Abstract
Introduction
Radiation-induced pneumonitis and fibrosis have restricted radiotherapy for lung cancer. In a pre-clinical lung tumor model, soy isoflavones showed the potential to enhance radiation damage in tumor nodules and simultaneously protect normal lung from radiation injury. We have further dissected the role of soy isoflavones in the radioprotection of lung tissue.
Methods
Naïve Balb/c mice were treated with oral soy isoflavones for 3 days before and up to 4 months after radiation. Radiation was administered to the left lung at 12 Gy. Mice were monitored for toxicity and breathing rates at 2, 3 and 4 months after radiation. Lung tissues were processed for histology for in situ evaluation of response.
Results
Radiation caused damage to normal hair follicles, leading to hair loss in the irradiated left thoracic area. Supplementation with soy isoflavones protected mice against radiation-induced skin injury and hair loss. Lung irradiation also caused an increase in mouse breathing rate that was more pronounced by 4 months after radiation, probably due to late effects of radiation-induced injury to normal lung tissue. However, this effect was mitigated by soy isoflavones. Histological examination of irradiated lungs revealed a chronic inflammatory infiltration involving alveoli and bronchioles and a progressive increase in fibrosis. These adverse effects of radiation were alleviated by soy isoflavones.
Conclusion
Soy isoflavones given pre- and post-radiation protected the lungs against adverse effects of radiation including skin injury, hair loss, increased breathing rates, inflammation, pneumonitis and fibrosis, providing evidence for a radioprotective effect of soy.
Keywords: Soy, Radiation, Lung, Protection
INTRODUCTION
Radiotherapy for non-small cell lung cancer (NSCLC) is limited by radiation toxicity to normal lung tissue which results from an inflammatory process caused by damage to capillary endothelial cells and epithelial lung cells causing pneumonitis and fibrosis (1-4).
We are exploring the potential for biological/nutritional intervention using soy isoflavones to boost the success of radiotherapy and decrease its toxicity in NSCLC. This complementary approach could benefit patients with compromised lung functions including COPD in smokers and patients with larger or non-peripheral tumors, such as unresectable stage III locally advanced NSCLC, in which radiotherapy is limited by anticipated toxicity (1). Soy isoflavones are non-toxic dietary plant estrogens extracted from soy beans and anti-cancer agents, as demonstrated in controlled clinical trials (5). They can also act as anti-oxidants in normal tissues and protect them from treatment-induced toxicity (6). This protective effect was observed in our clinical trial for prostate cancer patients showing that soy isoflavone pills, taken in conjunction with radiotherapy, reduced radiation toxicity resulting in improved urinary, gastrointestinal and sexual functions (6).
The rationale for selecting soy isoflavones to combine with radiotherapy for NSCLC is based on our work demonstrating that these compounds have a differential effect on tumor versus normal tissue. In contrast to normal cells, critical survival pathways are constitutively activated in cancer cells, including DNA repair molecules, the nuclear factor NF-κB and hypoxia inducible factor HIF-1α transcription factors, that are responsible for promoting malignant behavior by transcription of proteins involved in tumor progression. These survival pathways are further upregulated in response to radiation and thereby, are implicated in radioresistance of cancer. We have consistently shown that soy isoflavones inhibited radiation-induced upregulation of these survival pathways resulting in greater cancer destruction both in vitro and in vivo, using orthotopic models of lung carcinoma, renal cell carcinoma and prostate cancer (7-14). In particular, in lung cancer studies, we previously reported that soy isoflavones enhanced radiation-induced cell killing of human NSCLC cells in vitro by increasing DNA damage and inhibiting DNA repair in addition to inhibiting NF-κB and HIF-1α (12). In contrast, normal cells do not express such activated malignant survival pathways and thus, are not affected directly by soy isoflavones. In normal tissues, radiation causes tissue damage resulting in inflammatory processes leading to pneumonitis and fibrosis. Initial studies suggested that soy inhibited the progression of radiation-induced inflammatory events in normal lung tissue. In an orthotopic murine model of lung cancer, soy isoflavones increased radiation-induced destruction of lung tumor nodules, but also mitigated the vascular damage, inflammation and fibrosis, caused by radiation injury to lung tissue (7). These studies suggested that soy isoflavones have the dual potential to enhance radiation damage in lung tumors and simultaneously protect normal lung from radiation injury.
