Abstract
N-(3-oxododecanoyl)-L-homoserine lactone (3OC12-HSL) is a quorum-sensing molecule produced by gram-negative microbial pathogens such as Pseudomonas aeruginosa (PAO1). 3OC12-HSL is involved in the regulation of bacterial virulence factors and also alters the function of the host immune cells. Others and we have previously shown that paraoxonase 2 (PON2), a member of the paraoxonase gene family expressed in immune cells, hydrolyzes 3OC12-HSL. In this study, we examined i) whether macrophage PON2 participates in 3OC12-HSL hydrolysis, ii) the effect of PON2 deficiency in acute PAO1 infection in mice and iii) the effect of 3OC12-HSL on PON2 deficient (PON2-def) macrophages. When compared to wild type macrophages, both intact cells and membrane-enriched protein lysates obtained from PON2-def macrophages show a marked impairment in their ability to hydrolyze 3OC12-HSL. PON2 expression (message and protein) is not altered in response to 3OC12-HSL in macrophages. 3OC12-HSL treated PON2-def macrophages showed i) an increase in ER stress and oxidative stress, ii) defective phosphatidylinositol 3-kinase (PI3 kinase)/AKT activation, and iii) reduced phagocytosis function. Moreover, the nitration to phosphorylation ratio of Tyr458 in p85 protein, the regulatory subunit of PI3-kinase that has been correlated with the phagocytosis function of macrophages, was increased in PON2-def macrophages. Antioxidant treatment reversed the effects of PON2 deficiency in macrophage phagocytosis function. Furthermore, following administration of 1.6×107CFU of PAO1, bacterial clearance was significantly reduced in the lungs (5.7 fold), liver (2.5 fold), and spleen (14.8 fold) of PON2-def mice when compared to wild type mice. Our results suggest that PON2 plays an important role in innate immune defense against PAO1 infection.
Keywords: Paraoxonase 2, Pseudomonas aeruginosa, Quorum sensing, Mitochondrial oxidative stress, Endoplasmic reticulum stress
1. Introduction
Pseudomonas aeruginosa (PAO1) is an opportunistic pathogen that causes a wide variety of acute infections in immune-compromised patients, as well as chronic respiratory infections in patients suffering from cystic fibrosis, cancer, and other chronic respiratory diseases [1]. To facilitate the establishment of infection, PAO1 produces cell-associated and extracellular virulence factors, which are regulated by quorum sensing (QS) [2]. QS is a mechanism wherein small diffusible molecules, specifically acyl homo serine lactones (AHL), are produced and detected by the organisms’ surrounding molecules. As the number of bacteria increase, intracellular levels of AHLs achieve a threshold concentration, including the activation of targeted transcriptional regulators [3]. This process is well described for the formation of biofilm in chronic infection by PAO1 and 3OC12-HSL; a member of the AHL family is shown to be the primary QS molecule [4]. Biofilms typically display a marked resistance to antibiotic killing and therefore infections associated with biofilm-forming bacteria are difficult to eradicate[5]. 3OC12-HSL is shown to induce stress signaling in immune cells, reduce immune cell function, and improve bacterial survival. Hence, inactivation of these molecules or its effect on host cells is a therapeutic target for PAO1 associated disease.
Paraoxonases, a family of Ca2+dependent esterases consisting of PON1, PON2 and PON3[6], hydrolyze 3OC12-HSL[7]. The PON gene family is located on chromosome 6 in mice and on chromosome 7 in humans [8]. In humans, PON1 mRNA expression is limited to the lung and liver [8]. In contrast, hPON2 is ubiquitously expressed including kidney, liver, lung, placenta, small intestine, spleen, stomach, testis, and vascular cells. In humans, PON3 is restricted to the lung, liver, and kidney [8]. In addition, hPON1 and hPON3 are associated with HDL in the circulatory system whereas hPON2 protein is undetectable in HDL, LDL, or cell supernatants; it is, however, associated with intracellular membrane fractions [9]. A property shared by all PON proteins is the capacity to hydrolyze lactones [7]. In particular, AHLs have been identified as substrates common for all PON proteins, with PON2 exhibiting the highest specific activity [7].
Macrophages play a central role in response to extracellular and intracellular pathogens [10]. Our previous studies have shown that PON2 is expressed in this immune effector cell and plays an important role in macrophage function under chronic inflammatory conditions [11]. Based on this evidence, we hypothesized and tested whether PON2-def influences the innate immune response in an acute PAO1 infection model.
2. Materials and Methods
2.1 Animals
Male PON2-def mice on the C57BL6/J background and littermate controls, 8 to 10 weeks old, were used in all experiments. The Animal Research Committee at the University of California, Los Angeles approved all experiments.
