Abstract
Purpose
Our objective was to develop novel nanocarriers (protected graft copolymer, PGC) that improve the stability of heparin binding EGF (HBEGF) and gastrin and then to use PGC-formulated HBEGF (PGC-HBEGF) and Omeprazole (+/− PGC-gastrin) for normalizing fasting blood glucose (FBG) and improving islet function in diabetic mice.
Method
HBEGF, PGC-HBEGF, Omeprazole, Omeprazole+PGC-HBEGF, Omeprazole+PGC-gastrin+PGC-HBEGF and epidermal growth factor (EGF)+gastrin were tested in multiple low dose streptozotocin diabetic mice.
Results
Omeprazole+PGC-HBEGF normalized FBG and is better than EGF+gastrin at improving islet function and decreasing insulitis. Groups treated with Omeprazole, Omeprazole+PGC-HBEGF, or EGF+gastrin have significantly improved islet function versus saline control. All animals that received PGC-HBEGF had significantly reduced islet insulitis versus saline control. Non-FBG was lower for Omeprazole+PGC-gastrin+PGC-HBEGF but Omeprazole+PGC-HBEGF alone showed better FBG and glucose tolerance.
Conclusions
Omeprazole+PGC-HBEGF provides a sustained exposure to both EGFRA and gastrin, improves islet function, and decreases insulitis in multiple low dose streptozotocin diabetic mice. Although HBEGF or EGF elevates non-FBG, it facilitates a reduction of insulitis and, in the presence of Omeprazole, provides normalization of FBG at the end of treatment. The study demonstrates Omeprazole and PGC-HBEGF is a viable treatment for diabetes.
Keywords: type 1 diabetes, growth factors, nanocarrier, insulitis, islet function
INTRODUCTION
The hallmark of diabetes is hyperglycemia which can result from the depletion or lack of insulin producing β-cells in the pancreas, or a decrease of sensitivity of the body’s cells to insulin, or both. For type 1 diabetes (T1D), without β-cells or with very few β-cells, as reflected by the absence or very low level of blood insulin, the treatment has been frequent insulin injection for over half a century now. Transplantation of insulin-producing pancreatic islet cells has been shown to be effective in partially reversing T1D in patients but a chronic immunosuppressive regimen is needed to prevent rejection. Toxicity associated with immunosuppressive therapy contributes to a decrease in transplanted islet viability through interference with islet cell division. In addition, this approach is limited to a subgroup of diabetics and by the availability of donor islets. Regenerating missing or insufficient β-cells and/or shutting down the underlying autoimmune disease that attacks β-cells are recent major research goals. These goals are motivated by advances in the understanding of growth factors and hormones involved in β-cell regeneration and the development of various agents that are able to modulate the autoimmune disease. The measure of remaining β-cell mass correlates positively with and is directly related to insulin staining of the pancreas (1) and the latter correlates negatively with fasting blood glucose level (2). In this study, we used insulin staining and fasting blood glucose as reporters for an increase in β-cell mass or regeneration as a result of treatment, irrespective of whether regeneration was a result of 1) β-cell proliferation, 2) trans-differentiation of cells of the pancreatic duct into β-cells, 3) trans-differentiation of glucagon-producing α-cells into β-cells, or any combination thereof (see Fig. 1). Evidence exists indicating that epidermal growth factor receptor agonist (EGFRA) is a trans-differentiation factor that primes specific cells in the pancreas to differentiate into β-cell precursors, while gastrin facilitates β-cell maturation and proliferation (1–3). In fact, combination therapy of EGFRA and gastrin increases β-cell mass and reverses hyperglycemia in diabetic mice (1–3). Because blood half-lives of gastrin and EGFRA are only a few minutes (4–6), infusion or multiple daily administrations have been required to demonstrate 1) increase in β-cell mass, 2) reversal of diabetes, 3) islet regeneration, and 4) potential induction of immune-tolerance (1–3). The fact that EGFRA and gastrin regenerate β-cells and induce immune tolerance (2, 6) raises the exciting possibility of a cure for diabetes without transplantation and immune suppression. The practical human application of this treatment is limited by the lack of blood stability of gastrin and EGF as well as the gastric hyperacidity induced by gastrin. Although this combination treatment has been approved by the FDA for a Phase 1 clinical trial, the involvement of EGF in various cancers is a concern. This may limit the duration of treatment and possible re-treatment of patients using this combination therapy. To alleviate future risks or safety concerns, we selected soluble heparin-binding EGF (HBEGF) for this study; HBEGF has no membrane binding region which has been implicated in the self-sustaining cycle of tumor growth. We chose to use Omeprazole, an over-the-counter proton pump inhibitor that prevents stomach hyperacidity, and showed that it can increase blood gastrin in a sustained manner. The safety of Omeprazole has been established by over three decades of use. However, before human use, new safety studies must be done for Omeprazole combined with HBEGF to establish the boundary of safety compared to duration and frequency of treatment. In this study, we evaluated whether endogenous gastrin induction using Omeprazole can increase β-cell mass; use of Omeprazole has the advantage of eliminating stomach hyperacidity, a side effect of exogenous gastrin administration. In fact, a recent retrospective human study (7) showed that type 2 diabetes (T2D) patients using Omeprazole have significantly lower HbA1c (a surrogate for a two-week blood glucose average) than those patients who do not; in the present study we found similar results in diabetic mice. The present study also showed: 1) development of nanocarriers made of protected graft co-polymer (PGC) that bind HBEGF and gastrin; 2) formulated PGC-HBEGF and PGC-gastrin that have extended blood stability compared to unformulated HBEGF and gastrin; 3) Omeprazole induces sufficient endogenous gastrin elevation (15 to 120-fold) to replace gastrin or PGC-gastrin administration; and 4) once a day treatment with PGC-HBEGF and Omeprazole normalizes FBG, improves islet function, and decreases insulitis in multiple low dose streptozotocin diabetic mice. In addition, we show that combined PGC-HBEGF and Omeprazole, given once a day, is equivalent to, or better than, a combination of EGF and gastrin, given three times a day, at providing sustained exposure to EGFRA and gastrin, improving glucose homeostasis, improving islet function, and decreasing insulitis in multiple low dose streptozotocin diabetic mice.
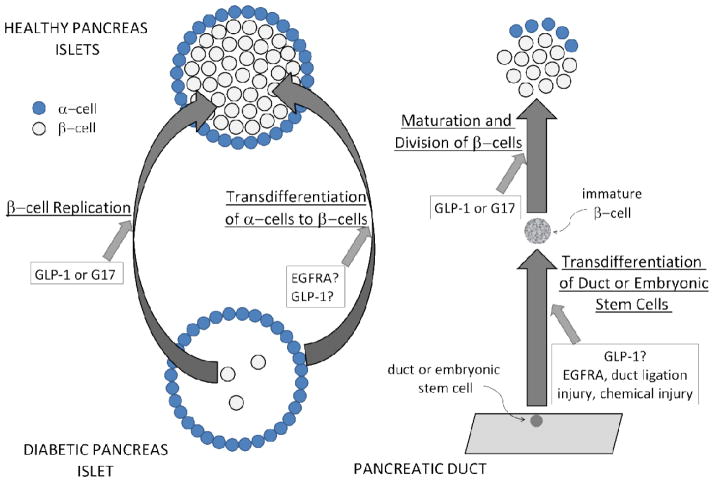
Figure 1.
Shown are various pathways proposed for β-cell regeneration, depending on the experimental model used. Regeneration of β-cells can be a result of 1) β-cell proliferation, 2) trans-differentiation of glucagon-producing α-cells into β-cells, 3) trans-differentiation of cells of the pancreatic duct into β-cells, or any combination. The agents that have been shown experimentally to cause these changes are indicated and those with question marks have been shown to occur but the mechanism remains controversial because of the possible contribution of other mechanisms.
