Abstract
Despite its proximity to the fecal flora, the urinary tract is considered sterile. The precise mechanisms by which the urinary tract maintains sterility are not well understood. Host immune responses are critically important in the antimicrobial defense of the urinary tract. During recent years, considerable advances have been made in our understanding of the mechanisms underlying immune homeostasis of the kidney and urinary tract. Dysfunctions in these immune mechanisms may result in acute disease, tissue destruction and overwhelming infection. The objective of this review is to provide an overview of the innate immune response in the urinary tract in response to microbial assault. In doing so, we focus on the role of antimicrobial peptides – a ubiquitous component of the innate immune response.
Keywords: Urinary Tract Infection, Pyelonephritis, Innate Immunity, Antimicrobial Peptides, Toll-like Receptors, Chemokines, Cytokines
Introduction
Urinary tract infections (UTI) are one of the most common and serious bacterial infections encountered by pediatricians [1]. Estimates on the cumulative incidence of UTIs in American children indicate that up to 180,000 of the annual birth cohort will be diagnosed with a UTI by 6 years of age (3–7% of girls and 1–2% of boys) [2,3]. Children diagnosed with UTIs account for over 500,000 emergency department visits, 50,000 hospitalizations and 1 million annual office visits, resulting in more than $8 billion of healthcare expenditure [4,5]. Long-term complications of UTIs include renal scarring, hypertension and chronic kidney disease. No treatment strategy to date has proven to be particularly effective in the prevention of UTI sequelae. Moreover, antibiotic resistance in uropathogenic bacteria has been increasing, in large part due to antibiotic overuse. Therefore, it is imperative that we seek a greater understanding of UTI pathogenesis and how the body defends the urinary tract against microbial insult so that we can learn to augment endogenous defense mechanisms to develop new, targeted UTI treatment options to improve patient care and reduce healthcare costs and co-morbidities.
Escherichia coli (E. coli) is the bacterial pathogen most frequently responsible for UTI and pyelonephritis. Uropathogenic E. coli (UPEC) are postulated to originate in the rectal flora, spread across the perineum, and enter the bladder via the urethra. Before invading the urothelium, UPEC must overcome several intrinsic characteristics of the urinary tract to cause an infection. Proposed functional mechanisms contributing to defense of the urinary tract include barrier formation, mucous production, the urinary microbiome, regular bladder emptying, urine flow and alterations in urine characteristics (Table 1) [6–9].
Table 1.
Urine parameters influencing UTI susceptibility
| Urine parameter | UTI relevance |
|---|---|
| pH | Optimal bacterial growth in urine occurs between a pH 6–7. |
| Urea | High urea concentrations are associated with inhibition of bacterial growth. |
| Glucose | Glucosuria can promote bacterial growth. |
| Calcium | Idiopathic hypercalcuria increases the risk of UTIs. |
| Iron | Increased iron promotes bacterial growth in urine. |
| Osmolality | Bacterial growth is inhibited when the urine osmolality is < 200 mosm/L or > 1200 mosm/L |
The microbial virulence of UPEC has been linked to many factors [10–12]. The most prominent is Type I fimbriae, which are filamentous bacterial appendages that are capped by FimH, a mannose-binding adhesion protein. Type 1 fimbriae promote tight bacterial binding to the matrix of uroplakin complexes on the surface of superficial bladder epithelial cells [11,13]. Similarly, the presence of flagella enhances virulence by permitting the organism to migrate toward the urothelium against the flow of the urinary stream [12]. After binding, UPEC invades the uroepithelium where it may either establish a state of commensalism or cause a severe, symptomatic infection characterized by a rapid innate host response with cytokine secretion, recruitment of immune cells to the site of infection and successful elimination of bacteria, or progressive disease with acute tissue destruction [14]. The antibacterial defense of the urinary tract relies almost entirely on innate immunity.
The highly conserved innate immune response provides front-line defense against microbial insult and leads to subsequent activation of the adaptive immune system. In contrast to the adaptive immune response, which develops over a period of days and confers long-lasting immunity, the innate immune response provides immediate defense against infection. The innate immune response is not based on the recognition of specific antigens and, therefore, is less specific than the adaptive immune system. Innate immunity encompasses a variety of diverse components that are constitutively active or rapidly induced – including barriers like epithelial surfaces and intracellular tight junctions, sensors of commensal and pathogenic microorganisms, cells that release inflammatory mediators and cytokines, phagocytic cells, inflammation-related serum proteins, and antimicrobial molecules [15,16]. These elements work in concert to ensure that the magnitude of the host response reflects the severity of the microbial threat (Figure 1). In the urinary tract, alterations in these immune mechanisms may result in acute disease that can lead to uroepithelial tissue destruction, parenchymal scarring or overwhelming infection.
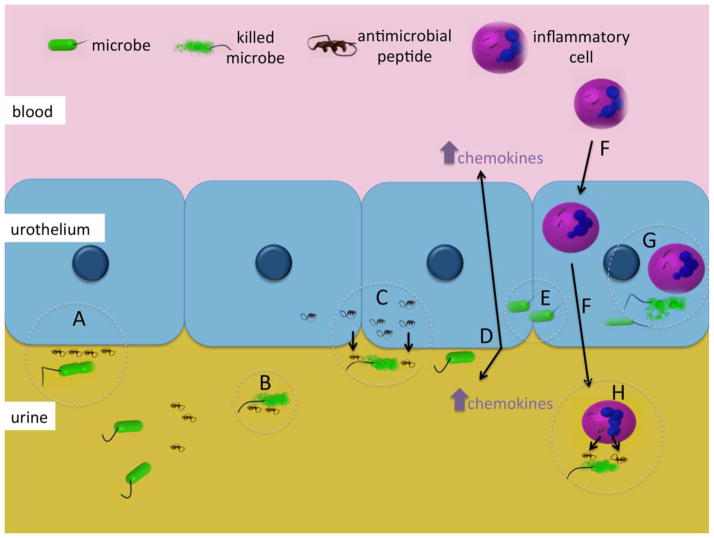
Figure 1. Innate immune mechanisms in the urinary tract.
Microbes enter the urinary tract and encounter constitutively expressed AMPs that can inhibit attachment to the urothelium (A) or cause bacterial lysis (B). If bacteria attach to the urothelium, they can induce AMP production, which results in destruction of adherent bacteria (C). When bacteria bind and invade the urothelium, they can elicit the production of chemokines (D/E) that attract inflammatory cells across the urothelium (F). These cells control infection by phagocytosis (G) and secretion of intracellular AMPs (H) [14,45,46].
