Abstract
Using specific riboprobes, we characterized the expression of VGLUT1-VGLUT3 transcripts in lumbar 4-5 (L4-5) DRGs and the thoracolumbar to lumbosacral spinal cord in male BALB/C mice after a 1- or 3-day hindpaw inflammation, or a 7-day sciatic nerve axotomy. Sham animals were also included. In sham and contralateral L4-5 DRGs of injured mice, VGLUT1-, VGLUT2- and VGLUT3 mRNAs were expressed in ~45%, ~69% or ~17% of neuron profiles (NPs), respectively. VGLUT1 was expressed in large and medium-sized NPs, VGLUT2 in NPs of all sizes, and VGLUT3 in small and medium-sized NPs. In the spinal cord, VGLUT1 was restricted to a number of NPs at thoracolumbar and lumbar segments, in what appears to be the dorsal nucleus of Clarke, and in mid laminae III-IV. In contrast, VGLUT2 was present in numerous NPs at all analyzed spinal segments, except the lateral aspects of the ventral horns, especially at the lumbar enlargement, where it was virtually absent. VGLUT3 was detected in a discrete number of NPs in laminae III-IV of the dorsal horn. Axotomy resulted in a moderate decrease in the number of DRG NPs expressing VGLUT3, whereas VGLUT1 and VGLUT2 were unaffected. Likewise, the percentage of NPs expressing VGLUT transcripts remained unaltered after hindpaw inflammation, both in DRGs and the spinal cord. Altogether, these results confirm previous descriptions on VGLUTs expression in adult mice DRGs, with the exception of VGLUT1, whose protein expression was detected in a lower percentage of mouse DRG NPs. A detailed account on the location of neurons expressing VGLUTs transcripts in the adult mouse spinal cord is also presented. Finally, the lack of change in the number of neurons expressing VGLUT1 and VGLUT2 transcripts after axotomy, as compared to data on protein expression, suggests translational rather than transcriptional regulation of VGLUTs after injury.
Keywords: VGLUTs, DRGs, hybridization, axotomy, hindpaw inflammation, spinal cord
Glutamate has an essential role in sensory neuron signaling, and it also participates in both acute and chronic pain mechanisms (Chiosa and Gane, 1956; Curtis et al., 1960; Karim et al., 2001; Minami et al., 2001; Sung et al., 2003; Binns et al., 2005; Liaw et al., 2005; Tao et al., 2005; Moechars et al., 2006). Presence of glutamate in dorsal root ganglion (DRG) neurons and their projections was first identified by Rustioni and Weinberg (1989). Recently, vesicular glutamate transporters (VGLUTs), the proteins responsible for the uptake of glutamate into synaptic vesicles, have been identified and characterized in numerous neuron types in both the central (CNS) and peripheral nervous systems (PNS) (see Kaneko and Fujiyama, 2002; Fremeau, Jr. et al., 2004; Seal and Edwards, 2006; Takamori, 2006). Because their expression is directly related to the glutamatergic phenotype of neurons, VGLUTs have emerged as the definitive markers to histochemically identify neurons that use glutamate as a neurotransmitter (see Brumovsky et al., 2011a and references therein). Three subtypes have been described thus far, VGLUT1, (Ni et al., 1994; Bellocchio et al., 2000; Takamori et al., 2000), VGLUT2 (Aihara et al., 2000; Fremeau, Jr. et al., 2001; Takamori et al., 2001; Bai et al., 2001; Hayashi et al., 2001; Herzog et al., 2001; Sakata-Haga et al., 2001; Varoqui et al., 2002) and VGLUT3 (Fremeau, Jr. et al., 2002; Gras et al., 2002; Schäfer et al., 2002).
Rodent DRG neurons are divided between those that innervate visceral and non-visceral tissues (see Robinson and Gebhart, 2008 and references therein). Initial studies focused on the immunohistochemical expression of VGLUT1 and VGLUT2 in rat DRG neurons projecting to non-visceral tissues (lumbar (L) 4 and 5), showing that VGLUT1 was primarily expressed in large non-peptidergic neurons whereas VGLUT2 was expressed in neurons of all sizes, including peptidergic ones (Tong et al., 2001; Oliveira et al., 2003; Hwang et al., 2004). These observations found a correlate in mouse (Brumovsky et al., 2007; Scherrer et al., 2010) and guinea pig (Morris et al., 2005) DRG neurons, as well as those targeting visceral organs such as the colorectum (Brumovsky et al., 2011a) or the urinary bladder (Brumovsky et al., 2012). The identification of VGLUT3 in DRGs has been more difficult, mainly because none of the commercially available antibodies against VGLUT3 efficiently labels these neurons or their projections. However, the recent generation of transgenic mice where reporter proteins such as the enhanced green fluorescent protein (EGFP) are under control of the VGLUT3 promoter (VGLUT3-EGFP), demonstrated the presence of VGLUT3 in subpopulations of sciatic nerve- (Seal et al., 2009; Lou et al., 2013) and urinary bladder-projecting (Brumovsky et al., 2012) DRG neurons.
VGLUTs are also immunohistochemically detected in the neuropil (but not in cell bodies) of the rodent spinal cord and exhibit different patterns of distribution, including dorsal vs. ventral or lateral horns (Varoqui et al., 2002; Oliveira et al., 2003; Todd et al., 2003; Olave and Maxwell, 2003; Li et al., 2003a; Alvarez et al., 2004; Hwang et al., 2004; Landry et al., 2004; Morris et al., 2005; Persson et al., 2006; Llewellyn-Smith et al., 2007; Seal et al., 2009). Some of the immunoreactivity to VGLUTs in the spinal cord represents transporter molecules synthesized in DRG neurons and then axonally transported through the central branches of dorsal roots to the spinal cord. This was first suggested by Varoqui et al. (2002), and later demonstrated by Todd et al. (2003), showing that VGLUT1 and VGLUT2 proteins are present in transganglionically labeled primary afferent terminals in the dorsal horn of the spinal cord of rat. In support, dorsal rhizotomy, a procedure normally utilized to unveil the contribution of molecules produced by DRG neurons and actively transported to the spinal cord, results in a dramatic (although not complete) decrease in VGLUT1-like-immunoreactivity (Li) in nerve fibers in the ventral and to some extent also in the dorsal horn of rats (Oliveira et al., 2003; Li et al., 2003a; Alvarez et al., 2004) and mice (Brumovsky et al., 2007). A decrease, although restricted to the superficial laminae of the dorsal horn, has been described for VGLUT3 as well as for VGLUT3-EGFP in transgenic mice (Seal et al., 2009). In contrast, and with one exception in the rat showing an ipsilateral decrease (Li et al., 2003a), dorsal rhizotomy fails to alter the immunoreactivity of VGLUT2, both in rat (Oliveira et al., 2003; Alvarez et al., 2004) and mouse (Brumovsky et al., 2007). These observations suggest presence of more than one source VGLUT proteins detected in the spinal cord. In fact, brainstem- (Oliveira et al., 2003; Du et al., 2012) and cortical-derived (Fremeau, Jr. et al., 2001; Persson et al., 2006; Du et al., 2012) descending fibers express VGLUT1 and/or VGLUT2.
Analyses of VGLUT transcripts in DRGs and spinal cord also have received attention. Thus, the use of oligo- (Oliveira et al., 2003) and riboprobes (Landry et al., 2004) in the adult rat revealed that VGLUT1 (Oliveira et al., 2003; Landry et al., 2004) and VGLUT2 (Landry et al., 2004) transcripts are expressed by a considerable number of neurons in L4-5 DRGs. VGLUT3 mRNA was also identified in rat DRGs by means of RT-PCR (Gras et al., 2002). More recently, the presence of VGLUT3 transcripts has been reported (but not quantified) in neonatal mouse DRG neurons (Lou et al., 2013). In the spinal cord, neuronal expression for all VGLUTs has been described in adult rats (Oliveira et al., 2003; Landry et al., 2004; Llewellyn-Smith et al., 2007), neonatal rats (Kullander et al., 2003), as well as in neonatal mice (Lou et al., 2013). However, the pattern of distribution for each VGLUT differs among studies (Oliveira et al., 2003; Kullander et al., 2003; Landry et al., 2004; Llewellyn-Smith et al., 2007; Lou et al., 2013).
To date, neither a detailed description of the transcript expression of VGLUTs in mouse DRG and spinal cord neurons is available, nor knowledge of possible changes in expression after peripheral nerve injury or inflammation. Thus, in the present study, we have characterized the expression of the three VGLUTs in lumbar DRG neurons and the thoracolumbar, lumbar and lumbosacral spinal cord of BALB/c mice by means of in situ hybridization. We used selective riboprobes that have been validated in their specificity to identify VGLUTs in peripheral neurons (Brumovsky et al., 2011a). Animals with a 1- or 3-day hindpaw inflammation or a 7-day axotomy of the sciatic nerve were included in the analysis. Portions of these data have been reported in abstract form (Vieytes et al., 2013).
EXPERIMENTAL PROCEDURES
Animals
The experiments were performed on fifty-one male BALB/C mice (b.wt. 20-30 g). The animals were maintained under standard conditions on a 12-hour day/night cycle (light on at 7AM), with water and food ad libitum. All experiments were performed following the Society for Neuroscience and the International Association for the Study of Pain guidelines for the use of animals in research, and approved by the institutional ethics committees (University of Pittsburgh and Austral University).
Axotomy (Axo)
Under aseptic conditions, mice were anesthetized with Isoflurane (Hospira Inc., Lake Forest, IL), and a complete transection of the right sciatic nerve was performed. Briefly, the right sciatic nerve was exposed mid-thigh and a 5- to 8-mm-long segment dissected free from the surrounding tissue, strongly ligated and transected distal to the ligation. In all cases, a 5-mm-long segment was resected distal to the transection. Finally, muscles and skin were sutured in layers, the skin incision treated with dibucaine ointment (1%, Perrigo, Allegan, MI), and subcutaneous buprenorphine (0.1 mg/kg, Bedford Labs, Bedford, OH) given for postoperative analgesia. Mice were allowed to recover in a warm environment under close observation.
Sham mice were treated in the same way as described above, with the exception that the sciatic nerve was only exposed and dissected free from the surrounding connective tissue, but it was not transected.
