Abstract
Multimodality imaging has made great strides in the imaging evaluation of patients with a variety of diseases. Positron emission tomography/computed tomography (PET/CT) is now established as the imaging modality of choice in many clinical conditions, particularly in oncology. While the initial development of combined PET/magnetic resonance imaging (PET/MRI) was in the preclinical arena, hybrid PET/MR scanners are now available for clinical use. PET/MRI combines the unique features of MRI including excellent soft tissue contrast, diffusion-weighted imaging, dynamic contrast-enhanced imaging, fMRI and other specialized sequences as well as MR spectroscopy with the quantitative physiologic information that is provided by PET. Most evidence for the potential clinical utility of PET/MRI is based on studies performed with side-by-side comparison or software-fused MRI and PET images. Data on distinctive utility of hybrid PET/MRI are rapidly emerging. There are potential competitive advantages of PET/MRI over PET/CT. In general, PET/MRI may be preferred over PET/CT where the unique features of MRI provide more robust imaging evaluation in certain clinical settings. The exact role and potential utility of simultaneous data acquisition in specific research and clinical settings will need to be defined. It may be that simultaneous PET/MRI will be best suited for clinical situations that are disease-specific, organ-specific, related to diseases of the children or in those patients undergoing repeated imaging for whom cumulative radiation dose must be kept as low as reasonably achievable. PET/MRI also offers interesting opportunities for use of dual modality probes. Upon clear definition of clinical utility, other important and practical issues related to business operational model, clinical workflow and reimbursement will also be resolved.
Medicine is evolving toward personalized care. This notion entails an amalgamation of data from multiple sources. Molecular imaging contributes significantly to the personalized medicine through providing noninvasive spatiotemporal information on physiologic and pathologic processes that then can be used not only for accurate diagnosis and determination of extent of disease but also for rational targeted therapy and treatment monitoring (1). Multimodality imaging contributes significantly in this new paradigm that in conjunction with other important data (e.g. genetic, clinical) can pave the way toward personalized medicine.
Integrated positron emission tomography/computed tomography (PET/CT) with the most common PET radiotracer, 18F-fluorodeoxyglucose (FDG) is now established as an important milestone in the imaging evaluation of patients with variety of disorders, particularly cancer (2). Nearly a decade after the introduction of PET/CT, clinical combined PET/magnetic resonance imaging (PET/MRI) systems have become available commercially. It is interesting to note that PET/MRI systems were developed first in the pre-clinical arena (3–6). With the success of clinical PET/CT and the unique features offered by MRI (e.g. excellent soft tissue contrast, options for multiparametric and functional imaging such as dynamic contrast enhancement or DCE and diffusion-weighted imaging or DWI, and lack of ionizing radiation), it was anticipated that clinical PET/MRI system will eventually appear in the market (7–13).
This article reviews the currently available clinical hybrid PET/MRI systems from the point of view of potential competitive advantages that they may provide over PET/CT camera systems and discuss the challenges ahead for mainstream placement of these systems in the care of patients under the paradigm of personalized medicine.
Clinical PET/MRI Systems
The first integrated clinical PET/MRI scanner (Brain PET, Siemens) was tailored to brain imaging in which a PET insert for a standard 3.0-T MRI scanner was devised (14). Recently, the 3 major imaging system vendors have come up with different approaches for performing whole-body PET/MRI. Torigian et al provides an excellent review of the technical details and features of each of these 3 approaches (15)(Figure1).
Figure 1.
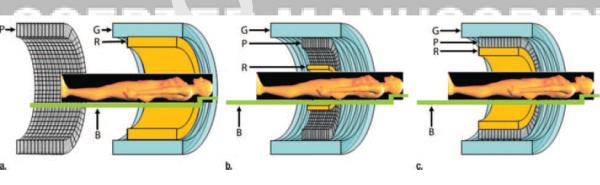
Schematic cross-sectional views of potential designs for combined PET/MR imaging systems: (a) tandem design with MR and PET mounted back-to back (similar to that in PET/CT instrumentation) to allow sequential rather than simultaneous acquisition, (b) insert design with PET imager (P) inserted between radiofrequency coil (R) and gradient set (G) of MR imager, and (c) fully integrated design with two imagers in same gantry. Radiofrequency coil, gradient set, PET imager and patient bed (B) are shown for all configurations. Reproduced with permission from Ref. 15.
The sequential approach has been implemented by GE Health Care (Milwaukee, WI). and Philips Healthcare (Best, The Netherlands). The GE approach is essentially using two stand-alone systems in two separate but adjacent rooms that can be used either separately for performing PET or MRI studies or be used to shuttle a common mobile patient bed from one scanner room to the other scanner room. Attenuation correction of PET data is CT-based. This trimodality approach is the simplest in the sense of least technical hurdles with scanner integration and least challenges with workflow. Such a system allows for the independent use of the magnet system for clinical MR only imaging while PET imaging is being acquired on different patients. However, patient motion artifact between the 2 separate scans may be problematic (16).
The Philips approach is also a sequential approach that uses the Ingenuity time-of-flight PET scanner and a 3.0-T MRI placed next to each other in tandem in a large room with a patient bed that can be rotated 180° to transfer the patient from one scanner to the other scanner (17, 18). This system uses a MR-based attenuation correction scheme. Similar to the GE approach, the serial scanning approach by Philips is associated with less technical issues (e.g. shielding) but is probably more prone to patient motion artifact and is incapable of parallel data acquisition.
The Siemens scanner (Biograph mMR) is a fully integrated single gantry system with the PET detector ring placed within the 3.0-T MRI main magnet (19–21). This simultaneous acquisition approach offers potentially exquisite structural and functional images (Figure 2). Significant technical challenges had to be overcome including design and implementation of MRI-compatible PET photodetectors (avalanche photodiodes) and techniques for maintaining magnetic field homogeneity and novel MR-based attenuation correction of PET data. Comprehensive review of the work-in-progress regarding the various approaches (segmentation, atlas or template-based), including advantages and disadvantages, to MR-based attenuation correction of PET imaging data have been published elsewhere (22–27).
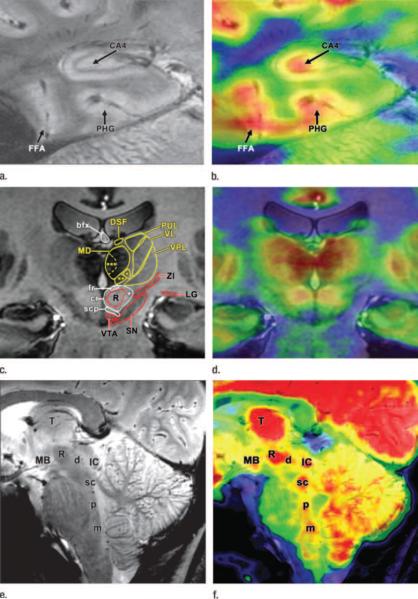
Figure 2.
