Abstract
Pseudoxanthoma elasticum (PXE) is an autosomal recessive disorder manifesting with ectopic connective tissue mineralization, caused by mutations in the ABCC6 gene, ~35% of all mutations being premature termination mutations. In this study, we investigated the therapeutic potential of the nonsense codon read-through-inducing drug, PTC124, in treating PXE. The ability of this drug to facilitate read-through of nonsense mutations was examined in HEK293 cells transfected with human ABCC6 expression constructs harboring seven different PXE associated nonsense mutations, and evaluated by immunofluorescence and In-Cell ELISA. Our data demonstrated that PTC124 did not exhibit cell toxicity in concentrations up to 40 µg/ml, and the facilitated read-through was not only dose dependent but also sequence context dependent. Considering the redundancy of the genetic code, it was postulated that in case of the most common recurrent nonsense mutation, p.R1141X, the read-through may result in substitution of the arginine 1141 by either glycine, tryptophan or cysteine. Their potential pathogenicity was tested in a recently developed zebrafish mRNA rescue assay, and demonstrated that all three mRNA transcripts were able to rescue abcc6a morpholino-induced phenotype of zebrafish. Thus, our results suggest that read-through of nonsense mutations in ABCC6 by PTC124 may have potential for pharmacologic treatment of PXE.
INTRODUCTION
Pseudoxanthoma elasticum (PXE) is a multi-system heritable disorder characterized by ectopic mineralization in the skin, eyes, and the vascular systems (Neldner, 1988; Uitto et al., 2010). The early cutaneous findings consist of yellowish papules on the predilection sites, i.e., sides of the neck and antecubital fossae, coalescing into inelastic plaques of leathery skin which can extend to involve the entire integument. While the skin findings are primarily of cosmetic concern, they signify the possibility of development of serious, debilitating, and occasionally life-threatening complications in the eyes and the cardiovascular system. The characteristic ophthalmologic finding in patients with PXE is the presence of angioid streaks, and after the age of 30 years their prevalence approaches 100%. Angioid streaks derive from breaks upon aberrant mineralization of the elastic lamina of Bruch’s membrane, which separates the pigmented layer of the retina from the choroid of the eye. These fractures lead to neovascularization from choriocapillaris, and subsequent leakage of newly formed vessels leads to hemorrhage and scarring. These progressive pathologic changes cause loss of visual acuity and eventually lead to central blindness if left untreated (Finger et al., 2009; Georgalas et al., 2011). The cardiovascular involvement, primarily reflecting mineralization of medium-sized arterial blood vessels, manifests with hypertension, intermittent claudication, bleeding from the gastrointestinal vessels, and, occasionally, myocardial infarcts and strokes.
PXE is caused by mutations in the ABCC6 gene which encodes a transmembrane efflux transporter protein, ABCC6, expressed primarily in the liver and the kidneys (Belinsky and Kruh, 1999). The precise function of this protein remains to be disclosed, however, the critical role of the ABCC6 gene in the pathogenesis of PXE has been confirmed by development of transgenic mice through targeted ablation of the corresponding mouse gene (Gorgels et al., 2005; Klement et al., 2005). Close to 600 distinct mutations representing well over 1000 mutant alleles have so far been encountered in the ABCC6 gene (http://www.ncbi.nlm.nih.gov/lovd/home.php?select_db=ABCC6). While the repertoire of these distinct mutations is varied, approximately 35% of all mutations are nonsense mutations which result in synthesis of truncated, non-functional ABCC6 protein. The most common recurrent nonsense mutation is p.R1141X which accounts for ~30% of all pathogenic PXE mutations in Caucasian patient populations.
Recently, pharmaceutical compounds have been developed to read through premature termination codons which allow synthesis of full-length, potentially functional protein. These include 1,2,4-oxadiazole, known as PTC124, a non-aminoglycoside nonsense mutation suppressor (Du et al., 2008; Peltz et al., 2013; Welch et al., 2007). The pharmacologic safety of this molecule has already been established, and clinical trials have been initiated to examine its effectiveness in treatment of cystic fibrosis and muscular dystrophy in cases caused by nonsense mutations (Finkel, 2010; Hirawat et al., 2007; Wilschanski et al., 2011). In this study, we have examined the efficacy of PTC124 in suppressing a number of nonsense mutations in the ABCC6 gene.