2.2 Membrane-Enriched Tissue and Cell Homogenates
Wild type and PON2-def mice were injected intraperitoneally with 1.5 ml of a solution consisting of 3% thioglycollate broth. Three days later, macrophages were collected from the peritoneal cavity. Cells were homogenized on ice in 1 volume of 25 mM Tris, pH 7.4, containing 1 mM CaCl2 with 10 passes on a Polytron homogenizer followed by 10 passes on a potter-elvehjem homogenizer. Samples were centrifuged at 17,000 × g for 30 minutes at 4°C and pellets re-suspended in extraction buffer containing 25 mM Tris HCl, pH 7.4, 1 mM CaCl2, and EDTA-free protease inhibitors (Roche, San Francisco, CA). The following day, samples were centrifuged at 2000 × g for 5 minutes and supernatants collected and stored at 4°C.
2.3 3OC12-HSL inactivation Bioassay
Reactions were carried out at room temperature in a 50 μL volume of 25 mM Tris-HCl, pH 7.4, 1 mM CaCl2 containing 10 μg of macrophage crude membrane extracts and 0.5 μM 3OC12-HSL. Reactions were stopped with an equal volume of acetonitrile, and 0.01 mL of a 1:100 dilution was used to measure 3OC12-HSL by a quantitative bioassay using E. coli MG4 (pKDT17) as described previously (13). 3OC12-HSL and E. coli MG4 were kindly provided by K. Janda (The Scripps Research Institute, San Diego, CA) and E. Greenberg (University of Iowa), respectively.
2.4 Measurement of oxidative stress, glutathione, and phagocytosis in macrophages
Peritoneal macrophages isolated from experimental animals were treated with 3OC12-HSL as described under the corresponding figure legends. 3OC12-HSL concentrations used were based on previous reports [12, 13]. Mitochondrial enriched fractions were isolated as described previously[14] and superoxide assays were carried out by incubating 4 μg of macrophage mitochondria with 5 μM dihydroethidium in a 96-well plate format at 37 °C for 60 min. The reaction was stopped by the addition of 0.6% triton and ethidium bromide/DNA fluorescence measured (excitation at 544 nm and emission at 612 nm) using a BMG Labtech fluorescence microplate reader [15]. 2′,7′-dichlorofluorescein (DCF) assay was used to quantify intracellular oxidative stress as previously described [16]. Briefly, peritoneal macrophages from wild type or PON2-def mice were cultured onto 96-well plates (5 × 104 cells/well) and loaded with 100 μM 2′,7′-dichlorodihydrofluorescein diacetate (Invitrogen, Invitrogen-Molecular Probes, Grand Island, NY, USA) for 1 hr at 37°C. Cells were then washed with Krebs-Ringer buffer and treated with 50 μM 3OC12-HSL in Krebs-Ringer buffer. Fluorescence was measured at the indicated times using a fluorescence microplate reader (Spectra Max Gemini XS, Molecular Devices) with an excitation wavelength at 485 nm and an emission wavelength at 530 nm. After treatment with 3OC12-HSL, macrophage phagocytosis function was assayed using a kit according to the manufacturer’s protocol (Invitrogen, Molecular probe, Grand Island, NY). Glutathione assay was performed according to the manufacturer’s protocol (Cayman Chemical Company, Ann Arbor, Michigan).
2.5 Analysis of gene expression
RNA was extracted using an RNeasy Plus kit (Qiagen, Valencia, CA) and cDNA synthesized using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). ER stress markers CHOP, GRP78 and ERO1-α expression levels were determined by RT-PCR using an iCycler thermal cycler (Biorad, Hercules, CA).
2.6 Assessment of TNF-α Protein Levels
Protein levels of TNFα were determined using an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA).
2.7 Immunoblotting and immunoprecipitation
Macrophages were isolated from experimental groups and treated with 3OC12-HSL as described under the figure legends. Total protein (50 ug) were resolved by 4–15% SDS-PAGE, transferred onto nitrocellulose membranes and blocked in Tris-buffered saline with 0.1% Tween 20 (TBST) containing 5% (w/v) nonfat milk for 1 hour. The following primary antibodies were used at the dilution indicated Anti-PON2 antibody (1:500; Genescript, Piscataway, NJ), anti-CHOP antibody (1:250; Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-ERO1-α antibody (Novus Biologicals, Littleton, CO), GRP78 antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, USA) at 1: 2000, Phospho-AKT (Ser473) and total AKT antibodies were used at 1: 1000 dilution (Cell Signaling technology, Danvers, MA), and mouse β-actin antibody was diluted at 1:5000 (Sigma Aldrich, St. Louis, MO). For the phagocytosis experiments, macrophages were treated with 3OC12-HSL and phagocytosis was measured (as described in the manufacturer’s protocol, Invitrogen, Molecular probe, Grand Island, NY) for different time points as described under the figure legend. Cells were washed with PBS buffer and lysed with lysate buffer containing 1% Triton X-100. 250μg total cell lysates from each treatment were incubated overnight with 25μl anti-nitro-tyrosine agarose beads (Millipore, MA). The immunoprecipitated products were washed 3 times with PBS, resolved on SDS-PAGE gels and blotted with p85-PI3K antibodies (1: 1000 dilution; Cell Signaling technology, Danvers, MA) or probed with nitrotyrosine antibody. Primary antibodies were diluted in TBS containing 3% milk protein at 4°C overnight followed by incubation with appropriate secondary antibody (1:5000) coupled to horseradish peroxidase and proteins were illuminated using an ECL Plus Western blotting kit (GE Healthcare, UK).