MR and fused PET/MR high-spatial resolution images of three 23-year-old volunteers, two men and one woman, acquired with the same PET imager (Siemens Biograph mMR; Siemens Medical Solutions, Erlangen, Germany). (a) In first male volunteer, coronal 7-T T2*-weighted gradient-echo MR image (750/21; flip angle, 30°) and (b) fused PET/MR image of hippocampal region show subhippocampal resolution separating metabolic function of region cornu ammonis 4 (CA4) from surrounding structures including parahippocampal gyrus (PHG) and fusiform face area (FFA). (c) In second male volunteer, coronal 7-T T1-weighted three-dimensional magnetization-prepared rapid-acquisition gradient-echo MR image (4000/5.3/1000; flip angle 10°) and (d) fused PET/MR image of thalamic region show subthalamic resolution, allowing for structural and anatomic quantification of individual nuclei. Thalamic nuclei (yellow): centromedian thalamic nucleus (*), parafascicular thalamic nucleus (**), magnocellular part of medial dorsal thalamic nucleus (***), medial dorsal thalamic nucleus (MD), dorsal superficial nucleus (DSF), pulvinar (PUL), ventral lateral thalamic nucleus (VL), ventral posterior lateral thalamic nucleus (VPL). Fiber tracts (white): superior cerebellar peduncle (scp), capsule of red nucleus (cr), fasciculus retroflexus (fr), body of fornix (bfx), cerebellorubrothalamic fibers (1). Other structures (red): lateral geniculate nucleus (LG), zona incerta (ZI), substantia nigra (SN), ventral tegmental area (VTA), red nucleus (R). (e) In female volunteer, midline sagittal 7-T T2*-weighted gradient-echo MR image (750/16.8; flip angle, 30°) and (f) fused PET/ MR image through brainstem show detailed anatomy and metabolic function of raphe nuclei. Raphe nuclei: dorsal (d), superior central (sc), pontine (p), medullary (including magnus, obscurus, and pallidus) (m). mamillary body (MB), thalamus (T), red nucleus (R), inferior colliculus (IC). Reproduced with permission from Ref. 15.
Current Challenges
As with introduction of any new diagnostic imaging technique, there are many challenges that will need to be addressed. PET/MRI systems appear to have been technically developed ahead of detailed considerations for many important practical issues including identification of clinical applications that would benefit exclusively from simultaneous imaging acquisition or dual-modality imaging probes, clinic workflow, optimized scan protocols, artifact handling, regulatory requirements, risks and safety considerations, training and credentialing needs for image interpretation, comparative effectiveness, cost-utility, and study reimbursement issues (28–32). Many of these challenges are currently being worked out. The reader is directed to an excellent review that summarizes the workflow and scan protocol consideration for integrated whole-body PET/MRI in patients with cancer (33–36). With regards to quantitative accuracy of PET, preliminary evidence suggests that MR contrast agents will not have adverse effects and that PET-derived semi-quantitative values are highly correlated to those values obtained from PET/CT (37, 38). The issue of credentialing has begun to be addressed in the US by a joint task force from the American College of Radiology and the Society of Nuclear Medicine and Molecular Imaging. Much work will be needed with regards to comparative effectiveness in various clinical settings which will then provide evidence for implementation of reimbursement by payers justifying broad-spectrum purchase and clinical use of PET/MRI systems.
Potential Clinical Applications of Integrated PET/MRI
While it is commonly agreed upon that combination of both PET and MRI can be useful in the imaging evaluation of various pathologic states, the need for simultaneity is still in debate. Nevertheless, PET/MRI provides unparalleled structural, metabolic, and functional (Figure 3) information which can significantly impact diagnostic evaluation and affect clinical decision-making, patient management, and potentially patient outcome. It may also enhance patient convenience by providing a “one-stop shop” diagnostic imaging work-up, reducing patient anxiety, total scan time, potential recalls for repeat scanning. In this section, we briefly review the current evidence on use of PET/MRI in various disease processes that may pave the way for identification of unique clinical situations where an integrated PET/MRI would be the most appropriate and accurate imaging modality for diagnostic evaluation. However, at the outset, it may be that simultaneous PET/MRI may be best suited for clinical situations that are disease-specific, organ-specific, or related to diseases of the children or those patients in whom cumulative radiation dose is desired to be kept as low as reasonably achievable. Here we briefly provide general information on how and why simultaneous PET/MRI may be useful in various clinical settings.
Figure 3.
A 51-year-old man with suspected intrathoracic sarcoidosis had an arachnoid cyst incidentally identified by FDG PET/CT. A, Axial fusion images of integrated FDG PET/MRI show normal glucose metabolism adjacent to the cortex of a voluminous arachnoid cyst. B, Functional MRI obtained after right (green) versus left (red) hand finger tapping demonstrates large areas of activation between inferior frontal gyrus and postcentral gyrus. There was no neurological deficit due to this functional reorganization. Functional MRI superimposed on morphological T1-weighted 3-dimensional MRI demonstrate apparent extracortical overflow of the activation area in the right hemisphere presumed secondary to increased blood oxygen levels in large superficial cortical veins draining the very thin motor cortex. Pink areas represent artifacts associated with inhomogeneities in the magnetic correction field map. Reproduced with permission from Lippincott Williams & Wilkins; Hubele F, Imperiale A, Kremer S, Namer IJ. Clin Nucl Med 2012; 37:982–983.
Neuropsychiatric
Catana et al summarized the potential clinical application of PET/MRI in patients with various neurological disorders (39). As noted by these authors, the simultaneous acquisition of PET and MRI data may be quite useful in translational neuropsychiatric research by providing an opportunity to collect comprehensive quantitative imaging-based data on a number of physiologic events (e.g. perfusion, metabolism, oxygenation, etc).
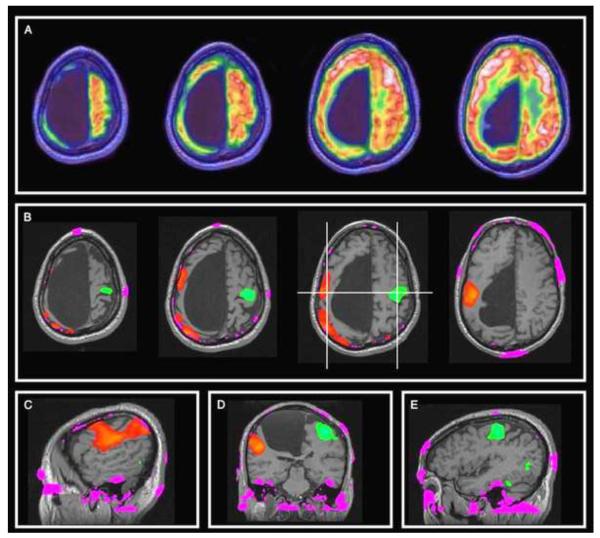
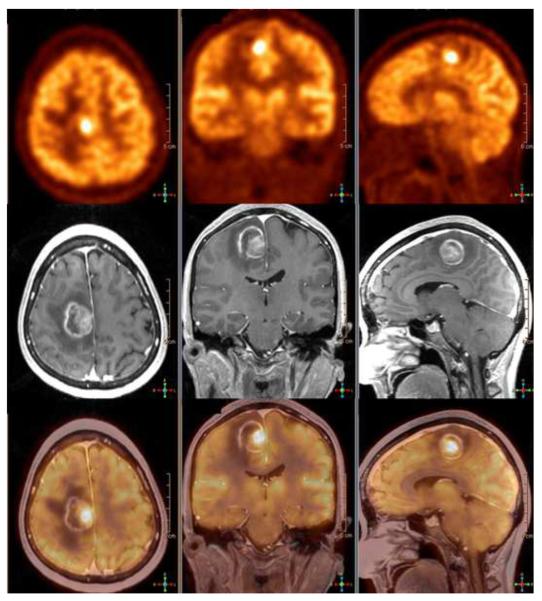
An area of growing interest in diagnostic imaging is in dementia work-up. This is further fueled by recent approval for use of PET agents for amyloid imaging (40). PET allows assessment of the relevant pathophysiology while MRI provides exquisite information on brain substructures as well as unique information such as diffusion tensor imaging, functional MRI, and default mode network (41–48). It is presumed that simultaneous PET/MRI can provide much convenience to these patients who are often elderly and may not tolerate repeated or long imaging sessions (49) (Figure 4). It can also help in deciphering the pathophysiology of the sequence of events that occurs in neurodegeneration in various dementia categories allowing not only for accurate differential diagnosis but also for objective evidence for clinical decision-making for a particular course of therapy.
Figure 4.
89-year-old woman with probable Lewy body dementia. MR images show diffuse atrophy, without regional specificity, whereas FDG PET shows significant posterior cortical hypometabolism, with preservation of posterior cingulate and precuneus metabolism, typically associated with Lewy body dementia. DaTSCAN results (not shown) were supportive for Lewy body disease. Reproduced with permission from Ref. 49.
