Abstract
Rationale
Alcohol is usually consumed in social contexts. However, the drug has been studied mainly under socially isolated conditions, and our understanding of how social setting affects response to alcohol is limited.
Objectives
The current study compared the subjective, physiological and behavioral effects of a moderate dose of alcohol in moderate social drikers who were tested in either a social or an isolated context, and in the presence of others who had or had not consumed alcohol. Methods: Healthy men and women were randomly assigned to either a social group tested in pairs (SOC; N=24), or an isolated group tested individually (ISO; N=20). They participated in four sessions, in which they received oral alcohol (0.8 g/kg) or placebo on two sessions each, in quasi randomized order under double blind conditions. In the SOC condition, the drug conditions of the co-participants were varied systematically: On two sessions both participants received the same substance (placebo or alcohol) and on the other two sessions one received alcohol while the other received placebo. Cardiovascular measures, breath alcohol levels and mood were assessed at regular intervals, and measures of social interaction were obtained in the SOC group.
Results
Alcohol produced greater effects on certain subjective measures in the SOC condition compared to the ISO condition, including feelings of intoxication and stimulation, but not on other measures such as feeling sedated or high, or on cardiovascular measures. Within the SOC condition, participants rated themselves as more intoxicated when their partner received alcohol, and paired subjects interacted more when at least one participant received alcohol.
Conclusions
The presence of others enhances some of the subjective and behavioral effects of alcohol, especially the presence of another intoxicated individual. This enhancement of alcohol effects may explain, in part, why it is used in a social context.
Keywords: social interaction, social behavior, mood, alcohol, humans
INTRODUCTION
Anecdotal and epidemiological evidence indicate that drugs – including alcohol, nicotine, and amphetamines – are commonly used in social contexts (Acosta et al. 2008; Halkitis et al. 2005; Rodgers et al. 2006; Single et al. 1993). At least one systematic large-scale survey of consumption in Canada and the United States provides supporting evidence that alcohol is most often consumed in social settings, such as restaurants, bars, and in-home social gatherings (Kairouz and Greenfield 2007). In a systematic study using event-contingent recordings in the natural setting, Aan het Rot et al (2008) also reported that use of alcohol in social settings is usually associated with enhanced mood states and increased perception of agreeableness in others. Whether, and how, social contexts influence the use and abuse of drugs is not fully understood. The presence of others may facilitate drug use, and, conversely, drugs themselves may also alter social function. Our knowledge of these bi-directional relationships is limited in part because most laboratory drug challenge studies are conducted under socially isolated conditions. We have initiated a series of studies investigating the effects of drugs, in this case alcohol, in a social compared to an isolated setting.
There is evidence from both nonhuman and human studies that the mere presence of other individuals affects responses to drugs. In several studies, laboratory animals self-administer drugs of abuse at a higher rate when conspecifics are present (Burkett and Young 2012; Hostetler and Ryabinin 2012; Neisewander et al. 2012; Smith 2012). Further, in both rats and prairie voles the drug state of another animal can increase consumption; these animals consume more alcohol in the presence of a conspecific that also received alcohol (Anaker and Ryabinin 2010; Hostetler et al. 2012). It has also been reported that rats show a greater preference for both cocaine and nicotine when they are tested in an environment that has been previously associated with social interaction (Thiel et al. 2008, 2009). In humans, alcohol produces more positive mood effects when consumed in the presence of others (Doty and de Wit 1995; Sher 1985). For example, Doty and de Wit (1995) found that alcohol (0.5 and 0.8 g/kg) produced more “positive” subjective effects (e.g., “high” and “drug liking”) in participants who were tested in social groups, compared to those who were tested alone, and participants in the social condition also consumed more alcohol when given the chance. However, the processes by which social variables influence drug responses are not fully understood.
Drugs can also, in turn, influence social behavior. Recently, Sayette and colleagues (2012a) examined the effects of alcohol on small group behavior, and their participants (N=720) reported that alcohol increased social bonding within the groups. Additionally, several drugs of abuse increase verbal behavior (Wardle et al. 2012; Marrone et al. 2010; Higgins and Stitzer 1988, 1989), and Higgins and Stitzer (1988) showed that amphetamine increased participants’ preference for social interaction over a monetary reward. These findings suggest that drugs can enhance the value of social interaction. These pro-social psychological effects of alcohol and other psychoactive drugs may, in turn, make the drug experience more pleasurable and more reinforcing when there are others present. Therefore, as a secondary measure in the present study we also investigated the effects of alcohol on social interactions.
Responses to drugs may be affected simply by the presence of others, but also by the behaviors of the others who are present. In particular, usually drugs are consumed in the presence of others who are using drugs, raising the possibility that an individual drug user may be influenced by the behavior of other drug-affected individuals. In laboratory animals, the drug consumption of a companion animal can influence drug self-administration. For example, Smith (2012) recently reported that rats consumed more cocaine in the presence of another animal that was also self-administering cocaine, but not in the presence of a non-self-administering conspecific. In humans, several older studies found that social drinkers consumed more alcohol in the presence of heavier drinkers (Caudill and Marlatt 1975; Cooper et al. 1979; Watson and Sobell 1982), an effect often attributed to “modeling”, or mimicing the pattern of consumption. However, to our knowledge there is little information about whether the drug state of another individual alters the acute responses to alcohol.
The present study had three goals. First we aimed to compare the subjective and behavioral effects of alcohol (0.8 g/kg) in social drinkers tested alone or in the presence of another person. Second, we aimed to investigate whether the drug state (alcohol vs placebo) of the co-participant influenced responses to alcohol (or placebo). Third, as a secondary goal we monitored the effects of alcohol on social interaction, using videotaped and self-report measures of social interaction in participants tested in the social condition. We hypothesized that participants would report greater subjective effects of alcohol in the social compared to the isolated condition, and that, in the social condition, they would report greater effects when the co-participant had received alcohol. We also expected that alcohol would increase social interaction.
METHODS
Participants
Healthy men and women (N=44) aged 21–35 and nonproblem social drinkers, were recruited via newspaper, community bulletin board, and online advertisements. They were eligible if they consumed an average of 10–30 standard drinks per week, with at least one binge-drinking episode (i.e., 4 or 5 drinks/occasion for women and men respectively) in the past month. After initial telephone screening participants underwent an in-person psychiatric evaluation and medical examination including an electrocardiogram and physical examination. Exclusion criteria were less than high school education, lack of fluency in English, BMI outside 18–30 (to limit the volume of alcohol administered), and cardiovascular or neurological disorders or current Axis I psychiatric disorder (DSM-IV; APA 1994). Participants were also excluded if they smoked more than 10 cigarettes per day.
Participants provided written informed consent prior to participation. They were told that the purpose of the study was to evaluate the factors that influence the effects of drugs. In order to minimize the influence of expectanicies, participants were told they could receive a stimulant, a sedative, an opioid, alcohol, or placebo. The study was approved by the Institutional Review Board at the University of Chicago in accordance with the Code of Federal Regulations (Title 45, Part 46) adopted by the National Institutes of Health and the Office for Protection from Research Risks of the US Federal Government. The study was conducted in accordance with the Helsinski Declaration of 1964 (revised 1989) and the National Advisory Council on Drug Abuse Recommended Guidelines for the Administration of Drugs to Human Subjects.