Hindpaw inflammation (CFA)
Lightly anesthetized mice (Isoflurane) received an injection of ~20 μl of a 1:1 solution of Complete Freunds Adjuvant in saline (CFA; Sigma, St. Louis, MO) into the plantar surface of the left hindpaw, using a 1-ml syringe and a 27G needle. Animals recovered in a warm environment, and were allowed to survive for 24 or 72 hours before sacrifice (1- or 3-day CFA, respectively). Sham animals (n = 16) received an injection of ~20 μl of saline solution.
Riboprobe in situ hybridization
Male, 7-week old, BALB/c mice were used for in situ hybridization analyses (VGLUT1 and VGLUT2, Axo = 4, Sham-Axo = 4; 1-day CFA = 3, 1-day Sham-CFA = 3; 3-day CFA= 4, 3-day Sham-CFA= 3; VGLUT3: Axo = 5, Sham-Axo = 5; 1-day CFA = 5, 1-day Sham-CFA = 5; 3-day CFA= 5, 3-day Sham-CFA= 5). Seven days after Axo and 1 or 3 days after hindpaw inflammation, mice were sedated using CO2 before rapid decapitation. Contra- and ipsilateral L4 and L5 DRGs and the thoracolumbar (TL), lumbar (L) and lumbosacral (LS) segments of the spinal cord were quickly dissected, embedded in O.C.T. (Tissue-Tek, Sakura, Torrance, CA) and frozen over dry ice. Sections of DRGs and the spinal cord were cut in a cryostat (14- or 20-μm thickness, respectively), thaw-mounted onto Superfrost Plus (Fisher Scientific, Waltham, MA) glass slides and stored at −20°C until hybridization. Adjacent sections through the different tissues were processed for in situ hybridization for the localization of VGLUT1, VGLUT2 and VGLUT3 mRNAs. In all cases 35S-labeled cRNA probes were used as previously described in detail (Seroogy and Herman, 1997; Brumovsky et al., 2011a; Brumovsky et al., 2011b; Hemmerle et al., 2012).
Sense and antisense cRNA probes complementary to the coding region of mouse VGLUT1 (nucleotides 855–1788; GenBank accession number XM_133432.2), VGLUT2 (nucleotides 848–2044; GenBank accession number NM_080853.2) (Nakamura et al., 2007) and VGLUT3 (nucleotides 1511-1768; GenBank accession numbers BB650431 and BB396899; Schäffer et al., 2002) were prepared by in vitro transcription, after linearization of cDNA constructs, using proper RNA polymerases (T3, T7, or SP6) and 35S-UTP (PerkinElmer, Chelton, CT). After drying and fixation, sections were incubated for 18–24 hours at 60°C in hybridization cocktail containing the 35S-labeled cRNA probe at a concentration of 1.0 × 106 cpm/50 μl per slide (Seroogy and Herman, 1997; Brumovsky et al., 2011a; Brumovsky et al., 2011b; Hemmerle et al., 2012).
For posthybridization treatment, sections were washed several times in 4 x SSC (1x SSC ¼ 0.015 M sodium citrate, 0.15 M sodium chloride at pH 7.0) containing 10 mM sodium thiosulfate at 37°C. The sections were then incubated in ribonuclease A (0.05 mg/mL) for 30 minutes at 45°C. This was followed by several washes in decreasing concentrations of SSC (2x, 0.5x, and 0.1x) at 37°C. All but the final wash also contained 10 mM sodium thiosulfate. After posthybridization, a brief rinse in distilled water (dH2O), a dip in 95% ethanol, and air-drying, sections were dipped in NTB nuclear track emulsion (Kodak, Rochester, NY; 1:1 in dH2O), air-dried, and exposed in sealed slide boxes at 4°C. Sections hybridized with the VGLUT1 or VGLUT2 riboprobes were exposed for 5-7 days, whereas those hybridized with the VGLUT3 riboprobe were exposed for 3-5 weeks (DRGs and the spinal cord). D19 (Kodak) and Rapidfix (Kodak) were used for developing and fixation of the dipped slides, respectively. Finally, all sections were counterstained with cresyl violet (Sigma) and coverslipped with DPX mounting solution (Fluka, Buchs, Switzerland) (Seroogy and Herman, 1997; Brumovsky et al., 2011a; Brumovsky et al., 2011b; Hemmerle et al., 2012).
Specificity controls included the pretreatment of some sections with ribonuclease A (0.05 mg/ml) for 30 minutes at 45°C before hybridization with the 35S-labeled cRNA probes; hybridization using sense-strand 35S-labeled riboprobes against each VGLUT; and incubation of several tissue sections in hybridization cocktail that lacked the radioactive probe as a chemography control. No specific labeling was observed under any of these conditions (Seroogy and Herman, 1997; Brumovsky et al., 2011a; Brumovsky et al., 2011b; Hemmerle et al., 2012).
Microscopy and image processing
All sections were examined with a Nikon Eclipse E800 microscope equipped with appropriate objectives and filters for bright- and dark-field visualization, and a DS U2 Nikon Digital Camera (Nikon, Tokyo, Japan).
Resolution, brightness and contrast of the images were optimized using the Adobe Photoshop CS3 software (Adobe System Inc., San Jose, CA).
Quantification and statistical analysis
Quantification was performed on L4-5 DRGs processed by in situ hybridization, in sham, 7-day Axo, 1- or 3-day CFA mice, comparing contra- and ipsilateral sides. Every fourth DRG section was used to quantify the number of VGLUT1-, VGLUT2- or VGLUT3 mRNA-positive neuron profiles (NPs), as well as the total number of cresyl violet-stained NPs. In total, between 9 and 13 sections per DRG were used for quantification. Only nucleated NPs with a density of accumulation of silver grains 5 times greater than background were counted as positively labeled NPs. Percentages of VGLUT-expressing NPs were obtained by counting, within the total number of cresyl violet-stained NPs, those expressing VGLUT1-3. Because of the quantification method utilized in this study, it is possible large diameter NPs were overestimated, and small NPs suffered a reduction in the opportunity to be counted (Guillery, 2002). In such case, and in particular for VGLUT2 and VGLUT3 mRNA-expressing DRG NPs, it is possible that the percentages presented here were underestimated. However, the intention of this study is only to provide with estimated numbers of the expression of each VGLUTs, and their differential distributions in DRG NPs. Establishing the absolute number of DRG neurons expressing each VGLUT in this study would require neurostereology (Mayhew and Gundersen, 1996), a method that is not easily applied to sections processed by in situ hybridization methods.
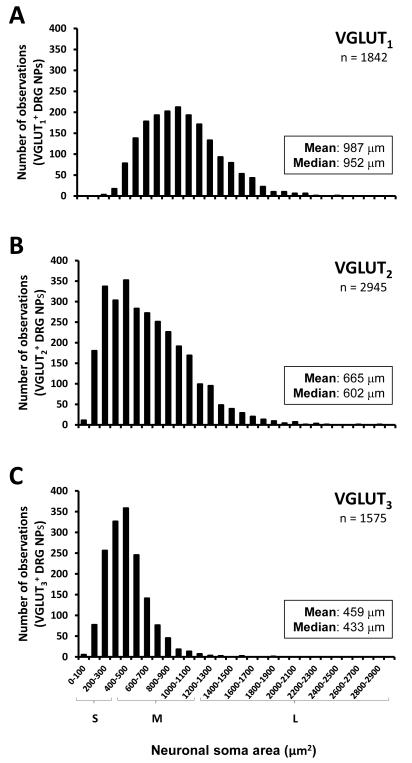
For the evaluation of neuronal size distribution, the cell body diameters of a representative sample of nucleated NPs expressing each VGLUT (VGLUT1, 1842 cells; VGLUT2, 2945 cells; VGLUT3, 2205 cells) in contralateral DRGs of all sham mice were measured using the public domain NIH program ImageJ (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). All values, separated by each VGLUT type, were pooled into an histogram, and the NP size distribution was categorized as follows (Brumovsky et al., 2007): 1) small, <400 μm2; 2) medium, 400-1225 μm2; and 3) large, >1225 μm2.
Data are presented as mean ± SEM and were statistically analyzed using one-way ANOVA, followed by the posthoc Tukey’s Multiple Comparison Test; P < 0.05 was considered significant. Mean and median size of DRG neurons expressing each VGLUT was also presented for comparison.
RESULTS
VGLUT1-3 mRNA expression in DRG NPs
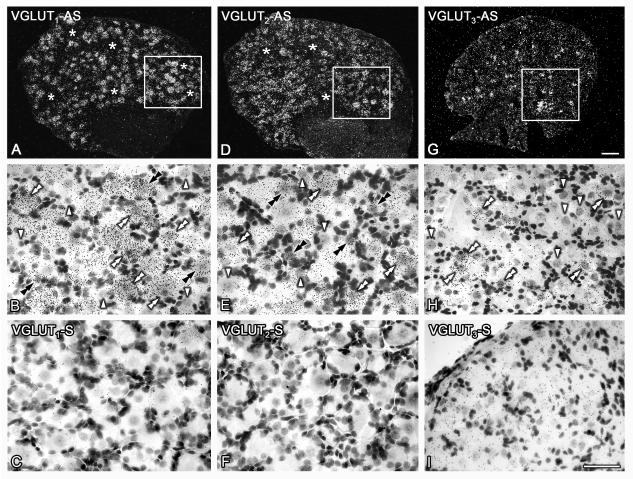
Presence of the transcript for each VGLUT was detected in uninjured DRG NPs, albeit showing different proportions. Thus, when taking into account the global expression of each VGLUT in contralateral L4-5 DRGs of sham mice, VGLUT1 transcript was expressed in ~44.7 ± 4.2%, VGLUT2 transcript was present in ~69.1 ± 3.7% NPs, and VGLUT3 mRNA was detected in ~17.1 ± 0.7% NPs (Table 1; Figure 1A, D, G). Most NPs expressing VGLUT1 mRNA were medium-sized and large, ranging from 247 to over 2469 μm2, with an mean size of 987 μm2 and a median size of 952 μm2; a large proportion of these NPs were counted between 500 and 1200 μm2 (Figs. 1B; 2A). NPs expressing VGLUT2 mRNA showed a widespread distribution, ranging from 54 to 2810 μm2 and with an mean size of 665 μm2 and a median size of 602 μm2; most NPs were counted between 200 and 1100 μm2 (Figs. 1E, 2B). Finally, VGLUT3 mRNA was detected in small and medium-sized NPs, ranging from 64 to 1811 μm2 and with an mean size of 459 μm2 and a median size of 433 μm2; the majority of NPs were counted between 200 and 600 μm2 (Figures 1F; 2C).
