Abstract
Background
The infarct sparing effects of exercise are evident following both long-term and short-term training regimens. Here we compared the infarct-lowering effects of nitrite therapy, voluntary exercise, and the combination of both following myocardial ischemia-reperfusion (MI/R) injury. We also compared the degree to which each strategy increased cardiac nitrite levels, as well as the effects of each strategy on the nitrite reductase activity of the heart.
Methods and Results
Mice subjected to voluntary wheel running (VE) for 4 weeks displayed an 18% reduction in infarct size when compared to sedentary mice, whereas mice administered nitrite therapy (25 mg/L in drinking water) showed a 53% decrease. However, the combination of VE and nitrite exhibited no further protection than VE alone. Although the VE and nitrite therapy mice showed similar nitrite levels in the heart, cardiac nitrite reductase activity was significantly reduced in the VE mice. Additionally, the cardiac protein expression of myoglobin, a known nitrite reductase, was also reduced after VE. Further studies revealed that cardiac NFAT activity was lower after VE due to a decrease in calcineurin activity and an increase in GSK3β activity.
Conclusion
These data suggest that VE downregulates cardiac myoglobin levels by inhibiting calcineurin/NFAT signaling. Additionally, these results suggest that the modest infarct sparing effects of VE are the result of a decrease in the hearts ability to reduce nitrite to nitric oxide during MI/R.
Keywords: exercise, nitrite, myocardial ischemia-reperfusion, calcineurin/NFAT signaling, myoglobin, nitric oxide
1. Introduction
Despite the well documented beneficial cardiovascular effects of exercise [1, 2], the signaling mechanisms, particularly in the heart, that mediate these effects have not been fully elucidated [3]. However, growing evidence from animal studies suggest that exercise leads to the activation and/or upregulation of numerous proteins that are classically associated with cytoprotective signaling cascades [4]. For instance, exercise increases components of the endogenous antioxidant defenses, increases the expression of heat-shock proteins, activates AMP-activated protein kinase, increases the expression and activity of endothelial nitric oxide synthase (eNOS) resulting in an increase in nitric oxide (NO) levels, and activates ATP-sensitive potassium channels [5]. Additionally, we recently discovered a novel role for β3-adrenergic receptors (β3-ARs) in exercise-mediated cardioprotection, as we found that β3-ARs play a critical role in regulating the phosphorylation of eNOS and the generation of NO in response to exercise [6]. Based on the existing evidence in the literature that the upregulation and/or activation of any of these proteins results in robust cardioprotection, it was rather surprising that our recent study found that 4 weeks of voluntary wheel running reduced infarct size by only 23% [6].
Previously, we reported that exercise increased the cardiac levels of the NO metabolites, nitrite and nitrosothiols, and that the generation and storage of these metabolites play an important role in the infarct sparing effects of exercise [6]. Nitrite is a storage reservoir of NO in blood and tissue that is reduced to NO during ischemia [7]. Nitrite reductase activity in mammalian tissue has been linked to several proteins including deoxyhemoglobin [8] and xanthine oxidase [9]. However, emerging evidence suggest that myoglobin is the major reductase in the heart [10, 11]. Nitrosothiols, which are formed by the ubiquitous redox-related modification of cysteine thiols in a process known as nitrosylation, have emerged as one of the most important mechanisms by which NO imparts its cellular effects [12]. Previously we have reported that oral nitrite therapy provides significant cardioprotection (48% reduction in infarct size) against acute myocardial ischemia-reperfusion (I/R) injury by increasing the steady-state levels of both nitrite and nitrosothiols in the heart [13]. Based on this evidence, one can speculate that exercise training does not increase the cardiac levels of nitrite to the same extent as oral nitrite therapy and/or alters the ability of the heart to reduce nitrite to NO.
To address these issues, we investigated if supplementing mice with nitrite in their drinking water during voluntary exercise (VE) training could provide additional infarct sparing effects against acute myocardial I/R injury. Furthermore, we also compared the degree to which each strategy increased cardiac nitrite levels, as well as the effects of each strategy on the nitrite reductase activity of the heart. Here, we report that the nitrite reductase activity of the heart is diminished after VE due to a decrease in myoglobin levels. These results uncover a previously unrecognized mechanism to account for the modest infarct lowering effects of VE.
2. Materials and Methods
2.1. Animals
Six strains of mice were utilized in this study: (1) Male C57BL6/J mice (Jackson Labs, Bar Harbor, ME; 8–10 weeks of age); (2) Male NFAT-luciferase reporter mice (10–12 weeks of age); (3) Male Calcineurin Aβ deficient, (4) Male NFATc2 deficient; (5) Male NFATc4 deficient; and (6) Male NFATc2/NFATc4 deficient mice. The generation of NFAT-luciferase reporter mice [14] has been described previously. The generation of Calcineurin Aβ [15], NFATc2 [16], NFATc4 [17], and NFATc2/NFATc4 [18] global non-conditional deficient mice have been described previously. All experimental procedures were approved by the Institute for Animal Care and Use Committee at Emory University School of Medicine and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 86–23, Revised 1996) and with federal and state regulations.
2.2. Materials
DETA/NO, 2,2′-(Hydroxynitrosohydrazino)bis-ethanamine (DETANO) was purchased from EMD Millipore. DETANO was dissolved in saline and administered using a 32-gauge needle at a dose of 0.5 mg/kg (final volume of 50 μL) as an injection into the LV lumen at the time of reperfusion. Saline was administered in the same manner for the vehicle groups.
2.3. Voluntary Exercise Protocol
Mice were placed in custom designed cages fitted with running wheels (Mini Mitter, Bend, OR) for a period up to 4 weeks. Running distances were monitored daily. After the exercise-training period, the running wheel was removed from the cage and the mice were allowed to rest for a 24-hour period before further experimentation was conducted.
2.4. Myocardial Ischemia-Reperfusion Protocol and Myocardial Injury Assessment
Surgical ligation of the left coronary artery (LCA), myocardial infarct size determination, and Troponin-I measurements were performed similar to methods described previously [19].
2.5. NO Metabolite Measurements
Nitrite concentrations were quantified by ion chromatography (ENO20 Analyzer, Eicom). Tissue nitrosothiol compounds were quantified using group specific reductive denitrosation by iodine-iodide with subsequent detection of the NO liberated by gas-phase chemiluminescence. Nitrosyl–heme (NO-heme) levels were determined by parallel injection of replicate aliquots of tissue homogenates into a solution of 0.05M ferricyanide in PBS at pH 7.5 and 37°C. All NO analysis procedures have been previously described in detail [13].