2.8 Acute Systemic Infection
PAO1 strain grown to mid-logarithmic phase in Luria broth was harvested by centrifugation at 1500 × g for 15 minutes and washed twice in pyrogen-free 0.9% NaCl. Bacteria were suspended in 10 mL of 0.9% NaCl and the number of bacteria determined by serial dilution in sterile isotonic saline and cultured onto Luria agar. Mice (n=9–10) were administered roughly 1×107 colony forming units (CFU) of PAO1 into the peritoneal cavity. Mice were sacrificed 3 hours following infection; blood and organs were collected and immediately processed as described below.
2.9 Determination of Bacterial Outgrowth
Serial 10-fold dilutions in sterile isotonic saline were made of the homogenates and 50 μL volumes were plated onto LB agar and incubated at 37°C. CFU were counted after 24 hours. For cytokine measurements, tissue homogenates or serum were spun at 1,500 × g for 15 minutes at 4°C and supernatants were filtered through a 35 μm-pore-size filter (Becton Dickinson, Lincoln Park, NJ) and frozen at −20°C until cytokine measurement.
3. Results
3.1 Quorum quenching is impaired in macrophages from PON2-def mice
Macrophages are vital immune cells that contribute to innate immune defense whose function influences the quality, duration, and magnitude of most inflammatory reactions [17]. PON2 is highly expressed in macrophages and has been shown to alter their activation state [18]. Therefore, we first evaluated the capacity of membrane extracts from PON2-def peritoneal macrophages to inactivate 3OC12-HSL. As shown in Fig.1, PON2-def membrane lysates (Fig.1A) as well as intact macrophages (Fig.1B) are impaired in 3OC12-HSL hydrolysis compared to wild type lysates and macrophages. PON2 mRNA (Fig.1C) and protein levels (Fig.1D) are not affected by 3OC12-HSL in wild type macrophages.
Fig.1. Quorum quenching is impaired in macrophages from PON2-def mice.
A) 4 μg of peritoneal macrophage membrane extract from PON2-def and wild type mice (n=3) were incubated with 0.5 μM 3OC12-HSL at different time points (0, 30, 60, 90,s and 120 minutes). The amount of 3OC12-HSL remaining was measured by a quantitative bioassay using E. coli MG-4 (pKDT17) as described previously (13). * P < 0.05 relative to wild type at all the indicated time points. B) 0.5 μM 3OC12-HSL was added to intact macrophages containing DMEM medium. At different times points (0, 30, 60, 90, and 120 minutes) medium was collected and the amount of 3OC12-HSL remaining was measured as described previously (13). C) Peritoneal macrophages were isolated from experimental groups and treated with 3OC12-HSL for indicated time periods (0, 60, 120, and 240 minutes) and PON2 gene expression was evaluated by quantitative RT-PCR. D) Total proteins were extracted from macrophages of experimental groups after treated with 50 μM 3OC12-HSL for the indicated time periods as above and PON2 protein was detected as described in the material and methods.
3.2 Bacterial clearance is impaired in PON2-def mice following acute PAO1 infections
To determine whether PON2 deficiency altered the innate immune response in an in vivo acute systemic infection model, approximately 10-week old mice were administered 1.6×107CFU of PAO1 into their peritoneal cavity and sacrificed 3 hours post-infection. Bacterial clearance was significantly decreased in organs collected from PON2-def mice, relative to wild type mice. Bacterial colonization was increased by 2.5 fold in the liver, 5.7-fold in the lungs, and 14.8 fold in the spleen of PON2-def mice. (Fig. 2A–2C).
Fig.2. Bacterial clearance is impaired in PON2-def mice following acute PAO1 infections.
PAO1 was administered by intraperitoneal inoculation to PON2-def and wild type mice (n= 9–10). Three hours late, the mice were sacrificed and the organs were collected. PAO1 colony forming units (CFU) in each organ were measured as described under materials and methods. Mean (± SD) CFU in the indicated organs are shown; (A) liver, (B) lungs, and (C) spleen. * p < 0.05 relative to organs from wild type mice.
3.3 Pro inflammatory response in PON2-def mice following PAO1 infection
To determine whether PON2 deficiency altered the proinflammatory response, we quantified the levels of TNFα in the serum and liver of PON2-def mice following PAO1 infection. No changes in serum TNFα protein levels were detected 3 hours post-infection (Fig. 3A). However, a significant 3-fold increase in liver mRNA levels was observed in PON2-def mice, relative to wild type (Fig.3B).
Fig.3. Pro-inflammatory cytokine response is altered in PON2-def mice following PAO1 infection.
TNFα concentrations in (A) serum and (B) liver homogenates of PON2-def and wild type mice (n=9–10) 3 hours after intraperitoneal inoculation with 107 CFU of P. aeruginosa (PAO1). Data are means ± SD. * p < 0.05.
