Abstract
Thyroid cancer exists in several forms. Differentiated thyroid cancers include papillary and follicular histologies. These tumors exist along a spectrum of differentiation, and their incidence continues to climb. A number of advances in the diagnosis and treatment of differentiated thyroid cancers now exist. These include molecular diagnostics and more advanced strategies for risk stratification. Medullary cancer arises from the parafollicular cells and not the follicular cells. Therefore, diagnosis and treatment differs from differentiated thyroid tumors. Genetic testing and newer adjuvant therapies has changed the diagnosis and treatment of medullary thyroid cancer. This review will focus on the epidemiology, diagnosis, work-up, and treatment of both differentiated and medullary thyroid cancers, focusing specifically on newer developments in the field.
Keywords: thyroid cancer, papillary thyroid cancer, follicular thyroid cancer, medullary thyroid cancer, diagnosis, treatment, epidemiology
Introduction
Thyroid cancers exist in several forms. Providers caring for patients with thyroid cancer must tailor the evaluation, treatment, and surveillance to the specific type and each individual patient's risk factors for recurrence and mortality. Newer molecular diagnostics and targeted therapies offer patients the potential for tailored, or personalized thyroid cancer therapy. However, the risk of complications versus any potential benefit must be weighed carefully since most thyroid cancers behave indolently, and complications could be life-long (1, 2).
The most common type of thyroid cancer is papillary thyroid cancer (PTC), comprising 80% of all cases. The second most common type is follicular thyroid cancer (FTC) that accounts for 10–20% of all cases. Together, papillary and follicular cancers are termed differentiated thyroid cancer (DTC), and both arise from the thyroid follicular cells. Medullary thyroid cancer (MTC), on the other hand, arises from the parafollicular C cells. This neuroendocrine thyroid tumor represents 6–8% of all thyroid cancer cases and occurs in familial and sporadic forms. Finally, anaplastic thyroid cancer is one of the most aggressive and rapidly fatal cancers. It can develop from DTC that de-differentiates over time or it also arises de-novo (3–6). The first part of this review will consider the spectrum of DTC, and the second section will cover MTC.
Differentiated Thyroid Cancer (DTC)
Epidemiology
Thyroid cancer is the most rapidly increasing malignancy in the U.S. for both men and women. Although the incidence has of DTC has increased worldwide, there was a 2.4-fold increase in DTC incidence in the U.S. between 1973 and 2002, and the incidence has continued to rise over the last decade (7, 8). This increasing incidence is attributed to improved detection of smaller (<2 cm) tumors via more frequent and better ultrasound detection and fine needle aspiration (FNA) biopsies (9). Another theory to explain the increasing incidence of DTC is the increased pathologic reporting of incidental microcarcinomas (tumors < 1 cm) in thyroids removed for benign disease. Autopsy studies support this notion, since incidental microcarcinomas are discovered in 10 to 30% of the population (10–12). Further analysis of the increasing incidence of thyroid cancers revealed that although the incidence of microcarcinomas increased by 19.3% per year between 1983 and 2006, the incidence of tumors 1–2 cm, 2–5 cm, and >5 cm in size increased by 12.3%, 10.3%, and 12.0% per year, respectively, over that same period (13). This suggests that better detection of smaller tumors does not fully account for the overall rising incidence of DTC. Possible explanations for the rising incidence include increased iatrogenic, or “diagnostic” radiation exposure, changes in the population's body mass index, fertility drugs, or changes in menstrual cycles (14–17). These potential causative factors need further exploration in the U.S. population. Regardless of the reason behind the rising incidence of DTC, the mortality rate remains largely unchanged at 0.5 per 100,000 population despite greater increases in incidence (8, 18).
Etiology
External radiation exposure to the head and neck region is one of the most well known causes of thyroid cancer. Historically, patients received radiation treatments for enlarged tonsils or acne. Today, patients with cancer such as Hodgkin's disease might still receive mantle radiation. In addition, children exposed to radioactive fallout from the Chernobyl accident have demonstrated an increased incidence of thyroid cancer (19, 20). Children, especially females, are particularly sensitive to external radiation with the greatest risk for exposures before adolescence. This occurs with exposure to either radioactive isotopes, as in nuclear accidents, or external beam radiation used for medical diagnostics or treatment. Originally, radiation doses of 1 to 10 Gy were considered causative, but further evaluation of patients exposed to Chernobyl fallout demonstrated a linear dose-response relationship. Several studies have demonstrated an exposure odds ratio of 5.2 per Gy even with lower doses (21–23). Epidemiologic studies report that 7–9% of patients who received 5–10 Gy external beam radiation develop thyroid cancer (24). A lag time of 10 to 20 years usually exists between exposure and diagnosis of thyroid cancer, although much shorter periods have been reported (24).
Another environmental etiology for thyroid cancer is dietary iodine content. PTC occurs with higher frequency in regions with high dietary iodine content such as the Iceland and the Pacific Rim (25). Iodine deficient countries, in contrast, have a higher incidence of FTC. Many factors confound these studies linking changes in DTC frequencies to iodine intake. Other dietary factors such as selenium, goitrogen and carcinogen intake likely play causative roles (26). However, it is difficult to isolate these factors from genetic and environmental factors that also likely contribute to thyroid carcinogenesis. Finally, the TSH level has also been identified as a cancer risk. A higher TSH level in patients with thyroid nodular disease is associated with both a greater risk of DTC and advanced tumor stage (27).
In the last 10 to 15 years, the information on genetic etiologies of DTC has seen an exponential growth. Both inherited and acquired genetic lesions have been studied. DTC occurs in inherited syndromes such as Gardner's, Cowden's, and Werner's syndromes (28, 29). Familial non-medullary thyroid cancer (FNMTC) is defined when two or more first-degree relatives are diagnosed with DTC in the absence of another syndrome. It exhibits autosomal dominant behavior with incomplete penetrance and variable expressivity (29–31). Several candidate genes for FNMTC have been studied, including TCO1, MNG1, fPTC/PRN, and NMTC1, but a single responsible gene has yet to be definitively identified (32–35). Compared to sporadic DTC, FNMTC is more aggressive with increased recurrence, local invasion, multicentricity, and lymph node metastases. FNMTC should be suspected in the setting of two or more family members with DTC and/or younger family members (20's – 30's) with more advanced cancers (i.e, lymph node metastases). More recently, Mazeh and colleagues demonstrated that even patients with just one family member affected by DTC were similar to FNMTC patients in terms of age, tumor size, tumor multicentricity, lymph node metastases, local invasiveness, and disease recurrence (36). Hence, inherited forms of DTC may prove more common than previously thought, and current definitions may change with further study. Currently, FNMTC remains difficult to distinguish from sporadic DTC in the absence of a validated genetic test and therefore no screening recommendations exist (36).
Increasing investigation into acquired genetic lesions that can distinguish carcinoma from benign nodules has greatly expanded our knowledge of the molecular pathogenesis of DTC. A number of different molecular markers have been identified in DTC. Here, four molecular markers with the most literature in human studies will be discussed. Some centers are beginning to utilize these markers in clinical practice as we will discuss in the diagnosis section. One such marker is the RET and papillary thyroid cancer (RET/PTC) rearrangement. For example, 70% of cancers found in Chernobyl survivors carried a RET/PTC rearrangement. The fusion of the tyrosine kinase encoding domain of the RET protein with a heterologous group of genes occurs in 20–40% cases (37). RET/PTC rearrangements are common in small, multifocal PTCs accompanied by an inflammatory infiltrate, often seen in individuals exposed to ionizing radiation (38).
BRAF is a member of the RAF-MEK-ERK serine/threonine kinase-signaling cascade, and a BRAF mutation is found in 40% of PTC cases (39). The BRAF point mutation, or mutations in another member of this signaling pathway, RAS, are frequent in cases of poorly differentiated PTC or anaplastic thyroid cancer (ATC) (40). Most BRAF mutations keep the protein in its active form, resulting in constitutive activation of the RAF-MEK-ERK signaling cascade and continued mitogenic activity. The Ras proteins are plasma membrane GTPases activated by growth factor receptors. Mutations that result in constitutive activation of RAS lead to oncogenesis. RAS mutations occur in 20–50% of follicular cancers.
Similar to the RET/PTC re-arrangement, another chromosomal translocation occurs in FTC. The promoter region of the gene encoding paired box 8 (PAX8) fuses with the coding sequence of the peroxisome proliferator-activated receptor γ (PPARγ) gene in 35% of FTCs (40–42). The intracellular signaling consequences of the PAX8-PPARγ rearrangement remain unclear. RAS mutations are also highly prevalent in FTC, but Nikiforova et al found that RAS and PAX8-PPARγ rearrangements are mutually exclusive, suggesting that these are two distinct molecular pathways for FTC development (40).
Classification and Prognosis
DTCs are broadly categorized as papillary or follicular. In general, well-differentiated PTC has an excellent prognosis with five year survival greater than 97% (8). Smaller tumors carry a better prognosis than larger tumors. PTC less than 1 cm in size are called papillary microcarcinomas, and have been reported in 10–30% of autopsy studies (10, 43, 44). In the past, these tumors were incidentally detected in thyroidectomy specimens, but they are now detected with increasing frequency by high-resolution ultrasound. They are believed to have an excellent prognosis, but some may behave more aggressively than previously appreciated, and management remains controversial (45, 46). In general, however, microcarcinomas hold an excellent prognosis with 10 and even 15-year disease specific survival rates exceeding 99% (45). Risk factors of mortality from a microcarcinoma include age older than 45 years, male sex, minority racial group, lymph node metastases, extrathyroidal extension or superficial location, intraglandular spread or multifocality, peri-tumoral fibrosis, and BRAF positivity. Several scoring systems that incorporate these factors have been developed to risk-stratify patients with microcarcinomas (45, 47).
Aside from size, age is another important determinant of prognosis in DTC. Older patients tend to have more poorly differentiated, aggressive variants. In these cases, death results from local invasion and extensive metastases. Therefore, age, the completeness of resection, and extrathyroidal extension also prognostic indicators employed in many staging systems for DTC (48, 49).
One of the greatest risk factors for disease-specific mortality is the presence of distant metastases (50–52). While the importance of distant metastases is rarely disputed, the importance of lymph node metastases in determining DTC-specific survival remains controversial. Lymph node involvement is common in PTC, but the precise incidence of lymph node metastases depends on how it is defined. Palpable disease in the lymph nodes is present in 5–10% of patients with PTC, but ultrasound detects pathologic lymph nodes in 30% of patients. Only 2% of patients with FTC have lymph node metastases because the route of spread is hematogenous rather than lymphatic, but treatment guidelines and retrospective studies frequently consider PTC and FTC together. Routine histologic examination of lymph nodes detects DTC in 20–50% of patients, but when PCR is performed, up to 90% of patients with DTC will have lymph nodes with microscopic disease. Historically, lymph node involvement was felt to increase local recurrence without affecting survival, and therefore surgeons took a conservative approach to lymph node dissection for DTC. Wada et al demonstrated that patients with pathologically positive lymph nodes had a recurrence rate of 16.3% compared to 0% in patients without pathologic lymph nodes (53). Whether metastatic lymph nodes are clinically evident preoperatively appears to be an important factor determining recurrence. For example, Ito and colleagues found that if metastatic lymph nodes were not seen preoperatively, then the risk of nodal recurrence was only 1.5%. Of note, in this study of 590 patients with microcarcinomas, 40% of patients had lateral neck lymph node metastases identified histologically after prophylactic neck dissection (54). Hence, lymph node metastases do affect recurrence, and clinically apparent nodes are more important than pathologically positive nodes. The impact of lymph node involvement on survival is less clear. Large series and population-based studies suggest that there is a small but significant affect on survival (55–57).
Most current staging systems consider lymph node metastases a binary factor, classifying patients by the presence or absence of diseased lymph nodes, or the anatomic location of these nodes (Table 1 and 2). The lymph node ratio (number of metastatic nodes divided by the total number of nodes harvested) is a method of further risk-stratifying patients with lymph node disease (57–60). The lymph node ratio is helpful for clinicians caring for patients with PTC because it indicates the adequacy of lymph node dissection and the extent of disease. Although the lymph node ratio does independently impact disease-specific survival (57), it is probably most helpful in assessing recurrence risk and tailoring postoperative treatment and follow-up accordingly (58).
Table 1.
Prognostic Schemes for Differentiated Thyroid Cancer
| Prognostic Variable | EORTC (1979) | AGES (1987) | AMES (1988) | MACIS (1993) | OSU (1994) | MSKCC (1995) | NTCTCS (1998) | Clinical Class (1990) |
|---|---|---|---|---|---|---|---|---|
| Patient Factors | ||||||||
| Age | X | X | X | X | - | X | X | |
| Sex | X | - | X | - | - | - | - | |
| Tumor Factors | ||||||||
| Size | - | X | X | X | X | X | X | |
| Multicentricity | - | X | - | - | X | - | X | |
| Grade | - | X | - | - | - | X | - | |
| Histology | X | Y | X | Y | - | X | X | X |
| ETE | X | X | X | X | X | X | X | |
| + LNs | - | - | - | - | X | X | X | X |
| Distant mets | X | X | X | X | X | X | X | X |
| Operative Factors | ||||||||
| Incomplete Resection | - | - | - | X | - | - | - | |
X = variable used in staging system
Y = variable used in staging system designed for PTC only
- = variable not used in staging system
EORTC = European Organization for Research on Treatment of Cancer
AGES = patient age, histologic grade of the tumor, tumor extent (extrathyroidal extension), and size of primary tumor
AMES = patient age, presence of distant metastases, extent and size of primary tumor
MACIS = metastasis, patient age, completeness of resection, local invasion, and tumor size
OSU = Ohio State University
MSKCC = Memorial Sloan-Kettering Cancer Center
NTCTCS = National Thyroid Cancer Treatment Cooperative Study
Table 2.
