Abstract
Adolescence is an important period for initiation of smoking and manifestation of depression, which are often comorbid. Researchers have examined associations between depressive symptoms and smoking to elucidate whether those with increased depressive symptoms smoke more to self-medicate, whether those who smoke experience increased subsequent depressive symptoms, or both. Collectively, there have been mixed findings; however, studies have been limited by a) cross-sectional or short-term longitudinal data, or b) the use of methods that test associations, or only one direction in the associations, rather than a fully-reciprocal model to examine directionality. This study examined the associations between smoking and depressive symptoms in a sample of adolescent girls using latent dual change scores to model 1) the effect of smoking on change in depressive symptoms, and simultaneously, 2) the effect of depressive symptoms on change in smoking across ages 11 to 20. Data were from a cohort-sequential prospective longitudinal study (N = 262). Girls were enrolled by age cohort (11, 13, 15, and 17 years) and were primarily White (61%) or African American (31%). Data were restructured by age. Every 6 months, girls reported depressive symptoms and cigarette use. Results indicated that, controlling for socio-demographic characteristics, higher levels of smoking predicted a greater increase in depressive symptoms across adolescence. These findings suggest that a higher level of cigarette smoking does contribute to more depressive symptoms, which has implications for prevention of depression and for intervention and future research.
Keywords: smoking, depressive symptoms, adolescent, longitudinal, latent dual change score model
Adolescence is an important period for the transition into nicotine use and dependence (Bachman, Johnston, O’Malley, & Schulenberg, 1996; Chassin, Presson, Rose, & Sherman, 1996; Schulenberg & Maslowsky, 2009), risk for increased depressive symptoms (Ge, Natsuaki, & Conger, 2006; Hankin, 2006), and the onset of depression (Angold, Costello, Erkanli, & Worthman, 1999; Angold, Costello, & Worthman, 1998; Stice, Presnell, & Bearman, 2001).The co-occurrence of smoking and depression has contributed to an extensive examination of the associations between these two constructs; however, uncertainty about the direction of these associations remains. The majority of research on the links between smoking and depression fall into one of two categories: research testing the hypothesis that depressed individuals engage in smoking as a way to self-medicate, and research testing the hypothesis that smoking contributes to depressive symptoms, through a variety of mechanisms. Addressing the former of these competing hypotheses, Maslowsky and Schulenberg (in press) found that depressive symptoms were a risk factor for cigarette and other substance use in national samples of adolescents, and Kim and colleagues reported that adolescent depression is a significant predictor of the likelihood to transition to daily smoking (Kim, Fleming, & Catalano, 2009). Similarly, among infrequent adolescent smokers (i.e., less than daily smoking), higher levels of depressive symptoms have been associated with an increased likelihood of nicotine dependence in young adulthood (McKenzie, Olsson, Jorm, Romaniuk, & Patton, 2010). In another study of 12–13 year old boys and girls followed for 5 years, Chaiton and colleagues explored whether individuals who reported smoking to help improve their mood experienced decreases in depressive symptoms (Chaiton, Cohen, O’Loughlin, & Rehm, 2010). The results suggested that, while girls experienced more depressive symptoms overall, there was no significant decline in depressive symptoms associated with smoking for either gender; however, the authors posit that smoking may help with the maintenance (i.e., preventing additional increases) of depressive symptoms.
In support of the latter hypothesis, smoking has also been identified as a risk factor for depression (Hanna & Grant, 1999; Wu & Anthony, 1999). Munafò and colleagues reported that among adolescents who had never smoked at baseline, engaging in smoking was associated with higher levels of depressive symptoms approximately one year later (Munafò, Hitsman, Rende, Metcalfe, & Niaura, 2008). In a longitudinal study of girls from ages 5 to 14, initiation of cigarette smoking was associated with an increase in depressive symptoms, and this effect was consistent across varying trajectories of depressive symptoms (e.g., increasing, decreasing; Marmorstein et al., 2010). There is also evidence for the potential decline in depression among those adolescent smokers who quit smoking, as compared to current smokers, adding further support to the possibility that smoking may contribute to depressive symptoms (Martini, Wagner, & Anthony, 2002). Finally, Goodman and Capitman (2000) reported that among adolescents who were not depressed at the beginning of the study, engaging in smoking predicted increased depression approximately one year later. However, among those who were not smokers at the beginning of the study, depression did not significantly predict smoking at the later time-point.
Importantly, the design of the studies reviewed above limits their ability to address competing hypotheses regarding the direction of this association. Specifically, it is still not clear whether depressive symptoms predict increased smoking, increased smoking predicts increased depressive symptoms, or both associations are occurring simultaneously in a reciprocal manner. Moon and colleagues (2010) have conducted one of the few studies to test bidirectional associations between smoking and depression. Using the Add Health data (middle and high school students, Wave 1 mean age of 16), they reported that higher levels of depression in Wave 1 was associated with smoking status (never, experimenter, consistent) in Wave 2 (approximately 1 year later) and that smoking status in Wave 1 was associated with depression in Wave 2 (Moon, Mo, & Basham, 2010). Effects by gender were not tested in these analyses.