Design
After an initial orientation session, participants completed four outpatient sessions on separate days, during which they received placebo and 0.8 g/kg alcohol, either alone or with a partner who also received either placebo or alcohol. Participants were randomly assigned to either: 1) a social condition (SOC; N=24), in which two same-sex individuals participated together in the same room; or 2) an isolated condition (ISO; N=20), in which participants were tested alone. In the SOC condition subjects participated with a new partner who they had not met before on each session. The paired subjects participated in the following four conditions, in randomized order: i) both members received alcohol, ii) both received placebo, iii) and iv) one received alcohol, the other placebo (See Table 1). In the ISO condition, participants received alcohol on two sessions and placebo on two sessions in a randomized order. Participants completed questionnaires and physiological measures were monitored at regular intervals during the 4-hour sessions. After completing all sessions participants were informed about experimental and drug conditions.
Table 1.
Study design
| Session 1 | Session 2 | Session 3 | Session 4 | |
|---|---|---|---|---|
| Isolated group (N=20) | Placebo | Alcohol | Placebo | Alcohol |
| Social group (N=24) | ||||
| Actor Dose (Partner Dose) |
Placebo (Placebo) |
Alcohol (Placebo) |
Placebo (Alcohol) |
Alcohol (Alcohol) |
Note: Alcohol dose = 0.8 g/kg.
SOC participants were matched with a different same-sex co-participant for each of the four sessions. Dosing order was varied across participants.
Procedure
Sessions were conducted between 3:00 pm and 8:00 pm, at least 48 hrs apart. Participants fasted from noon to standardize alcohol absorption. Upon reporting to the laboratory, they provided urine and breath samples to confirm abstinence from alcohol (as measured by an Alco-Sensor III Breathalyzer, Intoximeters Inc., St Louis, MO), amphetamine, cocaine and opiates (as measured by urine toxicology: Ontrak TesTstik, Roche Diagnostic Systems Inc., Somerville, NJ), and marijuana (as measured by a saliva test: Oratect, Branan Medical Corp., Irvine, CA), and women were tested for pregnancy (none tested positive). Sessions were rescheduled if the participant tested positive for drugs. At 3:20 pm, baseline (pre-drink) measures of heart rate, blood pressure, and breath alcohol levels (BAL) were obtained, and participants completed self-report mood and drug effects questionnaires (see below). From 3:30–3:45 pm, participants consumed beverages containing either alcohol or placebo (described below), and post-beverage physiological (i.e., heart rate, blood pressure, BAL) and subjective measures were obtained at 4:00, 4:15, 4:45, 5:15, 5:45, 6:45, and 7:45 pm. At 4:45 pm all participants completed a 5-min conversational talking task (described below). At 4:50 pm, participants in the SOC condition completed a brief questionnaire rating the perceived emotional responsiveness and attractiveness of their co-participants. All questionnaires were completed confidentially and participants were asked not to discuss their drug effects with their partners. Participants in the SOC condition were video taped to record their interactions. During times when no measures were scheduled the participants were allowed to relax and watch movies, read, or play board games. At 8:00 pm, they completed the End of Session Questionnaire (ESQ: see below), and were discharged if they passed a field sobriety test and their BALs were below 0.04mg% (as per National Institute of Alcoholism and Alcohol Abuse Guidelines).
Physiological measures
Heart rate, blood pressure and BAL were measured at regular intervals throughout the sessions. Heart rate and blood pressure were measured using portable monitors (Life Source, A&D, Tokyo, Japan). BAL was measured using a Breathalyser (Alco-sensor III; Intoximeters, St. Louis, MO).
Subjective Effects and ESQ
Participants completed subjective effect questionnaires before and at regular intervals after beverage administration. The questionnaires were selected to provide a complete profile of the mood and somatic effects of alcohol, and are sensitive to the effects of alcohol (Holdstock and de Wit 2001). The drug-effect questionnaire (DEQ), a visual analogue questionnaire (0 to 100 mm; not at all to extremely) to assess the extent to which they experienced the effects of the drug: ‘Feel Drug’, ‘Feel High’, ‘Like Drug’, ‘Dislike Drug’, and ‘Want More’ (Fischman and Foltin 1991; Justice and de Wit 2000; Morean et al. 2013). The visual analog scale questionnaire (VAS) consisted of adjectives describing effects of alcohol (i.e., ‘I feel…’ ‘Drunk,’ ‘Dizzy,’ ‘Stimulated,’ ‘Sedated’) and mood states (i.e., ‘I feel…’ ‘Sociable,’ ‘Lonely,’ ‘Playful,’ ‘Friendly’). Each adjective was presented with a 100-mm line labeled ‘not at all’ at one end and ‘extremely’ at the other end. The 52-item version of the Addiction Research Center Inventory (Martin et al. 1971) included 5 scales reflecting typical drug responses including stimulant-like (Amphetamine: A; Benzedrine Group: BG), euphorigenic (Morphine-Benzedrine Group: MBG), sedative (Pentobarbital-Chlorpromazine-Alcohol Group: PCAG) and dysphoric (LSD). The ESQ queries participants about the identity of the drug that they received (stimulant, sedative, opioid, alcohol, or placebo), about the strength of the drug experience (Likert scale: 1–5), and how much they would like to take the drug again (visual analog scale: 1–100).
Social Interaction Measures
Video recordings of social interaction
SOC condition sessions were digitally recorded and coded by research assistants who were blind to the drug condition. Research assistants recorded whether participants were interacting or not: Reading, silently watching a movie, and sleeping were labeled noninteractive, whereas playing games and conversing were labeled interactive. Interactive behavior was further categorized as either verbal or non-verbal (Haney et al. 2001; Kirkpatrick et al. 2012). Behavior was coded every 2.5 min throughout the 4-hour session, except at times were participants were completing questionnaires or the Talking Task (see below). The dependent variables were the proportion of 2.5-min intervals during which participants were interacting and the proportion during which they were verbalizing. The video recordings were coded by two separate raters and the inter-rater reliability was over 90%.
Talking task
Participants in both the SOC and ISO conditions participated in a 5-min conversational talking task, 60 min after consuming the beverage. In this task, adapted from Janowsky et al (1984; Janowsky 2003), participants talked for 5 min about a significant person in their lives, whose names were obtained during the orientation session. Participants in the SOC condition talked to their co-participant, and participants in the ISO condition talked to the research assistant. Results from this task will be reported separately.
Evaluations of the co-participant and self (SOC condition only)
In the SOC condition, participants completed the Interpersonal Attraction Questionnaire (AQ; McCroskey and McCain 1974) and two versions of the Perceived Responsiveness Questionnaire (PRQ Other and PRQ Self; Reis 2003) following the talking task. The AQ is a self-report questionnaire that assesses the perceived social attractiveness and physical attractiveness of another person, in this case the co-participant, on a Likert scale (1–5). The PRQ Other is a self-report questionnaire assessing the level of attention, interest, understanding, and empathy of another person (the co-participant) on a Likert scale (1–7). The PRQ Self asked the same questions about the participant’s own level of responsiveness toward the co-participant. At the end of each session, participants also completed the Social Interaction Questionnaire (SIQ: aan het Rot et al. 2006), on which they rated their perception of their own levels of social affiliation, defined by agreeable and quarrelsome behaviors, and social power or status, defined by dominant and submissive behaviors.