To evaluate further the role of soy isoflavones on moderating adverse effects of radiation on lung tissue, we have now investigated the effect of soy isoflavones on the damage caused by a high radiation dose in normal lung in naïve mice not bearing tumors. These conditions were selected to detect significant tissue damage by high radiation doses, which have been shown to result in greater toxicity in clinical studies (15). We report that supplementation with soy isoflavones pre- and post-radiation clearly attenuated skin injury and hair loss caused by radiation, a cogent evidence for the radioprotective effect of soy isoflavones on normal tissues. Furthermore, soy isoflavones protected mice from radiation-increased breathing rate. Histological observation of lung tissues confirmed that soy isoflavones protected normal lung structures against radiation-induced inflammation, damage and fibrosis.
MATERIALS AND METHODS
Mice
Female Balb/c mice of 5-6 week old (Harlan, Indianapolis, IN) were housed and handled in animal facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). The animal protocol was approved by Wayne State University Institutional Animal Care and Use Committee (IACUC).
Soy Isoflavones
The soy isoflavones mixture G-4660 used is a pure extract of 98.16% isoflavones from soybeans consisting of 83.3% genistein, 14.6% daidzein and 0.26% glycitein (manufactured by Organic Technologies and obtained from NIH). The soy isoflavones mixture was dissolved in 0.1 mol/L Na2CO3 and mixed with sesame seed oil at a 2:1 ratio just prior to treatment to facilitate gavage and avoid irritation of the esophagus by Na2CO3 (7). Mice were orally treated with 5 mg or 1mg per day of soy isoflavones (250 or 50 mg/kg body weight/day) by gavage. Control mice received the vehicle alone.
Lung Irradiation
Radiation was delivered to the left lobe of the lungs. Three anesthetized mice, in jigs, were positioned under a 6.4 mm lead shield with 3 cut-outs in an aluminum frame mounted on the X-ray machine to permit selective irradiation of the left lung in 3 mice at a time, as previously described (7). The radiation dose to the lung and the scattered dose to areas of the mouse outside of the radiation field were carefully monitored. To minimize backscattering of radiation, the bottom of the aluminum frame that holds the jigs was hollowed out and the backplate of the jig was thinned to 1.6mm thickness. Under these conditions and the lead shielding, the X ray dose to the shielded regions was reduced to 1% of the dose to the irradiated field. The dose rate was 101.0 cGy/min and HVL was 2 mm Cu. Photon irradiation was performed at a dose of 12 Gy with a Siemens Stabilipan X-ray set (Siemens Medical Systems, Inc) operated at 250 kV, 15 mA with 1 mm copper filtration at a distance of 47.5 cm from the target.
Experimental protocol
Mice were pre-treated with oral soy isoflavones each day for 3 days at a dose of 5mg/day (equivalent to 250mg/kg). Then, the left lung was selectively irradiated with 12 Gy. Soy treatment was continued on a daily basis for 5 more days at 5mg/day. Then mice were treated with a lower dose of 1mg/day (equivalent to 50mg/kg), given daily 5 days a week for up to 18 weeks. The rationale for giving a higher dose of soy isoflavones for pre-treatment and just after radiation is to optimize the effect of soy, based on previous studies (8, 10).
Hair loss monitoring of mice treated with soy isoflavones and lung irradiation
Mice were treated with soy isoflavones and left lung irradiation as described above (section of experimental protocol). After radiation, mice were treated with soy given daily, 5 days a week, for 5 more weeks. Four experimental treatment groups consisting of 13 mice per group included control mice treated with vehicle alone, mice treated with soy alone, mice treated with radiation alone and mice treated with soy and radiation. Mice were monitored three times a week for signs of radiation-induced injury. The area showing hair loss was measured in two dimensions. Hair loss in the radiated field was scored at 100% for bare skin, to 75, 50 and 25% based on the density of hair and the size of area showing hair loss.
Breathing rate measurements of mice treated with soy isoflavones and lung irradiation
Mice were treated with soy isoflavones and left lung irradiation as described above (section of experimental protocol). After radiation, mice were treated with soy given daily, 5 days a week, for up to 18 weeks. Four experimental treatment groups consisting of 8 mice per group included control mice treated with vehicle alone, mice treated with soy alone, mice treated with radiation alone and mice treated with soy and radiation. Mice were monitored for up to 4-5 months and their breathing rate was measured using a mouse CollarClip Sensor according to manufacturer instructions (STARR Life Sciences, Corp.; Oakmont, PA) (16-18). The breathing rate of 8 mice per treatment group was measured at late time points of about 2, 3, and 4 months (days 59, 86 and 130) after radiation. Data were collected on conscious mice during 10 minutes for each mouse. Breathing rates were measured when mice were relatively calm and no error code was shown on the instrument (16). These rates were computed to estimate the average breathing rate of each mouse.
