Abstract
Background
Starting lifelong antiretroviral therapy (ART) in HIV-infected pregnant women may decrease HIV progression and transmission but adherence after delivery may be difficult, especially for asymptomatic women. We evaluated disease progression among HIV-infected women not on ART with CD4+ lymphocyte counts above 200 cells/uL at delivery.
Methods
We analysed risk of death, progression to AIDS (stage IV or CD4 < 200 cells/uL), or to CD4+ count < 350 one year after delivery among postpartum women enrolled to a prevention of breastfeeding transmission trial using Kaplan-Meier methods. In the primary analysis, women were censored if ART was initiated.
Results
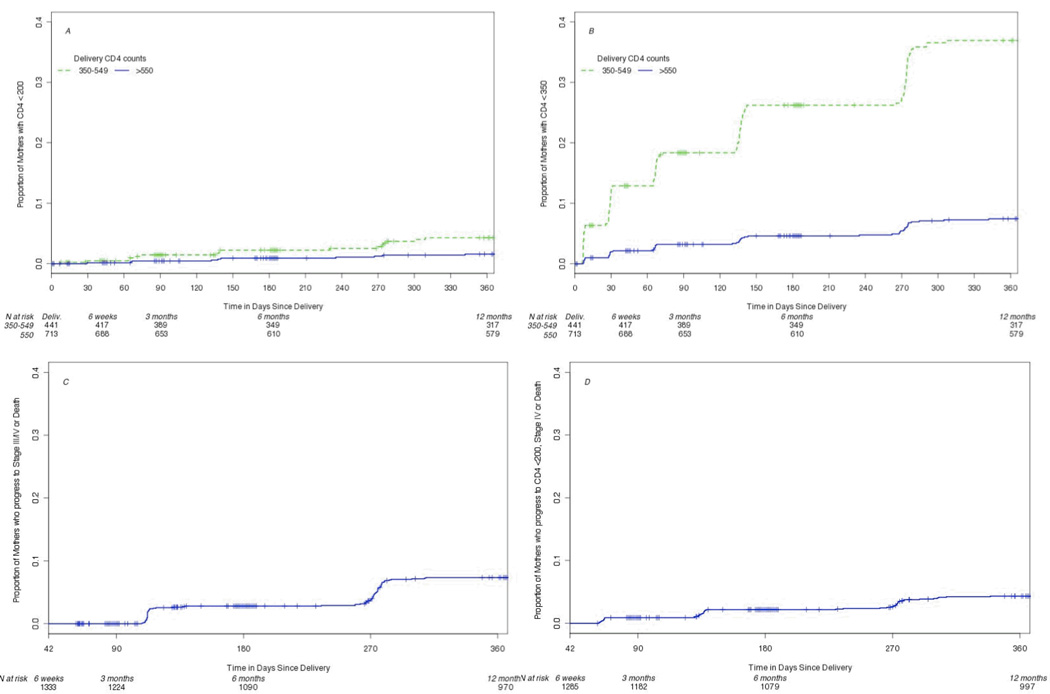
Among 1285 women who were < WHO stage IV at 6 weeks postpartum, 49 (4.3%) progressed to stage IV/CD4 < 200 cells/uL or death by one year. Progression to CD4 < 200 or death occurred among 16 (4.3%) of 441 women with CD4 count of 350–549 and 10 (1.6%) of 713 with CD4 counts > 550 at delivery. CD4 < 350 by 12 months postpartum occurred among 116 (37.0%) of 350 women with CD4 count 400–549 and 48 (7.4%) of 713 > 550 at delivery.
Conclusions
Progression to AIDS or CD4 count < 350 is uncommon through one year postpartum for women with CD4 counts over 550 at delivery, but occurred in over one third of those with CD4 counts under 550. ART should be continued after delivery or breastfeeding among women with CD4 counts < 550 if follow up and ARV adherence can be maintained.