In this review, we focus on how the innate immune response maintains urinary tract sterility. In doing so, we describe how microbial insult triggers toll-like receptors to activate host response mechanisms through chemokines, cytokines and inflammatory cells. Additionally, we focus on the role of antimicrobial peptides and discuss how they may be utilized as a promising new class of endogenous antibiotics that may overcome the limitations of traditional therapies and the emerging problems of antibiotic resistance. This review complements the 2012 review in this journal by Ragnarsdottir et al where they described how bacterial virulence factors and genetic variations in the host immune response influence UTI susceptibility [10].
Toll-like receptors and their importance in urinary tract defense
If uropathogens breach the physical barriers of the urothelium, they are recognized by toll-like receptors (TLR) that mobilize the immune responses of the bladder and kidney epithelial cells [17]. TLRs are transmembrane proteins with multiple leucine-rich repeats that are responsible for the recognition of pathogen-associated molecular patterns (PAMPs) – highly conserved molecules expressed by invading pathogens. The activation of TLRs by uropathogens activates several signaling pathways that induce a robust immune response through the expression of pro-inflammatory cytokines, chemokines, interferon signaling and the production of antimicrobial peptides (Figure 2). The common TLRs encountered in the urinary tract include TLR2 (recognizes bacterial lipoteichoic acid or lipoprotein), TLR3 (recognizes double stranded RNA), TLR4 (recognizes lipopolysaccharides), TLR5 (recognizes flagellin), TLR9 (recognizes unmethylated DNA of bacteria and viruses) and TLR11 (recognizes parasites) [16,18].
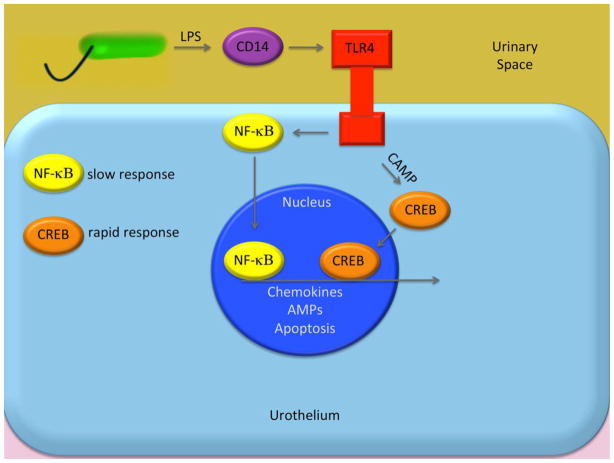
Figure 2. Signaling pathway of toll-like receptors in urinary tract infection.
Activation of TLRs expressed on the cell membrane by a bacterial ligand sets in motion a series of processes that leads to the release of inflammatory chemokines, cytokines and AMPs [14,19].
Among the TLRs that are expressed by the urothelium, only TLR4, TLR5, and TLR11 have been shown to participate in vivo in defense against bacterial infection [18,19]. Perhaps the most studied of the TLRs is TLR4, which is expressed on the epithelial cells of the kidney and bladder. TLR5 is predominantly expressed on bladder cells whereas TLR11 is primarily on kidney cells. Animal models that do not express TLR4, TLR5, TLR11 or have defective forms of these receptors demonstrate that these animals have difficulty clearing infection in different regions of the urinary tract [19–25].
Svanborg et al have extensively studied and reviewed the role of TLR4 in the context of UTI [10,22,26,27]. The importance of TLR4 in the defense against UTI in humans was confirmed in patients with asymptomatic bacteriuria, who exhibit a marked decrease in TLR4 expression [19,28]. Impaired bacterial clearance associated with defective TLR4 is likely a result of limited local cytokine, chemokine and neutrophil immune response, along with decreased ability of the receptor to mediate other antimicrobial activities in the urinary tract [18]. Additional studies suggest that TLR4 is also involved in bladder cytoprotection. When TLR4 is activated, it stimulates changes in renal cyclooxygenase-2, which is responsible for the formation of inflammatory prostanoids (i.e. prostaglandin) that participate in the regulation of bladder mucous barrier and cytoprotection [18,29].
The cytokine cascade and microbial clearance
TLR activation by uropathogens mobilizes a robust urothelial cytokine response. This cytokine response serves as the signal for recruitment of other aspects of the innate immune system and the inflammatory response. TLR4 activation by Gram-negative bacteria leads to increased transcription of IL-6 and IL-8 through multiple mechanisms [16]. IL-8 in turn acts as a chemoattractant for neutrophils by interacting with the receptors CXCR1 and CXCR2 on the surface of epithelial cells and neutrophils [10,30]. Furthermore, IL-8 promotes chemotaxis and transepithelial infiltration to infected sites. IL-6 activates B-cells to produce IgA and stimulates C-reactive protein production to further augment the inflammatory cascade [10]. IL-10 also increases in the urine of patients with UPEC cystitis, though its role during infection is not known. IL-10 deficient mice have higher bacterial loads in the bladder, urine and kidney compared to controls [31]. Other cytokines/chemokines have been implicated in various UTI models and patient populations including IL-1, RANTES/CCL5, MCP-1, TNF-α, IFN-α [10,32].
The roles of cytokine and cytokine receptor genes in UTI susceptibility have been elusive. While certain polymorphisms have been implicated in many different populations and patient cohorts, no global genetic variations in cytokines and their receptors have been identified as causative. Because of the multifactorial nature of the disease process and host response, many genetic variations have been implicated as associated with an increased risk for various infections of the urinary tract [10]. They include the following:
A SNP in the IL-8 promoter region was associated with an increased risk of severe kidney parenchymal infection [30]. This genotype was also associated with increased IL-8 levels in stool [33].
Genetic polymorphisms in the IL-8 receptors CXCR1 and CXCR2 result in decreased mRNA levels in patients with acute pyelonephritis or recurrent UTI [34,35]. Further, multiple variations have been associated with UTI susceptibility in children with VUR and premenopausal women [36].
RANTES polymorphism (also known as CCL5) that leads to higher transcriptional activity was associated with a lower risk of upper tract UTI. The exact mechanism of how this SNP is protective is not known [37].
In patients with rheumatoid arthritis being treated with methotrexate or the TNF inhibitor etanercept, a specific SNP in the pro-inflammatory cytokine TNF was associated with a statistically significant increased risk in UTI [38]. Interestingly, in ex vivo studies, TNF blockade inhibited TLR4 expression in dendritic cells. Whether etanercept inhibits TLR4 expression in uroepithelial cells is unknown.
