Abstract
Human arginase deficiency is characterized by hyperargininemia and infrequent episodes of hyperammonemia, which lead to neurological impairment with spasticity, loss of ambulation, seizures, and severe mental and growth retardation; uncommonly, patients suffer early death from this disorder. In a murine targeted knockout model, onset of the phenotypic abnormality is heralded by weight loss at around day 15, and death occurs typically by postnatal day 17 with hyperargininemia and markedly elevated ammonia. This discrepancy between the more attenuated juvenile-onset human disease and the lethal neonatal murine model has remained suboptimal for studying and developing therapy for the more common presentation of argianse deficiency. These investigations aimed to address this issue by creating an adult conditional knockout mouse to determine whether later onset of arginase deficiency also resulted in lethality. Animal survival and ammonia levels, body weight, circulating amino acids, and tissue arginase levels were examined as outcome parameters after widespread Cre-recombinase activation in a conditional knockout model of arginase 1 deficiency. One hundred percent of adult female and 70 percent of adult male mice died an average of 21.0 and 21.6 days, respectively, after the initiation of tamoxifen administration. Animals demonstrated elevated circulating ammonia and arginine at the onset of phenotypic abnormalities. In addition, brain and liver amino acids demonstrated abnormalities. These studies demonstrate that (a) the absence of arginase in adult animals results in a disease profile (leading to death) similar to that of the targeted knockout and (b) the phenotypic abnormalities seen in the juvenile-onset model are not exclusive to the age of the animal but instead to the biochemistry of the disorder. This adult model will be useful for developing gene- and cell-based therapies for this disorder that will not limited by by the small animal size of neonatal therapy and for developing a better understanding of the characteristics of hyperargininemia.
Keywords: Arginase deficiency, Hyperargininemia, Conditional knockout, Animal model
1.1 Introduction
Arginase deficiency is a rare single-enzyme liver defect in which arginase 1 (ARG1) is lost. This is the final enzyme in the urea cycle, the major pathway for the detoxification of ammonia in mammals. In the liver, ARG1 hydrolyzes arginine into ornithine, which re-enters the urea cycle as urea and then is excreted as waste. Multiple heterogeneous missense and nonsense mutations and deletions, resulting in an inability to detoxify ammonia and an elevation in arginine, have been described in diverse human populations [1-8]. Variability in penetrance is seen: affected children can present neonatally with severe hyperammonemia [4,9], but ARG1 deficiency usually presents in late infancy. The clinical presentation of hyperargininemia is quite distinct from those of other urea cycle disorders, and the occurrence of hyperammonemic encephalopathic episodes is uncommon. It usually presents with an insidious onset and manifests with a progressive neurological syndrome with microcephaly, spasticity, seizures, clonus, spastic diplegia, and failure to thrive [10] and rarely [4,9], if ever, is fatal in the perinatal or neonatal period.
The neurologic manifestations seen in arginase deficiency may arise from the accumulation of metabolites of arginine (that is, guanidino compounds) or may result from hyperargininemia itself; the exact cause and the proximate neurotoxin or mechanism of injury to the brain that causes the progressive neurological deterioration and mental retardation are not known. The neurologic impairment and developmental regression are associated with corticospinal [11] and pyramidal tract deterioration; despite having severe developmental delay, affected children typically avoid the catastrophic hyperammonemic crises characteristic of the other single-enzyme liver defects and therefore tend to survive into adulthood [12]. The lack of frequent episodes of hyperammonemia is likely but unproven to be due to an increase in the second form of arginase, arginase 2, which compensates for the lack of arginase 1 [12]; this enzyme is expressed in extrahepatic tissues, mainly in the kidney and brain [12,13]. Long-term therapy rests on the provision of a low-protein diet and administration of sodium benzoate and sodium phenyl butyrate. However, these therapies are incomplete and only palliative and not curative.
To model arginase deficiency, we previously generated ARG1-deficient mice in our laboratory by replacing the active site of the ARG1 gene, located on exon 4, with the neomycin resistance gene [14]. No ARG1 RNA was found on Northern blot nor was any cross-reacting material detected. These mice completely lack liver arginase 1 activity and therefore replicate the severe, neonatal human phenotype. In contrast to the juvenile-onset disease in which human patients can survive into adulthood, our arginase-deficient NIH-Swiss mouse model had a mean survival of 17 days, and the longest-surviving animal died at 21 days of life [15]. Biochemically and behaviorally severe hyperammonemia, as measured by both serum assay [15] and cognitive testing [16], was evident. Plasma ammonia levels of untreated ARG1-deficient mice were increased more than 10-fold, and serum arginine levels were markedly elevated [14]; their livers were abnormal and had histopathologic features similar to those seen in human arginase-deficient patients who died from hyperammonemia. We were able to palliate the disease by delivery of the arginase gene by using an adeno-associated virus (AAV) vector shortly after birth [15,16]; hepatic arginase levels rose to a sufficient level to metabolize arginine and prevent hyperammonemia and death. Treated animals survived to one year before euthanasia [16].
However, despite these encouraging results, no effective animal model replicating the later-onset juvenile arginase deficiency phenotype exists nor does an adult model in which alternative methods of therapy may be examined. To study the pathophysiology of arginase deficiency systematically in an adult animal model of the disease, we induced widespread arginase 1 deficiency in adult animals by using a conditional arginase 1 knockout mouse strain [17]. The goals of the present studies were to conditionally delete the arginase gene in adult mice via gavage administration of tamoxifen and investigate whether the human neonatal or juvenile phenotype would be replicated in adult animals and to define an adult animal model that could be used to develop therapeutic approaches for this disorder, which currently has no adequate therapeutic approach.
1.2 Materials and Methods
1.2.1 Mouse procedures
We generated mice that were hemizygous for a widespread Cre-recombinase (UBC-cre/ERT2; The Jackson Laboratory, Bar Harbor, ME, USA) with homozygosity for the Lox-P Arg1 insertion (at exons 7 and 8 of the ARG1 gene [17] [floxed]). By backcrossing and further mating, we produced a homozygous inducible gene deletion of arginase I with tamoxifen as the inducing agent. All mice were housed under specific pathogen-free conditions; chow (PicoLab Rodent Diet 20 [5053], calories 23.55% protein, 11.922% fat, carbohydrate 64.528%; LabDiet, St. Louis, MO, USA) and water were provided ad libitum. All mice were kept in accordance with the National Institutes of Health guidelines, and all experimental procedures were conducted in accordance with guidelines for the care and use of research animals at our institution. Scheduled blood sampling was taken from the retro-orbital plexus. Serum was frozen immediately and stored at −80°C until analysis. Tamoxifen (Sigma-Aldrich, St. Louis, MO, USA) (20 mg/mL) in corn oil was administered by gavage. Mice were euthanized by isoflurane, and blood and tissues were collected. Tissues were snap-frozen for later analysis and also placed in 4% paraformaldehyde for immunohistochemistry.
