Abstract
Primary liver cancer (PLC) is the third leading cause of cancer mortality globally. In endemic areas of sub-Saharan Africa and Asia PLC largely arises from chronic infection with hepatitis B virus (HBV) and ingestion of aflatoxins. While synergistic interactions between these two risk factors have been observed in cohort studies in China, here we determined the impact of agricultural reforms in the 1980s leading to diminished maize consumption and implementation of subsidized universal vaccination against HBV in the 2000s on PLC primary prevention. A population-based cancer registry was used to track PLC mortality in Qidong, China and was compared to the timeline of HBV immunization. Randomly selected serum samples from archived cohort collections from the 1980s to present were analyzed for aflatoxin biomarkers. Greater than 50% reductions in PLC mortality rates occurred across birth cohorts from the 1960s to the 1980s for Qidongese less than 35 years of age although all were born before universal vaccination of newborns. Median levels of the aflatoxin biomarker decreased from 19.3 pg/mg albumin in 1989 to undetectable (<0.5 pg/mg) by 2009. A population attributable benefit of 65% for reduced PLC mortality was estimated from a government facilitated switch of dietary staple from maize to rice; 83% of this benefit was in those infected with HBV. Food policy reforms in China resulted in a dramatic decrease in aflatoxin exposure, which, independent of HBV vaccination, reduced liver cancer risk. The extensive HBV vaccine coverage now in place augurs even greater risk reductions in the future.
Keywords: aflatoxin, primary prevention, liver cancer
Introduction
Primary liver cancer (PLC) is the third leading cause of cancer mortality worldwide with an estimated 696,000 deaths in 2008. Most PLC occurs in sub-Saharan Africa and southeast Asia; there are >370,000 deaths annually from PLC in the People’s Republic of China alone (1,2). The major etiological factors associated with PLC in China, as established from prospective cohort studies, are chronic infection with hepatitis B virus (HBV) and extended exposure to high levels of aflatoxin in the diet, especially from maize and peanuts (3). The largest study, comprising more than 18,000 men residing in Shanghai in the 1980s, examined HBV infection and aflatoxin exposure as independent and interactive risk factors for PLC (4). This nested case control study revealed a statistically significant increase in the relative risk (RR) of 7.3 (95% CI: 2.2, 24.4) for men who were chronically infected with HBV (HBsAg) but unexposed to aflatoxin. In men in whom urinary aflatoxin but not HBV biomarkers were detected the RR was 3.4 (95% CI: 1.1, 10.0). Furthermore, in men exhibiting both urinary aflatoxin biomarkers and positive HBsAg status, the RR was 59.4 (95% CI: 16.6, 212.0) (4). A subsequent cohort study in Taiwan confirmed these results (5). In light of this synergistic interaction, programs to eliminate either factor, or both, should have substantial impact on the burden of PLC.
Chronic infection with HBV has long been regarded as the major cause of PLC; thus, initiatives have been undertaken to implement universal immunization programs. More than 90% of countries now routinely vaccinate newborns against HBV, and approximately 70% are now delivering three immunization doses (3). Newborns are targeted because HBV transmission often occurs from mother to child at birth or during the perinatal period. In 1986, Taiwan became the first region to vaccinate all newborns against HBV. Since then, the number of HBV carriers in the juvenile population has declined dramatically. As of 2009, incidence of hepatocellular carcinoma was significantly lower among children aged 6–19 years in vaccinated versus antecedent unvaccinated birth cohorts (6). These results have heralded the expected benefits of global vaccination against HBV, projected as declines in hepatitis, cirrhosis and PLC.
Qidong, China, located at the mouth of the Yangtze River, is an endemic area for PLC (7). HBV vaccination of newborns in the rural townships was initiated during the 1980s, but did not become universal until 2002. Qidong is newly deposited river delta land unsuitable for the production of rice. Therefore, maize became the primary dietary staple and vector for aflatoxin exposure. Maize consumption in the 1970s, low socioeconomic status (i.e., occupation of “peasant”), and chronic infection with HBV were reported as major risk factors for PLC in neighboring Haimen County (8). In China, procurement practices tied rural household food consumption to local production, and in the commune system of the 1960s and 1970s, yields rather than quality were emphasized. Indeed, ducks and rats fed maize grown in Qidong in the 1970s exhibited very high incidence of liver cancer (7). Although China adopted institutional reforms after 1979 to shift from a planned economy to a market-oriented economy, importation from surrounding areas of foodstuffs, notably rice, was only permitted in rural Qidong beginning in 1985 (9). Rice typically harbors much lower levels of aflatoxin than maize. This study has delineated the changing dynamics of these key risk factors. Further, we have examined changes in PLC mortality by birth cohorts in Qidong in the context of two forms of primary prevention: HBV vaccination and reduction of dietary aflatoxin exposure.
Methods
Cancer Registry
The Qidong Cancer Registry, a population-based registry that collects information on all deaths and all cancer cases in the county, was established by the Qidong Liver Cancer Institute (QDLCI) in 1972 (10). Demographic data of the county are provided by the household register office of the local security bureau, which tracks citizens by sex and place of residence yearly.
