Abstract
Objective
Antidepressants may attenuate the effects of diet and exercise programs. We compared adherence and changes in body measures and biomarkers of glucose metabolism and inflammation between antidepressant users and non-users in a 12-month randomized controlled trial.
Methods
Overweight or obese, postmenopausal women were assigned to: diet (10% weight loss goal, N=118); moderate-to-vigorous aerobic exercise (225 minutes/week, N=117); diet+exercise (N=117); and control (N=87) in Seattle, WA 2005–2009. Women using antidepressants at baseline were classified as users (N=109). ANCOVA and generalized estimating equation approaches, respectively, were used to compare adherence (exercise amount, diet session attendance, and changes in percent calorie intake from fat, cardiopulmonary fitness, and pedometer steps) and changes in body measures (weight, waist and percent body fat) and serum biomarkers (glucose, insulin, homeostasis assessment-insulin resistance, and high-sensitivity C-reactive protein) between users and non-users. An interaction term (intervention × antidepressant use) tested effect modification.
Results
There were no differences in adherence except diet session attendance was lower among users in the diet+exercise group (P<0.05 vs. non-users). Changes in body measures and serum biomarkers did not differ by antidepressant use (Pinteraction>0.05).
Conclusion
Dietary weight loss and exercise improved body measures and biomarkers of glucose metabolism and inflammation independent of antidepressant use.
Keywords: Obesity, weight loss intervention, antidepressants, diet, exercise
Introduction
Antidepressant medications are frequently prescribed in the United States; prevalence of use in adults has increased more than threefold (Paulose-Ram et al., 2007). Several studies show that individuals taking antidepressants have increased risk for obesity or weight gain (Kivimaki et al., 2010; Patten et al., 2011; Rubin et al., 2010a; Serretti and Mandelli, 2010), diabetes (Kivimaki et al., 2010; Pan et al., 2012; Rubin et al., 2008; Rubin et al., 2010b), and cardiovascular disease (Cohen et al., 2000; Hamer et al., 2011b; Smoller et al., 2009).
It is not clear if antidepressant use has a physiological effect on weight loss efficacy and risk factors associated with chronic disease, or if the impact of antidepressants is tied to behavioral factors. In vitro studies show that fluoxetine (Garcia-Colunga et al., 1997), paroxetine (Fryer and Lukas, 1999), nefazodone (Fryer and Lukas, 1999), and venlafaxine (Fryer and Lukas, 1999), commonly prescribed antidepressants, non-competitively inhibit muscle nicotinic acetylcholine receptors, which may reduce energy expenditure. Paroxetine and sertraline inhibit insulin signaling in rat hepatoma cells (Levkovitz et al., 2007), suggesting potential direct effects of antidepressants on energy balance and insulin resistance. Among adults with type 2 diabetes, antidepressant users were more likely to have clinical and behavioral cardiovascular disease risk factors including high blood pressure or antihypertensive use, high cholesterol or lipid lowering drug use, high triglyceride or lipid lowering drug use, current smoking, and BMI ≥30kg/m2 (Rubin et al., 2010a).
Dietary weight loss and exercise interventions can reduce weight in patients with depression (Ludman et al., 2010; Pagoto et al., 2007; Richardson et al., 2005). Whether antidepressant use modifies intervention effects on weight is not well established, however. In a 24-month exercise and dietary weight loss intervention in 1632 overweight adult women, antidepressant users lost less weight vs. non-users (Linde et al., 2004). In 131 obese adults, participants with major depressive disorder lost less weight compared to those without the disorder (Pagoto et al., 2007), using the Diabetes Prevention Program (DPP) (Knowler et al., 2002). In 190 obese women with or without major depressive disorder attending a 12-month group-based caloric reduction and exercise weight loss intervention, there were no significant differences in weight loss between the two groups; those who attended ≥12 sessions reduced weight independent of depression status (Ludman et al., 2010).
Several dietary weight loss and exercise intervention studies have shown that individuals with depression have low adherence (Flegal et al., 2007; Somerset et al., 2011) and high dropout rates (Pagoto et al., 2007). Lower intervention adherence among antidepressant users may account for differences in weight loss between antidepressant users and non-users.
The primary aim of this analysis was to compare the effects of 12-month dietary weight loss and/or exercise interventions on body composition (weight, waist circumference, and percent body fat); and biomarkers of glucose metabolism (fasting glucose, insulin, and homeostasis assessment-insulin resistance [HOMA-IR]) and inflammation (high-sensitivity c-reactive protein [hs-CRP]) between overweight or obese postmenopausal women taking or not taking antidepressants. To our knowledge, no studies have tested whether antidepressant use modifies changes in biomarkers of glucose metabolism and hs-CRP, an inflammatory biomarker used to assess risk of coronary heart disease (Buckley et al., 2009). The secondary aim of this analysis was to compare adherence to the diet and exercise programs between those using and not using antidepressants.
Methods and Subjects
Study design and participants
The Nutrition and Exercise for Women (NEW) study was a 12-month, randomized controlled trial conducted from 2005–2009 at the Fred Hutchinson Cancer Research Center (FHCRC), Seattle, WA. The study examined the individual and combined effects of 12-months of reduced calorie diet and/or exercise interventions on breast cancer biomarkers. The primary outcome was serum estrone (Campbell et al., 2012). Secondary outcomes were additional sex hormones (Campbell et al., 2012), glucose metabolism (Mason et al., 2011), body composition (Foster-Schubert et al., 2011), quality of life (Imayama et al., 2011), and complete blood count (Imayama et al., 2012). In an ancillary study we assessed the interventions' effects on inflammatory biomarkers (Imayama et al., 2012). The trial was designed to have at least 80% power for a 0.05/3 level test to detect a difference of 10% in 12-month estrone changes for 3 primary comparisons. Because of funding limitation and expected adherence and retention after half of the women completed the trial, power calculations were repeated and the recruitment goal was changed from 503 to 439. The study procedures were reviewed and approved by the FHCRC Institutional Review Board. All participants provided signed Informed Consent.