1.2.2 Polymerase chain reaction genotyping
Genomic DNA was prepared from the tail tip by standard methods. Anion-exchange column-purified genomic DNA was subjected to polymerase chain reaction (PCR) for genotyping. Primer sets for our conditional arginase knockout (JAX strain C57BL/6-Arg1tm1Pmu/J) were oIMR9556 forward TGC GAG TTC ATG ACT AAG GTT and oIMR9557 reverse AAA GCT CAG GTG AAT CGG. PCR had the following cycle conditions: denaturation at 94°C for 30 seconds, annealing at 60°C for 1 minute, and elongation at 72°C for 1 minute for 35 cycles by using DNA polymerase (Takara, Mountain View, CA, USA, catalog number RR006A). Genotype for conditional Cre-Recombinase was also confirmed by using the following primers: (JAX strain Tg(UBC-cre/ERT2)1Ejb/J): transgene primer forward 1084: GCG GTC TGG CAG TAA AAA CTA TC; transgene primer reverse 1085: GTG AAA CAG CAT TGC TGT CAC TT; internal positive control forward 7338: CTA GGC CAC AGA ATT GAA AGA TCT; and internal positive control reverse 7339: GTA GGT GGA AAT TCT AGC ATC ATC C. Cycle parameters were denaturation at 94°C for 30 seconds, annealing at 51.7°C for 1 minute, and elongation at 72°C for 2 minutes for 35 cycles. To confirm that lox P sites were intact, conditional knockout primers and conditions were developed: forward primer 5′-ACC TGC TGG GAA GGT ACG ATG CT-3′ and reverse primer 5′-AGG CTA CCT CTC TGG ATA CCT TTG C-3′. Cycle parameters were denaturation at 95°C for 30 seconds, annealing at 60°C for 30 seconds, and elongation at 72°C for 1 minutes for 30 cycles (1100-base pair amplicon product).
1.2.3 Biochemical and ammonia analysis of serum
Serum amino acid analysis was performed on a Biochrom 30 HPLC amino acid analyzer (Biochrom Ltd., Cambridge, UK). In brief, 30 to 50 μL of serum was mixed with equal volumes of Biochrom Seraprep and Lithium dilution buffer. Protein was precipitated by centrifugation, and 10 μL of supernatant was injected into the analyzer. Physiological amino acid standard (Sigma-Aldrich) was used to calibrate and determine analyte concentration. Analysis was performed by using EZchrom Elite software (Agilent Technologies, Santa Clara, CA, USA). Ammonia was determined in serum samples, by reductive amination of 2-oxoglutarate and oxidation of NADPH, by employing a commercial kit (Sigma-Aldrich, catalog number AA0100) and using 20 μL of serum for each sample tested. Determination of guanidino compound levels and urea was accomplished as previously described [16].
1.2.4 Biochemical analysis of liver tissue
Tissue amino acid levels were measured in tamoxifen-treated conditional arginase knockout mice before (baseline) and after (experimental) administration. For each sample, the tissue was weighed and homogenized in water to a concentration of 0.2 g/mL. The supernatant was then removed and sonicated, 1 mL was removed, and 70 mg of sulfosalicylic acid was added. The samples were then centrifuged. Supernatants were removed and sent on dry ice to the laboratory of Stephen Goodman (University of Colorado Health Sciences Center, Denver, CO, USA) for amino acid analysis on a Beckman System 6300 Amino Acid Analyzer (Beckman Coulter, Inc., Fullerton, CA, USA) by using a 3-Lithium buffer method, as described previously [18], and values were presented as nanomoles per milliliter per 100 mg of starting tissue.
1.2.5 Immunohistochemistry for arginase expression
Tissues were removed from euthanized animals and placed in 4% paraformaldehyde for 18 to 24 hours. After being rinsed with tap water for 15 minutes, tissues were placed in 70% ethanol followed by routine processing and embedding in paraffin. Tissues on slides were deparaffinized and rehydrated with ethanol and xylene by routine procedures. Slides were removed from tap water and placed in a microwaveable vessel filled with sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) for antigen retrieval. Tissues were then permeabilized with 1× Tris-buffered saline (TBS) + 0.2% Triton X-100 for 5 to 10 minutes followed by 1× TBS + 0.025% Triton X-100 for 5 minutes. Tissues were blocked with protein blocker (Dako, Glostrup, Denmark, catalog number X0909) + 0.1% Tween 20 for 20 minutes. Primary antibody was applied to the sections and incubated overnight at 4°C: rabbit arginase I (H-52) antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, catalog number sc-20150) was diluted in protein blocker plus 0.1% Tween 20 at 1:50 ratio. After being rinsed twice for 5 minutes with 1×TBS 0.025% Triton, the slides were incubated in 0.3% H2O2 in TBS for 15 minutes followed by application of the secondary antibody (goat anti-rabbit IgG-HRP; Santa Cruz Biotechnology Inc., catalog number sc-2301; 1:100 in protein blocker + 0.1% Tween 20) and incubated for 1 hour at room temperature. After being rinsed three times with 1×TBS, slides were developed with an ImmPACT DAB kit (Vector Laboratories, Burlingame, CA, USA, catalog number SK-4105) for 3 minutes at room temperature. Slides were counterstained with hematoxylin and cover-slipped. Tissues were analyzed with an Olympus BX40 microscope, and images were captured with cellSens software (Olympus, Center Valley, PA, USA).
1.2.6 Real-time polymerase chain reaction for arginase expression
Total RNA was extracted from liver tissue by using an RNeasy Kit (Qiagen, Valencia, CA, USA) in accordance with the instructions of the manufacturer. cDNA synthesis was by reverse transcription by using the Thermoscript reverse transcription-polymerase chain reaction (RT-PCR) kit (Invitrogen/Life Technologies, Grand Island, NY, USA) in accordance with the instructions of the manufacturer. Determination of expression of exogenous arginase I by AAV compared with endogenous GAPDH expression was accomplished by real-time PCR. Samples were performed in duplicate by using SsoAdvanced SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA). Primers were as follows: CKO arginase forward primer: 5′-GAC AGG GCT CCT TTC AGG AC-3′; reverse primer: 5′-GCC AAG GTT AAA GCC ACT GC-3′; GAPDH forward primer: 5′-ACT CCA CTC ACG GCA AAT TC-3′; and reverse primer: 5′-TCT CCA TGG TGG TGA AGA CA-3′. Reaction conditions were 40 cycles of denaturation at 95°C for 5 seconds and annealing/extension at 66°C for 30 seconds. Gel imaging was carried out by performing RT-PCR for 30 cycles with representative samples and running on a 1.5% agarose gel.