MATERIALS AND METHODS
Synthesis of PGC
The PGC used for HBEGF was made by linking a chondroitin sulfate (mw=31–54 kiloDalton Pfaltz&Bauer, Waterbury, CT) backbone with multiple amino-terminated polyethylene glycol (amino PEG, mw=5 kiloDalton; Laysan Bio Inc. Arab, AL) chains at a weight ratio of 1:2 (chondroitin sulfate:PEG) using a N-hydroxysuccinimide and 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride facilitated reaction as previously described (8), see Fig. 2. PGC for gastrin was synthesized in a similar manner but with PEI (branched polyethyleneimine; mw=10 kiloDalton, Polysciences, Inc. Warrington, PA) as the backbone instead of chondroitin sulfate. The backbone amino groups of PEI were linked to, in order: 1) multiple carboxyl terminated PEG (Laysan Bio, Inc., Arab, AL) up to 44% amino saturation, 2) stearic acid up to 86% saturation, and 3) Iodomethane up to 100% saturation, using the same facilitated reaction as above for the first and second reactions, as previously described (8).
Figure 2.
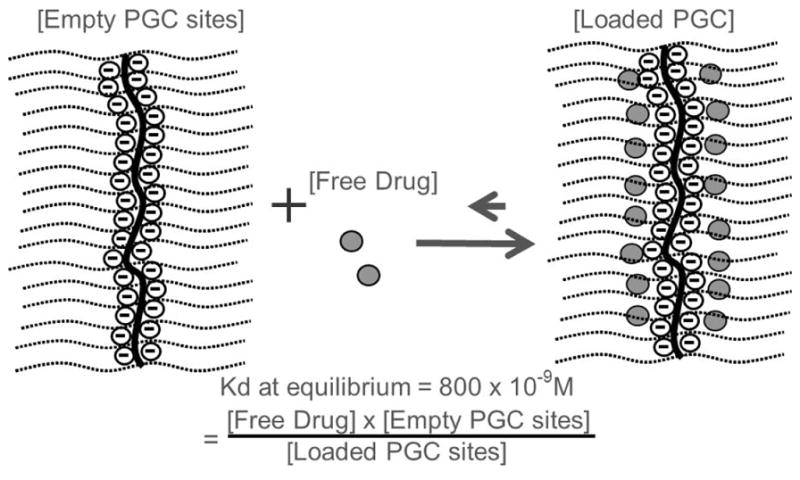
The structure of the Protected Graft Copolymer (PGC) used for HBEGF consists of a backbone polymer (central line), protective PEG chains (parallel lines), and a binding region shown by the circled negative charges along the chondroitin chain with 16 available sites; the peptide or free drug (dark circles) is in equilibrium, favoring the loaded PGC. The PGC used for gastrin 17 has a hydrophobic core similar to that previously published (8) but with different backbone. The average hydrodynamic diameter of this PGC is 20nm.
Binding of HBEGF and gastrin to PGC
For preliminary binding studies, PGC (0.1–2mg PGC for HBEGF; 1 and 4mg of PGC for gastrin) in 250ul buffered saline (100mM HEPES/100mM NaCl) was loaded and incubated for two hours at room temperature with 10ug of soluble HBEGF (Recombinant human HBEGF, 86 amino acids, 9.7 kiloDalton, DLQEADLDLL RVTLSSKPQA LATPNKEEHG KRKKKGKGLG KKRDPCLRKY KDFCIHGECK YVKELRAPSC ICHPGYHGER CHGLSL, PeproTech, Rocky Hill, NJ) or 20 and 8ug of stable human gastrin (gastrin, 17 amino acids, 2.08 kiloDalton, Pyr-GPWLEEEEEAYGWLDF-NH2, Anaspec, Freemont, CA). After 2 hours, the incubation mixture was processed as previously described (8) except that the analysis of bound and free was done using a HBEGF ELISA (R&D Systems, Minneapolis, MN) or gastrin EIA (Assay Designs-Enzo Life Sciences, Plymouth Meeting, PA). For the determination of dissociation constant (Kd), 2mg of PGC (or 0.25mg for gastrin) in 250ul buffered saline (100mM HEPES/100mM NaCl) was mixed with 5–120ug of HBEGF (or 12.5–75.0ug of gastrin) and incubated for two hours at RT (n=3) and processed as above. Using free and bound information, Scatchard plots were constructed from which the Kd and capacity of the carriers were calculated (slope is −1/Kd and x-intercept is capacity).
Pharmacokinetics of PGC-HBEGF, PGC-gastrin, and Omeprazole induction of endogenous gastrin
Animal use followed the “Principles of Laboratory Animal Care” in NIH publication #85-23, revised in 1985 and was approved by the Pacific Northwest Diabetes Research Institute animal use committee. Aqueous mixtures of PGC and HBEGF (0.5% loading or 30mg PGC with 0.15mg HBEGF) or PGC and gastrin (2% and 0.5% loading) were incubated for 2 hours and lyophilized. The lyophilized formulations and the HBEGF or gastrin control (without PGC) were dissolved in saline and injected subcutaneously (s.c.) into BALB/c mice (n=3) at 0.3mg HBEGF/Kg or 3mg gastrin/Kg. Blood samples were collected from the retro-orbital sinus at various times and the serum level of HBEGF or gastrin was measured as described above. Omeprazole in DMSO (50 and 100mg/kg) was injected intraperitoneally (i.p.) daily (9) for 1 week in BALB/c mice (n=5) and blood samples were collected retro-orbitally prior to the first injection (baseline control), and prior to the 2nd-5th Omeprazole injections. The blood level of endogenous gastrin was measured as described above.
Testing of the combined PGC-HBEGF and Omeprazole treatment in the multiple low dose streptozotocin diabetic mouse model
Male CD-1 mice, 4–5 weeks old, were given fresh Streptozotocin (40mg/Kg, i.p. injection) in 50mM Na-Citrate buffer, pH 4.5, for 5 consecutive days to induce chemical injury to the islet cells. The immune response that followed has been well documented in the literature, consistent with T1D. Non-FBG was monitored (Ascensia Contour Glucometer and test strips, Bayer, Mishawaka, IN) daily for 7 days and twice a week thereafter using tail-tip snip blood collection (5ul). The 28-day treatments started on day 8 after grouping the diabetic animals to have similar starting average glucose, see Table I. HbA1c was measured on days 11 and 31, prior to drug administration for that day, using a Bayer A1c Now+ (Sunnyvale, CA). After day 35, mice (n=5) were fasted for 16h and an intraperitoneal glucose tolerance test (IPGTT) was performed by injecting glucose (1.0 mg/g body weight) and monitoring glucose levels at various times over 2 hours to evaluate islet β-cell function. In addition, 5′-BrDU (Bromo-2′-deoxyuridine, 50 mg/kg) was injected along with glucose as a cell division marker (3). Animals were euthanized by CO2 inhalation and various organs were harvested for histopathology and immunohistochemical analysis.
Table I.
Treatment Groups. Group (Gr) 1 was normal mice and Gr 2–8 were multiple low dose streptozotocin diabetic mice.
| Group | Description | Dose | Frequency |
|---|---|---|---|
| 1 | Saline/DMSO non-diabetic control | match maximum volume | 1x/day |
| 2 | Saline/DMSO diabetic control | match maximum volume | 1x/day |
| 3 | unformulated HBEGF | 0.3mg HBEGF/Kg | 1x/day |
| 4 | PGC-HBEGF | 0.3mg HBEGF/Kg | 1x/day |
| 5 | Omeprazole | 50mg Omeprazole/Kg | 1x/day |
| 6 | Omeprazole+PGC-HBEGF | 50mg Omeprazole/Kg 0.3mg HBEGF/Kg |
1x/day |
| 7 | Omeprazole+PGC-HBEGF+PGC-gastrin | 50mg Omeprazole/Kg 0.3mg HBEGF/Kg 3mg gastrin/Kg |
1x/day |
| 8 | EGF+gastrin (literature control) | 0.1mg EGF/Kg 3mg gastrin/Kg |
3x/day |
Histology
Formalin fixed, paraffin-embedded sections (3×5um sections, 200um apart, per animal) of pancreata were stained with hematoxylin-eosin (2) for light microscopic evaluation. “Insulitis” or lymphocyte infiltration of the islets was evaluated. The average total number of islets with insulitis per total islets observed per section (n=15 sections) was determined blindly in each group. Insulin content was evaluated in adjacent serial sections (15 sections/group) via immunohistochemistry (2). The insulin content of the islets was scored blindly using the following scoring system: 0=no staining, 1=<50% β-cells staining in an individual islet, 2=50–75% β-cells staining, 3=75–90% β-cells staining, 4=>90% β-cells staining. BrDU immunostaining was performed as previously described (3). Stomachs were processed and surface areas of various regions were measured. Sections (3×5um sections, 200um apart, per animal) from the glandular area of the fundus and antrum were stained with hematoxylin-eosin. In addition to evaluation for tumors, sections were evaluated for lymphocytic inflammation, mucosal epithelial cell vacuolar degeneration and necrosis and glandular ectasia. These abnormalities are scored following: Minimal=1, Mild=2, Moderate=3, and Severe=4; for glandular ectasia: Rare=1 and Several=2.