Drug
The 0.8 g/kg alcohol dose was prepared in a 16% solution by volume with 95% alcohol and cranberry juice. The placebo beverage consisted of cranberry juice plus 1% alcohol added to mask the taste. For men, both beverages were prepared in a volume of 450 ml/70 kg and were divided into equal thirds (i.e., 150 ml/70 kg for each third), administered at 5-min intervals over 15 min (King et al. 2011). Doses for women were 15% less to adjust for sex differences in total body water (Frezza et al. 1990; King et al. 2011; Sutker et al. 1983). Chilled beverages were served in opaque, lidded cups and consumed through a drinking straw. Beverages were administered double-blind. This alcohol dose was chosen because it reliably produces subjective effects in moderate alcohol users (Doty and de Wit 1995).
Data Analysis
Overall data analysis strategy
First, we investigated the effects of the presence of another individual on responses to alcohol by comparing the effects of alcohol and placebo under the SOC and ISO conditions. Second, for the SOC condition we examined the influence of the co-participant’s drug state on acute response to alcohol and placebo. To distinguish the data obtained from the two participants in each SOC dyad, we will refer to the participant whose responses we are examining as the Actor and the co-participant as the Partner. All participants in the SOC condition contributed data as the Actor. Physiological and subjective measures are presented as change from baseline (i.e., pre-beverage). All analyses were conducted using IBM SPSS Statistics for Windows, Version 19.0 (Armonk, NY: IBM Corp., Released 2010).
Influence of social context on alcohol-related effects
In the comparison of the SOC versus ISO conditions, physiological and subjective-effects data from both placebo and alcohol sessions (four sessions total) were analyzed using multilevel linear models (MLMs). Independent (fixed) effects were dose (Placebo, Alcohol), group (ISO versus SOC), Sex, Time, and Session Order.
Influence of the Partners’ drug state on the Actors’ drug responses
To evaluate whether the responses to alcohol or placebo were affected by the drug state of the Partner, physiological measures, subjective effects, and behavioral data were analyzed using MLMs. Independent (fixed) effects were Actor dose (Placebo, Alcohol), Partner dose (Placebo, Alcohol), Sex, Time, and Session Order. For all statistical models within the SOC group, Sex did not alter any results and thus is not discussed. To account for possible interdependence between the co-participants’ data, Dyad (i.e., the unique Actor/Partner pairing) and Participant were used as random effects. In addition to calculating the main and interactive effects of Actor dose and Partner dose, MLMs provided the error terms needed to calculate planned comparisons designed to answer the following questions: 1) Does alcohol administered to the Partner alter the response to alcohol in the Actor (planned comparison = AlcoholActor/PlaceboPartner versus AlcoholActor/AlcoholPartner)?; and 2) Does alcohol administered to the Partner affect responses to placebo in the Actor (planned comparison = PlaceboActor/PlaceboPartner versus PlaceboActor/AlcoholPartner)?
For all analyses, p values were considered statistically significant at less than 0.05 with Bonferroni adjustments for multiple comparisons.
RESULTS
Sample Characteristics
In total, 44 research volunteers (45% Female; 1 Asian, 2 Black, 1 Hispanic, 4 Mixed, 36 White) completed the study. They were 23.6 ± 2.6 (mean ± SD) years old and had completed 15.1 ± 1.1 years of formal education. The mean weight was 71.6 kg (range 54.4–100.2 kg), mean height was 173 cm (range 157–191 cm) and mean BMI was 24.0 (range 18.7–30.0). All participants reported moderate alcohol use (mean 16.1 drinks/week; range 10–30). Fifteen currently smoked cigarettes (mean 3.2 cigarettes/week; range 1–10), 40 currently drank caffeine-containing beverages (mean 2.8 cups/day; range 1–8), and 30 currently smoked marijuana (mean 8.8 days/month; range 1–30). Table 2 provides demographic information by Group (i.e., SOC versus ISO). The ISO group had more years of education (p<0.05), but the groups did not differ on age, BMI, or drug use history. Several volunteers (8 in the ISO group; 5 in the SOC group) withdrew before completing the study; their data are not included.
Table 2.
Demographics by Setting Group
| Setting Group | ||||
|---|---|---|---|---|
| ISO (N=10 F; 10 M) |
SOC (N=10 F; 14 M) |
|||
| Mean | (SD) | Mean | (SD) | |
| Age (years) | 24.2 | (2.8) | 23.1 | (2.4) |
| Education (years) | 15.5 | (1.1) | 14.8 | (1.0)* |
| BMI | 23.7 | (2.5) | 24.2 | (2.9) |
| Current Drug Use | ||||
| Alcohol (drinks/week) | 15.2 | (4.9) | 16.9 | (6.0) |
| Caffeine (cups/day) | 3.2 | (1.9) | 2.4 | (1.3) |
| Marijuana (days/month) | 7.7 | (8.4) | 10.0 | (9.7) |
significantly different from ISO p < 0.05
Effects of Alcohol
Alcohol produced its typical subjective and physiological effects, including increases in heart rate, decreases in blood pressure and increases on almost all of the subjective rating scales, including both positive (e.g., liking) and negative (e.g., disliking scales) (Supplementary Table 1). Most of these effects peaked at 15 or 30 minutes after beverage ingestion, and dissipated over the 4-hour session. The time course of sedated followed a different time course (Fig 2), peaking later and lasting longer into the session. By the end of the sessions, participants correctly identified placebo beverages on 65% of session, and alcohol on about 80% of sessions.
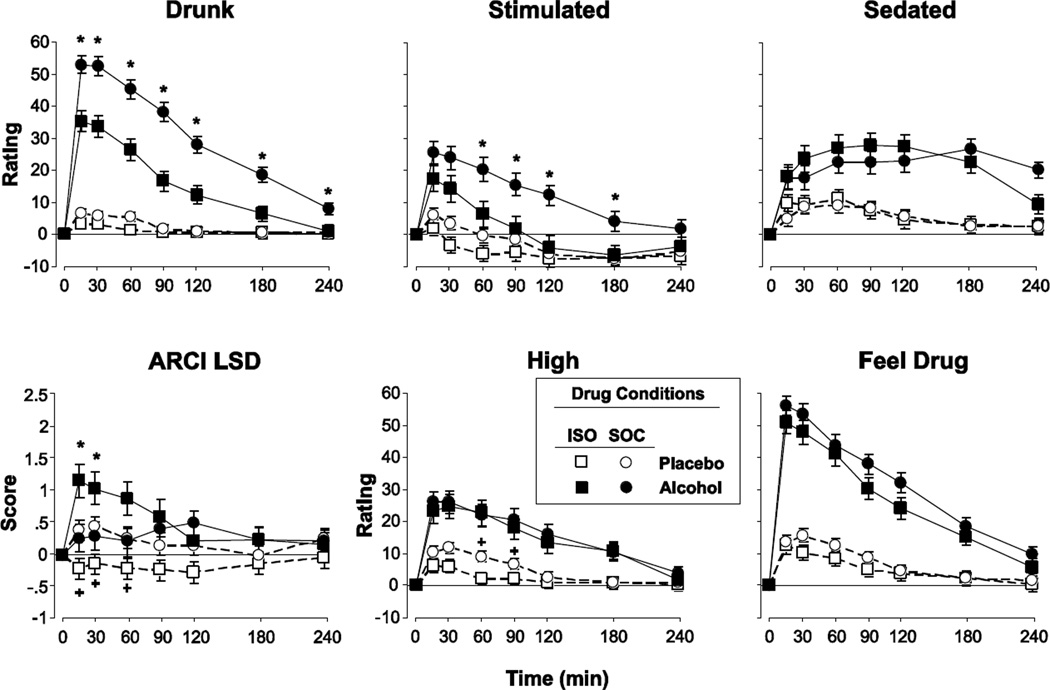
Figure 2.