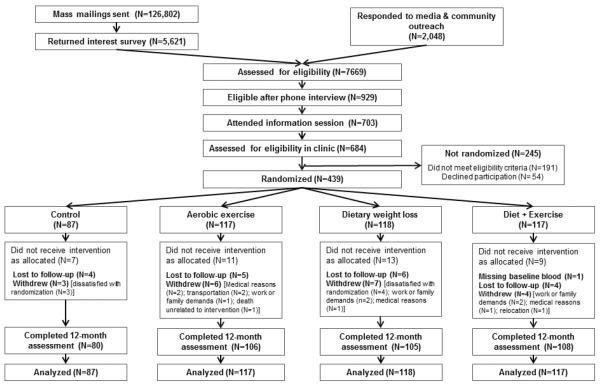
The study design, recruitment, and intervention methods have been reported elsewhere (Foster-Schubert et al., 2011). Participants were recruited from the greater Seattle area (Figure 1). Eligibility criteria included: 50–75 years of age; BMI ≥25.0 kg/m2 (if Asian-American ≥23.0 kg/m2); <100 minutes/week of moderate activity; postmenopausal; not taking postmenopausal hormone therapy for the past 3 months; no history of breast cancer, heart disease, diabetes mellitus, or other serious medical conditions; fasting glucose <126 mg/dL; non-smoking; ≤2 alcohol drinks/day; able to attend diet/exercise sessions at the intervention site; and a normal exercise tolerance test.
Figure 1.
CONSORT diagram of the Nutrition and Exercise for Women (NEW) trial, Seattle WA 2005–2009
Randomization and interventions
A total of 439 women were randomized to dietary weight loss with a goal of 10% weight reduction (N=118); moderate-to-vigorous intensity aerobic exercise for 45 minutes/day, 5 days/week (N=117); combined exercise and diet (N=117); or control (N=87). Computerized randomization was stratified by BMI (<30.0, ≥30.0 kg/m2) and race/ethnicity (non-Hispanic white, black, other). Permuted blocks allocated a smaller number of women to the control group. The sequence was concealed until the allocation was determined. Study staff enrolled and informed participants of group assignment. Other than statisticians, all study staff involved in assessments and investigators were blinded to randomization.
The dietary weight loss intervention was based on the DPP (Knowler et al., 2002) and the Action for Health in Diabetes trial lifestyle interventions (Ryan et al., 2003) with the following goals: caloric intake of 1200–2000 kcal/day based on weight, ≤30% calories from fat, and 10% weight loss by week 24, with weight maintenance thereafter. Dietitians with training in behavior modification conducted the diet intervention. Participants had 2–4 individual sessions with a study dietitian, attended weekly group sessions (5–10 women) until week 24, and completed daily food logs for at least 6 months or until they reached their weight loss goal. Afterwards they attended monthly group sessions and had e-mail/phone contact. The diet+exercise group attended separate diet sessions from the diet-only group.
The exercise goal was 45 minutes/day, 5 days/week of moderate-to-vigorous intensity exercise for 12 months. Participants attended 3 supervised sessions/week at the facility and exercised 2 days/week at home. They gradually increased exercise training to 70–85% of maximal heart rate (based on baseline VO2max treadmill test) for 45 minutes/session by week 7 and maintained thereafter. At each session participants wore Polar heart rate monitors (Polar Electro, New Hyde Park, NY, USA) and recorded exercise mode, duration, peak heart rate, and perceived exertion in facility and home activity logs. Activities with ≥4 METs were counted toward the prescribed exercise target (Ainsworth et al., 2000).
Exercise-only and diet-only participants were asked not to change their respective diet and exercise habits. Controls were asked not to change their diet or exercise habits. At the end of 12 months, controls were offered 4 group diet sessions and 8 weeks of supervised exercise sessions.
Measurements
Demographics, medication use, depressive symptoms, exercise and diet behaviors, cardiopulmonary fitness, pedometer counts, weight, waist circumference, and percent body fat were assessed at baseline and at 12 months. We used standardized questionnaires to collect demographic information. Participants brought current prescription and over-the-counter medication bottles to clinic visits. Participants regularly taking prescription antidepressant medications (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclics, and atypical antidepressants) at baseline were classified as antidepressant users. Because of concerns about a subgroup analysis using post-randomization factors, we defined user status according to baseline data (Yusuf et al., 1991).
Depression was measured using the validated Brief Symptom Inventory-18 (Derogatis, 2001; Derogatis and Melisaratos, 1983). Type, intensity and duration of exercise over the previous 3 months were assessed (Taylor et al., 1978). We used the 120-item food frequency questionnaire (FFQ) to assess usual dietary intake, and calculated percent of caloric intake from fat (Patterson et al., 1999). Cardiopulmonary fitness was assessed using a modified branching treadmill protocol (Foster-Schubert et al., 2011; Pate et al., 1991; Schauer and Hanson, 1987). Participants wore pedometers (Accusplit, Silicon Valley, CA, USA) for 7 consecutive days from which the mean steps/day was determined. Height, weight and waist circumference were measured and BMI was calculated as kg/m2 (Foster-Schubert et al., 2011). Body fat was measured by a dual-energy x-ray absorptiometry whole-body scanner (GE Lunar, Madison, WI, USA).
Twelve-hour fasting blood samples were collected at baseline and 12 months, with no exercise for 24 hours. The intra- and inter-assay coefficients of variations (CVs) for glucose were 1.1% and 3.5%, respectively. The intra-assay CV was 4.5% for insulin. We calculated HOMA-IR=fasting insulin(mU/L)×fasting glucose(mmol/L)/22.5 (Matthews et al., 1985). The lower detection limit was 0.2 mg/L for hs-CRP. Intra- and inter-batch CVs were 4.1% and 4.7%, respectively, for hs-CRP.
Change in percent of calorie intake from fat (FFQ) and diet session attendance were used as indicators of diet intervention adherence. Total minutes of moderate-to-vigorous exercise/week were calculated from the facility and home exercise logs. We used minutes/week of moderate-to-vigorous exercise, and 12-month changes in cardiopulmonary fitness and pedometer steps as indicators of exercise adherence.