Keywords: HIV, postpartum, disease progression
Background
A recent WHO programmatic update has suggested that all HIV-infected pregnant women should be considered for initiation of triple antiretroviral (ARV) regimens for prevention of mother to child transmission with consideration given to continuing all women on ARV’s for life once initiated, regardless of starting CD4+ lymphocyte count.1 The recommendation for use of triple ARV regimens, rather than zidovudine alone during pregnancy with additional peripartum ARV drugs for women with high CD4+ cell counts, is based on several considerations. Rapid initiation of combination ARV drugs without waiting for CD4 results in settings where CD4+ cell counts are often delayed assures treatment for women with low CD4+ lymphocyte counts who have the highest risk of transmission.2 Using the same regimen for all pregnant women and other adults allows for streamlining of drug supply and provider training. In addition, treating HIV-infected persons at higher CD4+ cell counts resulted in lower risk of transmission to sexual partners in one recent trial.3
However, the risks versus benefits of continuing triple antiretroviral regimens started among generally healthy, asymptomatic pregnant women with higher CD4+ lymphocyte counts after delivery or cessation of breastfeeding have not been evaluated adequately. The postpartum period is known in both resource rich and resource poor settings to be a high risk period for poor HIV treatment adherence, given demands of newborn care and frequent lack of disclosure with recent diagnosis during pregnancy.4 Especially in resource limited settings, there is increased potential for disruption in care as traditionally many women travel away from home after delivery for extended stays with family, frequently far from ARV treatment centers. In addition, resource constraints at the country level may preclude provision of long term therapy for women with higher CD4+ lymphocyte counts after cessation of breastfeeding without sacrificing treatment access for those who already meet WHO and ministry of health treatment criteria.
Rates of disease progression among asymptomatic HIV infected women during the first year after delivery have not been well documented in resource limited settings. Thus, the purpose of this analysis was to assess HIV immunologic and clinical disease progression during the first 12 months postpartum among asymptomatic HIV infected women with higher CD4 counts at delivery using data from a recently completed trial, HIV Prevention Trials Network (HPTN) 046.5
METHODS
HPTN 046 was a randomized, double-blinded, placebo-controlled trial of an extended nevirapine regimen in infants for prevention of transmission of HIV through breastfeeding conducted in Zimbabwe, South Africa, Uganda, and Tanzania.5 The protocol was approved by all relevant institutional review boards and regulatory bodies; and all women provided written consent for themselves and their infants before enrollment.
This analysis includes women who provided informed consent and were screened during the third trimester of pregnancy up to seven days postpartum whose infants were eligible and randomized. To be eligible, women had to have confirmed HIV-1 infection, be at least 18 years old, intend to breastfeed, and not have any serious medical condition that would interfere with breastfeeding or study participation. Their infants had to be HIV uninfected at three days of life under version 2.0 and at six weeks of age in version 3.0. Maternal care and follow up were the same in both versions. Maternal ARV regimens were not provided through the study but were available in accordance with the local standard of care, which primarily consisted of combination ARV therapy for women with CD4+ lymphocyte counts below 200 cells/uL and maternal and infant single dose nevirapine for those with higher CD4+ cell counts. Enrollment occurred between February, 2007 and March, 2010. Infants were enrolled at three days postpartum under version 2.0 and at six weeks postpartum in version 3.0 but all women were enrolled by seven days postpartum and had study visits at delivery or within seven days postpartum, at two and six weeks, and at three, six, and 12 months postpartum. At each visit an interim medical history and symptom-directed physical examination were completed. Maternal HIV disease staging was completed using the WHO staging system,6 and a complete blood count and CD4+ lymphocyte count were obtained in laboratories certified by the NIAID Division of AIDS Quality Assurance Program.
Statistical Analyses
In order to assess maternal disease progression over time, we used the Kaplan-Meier method to estimate the cumulative proportion of mothers who progressed to one of four surrogate disease endpoints at 6 and 12 months post-partum. Two of the endpoints described CD4+ lymphocyte decline from baseline at delivery: time to (1) CD4+ lymphocyte counts below 200 cells/uL and (2) CD4+ lymphocyte counts below 350 cells/uL. Women were censored at the time of ART initiation. The other two endpoints described progression to clinical AIDS or death from six weeks postpartum: (3) WHO Clinical Stage III or IV or death and a combined endpoint of (4) WHO Clinical Stage IV, CD4+ lymphocyte count below 200 cells/uL or death. Mothers were excluded from the analysis of the first two endpoints if they had delivery CD4+ lymphocyte counts below 200 cells/uL and 350 cells/uL respectively; while the third analysis excluded those mothers who were stage III or IV at six weeks postpartum or who had started ART prior to six weeks; and finally, the fourth analysis excluded those mothers who were Stage IV at six weeks, had taken ART prior to six weeks or had CD4+ lymphocyte counts below 200 cells/uL prior to six weeks. For the combined endpoints, three and four, time to event was calculated as the minimum event time if a mother had more than one of the qualifying outcomes. For the third endpoint, women were censored at time of ARV initiation after six weeks or at the last visit through 12 months postpartum if they did not die or progress to WHO Stage III or IV. For the fourth endpoint, women were censored at the time of ARV initiation after six weeks postpartum or at last CD4+ measure above the threshold of 200 cells/uL through 12 months postpartum if they did not die or progress to WHO Stage IV. Because ARV initiation is likely to be informative, a combined endpoint analysis was also performed where the time to event was the minimum visit in which the above thresholds (1–4) were crossed or ARV treatment was initiated. All participants in which the endpoint was not observed were censored at their last visit time.