While this is an important first step in testing possible reciprocality between smoking and depressive symptoms, it is difficult to draw conclusions with only two time points, because a minimum of 3 time points is needed to capture trajectories (Duncan & Duncan, 2004) so whether smoking predicts amount (i.e., intercept) or change (i.e., slope) in depressive symptoms (or the reverse) could not be examined. In another test of reciprocal effects, Hooshmand and colleagues (2012) estimated parallel latent growth curve models of the trajectories for both smoking and depression across four years, testing the effects of intercept of smoking on slope of depression and the reverse. The authors reported that the intercept of depression predicted the slope of smoking, where adolescents who had higher levels of depression in grade nine had a faster acceleration in smoking across high school. The reverse pathway was not significant, and the researchers concluded that smoking did not predict change in depression across high school. However, these models do not account for any effects of smoking after grade 9 on depression, and in grade 9, the majority of their sample was not smoking (Hooshmand, Willoughby, & Good, 2012). The effects of gender also were not discussed in that study. Similarly, Audrain-McGovern and colleagues (Audrain-McGovern, Rodriguez, & Kassel, 2009) reported that, across high school, the intercept of depression predicted the slope of smoking. They further found that the slope of smoking predicted the slope of depression, providing some evidence for bidirectional associations between smoking and depression. These findings suggest that depression is important for predicting smoking initiation, but that smoking is important for predicting increases in depression. Whether increasing depression is associated with increasing cigarette use is not known.
Taken together, the current literature addressing the relations between smoking and depressive symptoms is lacking longitudinal studies that begin prior to typical onset of smoking (i.e., ages 12–15; Riggs, Chih-Ping, Chaoyang, & Pentz, 2007) and include simultaneous measures of both cigarette use and depressive symptoms and how change in both trajectories impact one another across time. This type of design is necessary to test directionality and reciprocality in these relations. Understanding the nature and direction of these relations is important, as it has clear and practical clinical implications. The actions taken by health and educational professionals and others to intervene in these processes would be very different if smoking predicted change in depressive symptoms, as compared to cigarettes being used by depressed teens to self-medicate.
These limitations are addressed in the present study, which uses latent dual change score modeling (McArdle & Hamagami, 2001) to elucidate the direction of effects of smoking and depressive symptoms. The benefit of this model is that it estimates general associations across time (i.e., intercept and slope effects) as well as testing for relations between specific time points (e.g., whether smoking at time t predicts increases in depressive symptoms between times t and t+1). All of these hypotheses are tested within a single model, because latent dual change score modeling simultaneously tests the associations between trajectories of depressive symptoms and smoking (i.e., intercept and slope effects) and effects of depressive symptoms on changes in smoking and effects of smoking on changes in depressive symptoms. Further, these associations are tested across eight times of measurement, examining the relations across adolescence and early adulthood. Thus, the purpose of this study is two-fold: First, to test bidirectional change in smoking and depressive symptoms in girls across ages 11 to 20; second, to identify the persistence of this prediction across time of measurement.
Method
Participants
Data were collected in a cohort-sequential prospective longitudinal study of healthy girls (N = 262) recruited from a teen clinic in a large Midwestern children’s hospital and the surrounding community. The study was designed to examine the impact of depressive symptoms and substance use on reproductive and bone health in adolescent girls (blinded citations). Girls were enrolled by age cohort (11, 13, 15, and 17 years) and were primarily White (61%) or African American (31%). The first wave of measurement occurred between December 2003 and October 2007 and the final wave of data collection was completed in December 2010. Four annual visits were conducted. Exclusion criteria at baseline were (1) pregnancy or breastfeeding within 6 months of enrollment, (2) primary (< age 16) or secondary (< 6 cycles/year) amenorrhea, (3) body mass index at or below the first percentile or a weight above 300 pounds, (4) medication or illness that would influence bone density (a focus of the initial study), or (5) psychological disorders that would limit comprehension or compliance. Depressive symptoms were assessed at the annual visits and every 6 months for a total of 8 time-points, and smoking was assessed every 3 months, for a total of 14 time-points. For the purpose of this analysis, only the measures of smoking that were assessed at the same time as depressive symptoms will be used (i.e., every 6 months, 8 total assessments). Retention rates for this longitudinal study are quite high (90% present for at least two time-points). Of the girls participating, 66% were present at all times of measurement.
Procedures
The study was approved by the Institutional Review Board of the affiliated hospital. Parents provided consent and girls provided assent to participate. Annual interviews were conducted in-person at a Clinical Translational Research Center. Three, six, and nine months later, phone interviews were conducted. Smoking history and depressive symptoms were measured annually in-person and during six month phone interviews.
Measures
Smoking History
Lifetime smoking was measured using a graded categorical variable coded as 0 (never smoked), 1 (smoked one puff to 1 cigarette), 2 (smoked 1 cigarette), 3 (smoked 2 to 5 cigarettes), 4 (smoked 6 to15 cigarettes [half a pack]), 5 (smoked 16 to 25 cigarettes [about a pack]), 6 (smoked 26 to 99 cigarettes [more than 1 pack, but less than 5]), 7 (smoked 100 to 499 cigarettes [5 or more packs]), and 8 (smoked 500 or more cigarettes [25 or more packs]). Smoking history was adapted from Project SMART (Graham, Flay, & Johnson, 1984; Mayhew, Flay, & Mott, 2000).