Statistical analysis
Baseline characteristics between antidepressant users and non-users within each study group were compared using a t-test or χ2 test as appropriate. Numbers of participants who started using antidepressants (non-user at baseline and user at 12 months) and stopped using antidepressants (user at baseline and non-user at 12 months) among the study groups were compared using a Fisher's exact test. The association between antidepressant use and depression score (tertiles) at baseline was examined by χ2 test. Based on intention-to-treat principle, we analyzed the data using last observation carried forward (LOCF) for missing data at 12 months. We also repeated the analysis using available data (Supplementary Table 1–3). There were no substantial differences in the findings; thus, we report the result of LOCF analysis. Rates of dropouts and adherence to the diet and exercise sessions between antidepressant users and non-users within each intervention arm and across tertiles of baseline depression score in non-users were compared using a logistic regression model and analysis of covariance (ANCOVA), respectively. We used generalized estimating equations to compare changes from baseline to 12 months. An interaction term of intervention × antidepressant user status was included in the model to test effect modification by antidepressant use. As several interaction terms showed marginal statistical significance (p<0.20), and a previous report suggested possible modifying effects of antidepressant use on weight loss (Linde et al., 2004), we performed further analyses to investigate the effect size and statistical significance in each intervention arm vs. control within each antidepressant user status. Additionally, we compared 12-month weight loss between antidepressant users and non-users within each study arm using ANCOVA. Outcomes were also compared between users and non-users in all participants, intervention arms and control using ANCOVA adjusted for group assignment. To account for differences in intervention adherence, models were adjusted for intervention adherence variables that were significantly different between antidepressant users and non-users (i.e., diet session attendance). Models were further adjusted for depression symptoms to test for confounding effects. All analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Briefly, the diet, diet+exercise, and exercise groups decreased weight from baseline by 8.5% (p<0.01), 10.8% (p<0.01), and 2.4% (p=0.03) respectively (vs. controls, Foster-Schubert et al., 2011). All intervention groups decreased percent body fat (Δdiet=−4.2%; Δdiet+exercise=−5.9%; Δexercise=−1.6%; all p<0.01 vs. controls) (Foster-Schubert et al., 2011). Compared to controls (+1.1 cm), waist circumference decreased in diet (−4.4 cm, p<0.01), diet+exercise (−7.0 cm, p<0.01), and exercise (−2.0 cm, p=0.02) groups (Foster-Schubert et al., 2011).
Among the diet and diet+exercise groups, 41.5% (N=49) and 59.5% (N=69), respectively, achieved the 10% weight loss goal at 12 months. The exercise and diet+exercise groups completed respective means of 80.2% and 84.7% of the exercise goal (225 minutes/week). The exercise and diet+exercise groups increased pedometer counts by an average of 2415 and 3468 steps/day, respectively (both p<0.01 vs. controls). Aerobic fitness increased by 0.17 L/min in the exercise and 0.12 L/min in the diet+exercise groups (both p<0.01 vs. control). The diet and diet+exercise groups significantly reduced fasting glucose (Δdiet=−2.4%, Δdiet+exercise=−2.8%); insulin (Δdiet=−22.3%, Δdiet+exercise=−24.0%); HOMA-IR (Δdiet=−24.3%, Δdiet+exercise=−26.4%); and hs-CRP (Δdiet=−36.1%, Δdiet+exercise=−41.7%) compared to controls (p≤0.002). In the exercise group, there were no significant changes in these biomarkers (Imayama et al., 2012; Mason et al., 2011).
Baseline characteristics of participants
At baseline and 12-months, 109 (24.8%) and 85 (21.3%) women, respectively, were taking antidepressants (Supplementary Table 4). Of the 400 women who completed both baseline and 12-month assessments, 76 reported using antidepressants at both time-points, with 21 stopping them and 9 starting their use. There were no differences in the numbers of participants who started and stopped antidepressants during the study between the groups (p>0.05).
There were no significant baseline differences in demographics, anthropometrics, biomarkers of glucose metabolism and inflammation, and exercise and diet behaviors between antidepressant users and non-users within each study arm except for higher percent body fat among antidepressant users in the diet+exercise group (vs. non-users, p=0.04, Table 1). Antidepressant users had more depressive symptoms compared to non-users in both the diet-only and exercise-only groups (p<0.01). Prevalence of antidepressant use increased with increasing tertile of baseline depression score (p=0.006, Supplementary table 8).
Table 1.
Baseline characteristics of study participants stratified by baseline antidepressant use in Seattle, WA 2005–2009
| Control | Diet | Exercise | Diet + Exercise | |||||
|---|---|---|---|---|---|---|---|---|
| Users (N=19) | Non-users (N=68) | Users (N=26) | Non-users (N=92) | Users (N=29) | Non-users (N=88) | Users (N=35) | Non-users (N=82) | |
| Age, mean (SD) | 56.8 (4.4) | 57.6 (4.4) | 58.4 (6.1) | 58.0 (5.9) | 57.5 (5.5) | 58.3 (4.8) | 57.3 (3.4) | 58.4 (4.9) |
| Ethnicity, n (%) | ||||||||
| Non-Hispanic white | 17 (89.5) | 57 (83.8) | 21 (80.8) | 80 (87.0) | 27 (93.1) | 71 (80.7) | 30 (85.7) | 70 (85.4) |