2.6. Nitrite Reductase Activity Measurements
Tissue was homogenized in PBS. An aliquot of the homogenate was then placed in a reaction vessel connected to a NO chemiluminescence detector and purged with helium. NO generation was then measured in the presence and absence of nitrite (200 μM) as described previously [20]. In separate experiments, samples were incubated with myoglobin (25 μM) prior to placement in the reaction vessel. NO generation was then measured after the addition of nitrite.
2.7. Subcellular Fractionation and Western Blot Analysis
Subcellular Fractionation and Western blot analysis was performed as described previously [19].
2.8. Isolation of mRNA and Taqman qPCR
RNA was isolated using the RiboPure kit according to manufacturer’s instructions (Ambion). Reverse transcription was performed in a standard fashion with QuantiTect Reverse Transcription Kit (QIAGEN) supplemented with DNase treatment. Taqman qPCR was carried out according to the manufacturer’s instructions using probe sets for myoglobin and 18S. Analysis was carried out using the ΔΔ-CT method with 18S correction and reported as relative fold change versus sham.
2.9. NFAT Luciferase activity
Luciferase enzymatic activity in heart extracts was measured with a commercially available kit (luciferase assay system, Promega Corp.).
2.10. Calcineurin Activity Assay
Calcineurin activity was measured in heart samples with a commercially available kit (Calcineurin Cellular Activity Assay Kit, Calbiochem) according to the manufacturer’s instructions as previously described [21]. Calcineurin activity was measured as the dephosphorylation rate of a synthetic phosphopeptide substrate (RII peptide).
2.11. GSK-3β Activity
The activity of GSK-3β was measured in nuclear fractions prepared from heart tissue. The samples were first immunoprecipitated with a specific anti-GSK-3β antibody (Cell Signaling). An aliquot of the immunoprecipitated samples were incubated in a reaction buffer containing 12.5 mM Tris-HCl (pH 7.5), 2.5 mM β-glycerophosphate, 1 mM dithiothreitol, 0.05 mM Na3VO4, 5 mM MgCl2, 0.0625 mM ATP, and 10 μg of GSM (GSK-3β synthetic substrate peptide). The rate of ADP formed from the incorporation of ATP in the synthetic peptide was then measured with the ADP-Glo Kinase Assay kit (Promega) according to the manufacturer’s instructions. Activity was expressed as ADP generated (in picomoles) per minute per milligram of protein.
2.12. Xanthine Oxidase Activity
The activity of xanthine oxidase was measured in whole cell homogenates with a commercially available kit (Xanthine Oxidase Fluorometric Assay Kit, Cayman Chemical) according to the manufacturer’s instructions.
2.13. Echocardiography
Echocardiography was performed with a Vevo 2100 (Visualsonics) as previously described [22].
2.14. Statistics
All the data in this study are expressed as mean ± standard error (SEM). Differences in data between the groups were compared using Prism 5 (GraphPad Software, Inc) with Student’s paired 2-tailed t-test or one-way analysis of variance (ANOVA). For the one-way ANOVA, if a significant variance was found, the Tukey or Dunnett test was used as the post hoc analysis. For the echocardiography data, a 2-way repeated measures ANOVA with a Bonferroni test as the posthoc analysis was used. The p-value for these evaluations was adjusted by applying the Bonferroni correction for multiple comparisons. A p value less than 0.05 was considered statistically significant and p-values were two-sided.
3. Results
3.1. Nitrite supplementation does not enhance the cardioprotective effects of exercise training
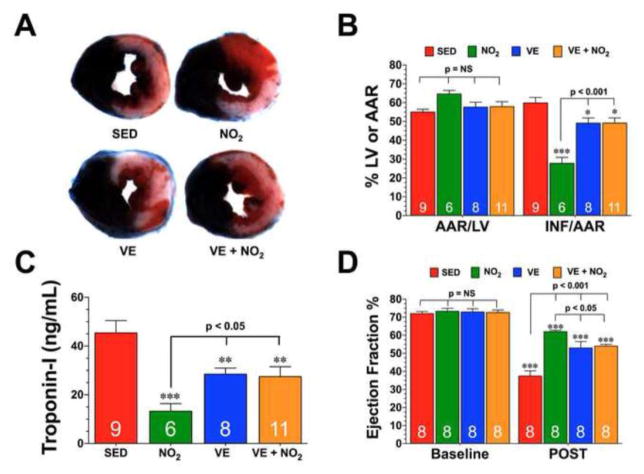
The cardioprotective effects of exercise training were tested in an in vivo murine model of I/R injury. For these experiments, mice were allowed to exercise voluntarily for a period of 4 weeks (VE). As shown in Supplemental Fig. I, the intensity of the exercise training remained constant throughout the 4-week training period with an average of 6.3±0.2 km/day. Mice that exercised displayed about a 21% increase in heart weight to body weight ratios and a 23% increase in heart weight tibia length ratios (Supplemental Figure 1B–C). At the end of the training period, mice were subjected to 45 minutes of LCA occlusion followed by 24 hours of reperfusion. Myocardial injury was then assessed by determining the extent of myocardial infarction and the levels of circulating Troponin-I (Fig. 1A–C). VE reduced myocardial infarct size relative to the area-at-risk (INF/AAR) by 18% (59.8±2.9% for SED vs. 49.1±2.8% for VE, p<0.05) and circulating Troponin-I levels by 37% (43.4±5.1% for SED vs. 28.5±2.5% for VE, p<0.01). Additional studies revealed that a 2-week training period and 8-week training period reduced INF/AAR in a similar manner as the 4-week training period (Supplemental Figure 1D).
Fig. 1. Nitrite Supplementation Does not Enhance the Cardioprotective Effects of Exercise Training.
Mice were housed in cages fitted with running wheels and allowed to exercise voluntarily for 4 weeks (VE). Control mice (sedentary, SED) were housed in cages without running wheels for the same durations as the VE mice. Groups of both SED and VE were also administered oral nitrite therapy (NO2, 25 mg/L in drinking water; NO2 and VE+NO2). (A) Representative mid-ventricular photomicrographs of hearts from each of the groups showing the degree of infarction following 45 minutes of left coronary artery occlusion and 24 hours or reperfusion. (B) Myocardial area-at-risk (AAR) as a percentage (%) of total left ventricle (LV) and infarct size (INF) as a percentage of area-at-risk (AAR) and (C) circulating Troponin-I levels (ng/mL) in each of the groups. (D) Ejection fraction (%) was calculated in separate groups of mice using echocardiography images at baseline and 7 days following myocardial ischemia (POST). Values are means ± S.E.M. Numbers inside the bars are the number of animals investigated. *p<0.05, **p<0.01, and ***p<0.001 vs. SED or Baseline.