Cohort Samples
Multiple screening programs for studies in cancer etiology, early detection and chemoprevention have been undertaken since the founding of the QDLCI. Repositories of serum samples have been preserved from the late 1980s onward. Qidong has been an agricultural region of 1,157 km2 with a current population of 1.12 million. In order to control for location, study samples were restricted to two townships: Daxin and HeZuo (~25 km apart). For aflatoxin-albumin adduct analyses, in order to control for changing age distributions in the population, we selected at random age-matched samples of 50 men and 50 women across the populations screened from studies conducted in 1995, 1999, 2003, 2009 and 2012 (11–13). In 1989 a survey was conducted throughout the region but only in men. Using the same random screening scheme for age-matched distributions, we identified 75 samples from this 1989 survey for analysis (14). In addition, 77 age-matched samples were also selected randomly and analyzed from a 1982 cohort of 1,070 men recruited from Fusui County, Guangxi, another PLC endemic area in China (15). The characteristics of these collections are summarized in Table 1. Also included in Table 1 are descriptions of two cohorts used for estimating prevalence of HBV infection in the Qidong population. Collections and overall analyses were approved by the Institutional Review Boards of the QDLCI and Johns Hopkins Bloomberg School of Public Health, in accord with assurances filed with and approved by the U.S. Department of Health and Human Services. All samples were obtained with informed consent.
TABLE 1.
Characteristics of random sub-samples selected to quantify aflatoxin-albumin adducts.
| Year | Village | Gender & Age Range | # Screened | Random Sub-sample: Age | # Assayed for Aflatoxin Biomarker | Reference |
|---|---|---|---|---|---|---|
| 1982 | “A”, Fusui, Guangxi | ♂ 30–64 | 1,070 | 45.0 [38.0, 53.0] | 77 ♂ | 15 |
| 1989 | HeZuo, Qidong | ♂ 30–59 | 494 | 45.0 [38.0, 54.0] | 75 ♂ | 14 |
| 1995 | Daxin, Qidong | ♂ & ♀ 25–65 | 1,006 | 44.3 [38.2, 50.8] | 50 ♂ | 11 |
| 1999 | Daxin, Qidong | ♂ & ♀ 25–65 | 1,407 | 45.1 [38.1, 53.0] | 50 ♂ 50 ♀ |
Not published |
| 2003 | HeZuo, Qidong | ♂ & ♀ 21–65 | 700 | 47.4 [39.3, 55.0] | 50 ♂ 50 ♀ |
12 |
| 2009 | HeZuo, Qidong | ♂ & ♀ 21–65 | 205 | 45.7 [38.7, 54.9] | 50 ♂ 50 ♀ |
13 |
| 2012 | Daxin, Qidong | ♂ & ♀ 25–65 | 206 | 47.3 [41.2, 54.0] | 50 ♂ 50 ♀ |
Not published |
Serum was analyzed for aflatoxin-albumin adducts using isotope dilution mass spectrometry with aflatoxin B1-D4-lysine as the internal standard and with a lower limit of detection of 0.5 pg aflatoxin-lysine/mg albumin (16). This internal dose biomarker, with a biological half-life of several weeks, integrates exposures over a several month period.
Statistical Analysis
For each of the site-periods, mixtures of two generalized gamma (GG) distributions (17) were used to describe the distributions of the aflatoxin-albumin adduct levels, whose likelihood function appropriately included the left censored observations corresponding to subjects with levels below the limit of detection (18). The GG distribution is characterized by three parameters: location (median), scale (interquartile ratio) and shape (tails) (18) and extends the classical approach of using normal models for log-transformed biomarkers (corresponding to the case of the shape parameters being equal to zero). Maximum likelihood methods were used to obtain estimates of the parameters of the mixtures and to formally test for the significance of the mixture against simply one GG distribution and the most parsimonious model was selected as the appropriate description of the biomarker distributions. In order to avoid overparametrization of mixtures when a substantial percent of observations were below the limit of detection, we used mixtures of lognormal (shape parameter = 0) and weibull (shape parameter r = 1) distributions.
To describe the benefit attributable to the reduction of aflatoxin exposure (defined as aflatoxin > 0.5 pg/mg albumin), we calculated the reduction of disease due to decreasing the exposure from 100% (in the 1980s) to p% (more recently). Specifically, using 18% as the steady rate of HBV positivity and among both aflatoxin unexposed and exposed individuals, the population attributable benefit (PAB) due to decreasing exposure to aflatoxin to p% from 100% is simply the differences in the rates of liver cancer relative to the rate when 100% were exposed. Namely, PAB= (1−p)×(0.82× (3.4−1)+0.18× (59.4−7.3))/(0.82×3.4+0.18×59.4). The contribution to the PAB due to the aflatoxin reduction among the HBV negative is directly proportional to the first summand in the numerator (0.82× (3.4−1)). In turn, the contribution to the PAB among the HBV positive is directly proportional to the second summand in the numerator (0.18× (59.4−7.3)).