3.4 PON2-def macrophages have reduced phagocytosis function in response to 3OC12-HSL
The immune defense is vital for the activation of the cellular effector functions, including the efficient uptake, the phagocytes, and intracellular killing by means of production of oxygen radicals. Recognition is accomplished by pattern recognition receptors, which are selective for groups of bacterial molecules, including lipopolysaccharides, lipoteichoic acid, or peptidoglycans. Recent study indicates that 3OC12-HSL is an “interkingdom” signaling molecule that also interacts with mammalian cells and alters the host cell function. Since macrophages are important for innate immune function, we asked whether 3OC12-HSL alters the phagocyte function of macrophage, and whether PON2 modulates it. To test this, macrophages from wild type and PON2-def mice were treated with 3OC12-HSL and their phagocytosis function was analyzed at different time points. As seen in Fig. 4A phagocytosis function is slightly but significantly increased in untreated PON2-def macrophages compared to untreated wild type macrophages. However, PON2-def macrophages show impaired phagocytosis compared to wild type macrophages at all time points tested following 3OC12-HSL treatment (Fig. 4A).
Fig.4. PON2-def macrophages have reduced phagocytosis function in response to 3OC12-HSL.

Peritoneal macrophages were isolated from experimental groups; treated with 50 μM 30C12-HSL for 0, 30, 60, and 90 minutes and phagocytosis assay was performed as described in manufacturer’s protocol at different times points. A) Phagocytosis function was analyzed (n= 3 independent experiments). Representative data are means ± SD. * p < 0.05. B) Total proteins were extracted from lysate and tyrosine phosphorylation of PI3K regulatory subunit P85 (Upper panel), and (C) total PI3K p85 subunit were analyzed by immunoblotting. D) Tyrosine-nitrated proteins were immunoprecipitated with nitrotyrosine antibody and subjected to western blotting with PIK P85 antibody (Top panel) or subjected to western blotting with nitrotyrosine antibody (bottom panel) as described in the methods. E) Peritoneal macrophages were isolated from experimental groups; treated with C4-HSL (50 μM) for 0, 30, 60, and 90 minutes and phagocytosis function was analyzed
Increases in the generation of phosphatidylinositol phosphates (PIP) within the phagocytic cup is an early and important event of phagocytosis. It has been reported that PI3 kinase catalyzes the production of PIPs by phosphorylating phosphatidylinositol. PI3 kinase is a heterodimer composed of a regulatory subunit (P110) and a catalytic subunit (P85). Phosphorylation of 85 is important for downstream effects including PIP formation. Hence we performed immuno blotting with phospho-PI3 kinase p85 (Tyr 458). In untreated cells there was a slight increase in the level phospho PI3 kinase in PON2-def macrophages compared to wild type macrophages. However, following 3OC12-HSL treatment, there was a decrease in phospho-PI3 kinase p85 in the macrophages of PON2-def mice compared to control macrophages (Fig. 4B). Similarly, we observed decreased AKT Phosphorylation in PON2-def macrophages upon treatment with 3OC12-HSL (Fig. 4C). The p85 subunit of PI3 kinase has been proposed as a direct target for tyrosine nitration. Hence, we examined the nitrotyrosine level in p85 subunit using immunoprecipitation using an anti-nitrotyrosine antibody followed by Western blotting using p85-PI3K p85 antibody or anti-nitrotyrosine antibody. As shown in Fig 4D, 3OC12-HSL treatment significantly increased the nitrotyrosine content of p85 (but not another nitrosylated 66 kDa protein) in PON2-def macrophages compared to control macrophages. As seen in Fig 4E, in contrast to 3OC12-HSL (Fig 4A) C4-HSL treatment neither induces nor inhibits the phagocytosis function in both wild and PON2-def mice over the course of the time points tested.
3.5 PON2-def macrophages treated with 3OC12-HSL harbor enhanced oxidative stress
Increase in superoxide is the major source for peroxynitrite formation and eventually leads to nitrosylation of many proteins especially targeted at the tyrosine residues. It has been reported that 3OC12-HSL treated bone marrow derived macrophages show dramatic morphological alterations in mitochondrial swelling and distension of the ER. These organelles are major sources of reactive oxygen species including superoxide which damage the organelles. In this juncture, we analyzed the role of PON2 in 3OC12-HSL mediated macrophage oxidative stress in an ex vivo model. Macrophages from PON2-def mice show increased mitochondrial superoxide levels under untreated condition and it was further increased upon treatment with 3OC12-HSL in time dependent manner compared to wild type macrophages (Fig. 5A). Similarly, total ROS was increased in the macrophages of PON2-def mice compared to control in both untreated as well as treated conditions (Fig. 5B). Compared to wild type macrophages, ER stress marker ERO-1α, GRP78 (Fig. 5C–D) was significantly increased in PON2-def macrophages after treatment with 3OC12-HSL at 60 and 90 min whereas CHOP was elevated at 90 min at the gene expression level (Fig. 5E) and protein level (Fig. 5F). In contrast to ROS, glutathione level was significantly decreased in PON2-def mice upon 3OC12-HSL treatment, but not under untreated condition (Fig. 5G).
Fig.5. Macrophage oxidative stress is increased in PON2-def mice in response to 3OC12-HSL.
