Abstract
Mucopolysaccharidosis VII (MPS VII) is due to deficient activity of the lysosomal enzyme β-glucuronidase (GUSB) and results in the accumulation of glycosaminoglycans (GAGs). This study determined the long-term effect of neonatal intravenous injection of a gamma retroviral vector (RV) on cardiac valve disease in MPS VII dogs. Transduced hepatocytes secreted GUSB into blood for up to 11 years at levels similar to or greater than those achieved with enzyme replacement therapy (ERT). Valve regurgitation and thickening were scored from 0 (normal) to +4 (severely abnormal). At 1 year, untreated MPS VII dogs had mitral regurgitation, mitral valve thickening, aortic regurgitation, and aortic valve thickening scores of 2.3±0.7, 2.3±0.6, 1.8±0.5, and 1.6±0.7, respectively, which were higher than the values of 0.6±0.1, 0.1±0.4, 0.3±0.8, and 0.1±0.4, respectively, in treated MPS VII dogs. Treated MPS VII dogs maintained low aortic regurgitation and aortic valve thickening scores for their lifetime. Although mitral regurgitation and mitral valve thickening scores increased to 2.0 at ≥8 years of age in the treated MPS VII dogs, older normal dogs from the colony had similar scores, making it difficult to assess mitral valve disease. Older treated dogs had calcification within the mitral and aortic valve annulus, while GUSB staining demonstrated enzyme activity within the mitral valve. We conclude that neonatal RV-mediated gene therapy reduced cardiac valve disease in MPS VII dogs for up to 11 years, and propose that neonatal initiation of ERT should have a similar effect.
Keywords: Lysosomal storage disease, mucopolysaccharidosis, gene therapy, cardiac disease, retroviral vector
1. Introduction
The mucopolysaccharidoses (MPS)1 are a group of 11 lysosomal storage diseases that are due to deficient activity of a lysosomal enzyme that contributes to the degradation of glycosaminoglycans (GAG), and have an overall incidence of 1:27,000 [1–2]. Clinical manifestations affect the heart, lung, bones, joints, eyes, ears, and brain [3]. Echocardiographic evidence of mitral valve (MV) or aortic valve (AV) disease occurs in 89% to 100% of all patients with MPS I and in 74% of MPS II, 66% of MPS III, 33% of MPS IV, and 100% of MPS VI patients [4–7]. Valve disease has also been reported in MPS VII [8–10], although the frequency cannot be calculated due to the low incidence of MPS VII. Valve disease occurs at a young age, as mitral regurgitation (MR) and/or aortic regurgitation (AR) can be found in infants [11] and was present in 42% and 82% of patients with various types of MPS at a mean age of 8 and 10 years, respectively [5–6]. Valve disease can result in the need for valve replacement [12], a major surgical procedure.
Current treatments for some types of MPS such as hematopoietic stem cell transplantation (HSCT) and enzyme replacement therapy (ERT) have not prevented cardiac disease. After HSCT, normal blood-derived cells secrete mannose 6-phosphate (M6P)-modified enzyme that can be taken up by nearby cells via the M6P receptor. Indeed, the need for cardiac surgery was reduced after HSCT in MPS I [13], although some patients have required valve replacement [14]. However, although valve thickening improved with HSCT, MR increased in 31% of MPS I patients at 12 years after performing HSCT at ~2 years of age [15]. Similarly, HSCT to MPS I or MPS VII dogs ameliorated, but did not prevent, cardiovascular disease [16–17]. ERT involves intravenous (IV) injection of enzyme, which can diffuse to tissues, and be transported to the lysosome via the M6P receptor. MR worsened or did not improve in MPS I and MPS VI patients at 2 to 6 years after starting ERT [18–21]. Early initiation of ERT was more effective at preventing cardiovascular disease in younger siblings than was initiation at an older age [22–24]. Likewise, a recent study in MPS I dogs demonstrated that initiation of ERT at birth was more effective at preventing cardiac manifestations at 1.5 years of age than was initiation at older ages, although the effect long-term was not determined [25].
Gene therapy is being tested in animals with MPS [26]. Neonatal IV injection of a gamma retroviral vector (RV) expressing the deficient enzyme resulted in transduction of liver, spleen, and blood cells, which secreted M6P-modified enzyme into blood, achieving stable levels of enzyme in serum for up to 11 years [27–29]. This reduced most cardiovascular manifestations of disease in MPS VII dogs at 1 year [30], but longer evaluation has not been reported in detail. Since dilatation of the ascending aorta developed at 6 years or older in MPS VII dogs that was associated with elastin fragmentation [31], it seemed possible that cardiac valve disease might similarly develop with time. Indeed, a similar gene therapy approach reduced, but did not prevent, cardiovascular manifestations of disease in MPS VI cats [32] and MPS I dogs [33]. The goal of this study was to evaluate the cardiac valves in gene therapy-treated MPS VII dogs at 6 to 11 years of age. These studies used the canine model of MPS VII with a missense mutation (R166H) in the β-glucuronidase (GUSB) gene [34] that results in accumulation of the GAGs heparan, dermatan, and chondroitin sulfates, which closely resembles the disease seen in humans [Online Mendelian Inheritance in Man (OMIM#253220)].