PET (with a variety of relevant tracers including FDG) and MRI have also been separately used for localization of epileptogenic focus in patients with intractable seizure (Figure 5). It is clear that if a combined PET/MRI can provide sufficient information on localization of the epileptogenic focus, that will probably be preferred by the patient and the provider rather than performing these studies in two imaging sessions, often much separated in time from each other, delaying diagnosis and therapy.
Figure 5.
8 year old girl with chronic seizures. Co-registered independently acquired 18F-FDG PET and 3D SPGR MR axial, coronal, and sagittal images demonstrate left open schizencephaly.
Another important clinical arena that simultaneous PET/MRI may be contributory is in detection of salvageable brain in patients with stroke. It would be desirable if the clinician can decide on appropriate treatment (short-term and long-term) based on a comprehensive “one-stop shop “imaging information on the presence and extent of stroke. This can be, as previously suggested, a disease-specific route for determining the diagnostic utility of integrated PET/MRI in this important and prevalent clinical setting.
Cardiac
There is a rich and long published history of the utility of either PET with various radiotracers or MRI with various scan protocols in the imaging evaluation of the heart for an array of disorders. The pivotal question is whether simultaneous PET/MRI may provide a competitive advantage over separate clinical examinations. The answer to this question is not clearly evident at this time. However, we know that combining PET and MRI diagnostic information can be quite useful in deciphering various cardiac disease entities (50).
MRI provides efficient, accurate, and reproducible detailed morphological, functional, perfusion, and viability results in a 30 to 60 minute examination without ionizing radiation. While coronary artery anatomy may be interrogated with MRI techniques, reliable results are not universally reported. PET may provide quantitative information on myocardial perfusion, contractility, viability, and adrenogeric function, potentially predicting outcomes long before there are morphologic or functional abnormalities. Not all patients however can be imaged with MRI (e.g. patients with pacemakers, implantable cardioverter defibrillators, etc). Some patients may not tolerate the relatively enclosed environment of the magnet bore in view of claustrophobia. Patients with severe renal disease may also not be able to receive MR contrasts that can lead to nephrogenic systemic fibrosis although this condition is much less common than the nephropathy induced by the iodinated CT contrast agents.
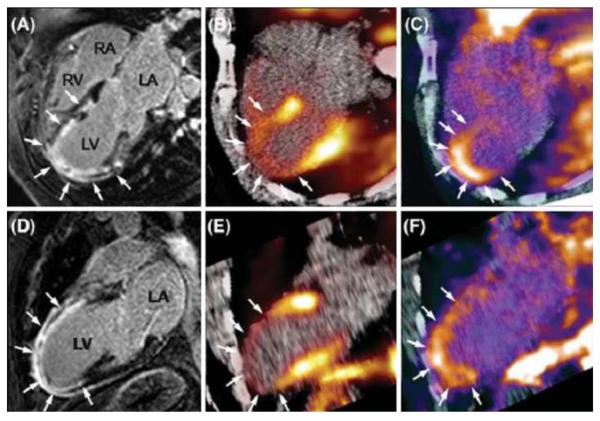
A possible workflow for combined PET/MRI cardiac study may last as long as 45 minutes which includes serial assessment of cardiac wall motion, attenuation correction sequence, assessment of coronaries and stress perfusion, another attenuation correction sequence followed by rest perfusion assessment and delayed enhancement and finally assessment for coronary arterial inflammation (50). This examination will provide comprehensive diagnostic information on pericardiac and cardiac morphology, valvular function, coronary arterial status (e.g. anatomy, stenosis, plaques), ischemia, infarction, myocardial viability, inflammation (myocarditis, pericarditis), cardiomyopathy, left ventricular wall motion and thickening, and metabolism (50, 51). There is also opportunity for specialized interrogation of other pathophysiologic events such as sympathetic innervation, angiogenesis, hypoxia, apoptosis, receptor expression and enzymatic activity (Figure 6). Additional interesting areas of investigation in the coming years would be related to PET/MRI assessment of response to gene therapy and evaluation of stem cell delivery, viability, and differentiation.
Figure 6.
Integration of delayed enhancement MRI, PET perfusion and αvβ3 integrin expression images in a patient with re-perfused myocardial infarction 2 weeks previously. A, D Four chamber view (A) and two chamber view (B) show delayed enhancement (arrows) extending from the anterior wall to the apical region. B, E Fusion of 13N-ammonia PET/MR images show severely reduced myocardial blood flow in the region of delayed enhancement (arrows). C, F Focal 18F-RGD activity colocalized to the infarcted area as seen by delayed enhancement MRI. 18F-RGD activity corresponds to the regions of severely reduced 13N-ammonia activity, reflecting the extent the of αvβ3 expression within the infarcted area noted on delayed contrast enhanced MR. Reproduced with permission from Springer: Nekolla SG, Martinez-Moeller A, Saraste A. PET and MRI in cardiac imaging: from validation studies to integrated applications. Eur J Nucl Med Mol Imaging 2009; 36 (Suppl 1):S121–S130.
Oncologic
Buchbender et al present a case-based summary of the potential utility of PET/MRI in patients with cancer (52, 53). As they note, PET/MRI may be highly accurate in T-staging of tumors for which MRI is routinely employed, such as head and neck cancer and primary bone and soft tissue tumors. For N-staging, the performance will probably be similar to PET/CT although use of the novel lymphotropic superparamagnetic nanoparticles may provide new opportunities for detection of nodal micrometastases that is not possible with PET/CT (54). For M-staging, the authors contend that MRI may provide higher accuracy for detection of lesions particularly in the brain, liver, and bone.
Kjaer et al reviewed their initial experience with the integrated PET/MRI system (Siemens Biograph mMR) that was installed at their facility in Copenhagen in December 2011 (55). A set of scanning protocols for specific organs (brain, head and neck, pelvis), patient population (e.g. children) and whole-body oncology are presented lasting from as short as 20 minutes (head and neck oncology) to as long as 45 minutes (whole-body oncology). The authors found that integrated PET/MRI improved delineation of brain tumors for radiation therapy planning and for defining post-surgical changes from viable tumor. For whole-body oncology they note that radiation exposure from CT can be avoided and that combined metabolic and diffusion-weighted imaging may improve assessment for treatment response.
Drzezga et al from Munich, Germany, also summarized their initial experience with the Siemens Biograph mMR scanner in 32 patients with a variety of cancers (19). In their protocol, PET/CT was first performed at about 86+/−8 minutes after FDG administration using a 2 minute per bed position followed by PET/MRI scanning at about 140+/−24 minutes post tracer injection using a 4 minute per bed position. They found that there was no significant difference between PET/CT and PET/MRI for detection of suspicious malignant lesions and that there was a high correlation between the mean standardized uptake values (SUVs) measured by either imaging modality for lesions (ρ=0.93) and background (ρ=0.92) activities.
In another investigation from Germany, 80 patients underwent single FDG injection, dual imaging protocol with PET/CT performed first (at mean of 73 minutes post injection and use of 3 minutes acquisition time per each of 7 bed positions) followed subsequently by PET/MRI (at mean of 172 minutes post injection and use of 10 minutes acquisition time per each of 4 bed positions)(56). MR-based attenuation correction was based on Dixon sequence. The concordance between PET/CT and PET/MRI for localization of lesions was 97.4%. No elevated noise and radiofrequency disturbances related to hardware cross talk between the PET and MR components were noted.
With the above overall summary on the usefulness of integrated PET/MRI system, we now briefly outline some of the studies that focused on specific tumor types.