Selected scores on subjective ratings for participants who were under Isolated conditions (ISO) or Social conditions (SOC) as a function of Dose and Time. Error bars represent one SEM. An * indicates significant difference in alcohol response between groups (p<0.05). A + indicates significant difference in placebo response between groups (p<0.05).
Comparison of alcohol-related effects in ISO and SOC
Physiological Effects
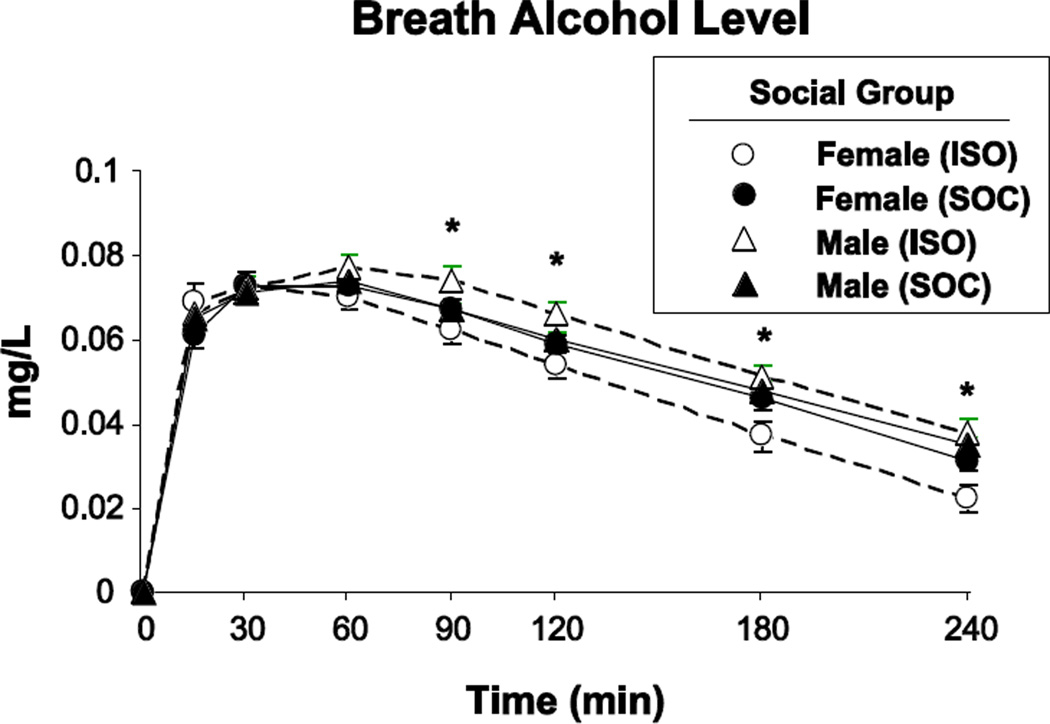
In the ISO group, men had higher BALs than women during the descending limb in men compared to women (Figure 1; significant Dose x Group x Sex x Time interaction; F[1, 1364.0] = 2.2, p < 0.05), probably due to random variation in body weight. Although BAL levels did not differ in ISO and SOC groups when data were collapsed across Sex (non-significant Dose x Social group x Time; F[1, 1364.0] = 1.1, p = 0.36), we included BAL as a covariate in all subsequent statistical models. Alcohol affected heart rate and blood pressure to a similar extent in both the ISO and SOC conditions.
Figure 1.
BAL in participants who were under Isolated conditions (ISO: N=20) or under Social conditions (i.e., dyads; SOC: N=24) as a function of Time and Sex. Error bars represent SEM. An * indicates significant difference between females and males within the ISO group (p<0.05).
Subjective Effects
The ISO and SOC groups differed in their subjective responses to alcohol on certain subjective measures (Figure 2). SOC participants reported higher ratings of ‘Drunk’, ‘Stimulated’, and ‘Social’ after alcohol compared to the ISO group (Figure 2 top left and middle panels; some data not shown; F[1, 89.1–120.6] = 7.7–20.6, p < 0.01 for all comparisons between groups). SOC participants also reported lower scores on the ARCI LSD scale (a measure of dysphoria) after alcohol and significantly higher LSD scores following placebo (Figure 2 bottom left panel; F[1, 951.3] = 3.3, p < 0.01). SOC participants also reported significantly greater ratings of ‘High’ after placebo (Figure 2 bottom middle panel; F[1, 52.2] = 4.2, p < 0.05) and greater ratings of ‘Friendly’ following both placebo and alcohol (data not shown; F[1, 979.6] = 2.7, p < 0.01). These group differences occurred at the time of peak alcohol effects but most group effects declined during the descending limb (except Drunk which remained high throughout the session). Notably, however, the effects of alcohol on most other measures, including ratings of feeling sedated, overall ratings of feeling a drug effect and liking the drug effect, did not differ under the SOC and ISO conditions (Supplementary Table 1; Fig 2 right panels).
On certain measures, alcohol effects differed in the SOC and ISO conditions only in women. In women but not in men, alcohol produced greater increases on ratings of ‘Friendly’ in the SOC condition compared to the ISO condition (significant Dose x Social group x Sex interaction; F[1, 79.5] = 7.4, p < 0.01). In addition, women in the SOC condition reported greater ARCI LSD scores and greater ratings of ‘High’ after placebo (significant Dose × Social group × Sex × Time interaction; F[1, 951.3–1038.9] = 2.2–2.3, p < 0.05 for both analyses).
End of Session Questionnaire
At the end of the sessions, the SOC and ISO groups did not differ on ratings of the strength of the effect, or whether they would take it again (Supplementary Table 1). The SOC and ISO groups were equally accurate in identifying the placebo (65% correct identifications), but the SOC group was slightly more accurate in identifying alcohol than the ISO group (92% vs 73%).