These studies demonstrate that certain genetic polymorphisms are associated with both an increased UTI risk and a protective effect in different patient cohorts. A seemingly paradoxical relationship exists between IL-8 and IL-8 receptors. For example, a variation that results in increased IL-8 levels is associated with increased UTI risk, yet variations that result in decreased transcription in the IL-8 receptors (CXCR1 and CXCR2) seem protective. These findings underscore the multifactorial nature of the innate immune response. In addition, they highlight the need for a greater understanding of UTI pathogenesis as we strive to develop better management and therapeutic strategies.
The leukocyte response to uropathogens
The host leukocyte response to uropathogens is incompletely characterized, particularly in the upper urinary tract. Moreover, the roles of leukocytes in human patients with acute and chronic UTI remain largely undefined. Our understanding of the role(s) of leukocytes in eradicating UTI is mainly inferred from studies of inbred mice inoculated transurethrally with large bacterial burdens of UPEC (107–109 colony forming units/milliliter). The murine model affords simultaneous characterization of leukocyte populations in blood, urine, bladder tissue and kidney tissue. Multiple studies using this model have demonstrated that innate myeloid cells play an important role in the clearance of uropathogens.
Several hours following UPEC inoculation, a neutrophil-predominant leukocyte population is present within the urinary space of the kidney and bladder [34,39]. Microbial challenge triggers neutrophil chemotaxis via the interaction of chemokines like IL-8 with its receptors, Cxcr1 and Cxcr2. Mice deficient in the IL-8 receptor exhibit delayed neutrophil transmigration across the urothelium, increased UTI susceptibility, renal abscess formation and bacteremia [34]. Similarly, neutrophil depletion by genetic disruption of chemokine/receptor interactions critical for chemotaxis or with the use of lineage specific antibodies results in increased bacterial burden in the bladder and kidneys [34,39]. The kidneys also contain a resident network of CD11c(+) dendritic cells (DC), which have been shown to infiltrate the bladder in response to UPEC inoculation [40]. To establish the relative importance of CD11c(+) DC in murine cystitis and pyelonephritis, DCs were conditionally depleted prior to transurethral UPEC challenge [40,41]. While DC depletion exerted no affect on UPEC burden in the bladder, the kidneys of these mice exhibited transiently higher bacterial burden, associated with decreased neutrophil recruitment [41]. Results from these studies clearly establish a central role for neutrophils in UPEC clearance.
To date, the role of T and B cells in UPEC clearance has not been well defined. Hopkins et al demonstrated that anti-E. coli neutralizing antibodies are induced following UTI – indicating that an adaptive immune response has been initiated [42]. Moreover, severe combined immunodeficiency mice devoid of functional T and B cells identified higher bacterial counts in bladder and kidneys seven days after inoculation compared to immunocompetent controls [43]. Finally, by labeling bacteria with the ovalbumin (OVA) peptide and the use of OVA tetramers to identify antigen specific CD4(+) T cells, Thumbikat et al demonstrated that a T cell response to bacteria occurs, is transplantable and plays a role in limiting infections, supporting a role for T lymphocytes in preventing recurrent UTI [44]. These findings may have relevance to individuals with UTI and acquired deficiencies in T and B cell function, such as patients with HIV/AIDS and kidney transplant recipients.
Antimicrobial peptides and urinary tract sterility
Antimicrobial peptides (AMPs), the primary effector of the innate immune system, are natural antibiotics produced by nearly all organisms [45,46]. AMPs are small cationic proteins expressed by phagocytic white cells and epithelial cells either constitutively or via induction by invading pathogens. Certain AMPs exhibit a narrow antimicrobial spectrum, while others show broad-spectrum antimicrobial activity against bacteria, enveloped viruses, fungi and protozoa. To date, we have an incomplete understanding of the antimicrobial mechanisms behind AMP function, limiting our understanding of how to develop AMPs as a novel class of antibiotics [45].
Current evidence indicates that the antimicrobial activity of AMPs relates to their net charge, secondary structure and amphipathicity [45,47,48]. As the peptide’s net charge increases, its electrostatic attraction to the negatively charged microbial membrane increases. Amphipathicity refers to the relationship between the AMP’s hydrophilic and hydrophobic amino acid residues – which allows it to remain in solution in hydrophilic environments as well as interact with hydrophobic cell membranes [46]. Increased amphipathicity, as estimated by the hydrophobic moment, allows an AMP to partition into the membrane lipid bilayer. Secondary structure refers to the dimensional topography of the peptide and can influence its antimicrobial activity. These properties govern how AMPs bind to microbial cell membranes and prevent bacterial attachment, stimulate other components of innate immunity, or permeabilize the cell membrane. Predominantly, AMPs act by disrupting the integrity of bacterial cell membranes through the interactions of their cationic domains with the negatively charged microbial cell surface components. However, some AMPs inhibit intracellular protein/DNA synthesis by membrane translocation (Figure 3). As long as AMPs retain their cationic charge and amphipathicity, they retain their antimicrobial activity [46].
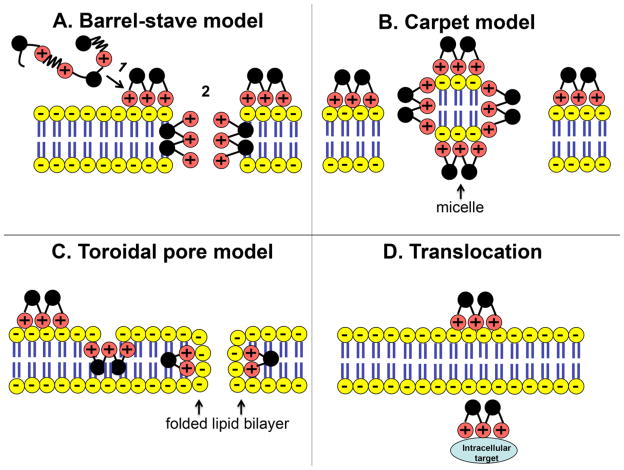
Figure 3. Proposed antimicrobial mechanisms of antimicrobial peptides.
AMPs can kill bacteria by disrupting the microbial membrane (A–C) or translocating across the membrane and binding to intracellular targets (D). Models of membrane disruption include the following: (A) Barrel-stave model: (A1) Cationic AMPs (+) bind to the negatively charged bacteria lipid bilayer (−) and disrupt the microbial membrane by forming an aqueous channel or “barrel-stave” (A2). (B) Carpet Model: AMPs blanket the microbial membrane and disrupt it by forming micelles. (C) Torodial Pore Model: AMPs bind to phospholipid head group on the microbial membrane allowing its hydrophobic portion to intercalate into the microbial membrane and cause the lipid bilayer to fold back on itself [50].