RESULTS
In vitro assay system for ABCC6 expression
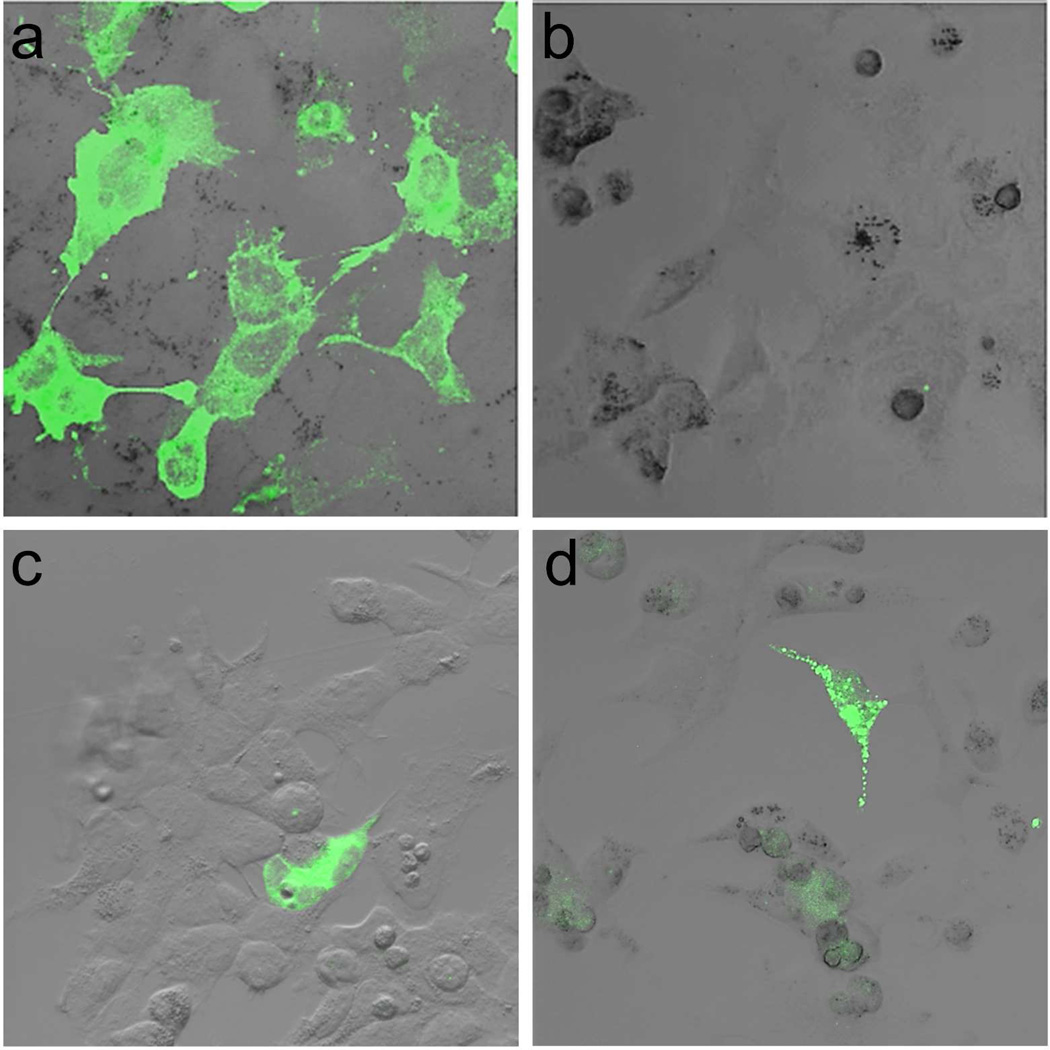
A total of 25 distinct nonsense mutations have been encountered in the ABCC6 gene in patients with PXE. In this study, we focused on seven of them, including the most common stop codon mutation, p.R1141X, in which the arginine at position 1141 (codon CGA) has been replaced by a stop codon (TGA) (Table 1). We first constructed an ABCC6 expression vector by cloning full-length human ABCC6 cDNA with a 3’-end DDK tag into the pCMV6-Entry vector. The expression of the full-length ABCC6 protein from this vector was examined by transfecting a number of different cell lines, including HEK293, NIH3T3, HeLa and MLE-10 cells, followed by immuno detection of the DDK tag at the carboxy terminal end of the newly synthesized protein using a specific antibody. While this vector was readily expressed in a number of cell lines tested, the best transfection and translation efficiency was noted in HEK293 cells (a human embryonic kidney epithelial cell line) (Figure 1a). We subsequently tested an expression vector containing the mutant ABCC6 cDNA into which different premature stop codon mutations were introduced by site-directed mutagenesis (Table 1). Transfection of the mutant constructs into HEK293 cells did not show any evidence of synthesis of full-length protein, although the transfection efficiency, as determined by parallel transfection with a CMV-EGFP construct, was comparable to that noted with the wild-type construct (Figure 1b).