RESULTS
PGCs bind to various EGFRAs and gastrin. Initially we synthesized and tested at least 20 different PGCs with a backbone of polylysine (8, 10, 11) for binding to three epidermal growth factors receptor agonists (EGFRAs) that bind to the same receptor (EGF, transforming growth factor alpha or TGF-a, and Heparin binding EGF or HBEGF). Over 95% of the carriers we tested did not bind any of the EGFRAs. The PGCs that did bind EGF, HBEGF, and TGF-alpha efficiently were DTPA-copper containing PGCs where each DTPA picks up two copper ions. When this copper chelate containing PGC was tested for EGF binding, it showed 0% free EGF at 0.5% loading (0.5mgEGF/100mg of PGC) with a Kd of 900nM and a capacity of ~15 EGF molecules per carrier molecule by Scatchard plot analysis. We also found that chondroitin sulfate-based PGC binds HBEGF with a Kd of 800nM and a capacity of 16 HBEGF molecules per PGC. The chondroitin sulfate-based PGC has additional low affinity sites for HBEGF with a Kd of 7uM and a capacity of 50 HBEGF per PGC but these sites will be of low significance in the high salt and protein environment in the blood. For this study, we chose to use the soluble HBEGF because it lacks a tumorigenic cytoplasmic domain (12–14) for better safety than the previously published EGF (2), a factor that must be considered if repeated treatments become necessary. We chose the chondroitin sulfate-based PGC for the animal studies because chondroitin sulfate has a history of human use for alleviating symptoms of arthritis. However, with copper being antibacterial by itself, the copper containing HBEGF formulation is potentially useful in treating diabetic foot ulcers since topical EGF, such as Regen-D®, has been shown to facilitate the healing of diabetic foot ulcers.
We also successfully formulated human gastrin using PGC with hydrophobic core made with 10 kiloDalton PEI as the backbone instead of previously described polylysine (8) and 44% of the amino groups linked to 5 kiloDalton PEG, 42% linked to stearic acid, and the remaining 14% were methylated. This PGC binds gastrin with a Kd of 1uM and a capacity of 15 gastrin molecules per PGC. However, because Omeprazole can increase gastrin in the blood, the use of PGC-gastrin may not be needed. The binding of HBEGF and gastrin to PGC is reversible, with a defined Kd. Because they are in solution, they were expected to provide a degree of automatic control of the amount of free drugs in the blood, limited only by blood components, such that when the concentration of free drug decreases the PGC will release more drug to satisfy the equilibrium based dissociation constant, Kd, between PGC and drug. Essentially this is a drug reservoir in the blood and the mechanism is as previously described (8).
PGC formulations of HBEGF and gastrin have significantly larger blood area under the curve, and longer half-life
As expected, when the PGC-formulated HBEGF and gastrin were subcutaneously administered into mice, a larger blood area under the curve and a longer half-life were observed (Fig. 3), consistent with results from other PGC-formulated peptides (8, 15, 16). PGC increased HBEGF blood area under the curve and half-life by 10-fold compared to unformulated HBEGF, and allowed for much greater/prolonged exposure (24h) of the animals to HBEGF from a single subcutaneous injection.
Figure 3.
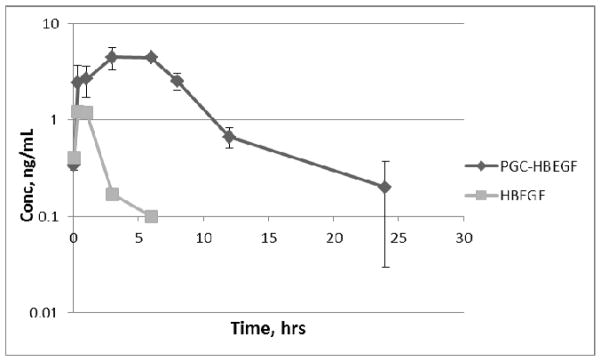
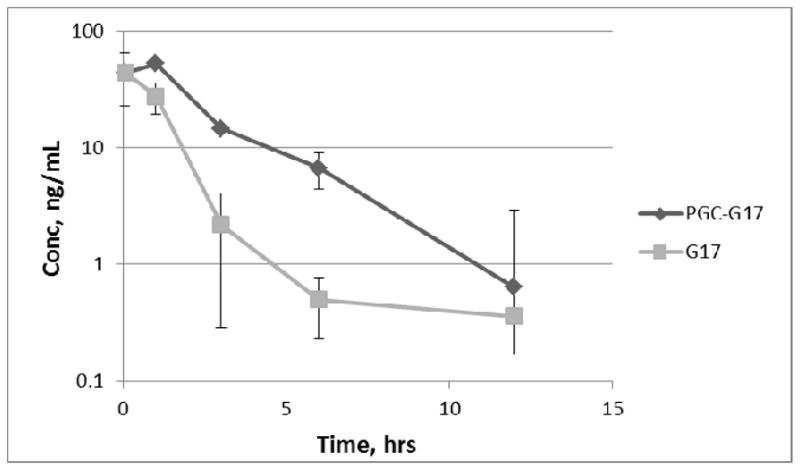
Serum HBEGF or gastrin (G17) in mice (n=3) after single s.c. dose with and without PGC (0.5% loading; Mean, ng/mL, error bars are SD). The limit of detection of the assay (LOD) is 0.02ng/ml; the endogenous background is below the limit of detection of the assay. The AUC of HBEGF increases from 3.90 to 40.98ng/ml*hr in the presence of PGC and the apparent half-life increases from 0.7 to 7hr. The AUC of gastrin increases from 70 to 166ng/ml*hr in the presence of PGC and the apparent half-life increases from 0.7 to 1.8hr.
Omeprazole provides a more sustained elevation of endogenous gastrin that is superior to the administration of a high dose of exogenous gastrin
When mice were given 50 and 100mg Omeprazole/kg daily for 1 week, the first 24 hour endogenous gastrin level in the blood increased by 4- and 37-fold (CV or 100*SD/mean of 9% and 67%, respectively) from the baseline (0.035ng/ml), respectively. Greater variability in the level of gastrin was observed during the first 24 hours perhaps because it takes some time for cells to reach the maximum synthetic capacity. On the fourth day blood gastrin levels of 100- and 126-fold (CV of 1% and 4.5%, respectively) over baseline were observed for animals that received 50 and 100mg Omeprazole/kg daily, respectively. The fourth day results also show that there is no tachyphylaxis from repeated administration of Omeprazole. The dose used in mice was lower than the previous report (136mg/Kg) (9) but achieved a much higher level of endogenous gastrin, likely due to the use of a freshly prepared solution of Omeprazole in DMSO each time. Omeprazole has a rat LD50 of greater than 2g/Kg and the standard human dose of Omeprazole to treat heartburn is 10–40mg/person making the use of Omeprazole in diabetes significantly safer and without the side effect of stomach hyperacidity from exogenous gastrin. Omeprazole provides a more sustained elevation of endogenous gastrin that is superior to the administration of high doses of exogenous gastrin, either PGC-formulated or unformulated.