Table 1.
Only VGLUT3 mRNA exhibits changes after axotomy of the sciatic nerve. Percentage of DRG neurons expressing either VGLUT1, VGLUT2 or VGLUT3 transcript, after 7-day sciatic nerve axotomy or 1- or 3-day CFA injuries. Sham animals were also included. To provide the global percentage of neurons expressing each VGLUT in normal conditions, the values from contralateral DRGs in sham animals were combined and averaged accordingly.
| VGLUT1 |
VGLUT2 |
VGLUT3 |
||||
|---|---|---|---|---|---|---|
| Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | |
| 7-day Axotomy | 42.1 ± 0.4 (n=4) | 42.5 ± 0.9 (n=4) | 66.5 ± 1.7 (n=4) | 69.3 ± 1.0 (n=4) | 12.8 ± 0.4 (n=5)* | 17.8 ± 1.6 (n=5) |
| Sham | 40.3 ± 1.0 (n=4) | 42.8 ± 1.4 (n=4) | 68.9 ± 1.0 (n=4) | 65.7 ± 1.8 (n=4) | 15.1 ± 0.5 (n=5) | 15.7 ± 1.1 (n=5) |
| 1-day Inflammation | 52.2 ± 0.6 (n=3) | 51.5 ± 1.6 (n=3) | 78.7 ± 1.6 (n=3) | 76.1 ± 0.8 (n=3) | 16.1 ± 0.9 (n=5) | 14.4 ± 0.3 (n=5) |
| Sham | 51.3 ± 2.4 (n=3) | 52.8 ± 0.8 (n=3) | 78.9 ± 1.7 (n=3) | 76.6 ± 2.0 (n=3) | 14.0 ± 0.7 (n=5) | 17.6 ± 1.4 (n=5) |
| 3-day Inflammation | 38.4 ± 0.8 (n=4) | 41.8 ± 1.8 (n=4) | 64.9 ± 1.8 (n=4) | 66.0 ± 2.5 (n=4) | 16.4 ± 0.9 (n=5) | 17.6 ± 1.3 (n=5) |
| Sham | 41.0 ± 1.2 (n=3) | 38.8 ± 0.9 (n=3) | 62.3 ± 3.2 (n=3) | 64.7 ± 1.8 (n=3) | 16.1 ± 1.2 (n=5) | 18.4 ± 0.6 (n=5) |
|
| ||||||
| Global Percentage | 44.7 ± 4.2 (n=10) | 69.1 ± 3.7 (n=10) | 17.1 ± 0.7 (n=15) | |||
Values are expressed as mean ± SEM.
(P<0.05) when comparing contra- vs. ipsilateral DRGs after a 7-day axotomy.
Figure 1.
VGLUTs transcripts are abundantly expressed in L4-5 DRGs. Dark- (A, D, G) and bright-field (B, C, E, F, H, I) photomicrographs of sections of contralateral DRGs from sham mice, hybridized with ‘antisense’ (A, B, D, E, G, H) and ‘sense’ (C, F, I) riboprobes against VGLUT1 (A-C), VGLUT2 (D-F) and VGLUT3 (G-I). Boxes in A, D, G are shown at higher magnification in B, E, H. (A, B) Many VGLUT1 mRNA-positive DRG NPs are observed (A), and these are usually large and medium-sized (white double arrowheads in D). Some small to medium-sized VGLUT1 mRNA-positive NPs are also found (black double arrowheads in D). Asterisks in (A) and arrowheads in (D) show NPs negative for VGLUT1. (B, E) Abundant VGLUT2 mRNA-positive NPs are present in DRGs (B). Many exhibit small size (black double arrowheads in E), although several others are large and medium-sized (white double arrowheads in E). Asterisks in (B) and arrowheads in (E) show NPs negative for VGLUT2. (C, F). A modest number of VGLUT3 mRNA-positive NPs is detected (C), usually being of small size (white double arrowheads in F). Arrowheads in (F) show NPs negative for VGLUT3. (G-I) Hybridization with the control ‘sense’ riboprobe for each VGLUT revealed no specific signal for VGLUT1, VGLUT2, or VGLUT3 (G, H, I, respectively). However, a higher background was noticed in sections hybridized with both antisense (C, H) and sense (I) VGLUT3 riboprobes, likely due to the longer exposure times necessary for VGLUT3. Scale bars = 100 μm (C=A, D; I=B, C, E, F, H).
Figure 2.
VGLUTs transcripts exhibit different size-distribution patterns. Graphs showing the size distribution of VGLUT1- (A), VGLUT2- (B) or VGLUT3 (C) mRNA-positive DRG NPs. (A) Most VGLUT1 mRNA-positive NPs are large to medium-sized. (B) VGLUT2 mRNA-positive NPs show a widespread distribution, including large, medium-sized and small NPs. (C) Most VGLUT3 mRNA-positive NPs are small to medium-sized.
Incubation with the sense sequence of each VGLUT resulted in no specific hybridization for VGLUT1 (Figure 1C), VGLUT2 (Figure 1F) or VGLUT3 (Figure 1I).
VGLUT1-3 mRNA expression in spinal cord
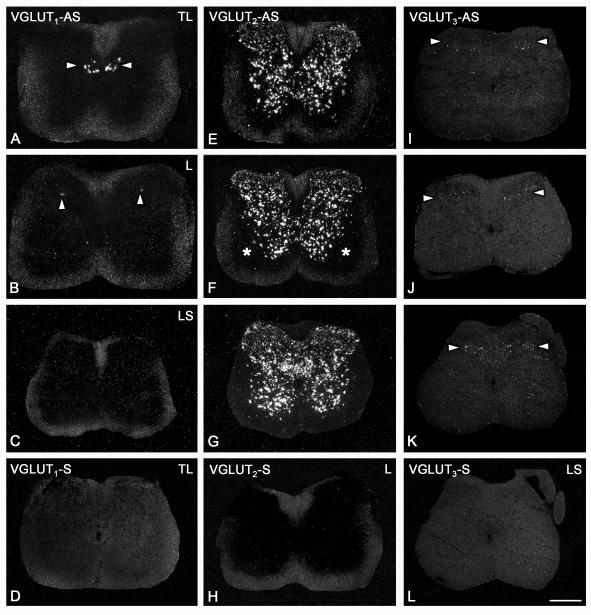
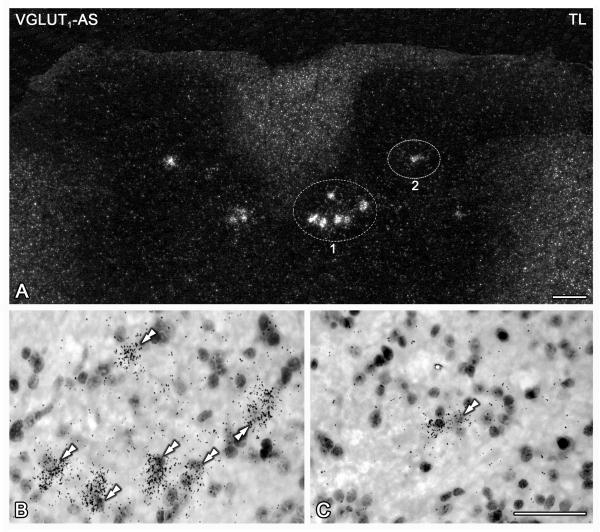
Presence of VGLUT1 mRNA was restricted to two locations in the analyzed segments of the spinal cord. In the thoracolumbar segment, a small population of VGLUT 1 mRNA-positive NPs was present almost exclusively in the dorsomedial part of the so-called intermediate zone of the dorsal horn (laminae IV-V) (Figure 3A). In addition, isolated VGLUT1 mRNA-positive NPs were detected in deep layers of the dorsal horn (laminae III-IV), at lumbar (Fig. 3B) or thoracolumbar (Fig. 4A-C) segments. In contrast, no VGLUT1 mRNA-positive NPs were observed in lumbosacral segments (Figure 3C).
Figure 3.
VGLUTs transcripts exhibit differential expressions across laminae and spinal cord levels. Dark-field photomicrographs of transverse sections of the spinal cord of sham-Axo mice at thoracolumbar (A, E, I, D), lumbar (B, F, J, H) and lumbosacral (C, G, K, L) segments, hybridized with antisense (A-C, E-G, I,K) and sense (D, H, L) riboprobes against VGLUT1 (A-D), VGLUT2 (E-H), or VGLUT3 (I-L). (A-C) A small number of VGLUT1 mRNA-positive neurons is detected in the dorsomedial aspect of the intermediate dorsal horn at thoracolumbar segments, possibly corresponding to the dorsal nucleus of Clarke (arrowheads in A). Occasional VGLUT1 mRNA-positive neurons are also detected in the medial part of the deep dorsal horn at lumbar segments (arrowheads in B). No neurons are detected at lumbosacral segments (C). (E-H) Abundant VGLUT2 mRNA-positive neurons are observed in the dorsal and ventral horns, and in area X at all analyzed spinal cord segments, with the exception of lamina IX, where virtually no VGLUT2 mRNA-positive neurons are detected (asterisks in F). (I-K) Only few weakly labeled VGLUT3 mRNA-positive neurons were detected in the spinal cord, and only in laminae III-IV of the dorsal horn, at all analyzed segments (arrowheads). (J-L) Hybridization with the control ‘sense’ riboprobe for each VGLUT resulted in no signal for VGLUT1, VGLUT2, or VGLUT3 (D, H, L, respectively). TL, thoracolumbar; L, lumbar; LS, lumbosacral. Scale bar: 100 μm (A-L).
Figure 4.
VGLUT1 mRNA is selectively expressed in spinal cord areas corresponding to the dorsal nucleus of Clarke. Dark- (A) and bright-field (B, C) photomicrographs of a section of the thoracolumbar spinal cord of sham mice, hybridized with the ‘antisense’ riboprobe against VGLUT1. Double arrowheads in (B) and (C) show VGLUT1 mRNA-positive neurons. (A-C) A small group of VGLUT1 mRNA-positive neurons is detected in, probably, the dorsal nucleus of Clarke (encircled area ‘1’ and double arrowheads in B). Additional isolated VGLUT1 mRNA-positive neurons are also found in the intermediate aspects of laminae IV-V of the dorsal horns (encircled area ‘2’ and double arrowheads in C). TL, thoracolumbar. Scale bars: 100 μm (A), 50 μm (C=B).