Figure 1. Expression of wild-type and mutant human ABCC6 cDNA, and demonstration that PTC124 induces read-through of a stop codon.
An expression vector (p.CMV6-Entry) containing full-length wild-type human ABCC6 cDNA (a) or a corresponding mutant cDNA harboring p.R1141X premature termination codon (b,c) containing a 3’-end sequence which encodes a DDK reporter peptide, or p.R1275X (d) were transfected into HEK293 cells in culture without (a,b) or with (c,b) PTC124 (10 µg/ml for 72 hours). The cell cultures were then stained with an anti-DDK antibody for the expression of full-length polypeptide. Note relatively low level of expression in some cells (asterisks).
PTC124 induces read-though of nonsense mutations in ABCC6
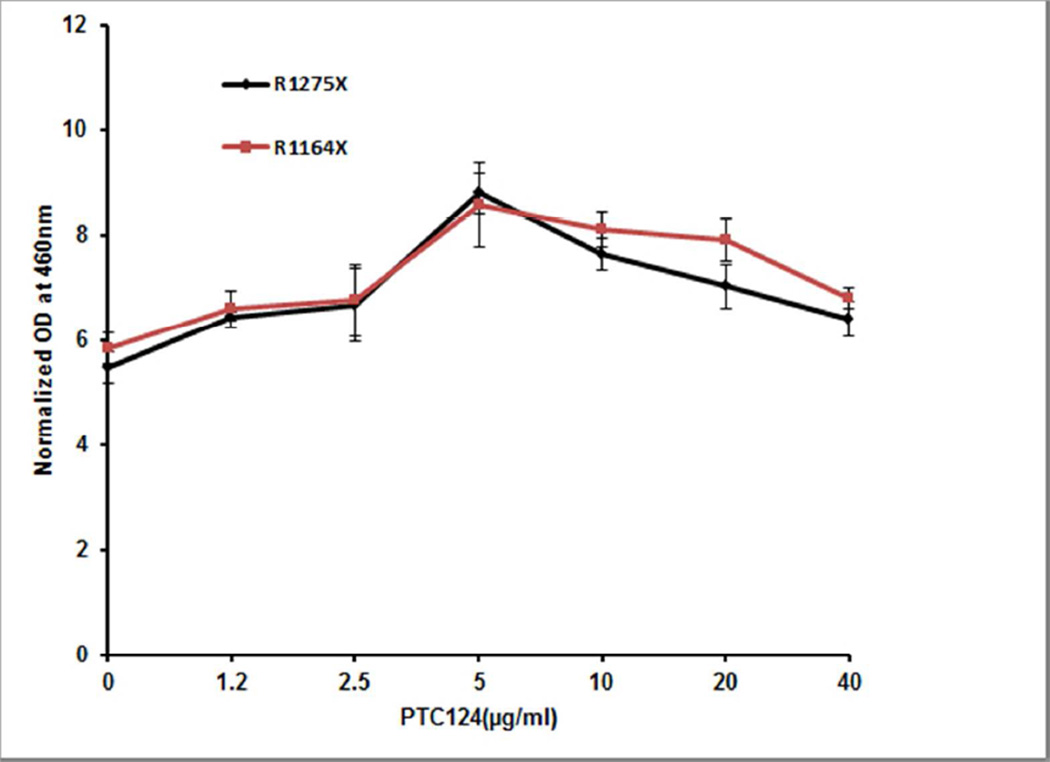
PTC124, a non-aminoglycoside premature termination codon suppressor, was then added to the incubation medium of cells transfected with mutant constructs. First, testing of PTC124 in different concentrations up to 72 hours of incubation indicated facilitation of the synthesis of full-length ABCC6 polypeptide, as determined by immunostaining with an anti-DDK antibody (Figure 1c,d). Testing of two mutant constructs, p.R1164X and p.R1275X, in the presence of varying concentrations of PTC124 indicated that the highest level of expression was noted with 5 µg/ml, as quantitated by In-Cell ELISA (Figure 2). Subsequently, all mutant ABCC6 expression constructs were tested for the read-through efficacy of PTC124 at 5 µg/ml at 48 or 72 hours of incubation. The results indicated varying degrees of enhancement with different mutant constructs at 48 hours, but in each case, the amount of full-length protein was increased when the incubation was continued up to 72 hours (Figure 3). Thus, PTC124 is able to induce the read-through of a number of termination codon mutations in ABCC6 in a time-dependent manner, but the degree of enhancement appears to be context dependent, influenced by the precise nucleotide sequence of the stop codon and the immediate 3’ nucleotide present in the sequence (Howard et al., 2000; Rowe and Clancy, 2009).
