TO THE EDITOR
Itch sensation is transmitted from the skin to the spinal cord by small-diameter, unmyelinated C fibers and thinly myelinated Aδ fibers (Schmelz, 2010; Ringkamp et al., 2011). In human skin, C fibers mediating pruritus are either mechanically insensitive, histamine-sensitive nerves or mechanically sensitive, polymodal nociceptors that are unresponsive or only weakly responsive to histamine (Schmelz, 2010; Ringkamp et al., 2011). In addition, data combined from human and nonhuman primate experiments identified mechanically sensitive Aδ fibers that contribute to histamine-induced and non-histamine, cowhage spicule–induced itch (Ringkamp et al., 2011). Histologic markers for itch-sensing nerves in human skin include the capsaicin receptor, transient receptor potential vanilloid 1 (TRPV1) (Schmelz, 2010; Ringkamp et al., 2011), and the vasoactive peptides calcitonin gene–related peptide (CGRP) or substance P (SP) (Davidson and Giesler, 2010). However, these histologic markers alone cannot differentiate Aδ and C fibers that perceive itch from those that sense pain. In rodent models, gastrin-releasing peptide receptor (GRPR), expressed in the dorsal horn lamina I, mediates a central nervous system itch–specific pathway (Sun and Chen, 2007; Sun et al., 2009). In mice, gastrin-releasing peptide (GRP), the ligand for GRPR, is expressed in both the skin and the dorsal root ganglion by peptidergic (i.e., containing CGRP and SP) nerves that express TRPV1 (Sun and Chen, 2007; Liu et al., 2009; Tominaga et al., 2009; Lagerstrom et al., 2010; Fleming et al., 2012). Although GRP is not likely to be the principal excitatory neurotransmitter activating GRPR-expressing spinal cord dorsal horn neurons, GRP-expressing neurons still may mediate itch-specific signals from the skin to the spinal cord in rodents (Lagerstrom et al., 2010; Akiyama et al., 2012). Accordingly, MrgprA3 expression defines a subset of mouse primary cutaneous sensory neurons that mediate itch, and 93% of MrgprA3-positive neurons coexpressed GRP (Liu et al., 2009; Han et al., 2012). Given the putative role of GRP-expressing nerves in mediating itch in these animal models, we sought to determine whether nondiseased human skin contains GRP-expressing nerves that show histologic features of primary afferent pruriceptors.
Disease-free human skin from 17 patients (nine women, mean age 66.9 years±14.7; eight men, mean age 61.2 years±9; Supplementary Table S1 online) was used for fluorescence immunohistochemistry on 80-μm-thick sections (see Supplementary Methods online). GRP(+) nerves (confirmed by costaining with protein gene product 9.5 (PGP9.5)) were identified in all patients and from all sites (Table 1, Supplementary Table S1 online). GRP(+) nerves were primarily located in the papillary dermis as free nerve endings terminating at the dermoepidermal junction (DEJ) (Figure 1), and in this anatomic skin compartment comprised between ∼5 and 15% of all PGP9.5(+) staining (Supplementary Table S1 online). GRP(+) nerves were not identified within the epidermis of human skin but were identified tightly associated with some appendageal structures, including hair follicles and eccrine glands (Supplementary Figure S1 online). Determining regional variation in GRP nerve density has relevance to future studies comparing diseased with nondiseased skin. After categorizing the samples into three anatomic locations (scalp/head/neck, trunk, and extremities), we found only modest site differences in the % of PGP9.5 nerve length that is GRP(+) at the DEJ (Table 1), indicating near-uniform anatomic distribution of GRP(+) nerves in human papillary dermis.
Table 1. Human papillary dermis nerve staining results.
| Human papillary dermal nerve staining | Head/neck/scalp | Trunk | Extremities |
|---|---|---|---|
| % Of PGP9.5(+) nerves that are also GRP(+)1 | 7.7, 3.8–12.12 | 7.8, 4.3–10.9 | 9.8, 7.6–16.62 |
| % Of PGP9.5(+) nerves that are also CGRP(+)1 | 8.4, 4.6–17.33 | 20.8, 13.1–30.43a | 17.9, 10.3–30.33b |
| % Of GRP(+) nerves that are also CGRP(+)1 | 36.3, 6.9–70.24 | 81.9, 42.6–97.34a | 67.2, 35.8–86.64b |
| % Of CGRP(+) nerves that are also GRP(+)1 | 20.4, 5.9–44.5 | 36.6, 22.5–40.1 | 35.7, 21.3–63.2 |
| % Of PGP9.5(+) nerves that are also SP(+)1 | 8.8, 4.2–14.7 | 5.1, 2.2–10.5 | 8.1, 7.2–15.2 |
| % Of GRP(+) nerves that are also SP(+)1 | 82.6, 37.3–100 | 57.7, 0.0–100 | 100, 74.2–100 |
| % Of SP(+) nerves that are also GRP(+)1 | 69.3, 46.6–100 | 42.5, 0.0–89.2 | 100, 88.3–100 |
| % Of GRP(+) nerves that are also C fibers1 | 48.1, 20.1–73.6 | 27.5, 11.3–48.4 | 12.9, 0–47.8 |
| % Of GRP(+) nerves that are also Aδ fibers1 | 51.9, 26.4–79.9 | 72.5, 51.6–88.9 | 87.1, 52.2–100 |
| % Of GRP(+) C fibers that are also SP(+)1 | 74.8, 40.6–100 | 52, 1–100 | 100, 17.3–100 |
| % Of GRP(+) Aδ fibers that are also SP(+)1 | 100, 59.3–100 | 100, 95.2–100 | 100, 100–100 |
Abbreviations: CGRP, calcitonin gene–related peptide; GRP, gastrin-releasing peptide; PGP9.5, protein gene product 9.5; SP, substance P.
