frequently since the early 20th century for a variety of investigations, but recently parabiosis has been used to demonstrate that circulatory factors differ between young and old animals.7 For example, the replication of hepatocytes,8 beta cells,9 skeletal muscle stem cells,8 and neuronal progenitors10 in old mice are increased by exposure to youthful circulation.
Loffredo et al.,5 use parabiosis to determine whether a specific type of cardiac hypertrophy related to aging is due to age-related differences in levels of a factor or factors in the blood. Previous studies have shown that left ventricular hypertrophy is associated with aging.2–4 Diastolic heart failure, heart failure with preserved ejection fraction, occurs in about ~50% of patients with heart failure.2,4 Diastolic heart failure is not associated with ventricular dilation, unlike systolic heart failure, which is heart failure with reduced ejection fraction. Most diastolic heart failure patients have ventricular and/or atrial hypertrophy and impaired relaxation and filling.2,4 Patients with diastolic heart failure are likely to be older, female, and hypertensive.4 Recent estimates indicate that the prevalence of diastolic heart failure is rising most likely due to an aging population.4
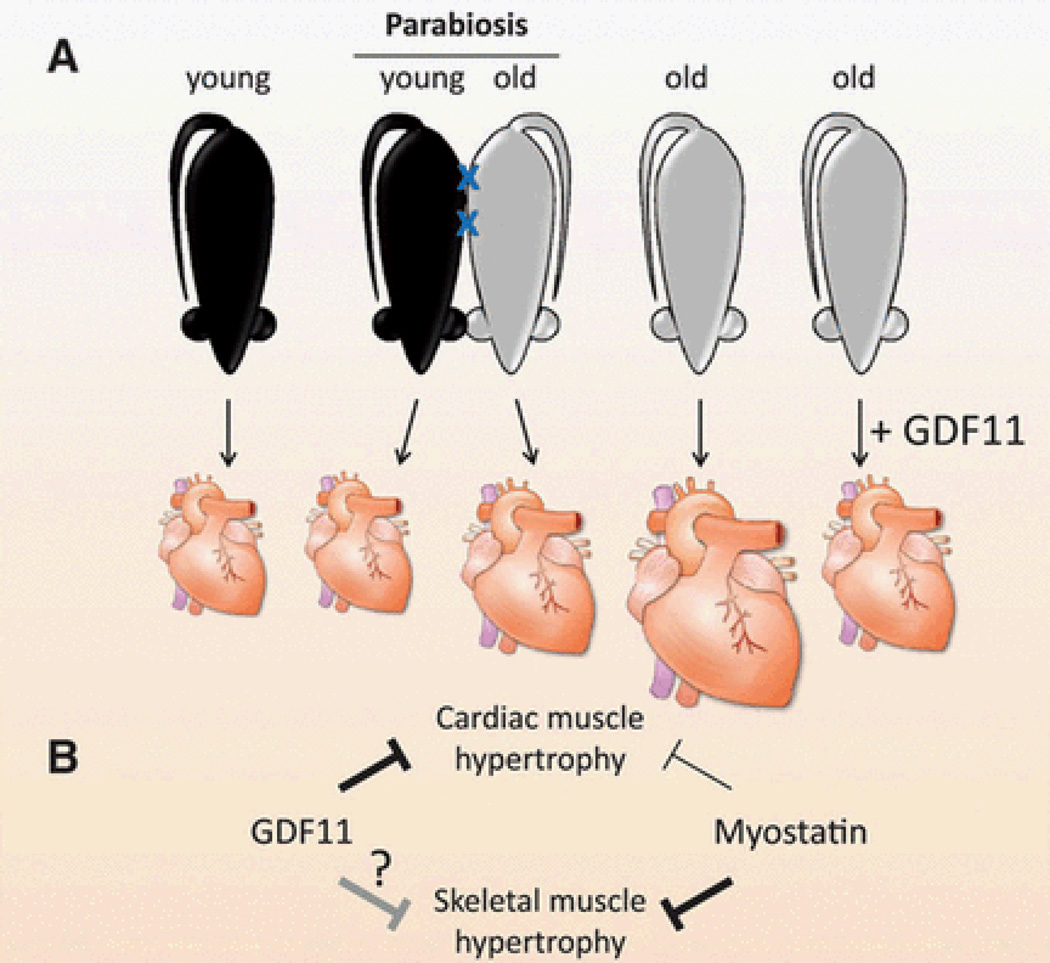
Leffredo et al.,5 generated parabiotic pairs of young with old (heterochronic) mice and compared their cardiac size with parabiotic pairs of mice of the same age (isochronic) and age-matched controls that did not undergo parabiosis. After only four weeks, cardiac hypertrophy was reversed in old heterochronically paired mice (Figure 1A). Heart weight (relative to tibia length) and cardiac myocyte area were decreased in old mice that were paired with young mice. Consistent with the altered morphometric phenotype, the expression of markers of cardiomyocyte hypertrophy were reduced while the expression of sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA-2), an ATPase important for relaxation, was increased in old hearts by heterochronic parabiosis. Commendably, the authors performed blinded morphometric analysis of cardiomyocyte area and carried out the parabiosis and morphometric analyses in both sexes. Several controls were used to confirm the conclusion that a circulating factor(s) from young blood reduced cardiac hypertrophy in old mice. Sham parabiosis, joining heterochronic pairs without shared circulation, had no effect of cardiac size of the older parabiont. This result demonstrated that