Influence of the co-participant’s drug state (SOC only)
Subjective and Physiological Effects
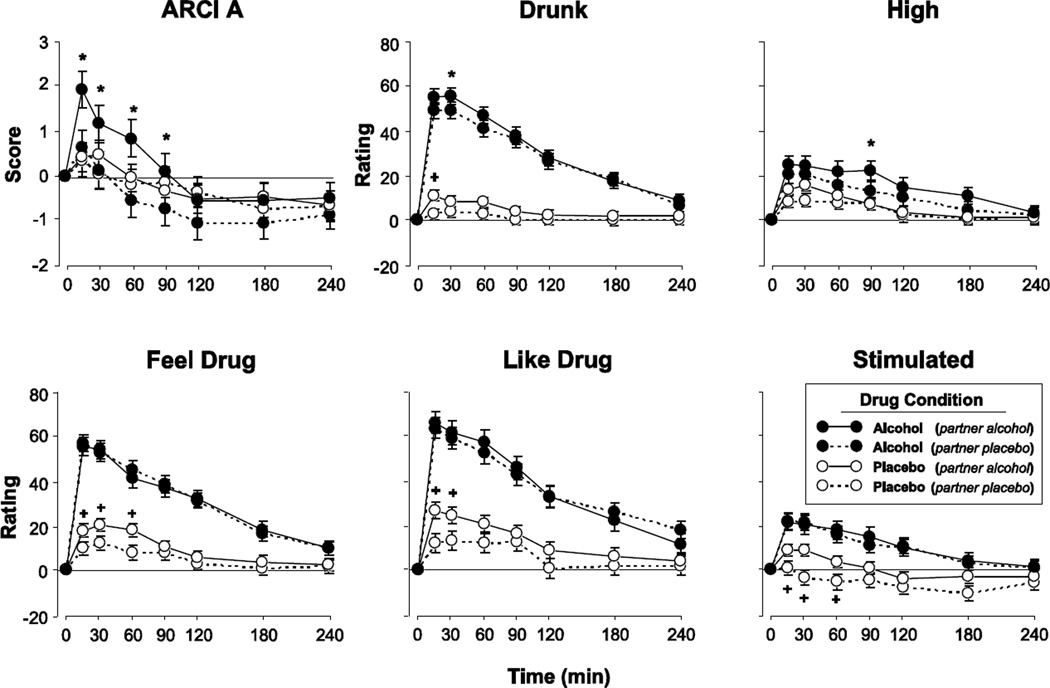
The drug condition of the Partner influenced subjective responses to alcohol on several measures (Figure 3). Alcohol produced a greater increase on the ARCI A scale when the Partner also received alcohol, compared to when the Partner received placebo (Figure 3 top left panel; F[1, 41.2] = 9.3, p < 0.01). Ratings of ‘Drunk’, ‘High’, and ‘Social’ were higher when the Partner received alcohol, regardless of whether or not the Actor received alcohol or placebo (Figure 3 top middle and right panels; some data not shown; Main effect of Partner Dose: F[1, 46.3–56.0] = 4.2–6.3, p < 0.05 for all analyses). Ratings of ‘Feel Drug’, ‘Like Drug’, ‘Stimulated’, and ‘Want More’ after placebo were higher when the Partner received alcohol, compared to placebo (Figure 3 bottom panels; some data not shown; F[1, 36.2–37.7] = 4.3–10.0, p < 0.05 for all comparisons). All of these observations are consistent with the idea that the presence of an intoxicated individual can produce, or increase, alcohol-related effects. The drug state of the Partner did not influence other subjective effects or physiological measures.
Figure 3.
Selected scores on subjective ratings for the social group as function of Time, when participants (i.e., Actors) received either alcohol or placebo in the presence of a co-participant (i.e., Partners) who had received either alcohol or placebo. The four conditions were: both Actor and Partner received placebo; Actor received alcohol while Partner received placebo; Actor received placebo while Partner received alcohol; both participants received alcohol. Error bars represent one SEM. A = 0.8 g/kg alcohol; P = placebo. An * indicates A/A significantly different from A/P (p<0.05). A + indicates P/A significantly different from P/P (p<0.05). ARCI A refers to a subscale the measures stimulant effects.
Social Interaction Measures
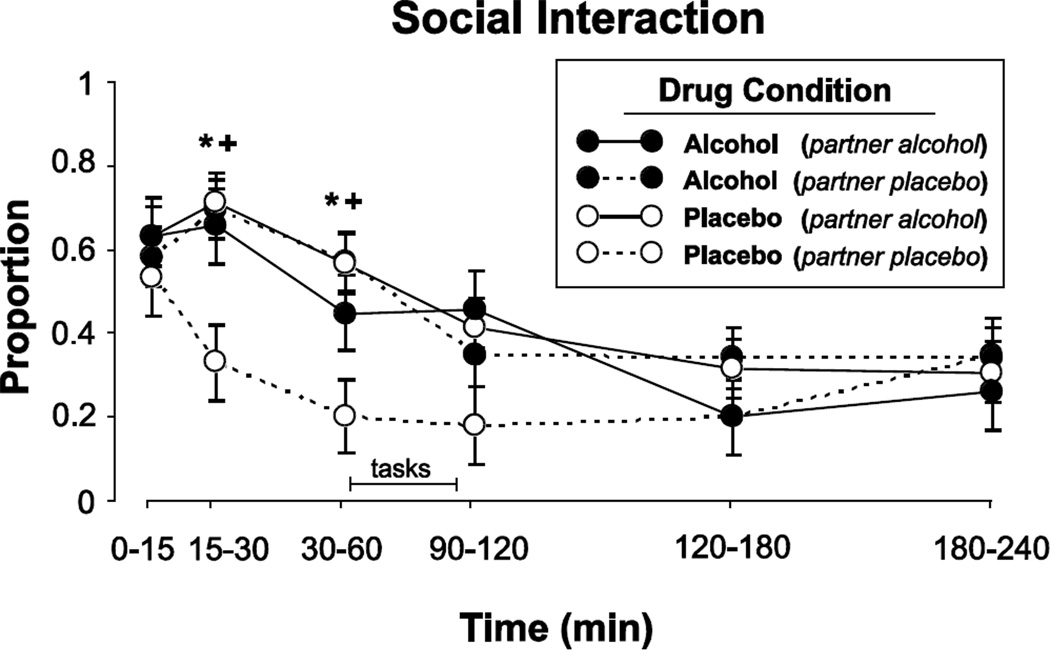
Alcohol increased participants’ level of social interaction during the ascending limb of the blood alcohol curve (Figure 4). Participants interacted more in the 60 minutes following the beverage if at least one of the pair received alcohol (F[1, 114.7– 116.8] = 10.5–11.8, p < 0.01 for all comparisons between 15 and 60 min after beverage). These effects were not apparent later in the session. Social interaction was highly correlated with verbal interaction, and the increased social interaction early in the session was primarily accounted for by an increase in talking (data not shown; F[1, 122.5–126.1] = 5.3–16.7, p < 0.05 for all comparisons). Participants rated their partners as being more emotionally responsive (PRQ Other) when the Partner received alcohol (Table 3; Main effect of Partner Dose: F[1, 51.30] = 4.5, p < 0.05) and they rated themselves as being more emotionally responsive (PRQ Self) when at least one of the pair received alcohol (Table 3; Main effects of Actor Dose and Partner Dose: F[1, 53.88–53.89] = 6.1–7.4, p < 0.05 for both tests). Finally, participants reported engaging in more agreeable behaviors (as measured by the SIQ) when they received alcohol, regardless of what the Partner received (Table 3; Main effect of Actor Dose: F[1, 69.53] = 4.5, p < 0.05). Neither alcohol nor the drug state of the partner altered ratings of attractiveness of the partner.