American Joint Committee on Cancer (AJCC] TNM Staging for Thyroid Cancer+
|
Primary Tumor (T)
| |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor ≤ 2 cm, limited to the thyroid |
| T1a | Tumor ≤ 1 cm, limited to the thyroid |
| T1b | Tumor >1 cm but < 2 cm, limited to the thyroid |
| T2 | Tumor >2 cm but < 4 cm, limited to the thyroid |
| T3 | Tumor > 4 cm, limited to the thyroid or minimal extrathyroid extension (sternothyroid or perithyroid soft tissues) |
| T4a * | Tumor of any size extending beyond thyroid capsule to invade subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve |
| T4b | Tumor invades prevertebral fascia or incases carotid artery or mediastinal vessels |
|
| |
|
Regional Lymph Nodes (N)
| |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph nodes metastasis |
| N1a | Metastasis to Level VI lymph nodes |
| N1b | Metastasis to unilateral, bilateral, or contralateral cervical [levels I, II, III, IV, or V) or superior mediastinal (level VII) lymph nodes |
|
| |
|
Distant Metastasis (M)
| |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| STAGE GROUPING: | |
|
| |
|
Differentiated Cancers& < 45 years old
| |
| Stage I | Any T Any N M0 |
| Stage II | Any T Any N M1 |
|
| |
|
Differentiated Cancer ≥ 45 years old
| |
| Stage I | T1 N0 M0 |
| Stage II | T2 N0 M0 |
| Stage III | T3 N0 M0 |
| T1 N1a M0 | |
| T2 N1a M0 | |
| T3 N1a M0 | |
| Stage IVA | T4a N0 M0 |
| T4a N1a M0 | |
| T1 N1b M0 | |
| T2 N1b M0 | |
| T3 N1b M0 | |
| T4a N1b M0 | |
| Stage IVB | T4b Any N M0 |
| Stage IVC | Any T Any N M1 |
|
| |
|
Medullary Carcinoma
| |
| Stage I | T1 N0 M0 |
| Stage II | T2 N0 M0 |
| T3 N0 M0 | |
| Stage III | T1 N1a M0 |
| T2 N1a M0 | |
| T3 N1a M0 | |
| Stage IVA | T4a N0 M0 |
| T4a N1a M0 | |
| T1 N1b M0 | |
| T2 N1b M0 | |
| T3 N1b M0 | |
| T4a N1b M0 | |
| Stage IVB | T4b Any N M0 |
| Stage IVC | Any T Any N M1 |
|
| |
|
Anaplastic Carcinomas
$
| |
| Stage IVA | T4a Any N M0 |
| Stage IVB | T4b Any N M0 |
| Stage IVC | Any T Any N M1 |
AJCC TNM Staging Manual, 7th ed., 2010
All anaplastic tumors are considered T4 tumors
Papillary and follicular carcinomas are considered differentiated thyroid cancers
A11 anaplastic carcinomas are considered Stage IV
PTC Subtypes
PTC has several subtypes spanning the spectrum of well-differentiated, classical papillary cancers to the most aggressive, anaplastic tumors. Well-differentiated tumors can be subdivided by their aggressiveness. Less aggressive variants include follicular, oxyphilic, and cribiform-morular. The most common subtype is follicular variant PTC (FVPTC) that has features of both PTC and FTC, but is classified as a PTC subtype. This subtype exhibits the overall follicular structure but with nuclear features of PTC. Often FVPTC is confused for follicular neoplasms or carcinomas (61, 62). Overall, the prognosis is intermediate between classical PTC and FTC. The prognosis also hinges upon whether the tumors are completely encapsulated or invasive (63). Since FVPTC histologically resembles follicular adenomas or carcinomas, immunohistochemical stains such as HBME-1 have proven particularly useful in making the pathologic diagnosis (64, 65).
It is important to recognize more aggressive variants of well-differentiated tumors. These include tall cell, columnar cell, solid, and diffuse-sclerosing variants. Tall cell variant of PTC, commonly encountered in older patients, are larger and more invasive with extrathyroidal extension and metastatic disease. While tall cell variant PTC is less iodine-avid than classical PTC, these tumors are typically PET positive. Histologically, these tumors have cells whose height is at least 2–3 times as tall as they are wide. Traditional criteria require at least 50% of the cells to exhibit these tall features (62). Necrosis, a high mitotic index, and nuclear pseudoinclusions are also prominent in these tumors (61). Recently, several different centers have noted that tall cell variant PTCs often harbor BRAF mutations, and molecular markers can be utilized to identify this subtype preoperatively (66–68). Such molecular diagnostics may become helpful since this subtype is believed to be under-diagnosed (62). In addition to tall cell variant, columnar cell, solid, and diffuse-sclerosing types also exhibit more aggressive behavior than classical PTC.
As follicular or papillary cancers progress or de-differentiate, their prognosis becomes much worse. Anaplastic cancers are at the least differentiated side of the spectrum, and represent one of the most aggressive cancers with a five year disease-free survival and cause-specific survival approaching zero (69). Anaplastic thyroid cancer can arise from well-differentiated tumors that de-differentiate or it can also develop de-novo(69). A group of tumors falls in between well-differentiated thyroid cancers and anaplastic cancers. These cancers, called poorly differentiated thyroid cancers, have a histologic appearance and behavior that is intermediate between well-differentiated PTC and anaplastic cancers (3). Although the literature remains inconsistent about what constitutes poorly differentiated cancer, the best definition comes from Burman et al that “poorly differentiated thyroid carcinoma is a concept proposed to include carcinomas of follicular thyroid epithelium that retain sufficient differentiation to produce scattered small follicular structures and some thyroglobulin, but generally lack the usual morphologic characteristics of papillary and follicular carcinoma” (70). These tumors include insular and large cell variants (3, 70). Patients with these types tend toward locally advanced disease with recurrence and metastases. Furthermore, de-differentiation of thyroid cancers leads to under expression or disordered assembly of the sodium-iodide symporter, decreasing the utility of radioactive iodine for treating or detecting metastatic disease (71). For these reasons, poorly differentiated thyroid cancers have a 51% disease-free survival and a 70% cause-specific survival at five years (3, 69).
Figure 1 provides an organizational scheme for classifying tumors of follicular cell origin by differentiation status.
Figure 1.
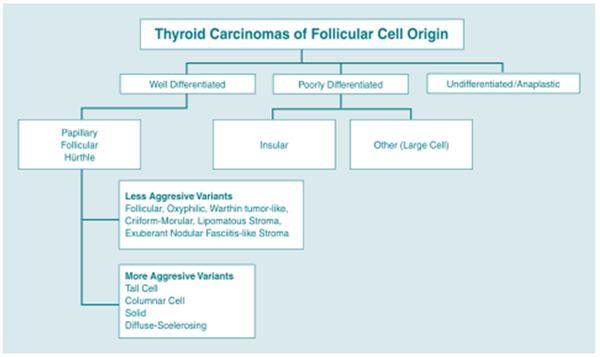
Classification of Follicular Cell Derived Tumors
FTC and Variants
It is worth noting some important ways in which FTC differs from PTC. Pure FTC carries a worse prognosis compared to PTC. Even when the disease is confined to the thyroid, mortality rates range from 5–15%, although survival time still extends decades as in PTC (72). In addition to the prognostic factors common to DTC staging systems (discussed below), prognosis in FTC especially depends on the degree of capsular and vascular invasion. Minimally invasive tumors are grossly contained within the thyroid, but have microscopic foci of invasion into the capsule. Invasive tumors carry a worse prognosis and invade the capsule and vasculature (73–75). For these reasons, FNA biopsy cannot distinguish a follicular adenoma from a carcinoma, and this has led to the investigation of molecular markers that can make this diagnosis as will be discussed below. Until molecular diagnostics are perfected, the current standard of care is to perform a diagnostic lobectomy in order to distinguish follicular adenoma from carcinoma (73, 76).
Hurthle cell or oncocytic tumors of the thyroid are often classified with follicular cancer as they are derived from the follicular cell. Hurthle cells arise from the follicular epithelium. Histologically, these cells feature large size, distinct cellular borders, plentiful granular cytoplasm, a large nucleus, and prominent nucleolus. Hurthle cells are found in a variety of conditions including nodular goiter, chronic lymphocytic (Hashimoto's) thyroiditis, and long-standing hyperthyroidism (77, 78). Both adenomas and carcinomas of the Hurthle cell can occur, and differentiating them by cytology is difficult as it is with follicular lesions (75, 79). As with follicular tumors, capsular and vascular invasion distinguish carcinoma from adenomas. Large Hurthle cell cancers (>2 cm) have a higher recurrence rate, ranging from 21 to 59% (79, 80). Furthermore, Hurthle cell cancers do not always concentrate iodine. For these reasons, Hurthle cell carcinoma carries a worse prognosis compared to other DTC. Adenomas carry an excellent prognosis after resection and less than 2.5% demonstrate malignant behavior, but resection is recommended for larger adenomas since size is a major predictor of malignancy (80, 81). In one series, 65% of Hurthle cell neoplasms 4 cm or greater in size were cancer on final pathology (79).
Thyroid Lymphoma
Primary lymphoma of the thyroid is not as common as DTC. Older females or patients with Hashimoto's thyroiditis are at highest risk for developing thyroid lymphoma (82, 83). These tumors typically present as a rapidly expanding mass causing pain and compressive symptoms. Flow cytometry of cytologic specimens can sometimes make the diagnosis, but might also be difficult to distinguish from advanced Hashimoto's thyroiditis. A core biopsy may therefore become necessary when this diagnosis is suspected in order to obtain enough tissue for flow cytometric analysis and/or analysis of antigen receptor gene rearrangements. Most are B cell lymphomas treated with chemotherapy and radiation. Surgery is occasionally necessary for palliation (83). Prognosis depends on the histologic subtype. Of note, up to 20% of patients suffering from generalized lymphoma can also have secondary involvement of the thyroid (84).
Staging Systems
Because of its unique extended survival period and the spectrum of disease behavior, a number of different staging or prognostic scoring systems have been developed for DTC (48, 85, 86). Table 1 summarizes the various staging systems for DTC.
Classical prognostic indicators for DTC include age and gender. Age is such an important factor in determining a patient's survival from DTC, that it is included in many of the staging systems for thyroid cancer. As with most other solid tumors, the tumor size also has prognostic significance, and most of the prognostic schemes also include tumor size (Table 1)
Because of their questionable effect on mortality, lymph node status is not included in all of the staging systems available for DTC. For example, the AGES system considers age, grade, extrathyroidal extension, and size (87). The AMES system uses age, distant (non-lymph node) metastases, extent of primary tumor, and size (88). Some, like the MACIS (metastases, age, complete excision, invasion, and size) system also account for the adequacy of surgical treatment (89). Alternatively, staging systems developed by the Ohio State University (90), the European Organization for Research and Treatment of Cancer (EORTC) (91), the National Thyroid Cancer Treatment Cooperative Study (NTCTCS) (92), Clinical Class (49), and the American Joint Committee on Cancer (AJCC) (93) all do consider lymph node status.
The AJCC is the most widely utilized staging system (Table 2). Also known as the TNM system since it considers tumor size and local extent (T), lymph node metastases (N), and distant metastases (M). Like many of the other thyroid cancer staging systems, it also considers age, with two different classifications for those younger and older than 45 years of age. In those under 45, patients with lymph node metastases are classified as stage I unless they have distant metastases (stage II) (93).
Diagnosis
Like any newly discovered mass elsewhere in the body, the workup of a thyroid nodule begins with a thorough history and physical exam. A strong family history of thyroid cancer or prior radiation exposure to the head and neck should raise the suspicion of thyroid cancer. Rapid growth with compressive symptoms may indicate that the thyroid nodule is thyroid lymphoma or a poorly differentiated thyroid cancer (3, 4, 24).
On physical exam, malignant nodules are harder and fixed while a nodule that is rubbery or soft and moves easily with deglutition suggests a benign nodule. Physical exam features alone do not ensure a benign diagnosis. Cervical lymphadenopathy also increases the likelihood that a thyroid nodule is malignant (4, 94).
Since the management of patients with hyperthyroidism differs from those with nonfunctional nodules, obtaining a thyroid stimulating hormone (TSH) measurement early in the workup of a thyroid nodule can efficiently identify patients with a nodule and hyperthyroidism. In the subset of patients with a suppressed TSH (hyperthyroid), an 123I scan can distinguish a solitary toxic nodule from a toxic multinodular goiter and Graves' Disease. A solitary hyper functioning nodule is rarely malignant, and FNA biopsy or further cancer workup is rarely necessary. Hyperfunctioning nodules are usually hypercellular, and will confuse the provider since cytology may suggest neoplasia, when the cellularity only reflects benign hyperplasia associated with excessive stimulation. The one exception is that functioning nodules in children do carry a higher risk of malignancy (95). If thyroid radionuclide scanning is undertaken, “cold” nodules should undergo FNA biopsy because 10–20% of “cold” nodules are malignant (96, 97). Nevertheless, thyroid radionuclide scanning is not recommended as part of the initial workup of adult patients with thyroid nodules as it generally does not alter the management.
Other laboratory tests can be helpful once the diagnosis of a certain type of thyroid cancer is made. For example, measuring serum thyroglobulin (Tg) in patients with DTC can assist with the long-term follow-up of patients treated for DTC (98–100). Although Tg can be elevated in patients with DTC, the test is not specific for diagnosing cancer. Elevations in Tg can occur in benign thyroid disorders, and The American Thyroid Association guidelines do not recommend routine preoperative Tg measurement for patients with DTC. After a total thyroidectomy, however, elevations in Tg can reliably indicate recurrent or metastatic disease (99). Different threshold Tg levels can indicate recurrence depending on the concomitant TSH level.
FNA biopsy remains the gold standard for evaluating thyroid nodules. Most clinical practice guidelines recommend FNA biopsy for nodules greater than 1 cm in largest dimension (96, 99, 101). When the FNA result is clearly benign or malignant, then the decision for further treatment including thyroidectomy becomes evident. The false-negative rate for FNA biopsy is 1–3% (Table 3). The false negative rate increases to 10–15% when the nodule is large (> 4 cm) (101–103). Other clinical scenarios where the clinician should not always trust a benign FNA result include patients with a family history of thyroid cancer, patients with a history of radiation exposure, and cystic nodules (104). Ultrasound-guidance can improve the accuracy of FNA biopsy because ultrasound can confirm the nodule is actually being sampled and target the most suspicious portions of the nodule (i.e., the wall of a cyst). This is especially true for non-palpable or posteriorly located nodules (105, 106).
Table 3.
The Bethesda System for Thyroid Cytopathology
| Category | Risk of Malignancy (%) | Recommended Management |
|---|---|---|
| Nondiagnostic or Unsatisfactory | 1–4 | Repeat FNA with U/S guidance |
| Benign | 0–3 | Clinical follow-up |
| Atypia of Undetermined Significance (AUS) or Follicular Lesion of Undetermined Significance (FLUS) | 5–15 | Repeat FNA* |
| Follicular Neoplasm or Suspicious for Follicular Neoplasm | 15–30 | Lobectomy |
| Suspicious for Malignancy | 60–75 | Lobectomy +/− frozen section or total thyroidectomy |
| Malignant | 97–99 | Total thyroidectomy |
FNA, fine needle aspiration
U/S, ultrasound
Lobectomy can also be considered depending on clinical or sonographic characteristics
FNA results are classified according to the Bethesda criteria that indicate the risk of malignancy (Table 3). One of the limitations of cytology in evaluating thyroid nodules is that it cannot distinguish between adenoma and carcinoma for follicular lesions (96, 102, 103). Therefore, lobectomy with permanent histology may be the best way to make a definitive diagnosis in follicular or indeterminate lesions. Many centers have turned to molecular analysis of FNA specimens to help distinguish follicular lesions. Cytology specimens are analyzed for a panel of mutations including BRAF, RAS, RET/PTC, and PAX8-PPARγ rearrangements (39–41, 69, 107). This will be discussed further in subsequent sections.