Results
Age Distributions over Time and Liver Cancer Mortality in Qidong
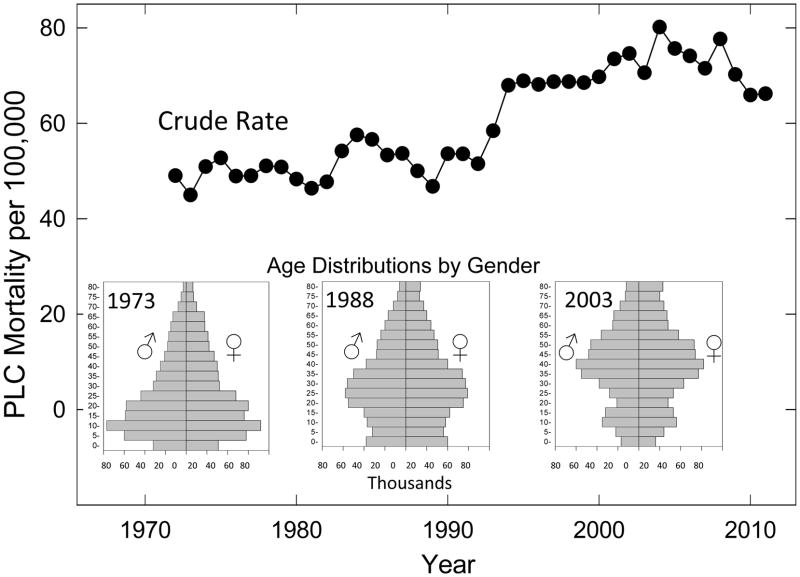
Figure 1 shows the dramatic changes of the age distributions in this population rising from 17.0% over 50 years of age in 1973 to 22.7% in 1988 and 34.2% in 2003. These changes contribute significantly to the crude rates of PLC per 100,000 residents of Qidong rising slightly from 1972 through 1990 at levels around 50/100,000 before increasing in the 1990s to a rate of about 75/100,000. This changing distribution underscores the importance of age-matching for the biomarker samples used in the study.
Figure 1. Burden of liver cancer in Qidong, China.
Annual crude mortality rates for PLC in Qidong from 1972 to 2011. Inserts. Qidong population pyramids for the years 1973, 1988 and 2003.
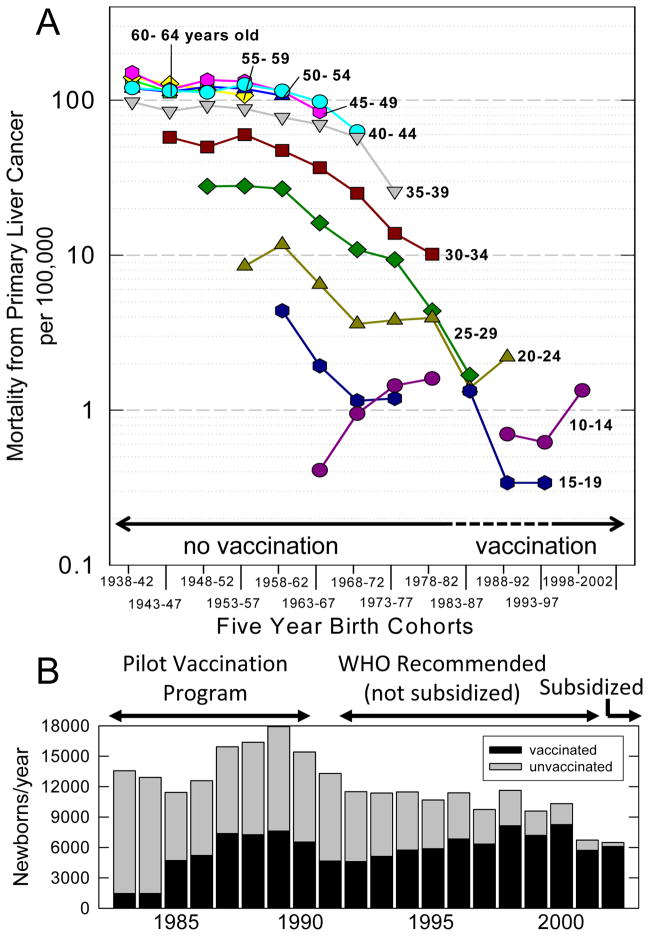
When mortality rates are examined within birth cohorts, there have been no significant changes in rates of mortality from PLC in Qidongese over age 40 over the past half century (Figure 2A). By contrast, 60–75% declines are seen in 20–24, 25–29 and 30–34 year olds born in 1973–77 as compared to 1958–62; all are birth years in which no newborns would have been immunized with HBV vaccine. Declines continue to be seen in the birth cohort of 1983–87, where relatively few newborns would have been immunized (Figure 2B).
Figure 2. Mortality rates from PLC in younger birth cohorts in Qidong and timeline of HBV vaccination.