the effect on cardiac size was due to a circulatory factor rather than some other effect such as altered physical activity levels or feeding. Also, parabiosis by itself did not affect cardiac weight or cardiac myocyte size as shown by analysis of the hearts from isochronic parabionts or unpaired controls. In addition, the authors ruled out differences in blood pressure as a cause by using tail-cuffs and intra-arterial catheters.
Figure 1.
Age-related cardiac hypertrophy is inhibited by a circulating growth factor, GDF11. A) Cardiac size is greater in old mice than in young mice. After four weeks of parabiosis with young mice, cardiac size in old mice is reduced demonstrating that a circulating factor that inhibits cardiac growth changes with aging. Systemic GDF11 levels are reduced during aging, and daily administration of GDF11 for 30 days reduced cardiac size in old mice similar to parabiosis with young mice. B) The related growth factors, GDF11 and myostatin, are inhibitors of muscle growth. GDF11 inhibits cardiac muscle hypertrophy, and raising GDF11 levels may help treat age-related cardiac hypertrophy. Myostatin inhibits skeletal muscle hypertrophy, and myostatin inhibitors are in clinical trials for increasing skeletal muscle mass. Some studies have shown that myostatin appears to inhibit cardiac growth as well, but it is unclear whether GDF11 can affect skeletal muscle growth.
To identify molecules found at different levels in the circulation of old versus young mice, Loffredo et al., used a proteomic approach.5 Of 13 proteins that were identified that differentiate old versus young plasma, GDF11, also called bone morphogenetic protein 11, was chosen for further study. GDF11 is expressed widely during development and in adulthood,11–13 and GDF11 knockout mice die soon after birth with defects in patterning of the axial skeleton14 and spinal cord15 due to the loss of expression in the primitive streak, a temporary developmental structure where cells acquire anterior/posterior positional identity. GDF11 mutants have kidney agenesis,11 changes in the number of islet cell types and progenitors,16,17 and an increased number of neurons in the olfactory epithelium18 and retinal ganglion.19 Loffredo et al.,5 suggest that the spleen may be the main source of GDF11 in circulation because of the relatively high gene expression of the growth factor in the spleen compared with other tissues. The authors also found that there was a decrease in GDF11 expression with aging in the spleen.