2. Materials and Methods
2.1 Materials
Materials were purchased from Sigma-Aldrich Chemical (St. Louis, MO) unless otherwise stated.
2.2 Animals and echocardiograms
National Institutes of Health (NIH) and United States Department of Agriculture guidelines for the care and use of animals in research were followed in the animal colony of the School of Veterinary Medicine, University of Pennsylvania. Ten MPS VII dogs were injected IV with 0.3 to 1×1010 transducing units (TU)/kg of the gamma RV designated hAAT-cGUSB-WPRE at 2 to 3 days after birth as reported previously [27–29] and are designated here as RV-treated. One MPS VII dog received hepatocyte growth factor (HGF) to attempt to potentiate hepatocyte replication, and thus transduction, prior to the injection of a higher dose (2×1010 TU/kg) of the same vector and is referred to as the HGF/RV-treated dog. Echocardiograms were performed by a board-certified veterinary cardiologist (MMS) using a Phillips Sonos 7500 echocardiographic machine. MR was scored from 0 (none), +1 (trace), +2 [regurgitant flow extends 1/3 of the way into the left atrium (LA)], +3 (regurgitant flow extends 1/3 to 2/3 of the way into the LA), and +4 (regurgitant flow extends more than 2/3 of the way into the LA). A score of +1 for AR indicates that there were a few pixels of AR with color flow, +2 that the color flow jet was larger than a few pixels but did not extend to the MV hinge point, which is the point of attachment of the anterior MV, +3 indicates that the color flow jet extends past the MV hinge point but not as far as the tip of the anterior MV leaflet, and +4 AR indicates that the color flow jet extends past the tip of the anterior MV leaflet. For post-mortem collection of samples, dogs received IV injections of 2 mg/kg of Propofol (Abbott, Chicago IL) and 80 mg/kg of sodium pentobarbital (Veterinary Laboratories, Lenexa, KS) in accordance with American Veterinary Medical Association guidelines, and hearts were dissected prior to freezing or after fixation.
2.3 Histopathology
For GUSB staining, 8 μm-thick frozen sections of MV were stained with naphthol AS-BI-β-D-glucuronide as described [29].
2.4 Statistics
The Mann-Whitney test compared non-continuous values between 2 groups, while Kruskal-Wallis One Way Analysis of Variance on Ranks (ANOVA on ranks) compared non-continuous values between 3 groups using Sigma Stat 12 software (Systat Software, Inc., Point Richmond, CA).
3. Results
3.1 Gene therapy procedure
The gene therapy procedure was reported previously [27–28] and was done as follows. Some MPS VII dogs were injected IV with 0.3 to 1×1010 TU/kg of the RV designated hAAT-cGUSB-WPRE at 2 to 3 days after birth, for which the human α1-antitrypsin (hAAT = SERPINA1) promoter directed expression of canine GUSB from an RV with a complete long-terminal repeat (LTR). Dogs that received RV without HGF (RV-treated) achieved stable expression of GUSB activity in serum for up to 10 years with lifetime averages that varied considerably from 108±61 U/ml (0.4-fold normal) to 4368±1199 U/ml (16-fold normal) in individual dogs, with an average of 884±1093 U/ml (3.6-fold normal), as previously reported [27–28]. The HGF/RV-treated dog M1287 received HGF in an attempt to increase hepatocyte replication and hence transduction prior to administration of 2×1010 TU/kg of RV, and achieved an average of 15,935±4,263 U/ml of GUSB activity in serum (65-fold normal) for 11 years [28]. Since cardiovascular parameters that were evaluated here did not differ overtly between the HGF/RV-treated dog M1287 and the dogs that received RV only, values for both groups were pooled and are referred to as treated dogs below. Treated MPS VII dogs had a median survival of 6.1 years, which was dramatically longer than the value of 0.4 years in untreated MPS VII dogs, as was recently discussed in more detail [28].
For the treated dogs, 11 animals were evaluated with echocardiograms at 6 months, and fewer animals were evaluated at older ages due to spontaneous deaths, euthanasia for clinical indications, planned deaths for the collection of tissues, or because the dogs that were treated more recently were too young. Supplementary Table 1 summarizes the dogs that were evaluated with echocardiography in this study. Occasionally, RV-treated dogs missed an evaluation at a particular age due to a failure to schedule the examination. The number of dogs of each gender was similar at 6 months and 1 year for normal and untreated MPS VII dogs. Phenotypically normal dogs that were evaluated at 4 or 8 years of age were mostly females, which are used for breeding in the colony. For the treated dogs, the animals evaluated at 6 years or older had an equal number of males and females. Among the younger treated dogs, there were more males than females, as treated MPS VII males were generated for breeding to maintain the colony.