Because pathogenic bacteria are susceptible to endogenous AMPs, AMPs have been considered as a possible therapeutic agent against drug resistant organisms. AMPs have many desirable features of a novel antibiotic class. 1) AMPs display antimicrobial activity at low micromolar concentrations. 2) Microbial resistance to AMPs is limited as microbes cannot significantly alter their cell wall targets or modify their cell wall composition. 3) AMPs overcome the shortfalls of antibiotics given their ability to permeabilize microbial membranes [49]. 4) AMPs show synergy with conventional antibiotics and may be engineered to augment their targets [50].
Despite their wide distribution throughout nature, very few AMPs have been described in the human kidney and urinary tract. AMPs described in the urinary tract include defensins, cathelicidin, hepcidin, and ribonuclease 7. Other proteins that possess antimicrobial properties found in the kidney and urinary tract include Tamm-Horsfall protein, lactoferrin, lipocalin and secretory leukocyte proteinase inhibitor (Figure 4) [45,46,51].
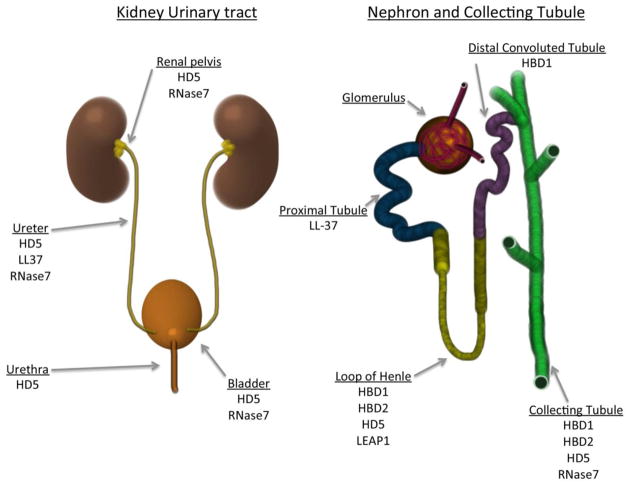
Figure 4. Defined antimicrobial peptides in the human kidney and urinary tract.
Left Panel: AMPs identified in the human lower and upper urinary tract.
Right Panel: AMPs identified in the nephron and collecting tubule of the human kidney [45].
Defensins
In humans and other mammals, defensins are one of the most studied families of AMPs. Defensins typically have broad-spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria, viruses, fungi and protozoa [52]. Along with their direct antimicrobial properties, defensins play a role in cell-mediated immunity as chemoattractants for immature dendritic cells [46]. Defensins are initially synthesized as preproproteins and undergo processing to become mature, biologically-active peptides. In humans, defensins are classified into one of two families depending on their disulfide- bridging pattern – the α-defensins or the β-defensins [53]. The clusters of genes encoding the α-defensin subfamily and the majority of the β-defensin subfamily are located on chromosome 8p22 and 8p23. Many defensin genes in these chromosome regions have variations in copy number ranging from 2 to 14 per diploid genome [45,54].
α-defensins
Human neutrophil peptides (HNPs) are α-defensins produced in the bone marrow within promyelocytes [55]. There are four identified HNPs, named HNP1 through HNP4. The HNPs are stored within the primary (azurophil) granules of neutrophils, where they provide non-oxidative antimicrobial activity. HNPs encounter pathogens after they are secreted onto the cell’s surface via degranulation or after a pathogen undergoes phagocytosis and the phagocytic vacuole fuses with the neutrophilic granule [45,46,55]. Regarding the urinary tract, Ihi et al demonstrated increased urinary levels of HNP1-3 in the setting of UTI [56]. Similarly, Tikhonov et al demonstrated that urinary HNP1 increased concomitantly with urinary IL-8 in patients with chronic pyelonephritis [57]. These findings may reflect the recruitment of neutrophils to the site of infection.
In contrast to the HNPs, mucosal epithelial cells produce human α-defensin 5 (HD5). The expression and function of α-defensin HD5 has mostly been reported in the small intestine where it is secreted by Paneth cells into the intestinal crypts and contributes to the balance of intestinal microbiotica [58]. HD5 has also been described in the male and female reproductive tracts, with evidence suggesting that it is inducible and important in eradicating infection [59,60]. HD5 has antibacterial activity against common uropathogenic Gram-positive bacteria and Gram-negative bacteria. HD5 also has antimicrobial activity against uropathogenic viruses like adenovirus and BK virus [61,62].
In the urinary tract, our research group has demonstrated that the gene encoding HD5, DEFA5, is constitutively expressed in the uroepithelium of the kidney, ureter and bladder. Expression increases from the upper urinary tract to the lower urinary tract, following the flow of the urinary stream [63]. Additionally, both gene expression and peptide production are induced with UTI. Secreted HD5 was not routinely detected in culture negative urine samples; however, HD5 levels significantly increased in urine samples infected with E. coli. Although HD5 did not reach urinary concentrations likely to be directly antimicrobial against common uropathogens, mucosal surface concentrations may be higher [63]. Other research groups have demonstrated that urinary HD5 is also detected in patients who have undergone ileal neobladder reconstruction and ileal conduit urinary diversion [64,65]. This data not only demonstrates an apparent role of HD5 in the innate immunity of the urinary tract, but it also suggests there may be potential to utilize HD5 as a diagnostic biomarker for UTI.
β-defensins
The human β-defensins are widely expressed in human epithelia and are active against Gram-positive and Gram-negative bacteria [45]. The β-defensin gene locus is subject to both copy number variations and single nucleotide polymorphisms, which may affect host antimicrobial defense. Of the β-defensins, human β-defensin-1 (HBD1) and human β-defensin-2 (HBD2) have been described in the human urinary tract. HBD1 mRNA is constitutively expressed by the epithelial lining of the loop of Henle, distal tubule and collecting duct. HBD1 peptide is constitutively detected in the urine [66]. Although urinary levels of HBD1 are insufficient to kill invading bacteria, HBD1 may provide a fast-acting antimicrobial coating of tubular lumens and prevent infection by inhibiting bacterial attachment to the urothelium and serving as a chemical shield [66]. Recent studies indicate that the redox-state of HBD1 significantly affects its antimicrobial potency, such that the reduced peptide is much more potent than the disulfide-linked oxidized form [67]. The redox state of HBD1 in the naïve and infected urinary tract has not been described. A role of HBD1 in urinary tract host defense is suggested by the finding that mice lacking the gene for beta defensin 1 (Defb1 −/−) have an increased probability of bacteriuria compared to non-mutant littermates [68]. Unlike HBD1, HBD2 is not constitutively expressed in non-infected kidneys. With pyelonephritis, HBD2 expression increases in the loops of Henle, distal tubules and collecting ducts of chronically infected kidneys [69].