Brain Tumors
It is interesting to note that the first application of combined PET/MRI was proposed for high quality anatomical, functional and metabolic imaging evaluation of the brain (14, 57). Boss et al reported on their initial experience with hybrid PET/MRI in patients with a number of different intracranial masses using 11C-acetate and 68-Gallium-DOTA-D-Phe1-Tyr-octreotide (68Ga-DOTATOC) as radiotracers (58). These investigators found no significant artifacts in the simultaneously acquired PET/MRI images with excellent agreement between computed tumor-to-reference tissue uptake ratios obtained from PET/MRI and PET/CT systems. Despite these early enthusiastic results, the exact role of simultaneously acquired PET/MRI (as opposed to fused images from different camera systems) in detection and localization of brain primary tumors and metastases needs further investigation. It is expected that MRI will contribute significantly in delineating cerebral lesions given its current major role in this clinical setting (Figures 7, 8). PET with a particular tracer can also provide clinically relevant information on tumor characterization and differentiation from post-therapy scar or for treatment planning (59). It is therefore generally reasonable to presume that PET/MRI will not only have a competitive advantage over PET/CT in this clinical arena but brain PET/MRI will probably be one of the main clinical applications of this combined imaging modality in the not too distant future.
Figure 7.
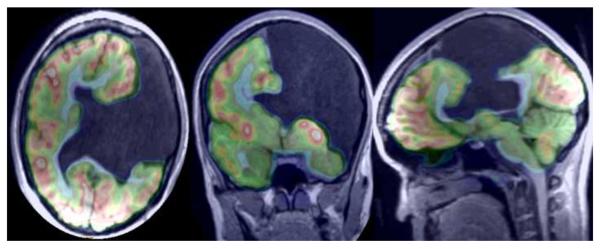
49 year old man with left leg weakness. Axial, coronal, and sagittal 18F-FDG PET (top row), contrast enhanced 3D SPGR (middle row) and co-registered PET/ MR images demonstrate an asymmetrical enhancing medial precentral gyrus lesion with a cystic component and prominent surrounding white matter edema. Hybrid images document significantly increased glucose metabolism within the most enhancing region, a Grade 4 glioma.
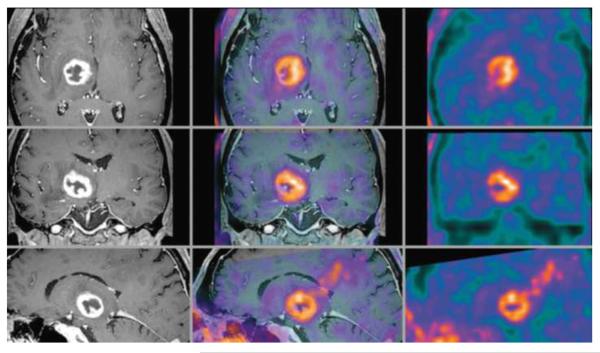
Figure 8.
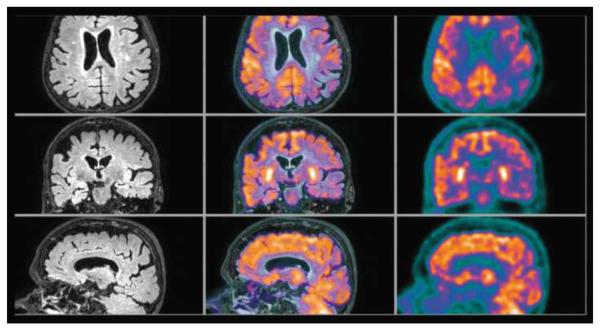
69-year-old man with right thalamic glioblastoma. Axial, coronal, and sagittal contrast enhanced T1 weighted MR (left column), inline hybrid 18F-fluoroethyltyrosine (FET) PET/MR (center column), and FET PET (right column) demonstrate significant ring contrast enhancement and intense FET uptake (estimated SUVmax is 3.4). Reproduced with permission from Ref. 49.
Head and Neck Cancer
MRI plays an important role in the imaging evaluation of head and neck due to its excellent soft tissue contrast that provides detailed anatomical information in a relatively small compartment. The utility of PET in head and neck cancer has also been established for detection of occult primary tumor, staging, restaging, treatment response assessment and prognostication (60, 61). Boss et al reported feasibility of performing simultaneous PET/MRI in a pilot study of 8 patients with head and neck cancer that demonstrated lack of recognizable artifacts from the PET insert and excellent agreement of metabolic ratios to those obtained from PET/CT (62). Platzek et al summarized their experience with the use of Philips Ingenuity time-of-flight PET/MRI scanner in 20 patients with head and neck squamous cell carcinoma (63). The patients first underwent a conventional PET (Siemens ECAT EXACT HR+) on average 64 minutes after FDG administration. PET/MRI was performed subsequently on average 177 minutes after tracer injection. The total PET/MRI scan time was 39 minutes. PET/MRI detected the tumor in 17 of 20 patients, 16 by either PET data set and 14 by MRI. The maximum SUVs of tumor sites from PET/MRI were significantly higher than those obtained from dedicated PET. This observation was thought to be related to temporal increase in tumor uptake level and possibly due to influence of the MRI-based attenuation correction scheme.
Lung Tumors
MRI is considered overall less sensitive than CT for detection of pulmonary lesions (64, 65). However, combination of PET metabolic information, use of certain MR sequences (half-Fourier, single-shot, turbo spin echo, and DWI) can provide clinically relevant information on primary and metastatic lung lesions including superior MRI-based delineation of tumor infiltration to adjacent tissues (66). Nevertheless, lung evaluation will probably not be the primary indication for combined PET/MRI but this assertion will need further investigation.
Breast Cancer
Specific data regarding the diagnostic performance of simultaneous PET/MRI of breast cancer is lacking. MR mammography is highly sensitive for detection of small tumor deposits but is limited by low specificity. PET also suffers from limited spatial resolution and can be falsely positive in non-malignant conditions (e.g. some fibroadenomas). However, as noted by Buchbender and colleagues, PET/MRI may be most useful in setting of evaluation for suspected tumor recurrence, response to neoadjuvant therapy, and prognosis (52). In the latter case, MRI can provide accurate delineation of the local tumor extent and PET supplies prognostically important information on tumor metabolism (67, 68).
Hepatic and Gastrointestinal Tumors
Information on the diagnostic utility of hybrid PET/MRI in hepatocellular carcinoma (HCC) is lacking (69, 70). However, based on what is already known about MRI and PET/CT in this clinical setting, it may be presumed that hybrid PET/MRI with use of an appropriate radiotracer (e.g. FDG for well-differentiate HCC and 11C-acetate or 18F-fluorocholine for poorly differentiated HCC) and multiparametric MRI (e.g. DCE, DWI) may provide a “one-stop shop” accurate imaging evaluation of HCC (71, 72). With regards to hepatic metastases, results from separately acquired PET/CT and MRI fusion images suggest that PET/MRI may have an advantage over PET/CT (73).
Beiderwellen and colleagues from Germany reported recently on their initial experience with simultaneous PET/MRI with 68Ga-DOTATOC in comparison to PET/CT in patients with gastroenterpancreatic neuroendocrine tumors (74). PET/MRI and PET/CT were essentially equivalent in their diagnostic performance for most lesions except that PET/MRI missed a number of lung and hypersclerotic bone lesions. It remains for future studies to elucidate whether PET/MRI may provide a competitive advantage over PET/CT in this clinical setting.
Prostate Cancer
MRI plays and important role in the clinical imaging evaluation of prostate gland (75, 76). There is also much active research that is currently underway for the deciphering the utility of PET with various radiotracers in different clinical phases of prostate cancer (77). Simultaneous PET/MRI may provide a valuable platform for accurate imaging assessment of the prostate gland providing opportunity for targeted biopsy reducing the current high rate of negative “blind” initial and repeated biopsies as well as facilitating the prospects for targeted focal therapy (male lumpectomy) (Figure 9). There may also be other unique applications in the imaging evaluation of patients with biochemical failure and evaluation of treatment response and prognosis in metastatic disease.