Peritoneal macrophages isolated from PON2-def (n=3) and wild type (n=3) mice were stimulated with 3OC12-HSL for different times points 0, 30, 60, and 90 minutes. (A) Mitochondrial enriched fraction was isolated from cells at the indicated time points and superoxide was quantified. (B) Total ROS was quantified using the DCF assay a described under methods. RNA was isolated from macrophages of experimental groups after treatment with 3OC12-HSL at the indicated time points and (C) ER01-α, (D) GRP78, (E) CHOP gene expression was analyzed using real time qPCR. (F) Immunoblots showing protein levels (in 50 μg of total lysates) of the corresponding genes from the same experiment. (G) Glutathione levels were measured (n= 3–4 independent experiments). Data are means ± SD. * p < 0.05.
3.6 Antioxidants rescue the impaired phagocytosis function in PON2-def macrophages
In order to examine whether ROS/RNS, play an important role for phagocytosis function, cells were pretreated with uric acid followed by 3OC12-HSL treatment and phagocytosis function was analyzed. As seen in Fig. 6A–B, phagocytosis function of macrophages was improved when the ROS levels were reduced by uric acid treatment in both wild type and PON2-def macrophages. Similar results observed when macrophages from wild type and PON2-def mice were pretreated with N-acetyl cysteine (data not shown)
Fig.6. Uric acid, an antioxidant reduces ROS and improves the phagocytosis function in PON2-def macrophages.
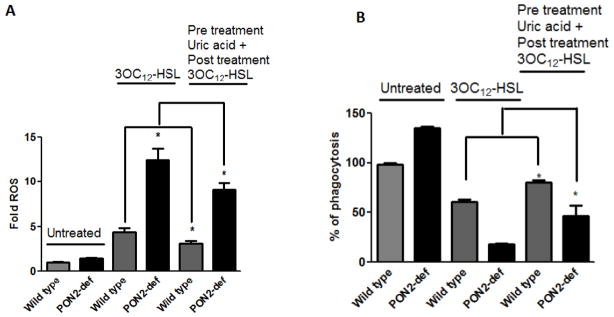
Peritoneal macrophages obtained from PON2-def and wild type littermate control mice were pretreated overnight with uric acid (10μM) followed by 3OC12-HSL for 90 minutes. A) Phagocytosis function and B) ROS levels were analyzed. Data are means ± SD. n= 3–4 independent experiments. * p < 0.05.
4. Discussion
PAO1 is ubiquitously present in the environment and is known to cause nosocomial infections. 3OC12-HSL, a quorum-sensing molecule secreted by PAO1, participates in bacterial colonization and inhibition of host immune response. Anti-quorum-sensing molecules are therapeutic targets for treating PAO1 associated diseases. PON2 has been shown to inactivate 3OC12-HSL in vitro. PON2 is expressed in immune cells; however, studies to date have only focused on the role of PON2 in atherosclerosis, neuronal disease and type 2 diabetes [18]. In this study, we examined the role of PON2 in innate immune response in an acute infection model. We demonstrate, for the first time, that 1) both intact cells and membrane-enriched protein lysates obtained from PON2-def macrophages are significantly impaired in their ability to inactivate 3OC12-HSL, 2), PAO1 is cleared dramatically slower in PON2-def mice compared to their control littermates, 3) PON2-def macrophages are defective in PI3 kinase/AKT activation leading to reduced phagocytosis function following 3OC12-HSL treatment, and 4) 3OC12-HSL induced ER stress and mitochondrial oxidative stress are elevated in PON2-def macrophages.
3OC12-HSL is a small lipid-based molecule that exhibits structural similarities to many eukaryotic hormones. Vikstrom et al. reported that 3OC12-HSL disrupts the barrier integrity of human epithelial Caco-2 cells [19]. A significant reorganization of actin cytoskeletons, lower trans epithelial electrical resistance, and reduced expression and phosphorylation state of key tight-junction proteins were demonstrated in gut epithelial cells exposed to 3OC12-HSL [19]. It has been documented that 3OC12-HSL inhibited the expression of two specific nucleotide receptors, P2Y2 and P2Y4, in airway epithelial cells lacking a functional cystic fibrosis trans membrane conductance receptor (CFTR). This effect was reversed when CFTR was expressed in an adenovirus vector [20]. Kravchenko et al. have shown that 3OC12HSL selectively impairs the regulation of NF-kB signaling in LPS activated bone marrow derived macrophages [21]. This impaired signaling is not a direct effect because in the presence of 3OC12HSL, purified IKK complex and a constitutive active form of its subunit IKK-β, neither promoted nor inhibited its kinase activity[21].