Cathelicidin
Human cathelicidin (LL-37), encoded by the gene CAMP, is expressed in circulating neutrophils, myeloid bone marrow cells and epithelial cells. Cathelicidin possesses antimicrobial activity against Gram-positive and Gram-negative bacteria as well as viruses, and it acts as a chemoattractant for neutrophils and monocytes by interacting with their fMLP-receptors. Chromek et al demonstrated that in the urinary tract cathelicidin is expressed in the proximal tubule and the urothelium of the renal pelvis and ureter. When challenged by uropathogenic E. coli, uroepithelial cells secrete cathelicidin into the urinary space/tubular lumen [70]. When mice deficient in Cramp (the ortholog of cathelicidin) were infected with UPEC, the uroepithelium was significantly compromised, suggesting that cathelicidin plays an important role as a first line of mucosal defense. During later stages of inflammation, invading leukocytes serve as the main source of the peptide. Thus, cathelicidin appears to participate in both epithelial antimicrobial defense, recruitment of immune cells and neutrophil killing of pathogens [70].
Hepcidin
Hepcidin, also known as liver-expressed antimicrobial peptide-1 (LEAP-1), is produced in the liver and excreted in the urine. Hepcidin has broad-spectrum antimicrobial activity and plays an important role in iron homeostasis. Genetically modified mice engineered to over express hepcidin died shortly after birth with severe iron deficiency, supporting a central role in iron regulation. Therefore, hepcidin participates in the innate immunity of the urinary tract by both direct antimicrobial activity and reduction of available iron, which is an essential nutrient for pathogens [45,71,72].
Ribonuclease 7
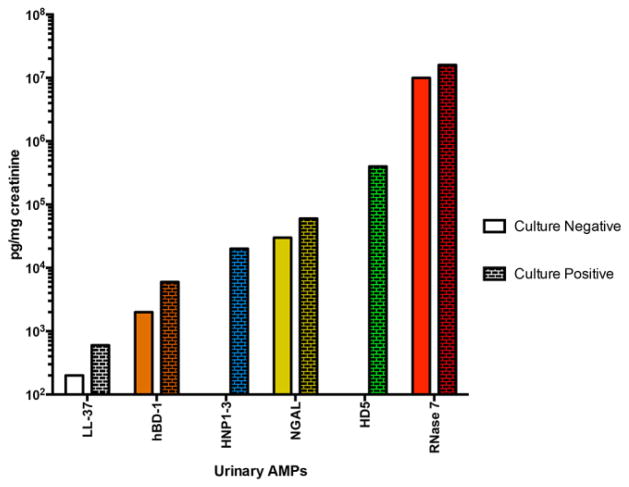
Recently, our research group has demonstrated that Ribonuclease 7 (RNase 7) is a potent AMP that contributes to maintaining sterility in the human urinary tract [73–75]. RNase 7 was first identified as an AMP in the epidermis [76]. The uroepithelium of the bladder and the ureter and the intercalated cells of the renal collecting tubule constitutively express RNase 7. Urinary levels of RNase 7 are significantly greater than other AMPs, reaching concentrations that are sufficient to kill bacteria (Figure 5). When urinary RNase 7 was neutralized in human urine specimens in vitro, urinary bacterial growth increases [75]. RNase 7 exhibits potent, rapid antimicrobial activity against common Gram-positive and Gram-negative uropathogens by disrupting microbial cell membranes at low micromolar concentrations [74]. Specifically, distinct regions of the peptide appear to be responsible for activity against various pathogens [77,78]. However, the mechanisms for RNase 7’s antimicrobial properties are not completely understood. Its bactericidal activity has been linked to its capacity to permeate and disrupt the bacterial cell membrane, which is an action independent of its ribonuclease activity. It has been stated that on a per molar basis, RNase 7 is the most potent human AMP [79].
Figure 5. Urinary antimicrobial peptide concentrations.
Mean urinary AMP levels in culture negative and culture positive urine samples [74].
Proteins with antimicrobial activity
In addition to the AMPs, there are other proteins described in the urinary tract that participate in host defense. Tamm-Horsfall protein (THP) is secreted by epithelial cells lining the loop of Henle [80]. THP is not antimicrobial itself, but it prevents attachment of certain bacteria to epithelia and thus facilitates bacterial washout. THP also activates dendritic cells. Similar to hepcidin, lactoferrin and lipocalin are proteins involved in restricting the availability of iron. Lactoferrin is expressed in the distal collecting tubule and decreases available iron by chelation. Lactoferrin also has direct antimicrobial activity by damaging microbial cell membranes. Lipocalin captures iron-laden siderophores, providing bacteriostatic activity against organisms that secrete siderophores to scavenge iron from the environment [14,46]. Finally, secretory leukocyte proteinase inhibitor (SLPI) is a major protease inhibitor that is produced in the distal tubules of the nephron. SLPI has some antimicrobial effects and up-regulates macrophage production of anti-inflammatory cytokines [51].
The role of the adaptive immune response in the urinary tract
Despite the innate immune response, bacteria still can persist in the urinary tract. Therefore, a more specific adaptive immune response ensues that protects the urinary tract. As outlined previously in this review, studies by Thumbikat et al demonstrated that both cell-mediated and humoral arms of the immune system are activated after UPEC infection that stimulates recruitment of activated T cells to the bladder and the production of specific IgG antibody in the serum and urine. As a result, their efforts demonstrated that mice were highly resistant to reinfection with a homologous UPEC strain [44]. Additionally, it has been postulated that the sensitized B lymphocyte cells migrate to the lamina propria from the lymphatics and differentiate into IgA secreting cells. However, it has been suggested that these antibodies inhibit bacterial colonization by lowering the bacterial adherence to the mucosa or assist in opsonization by WBCs. These results suggest that that urinary tract is able to mount an appreciable and protective adaptive immune response [16,81].
A summary of the immune mechanisms in the urinary tract
Simplistically, an ascending or aggressive infection in the urinary tract can develop once bacteria are able to ascend the urethra and evade the mechanical barriers that keep them from attaching to the uroepithelium – such as urine flow, mucus production and uroepithelial coating with THP or AMPs. Once overcoming these barriers, UPEC activates uroepithelial TLR4 that triggers a cascade of signals that activate pro-inflammatory pathways. Chemokines are released and leukocytes are recruited to the site of infection. Moreover, the uroepithelium and circulating leukocytes increase production of AMPs. Together, these mechanisms work in concert to help eradicate a UTI. In all likelihood, these mechanisms are constantly being utilized by our urinary tract to ward off invading pathogens without a single symptom or invasive infection.