Depressive Symptoms
Based on the age of participants, depressive symptoms were measured using either the Children’s Depression Inventory (CDI) or the Beck Depression Inventory (BDI); using t-scores, a score above 65 is considered clinically significant for both measures. The CDI is an established self-report measure of depressive symptoms in children ages 8 to 17 years (Kovacs et al., 1992). Responses to each item on the CDI range from 0 to 2, and participants are asked to consider their experiences in the past 2 weeks. Internal consistency of the CDI ranges from .71 to .89 (Kovacs, 2004); at the first time of measurement for the current study, the alpha is .89. Across time-points, t-scores in our study had an intraclass correlation coefficient (ICC) of .91. The BDI was administered during time-points 7–8 of the current study. This 21-item self-report questionnaire assesses depressive symptoms in those ages 13 years and older. Response options to items on the BDI range from 0 to 3, rated during a 2 week timeframe. Internal consistency ranges from .60 to .83 in non-clinical samples (Beck, Steer, & Garbin, 1988).
At the time of the study, there was no evidence to suggest how to compare t-scores from the CDI and BDI when attempting to estimate trajectories of depressive symptoms across adolescence; for that reason, the researchers chose to administer both the CDI and BDI at the same time during wave 3, so that t-scores within each participant could be compared. Based on results from another study using the same data, the best way to estimate depressive symptoms when the CDI and BDI were administered at the same time-point was to average standardized t-scores for in predictive models (blinded citation); we used this approach in the current analyses. In all other instances, the standardized t-scores of either the CDI or the BDI were used, depending on which measure was administered at that time point.
Participant Demographics
Socioeconomic status (SES) was assessed using parent report and the Hollingshead methodology (Hollingshead, 1975) where scores range from 10 to 66, and higher scores indicate higher SES. Race was also based on parent report and recoded as 1 (minority; 31% African American, 6% other) or 0 (white; 62%). To control for exposure to smoking, girls reported the total number of smokers in their household and the number of friends who smoke at least every week; these were included in analyses. Finally, pubertal timing, coded as early (0; 1 SD below the mean of this sample), on time (1), or late (2; 1 SD above the mean of this sample) calculated separately by race and based on self-reported age at menarche, was included to control for the effects of puberty on depressive symptoms. This coding is consistent with other studies of pubertal timing (Ge, Brody, Conger, & Simons, 2006a; Negriff, Dorn, & Huang, 2010).
Analytic Plan
To examine our first aim, data were restructured by age (11 to 20) to depict longitudinal trajectories of the CDI and the BDI t-scores by age. To assess the direction of associations between smoking and depressive symptoms, latent dual change score models (McArdle & Hamagami, 2001) were then estimated for smoking and depressive symptoms. Latent dual change score models, which are an extension of parallel growth curve models, use structural equation modeling (SEM) to assess the direction of relations between two variables (e.g., smoking and depressive symptoms) and include bidirectional latent intercept (level) and slope (rate of change) effects. In addition, these models add a third layer of prediction, where cross-lagged effects of level of one variable at a single time-point (e.g., smoking at age t) on a latent estimate of change in the other variable (e.g., change in depressive symptoms from age t to age t+1), and the reverse (i.e., depressive symptoms at t on change in smoking from t to t+1) are estimated. This provides a test of the effect of one variable on change in the other with the initial level of the latter controlled for, allowing for an explicit test of the directional effect. In this way, multiple potential pathways between depressive symptoms and smoking are tested simultaneously, and the relative strength of one direction in the presence of other directions is accounted for. Missing data were addressed using multiple imputation procedures – 100 datasets were imputed prior to re-structuring data (Graham, Olchowski, & Gilreath, 2007), using Mplus version 7.0 (Muthen & Muthen, 1998–2012). Imputation and integration algorithms were used to adjust for missingness resulting from restructuring data by age; as a result, standard fit statistics are not available. Results from each of the 100 datasets were aggregated and reported here. In multivariate analyses, maximum likelihood estimation with robust standard errors (MLR; Muthen & Muthen, 1998–2012) was used to accommodate non-normally distributed data.
To test the persistence of effects, our second aim used multiple regression analyses, where the metric of time was time of measurement rather than age. These analyses tested whether smoking at T1 predicted depressive symptoms at later time-points (or the reverse), and how far into the future associations continued to remain significant. All analyses used age, SES, smoking exposure, family structure, pubertal timing, and race as covariates.
Results
Univariate and bivariate analyses for depressive symptoms, smoking, and demographic variables at baseline are provided in Table 1. Skewness (1.46, SE = 0.15) and Kurtosis (2.20, SE = 0.30) statistics indicated a positively skewed distribution of depressive symptoms; non-normality persisted across times of measurement. Similarly, skewness (0.57, SE = 0.15) and Kurtosis (2.01, SE = .51) statistics for smoking indicated positively skewed data with non-normality persisting across times of measurement. Results from bivariate analyses indicate a significant and positive association between depressive symptoms and smoking. Further, independent variables were significantly associated with all demographic variables except race. As part of our preliminary analyses, we also examined stability in smoking and depressive symptoms across ages 11 to 20. Results indicated relative stability in both depressive symptoms (ICC = .70) and smoking (ICC = .94, p < .01) across time.
Table 1.