Lung tissue preparation for histological examination
Mice were treated with soy isoflavones and left lung irradiation as described above (section of experimental protocol). After radiation, mice were treated with soy given daily, 5 days a week, for up to 18 weeks. At different time points, mice were killed and lungs were perfused with 10% buffered formalin for fixation in situ. The lungs were resected and the irradiated left lung and the non-irradiated right lung were processed separately for paraffin embedding and sectioning (7). Sections were stained with hematoxylin-eosin (H&E). The extent of fibrosis was evaluated by staining lung sections with Masson’s Trichrome (NovaUltra Kit, IHCWORLD, Woodstock, MD) (7, 19). The extent of lung damage, inflammatory infiltration, pneumonitis and fibrosis were quantified using a scoring system on a scale from weak (+ −), moderate (+), strong (++) to heavy (+++).
Statistical analysis
For histological data analysis, differences in the surface area of hair loss and breathing rates among the various treatments groups were analyzed by two-tailed unpaired Student’s t test.
RESULTS
Cogent evidence demonstrating radioprotection by soy isoflavones: Decrease in radiation-induced hair loss
To determine if soy isoflavones could protect normal lung tissues from radiation-induced damage, mice were treated with radiation alone or combined with soy isoflavones for up to 7 weeks, as detailed in Materials and Methods. Mice were monitored daily for signs of toxicity. We observed hair loss in the left upper thoracic side which was irradiated. Hair loss was scored as 100%, 75%, 50% and 25% in the irradiated area based on the size of the injured area and the hair density (Fig 1). For example, in mice treated with radiation alone, a mouse with bared skin was scored as 100% hair loss and most of the mice showed 50-75% hair loss (Fig 1A). In contrast, a large number of mice in the experimental group treated with soy isoflavones prior to- and after radiation showed 0-25 % hair loss (Fig 1B). The proportion of mice with significant hair loss in the radiation group was 13 out of 13 mice with 92% of the mice showing 50% or greater hair loss (Fig 2A). However, in the experimental group treated with soy isoflavones and radiation, only 6 out of 13 mice had measurable hair loss mostly in the range of 25% (Fig 2A). Kinetics experiments showed that hair loss appeared as early as 3 weeks after radiation and all mice showed hair loss by 5 weeks after radiation (Fig 2B). The surface area with hair loss progressively increased with time in all mice treated with radiation only (Fig 2B). However, the kinetics of hair loss were much slower in mice treated with radiation combined with soy isoflavones beginning at 4-6 weeks after initiation of soy treatment (Fig 2C). The surface area of hair loss was also significantly smaller by 5-6 weeks compared to mice treated with radiation alone (p<0.01, Fig 2C).
Figure 1. Hair loss in irradiated thoracic area of mice treated with radiation or radiation combined with soy isoflavones.
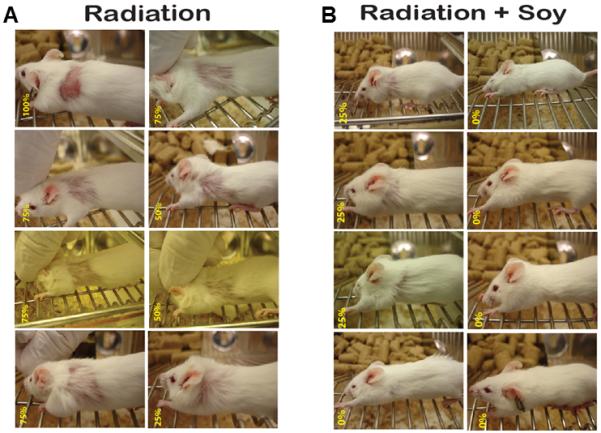
(A) Hair loss induced by radiation. Mice received 12 Gy radiation to the left lung. (B) Protection against radiation-induced hair loss by soy isoflavones. Mice were treated with soy isoflavones and 12 Gy radiation administered to the left lung. Soy was given 3 days prior radiation and continued 5 days a week after radiation. At about 7 weeks after initiation of soy treatment, hair loss was scored from 0-100% as shown in yellow numbers on the bottom left of each mouse picture. Eight representative mice per treatment group are shown.