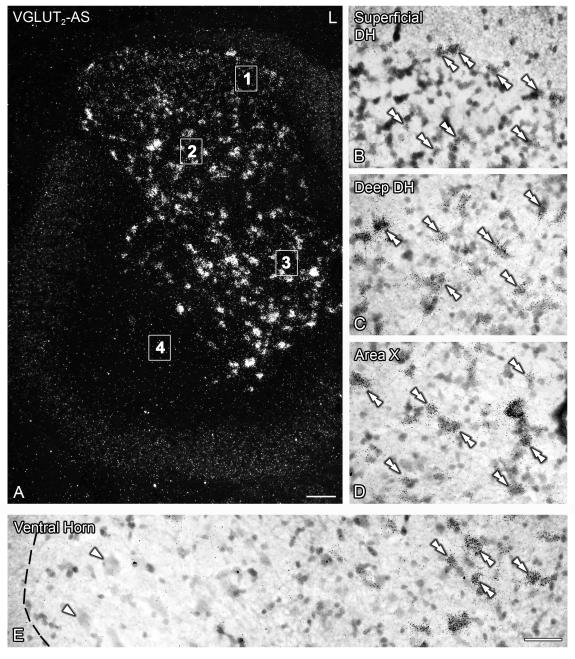
Numerous VGLUT2 mRNA-positive NPs were observed at all analyzed segments of the spinal cord. In the dorsal horn, neurons distinctly expressing VGLUT2 mRNA were detected in lamina I and deeper laminae (III-VI) (Figs. 3E-G; 5A-C). In contrast, laminae II-III exhibited a more diffuse VGLUT2 mRNA hybridization signal (Figs. 3E-G; 5A), virtually always corresponding to neuronal profiles (Fig. 5B, C). In area X and the medial aspects of the ventral horns, many VGLUT2 mRNA-positive neurons were detected (Figs. 3E-G; 5A, D, E). However, especially at the lumbar enlargement, the lateral aspects of the ventral horns appeared devoid of VGLUT2 mRNA-labeled neurons (Figs. 3F; 5A, E).
Figure 5.
VGLUT2 mRNA expression in the spinal cord is widespread and appears to exclude the area of motoneurons in the lumbar enlargement. Dark- (A) and bright-field (B-E) photomicrographs of a section of the lumbar spinal cord of sham mice, hybridized with the ‘antisense’ riboprobe against VGLUT2. Double arrowheads in (B-E) show VGLUT2 mRNA-positive neurons. Magnified images shown in (B-E) have been taken from the areas indicated with numbers (1-4) in (A), respectively. (A-E) Many VGLUT2 mRNA-positive neurons are detected in the superficial (A, B) and deep (A, C) dorsal horn, area X (A, D) and the medial aspect of the ventral horn (A, E). Note the diffuse VGLUT2 mRNA signal in the superficial dorsal horn, always associated with somatic profiles (double arrowheads in B). Also note the absence of VGLUT2 mRNA-positive neurons in the lateral aspect of the ventral horn (E, white arrowheads). The dotted line in (E) represents the border between the gray and white matter. L, lumbar. Scale bar: 100 μm (A), 50 μm (E=B-D).
A small number of weakly labeled VGLUT3 mRNA-positive neurons were detected in thoracolumbar, lumbar and lumbosacral segments of the dorsal horn, mostly in laminae III-IV (Figs. 3I, K; 6A, B).
Figure 6.
VGLUT3 mRNA is discretely expressed in some neurons in the deep dorsal horn. Dark- (A) and bright-field (B) photomicrographs of a section of the lumbosacral spinal cord of sham mice, hybridized with the ‘antisense’ riboprobe against VGLUT3. Double arrowheads in (A,B) show VGLUT3 mRNA-positive neurons. (A, B) Only few VGLUT3 mRNA-positive neurons are detected, always localized to laminae III-V of the dorsal horn (box in A; shown at higher magnification in B). LS, lumbosacral. Scale bars: 100 μm (A), 50 μm (B).
In all cases in the spinal cord, incubation with each corresponding sense VGLUT riboprobe revealed no specific hybridization signal (Fig. 3D, H, L).
Effects of Axotomy and 1- or 3-day hindpaw inflammation
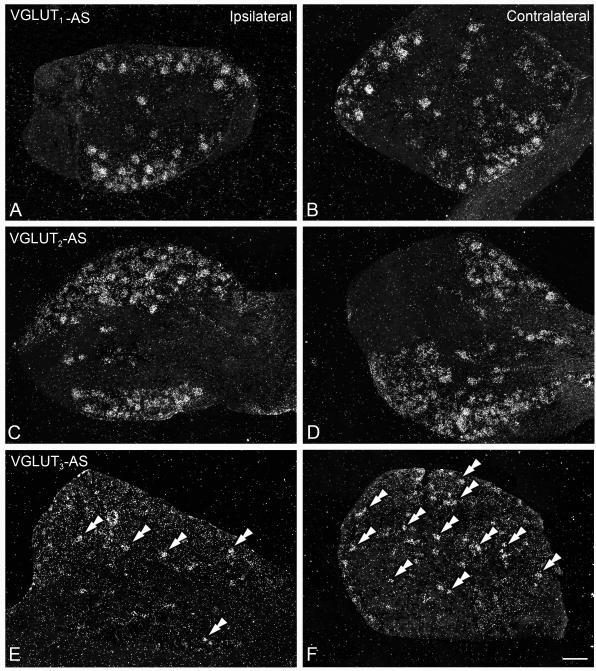
Whereas the number of VGLUT1 (Fig. 7A, B) and VGLUT2 (Fig. 7C, D) mRNA-positive L4-5 DRG NPs remained unaltered after axotomy of the sciatic nerve (Table 1), a discrete but significant reduction in the number of VGLUT3 mRNA-positive NPs was observed (one-way ANOVA, P=0.0334; 12.8 ± 0.4% ipsilateral AXO vs. 17.8 ± 1.6% contralateral AXO, Tukey’s, P<0.05; Table 1; Fig. 7E, F).
Figure 7.
Axotomy of the sciatic nerve induces downregulation of VGLUT3 mRNA expression in DRGs, leaving VGLUT1 and VGLUT2 unaffected. Dark-field photomicrographs of sections of ipsilateral (A, C, E) and contralateral (B, D, F) DRG of mice after a 7-day axotomy of the sciatic nerve, hybridized with ‘antisense’ riboprobes against VGLUT1 (A, B), VGLUT2 (C, D) or VGLUT3 (E, F). Double arrowheads in (E,F) show VGLUT3 mRNA-positive NPs. (A-F) A 7-day axotomy of the sciatic nerve does not alter the number of VGLUT1 or VGLUT2 mRNA-positive NPs in the ipsilateral DRG, as compared to the contralateral ones (A vs. B and C vs. D, respectively). In contrast, a decrease in the number of VGLUT3 mRNA-positive NPs was detected in ipsilateral DRG (E), as compared to the contralateral DRG (F). Scale bar: 100 μm (F=A-E).
No obvious changes were observed in the percentage of VGLUT1- or VGLUT2 mRNA-positive L4-5 DRG NPs after 1- or 3-day hindpaw inflammation (Table 1). The same outcome was obtained with VGLUT3, although one-way ANOVA analysis of the percentage of VGLUT3 mRNA-expressing NPs after 1-day hindpaw inflammation resulted in a small statistical significance (P=0.0491), that was, however, not supported by posthoc analysis between groups. Likewise, none of the injuries resulted in evident changes in the number of NPs expressing any of the VGLUT mRNAs at the spinal cord level (data not shown).
DISCUSSION
In this study, we present a thorough analysis of the presence of VGLUT1-3 transcripts in L4-5 DRGs and spinal cord NPs of mouse, including the effects of axotomy of the sciatic nerve or hindpaw inflammation. We show: 1) presence of VGLUT1, VGLUT2 and VGLUT3 transcripts in different proportions of DRG NPs and with different size distributions, with VGLUT2 being the most abundant transporter; 2) a larger percentage of VGLUT1 mRNA-positive DRG NPs than previously reported in mouse for its corresponding protein (Brumovsky et al., 2007); 3) presence of all VGLUTs in the spinal cord, with VGLUT2 being most abundantly expressed, followed by VGLUT1, almost exclusively in NPs that likely belong to the dorsal nucleus of Clarke, and finally VGLUT3, weakly detected in some NPs in the deep dorsal horn, and in accordance with a recent study in neonatal mice (Lou et al., 2013); 4) a small but significant decrease in the percentage of DRG NPs expressing the VGLUT3 transcript after sciatic nerve axotomy; VGLUT1 and VGLUT2 transcripts remained unaltered; 5) lack of any detectable change in the number of neurons expressing any VGLUTs transcript after hindpaw inflammation.
VGLUT transcript expression in mouse DRGs
In the first in situ hybridization analysis of the expression of VGLUT1 in rat L4-5 DRGs, Oliveira et al. (2003) reported that “…Many DRG neurons were VGLUT1 mRNA positive, and they were primarily large…”, although no quantification was performed at that time. In the rat trigeminal ganglia, ~80% of VGLUT1-immunoreactive (IR) neurons were detected (Li et al., 2003b), and Landry et al. (Landry et al., 2004) reported that most L4-5 DRG neurons in the rat expressed VGLUT1 transcript. A few years later, Morris et al. (2005) reported in mouse L4-5 DRGs up to 37% of VGLUT1-IR neurons, a proportion comparable to the ~32% VGLUT1-IR neurons projecting to the mouse urinary bladder (Brumovsky et al., 2012). In the present study in mouse, we show that ~45% of L4-5 DRG neurons of large and medium size express VGLUT1 mRNA. However, in other immunohistochemical studies in mouse, only up to ~11% L4-5 DRG neurons expressing VGLUT1 (Brumovsky et al., 2007) were detected, similar to the percentage of DRG neurons projecting to the colorectum (~12%; Brumovsky et al., 2011a). Differences in the number of neurons expressing the VGLUT1 protein between DRG levels could result from differential protein and gene regulation at each level. More difficult to explain are the differences in protein and transcript expressions across studies, although they could relate to the use of various types of VGLUT1 antibodies/probes, immunohistochemical and in situ hybridization techniques or even mouse strains. Alternatively, it could also be speculated that not all VGLUT1 transcript is translated into protein, suggesting different regulatory mechanisms.