Previously, it has been reported that oral nitrite supplementation therapy provides cardioprotection in the setting of acute myocardial I/R injury [13]. Since, we only observed a minimal reduction in infarct size in response to exercise training, we sought to determine if the addition of nitrite could augment the cardioprotective effects of exercise. Oral nitrite therapy (NO2, 25 mg/L in drinking water) for 4 weeks reduced INF/AAR by 53% (Fig. 1B; 59.8±2.9% for SED vs. 27.8±3.1% for NO2, p<0.001) and circulating Troponin-I levels by 71% (Fig. 1C; 43.4±5.1% for SED vs. 13.3±3.2% for VE, p<0.001). However, the combination of VE and nitrite (VE+NO2) for 4 weeks exhibited no further protection than VE. Nitrite therapy did not alter the intensity of the exercise training and did not alter exercised-induced cardiac enlargement (Supplemental Fig. I).
The effects of VE and NO2 therapy on LV structure and function following myocardial I/R were evaluated in separate groups of mice using in vivo transthoracic echocardiography. For these experiments, mice were subjected to 45 minutes of myocardial ischemia and 7 days of reperfusion. Myocardial I/R increased LV end-diastolic diameter (LVEDD) and LV end-systolic diameter (LVESD) in all groups compared to their respective baseline readings (Supplemental Fig. IE–F). Both NO2 therapy and VE attenuated the increase in LV diameters. However, as with infarct size reduction, NO2 therapy resulted in a smaller increase, especially for LVESD. Following myocardial I/R, LV ejection fraction decreased in all groups (Fig. 1D; p<0.001 vs. Baseline). Again, both NO2 therapy and VE improved LV ejection fraction when compared with the SED group (p<0.001 vs. SED) with NO2 therapy providing the best improvement (p<0.05 vs. VE and VE+NO2). For both LV dimensions and LV ejection fraction, the combination of VE and nitrite therapy exhibited no further protection than VE.
3.2. Exercise training and nitrite supplementation increased the levels of NO metabolites
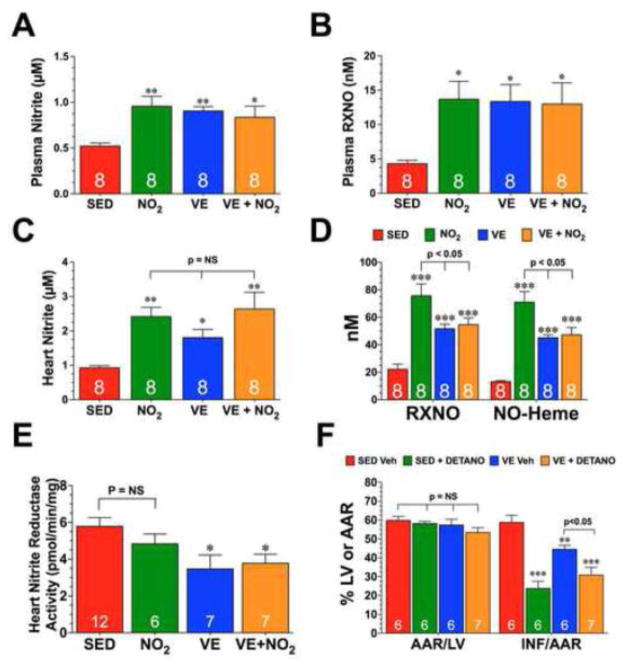
In an effort to determine why the combination of exercise training and oral nitrite supplementation did not provide more protection than exercise training alone, we first evaluated the storage of NO metabolites in these mice (Fig. 2). Both oral nitrite supplementation and exercise training increased the steady-state levels of plasma nitrite, plasma nitrosothiols (RXNO), and heart nitrite to the same extent. The addition of nitrite supplementation to exercise did little to alter the levels of plasma nitrite and nitrosothiols, nor the levels of heart nitrite. The levels of heart nitrosothiols and NO-Heme were increased in all three experimental groups when compared to SED controls. However, oral nitrite supplementation increased these levels to a greater extent than exercise training +/− nitrite supplementation.
Fig. 2. Exercise Training and Nitrite Supplementation Increase the Steady-State Levels of Nitrite and Nitrosothiols.
Steady-state levels of (A) plasma nitrite, (B) plasma nitrosothiols (RXNO), (C) heart nitrite, and (D) heart RXNO and nitrosyl-heme (NO-Heme) from SED, NO2, VE, and VE+NO2 mice. (E) Nitrite reductase activity from the hearts of SED, NO2, VE, and VE+NO2 mice. (F) Myocardial area-at-risk (AAR) as a percentage (%) of total left ventricle (LV) and infarct size (INF) as a percentage of area-at-risk (AAR) and (F) circulating Troponin-I levels (ng/mL) from SED and VE mice treated with the NO donor, DETANO, or vehicle at the time of reperfusion. Values are means ± S.E.M. *p<0.05, **p<0.01, and ***p<0.001 vs. SED or SED Veh.
Exercise training has previously been reported to alter the phosphorylation of endothelial nitric oxide synthase (eNOS) [6, 23]. Here we found that exercise training resulted in a similar increase in the phosphorylation of eNOS at serine residue 1177 (eNOS-PSer1177; phosphorylation here increases enzyme activity) in both the VE and VE+NO2 groups when compared to the SED group (Supplemental Fig. II). NO2 supplementation did not have any affect on the phosphorylation status of eNOS. No changes in the expression of total eNOS, nNOS, or iNOS were noted in any of the experimental groups compared to the SED group.
3.3. Exercise training reduces the ability of the heart to reduce nitrite to NO
The findings mentioned above regarding the changes in NO metabolites led us to evaluate the nitrite reductase capacity of the heart after VE training. Nitrite is reduced to NO under ischemic conditions to provide an alternative source of NO when NOS is inactive because of decreased oxygen saturation and substrate delivery [13]. Therefore, we next evaluated the effects of exercise training +/− nitrite supplementation on the ability of the heart to reduce nitrite to NO. For these experiments, NO generation was measured by chemiluminescence in heart homogenates taken from each group in the presence and absence of nitrite (200 μM). Consistent with previous studies [13], when nitrite was added to the homogenate under anaerobic conditions NO was detected, whereas NO was not generated in the absence of nitrite. As seen in Fig. 2E, cardiac nitrite reductase activity was significantly reduced in the VE and VE+NO2 groups when compared to the SED group or NO2 groups (p<0.05), whereas no difference was observed between the SED and NO2 groups. These results confirm the above-mentioned findings and further lend support to the idea that VE diminishes the capacity of the heart to reduce nitrite to NO.