| Education, n (%) | ||||||||
| College degree | 13 (68.4) | 46 (67.6) | 16 (61.5) | 60 (65.2) | 12 (41.4) | 58 (65.9) | 26 (74.3) | 56 (68.3) |
| Marital status, n (%) | ||||||||
| Married/living with a partner | 12 (63.2) | 47 (69.1) | 15 (57.7) | 64 (69.6) | 15 (57.7) | 51 (58.0) | 21 (60.0) | 49 (60.5) |
| Anthropometrics, mean (SD) | ||||||||
| Body mass index, kg/m2 | 30.8 (3.9) | 30.7 (3.9) | 31.4 (3.4) | 30.9 (4.1) | 31.3 (4.4) | 30.5 (3.5) | 31.4 (4.0) | 30.8 (4.4) |
| Waist circumference, cm | 93.7 (9.7) | 95.2 (10.4) | 97.9 (10.3) | 93.7 (10.0) | 94.5 (10.4) | 95.2 (10.1) | 95.5 (10.3) | 92.9 (9.7) |
| Percent body fat, % | 48.0 (5.0) | 47.8 (4.4) | 47.5 (3.5) | 47.6 (4.6) | 47.9 (4.1) | 47.9 (4.2) | 49.3 (4.9)* | 47.4 (4.3) |
| Cardiopulmonary fitness, mL/kg/min | 23.0 (3.5) | 23.1 (4.3) | 22.8 (2.9) | 22.6 (4.0) | 22.5 (3.1) | 22.4 (4.4) | 22.5 (5.0) | 24.0 (3.7) |
| Biomarkers, mean (SD) | ||||||||
| Fasting glucose, mg/dL | 96.4 (6.0) | 97.2 (8.9) | 96.9 (8.9) | 96.8 (8.6) | 95.4 (10.5) | 96.0 (7.2) | 95.2 (7.7) | 96.4 (7.8) |
| Insulin, mU/L | 12.7 (4.6) | 13.6 (6.9) | 14.3 (8.3) | 12.8 (8.9) | 13.9 (13.2) | 12.1 (5.4) | 11.7 (5.7) | 12.9 (9.6) |
| HOMA-IR | 3.0 (1.2) | 3.3 (1.8) | 3.4 (2.1) | 3.1 (2.3) | 3.4 (3.7) | 2.9 (1.3) | 2.7 (1.4) | 3.1 (2.5) |
| C-reactive protein, mg/L | 2.61 (2.30) | 3.40 (3.80) | 5.15 (6.00) | 3.69 (3.28) | 4.29 (5.22) | 3.69 (3.39) | 3.05 (2.58) | 3.12 (2.70) |
| Exercise & Diet habits, mean (SD) | ||||||||
| Physical activity, min/week | 14.6 (14.7) | 26.4 (45.7) | 25.9 (35.1) | 35.8 (48.0) | 49.1 (47.3) | 34.0 (42.1) | 23.3 (49.0) | 38.0 (42.2) |
| Physical activity, min/week, median (25th – 75th percentile) | 13.8 (0.0 – 23.0) | 1.4 (0.0 – 36.7) | 14.5 (0.0 – 55.1) | 13.8 (0.0 – 66.6) | 41.3 (9.2 – 76.9) | 25.2 (0.0 – 54.0) | 0.0 (0.0 – 23.0) | 23.0 (0.0 – 67.3) |
| Pedometer counts, steps/day | 5035 (2029) | 5765 (2403) | 5146 (2086) | 5652 (2302) | 5424 (2190) | 5886 (2111) | 6171 (2516) | 5903 (2354) |
| Daily calorie intake, kcal | 2158 (602) | 1945 (682) | 2084 (676) | 1831 (651) | 2022 (581) | 1974 (595) | 2027 (708) | 1830 (599) |
| % calorie intake from fat, % | 36.1 (5.8) | 35.4 (7.2) | 31.6 (5.6) | 33.5 (6.4) | 33.9 (6.7) | 33.5 (7.0) | 33.8 (5.7) | 36.0 (7.9) |
| Depression, mean (SD) | ||||||||
| Depression symptoms | 50.6 (8.6) | 47.3 (9.0) | 53.6 (9.0)† | 48.2 (9.7) | 52.3 (10.2)† | 47.0 (8.7) | 49.5 (9.3) | 47.7 (8.4) |
| Antidepressants use, n (%) | ||||||||
| SSRI | 14 (73.7) | --- | 13 (50.0) | --- | 17 (58.6) | --- | 21 (60.0) | --- |
| SNRI | 1 (5.3) | --- | 3 (11.5) | --- | 5 (17.2) | --- | 6 (17.1) | --- |
| Atypical antidepressant | 7 (36.8) | --- | 12 (46.2) | --- | 7 (24.1) | --- | 7 (20.0) | --- |
| Tricyclic antidepressant | 0 (0.0) | --- | 4 (3.8) | --- | 4 (13.8) | --- | 5 (14.3) | --- |
SSRI: Selective serotonin reuptake inhibitor SNRI: Serotonin–norepinephrine reuptake inhibitor
p<0.05 for the differences between antidepressants users and non-users within each intervention group
p<0.01 for the differences between antidepressants users and non-users within each intervention group
Adherence to intervention between antidepressant users and non-users
The number of dropouts between antidepressant users and non-users did not differ in any intervention groups (p>0.05, Table 2). We observed no differences in 12-month change in cardiopulmonary fitness and weekly minutes of moderate-to-vigorous exercise between antidepressant users and non-users (p>0.05).
Table 2.
Adherence to a 12-month lifestyle intervention among antidepressant users and non-users in Seattle, WA 2005–2009
| Diet | Exercise | Diet + Exercise | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Users | Non-users | Users | Non-users | Users | Non-users | ||||
| (N=26) | (N=92) | (N=29) | (N=88) | (N=35) | (N=82) | ||||
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | ||||
| Drop outs | 1.07 (0.21 to 4.21) | ref | 0.92 | 1.15 (0.29 to 4.67) | ref | 0.84 | 4.39 (0.99 to 19.6) | ref | 0.052 |
| Mean (SD) | Mean (SD) | p-value | Mean (SD) | Mean (SD) | p-value | Mean (SD) | Mean (SD) | p-value | |
| Exercise Adherence | |||||||||
| ΔCardiopulmonary fitness, L/min* | --- | --- | --- | 0.18 (0.17) | 0.16 (0.40) | 0.77 | 0.14 (0.34) | 0.12 (0.35) | 0.92 |
| Exercise adherence, min/week | --- | --- | --- | 160.2 (65.1) | 164.3 (72.7) | 0.78 | 157.4 (79.2) | 177.9 (53.6) | 0.13 |
| ΔPedometer counts, steps/day* | --- | --- | --- | 1646 (2709) | 2636 (2750) | 0.10 | 2573 (2885) | 3796 (2996) | 0.06 |
| Diet Adherence | |||||||||
| ΔCalorie intake from fat, %* | −3.1 (6.1) | −6.5 (7.2) | 0.08 | --- | --- | --- | −5.7 (6.3) | −7.7 (7.8) | 0.48 |
| Diet session attendance, percent of sessions attended | 91.2 (29.0) | 89.5 (29.0) | 0.77 | --- | --- | --- | 84.3 (25.1) | 95.6 (21.0) | 0.03 |
P values compared drop out and exercise and dietary adherence between antidepressant users and non-users.
ANCOVA models for changes in cardiopulmonary fitness, pedometer counts, percent calorie intake from fat were adjusted for baseline values.
Diet session attendance was higher among antidepressant non-users in the diet+exercise group (vs. users, p=0.03),which attenuated after adjusting for depressive symptoms at baseline and 12 months (vs. users, p>0.05, results not shown).
Among non-users there were no differences in adherence by tertile of baseline depression symptom except for a smaller reduction in %calorie intake from fat in the highest tertile in the diet+exercise group (vs. lowest or middle tertile, Supplementary table 9).