Acknowledgments
JDS is supported by the National Institute of Health Grant K08 DK094970-01. ALS and DSH are supported by the National Institute of Health Grant 1RC4DK090937-01.
Abbreviations
- UTI
Urinary Tract Infection
- UPEC
Uropathogenic E. coli
- VUR
Vesicoureteral Reflux
- TLR
Toll-like Receptor
- DC
Dendritic Cells
- AMP
Antimicrobial Peptides
- HNP
Human Neutrophil Peptides
- HD5
Human Alpha-Defensin 5
- HBD1
Human Beta Defensin 1
- HBD2
Human Beta Defensin 2
- RNase 7
Ribonuclease 7
- THP
Tamm-Horsfall protein
- SLPI
Secretory Leukocyte Protease Inhibitor
Footnotes
Disclosure
All the authors in this manuscript declared no competing interests.
References
- 1.Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatrics. 2001;108:311–316. doi: 10.1542/peds.108.2.311. [DOI] [PubMed] [Google Scholar]
- 2.Beetz R. May we go on with antibacterial prophylaxis for urinary tract infections? Pediatr Nephrol. 2006;21:5–13. doi: 10.1007/s00467-005-2083-6. [DOI] [PubMed] [Google Scholar]
- 3.Chesney RW, Carpenter MA, Moxey-Mims M, Nyberg L, Greenfield SP, Hoberman A, Keren R, Matthews R, Matoo TK. Randomized Intervention for Children With Vesicoureteral Reflux (RIVUR): background commentary of RIVUR investigators. Pediatrics. 2008;122(Suppl 5):S233–239. doi: 10.1542/peds.2008-1285c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman AL. Urologic diseases in North America Project: trends in resource utilization for urinary tract infections in children. J Urol. 2005;173:949–954. doi: 10.1097/01.ju.0000152092.03931.9a. [DOI] [PubMed] [Google Scholar]
- 5.Spencer JD, Schwaderer A, McHugh K, Hains DS. Pediatric urinary tract infections: an analysis of hospitalizations, charges, and costs in the USA. Pediatr Nephrol. 2010;25:2469–2475. doi: 10.1007/s00467-010-1625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobel JD. Pathogenesis of urinary tract infection. Role of host defenses. Infect Dis Clin North Am. 1997;11:531–549. doi: 10.1016/s0891-5520(05)70372-x. [DOI] [PubMed] [Google Scholar]
- 7.Brading AF, Turner WH. The unstable bladder: towards a common mechanism. Br J Urol. 1994;73:3–8. doi: 10.1111/j.1464-410x.1994.tb07447.x. [DOI] [PubMed] [Google Scholar]
- 8.Asscher AW, Sussman M, Waters WE, Davis RH, Chick S. Urine as a medium for bacterial growth. Lancet. 1966;2:1037–1041. doi: 10.1016/s0140-6736(66)92023-x. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, Fitzgerald M, Mueller ER, Schreckenberger P, Dong Q, Nelson DE, Brubaker L. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50:1376–1383. doi: 10.1128/JCM.05852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragnarsdottir B, Svanborg C. Susceptibility to acute pyelonephritis or asymptomatic bacteriuria: host-pathogen interaction in urinary tract infections. Pediatr Nephrol. 2012;27:2017–2029. doi: 10.1007/s00467-011-2089-1. [DOI] [PubMed] [Google Scholar]
- 11.Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci U S A. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright KJ, Seed PC, Hultgren SJ. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect Immun. 2005;73:7657–7668. doi: 10.1128/IAI.73.11.7657-7668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 14.Weichhart T, Haidinger M, Horl WH, Saemann MD. Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur J Clin Invest. 2008;38(Suppl 2):29–38. doi: 10.1111/j.1365-2362.2008.02006.x. [DOI] [PubMed] [Google Scholar]
- 15.Underwood MA, Bevins CL. Defensin-barbed innate immunity: clinical associations in the pediatric population. Pediatrics. 2010;125:1237–1247. doi: 10.1542/peds.2009-3289. [DOI] [PubMed] [Google Scholar]
- 16.Song J, Abraham SN. Innate and adaptive immune responses in the urinary tract. Eur J Clin Invest. 2008;38(Suppl 2):21–28. doi: 10.1111/j.1365-2362.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 17.Backhed F, Soderhall M, Ekman P, Normark S, Richter-Dahlfors A. Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ- and cell-specific expression of Toll-like receptors within the human urinary tract. Cell Microbiol. 2001;3:153–158. doi: 10.1046/j.1462-5822.2001.00101.x. [DOI] [PubMed] [Google Scholar]
- 18.Gluba A, Banach M, Hannam S, Mikhailidis DP, Sakowicz A, Rysz J. The role of Toll-like receptors in renal diseases. Nat Rev Nephrol. 2010;6:224–235. doi: 10.1038/nrneph.2010.16. [DOI] [PubMed] [Google Scholar]
- 19.Song J, Abraham SN. TLR-mediated immune responses in the urinary tract. Curr Opin Microbiol. 2008;11:66–73. doi: 10.1016/j.mib.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagberg L, Hull R, Hull S, McGhee JR, Michalek SM, Svanborg Eden C. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun. 1984;46:839–844. doi: 10.1128/iai.46.3.839-844.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen-Nissen E, Hawn TR, Smith KD, Nachman A, Lampano AE, Uematsu S, Akira S, Aderem A. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J Immunol. 2007;178:4717–4720. doi: 10.4049/jimmunol.178.8.4717. [DOI] [PubMed] [Google Scholar]
- 22.Samuelsson P, Hang L, Wullt B, Irjala H, Svanborg C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect Immun. 2004;72:3179–3186. doi: 10.1128/IAI.72.6.3179-3186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chassin C, Tourneur E, Bens M, Vandewalle A. A role for collecting duct epithelial cells in renal antibacterial defences. Cell Microbiol. 2011;13:1107–1113. doi: 10.1111/j.1462-5822.2011.01614.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 25.Scherberich JE, Hartinger A. Impact of Toll-like receptor signalling on urinary tract infection. Int J Antimicrob Agents. 2008;31(Suppl 1):S9–14. doi: 10.1016/j.ijantimicag.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 26.Ragnarsdottir B, Fischer H, Godaly G, Gronberg-Hernandez J, Gustafsson M, Karpman D, Lundstedt AC, Lutay N, Ramisch S, Svensson ML, Wullt B, Yadav M, Svanborg C. TLR- and CXCR1-dependent innate immunity: insights into the genetics of urinary tract infections. Eur J Clin Invest. 2008;38(Suppl 2):12–20. doi: 10.1111/j.1365-2362.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- 27.Svanborg-Eden C, de Man P, Jodal U, Linder H, Lomberg H. Host parasite interaction in urinary tract infection. Pediatr Nephrol. 1987;1:623–631. doi: 10.1007/BF00853600. [DOI] [PubMed] [Google Scholar]