In the present study, we found that the VGLUT2 mRNA is expressed by ~69% of mouse L4-5 DRG neurons, consistent with the percentages obtained in a recent study (~82%; Scherrer et al., 2010). In accordance, in a previous study in mouse we found that VGLUT2 protein is present in ~65% of L4-5 DRG neurons (Brumovsky et al., 2007). An even more abundant presence of VGLUT2-expressing NPs has also been show in mouse DRG (~90%; Scherrer et al., 2010) and rat trigeminal ganglia (~80%; Li et al., 2003b), and in mouse visceral DRG neurons, where more than 90% and up to 98% of urinary bladder- (Brumovsky et al., 2012) or colorectum-projecting (Brumovsky et al., 2011a) NPs express VGLUT2, respectively. VGLUT2-expressing DRG neurons span all cell soma sizes, although many of them are small and medium-sized (Brumovsky et al., 2007; 2011a; 2012; present study). Altogether, the abundance of VGLUT2 in a variety of peripheral sensory neurons supports the hypothesis that this is the main VGLUT in DRG neurons, and of its likely importance for glutamatergic neurotransmission.
We detected VGLUT3 transcript in ~17% of mouse L4-5 DRG NPs. This small percentage is in agreement with studies using transgenic mice (Seal et al., 2009; Lou et al., 2013). Thus, Seal et al. (2009) showed that 10-11% of L4-5 DRG or trigeminal ganglion neurons in the mouse express VGLUT3-EGFP. In a more recent study, using the reporter gene Tomato, ~19% of adult mouse L4-5 DRG neurons of small size were shown to express VGLUT3 (Lou et al., 2013). Interestingly, these authors also showed a transient vs. persistent expression of VGLUT3. Thus, the transporter was found in large and medium-sized myelinated DRG neurons during prenatal stages, it was downregulated during neonatal stages, and only the small neuron population remained VGLUT3-expressing during the adult life (Lou et al., 2013). Here, we not only detected VGLUT3 mRNA in small but also in medium-sized DRG NPs. It remains to be established whether the latter reflect some degree of persistence in the expression of the VGLUT3 (transcript and/or protein) from prenatal stages. Finally, visceral DRGs projecting to the mouse colorectum (Brumovsky et al., 2011a) or urinary bladder (Brumovsky et al., 2012) also present with low percentages of VGLUT3-expressing small DRG neurons (~10% and ~18%, respectively).
The abundance of VGLUT2 over the other two VGLUTs implies a certain degree of coexistence, a concept that was originally rejected (Fremeau, Jr. et al., 2001; Kaneko and Fujiyama, 2002; Schäfer et al., 2002), but is slowly gaining acceptance, for neurons both in the central (Boulland et al., 2004; Herzog et al., 2006) as well as the peripheral nervous systems (Li et al., 2003b; Landry et al., 2004; Brumovsky et al., 2007). In fact, coexistence of VGLUT1 and VGLUT2-Lis has been shown in rat trigeminal ganglion neurons (Li et al., 2003b), and in rat (Landry et al., 2004) or mouse L4-5 DRG neurons (Brumovsky et al., 2007); preliminary results in our laboratory also indicate that VGLUT2 and VGLUT3 largely coexpress in L4-5 DRGs (unpublished results). Moreover, the overwhelming expression of VGLUT2 in colorectal (Brumovsky et al., 2011a) and urinary bladder (Brumovsky et al., 2012) DRG neurons observed in mouse strongly supports coexpression with other VGLUTs in visceral neurons. In conclusion, DRG neurons may be provided with at least two different VGLUTs, although subpopulations expressing only one type of VGLUT are also likely.
It has been suggested that VGLUT expression may be associated with different patterns of neurotransmitter release. Thus, VGLUT1 is normally expressed in CNS neurons with low probability release (climbing fibers in the cerebellum), whereas VGLUT2 would be associated to those with high probability (parallel fibers in the cerebellum) (Dittman and Regehr, 1998). To what extent the type of VGLUT can be associated with release probability also in DRG neurons will require further research. An interesting question, then, is also the significance of the presence of more than one VGLUT in DRG neurons for glutamate release.
VGLUT transcript expression in mouse spinal cord
In the present study in adult mouse, neurons expressing different VGLUT transcripts were detected in the spinal cord, confirming previous studies in rat (Oliveira et al., 2003; Landry et al., 2004; Llewellyn-Smith et al., 2007) and neonatal mouse (Kullander et al., 2003; Lou et al., 2013). Each VGLUT exhibited a differential distribution across the analyzed laminae and spinal segments. VGLUT1 was almost exclusively detected in the thoracolumbar spinal cord, in a subpopulation of neurons located in the dorsomedial part of the intermediate zone of the dorsal horn (laminae IV-V). Interestingly, this area is known to contain the dorsal nucleus of Clarke, the origin of the spinocerebellar pathways (Mann, 1973). Additional isolated VGLUT1 mRNA-positive neurons were also found in the medial aspects of the deep dorsal horn (laminae III-IV). Other neurons, including motoneurons or superficial dorsal horn neurons appeared to lack VGLUT1, in contrast to observations in rat, where VGLUT1 mRNA positive neurons are also reported in lamina I of the dorsal horn, as well as in what appear to be motoneurons (Landry et al., 2004). Differences in the type of rodent, in situ hybridization techniques or riboprobes may explain the discrepancy.
In addition to its presence in spinal neurons, an abundant and dorsal rhizotomy-susceptible, VGLUT1-IR neuropil is found both in the ventral and dorsal horns (Oliveira et al., 2003; Alvarez et al., 2004; Hughes et al., 2004; Brumovsky et al., 2007; Llewellyn-Smith et al., 2007). A great proportion of these fibers is contributed by large and medium-sized VGLUT1-expressing DRG neurons, and have been ascribed roles in proprioception and the regulation of motor function (Oliveira et al., 2003; Todd et al., 2003; Kullander et al., 2003; Alvarez et al., 2004; Landry et al., 2004; Brumovsky et al., 2007; Llewellyn-Smith et al., 2007). Compelling evidence for a role in proprioception is the identification of numerous fibers in the ventral horn exhibiting VGLUT1-Li and colocalizing with parvalbumin, a marker of muscle proprioceptors, and known to participate in primary afferent-motoneuron contacts (see Alvarez et al., 2004; Hughes et al., 2004). It should be noted, however, that pyramidal cells in the neocortex express VGLUT1 mRNA (Fremeau, Jr. et al., 2001) and numerous rat corticospinal tract nerve fibers terminating in the ventral horns exhibit VGLUT1-Li (Persson et al., 2006; Du et al., 2012), thus implying the participation of glutamate not only in proprioception (primary afferent input), but also in the supraspinal, cortical control of motoneurons.
VGLUT1-Li is also detected in low-threshold cutaneous and muscle mechanoreceptors terminating in the superficial and deep dorsal horn (Todd et al., 2003; Alvarez et al., 2004; Hughes et al., 2004). Interestingly, abundant VGLUT1-IR primary afferent fibers appear to terminate in the area occupied by Clarke’s nucleus (Oliveira et al., 2003; Alvarez et al., 2004; Brumovsky et al., 2007), which contains the second order neurons that give rise to the spinocerebellar pathway (Mann, 1973). If these fibers did in fact establish synaptic contact with Clarke’s neurons, it could be hypothesized that they belong to the propriocetive spinocerebellar pathway and that it altogether utilizes VGLUT1 and glutamate for the transmission of proprioceptive information to the cerebellum. In support, abundant VGLUT1-Li is detected in nerve fibers terminating in the anterior and posterior zones of the cerebellum (Gebre et al., 2012), known to receive input from spinocerebellar mossy fibers (see Sillitoe et al., 2010).
In contrast to VGLUT1, VGLUT2 exhibited a widespread expression, being detected not only in almost all laminae of the dorsal and ventral horns, but also across all analyzed spinal segments (thoracolumbar to lumbosacral). Our data confirm previous studies in rat (Oliveira et al., 2003; Todd et al., 2003; Llewellyn-Smith et al., 2007) and neonatal mouse (Kullander et al., 2003) on the synthesis and abundance of VGLUT2 in local spinal cord neurons. A diffuse VGLUT2 mRNA signal was observed in laminae II-III, even though it always appeared associated with neuronal somata. It has been suggested that a similar type of signal in the rat spinal cord related to the small size and possibly low transcript levels in these neurons (Oliveira et al., 2003). The virtual absence of VGLUT2 in lamina IX, especially noticeable at the lumbar enlargement, along with the absence of VGLUT1 (and VGLUT3), further suggests that motoneurons and other interneurons in this lamina, and least at this spinal segment, do not synthesize glutamate (Oliveira et al., 2003; Kullander et al., 2003; Llewellyn-Smith et al., 2007). Whether motoneurons, which do express other glutamatergic markers such as glutamate itself (Meister et al., 1993; Waerhaug and Ottersen, 1993) and/or the plasma membrane-bound neuronal EAAT-3 (Meister et al., 1993), utilize a yet undescribed VGLUT remains to be established.
The abundance and distribution of VGLUT2 in spinal cord neurons in the rat (Oliveira et al., 2003; Todd et al., 2003; Kullander et al., 2003; Landry et al., 2004; Llewellyn-Smith et al., 2007) and mouse (present study) suggests its expression in both interneurons (Oliveira et al., 2003; Todd et al., 2003) and projection neurons. In fact, immunohistochemical analysis of nerve terminals produced by interneurons in the spinal cord of rat demonstrated that many of such terminals, which express somatostatin, neurotensin, substance P and/or enkephalin, also express VGLUT2 (Todd et al., 2003). More definitive evidence is provided by electrophysiology studies in the rat, where functionally identified excitatory interneurons show presence of VGLUT2-Li (Maxwell et al., 2007; Schneider et al., 2007; Yasaka et al., 2010). Our data supports the existence of several populations of excitatory glutamatergic spinal interneurons utilizing VGLUT2, also in the mouse. In addition, studies showing VGLUT2-Li in the large lemniscal and spinothalamic terminals to the ventral posterior thalamic nuclei in the rat (Graziano et al., 2008), also suggest that at least a number of neurons expressing VGLUT2 mRNA in the rat and mouse dorsal horn are projection neurons. Moreover, coexpression of both VGLUT1- and VGLUT2-Lis in mossy fibers in the cerebellum (see Gebre et al., 2012) suggests that Clarke’s nucleus also expresses VGLUT2.