Imamura et al. reported that mucin 5AC mRNA and protein levels were increased in NCI-H292 human lung epithelial cells exposed to 3OC12-HSL [22]; thus, studies from various laboratories revealed that 3OC12-HSL alters host cell and tissue function. Present study suggests that 3OC12-HSL increases mitochondrial oxidative stress, ER stress, and inflammation in PON2-def macrophages. It has been reported that PON2 modulates the mitochondrial oxidative stress during adverse conditions in female mice in a proatherogenic apoE−/−/C57BL/6 background as well as in a cell culture model when treated with ER stress inducers; tunicamycin and thapsigargin. Current study revealed that PON2-deficiency in C57BL/6 background (apoE+/+) increases the ER stress in macrophages under 3OC12-HSL induced condition suggesting PON2-def alone can enhance the ER stress in macrophages. Transgenic PON3 animal model studies suggest that PON3 has protective properties for atherosclerosis and obesity only in male mice but not in female mice[23]. Current study together with our previous findings suggests that the anti-oxidant properties of PON2 are not gender specific [24–26].
It has been shown that intact tracheal cells do not hydrolyze the 3OC12-HSL in contrast to their cell lysates. Horke et al have shown that 3OC12-HSL down-regulates PON2 expression (both mRNA and protein) in EA.hy 926 cells. The same studies revealed that decreased expression of PON2 is at least partly mediated by increase in cytosolic Ca2+ induced by 3OC12-HSL [27]. In our study, i) a decreased expression of PON2 by 3OC12-HSL in peritoneal macrophages is not observed, and ii) both lysate and intact cells hydrolyze the 3OC12-HSL in macrophages. The most likely explanation for these differences is that 3OC12-HSL effects are cell type specific. Supporting our results, recently, Kim et al. reported that silencing the hPON2 in human aortic endothelial cells (HAEC) increases ROS levels and NF-kB mediated inflammation when exposed to 3OC12-HSL and over-expression of PON2 in HeLa cells reduces ROS levels and NF-kB mediated inflammatory genes [13]. In the same report, it was further demonstrated that both lysates and intact cells hydrolyze 3OC12-HSL in both cell types [13].
Our studies show that PON2-def mice have reduced bacterial clearance in an acute infection model. In the current study, we have chosen the early time point since later time point PON2-def mice die faster than wild type. Phagocytes such as neutrophils and macrophages represent a powerful defense system against invading microorganisms. Phagocytes produce ROS during phagocytosis or stimulation with a wide variety of agents. ROS/RNS contribute to appropriate macrophage inflammatory activation and is also required for effective phagocytosis and clearance of invading pathogens [28, 29]. However, phagocytosis can actively produce more highly reactive radicals and molecules, which rapidly damage or alter the biological macromolecules that are important for cell function. Prolonged exposure of macrophages to ROS alters their viability and phagocytosis function [30]. Indeed, PON2-def macrophages show modest superoxide level and are sufficient to induce the phagocytosis and during this infection process produce more ROS. Thus, ROS/RNS generation can further induce the generation of ROS/RNS via a mechanism proposed as “ROS/RNS induced ROS/RNS generation”. Oxidative stress resulting from such feed-forward loops can affect macrophage functions. Nitric oxide (NO) is a free radical that is an important biological signaling molecule under physiological condition. However, under pathophysiological conditions, nitric oxide alters several biological processes through the generation of reactive nitrogen species (RNS). It has been reported that RNS modulates the activation of PI3-kinase-Akt signaling in various pathological conditions. Our study suggest that increased superoxide or ROS forms peroxinitrate which compete with phosphorylation site of p85 leading to defective signaling and alters the phagocytes function. Supporting our finding, Morrow et al demonstrated that antioxidants preserve the phagocytosis function of macrophages after PAO1 infection [31].
Unexpectedly in a sepsis model, PON1 knockout mice protect against the PAO1 infection due to up regulation of PON2 and PON3 [32]. Nevertheless, overexpression of PON1 in Drosophila protect from PAO1 lethality [33]. In the current report, liver and lung of PON2-def mice show lower bacterial clearance following PAO1 infection in mice. Simanski et al [34]infected keratinocytes (either expressing control siRNA or PON2 siRNA) with PAO1 (1.5 × 103 CFU) and showed that silencing PON2 has more bacterial colonization when compared to control siRNA infected cells. Bacterial products and cytokines released in response to extravascular infection are known to influence atheroma-associated cells [35]. O’Connor et al [35] proposed the ‘echo hypothesis’ to explain the role of microbial infection and atherosclerosis. In conclusion, we suggest that PON2 plays a role in innate immune function via redox signaling mechanisms during early infection. It is possible that under chronic pathophysiological conditions, PON2 keep QS molecules under check and prevents bacterial colonization and modulates the pro-inflammatory pathways mediated by bacterial products. Studies are underway in our laboratory to address and understand the role of PON2 in 1) screen various bacteria including 3OC12-HSL-deficient pseudomonas strain and dissect the mechanisms in the impairment of bacterial clearance in PON2-def mice 2) biofilm formation in chronic infection model and 3) atherosclerosis in a chronic lung infection model.
Highlights.
3OC12-HSL is a quorum sensor produced by Pseudomonas aeruginosa (PAO1)
PAO1 clearance is significantly reduced in PON2-def mice
The phagocytosis function of PON2-def macrophages is inhibited by 3OC12-HSL
Increased nitration of PI3 kinase correlates with reduced phagocytosis function in PON2-def macrophages
Antioxidant treatment reversed the phagocytosis function in PON2-def macrophages
PON2 plays an important role in innate immune defense against PAO1 infection
Acknowledgments
We thank Yuen Lin Lee and Ani Shabazian for their expert technical assistance. This work was supported by the National Heart, Lung and Blood Institute grant 1RO1HL71776 (S.T.R).