A. Age-specific mortality from PLC per 100,000 by 5-year birth cohorts. Shaded area indicates the birth cohorts possibly (·····) or likely (——) to be affected by the introduction of HBV vaccination programs. There were no deaths from PLC among the 15–19 year olds in the 1978–1982 birth cohort. B. Number of newborns per year in Qidong (
 ) and enumerated (1983–1990) or estimated (1991–2002) number of newborns vaccinated against HBV (■).
) and enumerated (1983–1990) or estimated (1991–2002) number of newborns vaccinated against HBV (■).
Timeline for HBV Immunization in Qidong
A pilot safety study was done in 1983–1984 in eight high-risk Qidong townships (out of 45) using the Merck HepB vaccine within 24 hours of birth, and at 1 and 6 months after birth (19). In 1985 the program was expanded to 20 townships, and in 1987 to 26. Remaining townships were followed as control groups through 1990. Overall, about 97% coverage in the test townships was achieved in this limited vaccination program administered by the QDLCI (20). Figure 2B illustrates that 40% (40,605/102,566) of newborns in Qidong were vaccinated in the pilot study between 1983 and 1990. HBsAg prevalence in unvaccinated versus vaccinated children was 7.1% versus 1.7%, showing a 75% efficacy for this period (2). Given the low initial penetration into the population and the 75% efficacy of the vaccine, the likely impact of HBV immunization on PLC development in people born in Qidong prior to 1991 is modest. In 1992, in response to recommendations of the World Health Organization, the Chinese government endorsed but did not subsidize universal vaccination. Vaccine was produced by domestic plants, first providing plasma-derived vaccine that was replaced by recombinant DNA vaccine in 1997. Vaccination rate in the rural areas was poor until 2002, when the vaccine was subsidized for all newborns. However, parents were charged an injection fee until 2006. The coverage rate was about 82% for timely administration of the birth dose and 94% for the three doses, irrespective of timing, in Jiangsu Province (including Qidong) in 2002 (21). However, in economically disadvantaged populations throughout China, timely birth-dose coverage was estimated to be 29%, and only 71% completed the 3-dose series regardless of timing. Thus, vaccination rates presented in Figure 2B may overestimate the coverage of newborns in the rural areas of Qidong between 1991 and 2002. Also beginning in 2002, children aged 0–10 years could receive the vaccine. In summary, timely, comprehensive HBV vaccination of newborns occurred in rural Qidong only during the last decade.
To confirm that HBsAg positivity was stable in the birth cohorts prior to immunization, results were reviewed from two community-based screens conducted in Qidong townships in 1976 with 14,694 subjects (22) and between 2007 and 2009 with 31,700 subjects. The 30–34 year olds in the recent survey (born just prior to initiation of HBV vaccination programs) had a HBsAg prevalence of 17.8%, which is nearly identical to the prevalence of 18.2% reported in this same age group in the 1976 survey (22). Moreover, a 1987 survey of 30–39 year olds in two other townships in Qidong showed 17.4% to be positive (23).
Declining Aflatoxin Exposures
Annual surveys of aflatoxin contamination in maize in Qidong between 1973 and 1982 reported that 26 to 99% of acquired samples tested positive for aflatoxin at levels >20 ppb (7), the action level of the US Food and Drug Administration. Average annual per capita maize consumption ranged from 82–124 kg during 1973–1982 (7); most Qidong families at that time consumed maize as a major dietary staple. In 1985, a sharp transition occurred because of the new open policy of provisionment in China (9). By 1998, only 9% of families in the Qidong area ate any maize; < 1% ate 100 kg/year. Very little maize is consumed in the Qidong region in 2012. Conversely, the proportion of rural residents consuming some rice quickly reached 97.4% in 1986 and 99.2% in 1997 (24).