Maternal death incidence was calculated by dividing the number of deaths by the total person years through 12 months of follow-up and stratified by CD4+ lymphocyte counts at delivery.
RESULTS
From February, 2007 to March, 2010, 2025 women enrolled into HPTN 046, including 1430 (70.7%) with CD4+ lymphocyte counts above 350 cells/uL.(Table 1.) WHO clinical staging was available at six weeks postpartum in 1945 women; 1855 (96%) were stage I or II. Women without WHO clinical staging at six weeks had similar demographic characteristics and baseline CD4+ lymphocyte counts compared to those with staging (data not shown). Twenty-eight per cent of women received triple ART regimens during pregnancy. Other baseline characteristics are summarized in Table 1.
Table 1.
Maternal baseline characteristics (n= 1995)
| Characteristic | Number (%) |
|---|---|
| Enrollment site | |
| Chitungwiza, Zimbabwe | 664 |
| Kampala, Uganda | 705 |
| Dar es Salaam, Tanzania | 218 |
| Durban, South Africa | 408 |
| Median age, years (range) | 27 (18–46) |
| Marital status | |
| Never married/not living with partner | 596 (29%) |
| Married | 757 (37%) |
| Living with partner | 604 (30%) |
| Separated/divorced/widowed | 68 (3%) |
| Antiretrovirals received during pregnancy before delivery | |
| None or only intrapartum prophylaxis | 583 (25%) |
| One or two drugs | 868 (43%) |
| Three drugs | 574 (28%) |
| CD4+ lymphocyte count (cells/uL) (n= 2022) | |
| < 200 | 171 (9%) |
| 200–349 | 421 (21%) |
| 350–49 | 594 (29%) |
| ≥ 550 | 836 (41%) |
| WHO Stage assessed at six weeks postpartum (n= 1945) | |
| I | 1568 (81%) |
| II | 287 (15%) |
| III | 84 (4%) |
| IV | 6 (< 1%) |
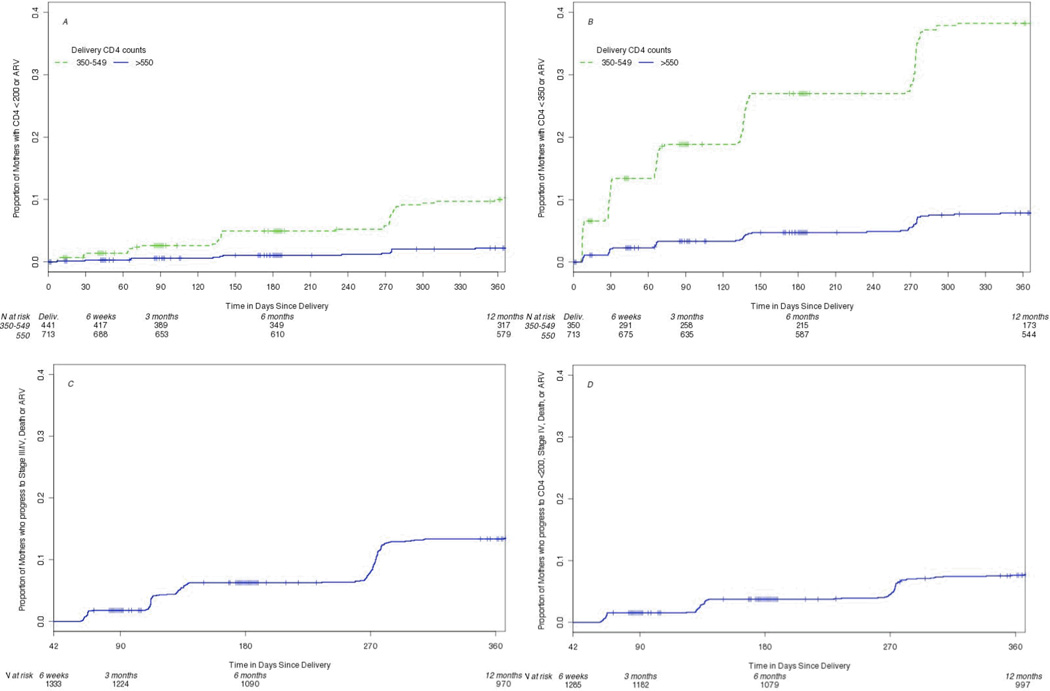
Maternal disease progression rates both with censoring at ARV initiation and with ARV initiation included in the endpoint are summarized in Table 2. Among women who had CD4+ cell count results available at delivery and postpartum sampling (Table 2A), 16 (4.3%) of those with counts between 350–549 cells/uL dropped to below 200 cells/uL by one year compared to 10 (1.6%) of those starting above 549 cells/uL. Among women who had a CD4+ lymphocyte count between 400–549 at delivery, 116 (37%) dropped under 350 cells/uL by one year after delivery while 48 (7.4%) of those with a CD4+ cell count of 550 cells/ul or higher at delivery dropped below this threshold. When adding ARV initiation to the endpoint rather than censoring, rates of the endpoint of CD4+ count < 200 cells/uL or ARV initiation more than doubled for women with CD4+ counts between 350–549 cells/uL but did not change appreciably for the endpoint including dropping to CD4+ count below 350 cells/uL or for those starting above a CD4+ count of 550 cells/uL. For women who started in stages