To demonstrate that GDF11 is a negative regulator of cardiac myocyte hypertrophy, Loffredo et al.,5 injected GDF11 protein daily for 30 days in aged mice using a blinded, randomized study design. Heart weight and cardiomyocyte area were reduced without affecting cardiac function (Figure 1A). Gene expression changes induced in hearts by GDF11 were similar to those of heterchronic parabiosis. The authors also tested the effects of GDF11 in a pressure-overload model that induces hypertrophy. In contrast to the hypertrophy accompanying aging, GDF11 had no effect on hypertrophy or fibrosis in this model demonstrating specificity of different hypertrophic pathways.
To determine whether the effects of GDF11 on cardiac hypertrophy could be direct, the α1-adrenergic receptor agonist phenylephrine was used to promote hypertrophy of neonatal cardiomyocytes in vitro. Previous work has shown that α1-adrenergic receptors are involved in pathological cardiac responses including hypertrophy.20 The authors found that phenylephrine-induced hypertrophy was inhibited by the addition of GDF11 in vitro. This result suggests that GDF11 in circulation directly affects cardiac myocytes and is consistent with its role in preventing excess hypertrophy. An effect of GDF11 on expression of hypertrophic markers or SERCA-2 on cardiac myocytes in vitro was not attempted.
The function of GDF11 in cardiac muscle described by Loffredo et al5 is analogous to that of another TGF-beta superfamily member, myostatin (MSTN), also known as GDF8 (Figure 1B). MSTN is a negative regulator of skeletal muscle hypertrophy, as demonstrated by a dramatic increase in skeletal muscle mass in a variety of animals and people with loss of function mutations.21 In addition, blocking postnatal MSTN signaling causes skeletal muscle fiber hypertrophy in mice.22 Consequently, a variety of MSTN or receptor inhibitors are in clinical trials for muscle wasting diseases including sarcopenia, the age-related loss of muscle mass and strength. Nevertheless, it is unclear whether MSTN levels in muscle change with aging.23 However, sarcopenia seems to proceed at the same rate in MSTN null mice compared to WT mice suggesting that MSTN does not regulate age-induced muscle loss at least in mice.24,25
At the molecular level, GDF11 and MSTN are similar. GDF11 is 90% identical by amino acid sequence to MSTN. Each of these proteins is secreted in latent complex that needs to be proteolytically processed to release the receptor binding form of the molecule. These proteins are also inhibited by the same secreted proteins and bind to the same receptors.22,26,27 These similarities raise the issue of whether these factors are functionally redundant with each other in cardiac or skeletal muscle. In other words - can GDF11 inhibit skeletal muscle growth and/or can MSTN inhibit cardiac muscle growth? These questions are important because levels of these factors need to be manipulated in opposite directions to treat cardiac hypertrophy or skeletal muscle wasting. In addition, clinical trials of anti-MSTN therapies have already commenced. In fact, administration of certain inhibitors that neutralize several TGF-beta family members causes further increases in skeletal muscle mass in MSTN knockout mice.22,28 These results clearly demonstrate that, in addition to MSTN, other TGF-beta family members may be involved in regulating skeletal muscle growth.
A few studies have examined the redundancy between GDF11 and MSTN. Mice that are null for both genes have more severe axial skeletal defects than GDF11 single knockout mice, suggesting that the factors might be functionally redundant for anterior/posterior patterning during development.29 Similarly, administration of ectopic GDF11 or MSTN inhibits myogenesis in developing chick limb muscle30,31 and mouse C2C12 myoblast cultures.32 On the other hand, GDF11 deletion specifically in skeletal muscle does not cause skeletal muscle hypertrophy in WT or MSTN null mice,29 possibly due to the availability of circulating GDF11 provided by other tissues. Thus, the factors seem to have the same function during early developmental patterning although a function for GDF11 in skeletal muscle has not been demonstrated in vivo.