Additional studies were then conducted to determine if the NO donor, DETANO, could augment the cardioprotective effects of exercise. Importantly, DETANO does not require an endogenous reductase to generate NO. For these experiments, DETANO was administered to SED (SED+DETANO) and VE (VE+DETANO) mice at the time of reperfusion. DETANO reduced INF/AAR by 60% in the SED group (Fig. 2F; 58.8±3.8% for SED vs. 23.8±3.8% for DETANO, p<0.001) and by 31% in the VE group (44.5±2.2% for VE vs. 30.9±4.1% for VE+DETANO, p<0.05). This data suggests that the VE heart is receptive to NO therapy and further supports the idea that VE reduces the capacity of the heart to reduce nitrite to NO.
3.4. Exercise training reduces the gene and protein expression of myoglobin by reducing the nuclear levels and activity of NFAT
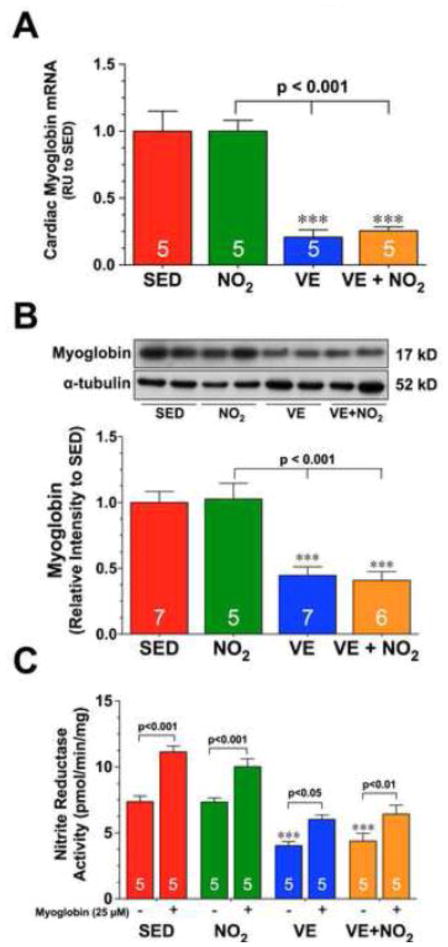
We next investigated if exercise training altered the expression of two known nitrite reductases, xanthine oxidase and myoglobin. Exercise training did not alter the protein expression or activity of xanthine oxidase in the heart (Supplemental Figure III). However, we did find that 4 weeks of exercise training significantly decreased the mRNA levels, as well as the protein expression of myoglobin in both the VE and VE+NO2 groups (Fig. 3A–B; p<0.001 vs. SED). NO2 supplementation did not have any effects on the expression of myoglobin. Additional experiments in which myoglobin (25 μM) was added to heart homogenates prior to their incubation in a reaction vessel with nitrite revealed enhanced nitrite reductase activity in all groups (Fig. 3C). More importantly, the addition of myoglobin to heart homogenates from the VE and VE+NO2 groups increased nitrite reductase activity to levels near those observed in the SED group without myoglobin. These results further support the idea that altering myoglobin levels can influence the nitrite reductase activity of the heart.
Fig. 3. Exercise Training Decreases the Expression of Cardiac Myoglobin.
(A) Gene expression and (B) representative immunoblots and densitometric analysis of myoglobin from the hearts of SED, NO2, VE, and VE+NO2 mice. (C) Nitrite reductase activity from the hearts of SED, NO2, VE, and VE+NO2 mice in the presence and absence of myoglobin (25 μM). Values are means ± S.E.M. *p<0.05 and ***p<0.001 vs. SED.
Analysis of skeletal muscle tissue taken from SED and VE mice revealed that VE slightly, although non-significantly, increased the mRNA and protein expression of myoglobin (Supplemental Fig. IVA–B). Nitrite reductase activity in the skeletal muscle of VE animals was also slightly increased (Supplemental Fig. IVC). This suggests that VE regulates the expression of myoglobin differently in the heart and skeletal muscle.
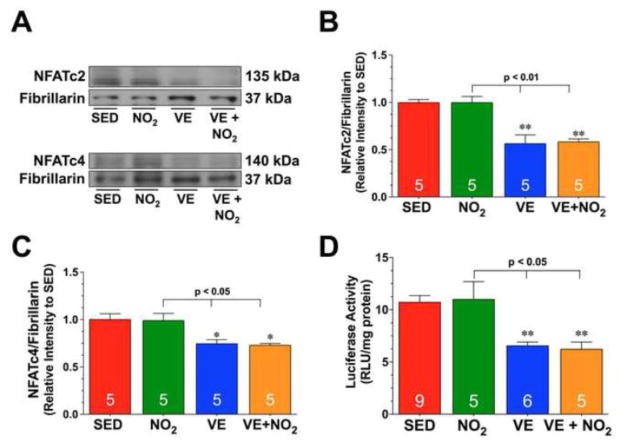
Myoglobin gene expression can be regulated by NFAT signaling [24–26]. Additional studies revealed that exercise training significantly decreased the nuclear expression of both NFATc2 and NFATc4 in the VE and VE+NO2 when compared to the SED groups (Fig. 4A–C; p<0.05 vs. SED and NO2). Further experiments were then conducted to determine if the changes in NFAT nuclear levels altered the activity of NFAT. For these studies, NFAT-Luciferase reporter mice were subjected to exercise training +/− NO2 supplementation. Analysis revealed a similar significant decrease in NFAT activity in the VE and VE+NO2 groups when compared to SED groups (Fig. 4D; p<0.01 vs. SED and p<0.05 vs. NO2). Again, NO2 supplementation did not have any effects on the expression or activity of NFAT.
Fig. 4. Exercise Training Decreases the Nuclear Expression and Activity of NFAT.
(A–C) Representative immunoblots and densitometric analysis of nuclear NFATc2 and NFATc4 from the hearts of SED, NO2, VE, and VE+NO2 mice. (D) Cardiac luciferase activity from the hearts of NFAT-Luciferase reporter mice divided into SED, NO2, VE, and VE+NO2 groups. Values are means ± S.E.M. *p<0.05 and **p<0.01 vs. SED.