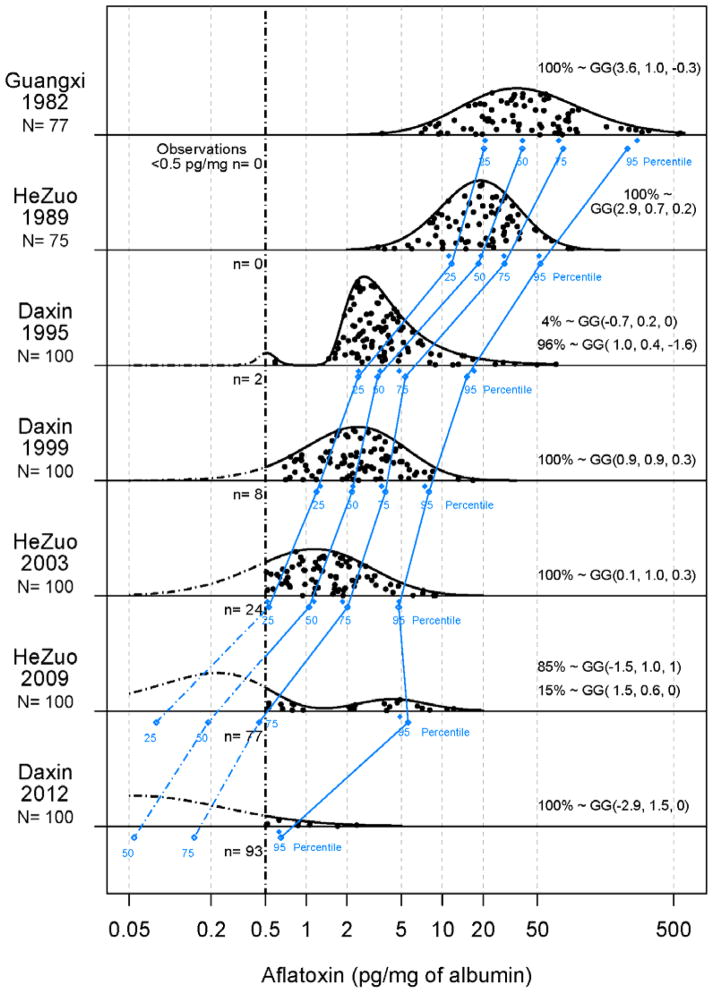
In order to describe accurately aflatoxin exposures over the past quarter century, frozen serum samples collected in the years of 1989, 1995, 1999, 2003, 2009 and 2012 from residents of two rural villages in Qidong were retrieved from collection banks (Table 1). Previous studies have shown the aflatoxin-albumin adduct to be stable in frozen serum samples for at least two decades (16). Testing of the samples from studies spanning more than 20 years indicates there has been a dramatic decline in levels of exposure from the 1980s to the present. Figure 3 shows the raw data as well as the fitted generalized gamma distributions, whose appropriateness is supported by the good agreement between the observed and expected percentiles. As shown in Figure 3, median levels of aflatoxin-albumin adducts from residents of the villages of Daxin or HeZuo declined from 19.3 pg/mg albumin in 1989, to 3.6 in 1995, to 2.3 in 1999, to 1.4 in 2003 and undetectable (i.e., <0.5 pg/mg) in 2009 (median estimated at 0.2) and 2012 (median estimated at 0.06). Only 23% and 7% of serum samples had levels above 0.5 pg/mg in 2009 and 2012, respectively. All samples tested in the 1989 cohort were positive. There were no differences in aflatoxin exposures by gender. From these exposure levels in 2009 and in 2012 we estimated that of the observed reduction in PLC mortality (see formula in methods section), 65% (currently) and 78% (anticipated) is due to decreasing aflatoxin exposure from 100% (proportion of the population with detectable aflatoxin-albumin adducts) to 23% and 7%, respectively. Due to the strong synergy between aflatoxin and HBV, the great majority (83%= (0.18× (59.4−7.3))/(0.82× (3.4−1)+0.18× (59.4−7.3))) of this reduction was attributable to decreasing aflatoxin exposure among those infected with HBV.
Figure 3. Distributions of levels of aflatoxin-albumin adducts.
(●) Adducts measured in randomly selected subsets of serum samples collected during screening programs (Table 1) in two high risk townships in Qidong (Daxin and HeZuo) as well as from another endemic area for PLC, Fusui, Guangxi: (-·-) Limit of detection (0.5 pg adduct per mg albumin). (
 ) Observed and (
) Observed and (
 ) predicted percentiles for adduct distributions. Parameters of generalized gamma (GG) distributions are indicated for each cohort.
) predicted percentiles for adduct distributions. Parameters of generalized gamma (GG) distributions are indicated for each cohort.
Although remote from Qidong, Fusui County, Guangxi is another endemic area for PLC where maize was also the dietary staple (15). Figure 3 also presents levels from older (1982) serum samples from that area. Levels of aflatoxin-albumin adducts were very high (median = 38.9 pg/mg albumin), and likely are representative of levels also occurring in Qidong during the 1970s and early 80s when moldy maize was a significant part of the rural diet there as well (25).
Discussion
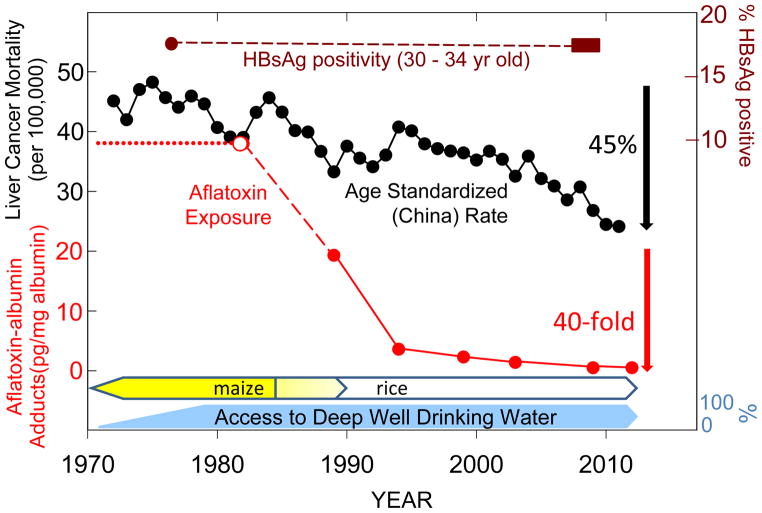
Changing patterns of cancer mortality, arising from population migration as well as implementation of screening, vaccination and tobacco control programs, provide powerful evidence of underlying etiologies and efficacies of preventive programs. However, there are few cancer registries in low-income nations that can provide detailed time-trend data, so much of this knowledge is gleaned about cancers common to the most economically developed countries. The birth cohort data from the Qidong Cancer Registry clearly indicate dramatic, and HBV vaccination-independent, declines in mortality rates from PLC in younger adults over the last three decades. Moreover, as shown in Figure 4, the age standardized (China) rate (CASR) of PLC mortality in Qidong exhibits an accelerating decline of nearly 45% since the early 1980s, reflecting a changing landscape of underlying risk factors. As validation of this registry, it, discouragingly, also presages the rising tide of cancers accompanying economic development as seen in other parts of the world; namely, breast, colon and lung cancers (26).