PGC-HBEGF+Omeprazole given once a day is better than EGF+gastrin given 3-times a day in improving glucose homeostasis, islet function, and reducing insulitis in multiple low dose streptozotocin diabetic mice
Because EGF and HBEGF can transiently increase non-FBG (17) during treatment, the ability to improve islet health (1–3) was evaluated post treatment by looking at FBG, IPGTT glucose area under the curve (gAUC), and islet histology. The group treated once a day with combined PGC-HBEGF and Omeprazole (Gr 6) had normal average FBG at the end of the treatment (Fig. 4 & 5) where 60% of animals were euglycemic (below 120mg/dl). In contrast, only 20% of animals in Gr 8 (unformulated EGF and gastrin given 3x/day) were euglycemic. Compared to vehicle control (Gr 2), positive control (Gr 8) treatment improved β-cell function consistent with the literature (1–3) but treatment once a day with combined PGC-HBEGF and Omeprazole (Gr 6) was even more effective (Fig. 4 & 5 and Table III). Later in the treatment, Gr 6 had significantly lower non-FBG and gAUC during the treatment period compared to Gr 2 (Table II). In contrast the positive control, HBEGF, and PGC-HBEGF groups all had similar non-FBG profiles during the treatment period, consistent with hyperglycemic effect of both HBEGF and EGF, confounded by the absence of gastrin to counteract this effect (Fig. 6 and Table II). At the end of the treatment, Gr 6 and Gr 5 had significantly improved islet function by IPGTT gAUC compared to Gr 2 (Table III, p=0.0052 and p=0.0135, respectively). Although higher, the average weights of all peptide-treated groups at the end of the treatment are not statistically different from the vehicle group. Power analysis indicated that a large standard deviation in weight (1.76g) would require n=15 animals for weight to be a useful predictor of metabolic health, unless weight changes are very dramatic which would likely reach significance even with n=8. BrDU staining in the exocrine pancreas, particularly near the duct, was higher in the combined Omeprazole and PGC-HBEGF treated group than normal and diabetic controls (not shown). Treatment with Omeprazole+PGC-HBEGF is also associated with a higher incidence of insulin staining in the exocrine pancreas than normal and diabetic controls (not shown). Association between these two observations (BrDU and insulin) in the exocrine pancreas will require a separate future study with sufficient power to reach a statistically valid conclusion. However, it is already established that HBEGF gene transduction promotes the proliferation of pre-existing β-cells and differentiation of duct cells into β-cells, resulting in an increase in β-cell mass and improved glucose tolerance (18).
Figure 4.
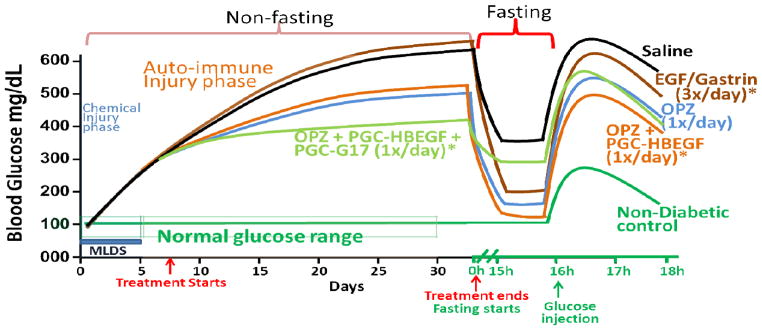
Diagram of the in vivo Blood Glucose level during and after the indicated treatment of multiple low dose streptozotocin diabetic mice. The diagram was derived from the best fit curve of the data and is for illustrative purposes only. CD1 mice (n=8/group; n=5/group for IPGTT) were treated with multiple low dose Streptozotocin (40mg/Kg daily for 5 days) to induce chemical injury followed by auto immune injury. PGC-HBEGF and HBEGF alone are similar to saline (not shown). Asterisks indicate susceptibility to hyperglycemic effect of HBEGF (17) during treatment while enhancing islet recovery. Proper evaluation of the effect of HBEGF containing treatment must be by FBG, IPGTT, and Histology after the treatment ends. It appears that too much gastrin improves blood glucose during treatment but can limit islet recovery based on FBG.
Figure 5.

Average fasting blood glucose (FBG; mean+/−SEM) value at Day 36; n=5 in this case because the other animals were used for other purpose. Groups are as in Table I. The group treated with Omeprazole+PGC-HBEGF (Gr 6) has significantly lower FBG than vehicle control Gr 2 (p<0.033). None of the other groups are significantly different from group 2 based on n=5. However, based on the FBG standard deviation and power analysis a much larger n is needed to have a power of 80% to not erroneously conclude a lack of significant difference between Gr 2 and Gr 5 or Gr 8 (p=0.061 and p=0.165, respectively). Considering the significant difference in IPGTT results between Gr 2 and Gr 5 or Gr 8 (Table III, p=0.014 and p=0.002, respectively), the differences in FBG between Gr 2 and Gr 5 or Gr 8 are likely to be significant at n= 6 or 10, respectively.
Table III.
Islet function: IPGTT gAUC (g/dl x min) and t-test p value compared to Gr 2, n=5, Group (Gr) descriptions are in Table I
| Group | Gr 1 | Gr 2 | Gr 3 | Gr 4 | Gr 5 | Gr 6 | Gr 7 | Gr 8 |
|---|---|---|---|---|---|---|---|---|
| mean | 19.8 | 74.9 | 73.2 | 64.1 | 53.4 | 47 | 65 | 61.3 |
| SEM | 0.59 | 1.2 | 8.5 | 7.9 | 6.1 | 6.4 | 6.3 | 2.3 |
| p value versus Gr 2 | <0.0001 | ----- | 0.8496 | 0.2252 | 0.0135a | 0.0052a | 0.1736 | 0.0019a |
IPGTT glucose area under the curve (gAUC) results showed that Gr 5, Gr 6, and Gr 8 have significantly lower gAUC than vehicle control Gr 2. Gr 7 which is similar to Gr 5 but received extra exogenous gastrin did not get the benefit of improved islet function (IPGTT) seen in Gr 5 but effectively counteracted the hyperglycemic effect of HBEGF during the treatment.
Table II.
Glucose homeostasis parameters showing Glycemia during the treatment, Group (Gr) descriptions are in Table I, n=8
| Group | Gr 1 | Gr 2 | Gr 3 | Gr 4 | Gr 5 | Gr 6 | Gr 7 | Gr 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Non-FBG near the end of treatment, day 31 | mg/dl, mean | 136 | 608 | 619 | 599 | 497 | 536 | 414 | 640 |
| SEM | 3 | 18 | 16 | 21 | 37 | 25 | 53 | 10 | |
| p value versus Gr 2 | <0.0001 | ----- | 0.6549 | 0.7497 | 0.0173a | 0.0348a | 0.0038a | 0.1425 | |
| Non-FBG gAUC during treatment, day 7–day 31 | mg/dl, mean | 3176 | 11614 | 12006 | 10863 | 9292 | 10526 | 8594 | 11266 |
| SEM | 44 | 758 | 318 | 516 | 918 | 439 | 1059 | 743 | |
| p value versus Gr 2 | <0.0001 | ----- | 0.6408 | 0.4265 | 0.0714 | 0.2346 | 0.0360a | 0.7479 | |
| HbA1c during treatment, week4-week1 | Δ HbA1c %, mean | −0.4 | 2.0 | 2.4 | 1.8 | 1.1 | 1.6 | 0.9 | 2.2 |
| SEM | 0.1 | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 | 0.4 | 0.3 | |
| p value versus Gr 2 | <0.0001 | ----- | 0.2860 | 0.5879 | 0.0067b | 0.2860 | 0.0275b | 0.5879 |
Omeprazole (Gr 5), Omeprazole+PGC-HBEGF (Gr 6), and Omeprazole+PGC-HBEGF+PGC-gastrin (Gr 7) treated animals have significantly lower non-FBG at day 31 than vehicle control (Gr 2). However, only Gr 7 (those that received the induction of endogenous gastrin, exogenous gastrin, plus PGC-HBEGF) have significantly lower glucose area under the curve during the treatment period, indicating that additional gastrin lowers blood sugar during the treatment but may have contributed to higher FBG after the treatment. In addition, early time points prior to the full effect of the treatment diluted the non-FBG glucose area under the curve (gAUC) results as seen by comparison with the non-FBG at day 31.
Gr 5 and Gr 7 have significantly less increase in HbA1c compared to Gr 2 control during the treatment period. Gr 6 and literature positive control Gr 8 did not show any decrease in HbA1c formation compared to vehicle control Gr 2 during the treatment, which may be due to a hyperglycemic effect of the formulations, despite improvement of islet function after the treatment as seen from IPGTT.
Note that Omeprazole alone (Gr 5) or Omeprazole+PGC-HBEGF+PGC-gastrin (Gr 7) showed reduced non-fasting blood glucose during treatment, indicating that gastrin can partially overcome the hyperglycemic effect of HBEGF.
Figure 6.