1.2.7 Determination of ornithine amino transferase expression
Relative OAT expression in the livers of vector-treated and untreated knockout mice was determined by quantitative RT-PCR [19,20]. RNA was isolated and cDNA was synthesized as above. Real-time PCR was then performed by using platinum SYBR Green (Invitrogen) and murine OAT-specific primers (forward 5′-GGG CTC TTG TGA AAC TCT GC-3′ and reverse 5′-AGA TGG GTC CGT TTC TCC TT-3′) for 40 cycles (95°C for 15 seconds, 56°C for 30 seconds, and 72°C for 15 seconds) after denaturing for 2 minutes at 95°C. GAPDH PCR was used to standardize the relative mOAT as above.
1.2.8 Western blotting
Tissue samples were homogenized with a 2-mL tissue grinder in RIPA buffer (Sigma-Aldrich) in the presence of Halt protease inhibitor cocktail (Pierce Biotechnology, Rockford, IL, USA). Twenty-five micrograms of centrifuge-clarified total protein was used in Western analysis and probed with polyclonal rabbit antisera against the murine ARG1 enzyme (Santa Cruz Biotechnology, Inc., catalog number SC-20150). Beta-tubulin antibody (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was used as a loading control and was detected by immunoblotting. The anti-ARG1 antibody was used at a dilution of 1:500, and the anti-β-tubulin antibody was used at a dilution of 1:500 in 1×TBST. Horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, Inc., catalog number sc-2301) was used as the secondary antibody and was visualized by enhanced chemiluminescent detection (Pierce Biotechnology).
1.2.9 Histologic quantification of tissue arginase expression
Sections of liver of affected and control animals were prepared via the immunohistochemical techniques described above and stained for arginase expression. A blinded reviewer received randomized slides and tabulated the number of arginase-positive cells per HPF [21]. Statistical comparisons between experimental and conditional knockout animals that did not receive tamoxifen (baseline) were made.
1.2.10 Enzymatic quantification of tissue arginase expression
Ten milligrams of liver was homogenized with 400 μL of ice-cold lysis buffer (0.1% Triton X-100 + HALT) followed by centrifugation at maximum speed at 4°C for 10 minutes. For wild-type and heterozygous mice, 10 μL of lysate is diluted with 90 μL of lysis buffer, whereas for the knockout samples, 100 μL of lysate without dilution is used directly; 50 μL of Tris-Cl (50 mM, pH 7.5) and 50 μL of MnCl2 (10 mM) are then added to the lysate, followed by incubation at 56°C for 10 minutes. With 5 μL of the above mixture, 25 μL of 0.5 M arginine pH 9.7 and 70 μL of ddH2O are added and incubated at 37°C for 1 hour. Then 400 μL of acid mixture (H2SO4 : H3PO4: H2O = 1:3:7 ratio) and 25 μL of 0.9% ISPF (9 mg ISPF + 100% ethanol) are added and incubated at 100°C for 45 minutes. The plate was then placed in the dark for 10 minutes as it cooled down to room temperature, and then a 200-μL aliquot in duplicate was placed in a 96-well plate, and the optical density was measured at 540 nm. Sample results were divided by total protein as determined previously [22]. To determine the final result, a urea standard starting at 300 μg/mL was made, followed by 2× serial dilution with ddH2O to 9.3 μg/mL, and final results were expressed as micrograms of urea per micrograms of total protein.
1.2.11 Statistical analysis
Survival curves were computed in each group of mice by using the Kaplan-Meier method and compared across groups by using the log-rank test. Animals were measured repeatedly across time, and between-group mean comparisons across age were carried out by using the repeated measures analysis of variance (ANOVA) model while taking into account that observations within the same animal are not independent. For weights, means were compared by using ANOVA after confirming the parametric normality and constant variance assumptions. We compared mean log ammonia level change from baseline between groups and within groups across time by using repeated measures ANOVA. For cell count, RT-PCR, and arginase analysis, means were compared across groups by using an unequal variance ANOVA model. The levels of serum and hepatic amino acids, OAT, and renal arginase were compared by t test. P values of less than 0.05 were considered significant. Calculations were made by using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
1.3 Results
1.3.1 Deletion of arginase expression in adult animals results in weight loss and death
Adult homozygous conditional arginase knockout mice (floxed ARG1) hemizygous for a ubiquitous Cre-recombinase deleter (herein referred to as experimental or floxed mice) were used for these experiments. Control animals (herein referred to as controls) were heterozygous for the conditional arginase knockout gene (with one copy of the wild-type arginase gene) and were hemizygous for Cre-recombinase. To conditionally delete arginase expression, we administered tamoxifen to the mice by gavage. Whereas control mice exhibited little weight loss over the 4-week (females) or 5-week (males) period of observation, the experimental mice showed substantial weight loss, up to 40% of pre-tamoxifen weight in some (P <0.0001 for both female and male experimental mice compared with controls; Fig. 1).
Fig. 1.
Weight of mice after administration of tamoxifen. Weight of mice at euthanasia or death after tamoxifen was administered. The graph shows the terminal weights of floxed arginase 1 (Arg1) mice compared with heterozygous lox-arginase mice (both hemizygous for Cre-recombinase); 100% is the baseline weight. Error bars represent standard deviation.
With loss of arginase expression in mice, death followed in all female animals (Fig. 2). Female mice were more susceptible to arginase deficiency (p=0.0002 compared to males); all female animals died by day 23 after initiation of tamoxifen administration (mean day of death: 21.0 ± 1.6 days; range 19 to 23 days; n = 10). Female control mice (n = 5) that received tamoxifen had some weight loss, but all survived (P <0.0001). Most male mice had a slightly prolonged survival (mean day of death: 21.4 ± 2.6 days; range 20 to 26 days; n = 7 of 10) compared with females. Phenotypically the animals became lethargic, including instability of gait and difficulty standing and righting themselves. None demonstrated evidence of seizure-like activity or findings consistent with spastic diplegia. Three male mice (herein referred to as intermediate mice) appeared phenotypically normal.
Fig. 2.
Survival of mice with tamoxifen administration. Survival in days between the experimental (floxed arginase, hemizygous Cre-recombinase) and control (heterozygous arginase, hemizygous Cre-recombinase) mice after tamoxifen administration. All female mice (n = 10) died 19 to 23 days after tamoxifen administration, whereas 70% (n = 7) of male mice died between days 20 and 26 (p=0.0002). The three surviving male mice retained hepatic arginase expression. f, female; m, male.