3.5. Exercise training upregulates calsarcin-1 and activates GSK3β
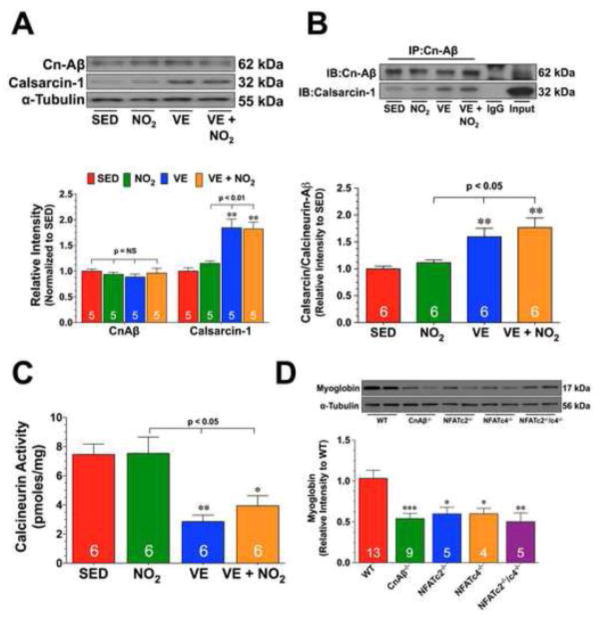
Experiments were then conducted to investigate potential mechanisms by which VE decreases NFAT nuclear levels. The nuclear localization of NFAT is controlled by pathways that either induce its nuclear import or nuclear export. Calcineurin (Cn) is a calcium-dependent serine-threonine phosphatase that controls NFAT nuclear localization through dephosphorylation [27]. Analysis revealed that exercise training did not alter the protein expression of CnAβ in either of the VE or VE+NO2 groups. However, an increase in the cardiac expression of the Cn inhibitor, calsarcin-1, was observed in both groups when compared to the SED groups (Fig. 5A; p<0.01 vs. SED and NO2). Further studies revealed an increased interaction of CnAβ with calsarcin-1 in the VE or VE+NO2 groups (Fig. 5B; p<0.05 vs. SED and NO2) and a decrease in calcineurin activity (Fig. 5C; p<0.05 vs. SED and NO2). Studies were then conducted to further demonstrate that Cn/NFAT signaling regulated the expression of myoglobin. For these experiments, we evaluated the expression of myoglobin in the hearts of mice deficient in CnAβ (CnAβ−/−), NFATc2 (NFATc2−/−), NFATc4 (NFATc4−/−), and NFATc2/NFATc4 (NFATc2−/−/NFATc4−/−). As seen in Fig. 5D, the protein expression of myoglobin was significantly decreased in the hearts of these mice when compared to wild-type hearts (p<0.05).
Fig. 5. Exercise Training Increases the Expression of Calsarcin-1.
(A) Representative immunoblots and densitometric analysis of calcineurin Aβ (CnAβ) and calsarcin-1 from the hearts of SED, NO2, VE, and VE+NO2 mice. (B) Representative immunoblots and densitometric analysis of the interaction of CnAβ with calsarcin-1 from the hearts of SED, NO2, VE, and VE+NO2 mice. For these experiments, heart homogenates were immunoprecipitated with a CnAβ antibody. The samples were then subjected to standard Western blot techniques and the membranes probed with CnAβ and calsarcin-1 antibodies to reveal the interaction. (C) Calcinuerin activity (pmoles/mg) from the hearts of SED, NO2, VE, and VE+NO2 mice. (D) Representative immunoblots and densitometric analysis of myoglobin from the hearts of Wild-type (WT), CnAβ deficient (CnAβ−/), NFATc4 deficient (NFATc4−/−), NFATc2 deficient (NFATc2−/−), and NFATc2/NFATc4 double deficient (NFATc2−/−/c4−/−) mice. Numbers inside the bars are the number of animals investigated. Values are means ± S.E.M. *p<0.05, **p<0.01, and ***p<0.001 vs. SED or WT.
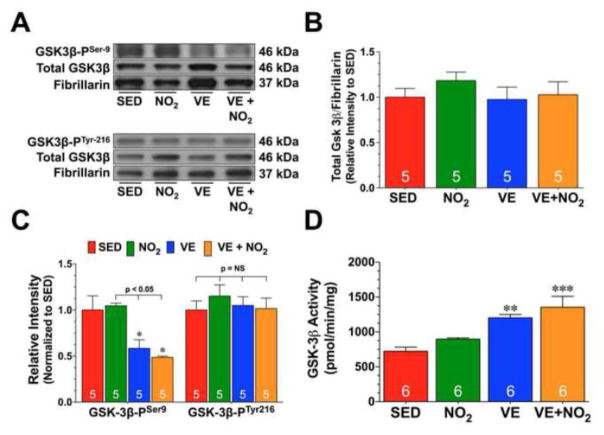
In contrast to the effects of Cn on NFAT, GSK3β induces the nuclear export of NFAT. In an effort to fully determine the effects of exercise training on GSK3β, we not only evaluated its nuclear expression but also evaluated its phosphorylation status. For these experiments, we exploited phosphorylation site-specific antibodies to probe immunoblots prepared from collected heart tissue. Exercise training did not alter the nuclear expression of GSK3β in either of the VE or VE+NO2 groups (Fig. 6A–B). Likewise, exercise training did not alter the phosphorylation of GSK3β at tyrosine residue 216 (Fig. 6A and 6C; GSK3β-PTyr216; phosphorylation here increases activity). However, exercise training did promote a significant decrease in the phosphorylation of GSK3β at serine residue 9 in both the VE or VE+NO2 groups (Fig. 6A and 6C; GSK3β-PSer9; phosphorylation here decreases activity; p<0.05 vs. SED and NO2), resulting in an increase in GSK3β activity (Fig. 6D; p<0.05 vs. SED).
Fig. 6. Exercise Training Suppresses GSK3.
β Inactivation.
(A) Representative immunoblots and densitometric analysis of (B) total GSK3β, (C) phosphorylated GSK3β at tyrosine residue 216 (GSK3β-PTyr216) and phosphorylated GSK3β at serine residue 9 (GSK3β-PSer9) in nuclear preparations from the hearts of SED, NO2, VE, and VE+NO2 mice. (D) GSK3β activity (pmol/min/mg) in nuclear fractions from the hearts of SED, NO2, VE, and VE+NO2 mice. Values are means ± S.E.M. *p<0.05, **p<0.01, and ***p<0.001 vs. SED.
4. Discussion
The main findings of the current study are as follows: (1) the addition of oral nitrite therapy to exercise training does not reduce infarct size following MI/R any more than exercise training alone; (2) exercise training reduces the nitrite reductase capacity of the heart by decreasing the gene and protein expression of myoglobin; (3) exercise training decreases NFAT nuclear levels and activity; (4) exercise training upregulates the expression of calsarcin-1 and increases its interaction with CnAβ; (5) exercise training alters the phosphorylation status and activity of GSK3β.