Not only can ultrasound improve the accuracy of FNA biopsy, but it is also an important tool in evaluating thyroid nodules as it is used to measure size and features of the nodule such as its borders, echogenicity, and vascularity. It can also identify additional non-palpable nodules. Ultrasound alone can increase the clinician's suspicion for malignancy if the nodule has fine microcalcifications, irregular borders, or chaotic vascular patterns. In addition, ultrasound evaluates the lymph nodes in both the central and lateral neck compartments which may prompt additional FNA biopsy of suspicious lymph nodes or alter the surgical plan (Figure 2). Small metastatic central compartment lymph nodes may not be seen on ultrasound because they are obscured by the overlying thyroid. Although ultrasound is highly operator-dependent, it is non-invasive and does not involve any radiation or contrast risk to the patient. High-resolution ultrasound can also demonstrate extracapsular invasion and subtle lymph node involvement (2, 105, 108–112). Therefore, ultrasound is the preferred method to evaluate the thyroid and cervical lymph nodes.
Figure 2.
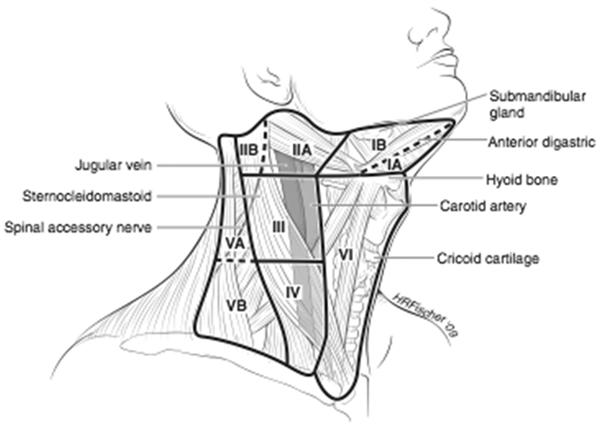
Lymph Node Compartments of the Neck
Very aggressive cancers that may invade local structures, extend into the chest, or demonstrate poorly differentiated cytology require careful preoperative planning. In this situation, CT becomes a helpful preoperative imaging study to help plan en-bloc resection of other organs aside from the thyroid, understand the extent of vascular involvement, determine if a thoracic incision is necessary, and plan for reconstruction (2, 3, 113, 114). When using CT, it is best to avoid the administration of iodinated contrast as this will reduce the ability of residual thyroid tissue or thyroid cancer to take up subsequent radioactive iodine for many months.
Imaging studies often identify thyroid nodules incidentally. Positron emission tomography (PET) scans detect thyroid masses during the workup and staging of other cancers. This subset of incidentally discovered thyroid nodules deserves special attention because up to 50% of FDG-avid thyroid nodules will contain thyroid cancer. Therefore, PET-positive thyroid nodules should be FNA biopsied. PET and/or CT scan are helpful for identifying lung or bone tumors in patients at risk for metastases, and this is especially true for patients with tumors that are not iodine-avid (2, 113).
Role of Molecular Markers
As discussed above, the identification of several molecular markers associated with either PTC or FTC has led to the development of several assays to improve upon FNA biopsy results. Specifically, these assays or gene panels target nodules with indeterminate cytology (follicular neoplasm, atypia, etc.) to determine whether these nodules are benign or malignant without the need for diagnostic surgery. For example, Nikiforov and colleagues recently developed a panel of mutations including BRAF, NRAS, HRAS, KRAS, and two different RET/PTC rearrangements. The detection of any one of these mutations was associated with a final histopathologic diagnosis of cancer in 88% of cases with a cytologic diagnosis of atypia and 87% of cases with cytologic diagnoses of follicular neoplasm/suspicious for a follicular neoplasm. The risk of cancer in nodules without any mutation was as high as 28% in patients with a cytologic diagnosis suspicious for malignancy, 14% of patients with follicular cytology, and 6% of patients with atypia (115). While these results are quite encouraging, the sensitivity of the molecular panel still leaves room for uncertainty and has not negated the need for diagnostic surgery to make a definitive diagnosis.
Rather than identifying the molecular signature of malignancy, another group has developed a different molecular test designed to identify the gene expression profile of a benign nodule. In a multicenter study, this test had a sensitivity of 92% and a specificity of 52%. The negative predictive value (predicts truly benign lesions) was 95%, 94%, and 85%, for the cytologic diagnoses of atypia, follicular neoplasm/suspicious for follicular neoplasm, and suspicious, respectively (116). This testing may be helpful for patients who wish to avoid surgery because of excessive surgical risk, but still leaves at least a 7% chance that a benign result truly is a cancer. At this point, it remains unclear how patients who avoid surgery because of a molecular profile should be followed. Furthermore, the natural history of follicular lesions is unknown. Until these questions are clarified, these molecular tests will play a limited role in clinical decision making for most patients.
Treatment
Treatment of DTC is multidisciplinary and involves a surgeon, endocrinologist, nuclear medicine specialist, and, occasionally, a radiation oncologist. This approach best serves patients with DTC and will be highlighted in the sections that follow.
Surgery
The extent of surgery for DTC remains controversial. This is especially true for small, encapsulated, well-differentiated tumors, and tumors less than one centimeter in size (microcarcinomas). The approach to microcarcinomas will be discussed further below, but for most DTC ≥ 1 cm diagnosed preoperatively, most clinicians recommend a total thyroidectomy (99). The rationale for total thyroidectomy is based on tumor biology and current treatment modalities. DTC, especially PTC, tends to be multicentric, with up to 80% of patients having multiple tumor foci and bilateral disease in 60% when a thorough pathologic examination of the contralateral lobe is performed (2, 6, 45). A total thyroidectomy as the initial procedure negates the need for re-operative surgery to remove the contralateral lobe should a recurrence become detected. Second, experienced thyroid surgeons can safely perform a total thyroidectomy with permanent complications such as recurrent laryngeal nerve injury and hypoparathyroidism occurring at a rate of less than 2% (114, 117). Radioactive iodine therapy for ablating microscopic disease becomes most effective when the thyroid remnant is small or absent. TG measurement and radioiodine whole body scanning are highly sensitive modalities for detecting recurrent or metastatic disease, but these two methods are most effective when all the thyroid tissue has been removed (2, 4).
Most low-risk cancers carry an excellent prognosis regardless of the extent of thyroidectomy, and there are no randomized prospective trials comparing total thyroidectomy to thyroid lobectomy in this group of patients. In addition, radioiodine may have limited utility in low-risk patients (6, 45). For these reasons, some favor thyroid lobectomy in low risk patients. For example, Shaha and associates have reported 20-year follow-up on 465 patients with low risk DTC. Although the lobectomy group had more local recurrence compared to the total thyroidectomy group (4% versus 1%), this was not statistically significant (118). Similarly, other groups have also failed to demonstrate any significant effect on survival (119–121). In contrast, large retrospective series have demonstrated improvement in recurrence for total thyroidectomy compared to lesser operations (122–125). In a frequently cited study, Mazzaferri and colleagues reported on 1355 patients with a mean follow-up of 15.7 years. Patients treated with total thyroidectomy experienced significant improvements in recurrence rate (26% vs. 40%, p < 0.02) and mortality rate (6% vs. 9%, p = 0.02) compared to lesser resections (122). While some have questioned the accuracy of risk-stratification and accounting for complications in these retrospective studies, current guidelines still recommend a total or near-total thyroidectomy for small (< 4 cm), unifocal, well-differentiated tumors with no lymph node metastases, or extrathyroidal extension (99).
Another hotly debated topic related to the extent of initial surgery for DTC is the role of prophylactic central neck dissection. Although the 2006 American Thyroid Association guidelines stated that routine prophylactic central neck dissection should be considered for patients with DTC (126), the most recent guidelines have been revised to recommend that “prophylactic central neck dissection may be performed, especially in patients with advanced primary tumors” and “total thyroidectomy without prophylactic central neck dissection may be appropriate for small (T1 or T2), non-invasive, clinically node negative patients” (99).
The central neck lymph nodes are also classified as level 6 lymph nodes and include the paratracheal, peri-thyroidal, and precricoid lymph nodes. These nodes are found along and behind the recurrent laryngeal nerve and frequently surround the lower parathyroid gland (Figure 2). Although the level 6 lymph nodes contain macroscopic disease in 10% of cases, when they are removed prophylactically, 32–69% of patients will have microscopic metastases (52, 127, 128).
Proponents of prophylactic central neck dissection argue that the initial operation is the safest time to remove central neck lymph nodes to prevent local recurrences and the complications associated with re-operative surgery in the central neck. Furthermore, the central neck nodes are difficult to evaluate with preoperative ultrasound when the thyroid remains in place. Wada et al. found the recurrence rate in patients treated with therapeutic lymph node dissection to be 21% while patients who underwent prophylactic neck dissection experienced a recurrence rate of only 0.43%. Importantly, those patients without clinically overt nodal disease who did not undergo prophylactic central neck dissection also experienced a very low recurrence rate of 0.65%. Hence, the absolute differences in recurrence are miniscule (53). Several other studies also support the concept that microscopically positive lymph nodes rarely progress to recurrence, especially after postoperative radioactive iodine ablation (129–131). Therefore, the debate regarding prophylactic central neck dissection is closely tied to the utility of radioactive iodine.
Clinically evident lymph node metastases place patients at higher risk for recurrence, and these patients benefit from therapeutic lymph node dissection. Prophylactic central neck dissection modestly reduces an already low recurrence rate, potentially eliminates or reduces the need for radioactive iodine, but is also associated with its own risks such as hypoparathyroidism. The risk benefit ratio may favor prophylactic central neck dissection in a subset of patients, but the putative risk factors that define such a subset remains unknown (2, 60, 132, 133). Some groups are currently using molecular markers to preoperatively risk stratify patients, and decide who might benefit from more aggressive surgery up front (134, 135).
Thyroidectomy still involves the same basic steps historically described, but newer technology and attention to cosmetics account for some more recent modifications of the basic technique. Traditionally, a Kocher collar incision was utilized, but this requires a very large dissection superiorly to reach the upper pole of the thyroid, placing the patient at risk for postoperative seroma. Intraoperative ultrasound can help assess the upper extent of the gland and place the incision appropriately. Often, the incision can be placed higher in the neck but hidden in a neck crease to allow a smaller but still cosmetically pleasing incision. Superior and inferior sub-platysmal flaps are raised to create a working space around the thyroid. Instead of traditional clamps and ties, most of the vasculature feeding the thyroid can now be managed using energy devices such as the Harmonic scalpel or Ligasure® (136, 137), but larger vessels still may require clips and/or ties. Before dividing any structures along the medial border of the gland, the recurrent laryngeal nerve must be identified and its course dissected. The nerve is found medial to the upper parathyroid gland and lateral to the lower parathyroid. The parathyroid glands must also be identified and dissected free from the thyroid on an intact vascular pedicle. Once the recurrent laryngeal nerve is identified, the branches of the inferior thyroid artery can be divided along the thyroid capsule.
In recent years, nerve monitoring devices have enabled surgeons to test the functionality of the recurrent laryngeal nerve intraoperatively. Reported rates of permanent recurrent laryngeal nerve injury when the surgeon visually identifies the nerve is less than 2% (138, 139). Even the largest trials have failed to show any significant prevention of nerve injury(140, 141). A multi-institutional prospective non-randomized study of 16,448 patients (29,998 nerves at risk) found no statistical difference in nerve injury rates when comparing patients treated with visual identification of the nerve alone compared to those treated with a combination of visual identification and nerve monitoring (142). One of the few prospective studies, Thomusch and colleagues reported on 8,534 patients (15,403 nerves at risk). They compared direct stimulation of the recurrent laryngeal nerve to indirect stimulation of the vagus nerve (the recurrent nerve is a distal branch of the vagus), and found that direct stimulation had a much lower sensitivity of predicting nerve palsy compared to indirect stimulation (45.9% vs. 99.6%) (143). Although nerve monitoring does not prevent nerve injury, many surgeons still use this technology to identify nerve palsies when they do occur. This last study suggests that when nerve monitoring is used in this fashion, it should not simply be used to stimulate the recurrent laryngeal nerve directly. The use of nerve monitoring remains quite controversial. Many experts feel that nerve stimulation is generally not necessary for the primary surgery on the thyroid, and may be more useful for reoperations.
Before passing the specimen off the field, the surgeon should examine it to make sure that there is no parathyroid tissue adherent to the gland. Any inadvertently removed parathyroid tissue can be finely minced and re-implanted into either the sternocleidomastoid or the strap muscles. Frozen section of a biopsy of this tissue can distinguish between fat, parathyroid, or lymph node; this will also avoid auto-transplanting cancer-bearing lymph nodes back into the patient. Two to three pockets are created within the muscle, and the minced parathyroid tissue is divided between these pockets. Each pocket should be marked with permanent suture so that it can easily be found in a re-operative setting.
Preoperative FNA or intraoperative frozen section can confirm that enlarged lymph nodes seen on ultrasound harbor metastatic disease. Cytologic or pathologic confirmation of lymph node metastases should prompt the surgeon to perform a compartment-oriented lymph node dissection. Lymph node sampling or “berry-picking” should be avoided as this leaves behind lymph nodes that likely contain microscopic disease which then become more difficult to excise in a re-operative setting.
Although “skip” metastases directly to the lateral compartment can occur in PTC, the central neck nodes (level VI) are usually the first nodes to receive drainage from the thyroid (Figure 2). The boundaries of the central neck are the carotid sheathes laterally, the hyoid bone superiorly, and the innominate artery inferiorly (144). Lymphadenectomy in this area requires skeletonizing the recurrent laryngeal nerve along its entire cervical course, and removing all the fibro-fatty tissue along the trachea. Frequently the lower parathyroid is invested in this tissue and becomes devascularized with this dissection (132).
A lateral neck dissection usually involves dissection of levels II, III, and IV (Figure 2). This dissection puts the spinal accessory, phrenic, vagus, cervical sensory, sympathetic trunk, hypoglossal, greater auricular, and the marginal mandibular branch of the facial nerves at risk. The extent of node dissection should be guided by preoperative and intraoperative ultrasound findings. Usually, the great vessels can be preserved, but more aggressive tumors can invade the internal jugular vein, and it should be sacrificed in this scenario. In addition, to nerve injury, chyle leak is another complication from performing lateral neck dissection (2, 114).