Changes in body composition between antidepressant users and non-users
The intervention effects on weight, waist circumference, and percent body fat were not modified by antidepressant user status in any intervention arm (Pinteraction>0.05, Table 3). Percent body fat and waist circumference were reduced regardless of antidepressant use in all intervention groups (p<0.05 vs. control). Weight was significantly reduced in both antidepressant users and non-users in all intervention groups (vs. controls, p<0.01), with the exception of non-users randomized to exercise alone (p=0.09). Adjusting for depressive symptoms at baseline and 12 months had negligible effects on the changes in body composition among both antidepressant users and non-users (results not shown).
Table 3.
12-month changes in anthropometric outcomes among antidepressant users and non-users in Seattle, WA 2005–2009
| Antidepressant users | Non-users | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| baseline | 12 months | Change | P value | baseline | 12 months | Change | P value | Pinteraction | |||
| N | Geo Means (95% CI) | Geo Means (95% CI) | Absolute (%) | N | Geo Means (95% CI) | Geo Means (95% CI) | Absolute (%) | ||||
| Weight, kg | |||||||||||
| Control | 19 | 83.2 (77.5–89.3) | 85.6 (80.0–91.7) | 2.5 (3.0) | Ref | 68 | 83.3 (80.5–86.3) | 81.8 (79.0–84.7) | −1.5 (−1.8) | Ref | |
| Diet | 26 | 84.9 (80.8–89.1) | 78.4 (74.0–83.2) | −6.4 (−7.6) | <0.0001 | 92 | 82.8 (80.4–85.2) | 75.0 (72.3–77.8) | −7.8 (−9.4) | <0.0001 | 0.18 |
| Exercise | 29 | 82.9 (78.5–87.6) | 82.8 (78.4–87.5) | −0.1 (−0.1) | 0.0006 | 88 | 82.7 (80.3–85.3) | 80.1 (77.6–82.7) | −2.7 (−3.2) | 0.09 | 0.19 |
| Diet + Exercise | 35 | 83.0 (79.5–86.8) | 75.4 (71.4–79.5) | −7.7 (−9.2) | <0.0001 | 82 | 81.3 (79.1–83.6) | 71.7 (69.4–74.0) | −9.6 (−11.9) | <0.0001 | 0.38 |
| Percent body fat, % | |||||||||||
| Control | 19 | 47.1 (44.9–49.5) | 48.1 (46.0–50.3) | 0.9 (2.0) | Ref | 68 | 47.0 (46.0–48.1) | 46.5 (45.2–47.8) | −0.5 (−1.2) | Ref | |
| Diet | 26 | 46.8 (45.5–48.2) | 43.5 (41.6–45.4) | −3.4 (−7.2) | <0.0001 | 92 | 46.8 (45.8–47.7) | 41.9 (40.5–43.5) | −4.8 (−10.3) | <0.0001 | 0.92 |
| Exercise | 29 | 47.1 (45.7–48.6) | 46.4 (44.9–47.9) | −0.8 (−1.6) | 0.002 | 88 | 47.1 (46.2–48.0) | 45.2 (44.0–46.3) | −1.9 (−4.1) | 0.005 | 0.71 |
| Diet + Exercise | 35 | 48.4 (46.8–50.0) | 43.8 (41.4–46.2) | −4.6 (−9.5) | <0.0001 | 82 | 46.7 (45.8–47.6) | 39.8 (38.3–41.3) | −7.0 (−14.9) | <0.0001 | 0.16 |
| Waist circumference, cm | |||||||||||
| Control | 19 | 93.2 (89.0–97.6) | 96.9 (93.0–101.0) | 3.7 (3.9) | Ref | 68 | 94.6 (92.2–97.1) | 94.8 (92.5–97.2) | 0.2 (0.2) | Ref | |
| Diet | 26 | 97.4 (93.6–101.0) | 92.8 (88.8–97.0) | −4.6 (−4.7) | <0.0001 | 92 | 93.2 (91.1–95.2) | 88.5 (86.2–90.9) | −4.7 (−5.0) | <0.0001 | 0.33 |
| Exercise | 29 | 94.0 (90.4–97.7) | 93.3 (90.2–96.6) | −0.6 (−0.7) | 0.005 | 88 | 94.7 (92.7–96.8) | 92.3 (90.2–94.5) | −2.4 (−2.6) | 0.01 | 0.37 |
| Diet + Exercise | 35 | 95.0 (91.7–98.4) | 89.1 (85.2–93.2) | −5.9 (−6.2) | <0.0001 | 82 | 92.5 (90.5–94.5) | 84.6 (82.4–86.9) | −7.9 (−8.5) | <0.0001 | 0.90 |
P values compared changes from baseline to 12 months in intervention group vs. controls within strata defined by antidepressant use.
P interaction compared differences in 12-month changes in intervention group vs. controls between antidepressant users and non-users.
All models were adjusted for diet session attendance.
Changes in biomarkers of glucose metabolism and inflammation
The diet and diet+exercise groups significantly reduced fasting glucose in antidepressant non-users (vs. controls, p<0.05), but not in users (Table 4). The diet and diet+exercise groups reduced insulin in both antidepressant users and non-users (p<0.01 vs. control). The exercise group reduced insulin among users only (p=0.03, vs. control). The diet and diet+exercise groups reduced HOMA-IR and hs-CRP in both antidepressant users and non-users (p<0.05, vs. control). All tests for interaction between anti-depressant use and intervention effects were nonsignificant, however.
Table 4.