Figure 4. Dietary exposures, but not HBV carrier status, are associated with declining PLC mortality in Qidong.
(●) Age standardized (China) rate of PLC in Qidong. (
 —
—
 ) Prevalence of positivity for HBsAg in 30–34 year old birth cohorts within two large scale community-based screening studies conducted in Qidong in either 1976 or 2007–2009. HBV vaccination was not available to any newborns in these two birth cohorts. Median levels of aflatoxin-albumin adducts determined from the distributions presented in Figure 4 from Qidong (
) Prevalence of positivity for HBsAg in 30–34 year old birth cohorts within two large scale community-based screening studies conducted in Qidong in either 1976 or 2007–2009. HBV vaccination was not available to any newborns in these two birth cohorts. Median levels of aflatoxin-albumin adducts determined from the distributions presented in Figure 4 from Qidong (
 ) or (
) or (
 ) Guangxi. See text for details regarding timelines for access to deep well drinking water and switch of dietary staple from maize to rice in Qidong.
) Guangxi. See text for details regarding timelines for access to deep well drinking water and switch of dietary staple from maize to rice in Qidong.
HBV and aflatoxin exposure are key risk factors in this endemic area while infection with HCV is not (27). Prevalence of infection with HBV is unchanged in Qidong adults to date (Figure 4). Thus, the two- to three-fold decrease in PLC mortality seen between the birth cohorts of the 1960s and 1980s cannot be explained by differential HBV infections alone. Aflatoxin exposures, on the other hand, have been reduced dramatically – the generalized gamma distributions of aflatoxin albumin adducts suggest upwards of a thousand-fold reduction since the early 1980s (Figure 3). Nonetheless, risk factors for PLC other than aflatoxin need to be considered to explain the decline in PLC. Economic development has come only recently to Qidong. In 1990, less than 2% of rural households owned a refrigerator; by 2009 more than 50% did (28). However, declines in the CASR for PLC in Qidong during the 1980s indicate that underlying risk factors diminished at or before this time. Some epidemiological studies have implicated drinking water obtained from ditches and ponds as an additional risk factor (9,29). Such water can be contaminated with microcystins, hepatotoxic peptides produced by algal blooms that may interact with aflatoxin to promote hepatocarcinogenesis. Exposures to microcystins were greatly reduced by the late 1970s through efforts of the Qidong government to provide rural residents access to deep well water, which is largely devoid of microcystins (29). Improvements in quality of drinking water preceded the reduction in aflatoxin exposure by a decade, perhaps accounting for the initial decline in CASR.
This study provides evidence on PLC causation; i.e., a drop in risk following a drop in aflatoxin exposure. We have shown here that reduction of aflatoxin exposure from 100% to 23% of samples positive for aflatoxin albumin adducts resulted in an estimated population attributable benefit (PAB) of 65% for reduction in the rate of PLC. Due to the strong synergy between aflatoxin and HBV, only 17% of the PAB was estimated to be due to the reduction of aflatoxin among those without HBV infection. Due to multiple sources of external data, it was not possible to calculate 95% confidence intervals of these effects.
In Taiwan, where economic development accelerated decades earlier than in Qidong, aflatoxin exposure also decreased. Wu and colleagues have estimated that the population attributable risk in Taiwan for PLC due to aflatoxin exposure in HBV infected populations has declined from 31% in the 1980s to 12% in the 1990s and 3% in the 2000s (30). Perhaps the greatest needs for aflatoxin control are elsewhere. In Africa, maize is often used as the pioneer crop in newly deforested and developed lands. In several regions of Africa maize has become by far the most important staple food, accounting for over 50% of calories and up to 60% of field plantings (31). In 2004, one of the largest documented aflatoxin poisoning outbreaks occurred in rural Kenya, resulting in 125 deaths. Aflatoxin-contaminated maize grown and consumed on family farms was the major cause. This outbreak marked the first time that biomarkers, namely aflatoxin-albumin adducts, were used to confirm the exposure in individuals (32).