- 28.Ragnarsdottir B, Samuelsson M, Gustafsson MC, Leijonhufvud I, Karpman D, Svanborg C. Reduced toll-like receptor 4 expression in children with asymptomatic bacteriuria. J Infect Dis. 2007;196:475–484. doi: 10.1086/518893. [DOI] [PubMed] [Google Scholar]
- 29.El-Achkar TM, Plotkin Z, Marcic B, Dagher PC. Sepsis induces an increase in thick ascending limb Cox-2 that is TLR4 dependent. Am J Physiol Renal Physiol. 2007;293:F1187–1196. doi: 10.1152/ajprenal.00217.2007. [DOI] [PubMed] [Google Scholar]
- 30.Cheng CH, Lee YS, Tsau YK, Lin TY. Genetic polymorphisms and susceptibility to parenchymal renal infection among pediatric patients. Pediatr Infect Dis J. 2011;30:309–314. doi: 10.1097/INF.0b013e3181ff84ff. [DOI] [PubMed] [Google Scholar]
- 31.Duell BL, Carey AJ, Tan CK, Cui X, Webb RI, Totsika M, Schembri MA, Derrington P, Irving-Rodgers H, Brooks AJ, Cripps AW, Crowley M, Ulett GC. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J Immunol. 2012;188:781–792. doi: 10.4049/jimmunol.1101231. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez JG, Sunden F, Connolly J, Svanborg C, Wullt B. Genetic control of the variable innate immune response to asymptomatic bacteriuria. PLoS One. 2011;6:e28289. doi: 10.1371/journal.pone.0028289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang ZD, Okhuysen PC, Guo DC, He R, King TM, DuPont HL, Milewicz DM. Genetic susceptibility to enteroaggregative Escherichia coli diarrhea: polymorphism in the interleukin-8 promotor region. J Infect Dis. 2003;188:506–511. doi: 10.1086/377102. [DOI] [PubMed] [Google Scholar]
- 34.Frendeus B, Godaly G, Hang L, Karpman D, Lundstedt AC, Svanborg C. Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. J Exp Med. 2000;192:881–890. doi: 10.1084/jem.192.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundstedt AC, Leijonhufvud I, Ragnarsdottir B, Karpman D, Andersson B, Svanborg C. Inherited susceptibility to acute pyelonephritis: a family study of urinary tract infection. J Infect Dis. 2007;195:1227–1234. doi: 10.1086/512620. [DOI] [PubMed] [Google Scholar]
- 36.Zaffanello M, Malerba G, Cataldi L, Antoniazzi F, Franchini M, Monti E, Fanos V. Genetic risk for recurrent urinary tract infections in humans: a systematic review. J Biomed Biotechnol. 2010;2010:321082. doi: 10.1155/2010/321082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centi S, Negrisolo S, Stefanic A, Benetti E, Cassar W, Da Dalt L, Rigamonti W, Zucchetta P, Montini G, Murer L, Artifoni L. Upper urinary tract infections are associated with RANTES promoter polymorphism. J Pediatr. 2010;157:1038–1040. e1031. doi: 10.1016/j.jpeds.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Hughes LB, Criswell LA, Beasley TM, Edberg JC, Kimberly RP, Moreland LW, Seldin MF, Bridges SL. Genetic risk factors for infection in patients with early rheumatoid arthritis. Genes Immun. 2004;5:641–647. doi: 10.1038/sj.gene.6364137. [DOI] [PubMed] [Google Scholar]
- 39.Haraoka M, Hang L, Frendeus B, Godaly G, Burdick M, Strieter R, Svanborg C. Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis. 1999;180:1220–1229. doi: 10.1086/315006. [DOI] [PubMed] [Google Scholar]
- 40.Engel D, Dobrindt U, Tittel A, Peters P, Maurer J, Gutgemann I, Kaissling B, Kuziel W, Jung S, Kurts C. Tumor necrosis factor alpha- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance. Infect Immun. 2006;74:6100–6107. doi: 10.1128/IAI.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tittel AP, Heuser C, Ohliger C, Knolle PA, Engel DR, Kurts C. Kidney dendritic cells induce innate immunity against bacterial pyelonephritis. J Am Soc Nephrol. 2011;22:1435–1441. doi: 10.1681/ASN.2010101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopkins WJ, Uehling DT, Balish E. Local and systemic antibody responses accompany spontaneous resolution of experimental cystitis in cynomolgus monkeys. Infect Immun. 1987;55:1951–1956. doi: 10.1128/iai.55.9.1951-1956.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopkins WJ, James LJ, Balish E, Uehling DT. Congenital immunodeficiencies in mice increase susceptibility to urinary tract infection. J Urol. 1993;149:922–925. doi: 10.1016/s0022-5347(17)36260-2. [DOI] [PubMed] [Google Scholar]
- 44.Thumbikat P, Waltenbaugh C, Schaeffer AJ, Klumpp DJ. Antigen-specific responses accelerate bacterial clearance in the bladder. J Immunol. 2006;176:3080–3086. doi: 10.4049/jimmunol.176.5.3080. [DOI] [PubMed] [Google Scholar]
- 45.Ali AS, Townes CL, Hall J, Pickard RS. Maintaining a sterile urinary tract: the role of antimicrobial peptides. J Urol. 2009;182:21–28. doi: 10.1016/j.juro.2009.02.124. [DOI] [PubMed] [Google Scholar]
- 46.Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol. 2007;18:2810–2816. doi: 10.1681/ASN.2007050611. [DOI] [PubMed] [Google Scholar]
- 47.Almeida PF, Pokorny A. Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry. 2009;48:8083–8093. doi: 10.1021/bi900914g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rathinakumar R, Walkenhorst WF, Wimley WC. Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: the importance of interfacial activity. J Am Chem Soc. 2009;131:7609–7617. doi: 10.1021/ja8093247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Splith K, Neundorf I. Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur Biophys J. 2011;40:387–397. doi: 10.1007/s00249-011-0682-7. [DOI] [PubMed] [Google Scholar]