Figure 2. Read-through efficiency of stop codon mutations p.R1275X and p.R1164X by PTC124 in different concentrations.
HEK293 cells were transfected with expression vectors containing full-length human ABCC6 cDNA harboring one of the two nonsense mutations. The cells were placed on medium containing PTC124 in concentrations indicated, and the read-through of full-length polypeptide was quantitated by an In-Cell ELISA with an anti-DDK antibody. The values represent determinations from three independent cultures (mean ± SEM).
Figure 3. Read-through efficiency of different nonsense mutations by PTC124 (5 µg/ml) at 48 and 72 hrs of incubation.
HEK293 cells were transfected with the mutant expression vectors as indicated in the legend to Figure 2. The nucleotide sequences of the stop codon mutations are shown in Table 1. The expression of full-length polypeptide was quantitated by an In-Cell ELISA.
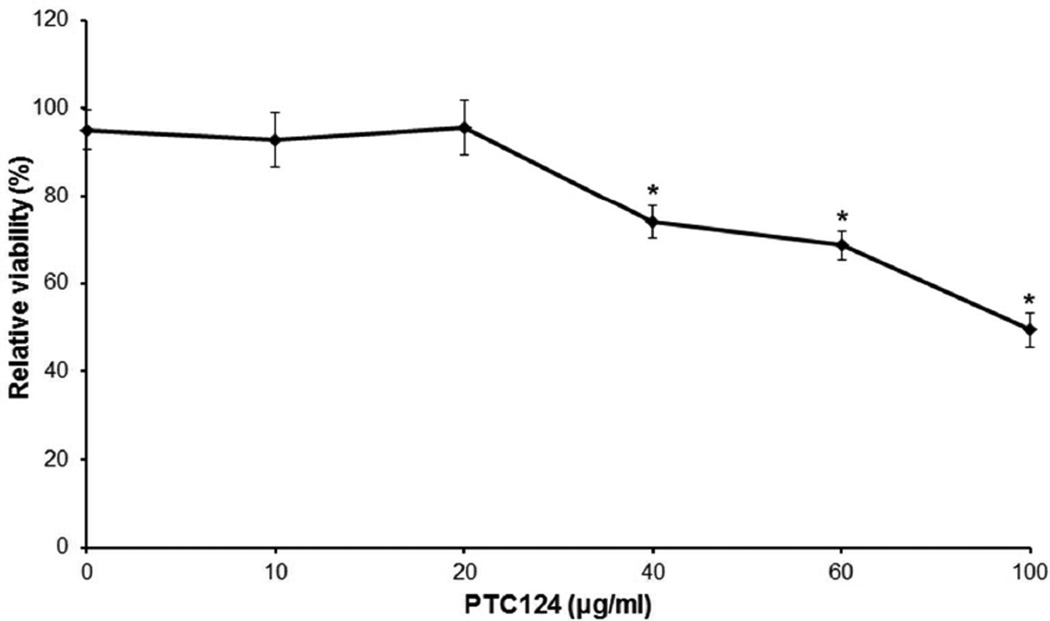
Since PTC124 was more efficient in inducing the read-through at 5 µg/ml than at 10 or 20 µg/ml concentration, subsequent experiments were performed to examine the toxicity of PTC124 in cell culture at different concentrations (0–100 µg/ml) up to 72 hours of incubation. The viability of HEK293 cells was determined by MTT colorimetric assay. The results indicated that PTC124 at the concentrations 0–20 µg/ml did not affect the viability of the cells in comparison to cells incubated with the vehicle (DMSO). At concentrations over 40 µg/ml, PTC124 elicited a statistically significant reduction of cell viability (p < 0.05) (Figure 4).
Figure 4. Demonstration that PTC124 is not toxic to the cells in culture up to 40 µg/ml.
HEK293 cells were incubated for 72 hrs with varying concentrations of PTC124, and the viability of cells was determined by MTT colorimetric assay. PTC124 in the concentration range of 0–20 µg/ml did not affect the viability of the cells after normalization to the viability of cells incubated with the corresponding concentration of vehicle (DMSO) in parallel. At concentrations 40, 60 and 100 µg/ml, PTC124 elicited a statistically significant reduction of cell viability (*P < 0.05; Student’s t-test) (mean ± SEM; n = 3).