Univariate and bivariate statistics for study variables at the first time of measurement in 262 adolescent girls.
| Depressive Symptoms (t score) | Smoking | Age (years) | Race | SES | Smoke Exposure | Pubertal Timing | |
|---|---|---|---|---|---|---|---|
| Smoking | .26** | - | - | - | - | - | - |
| Age | .20** | .62** | - | - | - | - | - |
| Race | −.03 | −.08 | .07 | - | - | - | - |
| SES | −.09 | −.22** | −.14* | −.31** | - | - | - |
| Smoke Exposure | .26** | .47** | .30** | .02 | −.34** | - | - |
| Pubertal Timing | .08 | .01 | .05 | −.01 | .03 | .03 | - |
|
| |||||||
| M (SD) or % | 46.27 (10.77) | 2.88 (3.11) | 14.93 (2.18) | White - 62.1% | 37.32 (13.67) | 2.38 (2.98) | Early: 14.9% On Time: 67.2% Late: 17.9% |
| N | 262 | 262 | 262 | 262 | 261 | 262 | 262 |
p< .05;
p< .01;
NOTE: Smoking coded as 1 (never smoked), 2 (smoked one puff to 5 cigarettes), 3 (smoked 6 to 99 cigarettes), 4 (smoked 100 or more cigarettes); Socioeconomic Status (SES); Smoke exposure based on the number of household members and friends teens reported as smokers (0 = None; 3 = 3 family members or friends smoke daily; etc.); Pubertal timing was categorized by early (0), on time (1), late (2). .
Bidirectional Change in Smoking and Depressive Symptoms across Ages 11 to 20
The model estimated level and change in depressive symptoms and smoking across adolescence. The trajectory for depressive symptoms was estimated with an initial level of 49.55 (SE = 3.83, p < .01), and a decreasing rate of change at −1.03 (SE = 3.52, p < .01). The trajectory for smoking was also estimated, with an initial level of 0.73 (SE = 0.81, p < .01) and an increasing rate of change across adolescence of 0.46 (SE = 0.80, p > .05). The levels and rates of change for depressive symptoms and smoking were not correlated.
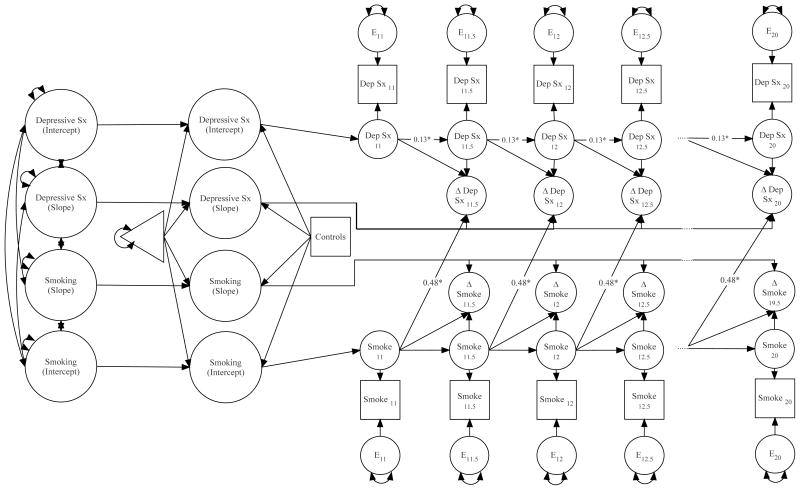
Results from latent dual change score models using smoking and depressive symptoms are provided in Table 2 and Figure 1. As reflected in Table 2, there was evidence to suggest that level of smoking at any age (e.g., age 11) predicted change in depressive symptoms over the next 6 months (e.g., from age 11 to age 11.5). Specifically, higher levels of smoking predicted increases in depressive symptoms at the subsequent time-point; the effect of depressive symptoms on change in depressive symptoms was also accounted for. This pattern persisted across ages 11 to 20, so these coefficients were constrained to be equal. The reverse direction, where depressive symptoms would predict change in smoking, was not significant (see Table 2). Specifically, when the effect of smoking at age 11 on change in smoking between age 11 and 11.5 was accounted for, depressive symptoms at age 11 did not predict change in smoking between ages 11 and 11.5 – these 6-month findings were also consistent across ages 11 to 20. Importantly, latent dual change score models build on a parallel growth model (described above) – Table 2 provides the associations between overall trajectories for level and change of smoking and depressive symptoms across ages 11 to 20 as estimated in the model. Among the correlations between levels and rates of change in smoking and depressive symptoms, none were significant. Of the covariates included in the model, only race was significant, where being White was associated with higher levels of smoking and less change in depressive symptoms1.
Table 2.
Cross-lagged effects of Smoking Predicting Change in Depressive Symptoms from Latent Dual Change Score Model with Smoking, Depressive Symptoms, and Controls.