Figure 2. Kinetics of hair loss in irradiated thoracic area of mice treated with radiation combined with soy isoflavones.

Mice were treated either with 12 Gy radiation administered to the left lung alone or combined with soy isoflavones treatment. Soy was given 3 days prior radiation and continued 5 days a week after radiation. (A) Hair loss scoring in mice treated with radiation or radiation and soy isoflavones. At about 7 weeks after initiation of soy treatment, hair loss was scored from 0-100% based on the density of hair and the size of area showing hair loss. The proportion of mice with hair loss is based on 13 mice per treatment group (inserted in text box). (B) (C) Kinetics of hair loss following radiation (B) or radiation combined with soy isoflavones treatment (C). Mice were monitored for hair loss three times a week after initiation of treatment. The area showing hair loss was measured in two dimensions and the surface area was plotted. Each dot in A, B and C panels represent one mouse.
Soy isoflavones mitigated the increased breathing rate induced by radiation
Lung tissue injury induced by radiotherapy leads to an inflammatory process which results in pneumonitis and fibrosis (20, 21). The presence of fibrosis in lung tissues leads to difficulties breathing causing increased breathing rate and these late effects of radiation toxicity occur in patients several months after radiation (3, 22). Increase in breathing rates was also documented in mice or rats treated with lung irradiation (2, 23-25). To evaluate the effect of soy isoflavones on breathing rates altered by radiation, we have measured the breathing rate of mice treated with lung irradiation, soy isoflavones and both combined. An increase in the breathing rate of mice receiving only lung irradiation was noticed by day 59 and 86 (Fig 3). The increased breathing rates were significantly more pronounced by 4 months (day 130) after radiation with a mean of 209±19 breaths/min compared to 170±8 breaths/min in control mice (p<0.001), probably due to late effects of radiation injury to lung tissue (Fig 3). However, this effect was mitigated by supplementation with soy isoflavones pre- and post-radiation causing a breathing rate in the lower range of 168±5 breaths/min (p<0.001, compared to radiation) that was comparable to that of untreated control mice (Fig 3).
Figure 3. Breathing rate of mice treated with soy and radiation.

Mice were treated either with soy or 12 Gy radiation (Rad) administered to the left lung alone or soy isoflavones combined with radiation (Soy + Rad). Soy was continued 5 days a week for up to 18 weeks. The breathing rate of 8 mice per treatment group was measured on day 59, 86 and 130 after initiation of soy treatment. Each dot represents the average breathing rate value obtained from one mouse (n=8 per group).
Histological observations in lungs treated with soy isoflavones and radiation
To assess in situ the effect of soy isoflavones and left lung irradiation on lung tissue structures, left lung and right lungs were obtained at about 2, 3 and 4 months following treatment with either therapy alone or both combined and processed for histology studies. At all time points, lungs from control mice showed normal blood vessels with integral basement membrane, endothelial lining with endothelial cells surrounded by normal alveoli (Fig 4A1). Normal structures of bronchioles lined by ciliated columnar epithelium and surrounded by normal thin alveolar septa were seen (Fig 4A2-3, Table 1). Following treatment with soy isoflavones at 2, 3 and 4 months, the structures of blood vessels (Fig 4B1,2), bronchioles and the thickness of alveolar were not altered and looked normal compared to untreated lungs (Fig 4B1-3, Table 1). In contrast, radiation to the left lung caused significant tissue damage which was clearly observed on day 64 and further increased at the later time points of day 87 and 134 (Table 1). The alveolar septa and vessel walls showed a chronic inflammatory infiltrate, indicative of pneumonitis causing a marked reduction in alveolar spaces compared to control or soy-treated lungs (Fig 4C1-3, Table 1). Clusters of inflammatory cells were observed in the vicinity of blood vessels and infiltrating into the alveoli (Fig 4C1,C3 and C1A,C3A), disrupting the integrity of the vessel wall (Fig 4C2). Focal areas of damaged alveolar tissues were replaced by a heavy infiltration of inflammatory infiltrates consisting mostly of histiocytes and lymphocytes (Fig. 4C2 and C2A, Table 1). Focal hemorrhages were seen, likely the result of vascular damage induced by radiation. These data suggest that radiation caused a long lasting and strong inflammatory response that became even more prominent by 4 months after radiation. These findings were not observed in non-irradiated right lungs of these mice. Irradiated left lungs from mice treated with soy isoflavones prior to- and after radiation showed mostly normal bronchioles, alveoli and blood vessels with minimal inflammatory infiltrate and hemorrhage showing milder pneumonitis compared to radiation-treated lungs (Fig 4D1-3, Table 1).