The presence of VGLUT2 in numerous spinal cord neurons also supports the concept that the abundant VGLUT2-Li normally observed in the gray matter of the spinal cord reported in rat (Oliveira et al., 2003; Li et al., 2003a; Alvarez et al., 2004; Landry et al., 2004) and mouse (Brumovsky et al., 2007) strongly depends on local sources. In support, dorsal rhizotomy fails to reduce VGLUT2-Li in the rodent spinal cord (Oliveira et al., 2003; Alvarez et al., 2004; Brumovsky et al., 2007), revealing only a minor contribution by DRG neurons (Todd et al., 2003; Alvarez et al., 2004). However, dorsal root ligation results in a modest accumulation of VGLUT2-Li (DRG side) in the mouse, implying low quantities of VGLUT2 being transported from the DRGs to the spinal cord (Brumovsky et al., 2007). Moreover, Todd et al. (2003) reported variable intensities (from low to high) of VGLUT2-Li in myelinated and unmyelinated primary afferent fibers. Therefore, a modest contribution of VGLUT2 by DRG neurons, overlapped by a strong local presence in spinal interneurons and projection neurons, may explain the apparent lack of change in VGLUT2-Li after dorsal rhizotomy. A similar hypothesis has been proposed for the expression of the neuropeptide tyrosine Y1 receptor in rat primary afferents (Brumovsky et al., 2002).
Gras et al. (2002) were the first to report, based on RT-PCR of tissue sample explants, the presence of VGLUT3 mRNA in the rat dorsal and ventral horns of the spinal cord. However, later studies showing lack of mRNA in situ hybridization signal of this transporter (Oliveira et al., 2003) suggested that VGLUT3 was not expressed by local spinal cord neurons in the rat. Such an assumption was strengthened by studies in transgenic mice, where VGLUT3-EGFP was virtually absent in the spinal cord (Seal et al., 2009). In contrast, in another study in adult rats, using non-radioactive riboprobe in situ hybridization, a very modest expression of VGLUT3 was reported in deep laminae of the dorsal horn, and in the ventral horn (Landry et al., 2004). In the present study in adult mice, VGLUT3 appeared as the least expressed transporter, and only weakly detected in some neurons in laminae III-IV of the dorsal horn. Interestingly, a recently published study demonstrates presence of VGLUT3 transcript in what appears to be two populations, one in the superficial and another in the deep layers of the dorsal horn of neonatal mice (Lou et al., 2013). It is possible that VGLUT3-expressing neurons in the superficial dorsal horn lost the capacity to synthesize the transporter in adult life, and only a limited number of neurons in deeper laminae remained capable of producing detectable quantities of the transporter, as shown here. It is not clear, if VGLUT3 transcript in these neurons is translated into protein. However, a virtual absence of VGLUT3-EGFP-IR neurons was reported in transgenic mice (Seal et al., 2009). In such case, the moderate VGLUT3-EGFP-Li detected in the superficial dorsal horn would be mostly dependent on the contribution from DRG neurons; in fact, dorsal rhizotomy completely abolished the presence of VGLUT3-EGFP-Li (Seal et al., 2009). However, it should be noted that nerve fibers exhibiting VGLUT3-Li have also been described in the sympathetic intermediolateral column, although their coexpression with serotonin supports a supraspinal origin (see Oliveira et al., 2003).
Effects of peripheral nerve injury and hindpaw inflammation on VGLUT transcripts
VGLUTs, in particular VGLUT2 (Moechars et al., 2006; Leo et al., 2009; Lagerström et al., 2010; Liu et al., 2010; Scherrer et al., 2010) and VGLUT3 (Seal et al., 2009; Yang et al., 2012; Lou et al., 2013), have been proposed as relevant to pain mechanisms during conditions such as peripheral nerve injury or tissue inflammation. Both types of injuries result in the down-regulation and up-regulation of numerous molecules involved in a variety of functions in DRG and sympathetic ganglia neurons, as well as motoneurons in the spinal cord (Costigan et al., 2002; Xiao et al., 2002; Ren et al., 2005; Anseloni et al., 2005; Hökfelt et al., 2007; Navarro et al., 2007; Hökfelt et al., 2013). Accordingly, the protein expression of VGLUTs appears to be challenged by peripheral nerve injury. Hughes et al., (2004) demonstrated that axotomy of the sciatic nerve in rat induces depletion of VGLUT1 protein in low-threshold cutaneous and muscle mechanoreceptors terminating in the dorsal and ventral horns. Such depletion most likely results from the dramatic decrease in VGLUT1-Li observed after axotomy of the sciatic nerve in large and medium-sized DRG neurons, as shown in mouse (Brumovsky et al., 2007). Axotomy of the sciatic nerve in the mouse also results in a moderate reduction in the number of VGLUT2-IR DRG neurons, although an increase of VGLUT2-Li was also detected in a subpopulation of small DRG neurons (Brumovsky et al., 2007). However, and in contrast to VGLUT1, these changes do apparently not translate into the expected decreases/increases in VGLUT2-Li at the lumbar segments of the spinal cord, probably due to a modest centrifugal transport through central projections and dense local VGLUT2 systems (see above) (Brumovsky et al., 2007).
Because changes in transcriptional regulation of VGLUTs induced by different pathological stimuli had been demonstrated in neurons in the CNS (see Smith et al., 2001; Kawasaki et al., 2005; Kawano et al., 2006), and based on the protein changes described above (Hughes et al., 2004; Brumovsky et al., 2007), we hypothesized that VGLUT transcripts would also exhibit alterations after peripheral nerve injury. However, here we show that the number of VGLUT1- or VGLUT2 mRNA-positive DRG (and spinal cord) neurons were unchanged in mice 7 days after sciatic nerve axotomy. Moreover, only a modest down-regulation in the number of VGLUT3 mRNA positive DRG neurons was observed after axotomy. Differences in the techniques (in situ hybridization vs. immunohistochemistry) and mouse strains (BalbC vs. NMRI mice) between studies could explain the discrepancy.
As described in previous sections, neonatal mice express VGLUT3 in medium-sized and large DRG neurons, and this disappears during adulthood (Lou et al., 2013). Because other molecules, for example the neuropeptide galanin also exhibit abundant prenatal expression that is lost during adulthood but evoked by peripheral nerve injury (Xu et al., 1996), an increase in the number of VGLUT3 mRNA-expressing large and medium-sized DRG NPs could have been expected. However, besides the down-regulation in small DRG neurons described in the present study, no evidence of VGLUT3 transcript up-regulation was obtained.
Despite the modest changes reported here in VGLUTs transcripts after peripheral nerve injury, the downregulation of VGLUT1- (Hughes et al., 2004) and VGLUT2-Lis (Brumovsky et al., 2007) and VGLUT3 mRNAs in DRG neurons (present study) may still reflect cellular efforts to counteract excess excitability at the spinal cord level, or even a protection mechanism against toxic glutamate levels in the extracellular space. In fact, in Drosophila, the overexpression of VGLUTs has been associated with increased spontaneous glutamate release, behavioral defects and premature death (Daniels et al., 2011). Alternatively, it could be speculated that, despite protein down-regulation of VGLUT1 and VGLUT2 protein after injury, DRG neurons maintain their transcription and contribute to the increased excitatory mechanisms at the spinal level. Such a scenario could be compatible with protein depletion at the neuronal cell body due to an increased centrifugal transport of synaptic vesicles containing VGLUTs, and use at peripheral and central terminals (Brumovsky et al., 2007). In support of this hypothesis, it has been observed that axotomy of the sciatic nerve in rats results in a reduction in the number of synaptic vesicles in the central terminals of axotomized primary afferents (Kapadia and LaMotte, 1987), including peptidergic ones (Zhang et al., 1995), suggesting increased clear synaptic vesicles (also containing VGLUTs) turnover.
Several lines of evidence support a role for glutamate in the periphery and during inflammatory processes. Thus, electrical stimulation of sciatic nerve A and/or C fibers, peripheral formalin and capsaicin treatments increase glutamate in the hindpaw extracellular space, likely due to its release by peripheral nerve endings arising in DRG somata (Agrawal and Evans, 1986; Evans et al., 1987; deGroot et al., 2000). Accordingly, exogenously administered glutamate activates peripheral glutamate receptors, resulting in depolarization of primary afferent C fibers and induction of pain-related behavior (Carlton et al., 1995; Jackson et al., 1995; deGroot et al., 2000; Tian et al., 2005). Interestingly, glutaminase, the enzyme catalyzing the synthesis of glutamate from the precursor glutamine, is up-regulated in rat DRG neurons during hindpaw inflammation (Hoffman and Miller, 2010; Miller et al., 2012). Therefore, it seemed plausible that tissue inflammation could drive changes in the transcript expression of VGLUTs. However, here we show that inflammation of the hindpaw also fails to induce detectable changes in the number of neurons expressing VGLUT1, VGLUT2 or VGLUT3 mRNAs, either in DRGs or the spinal cord. It remains to be established if the protein expression of VGLUTs is altered by hindpaw inflammation.
The lack of changes described above after peripheral nerve injury (with the exception of the modest down-regulation of VGLUT3 in DRG neurons) or inflammation may also reflect the essential role of synaptic vesicle/protein regulation and trafficking at the glutamatergic synapse (see Santos et al., 2009). An active and tight regulation of presynaptic vesicle and transmitter recycling occurs at the level of the synaptic cleft, to counteract depletion in situations of high activity (see Edwards, 2007; Chaudhry et al., 2008; Santos et al., 2009). Moreover, glutamate concentration, crucial in defining intravesicular glutamate content (see Edwards, 2007; Chaudhry et al., 2008), is 2 to 3 times higher in the terminals than in the cell body (see Featherstone, 2010). In support, the intraplantar inhibition of glutaminase reduces the inflammation-induced hindpaw edema and cFos expression in laminae I-II of the dorsal horn of rats, resulting in long-lasting analgesia (Hoffman and Miller, 2010). Altogether, it is possible that nerve injury and/or inflammation resulted in changes in the expression of synaptic vesicles, associated proteins (including VGLUTs) and neuronal glutamatergic machinery in general, contributing to a finer ‘tuning’ of pain mechanisms at the synaptic rather than at the cell body level.