I-III, 49 (4.3%) died or progressed to AIDS based on stage IV conditions or CD4+ lymphocyte count below 200 cells/uL by 12 months after delivery, censoring for ARV initiation (Table 2B). When including ARV initiation in the endpoint, 88 (7.7%) met progression criteria. By 12 months, 83 (7.4%) women starting in clinical stage I or II progressed to stage III or IV while 159 (13.4%) progressed to stage III or IV or initiated ARV. Of the 74 women initiating ARV by 12 months after delivery, 57 (77%) had nadir CD4+ lymphocyte counts below 200 cells/uL before initiation, 10 (14%) were between 200–300 cells/uL, and 7 (9%) remained above 300 cells/uL. The reasons for initiation of ARV at higher CD4+ counts were not documented.
Table 2.
Cumulative rates of (A) CD4 + cell decline or death stratified by CD4+ cell count at delivery and (B) progression to clinical AIDS or death at 6 and 12 months among women not on combination antiretroviral therapy before delivery
| A. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Endpoints: CD4 Decline or Death | ||||||||
|
1. Number (%) who drop below 200 cells/uL stratified by baseline CD4 count |
2. Number (%) who drop below 350 cells/uL stratified by baseline CD4 count |
|||||||
| Baseline | Delivery | Delivery | Delivery | Delivery | ||||
| CD4 count | 350–549 cells/uL | 550+ cells/uL | 400–549 cells/uL | 550+ cells/uL | ||||
| N | 441 | 713 | 350 | 713 | ||||
| Endpoint Reached | Cumulative Rate [95% CI] |
Endpoint Reached | Cumulative Rate [95% CI] |
Endpoint Reached | Cumulative Rate [95% CI] |
Endpoint Reached | Cumulative Rate [95% CI] |
|
| 6 months Postpartum |
Endpoint censored at ARV initiation | |||||||
| 9 | 2.2% [0.8%-3.7%] |
6 | 0.9% [0.2%-1.6%] |
86 | 26.2% [21.3%-30.9%] |
31 | 4.6% [3.0%-6.2%] |
|
| Endpoint includes ARV initiation | ||||||||
| 20 | 4.9% [2.8%-7.0%] |
7 | 1.1% [0.3%-1.8%] |
89 | 27.0% [22.0%-31.6%] |
32 | 4.7% [3.1%-6.3%] |
|
| 12 months Postpartum |
Endpoint censored at ARV initiation | |||||||
| 16 | 4.3% [2.2%-6.3%] |
10 | 1.6% [0.6%-2.5%] |
116 | 37.0% [31.3%-42.1%] |
48 | 7.4% [5.4%-9.4%] |
|
| Endpoint includes ARV initiation | ||||||||
| 39 | 10.3% [7.1%-13.3%] |
14 | 2.2% [1.1%-3.4%] |
121 | 38.2% [32.5%-43.4%] |
51 | 7.9% [5.8%-10.0%] |
|
| B. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Endpoints: Progression to Clinical AIDS or Death | ||||||||
|
3. Number (%) Stage I-II at baseline, progress to stage III or IV or death |
4. Number (%) Stage I-III at baseline, progress to stage IV, CD4 < 200 or death |
|||||||
| Baseline | 6 weeks postpartum | 6 weeks postpartum | ||||||
| CD4 count | Any | > 200 | ||||||
| N | 1333 | 1285 | ||||||
| Endpoint Reached |
Cumulative Rate [95% CI] |
Endpoint Reached | Cumulative Rate [95% CI] | |||||