There are multiple approaches to attenuating exposures to aflatoxins, including planting pest-resistant varieties of staple crops, reducing mold growth in harvested crops, improving storage methods following harvest, and using trapping agents that block the uptake of unavoidably ingested aflatoxins or agents that enhance its detoxication and elimination (33,34). Lower exposures to aflatoxin in North America and Europe result from dietary diversity as well as regulatory actions governing allowable levels of aflatoxin in foods entering interstate and international commerce. All approaches could have utility in high exposure areas. Nonetheless, the current results highlight the critical role of agricultural policies in reducing PLC risk in aflatoxin endemic areas.
PLC, like most chronic diseases, is multifactorial in origin. While it is logical to assume that universal vaccination against HBV is poised to eliminate liver cancer (6,35), the attributive evidence supporting this conclusion is not so clear. The vaccination follow-up studies in Taiwan (6), a region where aflatoxin has also been shown to be a synergistic co-factor (5) and where exposures have likely dropped antecedent to or congruent with vaccination (30), does not yet provide definitive evidence of the unilateral success of HBV vaccination in cancer prevention. With synergy, primary prevention leading to attenuation of either the dietary carcinogen or the viral carcinogen, or both, could account for risk reduction. Clearly, the experience in Qidong demonstrates that dietary change can effect rapid (within 1–2 decades), dramatic reductions in PLC mortality in cohorts of individuals not immunized against HBV. Certainly, HBV immunization is an essential effort for global prevention of liver disease but it will take more than a generation to reach full fruition of disease reduction, given the early age of viral transmission. Hence, prevention modalities for the 350 million HBV carriers (36) must not be neglected. Lack of a therapeutic vaccine against this virus, together with the world-wide poor prognosis for diagnosed PLC patients, means that the public health community must consider additional approaches. Reducing dietary exposures to the environmental carcinogen aflatoxin, through a variety of strategies discussed above, is likely to significantly reduce liver cancer risk, even in those already infected with HBV.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (P01 ES 006052) and the National Science and Technology Mega-Projects of China (No. 2008ZX10002-015, No. 2012ZX10002-008.
We thank Nancy E Davidson MD (University of Pittsburgh) and Gerald N Wogan PhD (Massachusetts Institute of Technology) for their thoughtful review of the manuscript and helpful comments.
Abbreviations
- PLC
primary liver cancer
- HBV
hepatitis B virus
- HBsAg
hepatitis B virus surface antigen
- RR
relative risk
- PAB
population attributable benefit
- QDLCI
Qidong Liver Cancer Institute
- GG
generalized-gamma
- CASR
China age standardized rate
Footnotes
Conflict of Interest Disclosures: No conflicts of interest.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Chen JG, Zhang SW. Liver cancer epidemic in China: past, present and future. Semin Cancer Biol. 2011;21:59–69. doi: 10.1016/j.semcancer.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian GS, Ross RK, Yu MC, Yuan JM, Gao YT, Henderson BE, et al. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 1994;3:3–10. [PubMed] [Google Scholar]
- 5.Chen CJ, Wang LY, Lu SN, Wu MH, You SL, Zhang YJ, et al. Elevated aflatoxin exposure and increased risk of hepatocellular carcinoma. Hepatology. 1996;24:38–42. doi: 10.1002/hep.510240108. [DOI] [PubMed] [Google Scholar]
- 6.Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccines: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348–55. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 7.Zhu YR, Chen JG, Huang XY. Hepatocellular carcinoma in Qidong County. In: Tang ZY, Wu MC, Xia SS, editors. Primary liver cancer. Beijing: China Academic Publishers, Springer-Verlag; 1989. pp. 204–22. [Google Scholar]
- 8.London WT, Evans AA, McGlynn K, Buetow K, An P, Gao L, et al. Viral, host and environmental risk factors for hepatocellular carcinoma: a prospective study in Haimen City, China. Interverology. 1995;38:155–61. doi: 10.1159/000150426. [DOI] [PubMed] [Google Scholar]
- 9.Lin JY. Rural reforms and agricultural growth in China. Am Econ Rev. 1992;82:34–51. [Google Scholar]
- 10.Chen JG, Zhu J, Zhang YH, Lu JH. Cancer survival in Qidong, China, 1992–2000. IARC Sci Publ. 2011;162:43–53. [PubMed] [Google Scholar]
- 11.Jacobson LP, Zhang BC, Zhu YR, Wang JB, Wu Y, Zhang QN, et al. Oltipraz chemoprevention trial in Qidong, People’s Republic of China: study design and clinical outcomes. Cancer Epidmiol Biomarkers Prev. 1997;6:257–65. [PubMed] [Google Scholar]