Even if GDF11 is shown to inhibit skeletal muscle growth in vivo, a difference in sensitivity between GDF11 and MSTN may allow doses of GDF11 to be used that do not cause skeletal muscle atrophy. Although muscle mass in mice is very sensitive to MSTN inhibitors, it seems to require very high concentrations of injected MSTN to achieve a significant decrease in skeletal muscle mass in WT mice.33,34 The MSTN concentrations injected in these in vivo studies are more than one order of magnitude greater than the GDF11 concentration used by Loffredo et al., to reduce cardiac mass in old mice5. Although, Loffredo et al.,5 did not analyze skeletal muscle size after GDF11 treatment, in future studies it will be necessary to analyze skeletal muscle mass in response to a systemic changes in GDF11.
In recent work, the role of MSTN in the heart has received considerable attention. However, there are conflicting reports regarding cardiac phenotype and function in MSTN knockout mice. Some researchers have found increased heart weight in knockout mice,43,45,46 although others have not.41,47,24 MSTN is expressed in the heart at much lower levels than in skeletal muscle.35 MSTN transcript or protein expression in the heart is increased by infarct,36 aortic restriction,37 volume-overload,38 and exercise,39 and in cardiac hypertrophy caused by overexpression of Akt in cardiomyocytes.40,41 Heineke et al.,37 proposed that the main effect of MSTN produced by the heart is to decrease skeletal muscle mass. They used a model of heart failure associated with skeletal muscle wasting, a risk factor for increased morbidity and mortality.42 By analyzing cardiac-specific MSTN deletion or overexpression lines of mice, Heineke et al., showed that this wasting was due to MSTN produced by the heart rather than skeletal muscle.37 However, they and others have found that heart weight is reduced slightly in transgenic mouse lines overexpressing MSTN in cardiac muscle as would be expected if MSTN is redundant to GDF11 in the heart.37,43,44
The latter result suggests that MSTN may have a direct effect on cardiac myocytes. As shown by Loffredo et al.,5 and others,41,48 MSTN activates similar signal transduction pathways in cardiac myocytes in culture as it does in skeletal muscle. Loffredo et al., demonstrated that, unlike GDF11, the recombinant mature MSTN peptide could not block phenylephrine-induced hypertrophy of rat neonatal cardiac myocytes, suggesting they are not redundant for this function.5 In contrast, Morissette et al., showed that adenoviral expression of full-length MSTN in rat neonatal cardiac myocytes efficiently blocks phenylephrine-induced hypertrophy.41 Furthermore, they showed that phenylephrine-induced cardiac hypertrophy is greater in MSTN knockout male mice than in WT mice,41 consistent with a marked effect of MSTN in inhibiting hypertrophy caused by α1-adrenergic stimulation in cardiac myocytes. The differing results of these in vitro experiments may be due to differences in effective doses of MSTN or the sensitivity to GDF11 compared to MSTN in this particular assay. Taken together, the effects of overexpression or deletion of MSTN on cardiac size are consistent with its role as a negative regulator of cardiomyocyte growth, although perhaps a relatively minor one when compared with GDF11.
Some therapeutic strategies to increase muscle mass promiscuously inhibit multiple TGF-beta superfamily members in addition to MSTN. For example, a soluble activin receptor type IIB (ACTRIIB or ACVR2B) binds several superfamily ligands including MSTN, GDF11, and the activins, and yields a greater increase in muscle mass than more specific anti-MSTN inhibitors.49 Activin A has been shown to regulate skeletal muscle mass in vivo similar to MSTN.28 Unfortunately, the soluble receptor approach has caused side effects such as nosebleeds, and the clinical trials have been halted.50 Another approach is to block one of the receptors that mediates skeletal muscle hypertrophy by using anti-ACTRIIB antibodies.51 Whether this strategy would stimulate cardiac hypertrophy by blocking GDF11 signaling in the heart depends on whether GDF11 function in cardiac myocytes requires ACTRIIB or another receptor. Finally, neutralizing monoclonal antibodies against MSTN that do not bind to GDF11 are one way to avoid blocking the hypertrophic effect of GDF11 inhibition on cardiac myocytes.