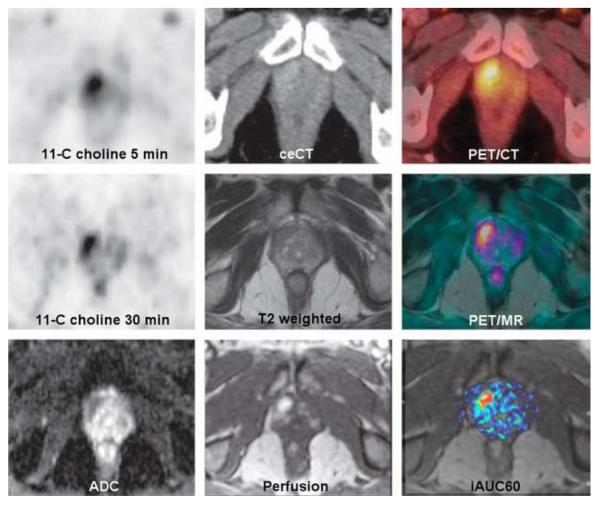
Figure 9.
A 70-year-old man with an elevated level of prostate-specific antigen (17.7 ng/mL) and prior negative transrectal biopsy. Top row, left to right: 11C-choline PET at 5 minutes, contrast enhanced CT, and hybrid PET/CT demonstrates slight enhancement of the right prostate apex with increased choline uptake. Middle row, left to right: 11C-choline PET at 30 minutes, T2 weighted MR, and fully integrated hybrid PET/MR (Siemens Biograph mMR; Siemens Medical Solutions, Erlangen, Germany) shows vague T2 increase and continued prominent choline uptake. Lower row, left to right: Apparent diffusion coefficient MR, early perfusion MR, and area under curve within 60 seconds documents restricted diffusion, increased perfusion with early wash-in of contrast agent. Re-biopsy of the ventral periphery of the right apex demonstrated high-grade prostatic intraepithelial neoplasia. Reproduced with permission from Lippincott Williams & Wilkins; Takei T, Souvatzoglou M, Beer AJ, Ambros J, et al. A Case of Multimodality Multiparametric 11C-Choline PET/MR for Biopsy Targeting in Prior Biopsy-Negative Primary Prostate Cancer. Clin Nucl Med 2012; 37:918–919.
Wetter et al reported their initial experience with simultaneous PET/MRI of the prostate gland using 18F-fluorocholine as the radiotracer in 8 patients with histologically proven prostate cancer, 2 patients with suspected cancer despite negative repeated biopsy results and 5 patients with suspected prostate cancer based on elevated or rising prostate specific antigen (78). High-resolution T2 weighed images localized the areas of elevated 18F-fluorocholine uptake and there was a high concordance between PET and DWI.
Gynecological Tumors
There are no data on the potential use of hybrid PET/MRI in gynecological cancers. However, it is recognized that the combination and excellent soft tissue contrast and diffusion-weighted imaging with MRI in combination with various radiotracers in conjunction with PET may provide a unique opportunity for PET/MRI in the imaging evaluation of female pelvis at lower radiation exposure in comparison to PET/CT. Limited data obtained from fused separate PET and MRI studies in this clinical setting are supportive of such supposition (79–81).
Soft tissue and Bone Tumors
Bouchbender et al summarize the data from fused PET and MRI in patients with bone and soft-tissue tumors as well as in lymphoma and melanoma (53). Based on these data, it appears that PET/MRI may have a competitive advantage over PET/CT in T-staging of primary bone and soft tissue tumors and in M-staging for hepatic and cerebral metastases. For N-staging, PET/MRI will probably yield similar performance to PET/CT. While data on simultaneous PET/MRI in this setting is lacking, single case reports or other evidence suggest a potentially major role for hybrid PET/MRI in the imaging evaluation of patients with lymphoma, melanoma, and bone and soft tissue tumors (82–88).
Musculoskeletal
PET/MRI provides an interesting opportunity to assess for musculoskeletal disorders in one setting. MRI is commonly used for assessment of joints, ligaments, tendons, cartilaginous structures, and bone marrow (89). PET with FDG has been shown to be useful in the imaging evaluation of infection including differential diagnosis of osteomyelitis and Charcot joint in patients with diabetes mellitus (90, 91). It is therefore reasonable to consider that combined PET/MRI may be helpful in a number of specific clinical situations such as assessment of arthridies and diabetic foot (92). However, whether simultaneity of this combined assessment would be beneficial in some way (clinical and financial) will need additional work.
Infection and Inflammation
Both PET with FDG and MRI have been shown to be useful for diagnostic evaluation of a variety of inflammatory and infectious processes (92–94). These may include spondylodiscitis, inflammatory bowel disease (Crohn's and ulcerative colitis), autoimmune inflammatory disorders (e.g. Sjogren's syndrome), sarcoidosis, arthritidies (rheumatoid arthritis, psoriatic arthritis, osteoarthritis), vasculitidies, systematic lupus erythematosus and metabolic syndrome. The inquiry remains whether simultaneous PET/MRI may a competitive advantage over separate imaging examinations if indeed both are considered to be performed in the same patient. At this time use of FDG PET in patients with infectious and inflammatory conditions are underutilized, despite proven potential utility, in view of lack of reimbursement and probably lack of awareness on the part of referring clinicians and even imaging specialists. The use of hybrid PET/MRI in this clinical setting, while overall rational, will need further evidence.
Pediatrics
At the outset, it appears logical that PET/MRI may be the multimodality of choice (over PET/CT) in view of less radiation exposure and all that is offered by MRI and PET for a comprehensive structural and metabolic assessment of pediatric patients. Unfortunately to date there are no relatively large-scale studies on the utility of the combined use of PET/MRI in children. However, small scale case studies or retrospective correlative investigations have demonstrated utility in specific clinical settings (95–98). Nevertheless, it is generally accepted that hybrid PET/MRI may play a significant role in the care of children, particularly with cancer, who may need to undergo repeated diagnostic imaging sessions and as such it is desirable to minimize their cumulative radiation exposure (99).
Conclusion
Clinical hybrid PET/MRI has become available and will probably be preferred over PET/CT in pediatrics or for evaluation of specific diseases, anatomical regions, or organs. PET/MRI also offers interesting opportunities for use of dual modality probes in both research and clinical arenas. It is expected that as exact clinical indications are defined in the near future, the other important logistical issues (clinical workflow, regulatory and reimbursement) will also be worked out.