In recent years, transaxillary approaches to thyroidectomy have been developed. There are several variations of these techniques including, trans-axillary endoscopic, robotic, and axillo-breast techniques (145–150). All of these techniques avoid a neck incision, and instead hide the incision in the crease between the axilla and the breast. Long-term data on the adequacy of resection using these approaches is lacking, and these techniques come with added complication risk such as brachial plexus injury (149).
Radioactive Iodine
Remnant ablation with radioactive iodine is the standard adjuvant treatment for selected patients with DTC. It can only be administered after a total or near-total thyroidectomy, otherwise the radioactive isotope will be absorbed by the remnant thyroid and not destroy any micro-metastatic disease as intended. Radioactive iodine is administered 1–3 months postoperatively as 131I as sodium iodide in an oral form whose half-life is 7–8 days. Consensus guidelines recommend a dose of 30–100 mCi for patients with low risk tumors and higher doses (100–200 mCi) for patients with residual disease, suspected microscopic disease, or more aggressive histologic subtypes (i.e., tall cell, columnar cell, or insular variants) (99, 151–153). In order to stimulate intracellular uptake of the isotope, the TSH concentration should be at least as high as 30 mU/L.
There are two methods for achieving such an elevation in TSH. The traditional method requires the patient to withdraw from thyroid hormone replacement over 4–6 weeks (4, 154). A newer method is to administer recombinant human TSH (rhTSH). rhTSH is administered in the form of intramuscular injections on two consecutive days followed by radioactive iodine on the third day. The advantage of this method is that the patient does not experience an extended period of hypothyroidism as with hormone withdrawal. However, long-term data on the effectiveness of rhTSH compared to traditional withdrawal are not established, although it appears effective for low-risk patients. The U.S. Food and Drug Administration (FDA) approved rhTSH for thyroid remnant ablation in patients who do not have evidence of metastatic disease (155, 156). In addition to making the TSH rise, clinicians should also prepare patients by instructing them to follow a low-iodine diet for 1–2 weeks prior to radioactive iodine treatment. This diet requires patients to avoid foods that contain iodized salt, dairy products, eggs, seafood, soybeans or soy-containing products, and foods colored with red dye #3 (152, 153). Its important to note that rhTSH is not approved for use in children.
While some studies show no benefit to radioactive iodine therapy (157, 158), other studies demonstrate a reduction in locoregional recurrences and distant metastases (122, 123). As with the controversy over the extent of thyroidectomy, the benefit of radioactive iodine for low risk patients remains unclear (154). The most recent ATA guidelines recommend remnant ablation for patients with T3 tumors or nodal disease. Selective use is recommended for 1–2 cm intrathyroidal tumors or T2 tumors. It is not recommended for intrathyroidal tumors less than or equal to 1 cm in size (99). The National Comprehensive Cancer Network (NCCN) guidelines require a more thorough evaluation for the extent of remaining disease after thyroidectomy with a radioiodine scan 1–12 weeks postoperatively. Radioactive iodine ablation is not recommended if the stimulated Tg is less than 1 ng/mL and the radioiodine scan is negative (151).
Recently, some studies have shown an increase in the risk of developing secondary malignancies after radioactive iodine therapy. This has been examined using the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database. Brown and colleagues found that patients treated for DTC had significantly higher rates of non-thyroid second primary malignancies than expected in the general population. Although the excess risk was relatively small, it was greater in the subset of patients who were treated with radioactive iodine (159). Iyer and associates specifically examined low risk patients (T1N0) treated with radioactive iodine and found that their excess absolute risk was 4.6 excess cases per 10,000 person years at risk (160). As discussed above, radioactive iodine clearly benefits patients with larger tumors and metastatic disease, but the increased risk of secondary malignancies in low risk patients where the long-term benefit of radioactive iodine is questionable means that careful patient selection is necessary.
Hematologic malignancies are the most common secondary malignancies after radioactive iodine, but there is also an association with kidney, breast, bladder, skin, and salivary gland cancers (161–163). The more commonly noted side effects after radioiodine treatment include dry mouth, mouth pain, salivary gland swelling (sialadenitis), altered smell and taste, conjunctivitis, and fatigue. Women should not be pregnant at the time of treatment nor should they become pregnant for at least 6 months following treatment. Similarly, men should avoid conception for at least 6 months following treatment (153, 162, 164, 165).
Thyroxine-suppression
Since all cells of follicular origin depend on TSH for growth, TSH suppression through the administration of supraphysiologic doses of levothyroxine (T4) remains an important strategy for maintaining disease-free survival and overall survival (166, 167). For high-risk patients with incomplete resection, tumor invasion into adjacent structures, or distant metastases, their physician should initially titrate levothyroxine dosing to a TSH < 0.1mU/L. Lower risk patients should be dosed to a TSH at or slightly below the lower limit of normal (0.1 – 0.5 mU/L) (99, 151). Once patients remain disease-free for at least two years, their TSH suppression can be liberalized to within the reference range. Patients with persistent disease should be kept at a TSH <0.1 mU/L indefinitely. TSH suppression carries risks of arrhythmias, anxiety, and osteoporosis. The risks and benefits should be carefully considered, particularly in older patients. Due to the risk of bone loss, the NCCN guidelines recommend daily calcium and vitamin D supplementation for patients on TSH suppression (151).
External-beam radiation
Although 131I is the preferred adjuvant therapy for thyroid carcinoma, external-beam radiation sometimes plays a role in treating this disease. Persistent, recurrent, anaplastic, or poorly differentiated tumors may fail to take up 131I. Treatment of anaplastic thyroid tumors almost always includes external beam radiation since these tumors often cannot be completely resected and do not concentrate iodine. Although no improvement in overall survival has ever been documented, external beam radiation is often given after resection of poorly differentiated tumors to reduce the risk of local relapse (168). The group at Memorial Sloan Kettering Cancer Center has found that up to 85% of poorly differentiated tumors display some iodine avidity, and therefore treatment with radioactive iodine may remain worthwhile. Patients with incompletely resected tumors, unresectable disease, and locoregional recurrence in a previously operated field may benefit from external beam radiation (3, 168, 169). External beam radiation is typically reserved as a last resort, after surgery and RAI have been exhausted.
Chemotherapy
Since radioactive iodine often can be effective treatment for well-differentiated tumors that have metastasized, cytotoxic chemotherapy has not been extensively evaluated for metastatic thyroid cancers. For large burden of disease, anaplastic cancers, or poorly differentiated tumors that are not iodine avid, chemotherapy becomes an important treatment component after surgery or if the tumor is not resectable. In these rare situations, chemotherapy confers minimal effects as these tumors hold a very poor prognosis. Historically, doxorubicin was the most effective single agent. Combination therapy with doxorubicin and cisplatin resulted in modest objective response rates (170, 171).
Newer, targeted therapies have shown some promise. Small molecule tyrosine kinase inhibitors (such as sorafenib or sunitinib) and antibodies (anti-VEGF) should be considered in the context of ongoing clinical trials (151, 172–174).
Mitogen-activated protein kinase (MAPK) inhibitors target specific oncogenic pathways in DTC progression. These small molecules are generally well-tolerated with low toxicity profiles. As discussed above, the BRAF gene is commonly mutated in thyroid cancer, and therefore, many of the targeted MAPK drugs block the Raf kinases for patients with RET or BRAF mutations (175, 176). Sorafenib is an orally administered multi-kinase inhibitor targeting BRAF, VEGF, RET, and c-kit. Two different phase II clinical trials enrolled patients with radioiodine resistant metastatic DTC, and reported that sorafenib stabilized disease progression and lowered serum thyroglobulin with minimal toxicity (177, 178).
Emerging therapies specifically target angiogenesis because DTC tumors express high levels of vascular endothelial growth factor (VEGF) receptors (179, 180). Multi-kinase inhibitors such as motesanib, vandetanib, sunitinib, and axitinib have shown early promise in patients with DTC (173, 181, 182). These drugs often target multiple VEGF receptors in addition to other signaling pathways such as c-Kit, RET, and PDGF.
Another mechanism targeted in anti-cancer therapy is the acetylation of N-terminal lysine residues on histones. Histone acetylation results in a more open chromatin configuration and gene transcription. Many different types of cancer cells have been found to have dysregulated histone acetyltransferase or histone deactylase (HDAC) enzymes (183–185). Several HDAC inhibitors including vorinostat, depsipeptide, and valproic acid have been shown to have an effect on thyroid cancer cells (186–188). For example, in thyroid carcinoma cell lines, valproic acid increased expression of the sodium-iodide symporter and radioiodine uptake (189–191). Many of these results come from in-vitro studies or early phase clinical trials, but do represent promising novel therapies with much lower toxicity than traditional chemotherapeutic agents.
Medullary Thyroid Cancer (MTC)
Medullary thyroid carcinoma (MTC) accounts for 6–8% of all thyroid cancers. Unlike DTC, MTC arises from the parafollicular C cells instead of the follicular epithelial cells. Hence, MTC is a neuroendocrine tumor, and shares some properties common among neuroendocrine cancers including secretion of peptide hormones such as calcitonin, serotonin, or vasoactive intestinal peptide. 25% of cases are due to germline genetic mutations, but most cases of MTC are sporadic. Hereditary cases occur either in isolation (familial medullary thyroid cancer) or as part of the multiple endocrine neoplasia syndrome type 2 (2A or 2B) (192–194).
Diagnosis
Although any thyroid nodule could potentially harbor MTC, historical features that may alert the physician to the potential for MTC include a family history of MTC, pheochromocytoma, hyperparathyroidism, or other manifestations of MEN-2 syndromes (5). As in evaluating all thyroid nodules, neck ultrasound and FNA play a major role in diagnosing MTC. Hereditary cases are often detected through genetic screening to identify germline mutations in the RET gene. Almost all sporadic cases present with a palpable neck mass and this could be either in the thyroid or a metastatic lymph node. Lymph nodes metastases occur in 35–50% of patients at initial diagnosis (195). Therefore ultrasound evaluation of the central and lateral neck compartments for suspicious lymph nodes becomes a crucial component to the initial diagnosis (196).
Since the parafollicular C cells are concentrated in the upper, posterior portion of each thyroid lobe, many MTCs arise in a posterior location, causing symptoms such as hoarseness or dysphagia due to compression of local structures. If there is any concern for vocal cord function, then direct laryngoscopy should be performed preoperatively (195). MTCs secrete cytokines and other peptides that can cause symptoms such as flushing, diarrhea, and weight loss (197).
FNA characteristics of MTC include the presence of stromal amyloid without thyroid follicles. Spindle shaped cells may be seen, and therefore MTC can be mistaken for parathyroid carcinoma or anaplastic thyroid cancer unless the specimen is stained for calcitonin, chromogranin A, or CEA—substances produced by MTC that confirm the diagnosis. A more sensitive technique than immunohistochemistry on cytology specimens is to measure the calcitonin level in the washout fluid from an FNA (198). Additionally, the presence of calcitonin messenger RNA (mRNA) has been performed when the cytologic or histologic diagnosis remains unclear (199). Histologically, MTCs form nests of cells with deposition of stromal amyloid that stains positively with Congo Red stain (195). Staining for chromogranin A can also help confirm the diagnosis.
Several serum markers can aid in the diagnosis of MTC, and are useful in following patients for recurrence and metastases. Calcitonin is commonly elevated in patients with MTC. Although a small percentage of normal patients will have some elevation in calcitonin, patients with a diagnosis of MTC usually exhibit levels above 100 pg/ml. In borderline cases, the diagnosis can be clarified by stimulating the calcitonin with either intravenous calcium gluconate or pentagastrin. Before the advent of genetic testing, these stimulated measurements were used to screen patients at high risk for MTC (200). The degree of calcitonin elevation correlates with tumor burden, with nodal metastases found at basal calcitonin levels of 10–40 pg/ml, and distant metastases found with calcitonin levels greater than 150 pg/ml. Patients with calcitonin levels over 3000 pg/ml are likely to have widely metastatic disease, and are unlikely to be cured despite aggressive surgery (201).
Preoperative measurement of serum Carcinoembryonic antigen (CEA) can also help risk stratify patients. Overall, CEA elevations occur in more than 50% of patients with MTC, but a preoperative serum CEA greater than 30 ng/ml highly predicts the inability to cure the patient with surgery (202). CEA levels above 100 ng/ml may signify extensive lymph node and distant metastases. Following CEA levels postoperatively can also monitor disease progression. An increasing CEA level in the presence of a stable calcitonin is associated with a worse prognosis as it may indicate tumor dedifferentiation and distant metastases. Other markers such as chromogranin A or serotonin can be elevated in patients with MTC as with many other neuroendocrine tumors, but calcitonin, chromogranin A, and CEA are the most useful for following MTC patients long-term (195, 203, 204)
Genetic testing plays an important part of the initial management because it identifies familial disease and risk stratifies patients with hereditary forms. Familial disease is characterized by germline mutations in the RET gene (205). A small percentage of apparently “sporadic” disease will also carry germline RET mutations, but truly sporadic cases frequently harbor somatic RET mutations. Commercial testing is performed through PCR amplification of the patient's germline DNA obtained from the patients white blood cells.
A spectrum of tumor aggressiveness exists among the various RET mutations, and the timing of prophylactic thyroidectomy is based on the specific mutation. Table 4 lists each mutation by codon and phenotype. Once a patient tests positive for a germline RET mutation, they should be carefully counseled regarding the risk to other family members and their children. At risk family members should be identified and also tested so that prophylactic thyroidectomy can be offered at the appropriate time (Table 5). While some overlap exists for genetic mutations associated with MEN-2A and familial MTC, distinct mutations are usually associated with MEN-2B (206, 207).
Table 4.