Figure 4. H & E staining of lung tissue sections from mice treated with soy, radiation and radiation + soy.

Mice were treated either with soy or 12 Gy radiation (Rad) administered to the left lung alone or soy isoflavones combined with radiation (Soy + Rad). Soy was continued 5 days a week for up to 19 weeks. Lung tissue sections obtained on days 64, 87, and 134 after radiation were processed for H& E staining. (A1-3) Control lung from untreated mice showing normal structures of lung tissues including vessels (V), bronchioles (B) and alveolar septa. (B1-3) Soy-treated lungs showing also normal morphology of lung structures on days 64, 87 and 134 of soy treatment. (C1-3) Radiation-treated left lung showing thickened alveolar septa and clusters of inflammatory infiltrates (IF) in the vicinity of blood vessels (C1, C3) and infiltrating into the alveoli (C2) as seen in C.1A, C.2A and C.3A enlarged areas. Focal areas of damaged alveoli tissues were replaced by a heavy infiltration of inflammatory infiltrates consisting mostly of histiocytes and lymphocytes (C2). (D1-3) Radiation + Soy treated lungs showing less thickening of alveolar septa and less disruptions in lung tissue with reduced inflammatory infiltrates compared to radiation alone. All magnifications 20X.
Table 1.
Summary and quantification of histological observations
| Lung damage | IF cells | Pneumonitis | Fibrosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D64 | D87 | D134 | D64 | D87 | D134 | D64 | D87D | D134 | D64 | D87 | D134 | |
| Control | ± | ± | + | ± | ± | + | ± | ± | + | ± | ± | + |
| Soy | ± | ± | + | ± ± | + | ± | ± | + | ± | ± | + | |
| Radiation | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | + | ++ | +++ |
| Rad + Soy |
± | ± | + | ± | ± | + | ± | ± | + | ± | ± | ++ |
Histological findings from the experience presented in Figures 4 and 5 were quantified. The extent of lung damage (disruption of alveoli, bronchioles, and vessels), inflammatory infiltration (IF cells), pneumonitis (thickened septa), and fibrosis were scaled from weak (±), moderate (+), strong (++), to heavey (+++).
Detection of fibrosis in lungs treated with soy and radiation
We have documented above the occurrence of radiation-induced pneumonitis as well as the beneficial effects of soy supplementation. Because pneumonitis is associated with development of fibrosis (4, 20), the effect of soy isoflavones on this late event was also evaluated by staining collagen fibers with the Masson’s Trichrome stain. The lungs from control and soy treated mice showed typical light blue staining of collagen fibers around vessels and bronchioles (Fig 5A1-2, Fig 5B1-2, Table 1) although the staining was more intense at a later time point on day 134 (Fig 5A3,B3, Table 1). Left lung irradiation caused an increase in collagen fibers supporting vessel walls and bronchioles seen on day 64 (Fig 5C1, Table 1) compared to control lungs. The intensity of collagen stain was further increased at later time points on days 87 and 134 (Fig 5C2-3, Table 1). By day 134, an intense blue stain of bronchovascular bundles was clearly observed (Fig 5C3, Table 1), suggesting progression of fibrosis. Lungs from mice treated with irradiation and soy isoflavones showed, within the bronchovascular bundles, an amount of collagen comparable to that observed in control lungs at the three time points tested (Fig 5D1-3, Table 1).
Figure 5. Soy inhibition of radiation-induced fibrosis in bronchovascular bundles.