A zebrafish system to test the functionality of ABCC6 polypeptides
While the precise mechanisms of PTC124 in facilitating read-through of stop codon mutations in the ABCC6 gene are currently unknown, it apparently replaces the stop codon with another amino acid through the mispairing of a near-cognate aminoacyl tRNA (Nilsson and Ryden-Aulin, 2003). In case of the p.R1141X mutation, considering the codon redundancy and possible combinations of the corrected mutations from the stop codon (TGA) to an amino acid, in addition to arginine (CGA) this position could be occupied either by cysteine (TGC), tryptophan (TGG) or glycine (GGA). Since a number of missense mutations have also been encountered in ABCC6 in patients with PXE, there is a possibility, at least theoretical, that the full-length polypeptide synthesized in the presence of PTC124 is nonfunctional.
In order to test the functionality of the full-length ABCC6 protein synthesized in the presence of PTC124, which instead of an arginine in position 1141 could contain cysteine, tryptophan or glycine, we utilized a zebrafish mRNA rescue system recently developed by us (Li et al., 2011). In this system, injection of 1 to 4-cell zebrafish embryos with a morpholino corresponding to the exon 7/intron 7 border of the abcc6a gene decreases the gene expression by up to 80% and induces a phenotype of pericardial edema and curled tail associated with early demise. Microinjecting the embryos with full-length human ABCC6 mRNA together with the morpholino rescues this phenotype (Li et al., 2010) (Figure 5). In contrast, injection of human ABCC6 mRNA harboring the p.R1141X stop codon mutation failed to reverse the phenotype (Figure 5, Table 2), suggesting that this mRNA rescue system can be utilized to check the pathogenicity of mutant human ABCC6 proteins, including those harboring the missense amino acids as a result of PTC124 read-through. We, therefore, engineered mutant human ABCC6 constructs which encode either cysteine, tryptophan or glycine in position 1141 using site-directed mutagenesis kit, and these mutant mRNAs were injected into zebrafish embryos in parallel to wild-type mRNA containing arginine in the corresponding position. Quantitation of embryonic lethality allowed us to determine whether the read-through ABCC6 protein is functional or not in rescuing the zebrafish phenotype. The results indicated that the lethality of the embryos injected with the abcc6a morpholino at day 1 post-fertilization was around 70% but this was reversed to the control level (~10–12%) by the human wild-type mRNA harboring arginine at position 1141 or as noted in embryos injected with universal standard control morpholino (Table 2). Similarly, the constructs harboring either cysteine, tryptophan or glycine in place of arginine reversed the morpholino induced lethality (Figure 5 and Table 2).
Figure 5. The zebrafish ABCC6 mRNA rescue system.
Injection of 1 to 4-cell zebrafish embryos with a morpholino targeting the abcc6a gene results in a profound phenotype consisting of pericardial edema and curled tail at 3 days post fertilization (b), as compared to control embryos injected with biologically inactive standard control morpholino (a). Injection of the wild-type (WT) human ABCC6 mRNA together with the morpholino essentially rescues the phenotype (c). Similarly, injection of human ABCC6 mRNA harboring glycine (e) or cysteine (f) at position 1141 reversed the morpholino induced phenotype. However, injection of human ABCC6 mRNA which harbors the p.R1141X mutation does not result in phenotypic rescue (d).
DISCUSSION
The overall goal of this study is to develop a pharmacologic treatment for a subset of patients with PXE, viz., those harboring nonsense mutations in one or both ABCC6 alleles. Our data, which demonstrated read-through of a stop codon in the presence of PTC124, attest to the feasibility of this approach. These data demonstrated significant expression of the full-length polypeptide under the in vitro culture conditions tested by PTC124, with the maximum effect at 5 µg/ml concentration. Increasing the concentration of PTC124 did not increase the read-through, consistent with findings by others (Welch et al., 2007). It should be noted that PTC124 at this concentration resulted only in partial correction of the ABCC6 expression. While the precise level of expression of ABCC6 needed in individual patients to prevent ectopic mineralization is currently unknown, at least 50% level, and possibly much lower, is sufficient as attested to by the fact that heterozygous carriers of a mutation do not develop mineralization phenotypes (Uitto et al., 2011). In further studies, the in vivo efficacy of PTC124 can be tested in a preclinical animal model of knock-in mice harboring human ABCC6 with p.R1141X mutations in both alleles on Abcc6−/− background.