| 6 months Postpartum |
Endpoint censored at ARV initiation | |||||||
| 34 | 2.8% [1.9%-3.7%] | 26 | 2.2% [1.3%-3.0%] | |||||
| Endpoint includes ARV initiation | ||||||||
| 78 | 6.3% [4.9%-7.6%] | 45 | 3.7% [2.7%-4.8%] | |||||
| 12 months Postpartum |
Endpoint censored at ARV initiation | |||||||
| 83 | 7.4% [5.8%-8.9%] | 49 | 4.3% [3.2%-5.5%] | |||||
| Endpoint includes ARV intiation | ||||||||
| 159 | 13.4% [11.4%-15.3%] | 88 | 7.7% [6.1%-9.2%] | |||||
Twelve (1%) of women who were not stage IV at their six week postpartum assessment developed 13 clinical stage IV endpoints by 12 months after delivery. These endpoints included five cases of extrapulmonary tuberculosis, two cases each of HIV wasting (> 10% weight loss plus either unexplained diarrhea for over 30 days or chronic weakness and unexplained fever for over 30 days), Pneumocystis jiroveci pneumonia, and extrapulmonary cryptococcosis, and one case each of central nervous system toxoplasmosis and symptomatic HIV-associated nephropathy/cardiomyopathy. Sixteen (0.8% ) of enrolled women died during the first year after delivery.(Supplemental Table 1) Cause of death was unknown in seven cases and had varied causes in the other nine. Only one (11%) of the nine deaths with known causes was potentially secondary to an AIDS-defining condition and only two women who died had a CD4+ cell count below 200 cells/uL at the visit before death. Maternal death rates per 100 person-years stratified by CD4+ lymphocyte count at delivery are shown in table 3. The rates of death did not differ significantly by CD4 stratum but numbers of events were low. Eleven (68.8%) of the deaths occurred among women with CD4+ lymphocyte counts over 350 cells/uL at the visit before their death.
Table 3.
Maternal death rates per 100 person-years stratified by CD4+ lymphocyte count
| CD4 at delivery | Deaths | Person-years | Death Rate (deaths/100py) |
95% CI |
|---|---|---|---|---|
| < 200 | 2 | 132.47 | 1.51 | (0.18, 5.45) |
| 200 – 349 | 2 | 353.08 | 0.57 | (0.07, 2.05) |
| 350 – 550 | 5 | 510.06 | 0.98 | (0.32, 2.29) |
| > 550 | 7 | 726.90 | 0.96 | (0.39, 1.98) |
DISCUSSION
The pregnant and postpartum HIV-infected women enrolled to this trial were representative of the women seen at clinical research sites in sub-Saharan Africa 7,8 and had a range of CD4+ lymphocyte counts at delivery, with 70% above 350 cells/uL, the current threshold for initiation of ARV therapy in many countries.9 Less than 5% had stage III or IV disease at baseline while 9% had CD4+ lymphocyte counts below 200 cells/uL, suggesting that symptoms do not reliably indicate those most in need of ARV therapy. In addition, with the current CD4+ count threshold of 350 cells/uL, symptoms will not identify many of those who need therapy.