In conclusion, these data further extend earlier (Tong et al., 2001; Varoqui et al., 2002; Oliveira et al., 2003; Todd et al., 2003; Kullander et al., 2003; Alvarez et al., 2004; Hwang et al., 2004; Landry et al., 2004; Morris et al., 2005; Persson et al., 2006; Brumovsky et al., 2007; Llewellyn-Smith et al., 2007) and more recent studies (Seal et al., 2009; Lou et al., 2013) on the expression of VGLUTs in non-visceral DRGs and the spinal cord, and reveal a very modest reaction to injury, only for VGLUT3 transcript and after peripheral nerve axotomy. Further research will be necessary to better understand the transcriptional and translational regulation of VGLUTs and their impact in normal as well as pathological conditions.
Mouse DRG neurons express VGLUTs mRNA in different proportions and size distributions
Many VGLUT2 mRNA-positive neurons are found at most laminae and spinal cord levels
VGLUT1 and VGLUT3 transcripts show a discrete expression in the mouse spinal cord
Only VGLUT3 mRNA downregulation is observed after sciatic nerve axotomy
Acknowledgements
We thank the generous donation of VGLUT1 and VGLUT2 DNA constructs by Dr. Akiya Watakabe, National Institute for Basic Biology, Okazaki, Japan. We also thank Tim McMurray and Michael Burcham for excellent technical assistance and Carly McCarthy for valuable comments on the manuscript. This study was supported by National Institutes of Health Awards NS035790 and DK093525 (GFG), the Selma Schottenstein Harris Laboratory for Research in Parkinson’s (KBS), by American Diabetes Association Innovation Award and the Competitive Medical Research Fund Award (RPS), and by IASP Early Career Research Award and an Austral University Grant (PRB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Agrawal SG, Evans RH. The primary afferent depolarizing action of kainate in the rat. Br. J. Pharmacol. 1986;87:345–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara Y, Mashima H, Onda H, Hisano S, Kasuya H, Hori T, Yamada S, Tomura H, Yamada Y, Inoue I, Kojima I, Takeda J. Molecular cloning of a novel brain-type Na(+)-dependent inorganic phosphate cotransporter. J Neurochem. 2000;74:2622–2625. doi: 10.1046/j.1471-4159.2000.0742622.x. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- Anseloni VC, He F, Novikova SI, Turnbach RM, Lidow IA, Ennis M, Lidow MS. Alterations in stress-associated behaviors and neurochemical markers in adult rats after neonatal short-lasting local inflammatory insult. Neuroscience. 2005;131:635–645. doi: 10.1016/j.neuroscience.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Bai L, Xu H, Collins JF, Ghishan FK. Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J. Biol. Chem. 2001;276:36764–36769. doi: 10.1074/jbc.M104578200. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr., Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Binns BC, Huang Y, Goettl VM, Hackshaw KV, Stephens RL., Jr. Glutamate uptake is attenuated in spinal deep dorsal and ventral horn in the rat spinal nerve ligation model. Brain Res. 2005;1041:38–47. doi: 10.1016/j.brainres.2005.01.088. [DOI] [PubMed] [Google Scholar]
- Boulland JL, Qureshi T, Seal RP, Rafiki A, Gundersen V, Bergersen LH, Fremeau RT, Jr., Edwards RH, Storm-Mathisen J, Chaudhry FA. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. doi: 10.1002/cne.20354. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Watanabe M, Hökfelt T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 2007;147:469–490. doi: 10.1016/j.neuroscience.2007.02.068. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Robinson DR, La JH, Seroogy KB, Lundgren KH, Albers KM, Kiyatkin ME, Seal RP, Edwards RH, Watanabe M, Hökfelt T, Gebhart GF. Expression of vesicular glutamate transporters type 1 and 2 in sensory and autonomic neurons innervating the mouse colorectum. J. Comp Neurol. 2011a;519:3346–3366. doi: 10.1002/cne.22730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky PR, Seal RP, Lundgren KH, Seroogy KB, Watanabe M, Gebhart GF. Expression of Vesicular Glutamate Transporters in Sensory and Autonomic Neurons Innervating the Mouse Bladder. J. Urol. 2012 doi: 10.1016/j.juro.2012.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky PR, Seroogy KB, Lundgren KH, Watanabe M, Hökfelt T, Gebhart GF. Some lumbar sympathetic neurons develop a glutamatergic phenotype after peripheral axotomy with a note on VGLUT(2)-positive perineuronal baskets. Exp. Neurol. 2011b;230:258–272. doi: 10.1016/j.expneurol.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky PR, Shi TJ, Matsuda H, Kopp J, Villar MJ, Hökfelt T. NPY Y1 receptors are present in axonal processes of DRG neurons. Exp. Neurol. 2002;174:1–10. doi: 10.1006/exnr.2001.7845. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci. Lett. 1995;197:25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Boulland JL, Jenstad M, Bredahl MK, Edwards RH. Pharmacology of neurotransmitter transport into secretory vesicles. Handb. Exp. Pharmacol. 2008:77–106. doi: 10.1007/978-3-540-74805-2_4. [DOI] [PubMed] [Google Scholar]
- Chiosa L, Gane P. Action of glutamic acid on the higher nervous activity of small laboratory animals (rats); considerations on the relations between the biochemical substratum and the higher nervous activity. Rev. Sci. Med. 1956;1:165–171. [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Phillis JW, Watkins JC. The chemical excitation of spinal neurones by certain acidic amino acids. J. Physiol. 1960;150:656–682. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Miller BR, DiAntonio A. Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol. Dis. 2011;41:415–420. doi: 10.1016/j.nbd.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deGroot J, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport. 2000;11:497–502. doi: 10.1097/00001756-200002280-00014. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J. Neurosci. 1998;18:6147–6162. doi: 10.1523/JNEUROSCI.18-16-06147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du BA, Shakya SS, Bannatyne BA, Jalicy SM, Linnen S, Maxwell DJ. Neurotransmitter phenotypes of descending systems in the rat lumbar spinal cord. Neuroscience. 2012;227:67–79. doi: 10.1016/j.neuroscience.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Evans RH, Evans SJ, Pook PC, Sunter DC. A comparison of excitatory amino acid antagonists acting at primary afferent C fibres and motoneurones of the isolated spinal cord of the rat. Br. J. Pharmacol. 1987;91:531–537. doi: 10.1111/j.1476-5381.1987.tb11246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone DE. Intercellular glutamate signaling in the nervous system and beyond. ACS Chem. Neurosci. 2010;1:4–12. doi: 10.1021/cn900006n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gebre SA, Reeber SL, Sillitoe RV. Parasagittal compartmentation of cerebellar mossy fibers as revealed by the patterned expression of vesicular glutamate transporters VGLUT1 and VGLUT2. Brain Struct. Funct. 2012;217:165–180. doi: 10.1007/s00429-011-0339-4. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El MS. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano A, Liu XB, Murray KD, Jones EG. Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. J. Comp Neurol. 2008;507:1258–1276. doi: 10.1002/cne.21592. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Otsuka M, Morimoto R, Hirota S, Yatsushiro S, Takeda J, Yamamoto A, Moriyama Y. Differentiation-associated Na+-dependent inorganic phosphate cotransporter (DNPI) is a vesicular glutamate transporter in endocrine glutamatergic systems. J. Biol. Chem. 2001;276:43400–43406. doi: 10.1074/jbc.M106244200. [DOI] [PubMed] [Google Scholar]
- Hemmerle AM, Dickerson JW, Herring NR, Schaefer TL, Vorhees CV, Williams MT, Seroogy KB. (+/−)3,4-methylenedioxymethamphetamine (“ecstasy”) treatment modulates expression of neurotrophins and their receptors in multiple regions of adult rat brain. J. Comp Neurol. 2012;520:2459–2474. doi: 10.1002/cne.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El MS. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J. Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Takamori S, Jahn R, Brose N, Wojcik SM. Synaptic and vesicular co-localization of the glutamate transporters VGLUT1 and VGLUT2 in the mouse hippocampus. J. Neurochem. 2006;99:1011–1018. doi: 10.1111/j.1471-4159.2006.04144.x. [DOI] [PubMed] [Google Scholar]
- Hoffman EM, Miller KE. Peripheral inhibition of glutaminase reduces carrageenan-induced Fos expression in the superficial dorsal horn of the rat. Neurosci. Lett. 2010;472:157–160. doi: 10.1016/j.neulet.2010.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Brumovsky P, Villar MJ. Retrograde cellular changes after nerve injury. In: Schmidt RF, Willis WD, editors. Encyclopedia of Pain. Springer; 2007. pp. 2122–2126. [Google Scholar]
- Hökfelt T, Zhang X, Villar MJ, Xu XJ, Wiesenfeld-Hallin Z. Central consequences of peripheral nerve damage. In: McMahon S, Koltzenburg M, Tracey I, Turk DC, editors. Textbook of pain. Elsevier; 2013. [Google Scholar]
- Hughes DI, Polgar E, Shehab SA, Todd AJ. Peripheral axotomy induces depletion of the vesicular glutamate transporter VGLUT1 in central terminals of myelinated afferent fibres in the rat spinal cord. Brain Res. 2004;1017:69–76. doi: 10.1016/j.brainres.2004.05.054. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Burette A, Rustioni A, Valtschanoff JG. Vanilloid receptor VR1-positive primary afferents are glutamatergic and contact spinal neurons that co-express neurokinin receptor NK1 and glutamate receptors. J Neurocytol. 2004;33:321–329. doi: 10.1023/B:NEUR.0000044193.31523.a1. [DOI] [PubMed] [Google Scholar]
- Jackson DL, Graff CB, Richardson JD, Hargreaves KM. Glutamate participates in the peripheral modulation of thermal hyperalgesia in rats. Eur. J. Pharmacol. 1995;284:321–325. doi: 10.1016/0014-2999(95)00449-u. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42:243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kapadia SE, LaMotte CC. Deafferentation-induced alterations in the rat dorsal horn: I. Comparison of peripheral nerve injury vs. rhizotomy effects on presynaptic, postsynaptic, and glial processes. J. Comp Neurol. 1987;266:183–197. doi: 10.1002/cne.902660205. [DOI] [PubMed] [Google Scholar]