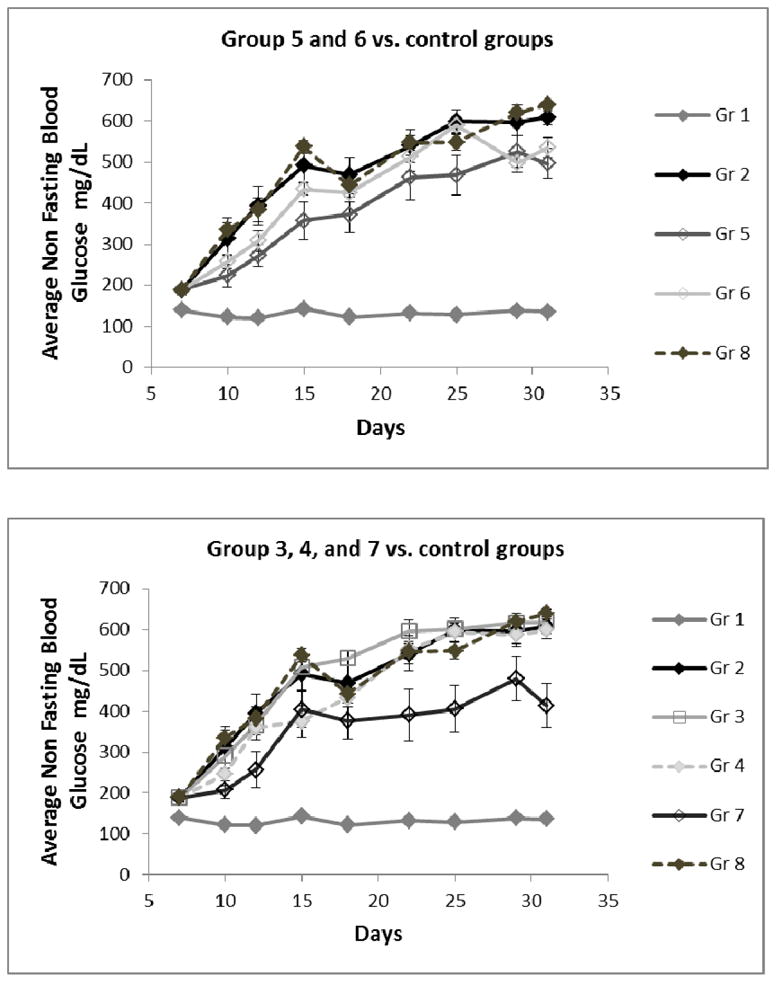
Average non-fasting blood glucose (non-FBG) values during the treatment period (n=8). Groups (Gr) are described in Table I.
During the treatment, a lower non-FBG is associated with the presence of Omeprazole or gastrin which appears to be counteracted by HBEGF or EGF
All groups that received Omeprazole have significantly lower average non-FBG near the end of the treatment (Table II, Gr 5 and Gr 6, p<0.04) compared to the saline treated group (Gr 2) and the group that received additional exogenous gastrin had the lowest non-FBG (Gr 7). The non-FBG of those that received HBEGF alone (Gr 3) or PGC-HBEGF alone (Gr 4) are close to saline with Gr 3 above saline and Gr 4 below saline, perhaps because in Gr 4 PGC limits the amount of hyperglycemic free HBEGF. When PGC-HBEGF is combined with Omeprazole (Gr 6), non-FBG drops significantly indicating that Omeprazole and/or gastrin is a good blood glucose lowering agent. The observation that HBEGF is a hyperglycemic agent is further supported by the fact that when Omeprazole alone (Gr 5) was supplemented with PGC-HBEGF (Gr 6) an increase in non-FBG was seen. When the treatment with Omeprazole and PGC-HBEGF (Gr 6) was supplemented with exogenous gastrin (Gr 7), a further drop in non-FBG was observed, consistent with the role of gastrin as observed in the Omeprazole only group, and, because of this consistency, it is unlikely to be due to the PGC that was used for gastrin. However, this supplementation with exogenous gastrin resulted in an increase in FBG, perhaps as a result of over-stimulation of β-cells that could compromise their overall health. This indicates that exogenous gastrin supplementation may not be advisable and the development of PGC for gastrin may not be a productive endeavor in the treatment of diabetes. The hyperglycemic effect of HBEGF is supported by previous observations (17, 19) but nevertheless Omeprazole with PGC-HBEGF (Gr 6) showed much improved β-cell function at the end of treatment with better FBG, IPGTT gAUC, and a reduction of insulitis, which is a better treatment outcome. This also confirms the beneficial role of EGFRA in islet health despite its hyperglycemic effect.
PGC alone had no impact on the alleviating diabetic condition
It is clear that the improvement of the diabetic condition is not caused by PGC because the comparison of HBEGF alone versus PGC-HBEGF (Gr 3 and Gr 4, respectively) shows little difference in non-FBG at day 31 or non-FBG gAUC during treatment (Table II) or FBG at Day 36 (Fig 5), and insulitis in Gr 4 is not lower than in Gr 3 (Table IV and Fig. 7). HBEGF and PGC-HBEGF treatment reduced insulitis compared to the Gr 2 control (Fig. 7 and Table IV), without a significant improvement in diabetic condition (Fig. 5 and Table III), thus PGC alone does not improve diabetic condition.
Table IV.
Blinded examination of the H&E-stained section of pancreas (mean number of islets with Insulitis per sectiona), Group (Gr) descriptions are in Table I. Groups that received EGFRA have significantly less insultis compared to the diabetic control.
| Group | Gr 1 | Gr 2 | Gr 3 | Gr 4 | Gr 5 | Gr 6 | Gr 7 |
|---|---|---|---|---|---|---|---|
| Mean # of islets with insulitis per sectiona | 0 | 0.38 | 0.02 | 0.17 | 0.30 | 0.17 | 0.13 |
| SEM | 0 | 0.08 | 0.02 | 0.03 | 0.07 | 0.05 | 0.05 |
| p value versus Gr 2 | <0.0001 | ----- | 0.0002 | 0.0204 b | 0.4580 | 0.0342 b | 0.0131 b |
Three sections per animal of at least 200um apart and 5 animals/group (15 sections) were stained with H&E and adjacent sections were stained for insulin, see Table V. Counting Islet insulitis was done blindly on 15 sections per group (see representative photos in Figure 7).
Groups that received HBEGF (Gr 3, 4, 6, & 7) have significantly less insulitis compared to vehicle control (Gr 2); PGC does not cause reduction of insulitis (Gr 3 vs. Gr 4) nor does it cause increase in non-FBG (Table II, Gr 3 vs. Gr 4: non-FBG, gAUC during treatment, and HbA1c) or FBG (Fig 5, Gr 3 vs. Gr 4).
Figure 7.
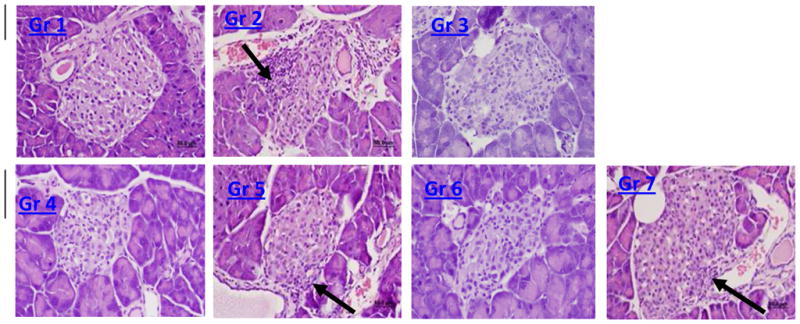
Representative H&E stain (40x; Groups as above, evaluated after IPGTT): Gr 1 has no insulitis, Gr 2 has insulitis (arrow), Gr 3 and Gr 4 have limited or no insulitis , Gr 5 has insulitis (arrow), Gr 6 has limited or no insulitis , and Gr 7 has very mild insulitis (arrow). Blind scoring data with statistical analysis are presented in Table IV.
The statistical power of the experimental design is limited to a dramatic effect that would be of practical significance
Insulin staining shows that the islet insulin content is significantly higher in both the Omeprazole alone (Gr 5) and combined Omeprazole and PGC-HBEGF (Gr 6) treated groups compared to saline (Table V). It should be noted that the design of the experiment is powered to detect only a dramatic effect that is substantial enough to make a practical difference in the treatment rather than any subtle effect. Such possible subtle effect which will not be detectable at n=5 include differences between Gr 3 vs. Gr 4, Gr 3 vs. Gr 7, Gr 4 vs. Gr 7, and Gr 5 vs. Gr 6. These comparisons will need larger numbers of samples to have appropriate statistical power and will be the subject of future study: the dissection of the contribution of each component in the formulation.