12-month changes in serum biomarkers among antidepressant users and non-users in Seattle, WA 2005–2009
| Antidepressant users | Non-users | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| baseline | 12 months | Change | P value | baseline | 12 months | Change | P value | Pinteraction | |||
| N | Geo Means (95% CI) | Geo Means (95% CI) | Absolute (%) | N | Geo Means (95% CI) | Geo Means (95% CI) | Absolute (%) | ||||
| Fasting glucose, mg/dL | |||||||||||
| Control | 19 | 96.2 (93.5–99.1) | 96.1 (93.2–99.0) | −0.2 (−0.2) | Ref | 68 | 96.8 (94.8–98.9) | 96.7 (94.9–98.6) | −0.1 (−0.1) | Ref | |
| Diet | 26 | 96.5 (93.2–99.9) | 95.9 (92.2–99.6) | −0.6 (−0.7) | 0.81 | 92 | 96.4 (94.6–98.2) | 93.5 (91.9−95.0) | −3.0 (−3.1) | 0.004 | 0.19 |
| Exercise | 29 | 94.9 (91.3–98.7) | 94.7 (91.3–98.2) | −0.2 (−0.2) | 0.99 | 88 | 95.7 (94.3–97.2) | 94.4 (93.0–95.9) | −1.3 (−1.3) | 0.23 | 0.55 |
| Diet + Exercise | 35 | 94.9 (92.4–97.5) | 92.8 (90.1–95.6) | −2.1 (−2.2) | 0.30 | 81 | 96.1 (94.4–97.8) | 92.7 (91.1–94.3) | −3.3 (−3.5) | 0.002 | 0.56 |
| Insulin, μU/mL | |||||||||||
| Control | 19 | 11.8 (9.9–14.1) | 13.6 (11.2–16.6) | 1.8 (15.3) | Ref | 68 | 12.0 (10.7–13.6) | 11.3 (10.0–12.7) | −0.8 (−6.3) | Ref | |
| Diet | 26 | 12.5 (10.3–15.3) | 9.6 (7.7–12.1) | −2.9 (−23.1) | <0.0001 | 92 | 10.6 (9.4–12.0) | 8.3 (7.4–9.3) | −2.3 (−22.0) | 0.004 | 0.14 |
| Exercise | 29 | 11.2 (9.1–13.9) | 10.6 (8.8–12.7) | −0.6 (−5.7) | 0.03 | 88 | 10.8 (9.8–12.0) | 9.9 (9.0–11.0) | −0.9 (−8.5) | 0.68 | 0.11 |
| Diet + Exercise | 35 | 10.4 (8.8–12.3) | 8.7 (7.4–10.2) | −1.7 (−16.2) | 0.0009 | 81 | 10.8 (9.5–12.3) | 7.9 (6.9–8.9) | −2.9 (−27.2) | 0.0002 | 0.51 |
| HOMA-IR (Homeostasis assessment - insulin resistance) | |||||||||||
| Control | 19 | 2.8 (2.3–3.4) | 3.2 (2.6–4.0) | 0.4 (15.1) | Ref | 68 | 2.8 (2.5–3.2) | 2.7 (2.4–3.0) | −0.2 (−6.4) | Ref | |
| Diet | 26 | 3.0 (2.4–3.6) | 2.3 (1.8–2.9) | −0.7 (−23.6) | 0.0002 | 92 | 2.5 (2.2–2.9) | 1.9 (1.7–2.1) | −0.6 (−24.4) | 0.002 | 0.25 |
| Exercise | 29 | 2.6 (2.0–3.3) | 2.5 (2.0–3.0) | −0.2 (−5.9) | 0.054 | 88 | 2.5 (2.3–2.8) | 2.3 (2.1–2.6) | −0.2 (−9.7) | 0.56 | 0.17 |
| Diet + Exercise | 35 | 2.4 (2.0–2.9) | 2.0 (1.7–2.4) | −0.4 (−18.0) | 0.002 | 81 | 2.5 (2.2–2.9) | 1.8 (1.6–2.0) | −0.8 (−29.7) | <0.0001 | 0.62 |
| High-sensitivity C-reactive protein, mg/L | |||||||||||
| Control | 19 | 1.7 (1.0–2.7) | 1.7 (1.1–2.6) | 0.0 (0.9) | Ref | 68 | 2.0 (1.5–2.6) | 2.0 (1.5–2.6) | 0.0 (0.8) | Ref | |
| Diet | 26 | 2.9 (1.9–4.4) | 1.7 (1.1–2.6) | −1.2 (−42.6) | 0.0002 | 92 | 2.5 (2.1–3.1) | 1.6 (1.3–1.9) | −0.9 (−36.4) | <0.0001 | 0.82 |
| Exercise | 29 | 2.7 (1.9–3.9) | 2.8 (2.0–4.1) | 0.1 (3.4) | 0.90 | 88 | 2.4 (2.0–3.0) | 2.2 (1.7–2.8) | −0.8 (−8.7) | 0.30 | 0.35 |
| Diet + Exercise | 35 | 2.2 (1.6–2.9) | 1.5 (1.0–2.1) | −0.7 (−33.2) | 0.02 | 81 | 2.1 (1.7–2.6) | 1.1 (0.9–1.4) | −1.0 (−46.4) | <0.0001 | 0.16 |
P values compared changes from baseline to 12 months in intervention group vs. controls within strata defined by antidepressant use.
P interaction compared differences in 12-month changes in intervention group vs. controls between antidepressant users and non-users.
All models were adjusted for diet session attendance.
To assess the effect of continuous antidepressant use, all analyses were repeated by comparing continuous antidepressant users (i.e., antidepressant use at both baseline and 12 months [N=76] versus non-users at both time points [N=294]). Except for a significant interaction in changes in fasting glucose between women who were continuous users and non-users in the diet group (Pinteraction=0.05, Supplementary Table 5–7), there were no substantial differences in the results.
Direct comparisons between antidepressant users and non-users
We also contrasted 12-month weight loss between antidepressant users and non-users within each study arm. We found a smaller weight loss among antidepressant users in the exercise (p=0.002, vs. non-users) and control groups (p=0.0008, vs. non-users), while there were no differences in the diet (p=0.20, vs. non-users) and the diet+exercise groups (p=0.35, vs. non-users; results not shown).
When all participants were combined, with adjustment for group assignment and diet session attendance, users had significantly lower decreases in weight, percent body fat and waist circumference (Supplementary table 10).
Discussion
In this study, we found no differences in most adherence variables and 12-month changes in body composition, biomarkers of glucose metabolism, and hs-CRP between antidepressant users and non-users during a 12-month diet and/or exercise intervention. Our findings suggest that when adherence is similar between antidepressant users and non-users, the dietary weight loss and exercise programs improve body composition, glucose metabolism, and inflammation independent of antidepressant use.