In summary, this exciting study suggests new therapeutic options for the treatment for age-related cardiac hypertrophy. However, future pre-clinical and clinical studies that target GDF11 or MSTN must be carefully designed to avoid unwanted side effects in skeletal or cardiac muscle or the other tissues that produce or respond to GDF11. Avoidance of such side effects, even if subtle, may be particularly important in the treatment of diseases that require long-term treatment. An interesting remaining question is whether declining GDF11 levels in the blood affect other organs in aging animals, but this remains to be seen. Overall, this study by Loffredo et al., provides additional evidence in support of the concept that the changes in the extracellular milieu can cause age-related dysfunction in a wide variety of organs. Elucidation of additional molecular differences in the circulating and local extracellular environments may yield more targets for anti-aging therapies in the future.
Acknowledgments
Sources of Funding
The author is supported by the Intramural Research Program of the NIDDK, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Under a licensing agreement between Pfizer and the Johns Hopkins University, A. C. M. is entitled to a share of royalty received by the University on sales of myostatin. A.C.M. also is a co-inventor on a GDF11 patent. The terms of these arrangements are being managed by the University in accordance with its conflict of interest policies.
References
- 1.Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of Heart Failure A Scientific Statement From the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117(19):2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises Part II: The Aging Heart in Health: Links to Heart Disease. Circulation. 2003;107(2):346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 4.Meyer T, Shih J, Aurigemma G. Heart Failure With Preserved Ejection Fraction (Diastolic Dysfunction) Ann Intern Med. 2013;158(1) doi: 10.7326/0003-4819-158-1-201301010-01001. ITC1–1. [DOI] [PubMed] [Google Scholar]
- 5.Loffredo FS, Steinhauser ML, Jay SM, et al. Growth Differentiation Factor 11 Is a Circulating Factor that Reverses Age-Related Cardiac Hypertrophy. Cell. 2013;153(4):828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finerty JC. Parabiosis in Physiological Studies. Physiol Rev. 1952;32(3):277–302. doi: 10.1152/physrev.1952.32.3.277. [DOI] [PubMed] [Google Scholar]
- 7.Conboy IM, Rando TA. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle. 2012;11(12):2260–2267. doi: 10.4161/cc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 9.Salpeter SJ, Khalaileh A, Weinberg-Corem N, Ziv O, Glaser B, Dor Y. Systemic regulation of the age-related decline of pancreatic beta-cell replication. Diabetes. 2013 Apr 29; doi: 10.2337/db13-0160. Published online before print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esquela AF, Lee S-J. Regulation of metanephric kidney development by growth/differentiation factor 11. Dev Biol. 2003;257(2):356–370. doi: 10.1016/s0012-1606(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 12.Gamer LW, Wolfman NM, Celeste AJ, Hattersley G, Hewick R, Rosen V. A novel BMP expressed in developing mouse limb, spinal cord, and tail bud is a potent mesoderm inducer in Xenopus embryos. Dev Biol. 1999;208(1):222–232. doi: 10.1006/dbio.1998.9191. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima M, Toyono T, Akamine A, Joyner A. Expression of growth/differentiation factor 11, a new member of the BMP/TGFβ superfamily during mouse embryogenesis. Mech Dev. 1999;80(2):185–189. doi: 10.1016/s0925-4773(98)00205-6. [DOI] [PubMed] [Google Scholar]
- 14.McPherron AC, Lawler AM, Lee S-J. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22(3):260–264. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- 15.Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32(6):997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- 16.Dichmann DS, Yassin H, Serup P. Analysis of pancreatic endocrine development in GDF11-deficient mice. Dev Dyn. 2006;235(11):3016–3025. doi: 10.1002/dvdy.20953. [DOI] [PubMed] [Google Scholar]