1.3.2 Loss of hepatic arginase expression in adult mice results in hyperammonemia and hyperargininemia
Blood was collected weekly; serum ammonia and amino acid concentrations (with particular attention paid to those amino acids implicated in the urea cycle) before and after the start of tamoxifen administration were determined. Circulating ammonia levels rose after Cre-recombinase induction (Fig. 3). The spike in serum ammonia corresponded temporally to profound phenotypic and behavioral changes in the animals. Serum ammonia levels in experimental animals, when compared with those of control animals who also received tamoxifen, were significantly elevated before euthanasia; ammonia levels in female mice (n = 7) increased from 116.1 ± 78.9 μmol/μL to 1,819.5 ± 2,258.2 μmol/μL by euthanasia (P = 0.05) after tamoxifen administration, whereas ammonia levels in male mice (n = 5) increased from 88.3 ± 45.5 μmol/μL to 1,572.2 ± 622.9 μmol/μL (P = 0.01). In control animals, ammonia levels were 111.9 ± 46.7 μmol/μL (n = 9) before tamoxifen administration and 155.1 ± 51.3 μmol/μL at euthanasia (P = 0.10).
Fig. 3.
Ammonia levels with arginase loss. Serum ammonia levels were measured weekly after tamoxifen administration was initiated. The groups presented are male and female floxed (experimental) mice and male and female control mice. Values are presented in micromoles per liter.
Similarly, circulating arginine levels did not become elevated until the onset of illness when a substantial elevation was present (Fig. 4). At this time, animals in the experimental group (n = 6) demonstrated serum arginine levels of 619.09 ± 74.29 μm/L whereas control animals (n = 6) demonstrated levels of 130.55 ± 51.09 μm/L (P = 0.0000001). Ornithine levels were low, there was a trend toward statistical significance (P = 0.07), and citrulline was elevated (117.22 ± 25.08 μm/L versus 61.30 ± 17.25 μm/L; P = 0.001) (Table 1). The mean glutamic acid level was one third and the proline level was approximately two thirds of the respective glutamic acid and proline levels for the experimental and control groups. In addition, histidine was unchanged, and glutamine concentrations were decreased by approximately one third. Concentrations of the branched-chain amino acids valine, isoleucine, and leucine were reduced to 0.2 to 0.4 times the concentrations found in the phenotypically normal control mice.
Fig. 4.
Serum arginine levels with arginase loss. Serum arginine levels were measured after tamoxifen administration was initiated. The groups presented are male and female floxed (experimental) mice and male and female control mice. Values are presented in micromoles per liter. The final time point for the floxed mice was when the mice were euthanized as in terminal crisis.
Table 1.
Serum amino acid levels at euthanasia in control and floxed conditional arginase 1 (ARG1) knockout mice that were administered tamoxifen.
| Monoamino, Monocarboxylic | Branched chain | Mercapto | Heterocyclic | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Alanine | Glycine | Isoleucine | Leucine | Valine | Cystine | Taurine | Proline | |
|
|
||||||||
| Control | 350.2 ± 104.8 | 246.2 ± 36.8 | 98.0 ± 28.9 | 166.8 ± 54.3 | 224.5 ± 64.6 | 6.2 ± 0.9 | 542.1 ± 189.2 | 94.4 ± 45.2 |
| Experimental | 159.0 ± 44.9 | 155.1 ± 44.9 | 61.3 ± 13.5 | 129.1 ± 20.0 | 187.6 ± 33.6 | 4.3 ± 0.8 | 637.7 ± 145.5 | 63.1 ± 25.4 |
| P value | 0.002 | 0.003 | 0.02 | 0.14 | 0.24 | 0.003 | 0.35 | 0.17 |
| Diamino, Monocarboxylic | Monoamino, Dicarboxylic | Hydroxy | Carboxamide | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Histidine | Lysine | Glutamic acid | Aspartic acid | Serine | Threonine | Asparagine | Glutamine | |
|
|
||||||||
| Control | 69.3 ± 12.2 | 235.2 ± 51.4 | 49.2 ± 32.0 | 10.8 ± 7.6 | 112.8 ± 31.5 | 141.4 ± 49.1 | 35.1 ± 8.2 | 670.1 ± 145.8 |
| Experimental | 66.9 ± 12.3 | 621.4 ± 106.9 | 16.4 ± 4.5 | 5.8 ± 1.2 | 76.4 ± 22.1 | 147.9 ± 54.4 | 59.4 ± 21.6 | 460.1 ± 106.4 |
| P value | 0.74 | 0.00001 | 0.03 | 0.05 | 0.04 | 0.83 | 0.03 | 0.02 |
| Aromatic | Thioether | Urea cycle-related | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Phenylalanine | Tyrosine | Methionine | Arginine | Citrulline | Ornithine | |
|
|
||||||
| Control | 99.8 ± 33.7 | 83.9 ± 25.9 | 82.9 ± 42.8 | 130.6 ± 51.1 | 61.3 ± 17.2 | 83.4 ± 46.9 |
| Experimental | 99.0 ± 10.6 | 70.3 ± 35.6 | 41.5 ± 18.8 | 619.1 ± 74.3 | 117.2 ± 25.1 | 40.0 ± 21.7 |
| P value | 0.96 | 0.47 | 0.06 | 0.0000001 | 0.02 | 0.01 |
Control and experimental values are presented as mean ± standard deviation in micrometers per liter.
Analysis of mean values for hepatic amino acid concentrations of mice (Table 2) revealed the following: monoamino monocarboxylic, branched-chain, and hydroxy amino acids along with proline, histidine, cystine, asparagine, and phenyalanine were not statistically different than those of control animals. Taurine, lysine, tyrosine, and methionine were increased. Glutamine was decreased, and as was similarly detected in serum, arginine and citrulline were elevated. Argininosuccinic acid was below the detection limit in the serum (data not shown) but was found to be elevated in the liver. Paradoxically, hepatic ornithine was increased over fivefold.
Table 2.
Hepatic amino acid levels at euthanasia in control and floxed conditional arginase 1 (ARG1) knockout mice that were administered tamoxifen.