While PTC124 has been shown to facilitate read-through of premature termination codons in a number of genes, the information on the amino acid that substitutes the stop codon is not known in case of ABCC6. It is conceivable that the presence of such an amino acid carried by a near-cognate tRNA (instead of the parent wild-type arginine in case of p.R1141X) could be pathogenic, essentially representing a missense mutation. To test the potential pathogenicity of such amino acid substitutions, we have developed an innovative zebrafish mRNA rescue system that will provide information as to whether the amino acid substitution in ABCC6 is pathogenic or not (Li et al., 2011). Our results showed that substitution of arginine at position 1141 by cysteine, tryptophan or glycine did not alter the functionality of the protein. It should be noted that this innovative zebrafish mRNA rescue system should be applicable to other proteins as well, an issue that has not been addressed in development of similar pharmacologic approaches for other heritable diseases.
PTC124 is an orally bioavailable compound that promotes read-through of nonsense mutations demonstrated in a number of cell culture and mouse model systems (Kayali et al., 2012; Peltz et al., 2013; Welch et al., 2007). The pharmacokinetics and safety of this compound in healthy adult volunteers have been documented in single dose and multi-dose studies with doses ranging from 10–50 mg per kg per dose twice per day for 14 days (Hirawat et al., 2007). PTC124 administered orally was well tolerated as a single dose up to 100 mg per kg. At higher doses, 150 and 200 mg per kg, PTC124 induced mild headache, dizziness, and gastrointestinal events. With multiple doses of 50 mg per kg per dose twice a day, no significant side effects were detected and there was no evidence of protein elongation due to non-specific ribosomal read-through of normal stop codons (Hirawat et al., 2007). Collectively, these studies suggested that PTC124 is safe for clinical trials.
Concomitant to these initial safety studies, the efficacy of PTC124 was tested in mouse models, including a model for cystic fibrosis harboring the CFTR-G542X stop codon mutation containing transgene (Du et al., 2008). Feeding these mice with PTC124 resulted in the appearance of human CFTR and restored 24–29% of its activity, i.e., cAMP-stimulated trans-epithelial chloride transport, in comparison to wild-type mice. These authors suggested that in light of its oral bioavailability, safety, toxicology profile in animal studies, and efficacy with other nonsense alleles, PTC124 has the potential to be an important therapeutic agent for the treatment of inherited diseases caused by nonsense mutations. Similar studies have demonstrated the efficacy of PTC124 in promoting dystrophin production in primary muscle cells from patients with muscular dystrophy and from mdx mice expressing dystrophin nonsense alleles (Finkel, 2010; Kayali et al., 2012). Based on these considerations, clinical trials have been initiated to test the efficacy of PTC124 for treatment of cystic fibrosis, a disease in which approximately 10% of the subjects harbor a premature stop codon mutation (Wilschanski et al., 2011). While the study on cystic fibrosis is ongoing, another study is currently recruiting patients with Duchenne’s muscular dystrophy caused by premature stop codon mutations in the dystrophin gene (see ClinicalTrials.gov). Finally, the feasibility of PTC124-mediated read-through of nonsense mutation in genes associated with a number of metabolic disorders (see e.g., (Ho et al., 2013; Sanchez-Alcudia et al., 2012; Tan et al., 2011) as well as in hereditary cancer syndromes (Bordeira-Carrico et al., 2012) has been tested.
It should be noted that while PXE is considered to be a rare disease (<200,000 affected individuals in the U.S.A.), its estimated prevalence, ~1:50,000, suggests that there are at least 7,000 affected individuals in the United States, and if the same prevalence is extrapolated to the global level, as many as 150,000 individuals are affected with PXE in the world (Uitto, 2012). Considering the fact that 100% of patients with PXE develop angioid streaks, and the majority of them encounter loss of visual acuity eventually leading to blindness if left untreated, successful therapy of these patients with PTC124 would potentially prevent blindness in thousands of individuals worldwide. Furthermore, prevention or amelioration of vascular complications would significantly reduce the morbidity and mortality associated with this, currently intractable, disorder.
MATERIALS AND METHODS
Reagents, plasmid and site-directed mutagenesis
PTC124 (Ataluren) was purchased from Selleckchem (Houston, TX) and dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich, Deisenhofen, Germany) just prior to use. A full-length wild-type human ABCC6 cDNA linked to a nucleotide sequence encoding DDK peptide tag at the 3’ end was cloned into pCMV6-Entry expression vector purchased from OriGene (RC211938, Rockville, MD). Using this construct as a template, seven different ABCC6 nonsense DNA-constructs were obtained by site-directed mutagenesis following the manufacturer’s instructions (Agilent, Santa Clara, CA).