Frequency of RET mutations associated with familial RET syndromes
| Exon | Codon | Amino Acid (wild type→mutant) | Phenotype | Frequency (%), MEN2 cases |
|---|---|---|---|---|
| 8 | 532,533,534 | Ins-Glu-Glu-Cys | FMTC | Rare |
| 533 | Gly-Cys | FMTC | ||
|
| ||||
| 10 | 609 | Cys-Arg | MEN 2A/FMTC | 0–1 |
| Cys-Gly | ||||
| Cys-Tyr | ||||
| 611 | Cys-Ser | MEN 2A/FMTC | 2–3 | |
| Cys-Arg | ||||
| Cys-Tyr | ||||
| Cys-Phe | ||||
| Cys-Trp | ||||
| 618 | Cys-Ser | MEN 2A/FMTC | 3–5 | |
| Cys-Arg | ||||
| Cys-Gly | ||||
| Cys-Tyr | ||||
| Cys-Ser | ||||
| 620 | Cys-Phe | MEN 2A/FMTC | 6–8 | |
| Cys-Ser | ||||
| Cys-Arg | ||||
| Cys-Gly | ||||
| Cys-Tyr | ||||
| Cys-Trp | ||||
|
| ||||
| 11 | 630 | Cys-Tyr | MEN 2A/FMTC | 0–1 |
| Cys-Ser | ||||
| Cys-Phe | ||||
| 634 | Cys-Ser | MEN 2A | 80–90 | |
| Cys-Arg | ||||
| Cys-Gly | ||||
| Cys-Tyr | MEN 2A/FMTC | |||
| Cys-Ser | MEN 2A/FMTC | 80–90 | ||
| Cys-Phe | ||||
| Cys-Trp | ||||
| 635,636,637,638 | Ins-Thr-Ser-Cys-Ala | MEN 2A/FMTC | Rare | |
| 637-638-639 | Ins-Cys-Arg-Thr | MEN 2A | ||
| 648 | Val-Ile | MEN 2A | ||
|
| ||||
| 13 | 768 | Glu-Asp | MEN 2A/FMTC | Rare |
| 790 | Leu-Phe | |||
| 791 | Tyr-Phe | |||
|
| ||||
| 14 | 804 | Val-Met | MEN 2A/FMTC | 0–1 |
| Val-Leu | ||||
|
| ||||
| 15 | 883 | Ala-Phe | MEN 2B | Rare |
| 891 | Ser-Ala | MEN 2A/FMTC | ||
|
| ||||
| 16 | 918 | Met-Thr | MEN 2B | 3–5 |
| 922 | Ser-Tyr | MEN 2B | Rare | |
Adapted from “Management of Medullary Thyroid Cancer” in The Handbook of Endocrine Surgery (RS Sippel, and H Chen, eds.)
Table 5.
`Codon-directed' timing of surgery based on ret mutations associated with hereditary MTC
| Risk level for MTC | ATA Risk level | Codon mutation | Age of prophylactic surgery |
|---|---|---|---|
|
| |||
| Level 3 (highest) | D | 883 | Within the first 6 months of life (preferably in the first year) |
| D | 918 | ||
| D | 922 | ||
|
| |||
| Level 2 (higher) | B | 611 | By age 5 |
| B | 618 | ||
| B | 620 | ||
| B/C | 634 | ||
|
| |||
| Level 1 (high) | B | 609 | By age 5–10 |
| B | 630 | ||
| A | 768 | ||
| A | 790 | ||
| A | 791 | ||
| A | 804 | ||
| A | 891 | ||
Adapted from “Management of Medullary Thyroid Cancer” in The Handbook of Endocrine Surgery (RS Sippel, and H Chen, eds.)
Treatment
Complete surgical excision is the treatment of choice for MTC. The minimum extent of surgery for patients with clinically apparent disease is a total thyroidectomy with bilateral central neck dissection. Eighty-one percent of patients with palpable disease have central neck lymph node metastases, and the addition of central neck dissection improves cure rates over total thyroidectomy alone in patients with clinically evident disease at presentation (208, 209). The initial approach to lateral neck lymph nodes continues to evolve. Historically, the initial surgical treatment included an ipsilateral lateral compartment neck dissection because up to 80% of patients will have ipsilateral nodal metastases (210). However, current guidelines recommend performing an ipsilateral lateral neck dissection if ultrasound or physical exam detects lymphadenopathy in the lateral neck, central compartment lymph nodes are involved, or when the primary tumor is greater than 1 cm (196). Contralateral lateral neck dissection is added when patients have bilateral tumors or there is extensive lymph node disease on the ipsilateral side. Because some patients often require extensive neck dissection, these procedures are often staged (195, 196). Unlike DTC where micro-metastatic disease can be effectively treated with radioactive iodine ablation, the only effective treatment for MTC is complete surgical resection. Therefore, all evident disease must be resected for the best long-term cure.
Prophylactic thyroidectomy is recommended for at risk family members in hereditary MTC. Current recommendations for the timing of prophylactic thyroidectomy balance the need to remove the at risk organ prior to it developing clinically apparent disease with the risks of surgery. In hereditary MTC, an age-related progression exists from C-cell hyperplasia to carcinoma, and ultimately nodal metastases. The optimal timing of prophylactic thyroidectomy depends on the risk level of the RET mutation (Table 5). In general, current guidelines recommend operating on children with MEN-2A and familial MTC by age 5 while those with MEN-2B should be operated on before 6 months of age (211, 212). Prophylactic surgery should consist of at least a total thyroidectomy. The role of prophylactic lymph node dissection in familial disease remains controversial. Lymph node metastases are present in 6% of screened patients (213), and therefore, some argue that prophylactic central lymph node dissection should be performed. Opponents to this approach state that with a normal preoperative ultrasound, normal calcitonin (basal and/or stimulated), and a normal CEA, the risk of occult nodal disease is very low and do not outweigh the risks of a central neck dissection such as permanent hypoparathyroidism (5, 208, 213). Because any complications resulting from a prophylactic surgery become lifelong problems for the patient, experienced surgeons should perform prophylactic surgery for MTC.
In general, prophylactic thyroidectomy does not need to include a central neck dissection for MEN-2A and FMTC as long as surgery is done prophylactically, before disease is clinically apparent. Because of the more aggressive nature of MTCs associated with MEN-2B, performance of a central neck dissection with prophylactic thyroidectomy is optional, depending on the timing, and the specific codon mutated (195, 196) Prior to proceeding with surgery, the surgeon should screen patients with hereditary disease for associated conditions such as pheochromocytoma (MEN-2A and B) and hyperparathyroidism (MEN-2A) (207, 212, 213).
All patients will require thyroid hormone replacement once the thyroid is removed, but TSH suppression is not required because the parafollicular C cells are not under TSH growth control. After surgery, the next phase of treatment is surveillance. This begins 2–3 months postoperatively with a new baseline calcitonin and CEA. If the calcitonin is undetectable, then these patients can be followed with yearly calcitonin measurements. Imaging is undertaken when the calcitonin level rises.
A spectrum of disease severity exists for both hereditary and sporadic MTC, and therefore, the natural history of MTC varies widely. Distant metastases in the lung, liver, or bone can arise and lead to death quite quickly. On the other hand, many patients live with a large tumor burden and very high calcitonin levels with few symptoms. Others suffer from intractable diarrhea. In this case, cytoreductive surgery or somatostatin analogs like octreotide can palliate severe symptoms (214).
Conventional chemotherapy regimens with doxorubicin, dacarbazine, capcitabine, and 5-fluorouracil only have demonstrated limited efficacy in patients with MTC. Newer, targeted therapies block the RET receptor tyrosine kinase or its multiple downstream pathways such as the extracellular signal-related kinase (ERK), phosphatidyl-inositol 3-kinase (PI3K)/Akt, p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase pathways (107, 195, 215). Some of these tyrosine kinase inhibitors inhibit multiple signaling pathways simultaneously (107, 216). These targeted therapies are currently being evaluated in multicenter trials (216, 217).
Recently, the U.S. Food and Drug Administration (FDA) approved one of these targeted therapies, vandetanib, for treatment of metastatic MTC. Vandetanib is a small molecule inhibitor of the vascular endothelial growth factor (VEGF) receptor, epidermal growth factor (EGF) receptor, and the RET tyrosine kinase(218). In a randomized controlled clinical trial, patients treated with vandetanib experienced a median progression-free survival of 22.6 months compared to 16.4 months in patients treated with placebo (216).
Acknowledgements
This study was supported by NIH T32 CA009614-23.
Footnotes
No disclosures or conflicts of interest.
References
- 1.Clark OH. Thyroid cancer and lymph node metastases. J Surg Oncol. 2011;103:615–8. doi: 10.1002/jso.21804. [DOI] [PubMed] [Google Scholar]
- 2.Sippel RS, Chen H. Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid. 2009;19(12):1373–80. doi: 10.1089/thy.2009.1606. [DOI] [PubMed] [Google Scholar]
- 3.Patel KN, Shaha AR. Poorly differentiated and anaplastic thyroid cancer. Cancer Control. 2006;13:119–28. doi: 10.1177/107327480601300206. [DOI] [PubMed] [Google Scholar]
- 4.Udelsman R, Chen H. The current management of thyroid cancer. Adv Surg. 1999;33:1–27. [PubMed] [Google Scholar]
- 5.Moo-Young TA, Traugott AL, Moley JF. Sporadic and familial medullary thyroid carcinoma: state of the art. Surg Clin North Am. 2009;89(5):1193–204. doi: 10.1016/j.suc.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarebczan B, Chen H. Multi-targeted approach in the treatment of thyroid cancer. Minerva Chir. 2010;65(1):59–69. PMCID: 2901507. [PMC free article] [PubMed] [Google Scholar]
- 7.Thyroid Cancer Home Page - National Cancer Institute [cited 2011 March 24th, 2011]; Available from: http://www.cancer.gov/cancertopics/types/thyroid.
- 8.SEER cancer statistics review 1975–2009. Bethesda, MD: [cited]; Available from: http://seer.cancer.gov/statfacts/html/thyro.html. [Google Scholar]
- 9.Davies L, Ouellette M, Hunter M, Welch HG. The increasing incidence of small thyroid cancers: where are the cases coming from? Laryngoscope. 2010;120(12):2446–51. doi: 10.1002/lary.21076. [DOI] [PubMed] [Google Scholar]
- 10.Bradly DP, Reddy V, Prinz RA, Guttuso P. Incidental papillary carcinoma in patients treated surgically for benign thyroid diseases. Surgery. 2009;146:1099–104. doi: 10.1016/j.surg.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Ishii T, Maeda K, Nakamura K, Hosoda Y. Cancer in the aged: an autopsy study of 940 cancer patients. J Am Geriatr Soc. 1979;27(7):307–13. doi: 10.1111/j.1532-5415.1979.tb06045.x. [DOI] [PubMed] [Google Scholar]
- 12.Bisi H, Fernandes VS, deCamargo RYA, Koch L, Abdo AH, de Brito T. The prevalence of unsuspected thyroid pathology in 300 sequential autopsies with special reference to the incidental carcinoma. Cancer. 1989;64:1888–93. doi: 10.1002/1097-0142(19891101)64:9<1888::aid-cncr2820640922>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery. 2010;148(6):1147–52. doi: 10.1016/j.surg.2010.10.016. discussion 52–3. [DOI] [PubMed] [Google Scholar]
- 14.Baker SR, Bhatti WA. The thyroid cancer epidemic: is it the dark side of the CT revolution? Eur J Radiol. 2006;60(1):67–9. doi: 10.1016/j.ejrad.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Engeland A, Tretli S, Akslen LA, Bjorge T. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer. 2006;95(3):366–70. doi: 10.1038/sj.bjc.6603249. PMCID: 2360634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannibal CG, Jensen A, Sharif H, Kjaer SK. Risk of thyroid cancer after exposure to fertility drugs: results from a large Danish cohort study. Hum Reprod. 2008;23(2):451–6. doi: 10.1093/humrep/dem381. [DOI] [PubMed] [Google Scholar]
- 17.Brindel P, Doyon F, Rachedi F, Boissin JL, Sebbag J, Shan L, et al. Menstrual and reproductive factors in the risk of differentiated thyroid carcinoma in native women in French Polynesia: a population-based case-control study. Am J Epidemiol. 2008;167(2):219–29. doi: 10.1093/aje/kwm288. [DOI] [PubMed] [Google Scholar]
- 18.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 19.Tuttle RM, Becker DV. The Chernobyl accident and its consequences: update at the millennium. Semin Nucl Med. 2000;30(2):133–40. doi: 10.1053/nm.2000.5412. [DOI] [PubMed] [Google Scholar]
- 20.Cetta F, Montalto G, Petracci M, Fusco A. Thyroid cancer and the Chernobyl accident. Are long-term and long distance side effects of fall-out radiation greater than estimated? J Clin Endocrinol Metab. 1997;82(6):2015–7. doi: 10.1210/jcem.82.6.9998. [DOI] [PubMed] [Google Scholar]
- 21.Cardis E, Kesminiene A, Ivanov V, Malakhova I, Shibata Y, Khrouch V, et al. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97(10):724–32. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 22.Davis S, Stepanenko V, Rivkind N, Kopecky KJ, Voilleque P, Shakhtarin V, et al. Risk of thyroid cancer in the Bryansk Oblast of the Russian Federation after the Chernobyl Power Station accident. Radiat Res. 2004;162(3):241–8. doi: 10.1667/rr3233. [DOI] [PubMed] [Google Scholar]
- 23.Cardis E, Hatch M. The Chernobyl accident--an epidemiological perspective. Clin Oncol (R Coll Radiol) 2011;23(4):251–60. doi: 10.1016/j.clon.2011.01.510. PMCID: 3107017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraker DL. Radiation exposure and other factors that predispose to human thyroid neoplasia. Surg Clin North Am. 1995;75(3):365–75. doi: 10.1016/s0039-6109(16)46627-2. [DOI] [PubMed] [Google Scholar]
- 25.Horn-Ross PL, Morris JS, Lee M, West DW, Whittemore AS, McDougall IR, et al. Iodine and thyroid cancer risk among women in a multiethnic population: the Bay Area Thyroid Cancer Study. Cancer Epidemiol Biomarkers Prev. 2001;10(9):979–85. [PubMed] [Google Scholar]
- 26.Knobel M, Medeiros-Neto G. Relevance of iodine intake as a reputed predisposing factor for thyroid cancer. Arq Bras Endocrinol Metabol. 2007;51(5):701–12. doi: 10.1590/s0004-27302007000500007. [DOI] [PubMed] [Google Scholar]
- 27.Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93(3):809–14. doi: 10.1210/jc.2007-2215. PMCID: 2266959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Hong SW, Shin SJ, Kim YM, Rhee Y, Jeon BI, et al. Papillary thyroid carcinoma associated with familial adenomatous polyposis: molecular analysis of pathogenesis in a family and review of the literature. Endocr J. 2004;51(3):317–23. doi: 10.1507/endocrj.51.317. [DOI] [PubMed] [Google Scholar]