| Predictor | Outcome | B | SE | Est/SE | P |
|---|---|---|---|---|---|
| Depressive Sx | Δ Depressive Sx | 0.13 | 0.06 | 0.67 | 0.03 |
| Smoking | Δ Depressive Sx | 0.48 | 0.22 | 0.73 | 0.03 |
| Smoking | Δ Smoking | 0.09 | 0.07 | 0.51 | 0.12 |
| Depressive Sx | Δ Smoking | −0.02 | 0.02 | −0.14 | 0.09 |
| Depressive Sx Int. | with Smoking Int. | 2.72 | 2.81 | 0.97 | 0.33 |
| Race | with Smoking Int. | −1.28 | 0.34 | −3.74 | 0.00 |
| SES | with Smoking Int. | −0.01 | 0.01 | −0.65 | 0.52 |
| Smoking Exposure | with Smoking Int. | 0.01 | 0.01 | 0.95 | 0.34 |
| Pubertal Timing | with Smoking Int. | −0.03 | 0.29 | −0.11 | 0.92 |
| Race | with Depressive Sx Int. | 1.69 | 1.65 | 1.03 | 0.31 |
| SES | with Depressive Sx Int. | −0.04 | 0.06 | −0.73 | 0.46 |
| Smoking Exposure | with Depressive Sx Int. | 0.48 | 0.39 | 1.23 | 0.22 |
| Pubertal Timing | with Depressive Sx Int. | −0.87 | 0.81 | −1.07 | 0.28 |
| Smoking Int. | with Smoking Slope | −0.12 | 0.23 | −0.51 | 0.61 |
| Depressive Sx Int. | with Smoking Slope | 1.17 | 0.73 | 1.61 | 0.11 |
| Depressive Sx Slope | with Smoking Slope | −0.10 | 0.18 | −0.53 | 0.60 |
| Race | with Smoking Slope | 0.14 | 0.08 | 1.71 | 0.09 |
| SES | with Smoking Slope | 0.00 | 0.00 | 0.29 | 0.78 |
| Smoking Exposure | with Smoking Slope | 0.03 | 0.05 | 0.52 | 0.61 |
| Smoking Int. | Depressive Sx Slope | −1.63 | 0.92 | −1.77 | 0.08 |
| Depressive Sx Int. | Depressive Sx Slope | −13.56 | 4.74 | −2.86 | 0.00 |
| Race | Depressive Sx Slope | 0.32 | 0.40 | 0.77 | 0.43 |
| SES | Depressive Sx Slope | 0.01 | 0.01 | 0.53 | 0.60 |
| Smoking Exposure | Depressive Sx Slope | 0.09 | 0.14 | 0.66 | 0.51 |
Note: Abbreviations include Symptoms (Sx); Intercept (Int); Socioeconomic Status (SES).
Figure 1.
Latent dual change score model evaluating effects of longitudinal smoking and depressive symptoms across ages 11 to 20. Only significant cross-lagged path shown. For full list of effects, see Table 2. *p < .05; Note: Symptoms (Sx); Depressive (Dep); smoking (Smoke); Change (Δ). 11, 11.5 and other numbers indicate age in years.
Amount of Time that Smoking Predicts Depressive Symptoms
To examine how many months into the future that smoking can influence depressive symptoms, a series of multiple regression analyses were conducted using data structured by time-point (rather than age). Smoking at the first time of measurement (T1) was used to predict depressive symptoms at 6-month intervals over a 2-year period. In all analyses, age, SES, exposure to smoking, pubertal timing, race, and depressive symptoms at T1 were included in the model as controls. As can be seen in Table 3, results indicate that smoking continues to significantly and positively predict depressive symptoms up to 18 months later; after 18 months smoking was not significant. An interaction between smoking and age was tested to elucidate whether smoking differentially predicted depressive symptoms at later time points based on age; this effect was not significant (p > .10).
Table 3.
Multiple regression analyses using smoking (T1) to predict depressive symptoms 6, 12, 18, and 24 months later in 262 adolescent girls.
| Model | Depressive Symptoms | |||
|---|---|---|---|---|
| 6 months post | 12 months post | 18 months post | 24 months post | |
| β | β | β | β | |
| T1 Smoking | 0.18** | 0.16* | 0.19* | 0.14 |
| T1 Age | −0.18** | −0.11 | 0.09 | −0.02 |
| T1 Race | 0.13** | 0.01 | 0.11 | 0.04 |
| T1 SES | −0.01 | 0.01 | 0.04 | 0.05 |
| T1 Smoke Exposure | −0.09 | 0.09 | 0.04 | 0.01 |
| T1 Pubertal Timing | −0.02 | 0.05 | −0.01 | −0.04 |
| T1 Depressive Sx | 0.72** | 0.68** | 0.48** | 0.44** |
| R2 | 0.56** | 0.53** | 0.29** | 0.23** |
| F | 44.36** | 32.94** | 12.10** | 8.59** |
| DF | 7, 242 | 7, 205 | 7, 210 | 7, 201 |
p < .05;
p < .01.
Note: Time 1 (T1); Socioeconomic status (SES); Symptoms (Sx).
Discussion
The current study is the first to use latent dual change score models to examine the directionality of the association between smoking and depressive symptoms. Using a sample of adolescent girls from age 11 to 20, the analyses sought to clarify the inconsistencies in the extant literature, with some studies supporting smoking as a precursor to depression (Hanna & Grant, 1999; Munafò et al., 2008; Wu & Anthony, 1999), others showing depression preceding initiation of smoking (Hooshmand et al., 2012; Kim et al., 2009; Maslowsky & Schulenberg, in press; McKenzie et al., 2010), as well as several finding reciprocal associations (Audrain-McGovern et al., 2009; Moon et al., 2010). Using sophisticated analyses that accounted for simultaneous bidirectional effects across adolescence, the current study provides support for smoking behavior leading to increases in depressive symptoms, but not the reverse (i.e. depressive symptoms leading to smoking). Of particular importance, the effects were invariant across the years, suggesting a robust effect across the second decade of life. This adds to the converging evidence that smoking is a risk for depressive symptoms and potentially for the development of depression (Goodman & Capitman, 2000; Hanna & Grant, 1999; Marmorstein et al., 2010; Martini et al., 2002; Munafò et al., 2008; Wu & Anthony, 1999).