To our knowledge, only one previous intervention study compared the differences in weight loss between antidepressant users and non-users (Linde et al., 2004). A 24-month randomized trial of 1632 overweight adults that compared low-cost telephone-based, mail-based, and usual care interventions for weight loss, reported a smaller weight loss among female antidepressant users (mean weight loss 0.91 kg) compared to non-users (mean weigh loss 2.68 kg) at 12 months (Linde et al., 2004). We did not observe significant differences in weight loss between antidepressant users and non-users; however, a closer observation of the 12-month changes in weight showed 1.4–2.6 kg differences in absolute weight changes between antidepressant users and non-users (1.4 kg in diet, 2.6 kg in exercise, 1.9 kg in diet+exercise and 4.0 kg in controls). Differences in our statistical approaches to compare the differences between antidepressant users and non-users may have partially contributed to conflicting findings with the prior study.
When we contrasted 12-month weight loss between antidepressant users and non-users within each study arm, we found a smaller degree of weight loss among antidepressant users in the exercise and control but not in the diet groups. Our statistical approach examined the intervention effects relative to controls. However, among controls, antidepressant users showed greater weight gain compared to non-users. Several studies have similarly shown that antidepressants users are more likely to gain weight compared to non-users (Kivimaki et al., 2010; Patten et al., 2011). Thus, the differences in natural body weight trajectory between antidepressant users and non-users may need to be considered when evaluating the effects of weight loss programs.
The diet and diet+exercise groups significantly reduced insulin, fasting glucose, HOMA-IR, and hs-CRP independent of antidepressant use. Observational studies have shown that antidepressant use is associated with higher CRP (Hamer et al., 2011a) and increased risk for diabetes (Ma et al., 2011; Pan et al., 2012; Rubin et al., 2008; Rubin et al., 2010b) and cardiovascular disease (Cohen et al., 2000; Hamer et al., 2011b; Smoller et al., 2009). An analysis of a cohort study of 4584 adults showed that antidepressant use at baseline (1997–1999) and follow-up (2003–2004) was associated with higher CRP compared to non-users at both time points (Hamer et al., 2011a). The biological mechanisms of how antidepressants increase the risk of diabetes and cardiovascular disease are not established. Our findings suggest that dietary weight loss and exercise interventions reduce weight and improve biomarkers of glucose metabolism and inflammation independently of antidepressant use given similar adherence to interventions.
Since antidepressant users may have reduced adherence, this underscores the need for more intensive strategies to increase adherence in behavioral intervention studies. Several studies (Flegal et al., 2007; Somerset et al., 2011), but not all (Ludman et al., 2010), have shown that depression is associated with low adherence to diet and/or exercise programs. Our antidepressant users had a mean depression score similar to the norm of non-depressed women (Derogatis, 2001), which may account for our observed, non-significant differences in most adherence variables between antidepressant users and non-users.
Although most adherence differences did not reach statistical significance, we observed a consistent tendency of smaller increases in pedometer counts and reductions in percent calorie intake from fat among antidepressant users compared with non-users in all intervention arms.
Our antidepressant users in the diet and diet+exercise groups reduced weight by 7.6% and 9.2% and hs-CRP by 42.6% and 33.2%, respectively. A review concluded that 5% weight loss has beneficial effects on health outcomes, including lipid profile and blood pressure control (Blackburn, 1995). Another review estimated that individuals with CRP>3.0mg/L have an estimated relative risk of 1.58 (95%CI=1.37–1.83) for coronary heart disease compared to those with CRP<1.0 mg/L (Buckley et al., 2009). The observed reduction of hs-CRP in the diet and diet+exercise programs could have a significant health benefits among antidepressant users and nonusers alike.
Strengths of this study includes a large sample size, study design allowing direct comparisons of three intervention arms, low dropout rate (9%), and valid measures to assess outcomes. There are several limitations. Our participants, even those taking antidepressants, had only mild or no symptoms of depression. The findings therefore cannot be generalized to patients with moderate or severe depression. The study was not large enough to differentiate types of antidepressant medications which may have different effects on weight change and other outcomes (Serretti and Mandelli, 2010). We did not take into account the length of antidepressant use. This was a secondary analysis of the trial; thus, we had limited power to detect differences in intervention effects between antidepressant users and non-users.
Conclusion
In this study, we found that antidepressant use does not interfere with the effects of dietary weight loss and exercise on body composition and biomarkers of glucose metabolism and inflammation. Because antidepressant use is associated with increased risk of obesity and obesity-related comorbidities, practitioners are encouraged to promote dietary weight loss and exercise programs to reduce risks of obesity, diabetes, and cardiovascular disease especially among individuals who are prescribed antidepressant medications.
Supplementary Material
Highlights
We compared intervention adherence and effects by antidepressant use.
There were no differences in most adherence variables between users and non-users.
Intervention effects on body measures did not differ by antidepressant use.
Intervention effects on serum biomarkers did not differ by antidepressant use.
Acknowledgements
The NEW trial was supported by R01 CA105204-01A1 and U54-CA116847 from National Cancer Institute (NCI). Part of this study was conducted at the University of Washington, Clinical Nutrition Research Unit supported by National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) 61–7015. While working on the trial, CMA was employed at the Ohio State University, and located to NCI following completion of her effort on the NEW trial. CM is and KC was supported by a fellowship from the Canadian Institutes of Health Research. AK was supported by NCI R25CA094880 at the time of this study and is currently supported by NCI 2R25CA057699. KEF is supported by 5KL2RR025015-03 from National Center for Research Resources, a component of the National Institute of Health (NIH) and NIH Roadmap for Medical Research. These funding agencies had no role in study design, data collection, data analysis and interpretation of the results, writing of the article and decision to submit it for publication.
Trial registration:
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Dr. McTiernan has received compensation as a consultant for Metagenics and holds stock in Merck.