One of the main goals of this study was to determine if VE increased nitrite levels to the same extent as oral nitrite therapy and to directly compare the infarct-lowering effects of each. We found that VE increased both the circulating and cardiac nitrite levels to the same extent as oral nitrite therapy. Despite having a similar increase in cardiac nitrite levels, mice subjected to VE displayed an 18% decrease in infarct size when compared to SED control mice, whereas mice receiving oral nitrite therapy alone displayed a 53% decrease in infarct size. The addition of oral nitrite therapy to VE training did not lead to a further increase in circulating or cardiac nitrite levels and surprisingly did not provide any further decrease in infarct size over VE alone. This suggests that the circulating and cardiac levels of nitrite found in the oral nitrite therapy and VE groups potentially represents the maximum increase in nitrite levels that can be achieved under these conditions, as the combination of both strategies failed to provide a further increase. Currently it is not known why the combination of nitrite and VE did not lead to a further increase in nitrite levels. It is possible that the skeletal muscle consumes the nitrite during the time when the mice are exercising or that the excess nitrite is simply excreted in the urine. Further studies aimed at investigating this issue are certainly warranted.
This data also indicates that VE alters something that reduces the effectiveness of oral nitrite therapy. These findings lead us to take a closer look at other NO metabolites levels in the heart and lead to the discovery that although the cardiac levels of nitrosothiols and NO-Heme were increased in the VE groups, the observed increase was not as robust as the oral nitrite therapy alone group. Further analysis revealed that VE decreased the nitrite reductase activity of the heart, which provides a plausible explanation as to why the cardiac levels of nitrosothiols and NO-Heme were lower in the VE groups compared to the oral nitrite therapy group. This is important because nitrite becomes a principal source of NO during myocardial ischemia since the production of NO from eNOS is decreased due to low oxygen tension, diminished substrate delivery, and increased oxidative stress [9]. Therefore, the decreased nitrite reductase activity could also explain why the VE groups did not display greater infarct-size lowering effects given that the cytoprotective effects of nitrite are dependent on NO generation [9, 28, 29].
Nitrite reductase activity in mammalian tissue has been attributed to nonenzymatic acid disproportionation, as well as to several protein, including components of the mitochondrial electron transport system, deoxyhemoglobin, and xanthine oxidase [30]. Recently, myoglobin has emerged as a major nitrite reductase in the heart under conditions of ischemia/hypoxia [10, 11]. Myoglobin is a rather interesting monomeric hemoprotein that has been a source of interest to physicians and scientists for almost a century [31, 32]. Classically, the physiological role of myoglobin was attributed to its oxygen storage capacity, but now it is known to be a physiological catalyst that has several cellular functions [32]. The finding that myoglobin is a nitrite reductase is interesting given that myoglobin has traditionally been considered a scavenger of NO [33]. For instance, Gödecke et al [34] elegantly demonstrated that the cardiac specific overexpression of inducible nitric oxide synthase does not induce cardiac dysfunction unless myoglobin levels are also diminished, suggesting that under normal conditions myoglobin serves as an important barrier to protect the heart from nitrosative stress. However, there is now clear evidence that during hypoxic conditions the NO generated from nitrite by myoglobin escapes autocapture and regulates mitochondrial respiration [11]. Furthermore, the administration of nitrite during myocardial I/R does not provide cardioprotection in myoglobin deficient mice due to diminished nitrite reduction to NO [10, 35].
The levels of myoglobin in the skeletal muscle of mammals are elevated as adaptations to deep-sea diving [36] and life at altitude [37]. Similarly, exercise training or sustained tonic muscle contractions also alter skeletal muscle myoglobin levels [24, 38, 39]. In the current study, we found that 4 weeks of VE slightly increased the expression of myoglobin in skeletal muscle. However, in contrast, we found that 4 weeks of VE downregulates the expression of myoglobin in the heart. To the best of our knowledge, this is the first study to report that exercise training alters the cardiac expression of myoglobin. Given that this change is (1) accompanied by a decrease in the nitrite reductase activity of the heart and (2) the addition of myoglobin to heart homogenates increases nitrite reductase activity, we speculate that the downregulation of myoglobin contributes to this deficiency. Also, based on the evidence that myoglobin is necessary for nitrite to protect the heart against I/R injury, our findings suggest that the downregulation of myoglobin contributes to the modest infarct lowering effects of VE training.
Another major finding of the current study relates to the potential mechanism by which VE alters the expression of myoglobin. Specifically, the current study provides important insights into the role of Cn/NFAT signaling. The promoter region of myoglobin contains two evolutionarily conserved NFAT response elements and NFAT signaling has been shown to regulate muscle-specific myoglobin expression [25, 26, 31, 40]. In the current study, we provide further evidence to support the role of NFAT in regulating myoblogin, as evidenced by the finding that the protein expression of myoglobin is reduced in the hearts of NFAT deficient mice. Previously, mice subjected to swimming exercise displayed a reproducible decrease in cardiac NFAT activity [41]. In agreement with this study, we provide evidence that 4 weeks of VE not only diminishes the nuclear expression of NFAT, but that it also decreases the activity of NFAT. Furthermore, we found that VE disrupts NFAT signaling in the heart by inhibiting Cn activity and by increasing GSK-3β activity. Together, these findings suggest that exercise training reduces NFAT activity in the heart under physiological conditions and that the decrease in myoglobin expression after VE is likely attributable to the reduction in NFAT activity. However, further work is needed to determine the exact mechanism(s) by which VE regulates Cn/NFAT signaling.
At first glance, the inhibition of Cn/NFAT signaling by VE training appears to be a potential mechanism by which exercise protects the heart against pathological stimuli. This is based on the extensive evidence that activation of Cn/NFAT signaling is associated with the development of pathological left ventricular remodeling following the induction of heart failure [21, 41, 42]. Even subjecting mice to treadmill or voluntary wheel running after the induction of heart failure decreases adverse left ventricular remodeling by reducing NFAT signaling [43, 44]. Furthermore, since exercise induces physiological hypertrophy the inhibition of pathological hypertrophic signaling fits. However, there is evidence that Cn/NFAT signaling plays an anti-apoptotic role in the setting of acute myocardial I/R. For example, CnAβ deficient hearts experienced more apoptotic cell death and displayed greater areas of infarction following myocardial ischemia when compared to wild-type hearts [45]. In contrast, mice that overexpress Cn displayed less apoptotic cell death in their hearts following I/R injury [46]. Therefore, it can be suggested that the inhibition of Cn/NFAT signaling in response to VE contributes to its modest infarct size lowering effects. Whether this is all related to the downregulation of myoglobin or if this occurs via myoglobin-independent mechanisms remains to be elucidated. Together these findings suggest that Cn/NFAT signaling may function as a double-edged sword, as its activation during early reperfusion antagonizes apoptosis, but long-term activation induces pathological hypertrophy and deleterious left ventricular remodeling. Therefore, future studies are needed to determine if VE protects the heart from pathological remodeling via an inhibition of Cn/NFAT signaling despite having only a modest effect on infarct size.