For some time researchers have recognized the co-occurrence between smoking and depression, but few studies have been designed to answer the question of directionality. Of those that examined temporal patterns, two studies of adolescents supported depression preceding smoking behavior (Audrain-McGovern et al., 2009; Hooshmand et al., 2012), while a third showed reciprocal effects (Moon et al., 2010). The present study provides the first evidence demonstrating that smoking is a temporal precursor to depressive symptoms using latent dual change score models. There is a theoretical basis for the possible models explaining the development and change in depressive symptoms and smoking across adolescence. First, depression may lead to smoking as a form of self-medication. Alternatively, others hypothesize that nicotine exposure affects depressive symptoms through central nervous system processes (Hall, 1993; Pomerleau, Turk, & Fertig, 1984)or thyroid function (Joffe, 1990).
In the current study we find only that smoking predicts depressive symptoms, not vice versa, limiting the evidence for the self-medication hypothesis. Importantly, our findings indicate that increased smoking at a given time point (t) predicts increases in depressive symptoms between time points t and t+1. However, the reverse pathway (i.e., depressive symptoms predicting smoking) was not significant, and overall estimates of level and change in depressive symptoms and smoking across ages 11 to 20 were not associated with one another once the relations between specific time points was taken into account. This, in combination with our findings that smoking predicts depressive symptoms for up to 18 months, seems to indicate that the timeframe within which smoking influences depressive symptoms is relatively short in duration. Said differently, it is not that people who increase the frequency of their smoking across adolescence also tend to follow a trajectory of increasing depressive symptoms.. Rather, people who increase smoking at a particular timepoint (e.g., age 16) tend to experience a greater increase in depressive symptoms six months later. This could explain some of the contradictory findings in the literature.
Our findings may also differ from those that support depression preceding smoking because of the number of time-points available in this data, which may allow us to capture the more discrete changes that occur during a short time span during adolescence. Moreover, our study began at age 11, well before the typical onset of smoking (Kim et al., 2009) and only included healthy girls, which are unique features not reflected in other studies. Further, there was relative stability in smoking in our sample – in a sample of adolescents or young adults with increased variability in smoking, the cross-lagged effects might differ. Lastly, there are potential genetic and family environment influences on both the development of depressive symptoms and the use of cigarettes. Some of these influences may overlap, such as low SES, poor school performance, low self-esteem, pubertal timing, and stressful life events (Ge, Best, Conger, & Simons, 1996; Ge, Conger, & Elder, 2001; Ge, Lorenz, Conger, Elder, & Simons, 1994; Ge et al., 2006b). Some studies have found that after adjusting for confounding variables there is still an association between smoking and depression, while others have not. For example, Fergusson and colleagues found that even after controlling for confounding factors (e.g. childhood adversity, novelty-seeking, parental smoking, deviant peer affiliations) there was still an association between major depression and smoking (Fergusson, Goodwin, & Horwood, 2003). However, others found that after accounting for rebelliousness the association between smoking and depressive symptoms were reduced (Maslowsky & Schulenberg, in press) or became non-significant (Albers & Biener, 2002). We included a number of potential confounding variables (e.g. SES, age, race, pubertal timing) bolstering confidence in our finding that smoking is associated with increased depressive symptoms six months later. Understanding the mechanisms of this predictive relationship, which is relatively robust across all of adolescence, deserves particular attention. The mechanisms are likely multi-level, including biological, psychological, and social mechanisms.
Our findings, that when smoking increases there is an associated greater increase in depressive symptoms six months later, have important implications for both smoking cessation and mental health. First, the findings provide yet another important reason to emphasize the prevention of cigarette use among adolescent girls, as it appears that even low levels of smoking might be affiliated with an uptake in depressive symptoms. Second, these findings speak to the importance of addressing both depressive symptoms and smoking behavior in girls who seek help for depression. Treating only the depressive symptoms may not be enough to counter the effect of continued cigarette use, when smoking and depression co-occur. Further, clinicians may need to be particularly vigilant about monitoring for depression in teens who smoke.
These findings were further strengthened by the companion analyses looking at the duration of effects of smoking on depressive symptoms, using time of measurement rather than across age. In regression analyses controlling for relevant confounds and depressive symptoms at baseline, smoking at baseline continued to positively and significantly predict depressive symptoms up to 18 months later – indicating that the duration of the effect of cigarette use on depressive symptoms is longer than the 6 months reflected in the first set of analyses. Thus, it may not be sufficient to examine smoking as a concurrent predictor of depressive symptoms. Rather, there may be some more proximal effects (i.e., within a 6 month period) and more distal effects (1.e. within an 18 month period) - indicating complexity in these relations, which likely reflects social or metabolic processes that operate beyond the immediate biological effects of nicotine.
While our findings do provide insight into the relations between smoking and depressive symptoms, we cannot conclusively determine cause in this study. It could be that a third construct (e.g., stress) may be causing both changes in smoking and in depressive symptoms, but that those changes are more immediate for smoking than for depressive symptoms, resulting in the patterns observed here. Future research should examine this further, perhaps within the framework of a randomized intervention or using techniques that allow for causal inference(e.g., propensity scores; Rosenbaum & Rubin, 1983).