- 17.Harmon EB, Apelqvist AA, Smart NG, Gu X, Osborne DH, Kim SK. GDF11 modulates NGN3+ islet progenitor cell number and promotes β-cell differentiation in pancreas development. Development. 2004;131(24):6163–6174. doi: 10.1242/dev.01535. [DOI] [PubMed] [Google Scholar]
- 18.Wu H-H, Ivkovic S, Murray RC, et al. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37(2):197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Wu HH, Lander AD, Lyons KM, Matzuk MM, Calof AL. GDF11 controls the timing of progenitor cell competence in developing retina. Science. 2005;308(5730):1927–1930. doi: 10.1126/science.1110175. [DOI] [PubMed] [Google Scholar]
- 20.Woodcock EA, Du X-J, Reichelt ME, Graham RM. Cardiac α1-adrenergic drive in pathological remodelling. Cardiovasc Res. 2008;77(3):452–462. doi: 10.1093/cvr/cvm078. [DOI] [PubMed] [Google Scholar]
- 21.Georges M. When less means more: Impact of myostatin on animal breeding. Immunol Endocr Metab Agents Med Chem. 2010;10(4):240–248. [Google Scholar]
- 22.Lee SJ. Extracellular regulation of myostatin: A molecular rheostat for muscle mass. Immunol Endocr Metab Agents Med Chem. 2010;10(4):183–194. doi: 10.2174/187152210793663748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson HA. Role of Myostatin Signaling in Aging: Applications for Age-Related Sarcopenia. Immun Endoc Metab Agents Med Chem. 2010;10:211–216. [Google Scholar]
- 24.Morissette MR, Stricker JC, Rosenberg MA, et al. Effects of myostatin deletion in aging mice. Aging Cell. 2009;8(5):573–583. doi: 10.1111/j.1474-9726.2009.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, McPherron AC. Myostatin Inhibition Induces Muscle Fibre Hypertrophy Prior to Satellite Cell Activation. J Physiol. 2012;590(9):2151–2165. doi: 10.1113/jphysiol.2011.226001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge G, Hopkins DR, Ho W-B, Greenspan DS. GDF11 forms a bone morphogenetic protein 1-activated latent complex that can modulate nerve growth factor-induced differentiation of PC12 cells. Mol Cell Biol. 2005;25(14):5846–5858. doi: 10.1128/MCB.25.14.5846-5858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McPherron AC. Metabolic functions of myostatin and GDF11. Immunol Endocr Metab Agents Med Chem. 2010;10(4):217–231. doi: 10.2174/187152210793663810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S-J, Lee Y-S, Zimmers TA, et al. Regulation of muscle mass by follistatin and activins. Mol Endocrinol. 2010;24(10):1998–2008. doi: 10.1210/me.2010-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McPherron AC, Huynh TV, Lee S-J. Redundancy of myostatin and growth/differentiation factor 11 function. Bmc Dev Biol. 2009;9(1):24. doi: 10.1186/1471-213X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamer LW, Cox KA, Small C, Rosen V. Gdf11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb. Dev Biol. 2001;229(2):407–420. doi: 10.1006/dbio.2000.9981. [DOI] [PubMed] [Google Scholar]
- 31.Amthor H, Huang R, McKinnell I, et al. The regulation and action of myostatin as a negative regulator of muscle development during avian embryogenesis. Dev Biol. 2002;251(2):241–257. doi: 10.1006/dbio.2002.0812. [DOI] [PubMed] [Google Scholar]
- 32.Souza TA, Chen X, Guo Y, et al. Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Mol Endocrinol. 2008;22(12):2689–2702. doi: 10.1210/me.2008-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stolz LE, Li D, Qadri A, Jalenak M, Klaman LD, Tobin JF. Administration of myostatin does not alter fat mass in adult mice. Diabetes Obes Metab. 2008;10(2):135–142. doi: 10.1111/j.1463-1326.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 34.Zimmers TA, Davies MV, Koniaris LG, et al. Induction of Cachexia in Mice by Systemically Administered Myostatin. Science. 