- 12.Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthene tetraols in a randomized clinical trial in He Zuo Township, Qidong, PRC. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–13. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 13.Egner PA, Chen JG, Wang JB, Wu Y, Sun Y, Lu JH, et al. Bioavailability of sulforaphane from two broccoli sprout beverages: results of a short term, cross-over clinical trial in Qidong, China. Cancer Prev Res. 2011;4:384–95. doi: 10.1158/1940-6207.CAPR-10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, et al. Screening for liver cancer: results of a randomized controlled trial in Qidong, China. J Med Screen. 2003;10:204–9. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- 15.Yeh F-S, Yu MC, Mo C-C, Luo S, Tong MJ, Henderson BE. Hepatitis B virus, aflatoxin and hepatocellular carcinoma in southern Guangxi, China. Cancer Res. 1989;49:2506–9. [PubMed] [Google Scholar]
- 16.Scholl PF, Groopman JD. Long-term stability of human aflatoxin B1 albumin adducts assessed by isotope dilution mass spectrometry and high-performance liquid chromatography-fluorescence. Cancer Epidemiol Biomarkers Prev. 2008;17:1436–9. doi: 10.1158/1055-9965.EPI-07-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox C, Chu H, Schneider MF, Muñoz A. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26:4352–74. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]
- 18.Chu HT, Kensler TW, Muñoz A. Assessing the effect of interventions in the context of mixture distributions with detection limits. Stat Med. 2005;24:2053–67. doi: 10.1002/sim.2079. [DOI] [PubMed] [Google Scholar]
- 19.Sun TT, Chu YR, Ni ZQ, et al. A pilot study on universal immunization of newborn infants in an area of hepatitis B virus and primary hepatocellular carcinoma prevalence with a low dose of hepatitis B vaccine. J Cell Physiol Suppl. 1986;4:83–90. [PubMed] [Google Scholar]
- 20.Zhu YR. Comprehensive prevention for the major etiological factors of liver cancer in Qidong. Bullet Chin Cancer. 1999;8:309–10. [Google Scholar]
- 21.Progress in hepatitis B prevention through universal infant vaccination 3 China, 1997—2006. Morb Mort Wkly Rep. 2007;56:441–45. [PubMed] [Google Scholar]
- 22.Chen JG, Lu JH, Zhu YR, Zhu J, Zhang YH. A thirty-one year prospective follow-up program on the HBsAg carrier state and primary liver cancer in Qidong, China. Zhonghua Liu Xing Bing Xue Za Zhi (Chin J Epidemiol) 2010;31:721–6. [PubMed] [Google Scholar]
- 23.Chen QX, Chen JG, Li JR, Gong Y. Prevalence of hepatic diseases from a sampling survey in two townships, Qidong. Zhonghua Liu Xing Bing Xue Za Zhi (China J Epidemiol) 1989;10:63. [Google Scholar]
- 24.Chen JG. Advances in the etiology and prevention for primary liver cancer. China J Can Prev Treat. 2003;10:1123–5. [Google Scholar]
- 25.Wang YB, Lan LZ, Ye BF, Xu YC, Liu YY, Li WG. Relation between geographical distribution of liver cancer and climate-aflatoxin B1 in China. Sci Sin B. 1983;26:1166–75. [PubMed] [Google Scholar]
- 26.Chen JG, editor. Cancer in Qidong, China (1972–2011) Beijing: Military Medical Scientific Press; 2013. pp. 1–346. [Google Scholar]
- 27.Ming L, Thorgeirsson SS, Gail MH, Lu P, Harris CC, Wang N, et al. Dominant role of hepatitis B virus and cofactor role of aflatoxin in hepatocarcinogenesis in Qidong, China. Hepatology. 2002;36:1214–20. doi: 10.1053/jhep.2002.36366. [DOI] [PubMed] [Google Scholar]
- 28. [23 July 2013, last date accessed];Jiangsu Province Statistical Yearbooks. http://chinadataonline.org/member/yearbook/default.asp?StartYear=1981&EndYear=2010&ybcode=JIANGSU#.
- 29.Ueno Y, Nagata S, Tsutsumi T, Hasegawa A, Watanabe MF, Park HD, et al. Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis. 1996;17:1317–21. doi: 10.1093/carcin/17.6.1317. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Chang C-CH, Marsh GM, Wu F. Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur J Cancer. 2012;48:2125–36. doi: 10.1016/j.ejca.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCann JC. Maize and Grace: Africa’s Encounter with a New World Crop, 1500–2000. Cambridge MA: Harvard University Press; 2005. [Google Scholar]
- 32.Azziz-Baumgartner E, Lindblade K, Gieseker K, Rogers HS, Kieszak S, Njapau H, et al. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ Health Perspect. 2005;113:1779–83. doi: 10.1289/ehp.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health problem. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kensler TW, Roebuck BD, Wogan GN, Groopman JD. Aflatoxin: A 50 year odyssey of mechanistic and translational toxicology. Toxicol Sci. 2011;120:28–48. doi: 10.1093/toxsci/kfq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colditz GA, Wolin KY, Gehlert S. Applying what we know to accelerate cancer prevention. Sci Transl Med. 2012;4:127rv4. doi: 10.1126/scitranslmed.3003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franceschi S, Raza SA. Epidemiology and prevention of hepatocellular carcinoma. Cancer Lett. 2009;286:5–8. doi: 10.1016/j.canlet.2008.10.046. [DOI] [PubMed] [Google Scholar]