Median, 25–75% quartiles.
P=0.0184, α=0.05.
P=0.005; 3–3bP=0.0037.
P=0.0058; 4–4bP=0.0294.
Figure 1.
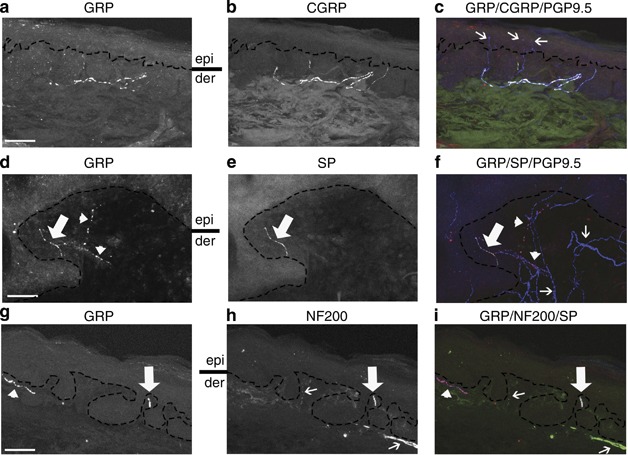
Characterization of gastrin-releasing peptide (GRP)-expressing nerves in the dermoepidermal junction (DEJ)/papillary dermis in human skin. Immunohistochemical triple staining showing (a, d, and g) GRP, (b) calcitonin gene–related peptide (CGRP), and (c) overlay of GRP (red), CGRP (green), and protein gene product 9.5 (PGP9.5) (blue), (e) substance P (SP), (f) overlay of GRP (red), SP (green), and PGP9.5 (blue), (h) neurofilament 200 (NF200), and (i) overlay of GRP (red), NF200 (green) and SP (blue). (c) White pseudocolor indicates GRP(+)/CGRP(+)/PGP9.5(+) staining. (c) Thin arrows highlight GRP(−)/CGRP(−)/PGP9.5(+) intraepidermal nerve fibers. (d–f) Thick arrows indicate GRP(+)/SP(+)/PGP9.5(+) staining; (d, f) Arrowheads highlight GRP(+)/SP(−)/PGP9.5(+) staining (pink pseudocolor, (f)). (f) Thin arrows highlight GRP(−)/SP(−)/PGP9.5(+) nerves. (g, i) Arrowhead indicates GRP(+)/NF200(−)/SP(+) staining (pink pseudocolor, (i)). (h, i) Thin arrow highlights GRP(−)/NF200(+)/SP(−) staining. (g–i) Thick arrows indicate GRP(+)/NF200(+)/SP(+) staining (white pseudocolor, (i)). (a, d, and h). der, dermis; epi, epidermis. Bars=50 μm.
Itch-sensing fibers are thought to be peptidergic, expressing SP and CGRP (Davidson and Giesler, 2010). We identified GRP expression in both CGRP(+) and CGRP(−) (Figure 1 and Supplementary Figure S1 online, Supplementary Table S2 online) and SP(+) and SP(−) nerves (Figure 1, Supplementary Table S3 online) at the DEJ. The majority of papillary dermal GRP(+) nerves were SP(+) (median 98.2, 25–75% quartiles, 32.8–100%), whereas these GRP(+) nerves were significantly less likely to be CGRP(+) (median 50.9, 25–75% quartiles, 18.8–80% P=0038). Similarly, GRP(+) neurons comprised a significantly larger proportion of SP(+) nerves at the DEJ (median 86.4, 25–75% quartiles, 40.0–100%) than of CGRP(+) nerves (median , 28.0, 25–75% quartiles, 8.1–43.3% P<0.0001). Finally, this GRP antibody colabeled with a peptidergic nerve fiber population in mouse skin (Supplementary Figure S2 online, Supplementary Table S4 online), similar to that previously reported (Sun and Chen, 2007; Liu et al., 2009; Tominaga et al., 2009; Lagerstrom et al., 2010; Fleming et al., 2012).