- Karim F, Bhave G, Gereau RW. Metabotropic glutamate receptors on peripheral sensory neuron terminals as targets for the development of novel analgesics. Mol. Psychiatry. 2001;6:615–617. doi: 10.1038/sj.mp.4000961. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Hoshi K, Kawano M, Nogami H, Yoshikawa H, Hisano S. Up-regulation of VGLUT2 expression in hypothalamic-neurohypophysial neurons of the rat following osmotic challenge. Eur J Neurosci. 2005;22:672–680. doi: 10.1111/j.1460-9568.2005.04240.x. [DOI] [PubMed] [Google Scholar]
- Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydstrom A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- Lagerström MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, Wood JN, Wallen-Mackenzie A, Kullander K. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M, Bouali-Benazzouz R, El MS, Ravassard P, Nagy F. Expression of vesicular glutamate transporters in rat lumbar spinal cord, with a note on dorsal root ganglia. J Comp Neurol. 2004;468:380–394. doi: 10.1002/cne.10988. [DOI] [PubMed] [Google Scholar]
- Leo S, Moechars D, Callaerts-Vegh Z, D’Hooge R, Meert T. Impairment of VGLUT2 but not VGLUT1 signaling reduces neuropathy-induced hypersensitivity. Eur J Pain. 2009;13:1008–1017. doi: 10.1016/j.ejpain.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Li JL, Fujiyama F, Kaneko T, Mizuno N. Expression of vesicular glutamate transporters, VGluT1 and VGluT2, in axon terminals of nociceptive primary afferent fibers in the superficial layers of the medullary and spinal dorsal horns of the rat. J Comp Neurol. 2003a;457:236–249. doi: 10.1002/cne.10556. [DOI] [PubMed] [Google Scholar]
- Li JL, Xiong KH, Dong YL, Fujiyama F, Kaneko T, Mizuno N. Vesicular glutamate transporters, VGluT1 and VGluT2, in the trigeminal ganglion neurons of the rat, with special reference to coexpression. J Comp Neurol. 2003b;463:212–220. doi: 10.1002/cne.10755. [DOI] [PubMed] [Google Scholar]
- Liaw WJ, Stephens RL, Jr., Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain. 2005;115:60–70. doi: 10.1016/j.pain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Liu Y, Abdel SO, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Martin CL, Fenwick NM, Dicarlo SE, Lujan HL, Schreihofer AM. VGLUT1 and VGLUT2 innervation in autonomic regions of intact and transected rat spinal cord. J. Comp Neurol. 2007;503:741–767. doi: 10.1002/cne.21414. [DOI] [PubMed] [Google Scholar]
- Lou S, Duan B, Vong L, Lowell BB, Ma Q. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J. Neurosci. 2013;33:870–882. doi: 10.1523/JNEUROSCI.3942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann MD. Clarke’s column and the dorsal spinocerebellar tract: a review. Brain Behav. Evol. 1973;7:34–83. doi: 10.1159/000124397. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Belle MD, Cheunsuang O, Stewart A, Morris R. Morphology of inhibitory and excitatory interneurons in superficial laminae of the rat dorsal horn. J Physiol. 2007;584:521–533. doi: 10.1113/jphysiol.2007.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Gundersen HJ. If you assume, you can make an ass out of u and me: a decade of the disector for stereological counting of particles in 3D space. J Anat. 1996;188:1–15. [PMC free article] [PubMed] [Google Scholar]
- Meister B, Arvidsson U, Zhang X, Jacobsson G, Villar MJ, Hokfelt T. Glutamate transporter mRNA and glutamate-like immunoreactivity in spinal motoneurones. Neuroreport. 1993;5:337–340. doi: 10.1097/00001756-199312000-00040. [DOI] [PubMed] [Google Scholar]
- Miller KE, Balbas JC, Benton RL, Lam TS, Edwards KM, Kriebel RM, Schechter R. Glutaminase immunoreactivity and enzyme activity is increased in the rat dorsal root ganglion following peripheral inflammation. Pain Res. Treat. 2012;2012:414697. doi: 10.1155/2012/414697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T, Matsumura S, Okuda-Ashitaka E, Shimamoto K, Sakimura K, Mishina M, Mori H, Ito S. Characterization of the glutamatergic system for induction and maintenance of allodynia. Brain Res. 2001;895:178–185. doi: 10.1016/s0006-8993(01)02069-8. [DOI] [PubMed] [Google Scholar]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Buist A, Cik M, van der SP, Kass S, Meert T, D’Hooge R, Rosenmund C, Hampson RM. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci. 2006;26:12055–12066. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JL, Konig P, Shimizu T, Jobling P, Gibbins IL. Most peptide-containing sensory neurons lack proteins for exocytotic release and vesicular transport of glutamate. J Comp Neurol. 2005;483:1–16. doi: 10.1002/cne.20399. [DOI] [PubMed] [Google Scholar]
- Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Ni B, Rosteck PR, Jr., Nadi NS, Paul SM. Cloning and expression of a cDNA encoding a brain-specific Na(+)-dependent inorganic phosphate cotransporter. Proc Natl Acad Sci U S A. 1994;91:5607–5611. doi: 10.1073/pnas.91.12.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olave MJ, Maxwell DJ. Axon terminals possessing the alpha 2c-adrenergic receptor in the rat dorsal horn are predominantly excitatory. Brain Res. 2003;965:269–273. doi: 10.1016/s0006-8993(02)04124-0. [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Persson S, Boulland JL, Aspling M, Larsson M, Fremeau RT, Jr., Edwards RH, Storm-Mathisen J, Chaudhry FA, Broman J. Distribution of vesicular glutamate transporters 1 and 2 in the rat spinal cord, with a note on the spinocervical tract. J. Comp Neurol. 2006;497:683–701. doi: 10.1002/cne.20987. [DOI] [PubMed] [Google Scholar]
- Ren K, Novikova SI, He F, Dubner R, Lidow MS. Neonatal local noxious insult affects gene expression in the spinal dorsal horn of adult rats. Mol. Pain. 2005;1:27. doi: 10.1186/1744-8069-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol Interv. 2008;8:242–253. doi: 10.1124/mi.8.5.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustioni A, weinberg RJ. The somatosensory system. 1989. pp. 219–321.
- Sakata-Haga H, Kanemoto M, Maruyama D, Hoshi K, Mogi K, Narita M, Okado N, Ikeda Y, Nogami H, Fukui Y, Kojima I, Takeda J, Hisano S. Differential localization and colocalization of two neuron-types of sodium-dependent inorganic phosphate cotransporters in rat forebrain. Brain Res. 2001;902:143–155. doi: 10.1016/s0006-8993(01)02290-9. [DOI] [PubMed] [Google Scholar]
- Santos MS, Li H, Voglmaier SM. Synaptic vesicle protein trafficking at the glutamate synapse. Neuroscience. 2009;158:189–203. doi: 10.1016/j.neuroscience.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, Basbaum AI. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci U S A. 2010;107:22296–22301. doi: 10.1073/pnas.1013413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SP, Walker TM. Morphology and electrophysiological properties of hamster spinal dorsal horn neurons that express VGLUT2 and enkephalin. J Comp Neurol. 2007;501:790–809. doi: 10.1002/cne.21292. [DOI] [PubMed] [Google Scholar]
- Schäfer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J. Biol. Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Seal RP, Edwards RH. The diverse roles of vesicular glutamate transporter 3. Handb. Exp. Pharmacol. 2006:137–150. doi: 10.1007/3-540-29784-7_7. [DOI] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroogy KB, Herman JP, Turner AJ, Bachelard HS. Neurochemistry—a practical approach. Oxford University Press; Oxford: 1997. In situ hybridization approaches to the study of the nervous system; pp. 121–150. [Google Scholar]
- Sillitoe RV, Vogel MW, Joyner AL. Engrailed homeobox genes regulate establishment of the cerebellar afferent circuit map. J. Neurosci. 2010;30:10015–10024. doi: 10.1523/JNEUROSCI.0653-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Vesicular glutamate transporter transcript expression in the thalamus in schizophrenia. Neuroreport. 2001;12:2885–2887. doi: 10.1097/00001756-200109170-00026. [DOI] [PubMed] [Google Scholar]
- Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J. Neurosci. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci. Res. 2006;55:343–351. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2) J Neurosci. 2001;21:RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Tao YX, Gu J, Stephens RL., Jr. Role of spinal cord glutamate transporter during normal sensory transmission and pathological pain states. Mol. Pain. 2005;1:30. doi: 10.1186/1744-8069-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YL, Guo Y, Cao DY, Zhang Q, Wang HS, Zhao Y. Local application of morphine suppresses glutamate-evoked activities of C and Adelta afferent fibers in rat hairy skin. Brain Res. 2005;1059:28–34. doi: 10.1016/j.brainres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgár E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ma J, Kirchgessner AL. Vesicular glutamate transporter 2 in the brain-gut axis. Neuroreport. 2001;12:3929–3934. doi: 10.1097/00001756-200112210-00015. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J. Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieytes CA, Lundgren KH, Malet M, Tomasella E, Seroogy KB, Gebhart GF, Hökfelt T, Brumovsky PR. Expression of vesicular glutamate transporters type-1 and -2 in lumbar 4 and 5 dorsal root ganglia - Effects of axotomy of the sciatic nerve or hindpaw inflammation. 42nd Society for Neuroscience Congress; 2013. Ref Type: Conference Proceeding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waerhaug O, Ottersen OP. Demonstration of glutamate-like immunoreactivity at rat neuromuscular junctions by quantitative electron microscopic immunocytochemistry. Anat. Embryol. (Berl) 1993;188:501–513. doi: 10.1007/BF00190144. [DOI] [PubMed] [Google Scholar]
- Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZQ, Shi TJ, Hökfelt T. Expression of galanin and a galanin receptor in several sensory systems and bone anlage of rat embryos. Proc. Natl. Acad. Sci U. S. A. 1996;93:14901–14905. doi: 10.1073/pnas.93.25.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-Q, Wei Y-Y, Leng Y-X, Zhong C-J, Zhang Y-S, Wan Y, Duan L-P. Vesicular Glutamate Transporter-3 Contributes to Visceral Hyperalgesia Induced by Trichinella spiralis Infection in Rats. Dig Dis Sci. 2012;57:865–872. doi: 10.1007/s10620-011-1970-x. [DOI] [PubMed] [Google Scholar]
- Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bean AJ, Wiesenfeld-Hallin Z, Xu XJ, Hokfelt T. Ultrastructural studies on peptides in the dorsal horn of the rat spinal cord--III. Effects of peripheral axotomy with special reference to galanin. Neuroscience. 1995;64:893–915. doi: 10.1016/0306-4522(94)00452-b. [DOI] [PubMed] [Google Scholar]