- 29.Loh KC. Familial nonmedullary thyroid carcinoma: a meta-review of case series. Thyroid. 1997;7(1):107–13. doi: 10.1089/thy.1997.7.107. [DOI] [PubMed] [Google Scholar]
- 30.Malchoff CD, Malchoff DM. Familial nonmedullary thyroid carcinoma. Cancer Control. 2006;13(2):106–10. doi: 10.1177/107327480601300204. [DOI] [PubMed] [Google Scholar]
- 31.Sippel RS, Caron NR, Clark OH. An evidence-based approach to familial nonmedullary thyroid cancer: screening, clinical management, and follow-up. World J Surg. 2007;31(5):924–33. doi: 10.1007/s00268-006-0847-1. [DOI] [PubMed] [Google Scholar]
- 32.Bonora E, Tallini G, Romeo G. Genetic Predisposition to Familial Nonmedullary Thyroid Cancer: An Update of Molecular Findings and State-of-the-Art Studies. J Oncol. 2010. 2010:385206. doi: 10.1155/2010/385206. PMCID: 2902056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bevan S, Pal T, Greenberg CR, Green H, Wixey J, Bignell G, et al. A comprehensive analysis of MNG1, TCO1, fPTC, PTEN, TSHR, and TRKA in familial nonmedullary thyroid cancer: confirmation of linkage to TCO1. J Clin Endocrinol Metab. 2001;86(8):3701–4. doi: 10.1210/jcem.86.8.7725. [DOI] [PubMed] [Google Scholar]
- 34.Bignell GR, Canzian F, Shayeghi M, Stark M, Shugart YY, Biggs P, et al. Familial nontoxic multinodular thyroid goiter locus maps to chromosome 14q but does not account for familial nonmedullary thyroid cancer. Am J Hum Genet. 1997;61(5):1123–30. doi: 10.1086/301610. PMCID: 1716029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKay JD, Lesueur F, Jonard L, Pastore A, Williamson J, Hoffman L, et al. Localization of a susceptibility gene for familial nonmedullary thyroid carcinoma to chromosome 2q21. Am J Hum Genet. 2001;69(2):440–6. doi: 10.1086/321979. PMCID: 1235316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazeh H, Benavidez J, Poehls JL, Youngwirth L, Chen H, Sippel RS. In patients with thyroid cancer of follicular cell origin, a family history of nonmedullary thyroid cancer in one first-degree relative is associated with more aggressive disease. Thyroid. 2012;22(1):3–8. doi: 10.1089/thy.2011.0192. [DOI] [PubMed] [Google Scholar]
- 37.Alberti L, Carniti C, Miranda C, Roccato E, Pierotti MA. RET and NTRK1 proto-oncogenes in human diseases. J Cell Physiol. 2003;195(2):168–86. doi: 10.1002/jcp.10252. [DOI] [PubMed] [Google Scholar]
- 38.Fusco A, Chiappetta G, Hui P, Garcia-Rostan G, Golden L, Kinder BK, et al. Assessment of RET/PTC oncogene activation and clonality in thyroid nodules with incomplete morphological evidence of papillary carcinoma: a search for the early precursors of papillary cancer. Am J Pathol. 2002;160(6):2157–67. doi: 10.1016/S0002-9440(10)61164-9. PMCID: 1850819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fugazzola L, Puxeddu E, Avenia N, Romei C, Cirello V, Cavaliere A, et al. Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: data from a multicentric Italian study and review of the literature. Endocr Relat Cancer. 2006;13(2):455–64. doi: 10.1677/erc.1.01086. [DOI] [PubMed] [Google Scholar]
- 40.Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW, 2nd, Tallini G, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88(5):2318–26. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 41.Fagin JA, Mitsiades N. Molecular pathology of thyroid cancer: diagnostic and clinical implications. Best Pract Res Clin Endocrinol Metab. 2008;22(6):955–69. doi: 10.1016/j.beem.2008.09.017. PMCID: 2615540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gianoukakis AG, Giannelli SM, Salameh WA, McPhaul LW. Well differentiated follicular thyroid neoplasia: impact of molecular and technological advances on detection, monitoring and treatment. Mol Cell Endocrinol. 2011;332(1–2):9–20. doi: 10.1016/j.mce.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 43.From G, Mellemgaard A, Knudsen N, Jorgensen T, Perrild H. Review of thyroid cancer cases among patients with previous benign thyroid disorders. Thyroid. 2000;10:697–700. doi: 10.1089/10507250050137789. [DOI] [PubMed] [Google Scholar]
- 44.Roti E, et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006;91:2171–8. doi: 10.1210/jc.2005-2372. [DOI] [PubMed] [Google Scholar]
- 45.Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254(4):653–60. doi: 10.1097/SLA.0b013e318230036d. [DOI] [PubMed] [Google Scholar]
- 46.Mazeh H, Chen H. Advances in surgical therapy for thyroid cancer. Nat Rev Endocrinol. 2011;7(10):581–8. doi: 10.1038/nrendo.2011.140. [DOI] [PubMed] [Google Scholar]
- 47.Niemeier LA, Akatsu HK, Song C, Carty SE, Hodak SP, Yip L, et al. A combined molecular-pathologic score improves risk stratification of thyroid papillary microcarcinoma. Cancer. 2012;118:2069–77. doi: 10.1002/cncr.26425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang BH, Chow SM, Lo CY, Law SC, Lam KY. Staging systems for papillary thyroid carcinoma: a study of 2 tertiary referral centers. Ann Surg. 2007;246:114–21. doi: 10.1097/01.sla.0000262785.46403.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71(2):414–24. doi: 10.1210/jcem-71-2-414. [DOI] [PubMed] [Google Scholar]
- 50.Baloch ZW, LiVolsi VA. Prognostic factors in well-differentiated follicular-derived carcinoma and medullary thyroid carcinoma. Thyroid. 2001;11(7):637–45. doi: 10.1089/105072501750362709. [DOI] [PubMed] [Google Scholar]
- 51.Eichhorn W, Tabler H, Lippold R, Lochmann M, Schreckenberger M, Bartenstein P. Prognostic factors determining long-term survival in well-differentiated thyroid cancer: an analysis of four hundred eighty-four patients undergoing therapy and aftercare at the same institution. Thyroid. 2003;13(10):949–58. doi: 10.1089/105072503322511355. [DOI] [PubMed] [Google Scholar]
- 52.Eustatia-Rutten CF, Corssmit EP, Biermasz NR, Pereira AM, Romijn JA, Smit JW. Survival and death causes in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2006;91(1):313–9. doi: 10.1210/jc.2005-1322. [DOI] [PubMed] [Google Scholar]
- 53.Wada N, Suganuma N, Nakayama H, Masudo K, Rino Y, Masuda M, et al. Microscopic regional lymph node status in papillary thyroid carcinoma with and without lymphadenopathy and its relation to outcomes. Langenbeck's Arch Surg. 2007;392:417–22. doi: 10.1007/s00423-007-0159-4. [DOI] [PubMed] [Google Scholar]
- 54.Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J Surg. 2004;28(5):498–501. doi: 10.1007/s00268-004-7192-z. [DOI] [PubMed] [Google Scholar]
- 55.Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106:524–31. doi: 10.1002/cncr.21653. [DOI] [PubMed] [Google Scholar]
- 56.Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005;71:731–4. doi: 10.1177/000313480507100907. [DOI] [PubMed] [Google Scholar]
- 57.Schneider DF, Chen H, Sippel RS. The impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2802-8. Epub ahead of print(Dec 23, 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider DF, Mazeh H, Chen H, Sippel RS. Lymph node ratio predicts recurrence in papillary thyroid cancer. Onocoligist. 2013 doi: 10.1634/theoncologist.2012-0240. Epub ahead of print(Jan 22, 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beal SH, Chen SL, Schneider PD, Martinez SR. An evaluation of lymph node yield and lymph node ratio in well-differentiated thyroid carcinoma. Am Surg. 2010;76:28–32. [PubMed] [Google Scholar]
- 60.Lang BH, Wong KP, Wan KY, Lo CY. Significance of metastatic lymph node ratio on stimulated thyroglobulin levels in papillary thyroid carcinoma after prophylactic unilateral central neck dissection. Ann Surg Oncol. 2012;19:1257–63. doi: 10.1245/s10434-011-2105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol. 2011;5(1):51–6. doi: 10.1007/s12105-010-0236-9. PMCID: 3037461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LiVolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011;24(Suppl 2):S1–9. doi: 10.1038/modpathol.2010.129. [DOI] [PubMed] [Google Scholar]
- 63.Rivera M, Ricarte-Filho J, Patel S, Tuttle M, Shaha A, Shah JP, et al. Encapsulated thyroid tumors of follicular cell origin with high grade features (high mitotic rate/tumor necrosis): a clinicopathologic and molecular study. Hum Pathol. 2010;41(2):172–80. doi: 10.1016/j.humpath.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004;28(10):1336–40. doi: 10.1097/01.pas.0000135519.34847.f6. [DOI] [PubMed] [Google Scholar]
- 65.Scognamiglio T, Hyjek E, Kao J, Chen YT. Diagnostic usefulness of HBME1, galectin-3, CK19, and CITED1 and evaluation of their expression in encapsulated lesions with questionable features of papillary thyroid carcinoma. Am J Clin Pathol. 2006;126(5):700–8. doi: 10.1309/044V-86JN-2W3C-N5YB. [DOI] [PubMed] [Google Scholar]
- 66.Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88(11):5399–404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 67.Tetzlaff MT, LiVolsi V, Baloch ZW. Assessing the utility of a mutational assay for B-RAF as an adjunct to conventional fine needle aspiration of the thyroid gland. Adv Anat Pathol. 2006;13(5):228–37. doi: 10.1097/01.pap.0000213044.23823.d3. [DOI] [PubMed] [Google Scholar]
- 68.Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93(10):3943–9. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 69.Wreesmann VB, Ghossein RA, Patel SG, Harris CP, Schnaser EA, Shaha AR, et al. Genome-wide appraisal of thyroid cancer progression. Am J Pathol. 2002;161(5):1549–56. doi: 10.1016/S0002-9440(10)64433-1. PMCID: 1850764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burman KD, Ringel MD, Wartofsky L. Unusual types of thyroid neoplasms. Endocrinol Metab Clin North Am. 1996;25(1):49–68. doi: 10.1016/s0889-8529(05)70312-1. [DOI] [PubMed] [Google Scholar]
- 71.Wang W, Macapinlac H, Larson SM, Yeh SD, Akhurst T, Finn RD, et al. [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography localizes residual thyroid cancer in patients with negative diagnostic (131)I whole body scans and elevated serum thyroglobulin levels. J Clin Endocrinol Metab. 1999;84(7):2291–302. doi: 10.1210/jcem.84.7.5827. [DOI] [PubMed] [Google Scholar]
- 72.Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338(5):297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 73.Brennan MD, Bergstralh EJ, van Heerden JA, McConahey WM. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1991;66(1):11–22. doi: 10.1016/s0025-6196(12)61170-7. [DOI] [PubMed] [Google Scholar]
- 74.Thompson LD, Wieneke JA, Paal E, Frommelt RA, Adair CF, Heffess CS. A clinicopathologic study of minimally invasive follicular carcinoma of the thyroid gland with a review of the English literature. Cancer. 2001;91(3):505–24. doi: 10.1002/1097-0142(20010201)91:3<505::aid-cncr1029>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 75.Harness JK, Thompson NW, McLeod MK, Eckhauser FE, Lloyd RV. Follicular carcinoma of the thyroid gland: trends and treatment. Surgery. 1984;96(6):972–80. [PubMed] [Google Scholar]
- 76.Grebe SK, Hay ID. Follicular thyroid cancer. Endocrinol Metab Clin North Am. 1995;24(4):761–801. [PubMed] [Google Scholar]
- 77.Nesland JM, Sobrinho-Simoes MA, Holm R, Sambade MC, Johannessen JV. Hurthle-cell lesions of the thyroid: a combined study using transmission electron microscopy, scanning electron microscopy, and immunocytochemistry. Ultrastruct Pathol. 1985;8(4):269–90. doi: 10.3109/01913128509141518. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez-Campora R, Herrero-Zapatero A, Lerma E, Sanchez F, Galera H. Hurthle cell and mitochondrion-rich cell tumors. A clinicopathologic study. Cancer. 1986;57(6):1154–63. doi: 10.1002/1097-0142(19860315)57:6<1154::aid-cncr2820570616>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 79.Chen H, Nicol TL, Zeiger MA, Dooley WC, Ladenson PW, Cooper DS, et al. Hurthle cell neoplasms of the thyroid: are there factors predictive of malignancy? Ann Surg. 1998;227(4):542–6. doi: 10.1097/00000658-199804000-00015. PMCID: 1191310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phitayakorn R, McHenry CR. Follicular and Hurthle cell carcinoma of the thyroid gland. Surg Oncol Clin N Am. 2006;15(3):603–23. ix–x. doi: 10.1016/j.soc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 81.Zhang YW, Greenblatt DY, Repplinger D, Bargren A, Adler JT, Sippel RS, et al. Older age and larger tumor size predict malignancy in hurthle cell neoplasms of the thyroid. Ann Surg Oncol. 2008;15(10):2842–6. doi: 10.1245/s10434-008-0079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holm L, Blomgren H, Lowhagen T. Cancer risk in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601–4. doi: 10.1056/NEJM198503073121001. [DOI] [PubMed] [Google Scholar]
- 83.Widder S, Pasieka JL. Primary thyroid lymphomas. Curr Treat Options Oncol. 2004;5(4):307–13. doi: 10.1007/s11864-004-0021-7. [DOI] [PubMed] [Google Scholar]
- 84.Kossev P, Livolsi V. Lymphoid lesions of the thyroid: review in light of the revised European-American lymphoma classification and upcoming World Health Organization classification. Thyroid. 1999;9(12):1273–80. doi: 10.1089/thy.1999.9.1273. [DOI] [PubMed] [Google Scholar]
- 85.Wong R, Bresee C, Braunstein G. Comparison with Published Systems of a New Staging System for Papillary and Follicular Thyroid Carcinomas. Thyroid. 2012 doi: 10.1089/thy.2012.0181. [DOI] [PubMed] [Google Scholar]
- 86.Dean DS, Hay ID. Prognostic indicators in differentiated thyroid carcinoma. Cancer Control. 2000;7(3):229–39. doi: 10.1177/107327480000700302. [DOI] [PubMed] [Google Scholar]
- 87.Hay ID, Grant CS, Taylor WF, McConahey WM. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102(6):1088–95. [PubMed] [Google Scholar]
- 88.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–53. [PubMed] [Google Scholar]
- 89.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114(6):1050–7. discussion 7–8. [PubMed] [Google Scholar]