Abbreviations
- AHL
Acyl homo serine lactones
- PON2-def
Paraoxonase 2-deficiency
- PI3 kinase
Phosphatidylinositol 3-kinase
- 3OC12-HSL
N-(3-oxododecanoyl)-L-homoserine lactone
- DCF
Dichlorofluorescein
- PAO1
Pseudomonas aeruginosa
- CFU
Colony Forming Units
- ER
Endoplasmic Reticulum
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annual review of genetics. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 2.Miller MB, Bassler BL. Quorum sensing in bacteria. Annual review of microbiology. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 3.Fuqua C, Greenberg EP. Listening in on bacteria: acyl-homoserine lactone signalling Nature reviews. Molecular cell biology. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 4.Winzer K, Williams P. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int J Med Microbiol. 2001;291:131–143. doi: 10.1078/1438-4221-00110. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Hinz AJ, Nadeau JP, Mah TF. Pseudomonas aeruginosa tssC1 Links Type VI Secretion and Biofilm-Specific Antibiotic Resistance. J Bacteriol. 2011;193:5510–5513. doi: 10.1128/JB.00268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 2004;37:1304–1316. doi: 10.1016/j.freeradbiomed.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Ng CJ, Shih DM, Hama SY, Villa N, Navab M, Reddy ST. The paraoxonase gene family and atherosclerosis. Free Radic Biol Med. 2005;38:153–163. doi: 10.1016/j.freeradbiomed.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Reddy ST, Devarajan A, Bourquard N, Shih D, Fogelman AM. Is it just paraoxonase 1 or are other members of the paraoxonase gene family implicated in atherosclerosis? Curr Opin Lipidol. 2008;19:405–408. doi: 10.1097/MOL.0b013e328304b64e. [DOI] [PubMed] [Google Scholar]
- 10.Silva MT. Neutrophils and macrophages work in concert as inducers and effectors of adaptive immunity against extracellular and intracellular microbial pathogens. Journal of leukocyte biology. 2010;87:805–813. doi: 10.1189/jlb.1109767. [DOI] [PubMed] [Google Scholar]
- 11.Bourquard N, Ng CJ, Reddy ST. Impaired hepatic insulin signalling in PON2-deficient mice: a novel role for the PON2/apoE axis on the macrophage inflammatory response. Biochem J. 2011;436:91–100. doi: 10.1042/BJ20101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere JC, Standiford TJ, Ishiguro M, Yamaguchi K. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun. 2003;71:5785–5793. doi: 10.1128/IAI.71.10.5785-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JB, Xia YR, Romanoski CE, Lee S, Meng YH, Shi YS, Bourquard N, Gong KW, Port Z, Grijalva V, Reddy ST, Berliner JA, Lusis AJ, Shih DM. Paraoxonase-2 Modulates Stress Response of Endothelial Cells to Oxidized Phospholipids and a Bacterial Quorum-Sensing Molecule. Arterioscl Throm Vas. 2011;31:2624–U2688. doi: 10.1161/ATVBAHA.111.232827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham JM. In: Isolation of mitochondria from tissues and cells by differential centrifugation Current protocols in cell biology/editorial board. Unit 33. Bonifacino Juan S, et al., editors. Chapter 3. 2001. [DOI] [PubMed] [Google Scholar]
- 15.Morten KJ, Ackrell BAC, Melov S. Mitochondrial reactive oxygen species in mice lacking superoxide dismutase 2 - Attenuation via antioxidant treatment. Journal of Biological Chemistry. 2006;281:3354–3359. doi: 10.1074/jbc.M509261200. [DOI] [PubMed] [Google Scholar]
- 16.Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. Journal of Biological Chemistry. 2001;276:44444–44449. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- 17.Cassetta L, Cassol E, Poli G. Macrophage Polarization in Health and Disease. Thescientificworldjo. 2011;11:2391–2402. doi: 10.1100/2011/213962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, Navab M, Fogelman AM, Lusis AJ, Young S, Reddy ST. Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins - Anti-atherogenic role for paraoxonase-2. Journal of Biological Chemistry. 2006;281:29491–29500. doi: 10.1074/jbc.M605379200. [DOI] [PubMed] [Google Scholar]
- 19.Vikstrom E, Tafazoli F, Magnusson KE. Pseudomonas aeruginosa quorum sensing molecule N-(3 oxododecanoyl)-L-homoserine lactone disrupts epithelial barrier integrity of Caco-2 cells. Febs Letters. 2006;580:6921–6928. doi: 10.1016/j.febslet.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 20.Saleh A, Figarella C, Kammouni W, Marchand-Pinatel S, Lazdunski A, Tubul A, Brun P, Merten MD. Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-L-homoserine lactone inhibits expression of P2Y receptors in cystic fibrosis tracheal gland cells. Infect Immun. 1999;67:5076–5082. doi: 10.1128/iai.67.10.5076-5082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Grauer DC, Lehmann M, Meijler MM, Janda KD, Ulevitch RJ. Modulation of gene expression via disruption of NF-kappaB signaling by a bacterial small molecule. Science. 2008;321:259–263. doi: 10.1126/science.1156499. [DOI] [PubMed] [Google Scholar]