Figure 4.
Mean proportion of time during the sessions when participants interacted as a function of Time. Drug conditions are the same as described in Figure 3. Error bars represent one SEM. A = 0.8 g/kg alcohol; P = placebo. An * indicates A/A significantly different from A/P (p<0.05). A + indicates P/A significantly different from P/P (p<0.05).
Table 3.
Alcohol-related effects on social interaction questionnaires
| Drug Condition | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (partner placebo) |
Placebo (partner alcohol) |
Alcohol (partner placebo) |
Alcohol (partner alcohol) |
||||||
| Mean | (SEM) | Mean | (SEM) | Mean | (SEM) | Mean | (SEM) | ||
| PRQ Other | 41.8 | (2.9) | 47.2 | (2.6) | 46.9 | (2.5) | 50.2 | (2.8) | |
| PRQ Self | 45.6 | (2.8) | 51.7 | (2.5) | 51.7 | (2.5) | 55.5 | (2.7) | |
| Attractiveness (AQ) | 89.4 | (3.9) | 96.3 | (3.3) | 98.4 | (3.3) | 95.3 | (3.8) | |
| Social Interaction (SIQ) | |||||||||
| Agreeable | 6.2 | (0.4) | 6.7 | (0.4) | 7.2 | (0.4) | 7.0 | (0.4) | |
| Quarrelsome | 0.3 | (0.2) | 0.7 | (0.2) | 0.4 | (0.2) | 0.4 | (0.2) | |
| Submissive | 3.0 | (0.3) | 2.3 | (0.3) | 3.0 | (0.3) | 2.5 | (0.3) | |
| Dominant | 5.1 | (0.5) | 5.6 | (0.4) | 5.8 | (0.5) | 5.6 | (0.5) | |
PRQ = Perceived Responsiveness Questionnaire
End of Session Questionnaire
The drug state of the partner did not affect most of participants’ responses on the ESQ. However, participants reported a greater overall strength of drug effects after placebo when their partner received alcohol (AlcoholActor/PlaceboPartner = 1.9 ± 0.12 versus PlaceboActor/PlaceboPartner = 1.3 ± 0.13: F[1, 82.40] = 12.5, p < 0.001). The placebo beverage was identified correctly on 75% of occasions when Partner also received placebo, but only 50% of occasions when the Partner received alcohol. Identification of the alcohol beverage was relatively high on both sessions in the SOC condition (Supplementary Table 2).
Validity checks
Order effects
There were no significant effects of Session Order for any outcome measure.
Effectiveness of placebo manipulation
Most participants correctly identified placebo and alcohol at the end of the sessions (Supplementary Table 2). The two groups identified placebo correctly on 65% of sessions. The ISO participants correctly identified alcohol on 73% of sessions whereas SOC participants correctly identified alcohol on 92% of occasions. Despite these identifications at the end of the sessions, when the drugs’ full pharmacological effects had come and gone, there was some evidence that the blinding was partially effective during the sessions: After placebo, participants (both groups) reported significantly increased ratings of “Feel Drug” 30, 60, 90 and 120 min after consuming the beverage (change from pre-beverage; p < 0.01).
DISCUSSION
In this study, the presence of another individual influenced the acute subjective and behavioral effects of alcohol in several ways. First, participants reported greater feelings of intoxication and stimulation on single-item measures after alcohol when they were tested in the presence of another person, compared to when they were alone. However, most other measures, including cardiovascular effects of alcohol, other measures of stimulation, and negative subjective effects such as sedation and dizziness, ratings were unaffected by the presence of another individual. Second, in the social condition, participants were modestly influenced by the drug state of the partner. When the co-participant also received alcohol, participants experienced greater subjective stimulation as measured by the composite ARCI A scale. Participants also reported feeling more intoxicated on both placebo and alcohol sessions when the partner received alcohol, and they interacted more when at least one participant had received alcohol. This effect on social interaction extends earlier reports that social factors influence subjective response to alcohol (Doty and de Wit 1995; Pliner and Cappell 1974; Sher 1985) and that alcohol facilitates social interaction (e.g., Sayette et al. 2012a, b). Thus, the acute subjective effects of alcohol can be influenced by both the presence of another individual and the drug state of others who are present.
The presence of another individual altered some, but not all, of the effects of alcohol. Participants in the social condition reported greater positive subjective effects on single-item ratings of intoxication and stimulation and fewer negative effects as measured by the ARCI LSD, compared to participants receiving alcohol alone. However, the social condition did not affect responses to alcohol on cardiovascular measures or on most other subjective measures, including standardized composite measures of stimulant drug effects (ARCI A and BG). The lack of effect of social condition on heart rate responses to alcohol is relevant to recent theories that greater heart rate increases after alcohol are related to greater sensitivity to alcohol reward (Conrod et al, 2001). Although it is not clear why participants reported more stimulation on the single-item scale than on the ARCI A or BG scales, it may be that the single-item scale was a more sensitive measure of alcohol-related stimulation in the between-groups comparisons. Thus, alcohol was perceived to be slightly more subjectively stimulating in the social condition, but social condition did not affect the cardiovascular response, indicating a dissociation between these measures.
The present findings are generally consistent with previous studies. In our previous study (Doty and de Wit 1995) alcohol produced stronger and more positive subjective effects when participants were tested under social conditions, compared to when they were tested individually. In that study, participants were tested in groups of 3 or 4 who received alcohol or placebo in mixed order. Therefore, there were usually 1 or 2 other alcohol-treated individuals in a single testing session, making it difficult to assess the influence of the drug state of the other individuals present. In the present study we controlled the drug state of the paired participants, and found that both the presence of another person and the drug state of the other person influenced the results. The latter finding is consistent with older studies indicating that the presence of an intoxicated individual can affect behavior even of sober individuals (Sher et al, 1985). Interestingly this finding is also consistent with self-administration data with rats (Smith 2012), indicating that the drug state of another animal substantially alters acute drug response. We had some evidence (Doty and de Wit, 1995) that the presence of other intoxicated individuals also increased alcohol consumption, but the effect of social setting on alcohol choice and consumption is complex, and remains to be examined more carefully in future studies.