Acknowledgments
Support: National Institutes of Health, National Cancer Institute, grants R01-CA111613 and R21-CA142426 (PI: H. Jadvar).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 2.Basu S, Alavi A. Unparalleled contribution of 18F-FDG PET to medicine over 3 decades. J Nucl Med. 2008;49(10):17N–21N. 37N. [PubMed] [Google Scholar]
- 3.Shao Y, Cherry SR, Farahani K, et al. Simultaneous PET and MR imaging. Phys Med Biol. 1997;42:1965–1970. doi: 10.1088/0031-9155/42/10/010. [DOI] [PubMed] [Google Scholar]
- 4.Tatsumi M, Yamamoto S, Imaizumi M, et al. Simultaneous PET/MRI body imaging in rats: initial experiences with an integrated PET/MRI scanner. Ann Nucl Med. 2013;27:225–232. doi: 10.1007/s12149-012-0581-8. [DOI] [PubMed] [Google Scholar]
- 5.Judenhofer M, et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14:459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 6.Wehrl HF, Judenhofer MS, Wiehr S, et al. Preclinical PET/MR: technological advances and ew perspectives in biomedical research. Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S56–68. doi: 10.1007/s00259-009-1078-0. [DOI] [PubMed] [Google Scholar]
- 7.Gaertner FC, Furst S, Schwaiger M. PET/MR: a paradigm shift. Cancer Imaging. 2013;13:36–52. doi: 10.1102/1470-7330.2013.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pichler B, et al. Positron emission tomography/magnetic resonance imaging: the next generation of multimodality imaging? Semin Nucl Med. 2008;38:199–208. doi: 10.1053/j.semnuclmed.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pichler BJ, Kolb A, Nagele T, et al. PET/MRI: paving the way for the next generation of clinical multimodality imaging applications. J Nucl Med. 2010;51:333–336. doi: 10.2967/jnumed.109.061853. [DOI] [PubMed] [Google Scholar]
- 10.Sauter AW, Wehrl HF, Kolb A, et al. Combined PET/MRI: one step further in multimodality imaging. Trends Mol Med. 2010;16:508–515. doi: 10.1016/j.molmed.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 11.von Schulthess GK, Schlemmer HP. A look ahead: PET/MRI versus PET/CT. Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S3–S9. doi: 10.1007/s00259-008-0940-9. [DOI] [PubMed] [Google Scholar]
- 12.Werner MK, Schmidt H, Schwenzer NF. MR/PET: a new challenge in hybrid imaging. AJR Am J Roentgenol. 2012;199:272–277. doi: 10.2214/AJR.12.8724. [DOI] [PubMed] [Google Scholar]
- 13.Schiepers C, Dahlbom M. Molecular imaging in oncology: the acceptance of PET/CT and the emergence of MR/PET imaging. Eur Radiol. 2011;21:548–554. doi: 10.1007/s00330-010-2033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlemmer HP, Pichler BJ, Schmand M, et al. Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology. 2008;248:1028–1035. doi: 10.1148/radiol.2483071927. [DOI] [PubMed] [Google Scholar]
- 15.Torigian DA, Zaidi H, Kwee TC, et al. PET/MR imaging: technical aspects and potential clinical applications. Radiology. 2013;267:26–44. doi: 10.1148/radiol.13121038. [DOI] [PubMed] [Google Scholar]
- 16.Veit-Haibarch P, Kohn FP, Weisinger F, et al. PET/MR imaging using a tri-modality PET/CT/MR system with a dedicated shuttle in clinical routine. MAGMA. 2013;26:25–35. doi: 10.1007/s10334-012-0344-5. [DOI] [PubMed] [Google Scholar]
- 17.Zaidi H, Ojha N, Morich M, et al. Design and performance evaluation of a whole-body Ingenuity TF PET/MRI system. Phys Med Biol. 2011;56:3091–3106. doi: 10.1088/0031-9155/56/10/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalemis A, Delattre BM, Heinzer S. Sequential whole-body PET/MR scanner: concept, clinical use, and optimization after two years in the clinic. The manufacturer's perspective. MAGMA. 2013;26:5–23. doi: 10.1007/s10334-012-0330-y. [DOI] [PubMed] [Google Scholar]
- 19.Drzezga A, Souvatzoglou M, Eiber M, et al. First clinical experience of integrated whole-body PET/MR: comparison to PET/CT in patients with oncological diagnoses. J Nucl Med. 2012;53:845–855. doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 20.Zaidi H, Del Guerra A. An outlook on future design of hybrid PET/MRI systems. Med Phys. 2011;38:5667–3689. doi: 10.1118/1.3633909. [DOI] [PubMed] [Google Scholar]
- 21.Delso G, Furst S, Jakoby B, et al. Performance measurement of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52:1914–1922. doi: 10.2967/jnumed.111.092726. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann M, et al. Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques. Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S93–S103. doi: 10.1007/s00259-008-1007-7. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Lee JS, Song I-C, et al. Comparison of segmentation-based attenuation correction methods for PET/MRI: evaluation of bone and liver standardized uptake value with oncologic PET/CT data. J Nucl Med. 2012;53:1878–1882. doi: 10.2967/jnumed.112.104109. [DOI] [PubMed] [Google Scholar]
- 24.Keller SH, Holm S, Hansen AE, et al. Image artifacts from MR-based attenuation correction in clinical, whole-body PET/MRI. MAGMA. 2013;26:173–181. doi: 10.1007/s10334-012-0345-4. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Moller A, Souvatzoglou M, Delso G, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50:520–526. doi: 10.2967/jnumed.108.054726. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Moller A, Nekolla SG. Attenuation correction for PET/MR: problems, novel approaches and practical solutions. Z Med Phys. 2012;22:299–310. doi: 10.1016/j.zemedi.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Keereman V, Mollet P, Berker Y, et al. Challenges and current methods for attenuation correction in PET/MR. MAGMA. 2012;26:81–98. doi: 10.1007/s10334-012-0334-7. [DOI] [PubMed] [Google Scholar]
- 28.Yankeelov TE, Peterson TE, Abramson RG, et al. Simultaneous PET-MRI in oncology: a solution looking for a problem. Magn Reson Imag. 2012;30:1342–1356. doi: 10.1016/j.mri.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brix G, Nekolla EA, Nosske D, et al. Risks and safety aspects related to PET/MR examination. Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S131–S138. doi: 10.1007/s00259-008-0937-4. [DOI] [PubMed] [Google Scholar]
- 30.Sattler B, Kochimsen T, Barthel H, et al. Physical and organizational provision for installation, regulatory requirement and implementation of a simultaneous hybrid PET/MR-imaging system in an integrated research and clinical setting. MAGMA. 2012;26:159–171. doi: 10.1007/s10334-012-0347-2. [DOI] [PubMed] [Google Scholar]
- 31.Chun SY, Reese TG, Ouyang J, et al. MRI-based nonrigd motion correction in simultaneous PET/MRI. J Nucl Med. 2012;53:1284–1291. doi: 10.2967/jnumed.111.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glaus C, Rossin R, Welch MJ, et al. In vivo evaluation of (64)Cu-labeled magnetic nanoparticles as a dual-modality PET/MR imaging agents. Bioconjug Chem. 21:715–722. doi: 10.1021/bc900511j. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Moller A, Eiber M, Nekolla SG, et al. Workflow and scan protocol considerations for integrated whole-body PET/MRI in oncology. J Nucl Med. 2012;53:1415–1426. doi: 10.2967/jnumed.112.109348. [DOI] [PubMed] [Google Scholar]
- 34.von Schulthess GK, Kuhn FP, Kaufmann P, et al. Clinical positron emission tomography/magnetic resonance imaging applications. Semin Nucl Med. 2013;43:3–10. doi: 10.1053/j.semnuclmed.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 35.von Schulthess GK, Burger C. Integrating imaging modalities: what makes sense from workflow perspective? Eur J Nucl Med Mol Imaging. 2010;37:980–990. doi: 10.1007/s00259-009-1378-4. [DOI] [PubMed] [Google Scholar]