- 90.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97(5):418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 91.Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, et al. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15(8):1033–41. doi: 10.1016/0014-2964(79)90291-3. [DOI] [PubMed] [Google Scholar]
- 92.Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST, Cooper DS, et al. Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83(5):1012–21. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 93.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Atlas. Springer; New York: 2010. [Google Scholar]
- 94.Scheumann GF, Gimm O, Wegener G, Hundeshagen H, Dralle H. Prognostic significance and surgical management of locoregional lymph node mestastases in papillary thyroid cancer. World J Surg. 1994;18:559–67. doi: 10.1007/BF00353765. [DOI] [PubMed] [Google Scholar]
- 95.Belfiore A, Garofalo MR, Giuffrida D, Runello F, Filetti S, Fiumara A, et al. Increased aggressiveness of thyroid cancer in patients with Graves' disease. J Clin Endocrinol Metab. 1990;70(4):830–5. doi: 10.1210/jcem-70-4-830. [DOI] [PubMed] [Google Scholar]
- 96.Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553–9. doi: 10.1056/NEJM199302253280807. [DOI] [PubMed] [Google Scholar]
- 97.Nagai GR, Pitts WC, Basso L, Cisco JA, McDougall IR. Scintigraphic hot nodules and thyroid carcinoma. Clin Nucl Med. 1987;12(2):123–7. doi: 10.1097/00003072-198702000-00010. [DOI] [PubMed] [Google Scholar]
- 98.Haugen BR, Ridgway EC, McLaughlin BA, McDermott MT. Clinical comparison of whole-body radioiodine scan and serum thyroglobulin after stimulation with recombinant human thyrotropin. Thyroid. 2002;12(1):37–43. doi: 10.1089/105072502753451959. [DOI] [PubMed] [Google Scholar]
- 99.Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–11214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 100.Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, et al. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 2003;88(4):1433–41. doi: 10.1210/jc.2002-021702. [DOI] [PubMed] [Google Scholar]
- 101.Gharib H, Papini E, Valcavi R, Baskin HJ, Crescenzi A, Dottorini ME, et al. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006;12(1):63–102. doi: 10.4158/EP.12.1.63. [DOI] [PubMed] [Google Scholar]
- 102.Lowhagen T, Granberg PO, Lundell G, Skinnari P, Sundblad R, Willems JS. Aspiration biopsy cytology (ABC) in nodules of the thyroid gland suspected to be malignant. Surg Clin North Am. 1979;59(1):3–18. doi: 10.1016/s0039-6109(16)41729-9. [DOI] [PubMed] [Google Scholar]
- 103.Crippa S, Mazzucchelli L, Cibas ES, Ali SZ. The Bethesda System for reporting thyroid fine-needle aspiration specimens. Am J Clin Pathol. 2010;134(2):343–4. doi: 10.1309/AJCPXM9WIRQ8JZBJ. author reply 5. [DOI] [PubMed] [Google Scholar]
- 104.Vriens MR, Sabanci U, Epstein HD, Ngai S, Duh QY, Siperstein AE, et al. Reliability of fine-needle aspiration in patients with familial nonmedullary thyroid cancer. Thyroid. 1999;9(10):1011–6. doi: 10.1089/thy.1999.9.1011. [DOI] [PubMed] [Google Scholar]
- 105.Cameci C, Jeffrey RB, McDougall IR, Nowels KW, Weigel RJ. Ultrasound-guided fine-needle aspiration biopsy of thyroid masses. Thyroid. 1998;8:283–9. doi: 10.1089/thy.1998.8.283. [DOI] [PubMed] [Google Scholar]
- 106.Carmeci C, Jeffrey RB, McDougall IR, Nowels KW, Weigel RJ. Ultrasound-guided fine-needle aspiration biopsy of thyroid masses. Thyroid. 1998;8(4):283–9. doi: 10.1089/thy.1998.8.283. [DOI] [PubMed] [Google Scholar]
- 107.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21(Suppl 2):S37–43. doi: 10.1038/modpathol.2008.10. PMCID: 2673022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poehls JL, Chen H, Sippel RS. Preoperative ultrasonography findings predict the need for repeated surgery in papillary thyroid cancer. Endocr Pract. 2012;18(3):403–9. doi: 10.4158/EP11221.OR. [DOI] [PubMed] [Google Scholar]
- 109.Luo J, McManus C, Chen H, Sippel RS. Are there predictors of malignancy in patients with multinodular goiter? J Surg Res. 2012;174(2):207–10. doi: 10.1016/j.jss.2011.11.1035. PMCID: 3323759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sippel RS, Elaraj DM, Poder L, Duh QY, Kebebew E, Clark OH. Localization of recurrent thyroid cancer using intraoperative ultrasound-guided dye injection. World J Surg. 2009;33(3):434–9. doi: 10.1007/s00268-008-9797-0. [DOI] [PubMed] [Google Scholar]
- 111.Al-Azawi D, Mann GB, Judson RT, Miller JA. Endocrine Surgeon-Performed US Guided Thyroid FNAC is Accurate and Efficient. World J Surg. 2012 doi: 10.1007/s00268-012-1592-2. [DOI] [PubMed] [Google Scholar]
- 112.Goldfarb M, Gondek SS, Lew JI. Clinic Based Ultrasound Can Predict Malignancy in Pediatric Thyroid Nodules. Thyroid. 2012 doi: 10.1089/thy.2011.0494. [DOI] [PubMed] [Google Scholar]
- 113.Kim MH, O JH, Ko SH, Bae JS, Lim DJ, Kim SH, et al. Role of [(18)F]-fluorodeoxy-D-glucose positron emission tomography and computed tomography in the early detection of persistent/recurrent thyroid carcinoma in intermediate-to-high risk patients following initial radioactive iodine ablation therapy. Thyroid. 2012;22(2):157–64. doi: 10.1089/thy.2011.0177. [DOI] [PubMed] [Google Scholar]
- 114.Yip L, Stang MT, Carty SE. Thyroid carcinoma: the surgeon's perspective. Radiol Clin North Am. 2011;49(3):463–71. vi. doi: 10.1016/j.rcl.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96(11):3390–7. doi: 10.1210/jc.2011-1469. PMCID: 3205883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367(8):705–15. doi: 10.1056/NEJMoa1203208. [DOI] [PubMed] [Google Scholar]
- 117.Mazeh H, Chen H. Advances in surgical therapy for thyroid cancer. Nat Rev Endocrinol. 2011;7:581–8. doi: 10.1038/nrendo.2011.140. [DOI] [PubMed] [Google Scholar]
- 118.Shaha AR, Shah JP, Loree TR. Low-risk differentiated thyroid cancer: the need for selective treatment. Ann Surg Oncol. 1997;4(4):328–33. doi: 10.1007/BF02303583. [DOI] [PubMed] [Google Scholar]
- 119.Wanebo H, Coburn M, Teates D, Cole B. Total thyroidectomy does not enhance disease control or survival even in high-risk patients with differentiated thyroid cancer. Ann Surg. 1998;227(6):912–21. doi: 10.1097/00000658-199806000-00015. PMCID: 1191404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sanders LE, Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg. 1998;133(4):419–25. doi: 10.1001/archsurg.133.4.419. [DOI] [PubMed] [Google Scholar]
- 121.Van Nguyen K, Dilawari RA. Predictive value of AMES scoring system in selection of extent of surgery in well differentiated carcinoma of thyroid. Am Surg. 1995;61(2):151–5. [PubMed] [Google Scholar]
- 122.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 123.Mazzaferri EL, Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981;70:511–8. doi: 10.1016/0002-9343(81)90573-8. [DOI] [PubMed] [Google Scholar]
- 124.Samaan NA, Schultz PN, Hickey RC, Goepfert H, Haynie TP, Johnston DA, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75(3):714–20. doi: 10.1210/jcem.75.3.1517360. [DOI] [PubMed] [Google Scholar]
- 125.Loh KC, Greenspan FS, Gee L, Miller TR, Yeo PP. Pathological tumor-nodemetastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab. 1997;82(11):3553–62. doi: 10.1210/jcem.82.11.4373. [DOI] [PubMed] [Google Scholar]
- 126.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–42. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 127.Shindo M, Wu JC, Park EE, Tanzella F. The importance of central compartment elective lymph node excision in the staging and treatment of papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. 2006;132(6):650–4. doi: 10.1001/archotol.132.6.650. [DOI] [PubMed] [Google Scholar]
- 128.Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237(3):399–407. doi: 10.1097/01.SLA.0000055273.58908.19. PMCID: 1514312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gemsenjager E, Perren A, Seifert B, Schuler G, Schweizer I, Heitz PU. Lymph node surgery in papillary thyroid carcinoma. J Am Coll Surg. 2003;197(2):182–90. doi: 10.1016/S1072-7515(03)00421-6. [DOI] [PubMed] [Google Scholar]
- 130.Bardet S, Malville E, Rame JP, Babin E, Samama G, De Raucourt D, et al. Macroscopic lymph-node involvement and neck dissection predict lymph-node recurrence in papillary thyroid carcinoma. Eur J Endocrinol. 2008;158(4):551–60. doi: 10.1530/EJE-07-0603. [DOI] [PubMed] [Google Scholar]
- 131.Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007;245(4):604–10. doi: 10.1097/01.sla.0000250451.59685.67. PMCID: 1877043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hughes DT, Doherty GM. Central neck dissection for papillary thyroid cancer. Cancer Control. 2011;18:83–8. doi: 10.1177/107327481101800202. [DOI] [PubMed] [Google Scholar]
- 133.Mazzaferri EL, Doherty GM, Steward DL. The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid. 2009;19:683–9. doi: 10.1089/thy.2009.1578. [DOI] [PubMed] [Google Scholar]
- 134.Melck AL, Yip L, Carty SE. The utility of BRAF testing in the management of papillary thyroid cancer. Oncologist. 2010;15(12):1285–93. doi: 10.1634/theoncologist.2010-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321(1):86–93. doi: 10.1016/j.mce.2009.10.012. PMCID: 2849928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zarebczan B, Mohanty D, Chen H. A Comparison of the LigaSure and harmonic scalpel in thyroid surgery: a single institution review. Ann Surg Oncol. 2011;18(1):214–8. doi: 10.1245/s10434-010-1334-3. PMCID: 3019290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Musunuru S, Schaefer S, Chen H. The use of the Ligasure for hemostasis during thyroid lobectomy. Am J Surg. 2008;195(3):382–4. doi: 10.1016/j.amjsurg.2007.12.009. discussion 4–5. [DOI] [PubMed] [Google Scholar]
- 138.Holt GR, McMurray GT, Joseph DJ. Recurrent laryngeal nerve injury following thyroid operations. Surg Gynecol Obstet. 1977;144(4):567–70. [PubMed] [Google Scholar]
- 139.Wagner HE, Seiler C. Recurrent laryngeal nerve palsy after thyroid gland surgery. Br J Surg. 1994;81(2):226–8. doi: 10.1002/bjs.1800810222. [DOI] [PubMed] [Google Scholar]
- 140.Dackiw AP, Rotstein LE, Clark OH. Computer-assisted evoked electromyography with stimulating surgical instruments for recurrent/external laryngeal nerve identification and preservation in thyroid and parathyroid operation. Surgery. 2002;132(6):1100–6. doi: 10.1067/msy.2002.128483. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 141.Hamelmann WH, Meyer T, Timm S, Timmermann W. [A Critical Estimation of Intraoperative Neuromonitoring (IONM) in Thyroid Surgery] Zentralbl Chir. 2002;127(5):409–13. doi: 10.1055/s-2002-31982. [DOI] [PubMed] [Google Scholar]
- 142.Dralle H, Sekulla C, Haerting J, Timmermann W, Neumann HJ, Kruse E, et al. Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery. 2004;136(6):1310–22. doi: 10.1016/j.surg.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 143.Thomusch O, Sekulla C, Machens A, Neumann HJ, Timmermann W, Dralle H. Validity of intra-operative neuromonitoring signals in thyroid surgery. Langenbecks Arch Surg. 2004;389(6):499–503. doi: 10.1007/s00423-003-0444-9. [DOI] [PubMed] [Google Scholar]
- 144.Carty SE, Cooper DS, Doherty GM, Duh QY, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19:1153–8. doi: 10.1089/thy.2009.0159. [DOI] [PubMed] [Google Scholar]
- 145.Kuppersmith RB, Holsinger FC. Robotic thyroid surgery: An initial experience with North American patients. Laryngoscope. 2011;121(3):521–6. doi: 10.1002/lary.21347. [DOI] [PubMed] [Google Scholar]
- 146.Lang BH, Lo CY. Technological innovations in surgical approach for thyroid cancer. J Oncol. 2010 doi: 10.1155/2010/490719. 2010. PMCID: 2926682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kang SW, Lee SC, Lee SH, Lee KY, Jeong JJ, Lee YS, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery. 2009;146(6):1048–55. doi: 10.1016/j.surg.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 148.Lee J, Nah KY, Kim RM, Ahn YH, Soh EY, Chung WY. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc. 2010;24(12):3186–94. doi: 10.1007/s00464-010-1113-z. [DOI] [PubMed] [Google Scholar]
- 149.Perrier ND, Randolph GW, Inabnet WB, Marple BF, VanHeerden J, Kuppersmith RB. Robotic thyroidectomy: a framework for new technology assessment and safe implementation. Thyroid. 2010;20(12):1327–32. doi: 10.1089/thy.2010.1666. [DOI] [PubMed] [Google Scholar]
- 150.Ryu HR, Kang SW, Lee SH, Rhee KY, Jeong JJ, Nam KH, et al. Feasibility and safety of a new robotic thyroidectomy through a gasless, transaxillary single-incision approach. J Am Coll Surg. 2010;211(3):e13–9. doi: 10.1016/j.jamcollsurg.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 151.Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8(11):1228–74. doi: 10.6004/jnccn.2010.0093. [DOI] [PubMed] [Google Scholar]
- 152.Reiners C, Dietlein M, Luster M. Radio-iodine therapy in differentiated thyroid cancer: indications and procedures. Best Pract Res Clin Endocrinol Metab. 2008;22(6):989–1007. doi: 10.1016/j.beem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 153.Mazzaferri EL, Kloos RT. Using recombinant human TSH in the management of well-differentiated thyroid cancer: current strategies and future directions. Thyroid. 2000;10(9):767–78. doi: 10.1089/thy.2000.10.767. [DOI] [PubMed] [Google Scholar]
- 154.Sawka AM, Thephamongkhol K, Brouwers M, Thabane L, Browman G, Gerstein HC. Clinical review 170: A systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004;89(8):3668–76. doi: 10.1210/jc.2003-031167. [DOI] [PubMed] [Google Scholar]