Although the current study demonstrates that cardiac myoglobin expression is diminished after VE and offers a potential mechanism, there are some limitations that need to be noted. First, we do not fully understand the physiological relevance of these findings. Given that myoglobin stores oxygen and facilitates its diffusion [32], the idea that cardiac myoglobin levels are decreased during exercise when vasodilation and maximal delivery of oxygen is needed is counterintuitive. However, genetic models have shown that a deficiency in myoglobin does not preclude mice from having a normal life with sufficient energy to exercise and procreate in a manner similar to mice with intact myoglobin [26, 47]. These mice adapt by upregulating a number of compensatory mechanisms, such as activation of hypoxia-inducible factor 1 (HIF-1) signaling, that counter the loss of myoglobin [48]. Although we did not evaluate the expression of HIF-1 in the current study, there is evidence to suggest that it is activated by exercise training [49]. Therefore, as with the genetic models, the heart of exercised mice may also adapt to life with diminished myoglobin by inducing similar adaptive mechanisms. Second, there is some evidence in the literature to suggest that in regards to cardiac contractility NO possesses both positive and negative inotropic effects depending on how much NO is present [50]. Therefore, during exercise when eNOS is maximally activated local NO production is already quite high [6], the downregulation of myoglobin could be part of a negative feedback loop that keeps NO signaling in check. Third, it is quite possible that our findings related to the limited infarct size lowering effects of VE, the reduction in myoglobin, and the reduction in nitrite reductase activity are unique to the mouse and may not accurately reflect what happens in larger mammals or humans after exercise. In regards to the infarct lowering effects, evidence in the literature indicates the degree of protection after exercise training ranges from 4–75% depending on the ischemic model studied (permanent ischemia or ischemia-reperfusion) and the training strategy employed (voluntary, treadmill, or swimming) [51]. Our findings are in line with similar studies that subjected rodents to chronic durations of training [5, 52]. There are several studies in the literature that evaluated the cardioprotective effects of exercise in larger mammals. However, these studies predominately evaluated the effects of short periods of exercise training making them hard to compare to our results. For instance, exercise (5 bouts of treadmill running for 5 minutes) immediately prior to coronary artery occlusion reduced infarct size by 78%. Therefore, future studies are definitely warranted to address the physiological relevance of our findings and to determine if these findings are relevant in larger mammals and humans.
In summary, this study provides novel evidence that the nitrite reductase activity of the heart is diminished after VE due to a decrease in myoglobin levels. Furthermore, we found that VE induces the downregulation of myoglobin by inhibiting Cn/NFAT signaling via the upregulation of calsarcin-1 and activation of GSK-3β. Together, these results suggest that the modest infarct sparing effects of VE are in part the result of a decrease in the hearts ability to reduce nitrite to NO during myocardial I/R. Exercise remains one of preventive medicines strongest approaches to combat the severity of myocardial infarction following ischemic injury, given that it is widely accessible and safe [53]. Therefore, continued investigation into the unknown signaling mechanisms of exercise are extremely important given their enormous health care implications [51]. While the results of the current study do not directly address therapeutic advances in the treatment of myocardial I/R injury, they do provide important insights into how the murine heart adapts to exercise training. Understanding how the heart adapts to exercise is important, because a better comprehension of the signaling cascades induced by exercise will hopefully provide the framework for developing therapeutic strategies designed to treat cardiovascular disease.
Supplementary Material
Highlights.
Chronic voluntary exercise (VE) reduces the nitrite reductase capacity of the heart
Chronic VE decreases the gene and protein expression of myoglobin
Chronic VE decreases calcineurin/NFAT signaling
These results uncover a mechanism for the modest infarct lowering effects of VE
Acknowledgments
Sources of funding
Supported by grants from the American Diabetes Association (7-09-BS-26) and the National Institutes of Health National Heart Lung and Blood Institute (NHLBI) (5R01HL098481-03) to J.W.C. and (5R01HL092141), (5R01HL093579), (1U24HL 094373), and (1P20HL113452) to D.J.L. This work was also supported by funding from the Carlyle Fraser Heart Center (CFHC) of Emory University Hospital Midtown.
Footnotes
Disclosures
D.J.L. has served on the Scientific Advisory Board of Theravasc, Inc. Theravasc is currently developing novel nitrite formulations for the treatment of peripheral arterial disease (PAD) and cardiovascular diseases. J.W.C. received research funding from Theravasc for a clinical study of nitrite therapy in PAD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Powers SK, Lennon SL, Quindry J, Mehta JL. Exercise and cardioprotection. Curr Opin Cardiol. 2002;17:495–502. doi: 10.1097/00001573-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation. 1999;99:963–72. doi: 10.1161/01.cir.99.7.963. [DOI] [PubMed] [Google Scholar]
- 3.Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, et al. C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–83. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezzerides V, Rosenzweig A. Saying yes to exercise and NO to cardiac injury. Circ Res. 2011;108:1414–6. doi: 10.1161/CIRCRESAHA.111.247122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, et al. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol. 2005;569:913–24. doi: 10.1113/jphysiol.2005.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvert JW, Condit ME, Aragon JP, Nicholson CK, Moody BF, Hood RL, et al. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–58. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci U S A. 2008;105:11430–5. doi: 10.1073/pnas.0800700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 9.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–8. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res. 2007;100:1749–54. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 11.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–61. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 12.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007;104:19144–9. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–86. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bueno OF, Brandt EB, Rothenberg ME, Molkentin JD. Defective T cell development and function in calcineurin A beta -deficient mice. Proc Natl Acad Sci U S A. 2002;99:9398–403. doi: 10.1073/pnas.152665399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xanthoudakis S, Viola JP, Shaw KT, Luo C, Wallace JD, Bozza PT, et al. An enhanced immune response in mice lacking the transcription factor NFAT1. Science. 1996;272:892–5. doi: 10.1126/science.272.5263.892. [DOI] [PubMed] [Google Scholar]