The state of intoxication of the Partner affected certain subjective responses of the participant, whether or not they received alcohol themselves. First, when participants received alcohol, they reported greater feelings of stimulation (i.e., increased ARCI A scores) when their Partner also received alcohol, compared to when their Partner received placebo. Second, participants reported feeling more ‘Drunk’, ‘High’, and ‘Social’ – regardless of whether they received alcohol or placebo – when their Partner had received alcohol. These results are consistent with data from previous studies, which suggest that social factors may alter the subjective, physiological and behavioral effects of a number of drugs including alcohol, amphetamine, and marijuana (Carlin et al. 1972; de Wit et al. 1997; Doty and de Wit 1995; Pliner and Cappell 1974). For example, Carlin and colleagues (1972) observed that participants self-reported greater marijuana-related intoxication and exhibited more cognitive performance disruptions in the presence of confederates who were trained to exhibit marijuana-like intoxication. Thus, the intensity of drug-related intoxication appears to be influenced by the perceived drug state of others. How this occurs is not clear, but it may involve modeling, social mimicry, expectancies, a desire to conform, or a disinhibition. These questions may be resolved in future studies in which confederates model different behaviors, or in which partners are treated with different classes of drugs.
Interestingly, the presence of an intoxicated partner also influenced the mood state of an individual who had received placebo. For instance, participants reported greater ratings of ‘Feel Drug’ and ‘Like Drug’ after placebo, when their co-participants received active drug. This is consistent with data from Sher (1985), who reported that sober participants tested in the presence of intoxicated participants reported levels of intoxication comparable to those reported by the alcohol-intoxicated participants. They also found that this effect was related to participants’ expectancies of how they typically experience alcohol-related effects. We did not assess participants’ expectancies of alcohol effects in our study. Participants were told that they might receive a stimulant, sedative, opioid, alcohol or placebo, and the placebo was made to resemble alcohol by adding a small amount of alcohol. Although, participants reported a significant increase in ‘Feel Drug’ during the placebo sessions, most participants correctly identified both alcohol and placebo at the end of the session after they had experienced the drugs’ full effects. Thus, there was some uncertainty about the identify of the drug during the sessions, but less uncertainty by the end of the sessions. The presence of an alcohol-treated partner had a modest effect on alcohol identification: In the SOC condition, 75% of participants labeled placebo correctly when they had a sober Partner, whereas 50% labeled it as placebo when their Partner was intoxicated (although it was not usually mis-identified as alcohol). It is possible that expectancies of any drug effect combined with an intoxicated co-participant increase subjective ratings. Future studies controlling participants’ expectancies more directly may differentiate the influence of expectancies from the direct influence of an intoxicated partner.
Alcohol increased both objective and subjective measures of social behavior. Alcohol increased the time the participants spent interacting and talking if at least one individual of the dyad received active drug. Similarly, alcohol in at least one of the pair increased ratings of emotional responsiveness by both individuals. We measured emotional responsiveness with a standardized rating scale of how emotionally sensitive participants considered themselves and their Partners to be (e.g. “…seems interested in what I am thinking and feeling”). However, these ratings of emotional responsiveness were significantly correlated with objective measures of how much participants interacted (r = 0.39), and so it is not clear whether this was an indirect consequence of the increased social interaction. Regardless, these findings are consistent with data showing that alcohol increases verbal behavior and enhances social bonding (Babor et al. 1983; Sayette et al. 2012a, 2012b). Indeed, in a recent study, Sayette and colleagues (2012a) reported that alcohol facilitated social bonding in previously unacquainted individuals. They suggested that the increase in social bonding might be related to the effect of increased positive affect, and reduced negative affect, on social interactions. Together with the current results, this suggests that alcohol produces prosocial effects that influence all those present, whether or not they are intoxicated.
Responses to alcohol, and most of the effects of social context on responses to alcohol, were similar in men and women. However, only women reported higher ratings of ‘Friendly’ following alcohol in the social condition compared to the isolated condition, suggesting that women may be more susceptible to the social enhancement effect than men. This sex difference was not observed on other measures in the comparisons between social and isolated settings. Contrary to some previous studies, we did not observe sex differences in responses to alcohol, overall. For example, Miller et al.(2009) reported that alcohol produced greater performance impairment and greater subjective intoxication in women than men, and Sayette et al (2012b) found that women exhibited more social behavior than men, although their social responses to alcohol were similar. The modest effects of sex in the current study may be related to the relatively small sample size of our study, or the testing conditions. It is also possible that greater social enhancement would be observed if participants were tested in opposite sex pairs.
The current study had several important limitations. First, our study was small (N=44) compared to other studies using as many as 720 participants (Sayette et al, 2012b). Second, we had only moderate success with the blinding of the alcohol and placebo beverages, and by the end of the session most participants correctly identified the placebo and alcohol beverages. Thus, participants’ expectancies of alcohol effects may have contributed to the observed social enhancement. In a future study, the inclusion of a control beverage (i.e., a known non-alcohol condition) might help to detect expectancy effects. Another limitation is the number and type of oucome measures that were obtained. We used a mix of single-item questionnaires and standardized instruments that have been validated for use with alcohol. It is possible that other scales, such as the more commonly used Biphasic Alcohol Effects Scale (Martin et al 1993) would yield slightly different results. Importantly, our measures of social interactions (i.e, ratings of videotapes) were less refined than measures used by other investigators (e.g., Sayette et al, 2012b). More detailed assessments of participants’ social interactions would shed light on the processes underlying the enhancement by alcohol. There are also limitations relating to the social context we created, and the activities that participants engaged in. For example, we allowed participants to watch television, which is difficult to classify as either a social or nonsocial activity. The social context differed from most naturalistic drinking conditions insofar as we tested pairs of strangers in an unfamiliar environment, and the alcohol dose was fixed. Finally, the significance of the study would be strengthened by the inclusion of a measure of alcohol consumption, to complement the changes in alcohol-induced mood states. Although many of the methodological features were necessary, and similar to other laboratory studies, the generalizability of the findings remains to be determined.
In conclusion, we have extended our understanding of the interaction between alcohol and social context in several ways. That is, we found that the presence of another individual enhanced some subjective responses to alcohol but not other subjective or cardiovascular effects. We also found that the presence of an intoxicated individual enhanced subjective responses to alcohol, and the mood state of a non-intoxicated participant. Alcohol increased verbal behavior when at least one of the pair received alcohol, and participants rated themselves and others as more emotionally responsive when at least one had consumed alcohol. Together, these findings further highlight the potential importance of social factors in acute drug effects and may partially explain why alcohol is used in a social context. Future studies may examine the extent to which these alterations in subjective states influence consumption of the drug.