Lung tissue sections obtained on days 64, 87, and 134 after radiation from the experiment described in Figure 4 were processed for Masson’s Trichrome staining for detection of fibrosis. (A1-3) Control lungs from untreated mice showing regular pattern of thin collagen fibers (stained in blue) around vessels (V) and bronchioles (B) in lung tissue. (B1-3) Lungs from soy-treated mice showing regular pattern of collagen staining like in control mice. (C1-3) Radiation (Rad) caused a marked increase in density of collagen fibers around vessels and bronchioles in left lung tissues which increased with time being extensive by day 134 after radiation. (D1-3) Lung tissues treated with radiation and soy (Rad +Soy) showed normal bronchovascular collagen fibers similar in density to that observed in control lungs on days 64 and 87. It should be noted that by day 134 of the experiment the lungs were more congested in all treatment groups with a higher density of bronchovascular collagen fibers, however there was a striking increase in fibrosis staining in radiation-treated lungs compared to control, soy and radiation + soy treated lungs. All magnifications 20X.
DISCUSSION
The process of lung injury caused by radiation is unique for this particular organ because of the clinical consequences of the pathological progression of normal lung tissue to inflamed lung and fibrotic tissue, thereby impeding normal breathing functions. Radiation pneumonitis is caused by an early inflammatory process triggered by damage to lung parenchyma, epithelial cells, vascular endothelial cells and stroma that involves induction of pro-inflammatory cytokines and chemokines which recruit inflammatory immune cells in the lung tissue (2, 3, 26, 27). This acute early pneumonitis actually progresses to a chronic inflammation mediated by cyclical phases of cytokines, chemokines and growth factors released in the tissue microenvironment (3, 28). These complex events culminate in the later stage of lung fibrosis which is due to excessive accumulation of collagen and other extracellular matrix components (3, 4, 28). These adverse events of radiotherapy affect patients’ breathing and their quality of life (1, 20, 22, 28).
In the current study, we have explored whether supplementation with natural and safe soy isoflavones could improve high dose radiotherapy for inoperable NSCLC by alleviating radiation injury to normal tissues. To focus our efforts on dissecting the effect of soy isoflavones on radiation injury to normal lung tissue, we selected to study this complementary approach in naïve mice, not bearing tumors.
Radiation caused damage to normal hair follicles, leading to hair loss in the irradiated left thoracic area. Supplementation with soy isoflavones pre- and post-radiation protected the majority of the mice against radiation-induced skin injury and hair loss or reduced the extent of hair loss. These original observations represent cogent evidence that soy isoflavones protect normal tissues from radiation-induced damage. Radiation-induced skin injury, probably due to damage to stem and progenitor cells, could progress from mild erythema to moist desquamation and ulceration and is still observed mostly in breast cancer patients as well as NSCLC patients because of the proximity between the skin and the target volume (15, 29, 30). Our findings on the protective effects of soy suggest that soy could be used to mitigate skin injury induced by radiation in patients. Other anti-oxidants including nitroxides such as tempol have shown efficacy in reducing alopecia in rodents and in a few patients treated with brain radiotherapy (31, 32). Other studies using mice showed the benefit of using plerixafor, a CXCR-4 antagonist, as a bone marrow stem cell mobilizer to improve both acute and late skin response to high doses of 25-30 Gy radiation (33).
The radiation induced acute inflammatory pneumonitis and late pulmonary fibrosis lead to compromised lung function including perfusion and gas exchange, affecting the breathing capacity of the patients (1, 20, 22, 28). This phenomenon expressed by increased breathing rates in patients was also reported in rats and mice by 2-4 months after doses of ≥ 10 Gy (2, 23, 25). In our studies, lung irradiation at 12 Gy caused an increase in breathing rate of mice that was more pronounced by 4 months after radiation probably due to late effects of radiation-induced injury to normal lung tissue. Supplementation with soy isoflavones prior to and after radiation blocked the increase in breathing rate. These findings are in agreement with recent studies in rats showing that the genistein isoflavone or EUK-207 superoxide dismutase (SOD) catalase mimetic delayed and suppressed radiation increased breathing rate, confirming a radioprotective effect of genistein, which is the most active component of soy (24). A natural extract of soy isoflavones, containing genistein, daidzein and glycetein isoflavones, was used for the current lung study because it was found to be more potent and safer than purified genistein (10). The increased breathing rate caused by radiation is associated with pneumonitis (20). Histological observations of the left lung treated with 12 Gy irradiation showed the occurrence of tissue damage along with an inflammatory response. Irradiated lung tissue showed thickening of alveolar septa that is indicative of pneumonitis, as well as damage to alveolar structures, bronchioles, and vessel integrity. Focal hemorrhages, probably due to vascular damage were seen. A heavy, chronic infiltration of inflammatory cells consisting of histiocytes and lymphocytes was observed involving vessels, bronchioles, and alveoli. These alterations continued to progress up to the later time points of 3-4 months after radiation. Concurrently, a progressive increase in fibrosis was also observed in the bronchovascular bundles, suggesting a long lasting and ongoing radiation-induced tissue damage over time. In contrast, lungs treated with radiation combined with soy showed milder pneumonitis and reduced fibrosis. A decrease in fibrosis caused by genistein or EUK-207 was also observed in irradiated lungs of naïve rats that could be associated with anti-oxidant and anti-inflammatory mechanisms involving inhibition of TGF-β1 expression which is a major fibrogenic cytokine (4, 24).