- 17.Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–75. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 18.Yang TT, Suk HY, Yang X, Olabisi O, Yu RY, Durand J, et al. Role of transcription factor NFAT in glucose and insulin homeostasis. Mol Cell Biol. 2006;26:7372–87. doi: 10.1128/MCB.00580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, et al. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–74. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med. 2008;45:468–74. doi: 10.1016/j.freeradbiomed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Rooij E, Doevendans PA, Crijns HJ, Heeneman S, Lips DJ, van Bilsen M, et al. MCIP1 overexpression suppresses left ventricular remodeling and sustains cardiac function after myocardial infarction. Circ Res. 2004;94:e18–26. doi: 10.1161/01.RES.0000118597.54416.00. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson CK, Lambert JP, Molkentin JD, Sadoshima J, Calvert JW. Thioredoxin 1 is essential for sodium sulfide-mediated cardioprotection in the setting of heart failure. Arterioscler Thromb Vasc Biol. 2013;33:744–51. doi: 10.1161/ATVBAHA.112.300484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, et al. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–7. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 24.Kanatous SB, Mammen PP, Rosenberg PB, Martin CM, White MD, Dimaio JM, et al. Hypoxia reprograms calcium signaling and regulates myoglobin expression. Am J Physiol Cell Physiol. 2009;296:C393–402. doi: 10.1152/ajpcell.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Z, Serrano AL, Schiaffino S, Bassel-Duby R, Williams RS. Regulatory elements governing transcription in specialized myofiber subtypes. J Biol Chem. 2001;276:17361–6. doi: 10.1074/jbc.M101251200. [DOI] [PubMed] [Google Scholar]
- 26.Wittenberg BA. Both hypoxia and work are required to enhance expression of myoglobin in skeletal muscle. Focus on “Hypoxia reprograms calcium signaling and regulates myoglobin expression”. Am J Physiol Cell Physiol. 2009;296:C390–2. doi: 10.1152/ajpcell.00002.2009. [DOI] [PubMed] [Google Scholar]
- 27.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–7. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 28.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–35. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 31.Kanatous SB, Mammen PP. Regulation of myoglobin expression. J Exp Biol. 2010;213:2741–7. doi: 10.1242/jeb.041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamga C, Krishnamurthy S, Shiva S. Myoglobin and mitochondria: a relationship bound by oxygen and nitric oxide. Nitric oxide: biology and chemistry/official journal of the Nitric Oxide Society. 2012;26:251–8. doi: 10.1016/j.niox.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunori M. Nitric oxide moves myoglobin centre stage. Trends Biochem Sci. 2001;26:209–10. doi: 10.1016/s0968-0004(01)01824-2. [DOI] [PubMed] [Google Scholar]
- 34.Godecke A, Molojavyi A, Heger J, Flogel U, Ding Z, Jacoby C, et al. Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J Biol Chem. 2003;278:21761–6. doi: 10.1074/jbc.M302573200. [DOI] [PubMed] [Google Scholar]
- 35.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2008;105:10256–61. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guyton GP, Stanek KS, Schneider RC, Hochachka PW, Hurford WE, Zapol DG, et al. Myoglobin saturation in free-diving Weddell seals. J Appl Physiol. 1995;79:1148–55. doi: 10.1152/jappl.1995.79.4.1148. [DOI] [PubMed] [Google Scholar]
- 37.Terrados N. Altitude training and muscular metabolism. Int J Sports Med. 1992;13 (Suppl 1):S206–9. doi: 10.1055/s-2007-1024641. [DOI] [PubMed] [Google Scholar]
- 38.Underwood LE, Williams RS. Pretranslational regulation of myoglobin gene expression. Am J Physiol. 1987;252:C450–3. doi: 10.1152/ajpcell.1987.252.4.C450. [DOI] [PubMed] [Google Scholar]
- 39.Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, et al. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 2001;20:6414–23. doi: 10.1093/emboj/20.22.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, et al. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–8. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 42.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliveira RS, Ferreira JC, Gomes ER, Paixao NA, Rolim NP, Medeiros A, et al. Cardiac anti-remodelling effect of aerobic training is associated with a reduction in the calcineurin/NFAT signalling pathway in heart failure mice. J Physiol. 2009;587:3899–910. doi: 10.1113/jphysiol.2009.173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, et al. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res. 2006;98:540–8. doi: 10.1161/01.RES.0000205766.97556.00. [DOI] [PubMed] [Google Scholar]
- 45.Bueno OF, Lips DJ, Kaiser RA, Wilkins BJ, Dai YS, Glascock BJ, et al. Calcineurin Abeta gene targeting predisposes the myocardium to acute ischemia-induced apoptosis and dysfunction. Circ Res. 2004;94:91–9. doi: 10.1161/01.RES.0000107197.99679.77. [DOI] [PubMed] [Google Scholar]
- 46.De Windt LJ, Lim HW, Taigen T, Wencker D, Condorelli G, Dorn GW, 2nd, et al. Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: An apoptosis-independent model of dilated heart failure. Circ Res. 2000;86:255–63. doi: 10.1161/01.res.86.3.255. [DOI] [PubMed] [Google Scholar]
- 47.Garry DJ, Ordway GA, Lorenz JN, Radford NB, Chin ER, Grange RW, et al. Mice without myoglobin. Nature. 1998;395:905–8. doi: 10.1038/27681. [DOI] [PubMed] [Google Scholar]
- 48.Grange RW, Meeson A, Chin E, Lau KS, Stull JT, Shelton JM, et al. Functional and molecular adaptations in skeletal muscle of myoglobin-mutant mice. Am J Physiol Cell Physiol. 2001;281:C1487–94. doi: 10.1152/ajpcell.2001.281.5.C1487. [DOI] [PubMed] [Google Scholar]
- 49.Ishikawa Y, Gohda T, Tanimoto M, Omote K, Furukawa M, Yamaguchi S, et al. Effect of exercise on kidney function, oxidative stress, and inflammation in type 2 diabetic KK-A(y) mice. Exp Diabetes Res. 2012;2012:702948. doi: 10.1155/2012/702948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pellegrino D, Shiva S, Angelone T, Gladwin MT, Tota B. Nitrite exerts potent negative inotropy in the isolated heart via eNOS-independent nitric oxide generation and cGMP-PKG pathway activation. Biochim Biophys Acta. 2009;1787:818–27. doi: 10.1016/j.bbabio.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Calvert JW, Lefer DJ. Role of beta-Adrenergic Receptors and Nitric Oxide Signaling in Exercise-Mediated Cardioprotection. Physiology (Bethesda) 2013;28:216–24. doi: 10.1152/physiol.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J Appl Physiol. 2003;95:2510–8. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- 53.Calvert JW. Cardioprotective effects of nitrite during exercise. Cardiovasc Res. 2011;89:499–506. doi: 10.1093/cvr/cvq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.