- 155.Ma C, Xie J, Liu W, Wang G, Zuo S, Wang X, et al. Recombinant human thyrotropin (rhTSH) aided radioiodine treatment for residual or metastatic differentiated thyroid cancer. Cochrane Database Syst Rev. 2010;(11):CD008302. doi: 10.1002/14651858.CD008302.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Klubo-Gwiezdzinska J, Burman KD, Van Nostrand D, Mete M, Jonklaas J, Wartofsky L. Radioiodine treatment of metastatic thyroid cancer: relative efficacy and side effect profile of preparation by thyroid hormone withdrawal versus recombinant human thyrotropin. Thyroid. 2012;22(3):310–7. doi: 10.1089/thy.2011.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16(12):1229–42. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 158.Chow SM, Law SC, Au SK, Leung TW, Chan PT, Mendenhall WM, et al. Differentiated thyroid carcinoma: comparison between papillary and follicular carcinoma in a single institute. Head Neck. 2002;24(7):670–7. doi: 10.1002/hed.10080. [DOI] [PubMed] [Google Scholar]
- 159.Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93(2):504–15. doi: 10.1210/jc.2007-1154. [DOI] [PubMed] [Google Scholar]
- 160.Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117(19):4439–46. doi: 10.1002/cncr.26070. PMCID: 3155861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen AY, Levy L, Goepfert H, Brown BW, Spitz MR, Vassilopoulou-Sellin R. The development of breast carcinoma in women with thyroid carcinoma. Cancer. 2001;92(2):225–31. doi: 10.1002/1097-0142(20010715)92:2<225::aid-cncr1313>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 162.Mandel SJ, Mandel L. Radioactive iodine and the salivary glands. Thyroid. 2003;13(3):265–71. doi: 10.1089/105072503321582060. [DOI] [PubMed] [Google Scholar]
- 163.Wiklund TA, Blomqvist CP, Raty J, Elomaa I, Rissanen P, Miettinen M. Postirradiation sarcoma. Analysis of a nationwide cancer registry material. Cancer. 1991;68(3):524–31. doi: 10.1002/1097-0142(19910801)68:3<524::aid-cncr2820680313>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 164.Sawka AM, Brierley JD, Tsang RW, Thabane L, Rotstein L, Gafni A, et al. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol Metab Clin North Am. 2008;37:457–80. doi: 10.1016/j.ecl.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 165.Almeida JP, Sanabria AE, Lima EN, Kowalski LP. Late side effects of radioactive iodine on salivary gland function in patients with thyroid cancer. Head Neck. 2010 doi: 10.1002/hed.21520. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 166.Cady B, Sedgwick CE, Meissner WA, Bookwalter JR, Romagosa V, Werber J. Changing clinical, pathologic, therapeutic, and survival patterns in differentiated thyroid carcinoma. Ann Surg. 1976;184(5):541–53. doi: 10.1097/00000658-197611000-00003. PMCID: 1345475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McGriff NJ, Csako G, Gourgiotis L, Lori CG, Pucino F, Sarlis NJ. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med. 2002;34(7–8):554–64. doi: 10.1080/078538902321117760. [DOI] [PubMed] [Google Scholar]
- 168.Brierley JD, Tsang RW. External-beam radiation therapy in the treatment of differentiated thyroid cancer. Semin Surg Oncol. 1999;16(1):42–9. doi: 10.1002/(sici)1098-2388(199901/02)16:1<42::aid-ssu8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 169.Tennvall J, Lundell G, Hallquist A, Wahlberg P, Wallin G, Tibblin S. Combined doxorubicin, hyperfractionated radiotherapy, and surgery in anaplastic thyroid carcinoma. Report on two protocols. The Swedish Anaplastic Thyroid Cancer Group. Cancer. 1994;74(4):1348–54. doi: 10.1002/1097-0142(19940815)74:4<1348::aid-cncr2820740427>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 170.Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, DeConti R. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. 1985;56(9):2155–60. doi: 10.1002/1097-0142(19851101)56:9<2155::aid-cncr2820560903>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 171.Williams SD, Birch R, Einhorn LH. Phase II evaluation of doxorubicin plus cisplatin in advanced thyroid cancer: a Southeastern Cancer Study Group Trial. Cancer Treat Rep. 1986;70(3):405–7. [PubMed] [Google Scholar]
- 172.Higgins MJ, Forastiere A, Marur S. New directions in the systemic treatment of metastatic thyroid cancer. Oncology (Williston Park) 2009;23(9):768–75. [PubMed] [Google Scholar]
- 173.Kim DW, Jo YS, Jung HS, Chung HK, Song JH, Park KC, et al. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab. 2006;91(10):4070–6. doi: 10.1210/jc.2005-2845. [DOI] [PubMed] [Google Scholar]
- 174.Haugen BR, Kane MA. Approach to the thyroid cancer patient with extracervical metastases. J Clin Endocrinol Metab. 2010;95(3):987–93. doi: 10.1210/jc.2009-2305. [DOI] [PubMed] [Google Scholar]
- 175.Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90(12):6373–9. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 176.Ouyang B, Knauf JA, Smith EP, Zhang L, Ramsey T, Yusuff N, et al. Inhibitors of Raf kinase activity block growth of thyroid cancer cells with RET/PTC or BRAF mutations in vitro and in vivo. Clin Cancer Res. 2006;12(6):1785–93. doi: 10.1158/1078-0432.CCR-05-1729. [DOI] [PubMed] [Google Scholar]
- 177.Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26(29):4714–9. doi: 10.1200/JCO.2008.16.3279. PMCID: 2653134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27(10):1675–84. doi: 10.1200/JCO.2008.18.2717. PMCID: 2668972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 180.Perez CA, Santos ES, Arango BA, Raez LE, Cohen EE. Novel molecular targeted therapies for refractory thyroid cancer. Head Neck. 2012;34(5):736–45. doi: 10.1002/hed.21755. [DOI] [PubMed] [Google Scholar]
- 181.Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359(1):31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 182.Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26(29):4708–13. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Workman JL, Kingston RE. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem. 1998;67:545–79. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- 184.Cress WD, Seto E. Histone deacetylases, transcriptional control, and cancer. J Cell Physiol. 2000;184(1):1–16. doi: 10.1002/(SICI)1097-4652(200007)184:1<1::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 185.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1(3):194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 186.Mitsiades CS, Poulaki V, McMullan C, Negri J, Fanourakis G, Goudopoulou A, et al. Novel histone deacetylase inhibitors in the treatment of thyroid cancer. Clin Cancer Res. 2005;11(10):3958–65. doi: 10.1158/1078-0432.CCR-03-0776. [DOI] [PubMed] [Google Scholar]
- 187.Luong QT, O'Kelly J, Braunstein GD, Hershman JM, Koeffler HP. Antitumor activity of suberoylanilide hydroxamic acid against thyroid cancer cell lines in vitro and in vivo. Clin Cancer Res. 2006;12(18):5570–7. doi: 10.1158/1078-0432.CCR-06-0367. [DOI] [PubMed] [Google Scholar]
- 188.Sandor V, Bakke S, Robey RW, Kang MH, Blagosklonny MV, Bender J, et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res. 2002;8(3):718–28. [PubMed] [Google Scholar]
- 189.Fortunati N, Catalano MG, Arena K, Brignardello E, Piovesan A, Boccuzzi G. Valproic acid induces the expression of the Na+/I- symporter and iodine uptake in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 2004;89(2):1006–9. doi: 10.1210/jc.2003-031407. [DOI] [PubMed] [Google Scholar]
- 190.Xiao X, Ning L, Chen H. Notch1 mediates growth suppression of papillary and follicular thyroid cancer cells by histone deacetylase inhibitors. Mol Cancer Ther. 2009;8(2):350–6. doi: 10.1158/1535-7163.MCT-08-0585. PMCID: 2673961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shen WT, Wong TS, Chung WY, Wong MG, Kebebew E, Duh QY, et al. Valproic acid inhibits growth, induces apoptosis, and modulates apoptosis-regulatory and differentiation gene expression in human thyroid cancer cells. Surgery. 2005;138(6):979–84. doi: 10.1016/j.surg.2005.09.019. discussion 84–5. [DOI] [PubMed] [Google Scholar]
- 192.Pinchot SN, Kunnimalaiyaan M, Sippel RS, Chen H. Medullary thyroid carcinoma: targeted therapies and future directions. J Oncol. 2009;2009:183031. doi: 10.1155/2009/183031. PMCID: 2798103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Williams ED. Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol. 1966;19(2):114–8. doi: 10.1136/jcp.19.2.114. PMCID: 473198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hill CS, Jr., Ibanez ML, Samaan NA, Ahearn MJ, Clark RL. Medullary (solid) carcinoma of the thyroid gland: an analysis of the M. D. Anderson hospital experience with patients with the tumor, its special features, and its histogenesis. Medicine(Baltimore) 1973;52(2):141–71. [PubMed] [Google Scholar]
- 195.Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13(5):539–47. doi: 10.1634/theoncologist.2007-0239. [DOI] [PubMed] [Google Scholar]
- 196.Chen H, Sippel RS, O'Dorisio MS, Vinik AI, Lloyd RV, Pacak K. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas. 2010;39(6):775–83. doi: 10.1097/MPA.0b013e3181ebb4f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Williams ED, Brown CL, Doniach I. Pathological and clinical findings in a series of 67 cases of medullary carcinoma of the thyroid. J Clin Pathol. 1966;19(2):103–13. doi: 10.1136/jcp.19.2.103. PMCID: 473194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Boi F, Maurelli I, Pinna G, Atzeni F, Piga M, Lai ML, et al. Calcitonin measurement in wash-out fluid from fine needle aspiration of neck masses in patients with primary and metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92(6):2115–8. doi: 10.1210/jc.2007-0326. [DOI] [PubMed] [Google Scholar]
- 199.Chin WW, Goodman RH, Jacobs JW, Wolfe HJ, Daniels GH, Habener JF. Medullary thyroid carcinoma identified by cell-free translation of tumor messenger ribonucleic acid in a patient with a neck mass and the syndrome of ectopic adrenocorticotropin. J Clin Endocrinol Metab. 1981;52(3):572–5. doi: 10.1210/jcem-52-3-572. [DOI] [PubMed] [Google Scholar]
- 200.Costante G, Meringolo D, Durante C, Bianchi D, Nocera M, Tumino S, et al. Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. J Clin Endocrinol Metab. 2007;92(2):450–5. doi: 10.1210/jc.2006-1590. [DOI] [PubMed] [Google Scholar]
- 201.Machens A, Schneyer U, Holzhausen HJ, Dralle H. Prospects of remission in medullary thyroid carcinoma according to basal calcitonin level. J Clin Endocrinol Metab. 2005;90(4):2029–34. doi: 10.1210/jc.2004-1836. [DOI] [PubMed] [Google Scholar]
- 202.Machens A, Dralle H. Pretargeted anti-carcinoembryonic-antigen radioimmunotherapy for medullary thyroid carcinoma. J Clin Oncol. 2006;24(20):e37. doi: 10.1200/JCO.2006.06.8171. author reply e8. [DOI] [PubMed] [Google Scholar]
- 203.de Groot JW, Kema IP, Breukelman H, van der Veer E, Wiggers T, Plukker JT, et al. Biochemical markers in the follow-up of medullary thyroid cancer. Thyroid. 2006;16(11):1163–70. doi: 10.1089/thy.2006.16.1163. [DOI] [PubMed] [Google Scholar]
- 204.Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107:2134–42. doi: 10.1002/cncr.22244. [DOI] [PubMed] [Google Scholar]
- 205.Wells SA, Jr., Chi DD, Toshima K, Dehner LP, Coffin CM, Dowton SB, et al. Predictive DNA testing and prophylactic thyroidectomy in patients at risk for multiple endocrine neoplasia type 2A. Ann Surg. 1994;220(3):237–47. doi: 10.1097/00000658-199409000-00002. discussion 47–50. PMCID: 1234374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kouvaraki MA, Shapiro SE, Perrier ND, Cote GJ, Gagel RF, Hoff AO, et al. RET proto-oncogene: a review and update of genotype-phenotype correlations in hereditary medullary thyroid cancer and associated endocrine tumors. Thyroid. 2005;15(6):531–44. doi: 10.1089/thy.2005.15.531. [DOI] [PubMed] [Google Scholar]
- 207.Ogilvie JB, Kebebew E. Indication and timing of thyroid surgery for patients with hereditary medullary thyroid cancer syndromes. J Natl Compr Canc Netw. 2006;4(2):139–47. doi: 10.6004/jnccn.2006.0014. [DOI] [PubMed] [Google Scholar]
- 208.Moley JF, DeBenedetti MK. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg. 1999;229(6):880–7. doi: 10.1097/00000658-199906000-00016. discussion 7–8. PMCID: 1420836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Greenblatt DY, Elson D, Mack E, Chen H. Initial lymph node dissection increases cure rates in patients with medullary thyroid cancer. Asian J Surg. 2007;30(2):108–12. doi: 10.1016/S1015-9584(09)60141-X. [DOI] [PubMed] [Google Scholar]
- 210.Machens A, Hauptmann S, Dralle H. Increased risk of lymph node metastasis in multifocal hereditary and sporadic medullary thyroid cancer. World J Surg. 2007;31(10):1960–5. doi: 10.1007/s00268-007-9185-1. [DOI] [PubMed] [Google Scholar]
- 211.Machens A, Dralle H. Genotype-phenotype based surgical concept of hereditary medullary thyroid carcinoma. World J Surg. 2007;31(5):957–68. doi: 10.1007/s00268-006-0769-y. [DOI] [PubMed] [Google Scholar]
- 212.Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19(6):565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 213.Tischler AS, Khan A, DeLellis RA. Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med. 2005;353(26):2817–8. doi: 10.1056/NEJMc052744. author reply -8. [DOI] [PubMed] [Google Scholar]
- 214.Chen H, Roberts JR, Ball DW, Eisele DW, Baylin SB, Udelsman R, et al. Effective long-term palliation of symptomatic, incurable metastatic medullary thyroid cancer by operative resection. Ann Surg. 1998;227(6):887–95. doi: 10.1097/00000658-199806000-00012. PMCID: 1191398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ball DW. Medullary thyroid cancer: therapeutic targets and molecular markers. Curr Opin Oncol. 2007;19(1):18–23. doi: 10.1097/CCO.0b013e32801173ea. [DOI] [PubMed] [Google Scholar]
- 216.Wells SA, Jr., Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–41. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.de Groot JW, Zonnenberg BA, van Ufford-Mannesse PQ, de Vries MM, Links TP, Lips CJ, et al. A phase II trial of imatinib therapy for metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92(9):3466–9. doi: 10.1210/jc.2007-0649. [DOI] [PubMed] [Google Scholar]
- 218.Langmuir PB, Yver A. Vandetanib for the treatment of thyroid cancer. Clin Pharmacol Ther. 2012;91(1):71–80. doi: 10.1038/clpt.2011.272. [DOI] [PubMed] [Google Scholar]