As with any study, we had some limitations, and future research should examine these processes more closely. Replication of this study should be conducted with larger sample sizes, along with inclusion of boys and adolescents from other socio-cultural backgrounds in order to enhance generalization. Further, whether these findings would replicate with a clinically depressed sample or with high-risk youth is unknown. Due to the age range of this sample, the tool used to measure depressive symptoms changed during the study (i.e., from the CDI to the BDI) which may artificially inflate error variances and difference scores, another limitation to this study. It is also possible that smoking is a proxy for another factor that shapes depressive symptom or that results could vary in a sample experiencing less stability in smoking behaviors. While we included all available control variables when they were theoretically relevant, it may be that an unmeasured confound is contributing to these findings. For example, nicotine dependence was not measured, and could explain the associations between smoking and depressive symptoms that were found.
Despite these limitations, we believe our findings, which took advantage of methods that would account for reciprocality in the associations between depressive symptoms and smoking along with relevant controls, speak to the importance of preventing and addressing cigarette use in adolescent girls to prevent increasing depressive symptoms. Such knowledge may be relevant to educators and clinicians in terms of addressing both prevention and intervention efforts with teens. Finally, determining the mechanisms of the impact of cigarette smoking on depressive symptoms during adolescence deserves attention, with theoretical and practical implications.
Acknowledgments
This research was supported in part by Grant Number R01 DA 16402, National Institute of Drug Abuse, NIH, PI: Lorah D. Dorn, PhD and by USPHS Grant # TR000077-04 from the National Center for Research Resources, NIH and by funds from the Bureau of Health Professions (BHPr), Health Resources and Services Administration (HRSA), Department of Health and Human Services (DHHS), under Grant # T32HP10027. We also thank the editor and two reviewers, who provided excellent feedback that contributed to the improvement of this paper.
Footnotes
A model using smoking in the past 30 days was also tested, and found not to be significant; this is likely due to the low variability of 30 day smoking – across ages, between 0% (ages 11 and 12) and 32% (age 17) reported any smoking in the past 30 days.
References
- Albers AB, Biener L. The role of smoking and rebelliousness in the development of depressive symptoms among a cohort of Massachusetts adolescents. Preventive Medicine. 2002;34(6):625–631. doi: 10.1006/pmed.2002.1029. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychological Medicine. 1999;29(5):1043–1053. doi: 10.1017/S0033291799008946. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman C. Puberty and depression: The roles of age, pubertal status, and pubertal timing. Psychological Medicine. 1998;28:10. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Kassel JD. Adolescent smoking and depression: evidence for self-medication and peer smoking mediation. Addiction. 2009;104(10):1743–1756. doi: 10.1111/j.1360-0443.2009.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman JG, Johnston LD, O’Malley PM, Schulenberg J. Transitions in drug use during late adolescence and young adulthood. In: Graber JA, Brooks-Gunn J, Petersen AC, editors. Transitions Through Adolescence: Interpersonal Domains and Context. 1996. pp. 111–140. [Google Scholar]
- Chaiton M, Cohen J, O’Loughlin J, Rehm J. Use of cigarettes to improve affect and depressive symptoms in a longitudinal study of adolescents. Addictive Behaviors. 2010;35(12):1054–1060. doi: 10.1016/j.addbeh.2010.07.002. http://dx.doi.org/10.1016/j.addbeh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Rose JS, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood: demographic predictors of continuity and change. Health Psychology. 1996;15(6):478–484. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC. An introduction to latent growth curve modeling. Behavior Therapy. 2004;35(2):333–363. doi: 10.1016/s0005-7894(04)80042-x. [DOI] [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychological Medicine. 2003;33(8):1357–1367. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- Ge X, Best KM, Conger RD, Simons RL. Parenting behaviors and the occurrence and co-occurrence of adolescent depressive symptoms and conduct problems. Developmental Psychology. 1996;32(4):717–731. [Google Scholar]
- Ge X, Brody GH, Conger RD, Simons RL. Pubertal maturation and African American children’s internalizing and externalizing symptoms. Journal of Youth & Adolescence. 2006a;35(4):528–537. doi: 10.1007/s10964-006-9046-5. [DOI] [Google Scholar]
- Ge X, Conger RD, Elder GH. Pubertal transition, stressful life events and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, Simons RL. Trajectories of stressful life events and depressive symptoms during adolescence. Developmental Psychology. 1994;30(4):17. [Google Scholar]
- Ge X, Natsuaki MN, Conger RD. Trajectories of depressive symptoms and stressful life events among male and female adolescents in divorced and nondivorced families. Developmental Psychology. 2006b;18:253–273. doi: 10.1017/S0954579406060147. [DOI] [PubMed] [Google Scholar]
- Goodman E, Capitman J. Depressive symptoms and cigarette smoking among teens. Pediatrics. 2000;106(4):748–755. doi: 10.1542/peds.106.4.748. [DOI] [PubMed] [Google Scholar]
- Graham JW, Flay BR, Johnson CA. Reliability of self-report measures of drug use in prevention research: evaluation of the Project SMART questionnaire via the test-retest reliability matrix. Journal of Drug Education. 1984;14(2):175–193. doi: 10.2190/cyv0-7dpb-djfa-ej5u. [DOI] [PubMed] [Google Scholar]