Cell culture and transfection
HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (Gibco BRL, Grand Island, NY), and incubated at 37°C under 5% CO2. Cells were transfected with different ABCC6 cDNA-constructs using Lipofectamine® 2000, as suggested by the manufacturer (Invitrogen, Grand Island, NY). After four hours of incubation the medium was replaced with fresh culture medium without or with PTC124 at different concentrations. At different time points the cells were evaluated for synthesis of ABCC6-DDK fusion protein either by immunofluorescence or in-cell ELISA. For each construct, at least three independent transfection experiments were performed.
Immunofluorescence
Immunofluorescence was performed on 4% paraformaldehyde-fixed cells (15 min at room temperature). Non-specific sites were blocked for 60 min in 2% bovine serum albumin, and cells were then incubated overnight at 4°C with mouse anti-DDK antibody (1:2000, OriGene). Following three washes in PBS, cells were incubated for 1 hour with Alexa Fluor® 488 conjugated goat anti-mouse secondary antibody. After triple washing with PBS, the cells were mounted with antifade reagent and stained with DAPI (Molecular Probes, Eugene, OR, USA). The stained cells were analyzed using a fluorescent microscope (Zeiss, Göttingen, Germany).
In-Cell ELISA
To quantify the efficiency of read-through, the treated cells in 96-well plate were evaluated by Colorimetric In-Cell ELISA Kit (Pierce Biotechnology, Rockford, IL) following the manufacturer’s protocol. The absorbance was measured at 460 nm within 30 minutes of stopping the reaction. The value was normalized to corresponding untreated controls and converted to percentage using the expression of the wild-type ABCC6-DDK construct as 100%.
Cell proliferation assay
For PTC124 cytotoxicity assay, HEK293 cells were seeded in 96-well plates in triplicate at a density of 2000 cells/well and incubated with PTC124 at concentrations from 0 to 100 µg/ml. The medium was changed daily. At 72 hours, cell proliferation was determined by a colorimetric assay using CellTitCellTiter 96® Non-Radioactive Cell Proliferation Assay Kit (Promega, Madison, WI) according to the manufacturer’s protocol. The spectrophotometric absorbance of each sample was measured at 495 nm using Biorad microplate reader (Hercules, CA)
Zebrafish mRNA rescue assay
To test the potential pathogenicity of missense substitutions after read-through of ABCC6 induced by PTC124, a zebrafish mRNA rescue assay were performed. Capped full-length human ABCC6 mRNA corresponding to wild-type, R1141X or putative read-through substitutions of argine1141 by cysteine, tryptophan or glycine was transcribed from an expression vector pCMV-Tag4B using T3 mMessage mMachine kit (Ambion, Austin, TX). The morpholino was injected into one- to four-cell-stage embryos either alone or in combination with the mRNA as above (2.4 mmol). The injected zebrafish embryos were followed for their phenotype and survival rate.
Statistical analysis
The data were expressed as mean ± SEM. Statistical analyses were performed using a two-sided Student’s t-test, and statistical significance was accepted at p < 0.05.
Acknowledgement
The authors thank Karine Amirikian for technical help. Carol Kelly assisted in manuscript preparation. Supported by NIH/NIAMS grants R01 AR28450 and R01 AR055225 (JU) and K08 AR 057099 (QJ). Dr. Shao was supported by T32 AR60715.
Footnotes
The authors declare no conflict of interest.