Table V.
Blinded examination of the insulin-stained section of pancreas (mean number of islets with Insulin per sectiona), Group (Gr) descriptions are in Table I. Groups that received EGFRA have significantly less insulin compared to the diabetic control.
| Group | Gr 1 | Gr 2 | Gr 4 | Gr 5 | Gr 6 | Gr 7 |
|---|---|---|---|---|---|---|
| Insulin Islet Score a; 0–4, mean | 4.0 | 3.0 | 2.50 | 3.73 | 3.70 | 3.6 |
| SEM | 0.0 | 0.24 | 0.19 | 0.12 | 0.24 | 0.21 |
| p value versus Gr 2 | 0.0003 | ----- | 0.1136 | 0.0111 b | 0.0486 b | 0.0703 c |
Three sections per animal of at least 200um apart and 5 animals/group (15 sections) were stained with H&E and adjacent sections were stained for insulin. Insulin Scoring System: 0=no staining, 1=<50% β-cells staining in an individual islet, 2=50–75% β-cells staining, 3=75–90% β-cells staining, 4=>90% β-cells staining.
Omeprazole treatment and Omeprazole+PGC-HBEGF have significantly higher insulin scores compared to the Gr 2 control.
Power analysis of the difference between Gr 2 and Gr 7 of 0.6 (with average standard deviation of 0.8 (0.21SEM*151/2)) indicates that we need at least 30 sections to rule out lack of significance with power of 80% and we only analyzed 15 sections. In other words, treatment with additional gastrin may have reduced the insulin content of the islet β-cells contributing to loss of analytical resolution.
No tumor was detected in the stomach upon histological examination at the end of the treatment period
An independent pathologist evaluated H&E stained tissue sections blindly after the short but effective 28d treatment (2.3y human equivalent); no abnormal growths or neoplasms were detected in tissue sections from the stomach or duodenum, the organs with the most cancer risk in T1D (20) and the target of gastrin-induced ECL cell hypertrophy/hyperplasia. No tumors were found in the other organs (not shown). Compared to controls, no significant increase in the total duodenum/stomach surface area was observed in any of the animals that received the formulations for 28 days. This result is consistent with a 30-week long study of HBEGF delivered using adenoviral vector (a more sustained delivery method) where no neoplasm was observed in liver, kidney, intestine, or muscle (18). This is also consistent with long-term use of Omeprazole in humans which does not cause neoplasm (21). Over-expression of EGF and gastrin for at least 14weeks in mice is safe. One may argue that cancer nodes in our specific treatment (Omeprazole+PGC-HBEGF) did not grow long enough to be detectable; this will be the subject of future studies. Elevation of gastrin, EGF, and/or HBEGF in any of the animals, if present, was not associated with morphologic changes in the gastric EC cells. We observed a 6% incidence of vacuolar degeneration of mucosal epithelial cells in all groups which was not associated with any particular treatment. The significance of this is unknown.
DISCUSSION
Brand et al (1) showed that one week of subcutaneous EGF/gastrin infusion (145ugEGF:260ug gastrin/Kg/day) in diabetic STZ rats (at 10 days after STZ treatment) prevented a further increase of FBG, from 360mg/dl to 363+/−46mg/dl, n=7, whereas the FBG of the untreated group increased from 360mg/dl to 499+/−26mg/dl, n=6. FBG of the treated group did not normalize (1) but deterioration was prevented along with improved glucose tolerance and increased β-cell mass with an increase in BrDU labeling (1). Similar and lasting improvement was found in Alloxan diabetic mice (3). Interestingly, for newly diagnosed NOD mice, an extremely low concentration of EGF/gastrin (1ugEGF/3ug gastrin/Kg/day given i.p. twice a day for 14 days) has also been reported to be effective in reversing diabetes (2, 22), indicating that perhaps NOD mice are easier to treat and require a lower dose of EGF/gastrin than either STZ- or Alloxan- diabetics. Similar results were obtained using Betacellulin, another EGFRA (23). Reversal to euglycemia is more difficult when done 8 days after STZ induction (1, 3). Our results were better even when animals were treated 8 days after the initial STZ treatment with a less frequently administered long acting PGC-HBEGF along with Omeprazole. This treatment normalizes FBG, improves islet function, and decreases insulitis, and is better than an EGF/gastrin combination in both efficacy and convenience, despite the hyperglycemic effect of HBEGF (17, 19). The present study shows that a combination of PGC-HBEGF and Omeprazole given once a day is in line with, if not better than, the EGF/gastrin infusion results of Brand et al. (1) and Rooman et al. (3). It is interesting that bariatric surgeries that eliminate the acid producing fundus from the food path improve the diabetic condition independent of weight loss (24, 25).
Both gastrin and GLP-1 are capable of inducing β-cell maturation, but they are not sufficient for neogenesis (1, 2, 26-28) without at least one trans-differentiation factor from any of the ErbB ligands (such as EGF, HBEGF, TGF-a, amphiregulin, betacellulin, epiregulin, epigen, and neuroregulin (1, 26, 29)). These factors are also released and facilitate repairs during duct ligation (27), partial pancreatectomy, cellophane wrapping of the gland, alloxan, STZ, and gamma interferon (2, 30, 31). Co-administration of ErbB ligand, along with GLP-1 or gastrin, causes neogenesis and proliferation of islet β-cells (1, 2, 26-28). However, it is critical to pick an ErbB ligand that is less likely to be associated with tumor formation. Unlike cell membrane bound HBEGF, the soluble HBEGF utilized in this study lacks an intracellular domain (12) that can initiate a self-sustaining signal of tumor formation. Use of soluble HBEGF will keep the signal potentially self-limiting once treatment is over. Our evaluation of various tissues (stomach, pancreas, kidney, liver, and spleen) indicated that the 28 day treatment did not cause neoplasm. In particular, neither neoplasm nor hyperplasia were observed in the stomach which is also the target of gastrin action (causing ECL cells hyperplasia). While T2D has increased risk of cancer in several organs (32) due to insulin and obesity, T1D cancer risk is mainly in the stomach due to sustained H. pylori induced inflammation (20). For T1D, treatment that reduces H. pylori induced inflammation should also reduce this risk; our data indicates that PGC-HBEGF reduces insulitis. The absence of neoplasm is consistent with a 30wk long study of HBEGF delivered using adenoviral vector (a more sustained delivery method) that looked at the effect on liver, kidney, intestine, and muscle (18). In addition, tissue injuries release ErB ligand and yet tissue injuries rarely lead to cancer (2, 27, 30, 31), perhaps because it is not sustained. Similarly, use of Omeprazole for over 30 years in humans does not cause neoplasm (21, 33, 34). Anticipated human treatment will occur over a few weeks and the risk of this treatment may not be greater than immune suppression treatments which lower resistance to infection and cancer.