- 36.Vargas MI, Becker M, garibotto V, et al. Approaches for the optimization of MR protocols in clinical hybrid PET/MRI studies. MAGMA. 2013;26:57–69. doi: 10.1007/s10334-012-0340-9. [DOI] [PubMed] [Google Scholar]
- 37.Lois C, Bezrukov I, Schmidt H, et al. Effect of MR contrast agents on quantiatiev accuracy of PET in combined whole-body PET/MR imaging. Eur J Nucl Med Mol Imaging. 2012;39:1756–66. doi: 10.1007/s00259-012-2190-0. [DOI] [PubMed] [Google Scholar]
- 38.Wiesmuller M, Qick HH, navalpakkam B, et al. Comparison of lesion detection and quantitation of racer uptake between PET from a simultaneously acquiring whole-body PET/MR hybrid scanner and PET from PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:12–21. doi: 10.1007/s00259-012-2249-y. [DOI] [PubMed] [Google Scholar]
- 39.Catana C, Drzezga A, Heiss W-D, et al. PET/MRI for neurologic applications. J Nucl Med. 2012;53:1916–1925. doi: 10.2967/jnumed.112.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Rieves D, Ganley C. Brain amyloid imaging--FDA approval of florbetapir F18 injection. New Engl J Med. 2012;367:885–7. doi: 10.1056/NEJMp1208061. [DOI] [PubMed] [Google Scholar]
- 41.Cho ZH, Son YD, Choi EJ, et al. In-vivo human brain molecular imaging with a brain-dedicated PET/MRI. MAGMA. 2013;26:71–79. doi: 10.1007/s10334-012-0329-4. [DOI] [PubMed] [Google Scholar]
- 42.Cho ZH, Son YD, Kim HK, et al. Observation of glucose metabolism in the thalamic nuclei by fusion PET/MRI. J Nucl Med. 2011;52:401–404. doi: 10.2967/jnumed.110.081281. [DOI] [PubMed] [Google Scholar]
- 43.Cho ZH, Son Y-D, Kim H-K, et al. Substructural hippocampal glucose metabolism observed on PET/MRI. J Nucl Med. 2010;51:1545–1548. doi: 10.2967/jnumed.110.076182. [DOI] [PubMed] [Google Scholar]
- 44.Chua TC, Wen W, Slavin MJ, et al. Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: a review. Curr Opin Neurol. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- 45.Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal memory system in mild cognitive impairment and Alzheimer's disease: insights from functional MRI studies. Neuropsychologia. 2008;46:1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greicius MD, Srivastava G, Reiss AL, et al. Default-mode network activity distinguishes Alzheimer's disease vrom healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heiss W. The potential of PET/MR for brain imaging. Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S105–S112. doi: 10.1007/s00259-008-0962-3. [DOI] [PubMed] [Google Scholar]
- 48.Boss A, Kolb A, Hofmann M, et al. Diffusion tensor imaging in a human PET/MR hybrid system. Invest Radiol. 2010;45:270–274. doi: 10.1097/RLI.0b013e3181dc3671. [DOI] [PubMed] [Google Scholar]
- 49.Garibotto V, Heinzer S, Vulliemoz S, et al. Clinical applications of hybrid PET/MRI in neuroimaging. Clin Nucl Med. 2013;38:e13–e18. doi: 10.1097/RLU.0b013e3182638ea6. [DOI] [PubMed] [Google Scholar]
- 50.Rischpler C, Nekolla SG, Dregely I, et al. Hybrid PET/MR imaging of the heart: potential, initial experiences, and future prospects. J Nucl Med. 2013;54:402–415. doi: 10.2967/jnumed.112.105353. [DOI] [PubMed] [Google Scholar]
- 51.Lee WW, Marinelli B, van der Laan AM, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59:153–163. doi: 10.1016/j.jacc.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchbender C, Heusner TA, Lauenstein TC, et al. oncologic PET/MRI, Part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J Nucl Med. 2012;53:928–938. doi: 10.2967/jnumed.112.105338. [DOI] [PubMed] [Google Scholar]
- 53.Buchbender C, Heusner TA, Lauenstein TC, et al. Oncologic PET/MRI, Part 2: Bone tumors, soft-tissue tumors, melanoma, and lymphoma. J Nucl Med. 2012;53:1244–1252. doi: 10.2967/jnumed.112.109306. [DOI] [PubMed] [Google Scholar]
- 54.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. New Engl J Med. 2003;348:2491–9. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 55.Kjaer A, Loft A, law I, et al. PET/MRI in cancer patients: first experiences and vision from Copenhagen. MAGMA. 2013;26:37–47. doi: 10.1007/s10334-012-0357-0. [DOI] [PubMed] [Google Scholar]
- 56.Quick HH, von Gall C, Zellinger M, et al. Integrated whole-body PET/MR hybrid imaging: clinical experience. Invest Radiol. 2013;48:280–289. doi: 10.1097/RLI.0b013e3182845a08. [DOI] [PubMed] [Google Scholar]
- 57.Schwenzer NF, Stegger L, Bisdas S, et al. Simultaneous PET/MR imaging in a human brain PET/MR system in 50 patients-current state of imaging quality. Eur J Radiol. 2012;81:3472–3478. doi: 10.1016/j.ejrad.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 58.Boss A, Bisdas S, Kolb A, et al. Hybrid PET/MRI of intracranial masses: initial experiences and comparison to PET/CT. J Nucl Med. 2010;51:1198–1205. doi: 10.2967/jnumed.110.074773. [DOI] [PubMed] [Google Scholar]
- 59.Thorwarth D, Henke G, Muller AC, et al. Simultaneous 68Ga-DOTATOC-PET/MRI for IMRT treatment planning for meningioma: first experience. Int J radiat Oncol Biol Phys. 2011;81:277–283. doi: 10.1016/j.ijrobp.2010.10.078. [DOI] [PubMed] [Google Scholar]
- 60.Blodgett TM, Fukui MB, Snyderman CH, et al. Combined PET-CT in the head and neck: part 1. Physiologic, altered physiologic, and artifactual FDG uptake. Radiographics. 2005;25:897–912. doi: 10.1148/rg.254035156. [DOI] [PubMed] [Google Scholar]
- 61.Fukui MB, Blodgett TM, Snyderman CH, et al. Combined PET-CT in the head and neck: part 2. Diagnostic uses and pitfalls of oncologic imaging. Radiographics. 2005;25:913–930. doi: 10.1148/rg.254045136. [DOI] [PubMed] [Google Scholar]
- 62.Boss A, Stegger L, Bisdas S, et al. Feasibilty of simultaneous PET/MR imaging in the head and upper neck area. Eur Radiol. 2011;21:1439–1446. doi: 10.1007/s00330-011-2072-z. [DOI] [PubMed] [Google Scholar]
- 63.Platzek I, Beuthien-Baumann B, Schneider M, et al. PET/MRI in head and neck cancer: initial experience. Eur J Nucl Med Mol Imaging. 2013;40:6–11. doi: 10.1007/s00259-012-2248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antoch G, Backisch A. Combined PET/MRI: a new dimension in whole-body oncologic imaging? Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S113–S126. doi: 10.1007/s00259-008-0951-6. [DOI] [PubMed] [Google Scholar]
- 65.Antoch G, Vogt FM, Freudenberg LS, et al. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA. 2003;290:3199–3206. doi: 10.1001/jama.290.24.3199. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt H, Brendle C, Schrami C, et al. Correlation of simultaneously acquired diffusion-weighted imaging and 2-deoxy-[18F]fluoro-2-D-glucose positron emission tomography of pulmonary lesions in a dedicated whole-body magnetic resonance/positron emission tomography system. Invest Radiol. 2013;48:247–255. doi: 10.1097/RLI.0b013e31828d56a1. [DOI] [PubMed] [Google Scholar]
- 67.Shamim SA, Torigian DA, Kumar R. PET, PET/CT, and PET/MRI assessment of breast cancer. PET Clin. 2008;3:81–393. doi: 10.1016/j.cpet.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 68.Aklan B, Paulus DH, Wenkel E, et al. Toward simultaneous PET/MR breast imaging: systematic evaluation and integration of a radiofrequency breast coil. Med Phys. 2013;40:024301. doi: 10.1118/1.4788642. [DOI] [PubMed] [Google Scholar]
- 69.Schwenzer NF, Schmidt H, Claussen CD. Whole-body MR/PET: applications in abdominal imaging. Addom Imaging. 2012;37:20–28. doi: 10.1007/s00261-011-9809-7. [DOI] [PubMed] [Google Scholar]