Supplementary Material
Acknowledgements
The assistance of Lindsey Davis, Justin Birnholz, and Michael Helzer are gratefully acknowledged. This research was supported by grant number DA002812 from the National Institute on Drug Abuse.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
REFERENCES
- Acosta MC, Eissenberg T, Nichter M, Nichter, Balster R. Characterizing early cigarette use episodes in novice smokers. Addictive Beh. 2008;33:106–121. doi: 10.1016/j.addbeh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Anacker AM, Ryabinin AE. Biological contribution to social influences on alcohol drinking: evidence from animal models. Int J Environ Res Public Health. 2010;7:473–493. doi: 10.3390/ijerph7020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- aan het Rot M, Moskowitz DS, et al. Social behaviour and mood in everyday life: the effects of tryptophan in quarrelsome individuals. J Psychiatry Neurosci. 2006;31:253–262. [PMC free article] [PubMed] [Google Scholar]
- Aan het Rot M, Russell JJ, Moskowitz DS, Young S. Alcohol in a social context: findings from event-contingent recording studies of everyday social interactions. Alcohol: Clin Exper Res. 2008;32:549–471. doi: 10.1111/j.1530-0277.2007.00590.x. [DOI] [PubMed] [Google Scholar]
- Babor TF, Berglas S, Mendelson JH, Ellingboe J, Miller K. Alcohol, affect, and the disinhibition of verbal behavior. Psychopharmacol (Berl) 1983;80:53–60. doi: 10.1007/BF00427496. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacol (Berl) 2012;224:1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin AS, Bakker CB, Halpern L, Post RD. Social facilitation of marijuana intoxication: impact of social set and pharmacological activity. J Abnorm Psychol. 1972;80:132–140. doi: 10.1037/h0033317. [DOI] [PubMed] [Google Scholar]
- Caudill BD, Marlatt GA. Modeling influences in social drinking: an experimental analogue. J Consult Clin Psychol. 1975;43(3):405–415. doi: 10.1037/h0076689. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology. 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Cooper AM, Waterhouse GJ, Sobell MB. Influence of gender on drinking in a modeling situation. J Stud Alcohol. 1979;40(7):562–570. doi: 10.15288/jsa.1979.40.562. [DOI] [PubMed] [Google Scholar]
- de Wit H, Clark M, Brauer LH. Effects of d-amphetamine in grouped versus isolated humans. Pharmacol Biochem Behav. 1997;57(1–2):333–340. doi: 10.1016/s0091-3057(96)00316-4. [DOI] [PubMed] [Google Scholar]
- Doty P, de Wit H. Effect of setting on the reinforcing and subjective effects of ethanol in social drinkers. Psychopharmacol (Berl) 1995;118(1):19–27. doi: 10.1007/BF02245245. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women: the role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Green KA, Mourgues P. Longitudinal investigation of methamphetamine use among gay and bisexual men in New York City: findings from Project BUMPS. J Urban Health. 2005;82:18–25. doi: 10.1093/jurban/jti020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology. 2001;155:171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Stitzer ML. Effects of alcohol on speaking in isolated humans. Psychopharmacol (Berl) 1988;95:189–194. doi: 10.1007/BF00174508. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Stitzer ML. Monologue speech: Effects of d-amphetamine, secobarbital and diazepam. Pharmacol Biochem Behav. 1989;34:609–618. doi: 10.1016/0091-3057(89)90567-4. [DOI] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Individual differences in responses to ethanol and d-amphetamine: a within-subject study. Alcohol Clin Exp Res. 2001;2:540–548. [PubMed] [Google Scholar]
- Hostetler CM, Anacker AM, Loftis JM, Ryabinin AE. Social housing and alcohol drinking in male-female pairs of prairie voles (Microtus ochrogaster) Psychopharmacology (Berl) 2012;224:121–132. doi: 10.1007/s00213-012-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Ryabinin AE. Love and addiction: the devil is in the differences: a commentary on “the behavioral, anatomical and pharmacological parallels between social attachment, love and addiction”. Psychopharmacology (Berl) 2012;224:27–29. doi: 10.1007/s00213-012-2858-y. [DOI] [PubMed] [Google Scholar]
- Janowsky DS. Depression and dysphoria effects on the interpersonal perception of negative and positive moods and caring relationships: effects of antidepressants, amphetamine, and methylphenidate. Current Psychiatry Reports. 2003;5:451–459. doi: 10.1007/s11920-003-0084-3. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Kraft A, Clopton P, Huey L. Relationships of mood and interpersonal perceptions. Comprehensive Psychiatry. 1984;25:546–551. doi: 10.1016/0010-440x(84)90035-x. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H. Acute effects of D-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav. 2000;66:509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Kairouz S, Greenfield TK. A comparative multi-level analysis of contextual drinking in American and Canadian adults. Addiction. 2007;102:71–80. doi: 10.1111/j.1360-0443.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Levin FR, Foltin RW, Hart CL. Acute and residual interactive effects of repeated administrations of oral methamphetamine and alcohol in humans. Psychopharmacology. 2012;219:191–204. doi: 10.1007/s00213-011-2390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone GF, Pardo JS, Krauss RM, Hart CL. Amphetamine analogs methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) differentially affect speech. Psychopharmacol (Berl) 2010;208:169–177. doi: 10.1007/s00213-009-1715-0. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McCroskey JC, McCain TA. The measurement of interpersonal attraction. Speech Monographs. 1974;41:261–266. [Google Scholar]
- Morean MEH, de Wit AC, King M, Sofuoglu SY, Rueger SS, O’Malley The Drug Effects Questionnaire: Psychometric support across three drug types. Psychopharmacology. 2013;227:177–192. doi: 10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Peartree NA, Pentkowski NS. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacol (Berl) 2012;224:33–56. doi: 10.1007/s00213-012-2853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliner P, Cappell H. Modification of affective consequences of alcohol: a comparison of social and solitary drinking. J Abnorm Psychol. 1974;83:418–425. doi: 10.1037/h0036884. [DOI] [PubMed] [Google Scholar]
- Reis HT. A self-report measure of perceived partner responsiveness. University of Rochester. 2003 [Google Scholar]
- Rodgers J, Buchanan T, Pearson C, Parrott AC, et al. Differential experiences of the psychobiological sequelae of ecstasy use: quantitative and qualitative data from an internet study. Journal of Psychopharmacology. 2006;20:437–446. doi: 10.1177/0269881105058777. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Creswell KG, Dimoff JD, Fairbairn CE, Cohn JF, Heckman BW, Kirchner TR, Levine JM, Moreland RL. Alcohol and group formation: a multimodal investigation of the effects of alcohol on emotion and social bonding. Psychol Sci. 2012a;23:869–878. doi: 10.1177/0956797611435134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Dimoff JD, Levine JM, Moreland RL, Votruba-Drzal E. The effects of alcohol and dosage-set on risk-seeking behavior in groups and individuals. Psychol Addict Behav. 2012b;26:194–200. doi: 10.1037/a0023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ. Subjective effects of alcohol: the influence of setting and individual differences in alcohol expectancies. J Stud Alcohol. 1985;46:137–146. doi: 10.15288/jsa.1985.46.137. [DOI] [PubMed] [Google Scholar]
- Single E, Wortley S. Drinking in various settings as it relates to demographic variables and level of consumption: Findings from a national survey in Canada. J Stud Alcohol. 1993;54:590–599. doi: 10.15288/jsa.1993.54.590. [DOI] [PubMed] [Google Scholar]
- Smith MA. Peer influences on drug self-administration: Social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacol (Berl) 2012;224:81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutker PB, Tabakoff B, Goist KC, Randall CL. Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacol Biochem Behav. 1983;18:349–354. doi: 10.1016/0091-3057(83)90198-3. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Garner MJ, Munafo MR, de Wit H. Amphetamine as a social drug: effects of d-amphetamine on social processing and behavior. Psychopharmacology (Berl) 2012;223:199–210. doi: 10.1007/s00213-012-2708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DW, Sobell MB. Social influences on alcohol consumption by black and white males. Addict Behav. 1982;7(1):87–91. doi: 10.1016/0306-4603(82)90031-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.