As both Aδ and C fibers mediate itch, we next examined whether GRP(+) fibers at the DEJ belonged to the Aδ fiber (neurofilament 22 (NF200)+) and/or the C fiber (NF200−) populations of nerves (see Methods). Combining the 17 subjects, GRP(+) nerves comprised 38.1% (median, 25–75% quartiles, 8.7–60.0%) of C fibers and 66.3% (median, 25–75% quartiles, 41.0–91.3%) of Aδ fibers (Figure 1, Table 1), with this difference being significant (χ2 7.63, DF1, Prob>χ2 0.0057). The majority of GRP(+) C fibers and GRP(+) Aδ fibers coexpressed SP (median 88.4, 25–75% quartiles, 34.3–100% median 100, 25–75% quartiles, 95.9–100%, respectively) (Figure 1).
Our identification, in human skin, of GRP-expressing C and Aδ fibers that coexpress either SP or CGRP makes these neurons candidate primary afferent pruriceptors. Moreover, putative broad GRPR expression in human skin, including keratinocytes, makes possible additional functions for this nerve population (Staniek et al., 1996). As for SP and CGRP nerves, GRP-expressing nerves comprised a minority of the nerve plexus in human papillary dermis and predominantly terminated at the DEJ without intraepidermal extension. Itch-specific signaling has been known to reside superficially in human skin (Magerl, 1996). However, whether human intraepidermal nerve fibers (IENFs) and/or papillary dermal nerves signal itch is not clear. Pain-sensing pathways, thought to reside deeper in the dermis, may block, at the level of the spinal cord, a coactivated superficial itch signal (Davidson and Giesler, 2010; Schmelz, 2010; Ross, 2011). This superficial itch signal in humans may reside preferentially at the DEJ, and not in IENFs, given the paradoxical loss of IENFS in some chronic pruritic conditions (Oaklander and Rissmiller, 2002; Maddison et al., 2008). In mice, molecular silencing of a population of TRPV1(+) primary nociceptive sensory neurons unmasked a chronic cutaneous scratching phenotype, possibly mediated by residual signaling from GRP(+) itch-sensing primary sensory neurons (Lagerstrom et al., 2010; Liu et al., 2010). Extending this work to humans, the location of GRP(+) nerves makes this population a prime candidate to selectively signal itch from the DEJ in some skin diseases where pain-only sensing nerves, such as in the epidermis, are preferentially lost—a hypothesis we are now actively testing.
The Boston University IRB approved this study and did not require patient consent for the use of de-identified and otherwise discarded surgical tissue; the study was conducted according to the Declaration of Helsinki Principles.
Acknowledgments
This study was supported in part by CTSA grant UL1-TR000157 from the NIH.
Glossary
- CGRP
calcitonin gene–related peptide
- DEJ
dermoepidermal junction
- GRP
gastrin-releasing peptide
- GRPR
gastrin-releasing peptide receptor
- IENF
intraepidermal nerve fibre
- NF200
neurofilament 200
- PGP9.5
protein gene product 9.5
- SP
substance P
- TRPV1
transient receptor potential vanilloid 1
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Akiyama T, Tominaga M, Davoodi A, et al. Roles for substance P and gastrin releasing peptide as neurotransmitters released by primary afferent pruriceptors. J Neurophysiol. 2012;109:742–748. doi: 10.1152/jn.00539.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MS, Ramos D, Han SB, et al. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol Pain. 2012;8:52. doi: 10.1186/1744-8069-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2012;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerstrom MC, Rogoz K, Abrahamsen B, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison B, Namazi MR, Samuel LS, et al. Unexpected diminished innervation of epidermis and dermoepidermal junction in lichen amyloidosus. Br J Dermatol. 2008;159:403–406. doi: 10.1111/j.1365-2133.2008.08685.x. [DOI] [PubMed] [Google Scholar]
- Magerl W.1996Neural mechanisms of itch sensation Tech Corner IASP Newsletteravailabel at: : http://www.iasp-pain.org/AM/Template.cfm?Section=Technical_Corner&Template=/CM/ContentDisplay.cfm&ContentID=2200
- Oaklander AL, Rissmiller JG. Postherpetic neuralgia after shingles: an under-recognized cause of chronic vulvar pain. Obstetrics Gynecol. 2002;99:625–628. doi: 10.1016/s0029-7844(01)01663-5. [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Schepers RJ, Shimada SG, et al. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31:14841–14849. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol. 2011;21:880–887. doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Schmelz M. Itch and pain. Neurosci Biobehav Rev. 2010;34:171–176. doi: 10.1016/j.neubiorev.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Staniek V, Misery L, Peguet-Navarro J, et al. Expression of gastrin-releasing peptide receptor in human skin. Acta Dermato-Venereologica. 1996;76:282–286. doi: 10.2340/0001555576282286. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, et al. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Ogawa H, Takamori K. Histological characterization of cutaneous nerve fibers containing gastrin-releasing peptide in NC/Nga mice: an atopic dermatitis model. J Invest Dermatol. 2009;129:2901–2905. doi: 10.1038/jid.2009.188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