This study did not address the reason why HBEGF causes a reduction or elimination of insulitis or whether the hyperglycemic effect of HBEGF can be dissociated from the elimination of insulitis and its ability to induce trans-differentiation. A previous adoptive transfer model study indicated that EGF and gastrin induce immunoregulatory cells that prevent autoimmunity (2). The mechanism responsible for this observation will be the subject of future studies. Whatever the mechanism, this approach offers exciting prospects for the treatment and/or prevention of T1D without the use of immunosuppressant drugs that can increase the risk of infection, such as anti-CD3, tacrolimus or rapamycin, with the latter two also inhibiting β-cell division (35-37). Although the comparison of insulitis between HBEGF and PGC-HBEGF treated groups showed that PGC does not cause the reduction of insulitis, the PGC seems to increase insulitis to some extent. Despite wide use of chondroitin sulfate to alleviate arthritis symptoms, it is possible that the chondroitin-containing PGC causes inflammation although it is likely that PGC reduces the anti-inflammatory effect of HBEGF because of lowering the amount of free HBEGF needed to suppress inflammation while prolonging the exposure. Paradoxically, hyperglycemia and inflammation facilitate islet cell regeneration (38) and a proper balance will be required to achieve full regeneration in addition to trans-differentiation of other cells into β-cells. In this study, both hyperglycemia and inflammation are affected by HBEGF but whether these effects are independent and distinct from the induction of trans-differentiation, and whether one can be suppressed while maintaining the other effect, is not clear. Previous studies indicated that prolongation of exposure by frequent administration or infusion is necessary for β-cell recovery. However, we do not know the level and duration of the exposure sufficient for each of the following: 1) trans-differentiating cells into to β-cells, 2) reducing autoimmunity to a less harmful and more beneficial level, and 3) the degree of beneficial hyperglycemia needed to assist gastrin-induced β-cell proliferation. In fact, the beneficial effect of an EGFRA can reverse at a higher bolus dose (23), perhaps because extreme hyperglycemia causes glucotoxicity in β-cells which outweighs suppression of inflammation that destroys β-cells. In the present study, the hyperglycemic effect of HBEGF is moderated by PGC and is clearly reduced by the Omeprazole-induced gastrin elevation. Perhaps those groups that experienced the strong hyperglycemic effect of HBEGF during treatment will have glucotoxicity while those that have been tempered by Omeprazole or PGC (by limiting the amount of free HBEGF) will not; the latter will have improved regeneration assisted by suppression of inflammation and trans-differentiation of other cells into β-cells. The literature control group (Gr 8), which also partially recovers, was treated with EGF and gastrin 3x/day. Gr 8 also showed hyperglycemia during treatment, which may also have been partially suppressed by gastrin 3x/day to minimize glucotoxicity and facilitate recovery.
PGC uses reversible binding of peptide/protein drugs to provide an automatic control of the amount of free drug in the blood such that when the concentration of free drug decreases, the PGC releases more drug to satisfy the Kd, thus acting as a drug reservoir/buffer in the blood. The complex is encased in a protective PEG polymer, shielding both the PGC and drug from elimination by the reticuloendothelial system and enzyme degradation (8, 39). This complex is large enough (15–30nm) to escape from glomerular clearance (4nm) that normally affects most drugs. Because of the size of PGC, there is preferential accumulation at sites of high vascular permeability such as inflamed pancreas (40). In fact, PGC has been used to deliver GLP-1 for once a week administration in diabetic-ZDF rats and has efficacy that is equivalent to exendin-4 administered twice a day (8); twice a week administration of PGC-GLP-1 is more effective than twice a day administration of exendin-4 (8).
The novel findings in this study include the use of Omeprazole as a substitute for gastrin, the use of PGC to improve blood stability of growth factors relevant to diabetes (EGF, HBEGF, gastrin), and the improved approach to treat diabetes using a combination of Omeprazole and PGC-HBEGF. This approach has the distinct advantage of replacing injectable labile gastrin with an FDA-approved orally available medication. Despite the hyperglycemic effect of HBEGF during treatment, we found that it is responsible for the reduction of insulitis while Omeprazole (or gastrin) is responsible for reducing blood glucose and increasing islet insulin content. A combination of PGC-HBEGF and Omeprazole normalizes FBG, improves islet function, and reduces insulitis in multiple low dose streptozotocin diabetic mice.
Acknowledgments
This work was supported by the SBIR Grant # DK084724 from National Institute of Diabetes and Digestive and Kidney diseases of the National Institute of Health. There has been no prior publication of the present study.
ABBREVIATIONS
- EGFRA
epidermal growth factor receptor agonist
- FBG
fasting blood glucose
- HBEGF
heparin-binding EGF
- Kd
dissociation constant
- non-FBG
non-fasting blood glucose
- PGC
protected graft copolymer
- T1D
type 1 diabetes
- T2D
type 2 diabetes
Footnotes
G.M.C. conceived the approach, designed the experiments, researched data, and wrote the manuscript. A.N.A. contributed to the design of the experiments, researched data, and reviewed/edited the manuscript. A.A.B. and J.L.L. researched data and reviewed/edited the manuscript. A.V.L. reviewed/edited the manuscript. E.M.B. contributed to the design of the experiments and reviewed/edited the manuscript. The authors thank Ms. Cynthia Jones for help with preparing the manuscript, and Mr. ManShun Lai and Dr. Sandra Reichstetter for technical assistance. G.M.C., A.N.A., C.C.J., M.S.L., S.R., and E.M.B. are employees of PharmaIN Corp.; A.A.B., J.A.L., and A.V.L. are employees of the University of Illinois.
Contributor Information
Akiko Nishimoto-Ashfield, Email: aashfield@pharmain.com.
Aryamitra A. Banerjee, Email: aryamitr@uic.edu.
Jennifer A. Landolfi, Email: landolfi@illinois.edu.
Alexander V. Lyubimov, Email: lyubimov@uic.edu.
Elijah M. Bolotin, Email: ebolotin@pharmain.com.
Literature cited
- 1.Brand SJ, Tagerud S, Lambert P, Magil SG, Tatarkiewicz K, Doiron K, et al. Pharmacological treatment of chronic diabetes by stimulating pancreatic beta-cell regeneration with systemic co-administration of EGF and gastrin. Pharmacol Toxicol. 2002;91(6):414–20. doi: 10.1034/j.1600-0773.2002.910621.x. [DOI] [PubMed] [Google Scholar]
- 2.Suarez-Pinzon WL, Yan Y, Power R, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin increases beta-cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes. 2005;54(9):2596–601. doi: 10.2337/diabetes.54.9.2596. [DOI] [PubMed] [Google Scholar]
- 3.Rooman I, Bouwens L. Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycaemia in C57Bl6/J mice treated with alloxan. Diabetologia. 2004;47(2):259–65. doi: 10.1007/s00125-003-1287-1. Epub 2003/12/11. [DOI] [PubMed] [Google Scholar]
- 4.Lev-Ran A, Hwang DL, Ben-Ezra J, Williams LE. Origin of urinary epidermal growth factor in humans: excretion of endogenous EGF and infused [131I]-human EGF and kidney histochemistry. Clinical and experimental pharmacology & physiology. 1992;19(10):667–73. doi: 10.1111/j.1440-1681.1992.tb00402.x. Epub 1992/10/01. [DOI] [PubMed] [Google Scholar]
- 5.Feng J, Mehta VB, El-Assal ON, Wu D, Besner GE. Tissue distribution and plasma clearance of heparin-binding EGF-like growth factor (HB-EGF) in adult and newborn rats. Peptides. 2005 doi: 10.1016/j.peptides.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes. 2008;57(12):3281–8. doi: 10.2337/db08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mefford IN, Wade EU. Proton pump inhibitors as a treatment method for type II diabetes. Med Hypotheses. 2009;73(1):29–32. doi: 10.1016/j.mehy.2009.02.010. Epub 2009/03/24. [DOI] [PubMed] [Google Scholar]
- 8.Castillo GM, Reichstetter S, Bolotin EM. Extending Residence Time and Stability of Peptides by Protected Graft Copolymer (PGC) Excipient: GLP-1 Example. Pharm Res. 2012;29(1):306–18. doi: 10.1007/s11095-011-0542-2. Epub 2011/08/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Hypergastrinemia in response to gastric inflammation suppresses somatostatin. Am J Physiol Gastrointest Liver Physiol. 2002;282(1):G175–83. doi: 10.1152/ajpgi.00287.2001. Epub 2001/12/26. [DOI] [PubMed] [Google Scholar]