Taken together, these observations confirm that soy isoflavones can modulate the inflammatory response caused by radiation and slow down the progressive tissue damage induced by radiation, which leads to impaired lung function. These findings in naïve mice are in agreement with our findings on radioprotection of normal lung tissues in the A549 lung tumor model (7) and demonstrate that soy can protect against radiation-induced injury to normal lung tissue. In our studies, the levels of isoflavones measured in the serum of mice treated with soy isoflavones reflected typical in vivo metabolism with significant levels of daidzein (1.6 μM) and genistein (1.7 μM) (10). These levels are comparable to plasma concentrations of 1–4 μM soy isoflavones measured in Asian populations consuming foods rich in soy isoflavones, in contrast to 10–30 nM levels found in Western populations (14).
Several studies on radioprotectors and mitigators to minimize radiation toxicity are ongoing and include radical scavengers and anti-oxidants, some of which such as amifostine, tempol and melatonin have been clinically tested (20, 32). However, limitations include their safety and tumor protection from radiation effect. The use of soy isoflavones as radioprotectors is attractive because they were proven to be safe in controlled human clinical trials (5). Our experimental studies in animal models suggest that the addition of soy to radiotherapy might improve the effect of radiotherapy on the tumor target and reduce the radiation dose-limiting toxicity of radiotherapy to the normal lung. If this proves to be the case, this simple, non-toxic, natural compound would radically improve the effectiveness of this new radiation treatment for inoperable NSCLC.
Acknowledgements
These studies were supported by the American Institute for Cancer Research grant #10A108 and by grant 1R21CA155518-01A1 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or National Institutes of Health. We thank Mohit Agarwal and Edwin David for excellent technical assistance.
Footnotes
Conflict of Interest: No financial disclosures.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schallenkamp JM, Miller RC, Brinkmann DH, et al. Incidence of radiation pneumonitis after thoracic irradiation: Dose-volume correlates. Int J Radiat Oncol Biol Phys. 2007;67:410–416. doi: 10.1016/j.ijrobp.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 2.Hill RP. Radiation effects on the respiratory system. BJR Suppl. 2005;27:75–81. [Google Scholar]
- 3.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 4.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97:149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Messina M, Kucuk O, Lampe JW. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J AOAC Int. 2006;89:1121–1134. [PubMed] [Google Scholar]
- 6.Ahmad IU, Forman JD, Sarkar FH, et al. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr Cancer. 2010;62:996–1000. doi: 10.1080/01635581.2010.509839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillman GG, Singh-Gupta V, Runyan L, et al. Soy isoflavones radiosensitize lung cancer while mitigating normal tissue injury. Radiother Oncol. 2011;101:329–336. doi: 10.1016/j.radonc.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillman GG, Wang Y, Kucuk O, et al. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol Cancer Ther. 2004;3:1271–1279. [PubMed] [Google Scholar]
- 9.Hillman GG, Wang Y, Che M, et al. Progression of renal cell carcinoma is inhibited by genistein and radiation in an orthotopic model. BMC Cancer. 2007;7:4. doi: 10.1186/1471-2407-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raffoul JJ, Banerjee S, Che M, et al. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int J Cancer. 2007;120:2491–2498. doi: 10.1002/ijc.22548. [DOI] [PubMed] [Google Scholar]
- 11.Raffoul JJ, Banerjee S, Singh-Gupta V, et al. Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007;67:2141–2149. doi: 10.1158/0008-5472.CAN-06-2147. [DOI] [PubMed] [Google Scholar]
- 12.Singh-Gupta V, Joiner MC, Runyan L, et al. Soy isoflavones augment radiation effect by inhibiting APE1/Ref-1 DNA repair activity in non-small cell lung cancer. J Thorac Oncol. 2011;6:688–698. doi: 10.1097/JTO.0b013e31821034ae. [DOI] [PubMed] [Google Scholar]