Given the standard of care existing at the sites during the HPTN 046 trial, asymptomatic women with CD4+ lymphocyte counts above 350 cells/uL did not meet WHO or country-specific criteria for ARV therapy. Within this context, we carefully monitored the women study participants for disease progression in order to initiate treatment if needed. This close follow up also provided the opportunity to help inform future treatment recommendations. Women who were asymptomatic (WHO clinical stage I or II at baseline) had a relatively low risk (7.4%) of progressing to symptomatic HIV disease within one year postpartum, emphasizing the need for CD4+ lymphocyte testing to target therapy for those individuals at highest risk for disease progression in settings where universal treatment is unaffordable. Among women with CD4+ cell counts between 400 and 549 cells/uL at baseline, 37% dropped to below 350 cells/uL at one year, indicating a need for ARV therapy and suggesting that women in this range should be offered continuation of therapy after cessation of perinatal transmission risk. These findings are similar to data from the multi-country MTCT-Plus Initiative, which found that women stopping a variety of ARV regimens for prevention of perinatal transmission had a 46% risk of dropping below 350 cells/uL by 24 months postpartum when the initial CD4+ cell count during pregnancy was 400–499 cells/uL.10 A study from Haiti found that women stopping antiretroviral prophylaxis at delivery with a CD4+ lymphocyte count between 350 and 499 cells/uL dropped to the threshold of 350 cells/uL requiring therapy at a median of 19 months after delivery compared to a median of 71 months to reach this threshold among women with CD4+ cell counts at or above 500 cells/uL at delivery.11 These data are also consistent with a study from Brazil, which showed that among women discontinuing ARV agents after delivery, the group with levels between 250–500 cells/uL had a risk of progression to stage II or III events that was 2.5 times higher than women with CD4+ counts above 500 cells/uL.12 The importance of CD4+ lymphocyte results for predicting progression were also shown in a study from Kenya demonstrating that CD4 counts and percentage during pregnancy were most predictive of mortality over the first two years postpartum, compared to total lymphocyte count, hemoglobin, HIV RNA level, or body mass index.8
Among women in the 350–550 cells/uL CD4+ lymphocyte group, continuation would also have benefits in reduction of heterosexual transmission to discordant partners. This conclusion is based on results from a large randomized trial of discordant couples which found that treating HIV-infected subjects with CD4+ lymphocyte counts between 350 and 550 cells/uL reduced linked transmissions to partners by 96% as compared to delaying therapy until the CD4+ lymphocyte count drops below 250 cells/uL or symptomatic illness occurred.3 While earlier treatment at higher CD4+ levels above 550 cells/uL may also reduce the risk of sexual transmission, the benefit in the group with higher counts has not been proven. Women beginning ARVs in pregnancy with CD4+ cell counts between 350 and 550 cells/uL should be counseled about the potential benefit of reduced sexual transmission when they are considering whether or not to continue ARVs after delivery.
Intensive counseling for adherence to the regimen must be provided for women who choose to continue ARVs after cessation of mother-to-child HIV transmission risk, as adherence has been shown to decrease markedly once the incentive of transmission prevention has ended. A recent meta-analysis reported a pooled estimate of adequate adherence (> 80% of doses) of 75.7% (95% CI 71.5–79.7%) during pregnancy compared to 53.0% (95% CI 32.8–72.7%, p= 0.005) during the postpartum period.4 This study included women from a range of low, middle, and high income countries. Likewise, in several reports from African sites, HIV- infected women were at particularly high risk for loss to follow up with 50% or more defaulting on their maternal child health appointments after delivery in some studies.13–16 Travel away from home to stay with family for several months following the birth of a child is common. In addition, many women have not disclosed their HIV status to partners or family members making adherence challenging, especially if residing with relatives.17 Poor adherence increases the risk of development of viral resistance and treatment failure.18 Concerns regarding development of resistance may be especially important given the current WHO recommendations for efavirenz-based first line therapies, which require a single mutation for resistance.9 Clearly, support for adherence and follow up after delivery will need to be intensified for women planning to continue ARV agents postpartum.
In our study, women with CD4+ lymphocyte counts of 550 cells/uL or higher had a lower risk of disease progression with only 7.4% dropping below 350 and 1.6% below 200 cells/uL by one year postpartum without ARVs. These results are consistent with data from the MTCT-Plus Initiative which demonstrated that 18.5% of women with initial CD4+ cell counts above 500 cells/uL during pregnancy progressed to counts below 350 cells/uL by 24 months after delivery.10 The group of women with CD4+ cell counts above 550 cells/uL would be expected to have a low risk of disease progression even with discontinuation of ARVs after pregnancy and breastfeeding, so could discontinue therapy if desired. These findings underscore the utility of CD4+ lymphocyte testing and suggest that in this group of women, more data are needed regarding the risks and benefits of continuing ARVs after risk of mother-to-child transmission of HIV has passed.
There is also a need for operational research that investigates better ways to achieve high levels of adherence to ARV drugs among both pregnant and postpartum women and to decrease loss to follow up among HIV infected pregnant women. In areas where implementation of universal treatment is currently not feasible for cost, supply, and logistic reasons, targeting therapy to groups at highest risk for disease progression and transmission to their infants based on their CD4+ cell counts remains necessary. Even with implementation of the strategy of starting lifelong therapy for all pregnant women, CD4+ lymphocyte testing may be helpful to target women with low CD4+ cell counts for increased support and provision of opportunistic infection prophylaxis. Events occurring among the postpartum women were rare but similar to those reported in the HPTN052 study discussed above, with deaths (0.8%) and extrapulmonary tuberculosis (0.2%) being the most frequent serious events in the current study.3 Causes of death were heterogeneous with only two cases occurring with proximate CD4+ lymphocyte counts under 200 cells/uL and only one case (probable meningitis) likely AIDS-defining. Other infections (malaria and hepatitis) and co-morbidities (anemia, diabetes, pancreatitis, cardiac failure) contributed to deaths, and some of these conditions may have been ameliorated by earlier ARV. Incomplete data on causes of death limit the conclusions that can be drawn.