References
- Belinsky MG, Kruh GD. MOAT-E (ARA) is a full-length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer. 1999;80:1342–1349. doi: 10.1038/sj.bjc.6690527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeira-Carrico R, Pego AP, Santos M, Oliveira C. Cancer syndromes and therapy by stop-codon readthrough. Trends Mol Med. 2012;18:667–678. doi: 10.1016/j.molmed.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Du M, Liu X, Welch EM, Hirawat S, Peltz SW, Bedwell DM. PTC124 is an orally bioavailable compound that promotes suppression of the human CFTR-G542X nonsense allele in a CF mouse model. Proc Natl Acad Sci U S A. 2008;105:2064–2069. doi: 10.1073/pnas.0711795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger RP, Charbel Issa P, Ladewig MS, Gotting C, Szliska C, Scholl HP, et al. Pseudoxanthoma elasticum: genetics, clinical manifestations and therapeutic approaches. Surv Ophthalmol. 2009;54:272–285. doi: 10.1016/j.survophthal.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Finkel RS. Read-through strategies for suppression of nonsense mutations in Duchenne/Becker muscular dystrophy: aminoglycosides and ataluren (PTC124) J Child Neurol. 2010;25:1158–1164. doi: 10.1177/0883073810371129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgalas I, Tservakis I, Papaconstaninou D, Kardara M, Koutsandrea C, Ladas I. Pseudoxanthoma elasticum, ocular manifestations, complications and treatment. Clin Exp Optom. 2011;94:169–180. doi: 10.1111/j.1444-0938.2010.00559.x. [DOI] [PubMed] [Google Scholar]
- Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, et al. Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet. 2005;14:1763–1773. doi: 10.1093/hmg/ddi183. [DOI] [PubMed] [Google Scholar]
- Hirawat S, Welch EM, Elfring GL, Northcutt VJ, Paushkin S, Hwang S, et al. Safety, tolerability, and pharmacokinetics of PTC124, a nonaminoglycoside nonsense mutation suppressor, following single- and multiple-dose administration to healthy male and female adult volunteers. J Clin Pharmacol. 2007;47:430–444. doi: 10.1177/0091270006297140. [DOI] [PubMed] [Google Scholar]
- Ho G, Reichardt J, Christodoulou J. In vitro read-through of phenylalanine hydroxylase (PAH) nonsense mutations using aminoglycosides: a potential therapy for phenylketonuria. J Inherit Metab Dis. 2013 doi: 10.1007/s10545-013-9602-6. [DOI] [PubMed] [Google Scholar]
- Howard MT, Shirts BH, Petros LM, Flanigan KM, Gesteland RF, Atkins JF. Sequence specificity of aminoglycoside-induced stop condon readthrough: potential implications for treatment of Duchenne muscular dystrophy. Ann Neurol. 2000;48:164–169. [PubMed] [Google Scholar]
- Kayali R, Ku JM, Khitrov G, Jung ME, Prikhodko O, Bertoni C. Read-through compound 13 restores dystrophin expression and improves muscle function in the mdx mouse model for Duchenne muscular dystrophy. Hum Mol Genet. 2012;21:4007–4020. doi: 10.1093/hmg/dds223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, et al. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–8310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Frank M, Thisse CI, Thisse BV, Uitto J. Zebrafish: a model system to study heritable skin diseases. J Invest Dermatol. 2011;131:565–571. doi: 10.1038/jid.2010.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sadowski S, Frank M, Chai C, Varadi A, Ho SY, et al. The abcc6a gene expression is required for normal zebrafish development. J Invest Dermatol. 2010;130:2561–2568. doi: 10.1038/jid.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988;6:1–159. doi: 10.1016/0738-081x(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Ryden-Aulin M. Glutamine is incorporated at the nonsense codons UAG and UAA in a suppressor-free Escherichia coli strain. Biochim Biophys Acta. 2003;1627:1–6. doi: 10.1016/s0167-4781(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Peltz SW, Morsy M, Welch EM, Jacobson A. Ataluren as an agent for therapeutic nonsense suppression. Annu Rev Med. 2013;64:407–425. doi: 10.1146/annurev-med-120611-144851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SM, Clancy JP. Pharmaceuticals targeting nonsense mutations in genetic diseases: progress in development. BioDrugs. 2009;23:165–174. doi: 10.2165/00063030-200923030-00003. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alcudia R, Perez B, Ugarte M, Desviat LR. Feasibility of nonsense mutation readthrough as a novel therapeutical approach in propionic acidemia. Hum Mutat. 2012;33:973–980. doi: 10.1002/humu.22047. [DOI] [PubMed] [Google Scholar]
- Tan L, Narayan SB, Chen J, Meyers GD, Bennett MJ. PTC124 improves readthrough and increases enzymatic activity of the CPT1A R160X nonsense mutation. J Inherit Metab Dis. 2011;34:443–447. doi: 10.1007/s10545-010-9265-5. [DOI] [PubMed] [Google Scholar]
- Uitto J. Rare heritable skin diseases: Targets for regenerative medicine. J Invest Dermatol. 2012;132:2485–2488. doi: 10.1038/jid.2012.334. [DOI] [PubMed] [Google Scholar]
- Uitto J, Bercovitch L, Terry SF, Terry PF. Pseudoxanthoma elasticum: progress in diagnostics and research towards treatment : Summary of the 2010 PXE International Research Meeting. Am J Med Genet A. 2011;155A:1517–1526. doi: 10.1002/ajmg.a.34067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J Invest Dermatol. 2010;130:661–670. doi: 10.1038/jid.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- Wilschanski M, Miller LL, Shoseyov D, Blau H, Rivlin J, Aviram M, et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur Respir J. 2011;38:59–69. doi: 10.1183/09031936.00120910. [DOI] [PubMed] [Google Scholar]