| Monoamino, Monocarboxylic | Branched chain | Mercapto | Heterocyclic | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Alanine | Glycine | Isoleucine | Leucine | Valine | Cystine | Taurine | Proline | |
|
|
||||||||
| Control | 594.1 ± 52.1 | 368.7 ± 54.5 | 50.5 ± 8.2 | 128.4 ± 24.7 | 102.45 ± 17.3 | 0 ± 0 | 976.2 ± 259. | 62.6 ± 13.5 |
| Experimental | 528.5 ± 135.1 | 352.6 ± 72.0 | 60.6 ± 19.2 | 145.4 ± 41.3 | 123.3 ±38.1 | 0 ± 0 | 1,397.9 ± 124 | 57.8 ± 17.6 |
| P value | 0.98 | 0.69 | 0.13 | 0.21 | 0.12 | 1.00 | 0.004 | 0.90 |
| Diamino, Monocarboxylic | Monoamino, Dicarboxylic | Hydroxy | Carboxamide | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Histidine | Lysine | Glutamic acid | Aspartic acid | Serine | Threonine | Asparag | Glutamine | |
|
|
||||||||
| Control | 69.4 ± 8.3 | 156.7 ± 16.8 | 231.4 ± 111.4 | 341.1 ± 68.6 | 155.9 ± 35.6 | 107.3 ± 19.2 | 69.5 ± 1 | 322.1 ± 98.5 |
| Experimental | 62.3 ± 16.3 | 529.5 ± 146.3 | 221.6 ± 85.7 | 199.8 ± 57.4 | 169.3 ± 45.1 | 122.9 ± 24.9 | 75.8 ± 1 | 190.3 ± 32.2 |
| P value | 0.96 | 0.00001 | 0.92 | 0.06 | 0.31 | 0.14 | 0.31 | 0.05 |
| Aromatic | Thioether | Urea cycle-related | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Phenylalanine | Tyrosine | Methionine | Arginine | Citrulline | Ornithine | ASA | |
|
|
|||||||
| Control | 62.1 ± 11.4 | 48.8 ± 8.9 | 38.4 ± 8.2 | 1.9 ± 0.5 | 4.4 ± 1.1 | 96.7 ± 20.0 | 0 ± 0 |
| Experimental | 63.7 ± 18.2 | 65.6 ± 17.9 | 58.6 ± 14.3 | 107.4 ± 73.5 | 81.8 ± 55.3 | 510.4 ± 112.3 | 6.40 ± 4.1 |
| P value | 0.47 | 0.03 | 0.01 | 0.001 | 0.002 | 0.0000003 | 0.08 |
Control and experimental values are presented as mean ± standard deviation in nanomoles per milliliter per 100 mg of tissue. ASA, argininosuccinic acid.
We examined whether ornithine amino transferase (OAT) was induced in the liver to explain the elevation in hepatic ornithine. Glutamate and pyrroline-5-carboxylate together with ornithine aminotransferase can synthesize ornithine. Relative ornithine expression was examined in controls (n = 5) and in the experimental group (n = 5) and found to have increased in the experimental mice by 2.04 times that of controls.
We also examined whether guanidino compounds, derived from arginine and speculated to be involved in the unique neuropathology of arginase deficiency, were abnormal in these animals (Table 3). Comparing experimental animals with baseline animals, we detected statistically significant increases in serum α-N-acetylarginine, arginine, creatinine, γ-guanidinobutyric acid, guanidinoacetic acid, homoarginine, and urea. Argininic acid and α-keto-δ-guanidinovaleric acid increased trending toward significance (P = 0.06 and P = 0.07, respectively). Guanidinosuccinic acid, creatine, β-guanidinopropionic acid, and methylguanidine were not significantly different between groups.
Table 3.
Serum guanidino compound levels at euthanasia in baseline and floxed conditional arginase 1 (ARG1) knockout mice that were administered tamoxifen.
| αKδ-GVA | GSA | CT | GAA | α-N-AA | ArgA | β-GPA | |
|---|---|---|---|---|---|---|---|
| Baseline (n = 6) | 0.20 ± 0.04 | 0.25 ± 0.07 | 126.95 ± 49.02 | 1.00 ± 0.21 | 0.04 ± 0.03 | 0.05 ± 0.01 | 0.000 ± 0.00 |
| Experimental (n = 11) | 5.82 ± 6.98 | 0.19 ± 0.15 | 175.05 ± 102.58 | 2.02 ± 1.14 | 0.19 ± 0.14 | 3.25 ± 3.84 | 0.002 ± 0.00 |
|
P value (between treated and littermate) |
0.07 | 0.38 | 0.30 | 0.05 | 0.03 | 0.06 | 0.51 |
| CTN | γ-GBA | Arg | HArg | MG | Urea, mM | |
|---|---|---|---|---|---|---|
| Baseline (n = 6) | 9.00 ± 1.21 | 0.10 ± 0.04 | 98.97 ± 12.77 | 0.22 ± 0.09 | 0.00 ± 0.00 | 6.04 ± 0.07 |
| Experimental (n = 11) | 12.77 ± 2.72 | 0.28 ± 0.17 | 435.36 ± 358.00 | 0.54 ± 0.31 | 0.05 ± 0.06 | 10.83 ± 3.95 |
| P value | 0.01 | 0.02 | 0.04 | 0.03 | 0.11 | 0.01 |
Values are given as mean ± standard deviation and are expressed as micrometers, except where noted otherwise. αKδ-GVA, α-keto-δ-guanidinovaleric acid; α-N-AA, α-N-acetylarginine; Arg, arginine; ArgA, argininic acid; CT, creatine; CTN, creatinine; GAA, guanidinoacetic acid; γ-GBA, γ-guanidinobutyric acid; GSA, guanidinosuccinic acid; HArg, homoarginine; MG, methylguanidine; β-GPA, β-guanidinopropionic acid.
1.3.3 Administration of tamoxifen results in loss of hepatic arginase 1 and induction of renal arginase
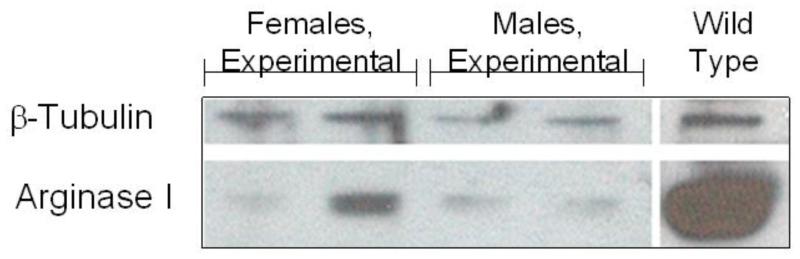
Hepatic arginase loss was examined by three methods: (a) immunohistochemistry of the liver (Fig. 5), (b) measurement of hepatic arginase activity (Fig. 6), and (c) Western blot of the liver (Fig. 7). Immunohistochemistry revealed a profound loss of arginase expression throughout the hepatic parenchyma in both male (n = 7) and female (n = 10) mice. Cell counts between groups demonstrated statistically significant declines in arginase-positive cells in the liver. Baseline animals (n = 5) had 200.6 ± 39.8 arginase-positive cells per high-power field (HPF), and as expected, the experimental mice had a significant decrease (34.7 ± 10.6 [n = 6] [P = 0.002] and 13.1 ± 10.6 [n = 8] [P = 0.0003]) in arginase-positive cells per HPF in males and females, respectively. Measurement of arginase activity in hepatic samples demonstrated a decline in urea production. The median enzymatic activity in hepatocytes in males declined from 12.51 ± 1.61 μg urea per μg total protein (n = 3) before tamoxifen administration to 0.76 ± 0.41 μg urea per μg total protein (n = 7) (P = 0.00004) after administration, whereas in females it declined from 9.60 ± 1.46 μg urea per μg total protein (n = 3) before tamoxifen administration to 0.85 ± 0.49 μg urea per μg total protein (n = 9) (P = 0.0001) after administration. By comparison, untreated targeted knockout mice, when moribund, demonstrated arginase activity of 0.17 ± 0.05 μg urea per μg total protein (n = 13) (data not shown). Control animals receiving tamoxifen demonstrated largely retained expression: 10.33 ± 1.98 μg urea per μg total protein for females (n = 5) (P = 0.6) and 7.53 ± 2.07 μg urea per μg total protein (n = 5) for males (P = 0.01). These findings were confirmed at the protein expression level by Western blot: affected animals had strikingly reduced arginase expression as compared with controls.