- 10.Castillo GM, Bolotin EM, Nishimoto-Ashfield A. PCT/US10/48145: Anionic-core Composition for delivery of therapeutic agents, and methods of making and using the same. USPTO. 2012 Jul 26;20120190097 Epub July 26, 2012. [Google Scholar]
- 11.Bolotin E. USPA # 2003/0224974 A1: Compositions for Delivery of Therapeutics and other Materials, and Methods of Making and Using the Same (now patent#7,138,105) 2003. [Google Scholar]
- 12.Wang X, Mizushima H, Adachi S, Ohishi M, Iwamoto R, Mekada E. Cytoplasmic domain phosphorylation of heparin-binding EGF-like growth factor. Cell Struct Funct. 2006;31(1):15–27. doi: 10.1247/csf.31.15. Epub 2006/03/25. [DOI] [PubMed] [Google Scholar]
- 13.Hieda M, Isokane M, Koizumi M, Higashi C, Tachibana T, Shudou M, et al. Membrane-anchored growth factor, HB-EGF, on the cell surface targeted to the inner nuclear membrane. J Cell Biol. 2008;180(4):763–9. doi: 10.1083/jcb.200710022. Epub 2008/02/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adam RM, Danciu T, McLellan DL, Borer JG, Lin J, Zurakowski D, et al. A nuclear form of the heparin-binding epidermal growth factor-like growth factor precursor is a feature of aggressive transitional cell carcinoma. Cancer Res. 2003;63(2):484–90. Epub 2003/01/25. [PubMed] [Google Scholar]
- 15.Reichstetter S, Castillo GM, Lai M, Nishimoto-Ashfield A, Banerjee A, Bogdanov A, et al. Protected Graft Copolymer (PGC) Basal Formulation of Insulin as Potentially Safer Alternative to Lantus(R) (Insulin-Glargine): A Streptozotocin-Induced, Diabetic Sprague Dawley Rats Study. Pharm Res. 2012;29(4):1033–9. doi: 10.1007/s11095-011-0646-8. Epub 2011/12/29. [DOI] [PubMed] [Google Scholar]
- 16.Reichstetter S, Castillo GM, Rubinsteinb I, Nishimoto-Ashfield A, Lai M, Jones CC, et al. Protected Graft Copolymer Excipient Leads to a Higher Acute Maximum Tolerated Dose and Extends Residence Time of Vasoactive Intestinal Peptide Significantly Better than Sterically Stabilized Micelles. Pharm Res. 2012 doi: 10.1007/s11095-012-0904-4. In Press. Epub 2012/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grau M, Tebar F, Ramirez I, Soley M. Epidermal growth factor administration decreases liver glycogen and causes mild hyperglycaemia in mice. Biochem J. 1996;315(Pt 1):289–93. doi: 10.1042/bj3150289. Epub 1996/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozawa J, Tokui Y, Moriwaki M, Li M, Ohmoto H, Yuan M, et al. Regenerative and therapeutic effects of heparin-binding epidermal growth factor-like growth factor on diabetes by gene transduction through retrograde pancreatic duct injection of adenovirus vector. Pancreas. 2005;31(1):32–42. doi: 10.1097/01.mpa.0000163177.59920.f8. Epub 2005/06/22. [DOI] [PubMed] [Google Scholar]
- 19.Cameron CM, Kostyo JL, Papkoff H. Nonmammalian growth hormones have diabetogenic and insulin-like activities. Endocrinology. 1985;116(4):1501–5. doi: 10.1210/endo-116-4-1501. Epub 1985/04/01. [DOI] [PubMed] [Google Scholar]
- 20.Zendehdel K, Nyren O, Ostenson CG, Adami HO, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst. 2003;95(23):1797–800. doi: 10.1093/jnci/djg105. Epub 2003/12/05. [DOI] [PubMed] [Google Scholar]
- 21.Bateman DN, Colin-Jones D, Hartz S, Langman M, Logan RF, Mant J, et al. Mortality study of 18 000 patients treated with omeprazole. Gut. 2003;52(7):942–6. doi: 10.1136/gut.52.7.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy S, Cheung CC, Chai RC, Rodrigues JA. Persistence of residual beta cells and islet autoimmunity during increasing duration of diabetes in NOD mice and experimental approaches toward reversing new-onset disease with bioactive peptides. Ann N Y Acad Sci. 2008;1150:171–6. doi: 10.1196/annals.1447.010. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Seno M, Yamada H, Kojima I. Betacellulin improves glucose metabolism by promoting conversion of intraislet precursor cells to beta-cells in streptozotocin-treated mice. Am J Physiol Endocrinol Metab. 2003;285(3):E577–83. doi: 10.1152/ajpendo.00120.2003. Epub 2003/08/06. [DOI] [PubMed] [Google Scholar]
- 24.Rizzello M, Abbatini F, Casella G, Alessandri G, Fantini A, Leonetti F, et al. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg. 2010;20(1):50–5. doi: 10.1007/s11695-009-0017-2. Epub 2009/11/17. [DOI] [PubMed] [Google Scholar]
- 25.Spector D, Shikora S. Neuro-modulation and bariatric surgery for type 2 diabetes mellitus. International journal of clinical practice Supplement. 2010;166:53–8. doi: 10.1111/j.1742-1241.2009.02279.x. Epub 2010/08/18. [DOI] [PubMed] [Google Scholar]
- 26.Brand SJ. USPN # 6992060B2: Prolonged efficacy of islet neogenesis therapy methods with a gastrin/CCK receptor ligand and an EGF receptor ligand composition in subjects with preexisting diabetes. 2006. [Google Scholar]
- 27.Rooman I, Lardon J, Bouwens L. Gastrin stimulates beta-cell neogenesis and increases islet mass from transdifferentiated but not from normal exocrine pancreas tissue. Diabetes. 2002;51(3):686–90. doi: 10.2337/diabetes.51.3.686. [DOI] [PubMed] [Google Scholar]
- 28.Song SY, Gannon M, Washington MK, Scoggins CR, Meszoely IM, Goldenring JR, et al. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology. 1999;117(6):1416–26. doi: 10.1016/s0016-5085(99)70292-1. [DOI] [PubMed] [Google Scholar]
- 29.Wilson KJ, Gilmore JL, Foley J, Lemmon MA, Riese DJ., 2nd Functional selectivity of EGF family peptide growth factors: implications for cancer. Pharmacol Ther. 2009;122(1):1–8. doi: 10.1016/j.pharmthera.2008.11.008. Epub 2009/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42(12):1715–20. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 31.Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development. 1993;118(1):33–46. doi: 10.1242/dev.118.1.33. [DOI] [PubMed] [Google Scholar]
- 32.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocrine-related cancer. 2009;16(4):1103–23. doi: 10.1677/ERC-09-0087. Epub 2009/07/22. [DOI] [PubMed] [Google Scholar]
- 33.Singh P, Indaram A, Greenberg R, Visvalingam V, Bank S. Long term omeprazole therapy for reflux esophagitis:follow-up in serum gastrin levels, EC cell hyperplasia and neoplasia. World J Gastroenterol. 2000;6(6):789–92. doi: 10.3748/wjg.v6.i6.789. Epub 2002/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ligumsky M, Lysy J, Siguencia G, Friedlander Y. Effect of long-term, continuous versus alternate-day omeprazole therapy on serum gastrin in patients treated for reflux esophagitis. J Clin Gastroenterol. 2001;33(1):32–5. doi: 10.1097/00004836-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Hyder A, Laue C, Schrezenmeir J. Effect of the immunosuppressive regime of Edmonton protocol on the long-term in vitro insulin secretion from islets of two different species and age categories. Toxicol In Vitro. 2005;19(4):541–6. doi: 10.1016/j.tiv.2005.01.005. Epub 2005/04/14. [DOI] [PubMed] [Google Scholar]
- 36.Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, et al. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008;8(6):1250–61. doi: 10.1111/j.1600-6143.2008.02234.x. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Talavera JC, Garcia-Ocana A, Sipula I, Takane KK, Cozar-Castellano I, Stewart AF. Hepatocyte growth factor gene therapy for pancreatic islets in diabetes: reducing the minimal islet transplant mass required in a glucocorticoid-free rat model of allogeneic portal vein islet transplantation. Endocrinology. 2004;145(2):467–74. doi: 10.1210/en.2003-1070. Epub 2003/10/11. [DOI] [PubMed] [Google Scholar]
- 38.Akirav EM, Baquero MT, Opare-Addo LW, Akirav M, Galvan E, Kushner JA, et al. Glucose and Inflammation Control Islet Vascular Density and {beta}-Cell Function in NOD Mice: Control of Islet Vasculature and Vascular Endothelial Growth Factor by Glucose. Diabetes. 2011 doi: 10.2337/db10-0793. Epub 2011/02/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bogdanov AA, Jr, Mazzanti M, Castillo G, Bolotin E. Protected Graft Copolymer (PGC) in Imaging and Therapy: A Platform for the Delivery of Covalently and Non-Covalently Bound Drugs. Theranostics. 2012;2(6):553–76. doi: 10.7150/thno.4070. Epub 2012/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medarova Z, Castillo G, Dai G, Bolotin E, Bogdanov A, Moore A. Noninvasive magnetic resonance imaging of microvascular changes in type 1 diabetes. Diabetes. 2007;56(11):2677–82. doi: 10.2337/db07-0822. [DOI] [PubMed] [Google Scholar]