- 70.Schlemmer HP, Pichler BJ, Krieg R, et al. An integrated MR/PET system: prospective applications. Abdom Imaging. 2009;34:668–74. doi: 10.1007/s00261-008-9450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Martino M, Marin D, Guerrisi A, et al. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology. 2010;256:806–816. doi: 10.1148/radiol.10091334. [DOI] [PubMed] [Google Scholar]
- 72.Jadvar H. Hepatocellular carcinoma and gastroenteropancreatic neuroendocrine tumors: potential role of other positron emission tomography radiotracers. Semin Nucl Med. 2012;42:247–254. doi: 10.1053/j.semnuclmed.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Yong TW, Yuan ZZ, Jun Z, et al. Sensitivity of PET/MRI images in liver metsatses from colorectal carcinoma. Hell J Nucl Med. 2011;14:264–268. [PubMed] [Google Scholar]
- 74.Beiderwellen KJ, Poeppel TD, Hartung-Knemeyer V, et al. Simultaneous 68Ga-DOTADOC PET/MRI in patients with gastroenteropancreatic neuroendocrine tumors: initial results. Invest Radiol. 2013;48:273–279. doi: 10.1097/RLI.0b013e3182871a7f. [DOI] [PubMed] [Google Scholar]
- 75.Wu LM, Xu JR, Ye YQ, et al. The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR Am J Roentgenol. 2012;199:103–110. doi: 10.2214/AJR.11.7634. [DOI] [PubMed] [Google Scholar]
- 76.Park H, Wood D, Hussain H, et al. Introducing parametric fusion PET/MRI of primary prostate cancer. J Nucl Med. 2012;53:546–551. doi: 10.2967/jnumed.111.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jadvar H. Molecular Imaging of prostate cancer: PET radiotracers. AJR Am J Roentgenol. 2012;199:278–291. doi: 10.2214/AJR.12.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wetter A, Lipponer C, Nensa F, et al. Simultaneous 18F choline positron emission tomography/magnetic resonance imaging of the prostate: initial results. Invest Radiol. 2013;48:256–262. doi: 10.1097/RLI.0b013e318282c654. [DOI] [PubMed] [Google Scholar]
- 79.Kim SK, Choi HJ, Park SY, et al. Additional value of MR/PET fusion compared with PET/CT in the detection of lymph node metastases in cervical cancer patients. Eur J Cancer. 2008;45:2103–2109. doi: 10.1016/j.ejca.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 80.Fiaschetti V, Calabria F, Crusco S, et al. MR-PET fusion imaging in evaluating adnexal lesions: a preliminary study. Radiol Med. 2011;116:1288–1302. doi: 10.1007/s11547-011-0720-7. [DOI] [PubMed] [Google Scholar]
- 81.Nakajo K, tatsumi M, Inoue A, et al. Diagnostic performance of fluorodeoxyglucose positron emission tomography/magnetic resonance imaging fusion images of gynecological malignant tumors: comparison with positron emission tomography/computed tomography. Jpn J Radiol. 2010;28:95–100. doi: 10.1007/s11604-009-0387-3. [DOI] [PubMed] [Google Scholar]
- 82.Vargas MI, Garibotto V, Viallon M, et al. Peripheral nerves, tumors, and hybrid PET-MRI. Clin Nucl Med. 2013;38:e40–42. doi: 10.1097/RLU.0b013e31824c6013. [DOI] [PubMed] [Google Scholar]
- 83.Punwani S, Taylor SA, Saad ZZ, et al. Diffusion-weighted MRI of lymphoma: prognostic utility and implications for PET/MRI? Eur J Nucl Med Mol Imaging. 2013;40:373–385. doi: 10.1007/s00259-012-2293-7. [DOI] [PubMed] [Google Scholar]
- 84.Tewfik JN, Greene GS. Fluorine-18-deoxyglucose positron emission tomography imaging with magnetic resonance and computed tomographic correlation in the evaluation of bone and soft-tissue sarcomas: a pictorial essay. Curr Probl Diagn Radiol. 2008;37:178–188. doi: 10.1067/j.cpradiol.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt GP, Kramer H, Reiser MF, et al. Whole-body magnetic resonance and positron emission tomography-computed tomography in oncology. Top Magn Reson Imaging. 2007;18:193–202. doi: 10.1097/RMR.0b013e318093e6bo. [DOI] [PubMed] [Google Scholar]
- 86.Wu X, Korkola P, Pertovaara H, et al. No correlation between glucose metabolism and apparent diffusion coefficient in diffuse large B-cell lymphoma: a PET/CT and DW-MRI study. Eur J Radiol. 2011;79:e117–21. doi: 10.1016/j.ejrad.2011.04.062. [DOI] [PubMed] [Google Scholar]
- 87.Rahmouni A, Luciani A, Itti E. MRI and PET in monitoring response in lymphoma. Cancer Imaging. 2005;5(Spec No A):S106–12. doi: 10.1102/1470-7330.2005.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laurent V, Trausch G, Bruot O, et al. Compartaive study of tow whole-body imaging techniques in the case of melanoma metastases: advantages of multi-contrast MRI examination including a diffusion-weighted sequence in comparison with PET/CT. Eur J Radiol. 2012;22:1537–1546. doi: 10.1016/j.ejrad.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 89.Chen K, Blebera J, Laredo JD, et al. Evaluation of musculoskeletal disorders with PET, PET/CT, and PET/MRI. PET Clin. 2008;3:451–463. doi: 10.1016/j.cpet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Basu S, Chryssikos T, Houseini M, et al. Potential role of FDG PET in the setting of diabetic neuroosteoarthropathy: can it differentiate uncomplicated Charcot's neuroarthropathy from osteomyelitis and soft tissue infection? Nucl Med Commun. 2007;28:465–472. doi: 10.1097/MNM.0b013e328174447f. [DOI] [PubMed] [Google Scholar]
- 91.Nawaz A, Torigian DA, Siegelemann ES, et al. Diagnostic performance of FDG PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomeylitis in the diabetic foot. Mol Imaging Biol. 2010;12:335–342. doi: 10.1007/s11307-009-0268-2. [DOI] [PubMed] [Google Scholar]
- 92.Miese F, Scherer A, Ostendorf B, et al. Hybrid 18F-FDG PET-MRI of the hand in rheumatoid arthritis: initial results. Clin Rheumatol. 2011;30:1247–1250. doi: 10.1007/s10067-011-1777-3. [DOI] [PubMed] [Google Scholar]
- 93.Glaudemans AWJM, Quintero AM, Signore A. PET/MRI in infectious and inflammatory diseases: will it be a useful improvement. Eur J Nucl Med Mol Imaging. 2012;39:745–749. doi: 10.1007/s00259-012-2060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maccioni F, Patak MA, Signore A, et al. New frontiers of MRI in Crohn's disease: motility imaging, diffusion-weighted imaging, perfusion MRI, MR spectroscopy, molecular imaging, and hybrid imaging (PET/MRI) Abdom Imaging. 2012;37:974–982. doi: 10.1007/s00261-012-9890-6. [DOI] [PubMed] [Google Scholar]
- 95.Salamon N, Kung J, Shaw SJ, et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology. 2008;71(20):1594–601. doi: 10.1212/01.wnl.0000334752.41807.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rubí S, Setoain X, Donaire A, et al. Validation of FDG-PET/MRI coregistration in nonlesional refractory childhood epilepsy. Epilepsia. 2011;52(12):2216–24. doi: 10.1111/j.1528-1167.2011.03295.x. [DOI] [PubMed] [Google Scholar]
- 97.Jissendi-Tchofo P, Pandit F, Vallée L, et al. Brain regional glucose uptake changes in isolated cerebellar cortical dysplasia: qualitative assessment using coregistrated FDG-PET/MRI. Cerebellum. 2012;11:280–8. doi: 10.1007/s12311-011-0309-7. [DOI] [PubMed] [Google Scholar]
- 98.Pfluger T, Melzer HI, Mueller WP, et al. Diagnostic value of combined (18)F-FDG PET/MRI for staging and restaging in pediatric oncology. Eur J Nucl Med Mole Imaging. 2012;39:1745–55. doi: 10.1007/s00259-012-2228-3. [DOI] [PubMed] [Google Scholar]
- 99.Fahey FH, Treves ST, Adelstein SJ. Minimizing and communicating radiation risk in pediatric nuclear medicine. J Nucl Med. 2011;52:1240–1251. doi: 10.2967/jnumed.109.069609. [DOI] [PubMed] [Google Scholar]