- 13.Singh-Gupta V, Zhang H, Banerjee S, et al. Radiation-induced HIF-1alpha cell survival pathway is inhibited by soy isoflavones in prostate cancer cells. Int J Cancer. 2009;124:1675–1684. doi: 10.1002/ijc.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillman GG, Singh-Gupta V. Soy isoflavones sensitize cancer cells to radiotherapy. Free Radic Biol Med. 2011;51:289–298. doi: 10.1016/j.freeradbiomed.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 15.Lo SS, Sahgal A, Chang EL, et al. Serious complications associated with stereotactic ablative radiotherapy and strategies to mitigate the risk. Clin Oncol (R Coll Radiol) 2013;25:378–387. doi: 10.1016/j.clon.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Davis IC, Lazarowski ER, Chen FP, et al. Post-infection A77-1726 blocks pathophysiologic sequelae of respiratory syncytial virus infection. Am J Respir Cell Mol Biol. 2007;37:379–386. doi: 10.1165/rcmb.2007-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannizzaro V, Berry LJ, Nicholls PK, et al. Lung volume recruitment maneuvers and respiratory system mechanics in mechanically ventilated mice. Respir Physiol Neurobiol. 2009;169:243–251. doi: 10.1016/j.resp.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Early MA, Lishnevsky M, Gilchrist JM, et al. Non-invasive diagnosis of early pulmonary disease in PECAM-deficient mice using infrared pulse oximetry. Exp Mol Pathol. 2009;87:152–158. doi: 10.1016/j.yexmp.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Para AE, Bezjak A, Yeung IW, et al. Effects of genistein following fractionated lung irradiation in mice. Radiother Oncol. 2009;92:500–510. doi: 10.1016/j.radonc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Williams JP, Johnston CJ, Finkelstein JN. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr Drug Targets. 2010;11:1386–1394. doi: 10.2174/1389450111009011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin P, Johnston CJ, Williams JP, et al. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 22.Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65:1075–1086. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 23.Travis EL, Down JD. Repair in mouse lung after split doses of X rays. Radiat Res. 1981;87:166–174. [PubMed] [Google Scholar]
- 24.Mahmood J, Jelveh S, Zaidi A, et al. Mitigation of Radiation-Induced Lung Injury with EUK-207 and Genistein: Effects in Adolescent Rats. Radiat Res. 2013;179:125–134. doi: 10.1667/RR2954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novakova-Jiresova A, van Luijk P, van Goor H, et al. Pulmonary radiation injury: identification of risk factors associated with regional hypersensitivity. Cancer Res. 2005;65:3568–3576. doi: 10.1158/0008-5472.CAN-04-3466. [DOI] [PubMed] [Google Scholar]
- 26.Hill RP, Zaidi A, Mahmood J, et al. Investigations into the role of inflammation in normal tissue response to irradiation. Radiother Oncol. 2011;101:73–79. doi: 10.1016/j.radonc.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–1293. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 28.Williams JP, Jackson IL, Shah JR, et al. Animal models and medical countermeasures development for radiation-induced lung damage: report from an NIAID Workshop. Radiat Res. 2012;177:e0025–0039. doi: 10.1667/rrol04.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen MF, Chen WC, Lai CH, et al. Predictive factors of radiation-induced skin toxicity in breast cancer patients. BMC Cancer. 2010;10:508. doi: 10.1186/1471-2407-10-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirova YM, Fromantin I, De Rycke Y, et al. Can we decrease the skin reaction in breast cancer patients using hyaluronic acid during radiation therapy? Results of phase III randomised trial. Radiother Oncol. 2011;100:205–209. doi: 10.1016/j.radonc.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Metz JM, Smith D, Mick R, et al. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin Cancer Res. 2004;10:6411–6417. doi: 10.1158/1078-0432.CCR-04-0658. [DOI] [PubMed] [Google Scholar]
- 32.Citrin D, Cotrim AP, Hyodo F, et al. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15:360–371. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JH, Kolozsvary A, Jenrow KA, et al. Plerixafor, a CXCR4 antagonist, mitigates skin radiation-induced injury in mice. Radiat Res. 2012;178:202–206. doi: 10.1667/rr2886.1. [DOI] [PubMed] [Google Scholar]