- 22.Imamura Y, Yanagihara K, Mizuta Y, Seki M, Ohno H, Higashiyama Y, Miyazaki Y, Tsukamoto K, Hirakata Y, Tomono K, Kadota J, Kohno S. Azithromycin inhibits MUC5AC production induced by the Pseudomonas aeruginosa autoinducer N-(3-oxododecanoyl) homoserine lactone in NCI-H292 cells. Antimicrob Agents Ch. 2004;48:3457–3461. doi: 10.1128/AAC.48.9.3457-3461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih DM, Xia YR, Wang XP, Wang SS, Bourquard N, Fogelman AM, Lusis AJ, Reddy ST. Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ Res. 2007;100:1200–1207. doi: 10.1161/01.RES.0000264499.48737.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devarajan A, Grijalva V, Bourquard N, Meriwether DD, Reddy ST. Coupling Mitochondrial Dysfunction To Endoplasmic Reticulum Stress Response: a Molecular Mechanism Leading To Atherosclerosis in Paraoxonase 2 Deficient Mice Free Radical. Bio Med. 2011;51:S39–S39. [Google Scholar]
- 25.Devarajan A, Bourquard N, Hama S, Navab M, Grijalva VR, Morvardi S, Clarke CF, Vergnes L, Reue K, Teiber JF, Reddy ST. Paraoxonase 2 Deficiency Alters Mitochondrial Function and Exacerbates the Development of Atherosclerosis Antioxid. Redox Sign. 2011;14:341–351. doi: 10.1089/ars.2010.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devarajan A, Grijalva VR, Bourquard N, Meriwether D, 3rd, Imaizumi S, Shin BC, Devaskar SU, Reddy ST. Macrophage paraoxonase 2 regulates calcium homeostasis and cell survival under endoplasmic reticulum stress conditions and is sufficient to prevent the development of aggravated atherosclerosis in paraoxonase 2 deficiency/apoE−/− mice on a Western diet. Mol Genet Metab. 2012;107:416–427. doi: 10.1016/j.ymgme.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horke S, Witte I, Altenhofer S, Wilgenbus P, Goldeck M, Forstermann U, Xiao JH, Kramer GL, Haines DC, Chowdhary PK, Haley RW, Teiber JF. Paraoxonase 2 is down-regulated by the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L-homoserine lactone and attenuates oxidative stress induced by pyocyanin. Biochem J. 2010;426:73–83. doi: 10.1042/BJ20091414. [DOI] [PubMed] [Google Scholar]
- 28.Fang FC. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 29.Splettstoesser WD, Schuff-Werner P. Oxidative stress in phagocytes - “The enemy within”. Microsc Res Techniq. 2002;57:441–455. doi: 10.1002/jemt.10098. [DOI] [PubMed] [Google Scholar]
- 30.King AJ, Sundaram S, Cendoroglo M, Acheson DWK, Keusch GT. Shiga toxin induces superoxide production in polymorphonuclear cells with subsequent impairment of phagocytosis and responsiveness to phorbol esters. J Infect Dis. 1999;179:503–507. doi: 10.1086/314579. [DOI] [PubMed] [Google Scholar]
- 31.Morrow DM, Entezari-Zaher T, Romashko J, 3rd, Azghani AO, Javdan M, Ulloa L, Miller EJ, Mantell LL. Antioxidants preserve macrophage phagocytosis of Pseudomonas aeruginosa during hyperoxia Free Radic. Biol Med. 2007;42:1338–1349. doi: 10.1016/j.freeradbiomed.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozer EA, Pezzulo A, Shih DM, Chun C, Furlong C, Lusis AJ, Greenberg EP, Zabner J. Human and murine paraoxonase 1 are host modulators of Pseudomonas aeruginosa quorum-sensing. FEMS microbiology letters. 2005;253:29–37. doi: 10.1016/j.femsle.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 33.Stoltz DA, Ozer EA, Taft PJ, Barry M, Liu L, Kiss PJ, Moninger TO, Parsek MR, Zabner J. Drosophila are protected from Pseudomonas aeruginosa lethality by transgenic expression of paraoxonase-1. Journal of Clinical Investigation. 2008;118:3123–3131. doi: 10.1172/JCI35147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simanski M, Babucke S, Eberl L, Harder J. Paraoxonase 2 Acts as a Quorum Sensing-Quenching Factor in Human Keratinocytes. Journal of Investigative Dermatology. 2012;132:2296–2299. doi: 10.1038/jid.2012.128. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor S, Taylor C, Campbell LA, Epstein S, Libby P. Potential infectious etiologies of atherosclerosis: a multifactorial perspective. Emerging infectious diseases. 2001;7:780–788. doi: 10.3201/eid0705.010503. [DOI] [PMC free article] [PubMed] [Google Scholar]