Fig. 5.
Arginase expression in hepatocytes by immunohistochemistry. A. Representative sections of immunohistochemically detected endogenous hepatic arginase expression. Images show expression of arginase in liver of (A) baseline animal (no tamoxifen administered), (B) control male (tamoxifen administered), (C) control female (tamoxifen administered), (D) experimental male (tamoxifen administered) with incomplete arginase deletion (‘intermediate’ male), (E) experimental male (tamoxifen administered), and (F) experimental female (tamoxifen administered) mice. B. Count of arginase expressing hepatocytes. Arginase-positive hepatocytes were compared between baseline animals and experimental females (n = 10) and experimental males (n = 7) after tamoxifen administration when animals were euthanized. Error bars represent standard deviation.
Fig. 6.
Hepatic arginase levels in mice administered tamoxifen by arginase assay. Liver tissue was collected from baseline (n = 6), control (n = 10), and floxed (male n = 7, female n = 10) mice at euthanasia. Floxed mice administered tamoxifen exhibited a significant loss of hepatic arginase activity. Error bars represent standard deviation. f, female; m, male.
Fig. 7.
Hepatic arginase expression in mice administered tamoxifen by Western blot. Total protein was isolated from mice administered tamoxifen at euthanasia. Western blot analysis showed that arginase expression in floxed arginase 1 (ARG1) mice was greatly reduced when assayed at euthanasia. GAPDH was used as a control for loading.
Measurement of arginase mRNA also demonstrated loss (Fig. 8); arginase 1 mRNA level in experimental female mice, at time of death or when found to be moribund, was 1.4% ± 1.2% of control females (n = 3) not receiving tamoxifen (P = 0.03); the corresponding level in males was 6.4% ± 1.0% of controls (n = 6) not receiving tamoxifen (P = 0.06). Control animals that did receive tamoxifen did demonstrate some decline in arginase 1 mRNA expression, as expected: male mice declined to 61.8% ± 8.4% of controls (P = 0.35), and females declined to 30.0% ± 9.7% of controls (P = 0.04).
Fig. 8.
Hepatic arginase mRNA in mice administered tamoxifen by quantitative reverse transcription-polymerase chain reaction (RT-PCR). A. Relative arginase expression in tamoxifen-treated mice was compared with baseline. Liver tissue was collected from baseline (n = 6) (no tamoxifen), control (n = 10), and experimental animals that received tamoxifen (females, n = 10; males, n = 7; male intermediate, n = 3). Arginase 1 (ARG1) expression by RT-PCR was compared and showed significantly lower levels of ARG1 expression in the liver. Error bars represent standard deviation. f, female; m, male. B. RT-PCR analysis of ARG1 gene expression. Lanes 1 and 2 represent control animals treated, whereas lanes 3 and 4 represent experimental animals; all treatment was with tamoxifen. The GAPDH gene was used as a housekeeping gene standard.
In adult mice, arginase II is expressed only in the kidney and intestine [23]. Therefore, we sought to examine whether arginase II was induced in the kidney of adult mice when arginase I was lost and plasma arginine levels were increased. In control male mice, average kidney arginase activity was 1.07 ± 0.08 μg urea per μg total protein (n = 5), whereas in the experimental male mice, arginase activity increased to 1.32 ± 0.23 μg urea per μg total protein (n = 5) (P = 0.057). In control female mice, arginase activity was 2.11 ± 0.31 μg urea per μg total protein (n = 5), whereas in experimental female mice, it increased to 3.25 ± 1.06 μg urea per μg total protein (n = 5) (P = 0.049).
1.3.4 Surviving male mice
Three experimental male mice (referred to as intermediate) did not develop symptoms of hyperargininemia or hyperammonemia after tamoxifen administration, whereas all females developed clinical symptoms and expired or were ill and euthanized (Fig. 2). Additional genotyping of these animals was performed to ensure that both loxP sites were intact, and this was confirmed; this was performed to ensure that there was not a loss of a loxP site that could explain persistence of hepatic arginase expression as recombination would then not have occurred. Whereas average ammonia level at 3 weeks in the affected experimental male mice (n = 6) was 1,258.61 ± 777.95 μmole/μL, the ammonia in these animals (n = 3) was 144.26 ± 109.01 μmole/μL. The median enzymatic activity in hepatocytes in males before tamoxifen administration was 12.51 ± 1.61 μg urea per μg total protein (n = 3) and, in these mice, declined to 1.06 ± 0.44 μg urea per μg total protein (8.5% of untreated levels), and there was no evidence of abnormal phenotype. The decline in arginase-positive cells is visible with immunohistologic examination of liver from these animals (Fig. 5A). Finally, expression of hepatic ARG1 mRNA was performed in these animals and was determined to be 16.6% ± 7.1% of baseline levels (Fig. 8).
1.4 Discussion
Previously, using standard gene replacement techniques, we introduced a null arginase 1 allele into the mouse genome. Mice homozygous for the null allele completely lacked the activity of arginase 1 and died at postnatal days 10 to 14 on the C57BL/6 background, exhibiting symptoms of severe hyperammonemia, including decerebrate posture, lethargy, and a high frequency tremor of the extremities, particularly the tail. Compared with their wild-type and heterozygous littermates, knockout mice exhibited significant alterations in serum concentrations of several amino acids involved in arginine metabolism, including the expected increase in arginine and also decreased levels of proline and ornithine. These ARG1-deficient animals also exhibited other common consequences of liver damage, including elevations of serum levels of glutamate, citrulline, and histidine and reductions of the branched-chain amino acids valine, isoleucine, and leucine. Knockout animals also exhibited a general reduction in the concentrations in plasma of most amino acids, possibly because they feed less as symptoms progress. The symptoms exhibited in these arginase 1-deficient mice were consistent with perturbation of the urea cycle. However, because of the early death (before weaning) of these mice, it is not possible to study the mechanism of brain injury in this model or to orally administer sodium phenylacetate, a diversion therapy used successfully in arginase 1-deficient humans to reduce ammonia.