2002;296(5572):1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 35.Breitbart A, Auger-Messier M, Molkentin JD, Heineke J. Myostatin from the heart: local and systemic actions in cardiac failure and muscle wasting. Am J Physiol - Heart Circ Physiol. 2011;300(6):H1973–H1982. doi: 10.1152/ajpheart.00200.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma M, Kambadur R, Matthews KG, et al. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999;180(1):1–9. doi: 10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 37.Heineke J, Auger-Messier M, Xu J, et al. Genetic Deletion of Myostatin From the Heart Prevents Skeletal Muscle Atrophy in Heart Failure. Circulation. 2010;121(3):419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shyu KG, Lu MJ, Wang BW, Sun HY, Chang H. Myostatin expression in ventricular myocardium in a rat model of volume-overload heart failure. Eur J Clin Invest. 2006;36(10):713–719. doi: 10.1111/j.1365-2362.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 39.Lenk K, Schur R, Linke A, et al. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur J Heart Fail. 2009;11(4):342–348. doi: 10.1093/eurjhf/hfp020. [DOI] [PubMed] [Google Scholar]
- 40.Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional Effects of Chronic Akt Activation in the Heart. J Biol Chem. 2002;277(25):22528–22533. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- 41.Morissette MR, Cook SA, Foo S, et al. Myostatin Regulates Cardiomyocyte Growth Through Modulation of Akt Signaling. Circ Res. 2006;99(1):15–24. doi: 10.1161/01.RES.0000231290.45676.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Georgiadou P, Adamopoulos S. Skeletal Muscle Abnormalities in Chronic Heart Failure. Curr Heart Fail Rep. 2012;9(2):128–132. doi: 10.1007/s11897-012-0090-z. [DOI] [PubMed] [Google Scholar]
- 43.Artaza JN, Reisz-Porszasz S, Dow JS, et al. Alterations in myostatin expression are associated with changes in cardiac left ventricular mass but not ejection fraction in the mouse. J Endocrinol. 2007;194(1):63–76. doi: 10.1677/JOE-07-0072. [DOI] [PubMed] [Google Scholar]
- 44.Reisz-Porszasz S, Bhasin S, Artaza JN, et al. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003;285(4):E876–E888. doi: 10.1152/ajpendo.00107.2003. [DOI] [PubMed] [Google Scholar]
- 45.Jackson MF, Luong D, Vang DD, et al. The aging myostatin null phenotype: reduced adiposity, cardiac hypertrophy, enhanced cardiac stress response, and sexual dimorphism. J Endocrinol. 2012;213(3):263–275. doi: 10.1530/JOE-11-0455. [DOI] [PubMed] [Google Scholar]
- 46.Rodgers BD, Interlichia JP, Garikipati DK, et al. Myostatin represses physiological hypertrophy of the heart and excitation–contraction coupling. J Physiol. 2009;587(20):4873–4886. doi: 10.1113/jphysiol.2009.172544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohn RD, Liang H-Y, Shetty R, Abraham T, Wagner KR. Myostatin does not regulate cardiac hypertrophy or fibrosis. Neuromuscul Disord. 2007;17(4):290–296. doi: 10.1016/j.nmd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKoy G, Bicknell KA, Patel K, Brooks G. Developmental expression of myostatin in cardiomyocytes and its effect on foetal and neonatal rat cardiomyocyte proliferation. Cardiovasc Res. 2007;74(2):304–312. doi: 10.1016/j.cardiores.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 49.Lee SJ, Reed LA, Davies MV, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci U S. 2005;102(50):18117–18122. doi: 10.1073/pnas.0505996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acceleron Pharma. Acceleron and Shire Conclude Collaboration on ACE-031. 2013 http://www.acceleronpharma.com/2013/05/acceleron-and-shire-conclude-collaboration-on-ace-031/ [Google Scholar]
- 51.Fournier B, Murray B, Gutzwiller S, et al. Blockade of the Activin Receptor IIB Activates Functional Brown Adipogenesis and Thermogenesis by Inducing Mitochondrial Oxidative Metabolism. Mol Cell Biol. 2012;32(14):2871–2879. doi: 10.1128/MCB.06575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]