- 50.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 51.Ohlsson S, Ljungkrantz I, Ohlsson K, Segelmark M, Wieslander J. Novel distribution of the secretory leucocyte proteinase inhibitor in kidney. Mediators Inflamm. 2001;10:347–350. doi: 10.1080/09629350120102389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 53.Liu L, Zhao C, Heng HH, Ganz T. The human beta-defensin-1 and alpha-defensins are encoded by adjacent genes: two peptide families with differing disulfide topology share a common ancestry. Genomics. 1997;43:316–320. doi: 10.1006/geno.1997.4801. [DOI] [PubMed] [Google Scholar]
- 54.Linzmeier RM, Ganz T. Human defensin gene copy number polymorphisms: comprehensive analysis of independent variation in alpha- and beta-defensin regions at 8p22-p23. Genomics. 2005;86:423–430. doi: 10.1016/j.ygeno.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 56.Ihi T, Nakazato M, Mukae H, Matsukura S. Elevated concentrations of human neutrophil peptides in plasma, blood, and body fluids from patients with infections. Clin Infect Dis. 1997;25:1134–1140. doi: 10.1086/516075. [DOI] [PubMed] [Google Scholar]
- 57.Tikhonov I, Rebenok A, Chyzh A. A study of interleukin-8 and defensins in urine and plasma of patients with pyelonephritis and glomerulonephritis. Nephrol Dial Transplant. 1997;12:2557–2561. doi: 10.1093/ndt/12.12.2557. [DOI] [PubMed] [Google Scholar]
- 58.Bevins CL. Paneth cell defensins: key effector molecules of innate immunity. Biochem Soc Trans. 2006;34:263–266. doi: 10.1042/BST20060263. [DOI] [PubMed] [Google Scholar]
- 59.Porter E, Yang H, Yavagal S, Preza GC, Murillo O, Lima H, Greene S, Mahoozi L, Klein-Patel M, Diamond G, Gulati S, Ganz T, Rice PA, Quayle AJ. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect Immun. 2005;73:4823–4833. doi: 10.1128/IAI.73.8.4823-4833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, Mok SC. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- 61.Dugan AS, Maginnis MS, Jordan JA, Gasparovic ML, Manley K, Page R, Williams G, Porter E, O’Hara BA, Atwood WJ. Human alpha-defensins inhibit BK virus infection by aggregating virions and blocking binding to host cells. J Biol Chem. 2008;283:31125–31132. doi: 10.1074/jbc.M805902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith JG, Nemerow GR. Mechanism of adenovirus neutralization by Human alpha-defensins. Cell Host Microbe. 2008;3:11–19. doi: 10.1016/j.chom.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Spencer JD, Hains DS, Porter E, Bevins CL, Dirosario J, Becknell B, Wang H, Schwaderer AL. Human alpha defensin 5 expression in the human kidney and urinary tract. PLoS One. 2012;7:e31712. doi: 10.1371/journal.pone.0031712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Porter EM, Poles MA, Lee JS, Naitoh J, Bevins CL, Ganz T. Isolation of human intestinal defensins from ileal neobladder urine. FEBS Lett. 1998;434:272–276. doi: 10.1016/s0014-5793(98)00994-6. [DOI] [PubMed] [Google Scholar]
- 65.Townes CL, Ali A, Robson W, Pickard R, Hall J. Tolerance of bacteriuria after urinary diversion is linked to antimicrobial peptide activity. Urology. 2011;77:509, e501–508. doi: 10.1016/j.urology.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 66.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Jr, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469:419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 68.Morrison G, Kilanowski F, Davidson D, Dorin J. Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect Immun. 2002;70:3053–3060. doi: 10.1128/IAI.70.6.3053-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lehmann J, Retz M, Harder J, Krams M, Kellner U, Hartmann J, Hohgrawe K, Raffenberg U, Gerber M, Loch T, Weichert-Jacobsen K, Stockle M. Expression of human beta-defensins 1 and 2 in kidneys with chronic bacterial infection. BMC Infect Dis. 2002;2:20. doi: 10.1186/1471-2334-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1681/01.asn.0000926856.92699.53. [DOI] [PubMed] [Google Scholar]
- 71.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 72.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 73.Wang H, Schwaderer AL, Kline J, Spencer JD, Kline D, Hains DS. Contribution of Structural Domains to Ribonuclease 7’s Activity Against Uropathogenic Bacteria. Antimicrob Agents Chemother. 2012;57:766–774. doi: 10.1128/AAC.01378-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spencer JD, Schwaderer AL, Wang H, Bartz J, Kline J, Eichler T, Desouza KR, Sims-Lucas S, Baker P, Hains DS. Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 2013;83:615–625. doi: 10.1038/ki.2012.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spencer JD, Schwaderer AL, Dirosario JD, McHugh KM, McGillivary G, Justice SS, Carpenter AR, Baker PB, Harder J, Hains DS. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int. 2011;80:174–180. doi: 10.1038/ki.2011.109. [DOI] [PubMed] [Google Scholar]
- 76.Harder J, Schroder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem. 2002;277:46779–46784. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- 77.Huang YC, Lin YM, Chang TW, Wu SJ, Lee YS, Chang MD, Chen C, Wu SH, Liao YD. The flexible and clustered lysine residues of human ribonuclease 7 are critical for membrane permeability and antimicrobial activity. J Biol Chem. 2007;282:4626–4633. doi: 10.1074/jbc.M607321200. [DOI] [PubMed] [Google Scholar]
- 78.Wang H, Schwaderer AL, Kline J, Spencer JD, Kline D, Hains DS. Contribution of Structural Domains to the Activity of Ribonuclease 7 against Uropathogenic Bacteria. Antimicrob Agents Chemother. 2013;57:766–774. doi: 10.1128/AAC.01378-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boix E, Nogues MV. Mammalian antimicrobial proteins and peptides: overview on the RNase A superfamily members involved in innate host defence. Mol Biosyst. 2007;3:317–335. doi: 10.1039/b617527a. [DOI] [PubMed] [Google Scholar]
- 80.Reinhart HH, Spencer JR, Zaki NF, Sobel JD. Quantitation of urinary Tamm-Horsfall protein in children with urinary tract infection. Eur Urol. 1992;22:194–199. doi: 10.1159/000474754. [DOI] [PubMed] [Google Scholar]
- 81.Svanborg-Eden C, Svennerholm AM. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1978;22:790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]