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention Science. 2007;8(3):206–213. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- Hall JE. U. S. D. o. H. a. H. Services, editor. Alcohol and the Endocrine System. Bethesda, MD: National Institutes of Health; 1993. Female reproductive system: Physiology and pathophysiology; pp. 117–138. Vol. NIH Publication No. 93-3533. [Google Scholar]
- Hankin BL. Adolescent depression: description, causes, and interventions. Epilepsy & Behavior. 2006;8(1):102–114. doi: 10.1016/j.yebeh.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Hanna EZ, Grant BF. Parallels to early onset alcohol use in the relationship of early onset smoking with drug use and DSM-IV drug and depressive disorders: findings from the National Longitudinal Epidemiologic Survey. Alcoholism: Clinical and Experimental Research. 1999;23(3):513–522. [PubMed] [Google Scholar]
- Hollingshead AB. A Four-Factor Classification of Social Status. New Haven, CT: Yale University CT Press; 1975. [Google Scholar]
- Hooshmand S, Willoughby T, Good M. Does the direction of effects in the association between depressive symptoms and health-risk behaviors differ by behavior? A longitudinal study across the high school years. The Journal of Adolescent Health. 2012;50(2):140–147. doi: 10.1016/j.jadohealth.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Joffe RT. Perspective on the thyroid and depression. Canadian Journal of Psychiatry. 1990;35(9):754–758. doi: 10.1177/070674379003500906. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Fleming CB, Catalano RF. Individual and social influences on progression to daily smoking during adolescence. Pediatrics. 2009;124(3):895–902. doi: 10.1542/peds.2008-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Beck AT, Dadds MR, Sanders MR, Morrison M, Rebgetz M. Child Depression Inventory. Childhood depression and conduct disorder: II An analysis of family interaction patterns in the home. 1992;101:505–513. [PubMed] [Google Scholar]
- Marmorstein NR, White H, Tammy C, Hipwell A, Stouthamer-Loeber M, Loeber R. Associations between first use of substances and change in internalizing symptoms among girls: Differences by symptom trajectory and substance use type. Journal of Clinical Child & Adolescent Psychology. 2010;39(4):545–558. doi: 10.1080/15374416.2010.486325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini S, Wagner FA, Anthony JC. The association of tobacco smoking and depression in adolescence: Evidence from the United States. Substance Use & Misuse. 2002;37(14):1853–1867. doi: 10.1081/ja-120014087. [DOI] [PubMed] [Google Scholar]
- Maslowsky J, Schulenberg JE. Interaction matters: Quantifying conduct problem by depressive symptoms interaction and its association with adolescent alcohol, cigarette, and marijuana use in a national sample. Development and Psychopathology. doi: 10.1017/S0954579413000357. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew KP, Flay BR, Mott JA. Stages in the Development of Adolescent Smoking. Drug and Alcohol Dependence. 2000;59(Suppl 1):S61–S81. doi: 10.1016/s0376-8716(99)00165-9. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data. In: Collins LM, Sayer AG, editors. New Methods for the Analysis of Change. American Psychological Association; 2001. pp. 139–175. [Google Scholar]
- McKenzie M, Olsson CA, Jorm AF, Romaniuk H, Patton GC. Association of adolescent symptoms of depression and anxiety with daily smoking and nicotine dependence in young adulthood: findings from a 10-year longitudinal study. Addiction. 2010;105(9):1652–1659. doi: 10.1111/j.1360-0443.2010.03002.x. [DOI] [PubMed] [Google Scholar]
- Moon S, Mo B, Basham R. Adolescent depression and future smoking behavior: A prospective study. Child & Adolescent Social Work Journal. 2010;27(6):405–422. doi: 10.1007/s10560-010-0212-y. [DOI] [Google Scholar]
- Munafò MR, Hitsman B, Rende R, Metcalfe C, Niaura R. Effects of progression to cigarette smoking on depressed mood in adolescents: evidence from the National Longitudinal Study of Adolescent Health. Addiction. 2008;103(1):162–171. doi: 10.1111/j.1360-0443.2007.02052.x. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus. 5. Los Angeles, CA: Muthen & Muthen; 1998–2012. [Google Scholar]
- Negriff S, Dorn LD, Huang B. Pubertal timing and smoking initiation in adolescent females: Differences by race. Nicotine & Tobacco Research. 2010;12(7):748–755. doi: 10.1093/ntr/ntq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF, Turk DC, Fertig JB. The effects of cigarette smoking on pain and anxiety. Addictive Behaviors. 1984;9:265–271. doi: 10.1016/0306-4603(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Riggs NR, Chih-Ping C, Chaoyang L, Pentz MA. Adolescent to emerging adulthood smoking trajectories: When do smoking trajectories diverge, and do they predict early adulthood nicotine dependence? Nicotine & Tobacco Research. 2007;9(11):1147–1154. doi: 10.1080/14622200701648359. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- Schulenberg JE, Maslowsky J. Monographs of the Society for Research in Child Development. 2009. Taking substance use and development seriously: Developmentally distal and proximal influences on adolescent drug use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Presnell K, Bearman SK. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Developmental Psychology. 2001;37(5):608–619. doi: 10.1037//0012-1649.37.5.608. [DOI] [PubMed] [Google Scholar]
- Wu L, Anthony JC. Tobacco Smoking and Depressed Mood in Late Childhood and Early Adolescence. American Journal of Public Health. 1999;89(12):1837. doi: 10.2105/ajph.89.12.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]