This is the first study in which the gene for a single urea cycle enzyme was successfully conditionally deleted in adult animals, and the gene has been found, in this model, to replicate the lethal neonatal phenotype of arginase deficiency. Death occurs about 3 weeks after tamoxifen induction with abnormal biochemical markers: serum ammonia levels of more than 10 times normal and markedly elevated arginine levels. Unlike in the germline deletion model on the C57Bl/6 background in which affected animals expire in the first 2 weeks of life, adult animals with a controllable, conditional deletion that replicates the neonatal-onset disease would be amenable to a much larger array of investigational techniques, including but not limited to ex vivo gene correction, viral vector-based gene therapy, and cellular or even whole organ transplantation along with the testing of potential oral therapeutics.
With induction of Cre-recombinase in floxed animals to spatiotemporally delete arginase 1 expression using the CreERT/loxP system, we detected a substantial decline in animal weight; some lost up to 40% of their initial weight. Tamoxifen is known to be able to cause a rearrangement of the gastric mucosa, including loss of greater than 90% of parietal cells and morphologic changes in the chief cell lineage in the gastric units [24]. Whereas the decline in weight in the floxed animals was dramatic and could be explained in part by alterations in gastric physiology, weight loss in control animals was substantially less (as little as 10%). In addition, our previous studies have demonstrated that targeted arginase knockout mice treated as neonates with an AAV vector expressing arginase 1 did not reach the weights of littermate controls [15,16] and remained with a lean body habitus. The mechanism causing these weight differences is not known.
While conditional gene targeting allowing for spatial and temporal control of genetic deletion has contributed to the increase in the number of genetically engineered mice, there have been unexpected findings regarding cre activity [25]. The parent of origin of the cre transgene can affect excision patterns. Both maternal or paternal inheritance has been demonstrated to affect cre excision patterns [26]. In addition, there have been reports of inconsistent cre recombination between littermates. Within a single litter, cre excision has varied from tissue specific to ubiquitous. These findings of cre activity variability based of maternal or paternal pattern of inheritance of the cre allele and inconsistent recombination between littermates may be the cause of the incomplete arginase 1 deletion and mosaicism found in the three male floxed animals that did not result in complete loss of hepatic arginase 1 expression.
Most of the differences revealed by comparison of mean amino acid levels in the serum of arginase 1 knockout mice with those of phenotypically normal littermates can be explained by the expected perturbations of arginine and ornithine metabolism caused by loss of arginase I activity. Although in the liver itself we see similar findings, there is one notable exception: there is a paradoxical increase in ornithine. Interestingly, we observed this finding previously in arginase-deficient mice that were corrected in the short term with a helper-dependent adenoviral vector [19]. These mice survived to day 27, and death has been attributed to loss of viral-based arginase expression with cellular division and cytoplasmic degradation of previously episomally located viral vector genomes. Hepatic ornithine was increased almost six times that of heterozygous mice in those studies. It appears likely that ornithine is being either overproduced or underutilized and that the low plasma level does raise the issue of some end-organ ornithine shortage. We have demonstrated that OAT mRNA does double in these animals compared with littermate controls; however, it is not clear whether this increase in OAT mRNA is enough to account for a sixfold increase in hepatic ornithine. In addition, it has been demonstrated in competition experiments that lysine can decrease the transport of ornithine through the plasma membrane as well as the mitochondrial inner membrane [27]. In our studies, lysine in both the liver and serum is elevated in the conditional arginase-deficient animals. We are still actively working to understand this “ornithine paradox”; however, hypothesizing that diminished liver ornithine itself is responsible for the development of hyperammonemia remains in question. Minimally this would need to be explained by a compartmentalization phenomenon: ornithine produced is not in the same periportal area of the liver where urea cycle function takes place.
As with the targeted knockout, guanidino compounds, long speculated to be at least partly involved in the unique neuropathology of hyperargininemia, were abnormal in these animals. All of the guanidino compounds analyzed, except for methylguanidine, changed in the same direction as we have previously seen when comparing control littermates with untreated Arg−/− mice [16] in the targeted model. Methylguanidine increased slightly in the present studies but declined in the targeted model, although neither change was statistically significant.
To develop an adult animal model of the disorder, we took the approach of using a global conditional knockout. In doing so, we demonstrate that the gene was silenced at the DNA level. Hepatic mRNA expression of arginase were 6.4% of normal in males and 1.4% of normal in females when animals demonstrated findings of central nervous system dysfunction, and hepatic protein expression of arginase at that time were 6.1% and 8.8% of normal levels in affected males and females, respectively. It is unclear why three healthy-appearing male animals were never completely induced with tamoxifen but had residual ARG1 mRNA of 16.6% and arginase activity of 8.5% of baseline animals.
It has been postulated that, for urea cycle disorder gene therapy, attaining 10% enzyme replacement is sufficient for normal urea cycle function. In aggregate, these findings support that statement and show that, most likely, minimal arginase enzymatic activity of as little as 9% for adult arginase 1-deficient mice maintained on a normal chow diet is necessary for survival. Adding carbohydrate and limiting protein intake may require less functional arginase, and studies addressing this question are presently under way. This unique conditional arginase knockout model will be used for developing new therapies for this disorder, answering remaining questions about the poorly understood central nervous system pathology of hyperargininemia, and elucidating the mechanism of the paradoxically decreased hepatic ornithine detected in these animals.
Highlights.
Development of a widespread conditional arginase knockout mouse model is possible.
Deletion of Arginase 1 in the adult mouse results in similar phenotype to the lethal murine neonatal model.
Hypargininemia and hyperammonemia are detected about three weeks after induction with tamoxifen.
While serum hypoornithinemia is present, there is paradoxical markedly elevated hepatic ornithine (6 times controls), not completely explained by the mildly elevated ornithine aminotransferase mRNA (2 times controls).
Renal arginase level (arginase II) increases with deletion of arginase 1 globally.
Minimal hepatic arginase I activity necessary for normal phenotype is approximately 9% for mice maintained on regular chow diet.
Acknowledgments
The authors thank Daniela Markovic for assistance with the statistical evaluation and both the Semel Institute for Neuroscience and the Intellectual and Developmental Disabilities Research Center at UCLA for their support. This work was supported by grants from the National Institutes of Health (5K08HD057555-05 and 1R01NS071076-03A1).
Abbreviations
- AAV
adeno-associated virus
- ANOVA
analysis of variance
- ARG
arginase
- HPF
high-power field
- OAT
ornithine amino transferase
- PCR
polymerase chain reaction
- RT-PCR
reverse transcription-polymerase chain reaction
- TBS
Tris-buffered saline.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statements
The authors declare no financial or other conflict of interest.