References
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3(Suppl 2):211–16. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:483–95. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Foster-Schubert KE, Alfano CM, Wang CC, Wang CY, Duggan CR, Mason C, Imayama I, Kong A, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30:2314–26. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med. 2000;108:2–8. doi: 10.1016/s0002-9343(99)00301-0. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory 18: Administration, scoring, and procedures manual. NCS Pearson, Inc.; Minneapolis: 2001. [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- Flegal KE, Kishiyama S, Zajdel D, Haas M, Oken BS. Adherence to yoga and exercise interventions in a 6-month clinical trial. BMC Complement Altern Med. 2007;7:37. doi: 10.1186/1472-6882-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, Bain CE, Wang CY, Blackburn GL, et al. Effect of Diet and Exercise, Alone or Combined, on Weight and Body Composition in Overweight-to-Obese Postmenopausal Women. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Antidepressants noncompetitively inhibit nicotinic acetylcholine receptor function. J Neurochem. 1999;72:1117–24. doi: 10.1046/j.1471-4159.1999.0721117.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Colunga J, Awad JN, Miledi R. Blockage of muscle and neuronal nicotinic acetylcholine receptors by fluoxetine (Prozac) Proc Natl Acad Sci U S A. 1997;94:2041–4. doi: 10.1073/pnas.94.5.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Batty GD, Marmot MG, Singh-Manoux A, Kivimaki M. Anti-depressant medication use and C-reactive protein: results from two population-based studies. Brain Behav Immun. 2011a;25:168–73. doi: 10.1016/j.bbi.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, David Batty G, Seldenrijk A, Kivimaki M. Antidepressant medication use and future risk of cardiovascular disease: the Scottish Health Survey. Eur Heart J. 2011b;32:437–42. doi: 10.1093/eurheartj/ehq438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayama I, Alfano CM, Kong A, Foster-Schubert KE, Bain CE, Xiao L, Duggan C, Wang CY, Campbell KL, et al. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:118. doi: 10.1186/1479-5868-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayama I, Ulrich CM, Alfano CM, Wang C, Xiao L, Wener MH, Campbell KL, Duggan C, Foster-Schubert KE, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 2012;72:2314–26. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimaki M, Hamer M, Batty GD, Geddes JR, Tabak AG, Pentti J, Virtanen M, Vahtera J. Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care. 2010;33:2611–6. doi: 10.2337/dc10-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitz Y, Ben-Shushan G, Hershkovitz A, Isaac R, Gil-Ad I, Shvartsman D, Ronen D, Weizman A, Zick Y. Antidepressants induce cellular insulin resistance by activation of IRS-1 kinases. Mol Cell Neurosci. 2007;36:305–12. doi: 10.1016/j.mcn.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Linde JA, Jeffery RW, Levy RL, Sherwood NE, Utter J, Pronk NP, Boyle RG. Binge eating disorder, weight control self-efficacy, and depression in overweight men and women. Int J Obes Relat Metab Disord. 2004;28:418–25. doi: 10.1038/sj.ijo.0802570. [DOI] [PubMed] [Google Scholar]
- Ludman E, Simon GE, Ichikawa LE, Operskalski BH, Arterburn D, Linde JA, Jeffery RW, Rohde P, Finch EA. Does depression reduce the effectiveness of behavioral weight loss treatment? Behav Med. 2010;35:126–34. doi: 10.1080/08964280903334527. [DOI] [PubMed] [Google Scholar]
- Ma Y, Balasubramanian R, Pagoto SL, Schneider KL, Culver AL, Olendzki B, Tinker L, Liu S, Safford M, et al. Elevated depressive symptoms, antidepressant use, and diabetes in a large multiethnic national sample of postmenopausal women. Diabetes Care. 2011;34:2390–2. doi: 10.2337/dc11-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason C, Foster-Schubert KE, Imayama I, Kong A, Xiao L, Bain C, Campbell KL, Wang CY, Duggan CR, et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41:366–75. doi: 10.1016/j.amepre.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Pagoto S, Bodenlos JS, Kantor L, Gitkind M, Curtin C, Ma Y. Association of major depression and binge eating disorder with weight loss in a clinical setting. Obesity (Silver Spring) 2007;15:2557–9. doi: 10.1038/oby.2007.307. [DOI] [PubMed] [Google Scholar]
- Pan A, Sun Q, Okereke OI, Rexrode KM, Rubin RR, Lucas M, Willett WC, Manson JE, Hu FB. Use of antidepressant medication and risk of type 2 diabetes: results from three cohorts of US adults. Diabetologia. 2012;55:63–72. doi: 10.1007/s00125-011-2268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate RR, Blair SN, Durstine JL, Eddy DL, Hanson P, Painter P, Smith LK, Wolfe LA. Guidelines for Exercise Testing and Prescription. 4th ed Lea & Febiger; Philadelphia, PA: 1991. [Google Scholar]
- Patten SB, Williams JV, Lavorato DH, Khaled S, Bulloch AG. Weight gain in relation to major depression and antidepressant medication use. J Affect Disord. 2011;134:288–93. doi: 10.1016/j.jad.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- Paulose-Ram R, Safran MA, Jonas BS, Gu Q, Orwig D. Trends in psychotropic medication use among U.S. adults. Pharmacoepidemiol Drug Saf. 2007;16:560–70. doi: 10.1002/pds.1367. [DOI] [PubMed] [Google Scholar]
- Richardson CR, Avripas SA, Neal DL, Marcus SM. Increasing lifestyle physical activity in patients with depression or other serious mental illness. J Psychiatr Pract. 2005;11:379–88. doi: 10.1097/00131746-200511000-00004. [DOI] [PubMed] [Google Scholar]
- Rubin RR, Gaussoin SA, Peyrot M, DiLillo V, Miller K, Wadden TA, West DS, Wing RR, Knowler WC. Cardiovascular disease risk factors, depression symptoms and antidepressant medicine use in the Look AHEAD (Action for Health in Diabetes) clinical trial of weight loss in diabetes. Diabetologia. 2010a;53:1581–9. doi: 10.1007/s00125-010-1765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RR, Ma Y, Marrero DG, Peyrot M, Barrett-Connor EL, Kahn SE, Haffner SM, Price DW, Knowler WC. Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care. 2008;31:420–6. doi: 10.2337/dc07-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RR, Ma Y, Peyrot M, Marrero DG, Price DW, Barrett-Connor E, Knowler WC. Antidepressant medicine use and risk of developing diabetes during the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care. 2010b;33:2549–51. doi: 10.2337/dc10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Schauer JE, Hanson P. Usefulness of a branching treadmill protocol for evaluation of cardiac functional capacity. Am J Cardiol. 1987;60:1373–7. doi: 10.1016/0002-9149(87)90622-9. [DOI] [PubMed] [Google Scholar]
- Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259–72. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Allison M, Cochrane BB, Curb JD, Perlis RH, Robinson JG, Rosal MC, Wenger NK, Wassertheil Smoller S. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women's Health Initiative study. Arch Intern Med. 2009;169:2128–39. doi: 10.1001/archinternmed.2009.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerset SM, Graham L, Markwell K. Depression scores predict adherence in a dietary weight loss intervention trial. Clin Nutr. 2011;30:593–8. doi: 10.1016/j.clnu.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA. 1991;266:93–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.