Availability of ARV therapy for women with CD4+ lymphocyte counts below 200 cell/uL in the current HPTN 046 study appears to have lowered the risk of death compared to earlier studies in similar populations. The HPTN 024 study, conducted among pregnant women in Malawi, Zambia, and Tanzania between 2001–2003, at a time when antiretroviral therapy was not widely available in those settings, reported death rates over the first year postpartum of 6.1/100 person-years among women with CD4+ lymphocyte counts below 200 cells/uL at delivery, 1.1/100 person-years with CD4+ cell count of 200–500 cells/uL, and 0.4/100 person-years for those with CD4+ cell counts over 500 cells/uL. No deaths occurred among 331 HIV-uninfected women in the study.19 No ARV therapy was available to women after delivery in that study. In a retrospective review of a cohort of women delivering in Malawi in 2008 who received postpartum ARV therapy if they had WHO stage III or IV disease or CD4+ lymphocyte count below 250 cells/uL, the mortality rate over 18–20 months postpartum was 4.24 deaths/100 person-years among 173 consecutive HIV-infected women and 0 among HIV-uninfected delivering at the same sites.20
There are certain limitations to these findings including incomplete data on causes of death and lack of longer term follow up. Data on use of hormonal contraception are not available, but unlikely to have influenced the findings, based on data from Kenya among postpartum women that found no differences in CD4+ cell count or HIV RNA changes with use of hormonal contraceptives compared to no use.21 Despite these limitations, there are also a number of strengths of the HPTN 046 study including the large number of participants followed in four African countries and excellent retention which allowed careful monitoring of serial CD4+ lymphocyte counts and endpoint ascertainment over the first year after delivery. Since WHO and in-country guidelines in place during the study did not recommend ARV treatment for asymptomatic individuals with CD4+ cell counts above 200 cells/uL, these data from HIV-infected women in the first year after delivery can help inform evolving guidelines about continuation of ARV after delivery.
Data from follow up of the HIV-infected women enrolled to the HPTN046 study are informative on the risk of disease progression among postpartum women in resource limited settings. Women with CD4+ lymphocyte counts between 4000 and 550 cells/uL in pregnancy had a one in three risk of progressing to indications for ARV therapy within one year of delivery based on current WHO recommendations. Given these findings, this group of women should be counselled regarding the benefits of continuing ARVs after the need for ARV’s for prevention of transmission is completed. Those who are interested in continuing ARV’s should be provided with support for disclosure to family members, treatment adherence, and regular follow up visits. Pending more data on long term outcomes from ongoing trials, women with CD4+ lymphocyte counts above 550 cells/uL could be presented with information regarding the potential benefits and risks of continuing ARVs and supported in their decision to stop or continue ARVs after the indication for prevention of mother-to-child transmission of HIV has ceased.
Supplementary Material
Figure 1.
Time to (A) CD4+ lymphocyte counts below 200 cells/uL, (B) CD4+ lymphocyte counts below 350 cells/uL, (C) WHO clinical stage III/IV or death, and (D) WHO clinical stage IV, CD4+ count < 200 cells/uL, or death.
Figure 2.
Time to ART initiation or (A) CD4+ lymphocyte counts below 200 cells/uL, (B) CD4+ lymphocyte counts below 350 cells/uL, (C) WHO clinical stage III/IV or death, and (D) WHO clinical stage IV, CD4+ count < 200 cells/uL, or death.
Acknowledgements
We thank the mothers and their children who participated in the study; the HPTN 046 study coordinators, counselors, clinicians, pharmacists, data quality and laboratory staff, and those responsible for recruitment and retention for their dedication and hard work on site; Thomas R. Fleming (University of Washington/Fred Hutchinson Cancer Research Center) for his contributions to study design and his strong support throughout study conduct; Scharla Estep (NIAID protocol pharmacist) for her help on pharmaceutical matters; and Avinash Shetty (Wake Forest University Health Sciences) for his contribution to study development and safety data review.
This study was supported by the HIV Prevention Trials Network (HPTN) and sponsored by the National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the National Institutes of Health, U.S. Department of Health and Human Services under award # U01 AI046749. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).The study products were provided for free by Boehringer-Ingelheim.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest and Source of Funding
No conflicts of interest were declared.