References
- 1.Carvalho DR, Brand GD, Brum JM, Takata RI, Speck-Martins CE, Pratesi R. Analysis of novel ARG1 mutations causing hyperargininemia and correlation with arginase I activity in erythrocytes. Gene. 2012;509:124–130. doi: 10.1016/j.gene.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Edwards RL, Moseley K, Watanabe Y, Wong LJ, Ottina J, Yano S. Long-term neurodevelopmental effects of early detection and treatment in a 6-year-old patient with argininaemia diagnosed by newborn screening. J. Inherit. Metab. Dis. 2009;32(Suppl 1):197–200. doi: 10.1007/s10545-009-1148-2. [DOI] [PubMed] [Google Scholar]
- 3.Grody WW, Klein D, Dodson AE, Kern RM, Wissman PB, Goodman BK, Bassand P, Marescau B, Kang S-S, Leonard JV, Cederbaum SD. Molecular genetic study of human arginase deficiency. Am. J. Hum. Genet. 1992;50:1281–1290. [PMC free article] [PubMed] [Google Scholar]
- 4.Jain-Ghai S, Nagamani SC, Blaser S, Siriwardena K, Feigenbaum A. Arginase I deficiency: severe infantile presentation with hyperammonemia: more common than reported? Mol. Genet. Metab. 2011;104:107–111. doi: 10.1016/j.ymgme.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee BH, Jin HY, Kim GH, Choi JH, Yoo HW. Argininemia presenting with progressive spastic diplegia. Pediatr. Neurol. 2011;44:218–220. doi: 10.1016/j.pediatrneurol.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Uchino T, Haraguchi Y, Aparicio JM, et al. Three novel mutations in the liver-type arginase gene in three unrelated Japanese patients with argininemia. Am. J. Hum. Genet. 1992;51:1406–1412. [PMC free article] [PubMed] [Google Scholar]
- 7.Uchino T, Snyderman SE, Lambert M, et al. Molecular basis of phenotypic variation in patients with argininemia. Hum. Genet. 1995;96:255–260. doi: 10.1007/BF00210403. [DOI] [PubMed] [Google Scholar]
- 8.Vockley JG, Tabor DE, Kern RM, et al. Identification of mutations (D128G, H141L) in the liver arginase gene of patients with hyperargininemia. Hum. Mutat. 1994;4:150–154. doi: 10.1002/humu.1380040210. [DOI] [PubMed] [Google Scholar]
- 9.Picker JD, Puga AC, Levy HL, et al. Arginase deficiency with lethal neonatal expression: evidence for the glutamine hypothesis of cerebral edema. J. Pediatr. 2003;142:349–352. doi: 10.1067/mpd.2003.97. [DOI] [PubMed] [Google Scholar]
- 10.Prasad AN, Breen JC, Ampola MG, Rosman NP. Argininemia: a treatable genetic cause of progressive spastic diplegia simulating cerebral palsy: case reports and literature review. J. Child Neurol. 1997;12:301–309. doi: 10.1177/088307389701200502. [DOI] [PubMed] [Google Scholar]
- 11.Oldham MS, VanMeter JW, Shattuck KF, Cederbaum SD, Gropman AL. Diffusion tensor imaging in arginase deficiency reveals damage to corticospinal tracts. Pediatr. Neurol. 2010;42:49–52. doi: 10.1016/j.pediatrneurol.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer R, Jenkinson CP, Vockley JG, Kern RM, Grody WW, Cederbaum S. The human arginases and arginase deficiency. J. Inherit. Metab. Dis. 1998;21(Suppl 1):86–100. doi: 10.1023/a:1005313809037. [DOI] [PubMed] [Google Scholar]
- 13.Vockley JG, Jenkinson CP, Shukla H, Kern RM, Grody WW, Cederbaum SD. Cloning and characterization of the human type II arginase gene. Genomics. 1996;38:118–123. doi: 10.1006/geno.1996.0606. [DOI] [PubMed] [Google Scholar]
- 14.Iyer RK, Yoo PK, Kern RM, et al. Mouse model for human arginase deficiency. Mol. Cell Biol. 2002;22:4491–4498. doi: 10.1128/MCB.22.13.4491-4498.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee EK, Hu C, Bhargava R, et al. Long-term survival of the juvenile lethal arginase-deficient mouse with AAV gene therapy. Mol. Ther. 2012;20:1844–1851. doi: 10.1038/mt.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EK, Hu C, Bhargava R, et al. AAV-based gene therapy prevents neuropathology and results in normal cognitive development in the hyperargininemic mouse. Gene Ther. 2013 Feb 7; doi: 10.1038/gt.2012.99. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Kasmi KC, Qualls JE, Pesce JT, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat. Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Lawler AM, Steel G, Sipila I, Milam AH, Valle D. Mice lacking ornithine-delta-aminotransferase have paradoxical neonatal hypoornithinaemia and retinal degeneration. Nat. Genet. 1995;11:185–190. doi: 10.1038/ng1095-185. [DOI] [PubMed] [Google Scholar]
- 19.Gau CL, Rosenblatt RA, Cerullo V, et al. Short-term correction of arginase deficiency in a neonatal murine model with a helper-dependent adenoviral vector. Mol. Ther. 2009;17:1155–1163. doi: 10.1038/mt.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ventura G, De Bandt JP, Segaud F, et al. Overexpression of ornithine aminotransferase: consequences on amino acid homeostasis. Br. J. Nutr. 2009;101:843–851. doi: 10.1017/S0007114508043389. [DOI] [PubMed] [Google Scholar]
- 21.Duarte S, Shen XD, Fondevila C, Busuttil RW, Coito AJ. Fibronectin-alpha4beta1 interactions in hepatic cold ischemia and reperfusion injury: regulation of MMP-9 and MT1-MMP via the p38 MAPK pathway. Am. J. Transplant. 2012;12:2689–2699. doi: 10.1111/j.1600-6143.2012.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu C, Busuttil RW, Lipshutz GS. RH10 provides superior transgene expression in mice when compared with natural AAV serotypes for neonatal gene therapy. J. Gene Med. 2010;12:766–778. doi: 10.1002/jgm.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cederbaum SD, Yu H, Grody WW, Kern RM, Yoo P, Iyer RK. Arginases I and II: do their functions overlap? Mol. Genet. Metab. 2004;81(Suppl 1):S38–44. doi: 10.1016/j.ymgme.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24. e27. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heffner CS, Pratt CH, Babiuk RP, et al. Supporting conditional mouse mutagenesis with a comprehenisive cre characterization resource. Nat. Comm. 2012;3:1–9. doi: 10.1038/ncomms2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckardt D, Theis M, Doring B, Speidel D, Willecke K, Ott T. Spontaneous ectopic recombination in cell-type-specific Cre mice removes loxP-flanked marker cassettes in vivo. Genesis. 2004;38:159–165. doi: 10.1002/gene.20011. [DOI] [PubMed] [Google Scholar]
- 27.Zollner H. Ornithine uptake by isolated hepatocytes and distribution within the cell. Int. J. Biochem. 1984;16:681–685. doi: 10.1016/0020-711x(84)90038-7. [DOI] [PubMed] [Google Scholar]