References
- 1.World Health Organization. Use of antiretroviral drugs for treating pregnant women and prevention HIV infection in infants: programmatic update. 2012 Apr; Available at http://www.who.int/hiv/pub/mtct/programmatic_update2012/en/
- 2.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Myiwa M, Thea DM. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to-child HIV transmission. AIDS. 2010;24:1374–1377. [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. for the HPTN 052 Study Team. Prevention of HV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-, middle, and high income countries: a systematic review and meta-analysis. AIDS. 2012;26 doi: 10.1097/QAD.0b013e328359590f. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coovadia HM, Brown ER, Fowler MG, et al. for the HPNT046 protocol tteam. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:221–228. doi: 10.1016/S0140-6736(11)61653-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. 2007 Available at http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf.
- 7.Fawzi WW, Msamanga GI, Hunter D, et al. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early childhood mortality. AIDS. 2002;14:1935–1944. doi: 10.1097/00002030-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 8.Brown ER, Otieno P, Mbori-Ngacha D, et al. Comparison of CD4 cell count, viral load, and other markers for the prediction of mortality among HIV-1-infected Kenyan pregnant women. J Infect Dis. 2009;199:1292–1300. doi: 10.1086/597617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. 2009 Nov; Available at http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf. [PubMed]
- 10.Ekouevi D, Abrams EJ, Schlesinger M, Myer L, Phanuphak N, Carter RJ for the MTCT-Plus Initiative. Maternal CD4+ cell count decline after interruption of antiretroviral prophylaxis for the prevention of mother-to-child transmission of HIV. PLos One. 2012;7:e43750. doi: 10.1371/journal.pone.0043750. 10 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coria A, Noel F, Bonhomee J, et al. Consideration of postpartum management in HIV-positive Haitian women: an analysis of CD4 decline, mortality, and follow-up after delivery. J Acquir Immune Defic Syndr. 2012;61:636–643. doi: 10.1097/QAI.0b013e31826abdd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilotto JH, Velasque LS, Friedman RK, et al. Maternal outcomes after HAART for the prevention of mother-to-child transmission in HIV-infected women in Brazil. Antiviral Ther. 2011;16:349–356. doi: 10.3851/IMP1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manzi M, Zahariah R, Teck R, et al. High acceptability of voluntary counseling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission progreamme in rural Malawi: scaling-up requires a different way of acting. Trop Med and Internatl Health. 2005;10:1242–1250. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang B, Losina E, Stark R, et al. Loss to follow-up in a community clinic in South Africa- roles of gender, pregnancy and CD4 count. S African Med J. 2011;101:253–257. doi: 10.7196/samj.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clouse K, Maskey M, Fox MP, Bassett J, Larson B. Delayed diagnosis of HIV and high rates of loss to follow-up among pregnant women receiving antenatal services at a primary healthcare clinic in Johannesburg, South Africa; Presented at the 19th Conference on Retroviruses and Opportunistic Infections; March, 2012; Seattle, WA. Abstract 1004. [Google Scholar]
- 16.Ahoua L, Ayikoru H, Gnauck K, et al. Evaluation of a 5-year programme to prevent mother-to-child transmission of HIV infection in Northern Uganda. J Trop Pediatr. 2010;56:43–52. doi: 10.1093/tropej/fmp054. [DOI] [PubMed] [Google Scholar]
- 17.Ekama SO, Herbertson EC, Addeh EJ, et al. Pattern and determinants of antiretroviral drug adherence among Nigerian pregnant women. J Pregnancy. 2012;2012:851810. doi: 10.1155/2012/851810. epub ahead of print February 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nachega JB, Marconi VC, van Zyl GU, et al. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disorders- Drug Targets. 2011;11:167–174. doi: 10.2174/187152611795589663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chilongozi D, Wang L, Brown L, et al. for the HIVNET 024 Study Team. Morbidity and mortality among a cohort of human immunodeficiency virus type 1-infected and uninfected pregnant women and their infants from Malawi, Zambia, and Tanzania. Pediatr Infect Dis J. 2008;27:808–814. doi: 10.1097/INF.0b013e31817109a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landes M, van Lettow M, Bedell R, et al. Mortality and health outcomes in HIV-infected and HIV-uninfected mothers at 18–20 months postpartum in Zomba district, Malawi. PLoS One. 2012;7:e44396. doi: 10.1371/journal.pone.0044396. 6 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson BA, Otieno PA, Mbori-Ngacha D, Overbaugh J, Farquhar C, John-Stewart GC. Hormonal contraception and HIV-1 disease progression among postpartum Kenyan women. AIDS. 2007;21:749–753. doi: 10.1097/QAD.0b013e328032790f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.