3.2 Echocardiographic evaluation of the mitral valve in MPS VII Dogs
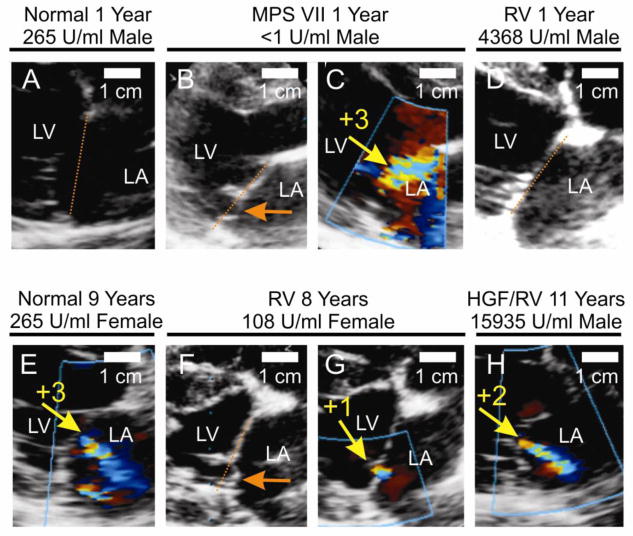
Echocardiograms of mitral valves are shown in Fig. 1. An untreated MPS VII dog had MV prolapse (MVP) at 1 year of age, as the posterior leaflet extended 3 mm into the LA, as shown in Fig. 1B. The same animal at the same age had severe MR with color Doppler (scored as +3, which means that the regurgitant flow extended 1/3 to 2/3 of the way into the LA), with the major jet of flow directed toward the anterior LA in Fig. 1C. MVP and MR were not observed at 1 year in the normal or RV-treated MPS VII dogs whose echocardiograms are shown in Fig. 1A and Fig. 1D, respectively. However, RV-treated dog M1332 had posterior leaflet MVP at 14 months, as reported previously [30], which was still present to a similar degree at 8 years, as shown in Fig. 1F, and was associated with trace MR (+1; Fig. 1G). HGF/RV-treated dog M1287 had +2 MR at 11 years (Fig. 1H), although MVP was not identified. However, a heterozygous normal dog from the colony had +3 severity MR (Fig. 1E) at 9 years without evidence of MVP, making it difficult to know if the MR present in treated dogs was related to age or the effect of MPS VII. For the untreated MPS VII dogs, it was difficult to determine what percentage had MVP at any age of evaluation, as many echocardiograms were of poor quality due to chest wall abnormalities from MPS VII. MVP was only detected in 1 RV-treated MPS VII dog.
Fig. 1. Echocardiogram analysis of MR.
Some normal (Normal) and MPS VII (MPS VII) dogs were untreated. Other MPS VII dogs were injected at 2–3 days after birth with the gamma RV without preceding hepatocyte growth factor (RV) or with the RV after administration of hepatocyte growth factor (HGF/RV), as described previously [27–29]. A–H. Representative images. Echocardiograms were obtained during systole at the indicated age for dogs of the indicated groups with color Doppler (panels A, C–E, and G–H) or without color Doppler (panels B and F). Panels B and C, and panels F and G, were obtained from the same dog at the same age. The left ventricle (LV) and left atrium (LA) are indicated. In some panels, the orange dotted line connects the mitral valve annulus on either side of the valve. MV prolapse (MVP) of the posterior leaflet is indicated with the orange arrows in panels B and F. The size marker is 1 cm. The yellow arrows in some panels identify the MR jet with color-flow Doppler, and the adjacent yellow numbers indicate the severity of MR. The average serum GUSB activity and gender are indicated; panel D has an image for M2165, panel F–G has images for M1332, and panel H has an image for M1287. Most of the images are from the posterior plane, which is the standard view to assess for MVP. However, the images shown in panels B and C were obtained from the anterior plane because of chest wall abnormalities. I. Mitral regurgitation scores. Dogs were scored for MR from normal (0) to severe (+4). ANOVA on ranks was used to compare values in the 3 groups at 0.5 and 1 years, while the Mann-Whitney rank sum test was used to compare values in MPS VII and RV-treated dogs at 2 years, and to compare values in treated and normal dogs at 7 years. * represents a p valve of 0.01 to 0.05 and ** represents a p value <0.01 for values in other groups as compared with those in untreated MPS VII dogs. The number of dogs evaluated in each group at each age is indicated on the graph.
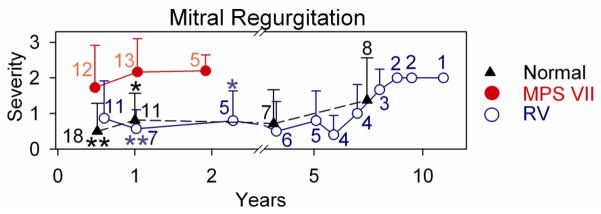
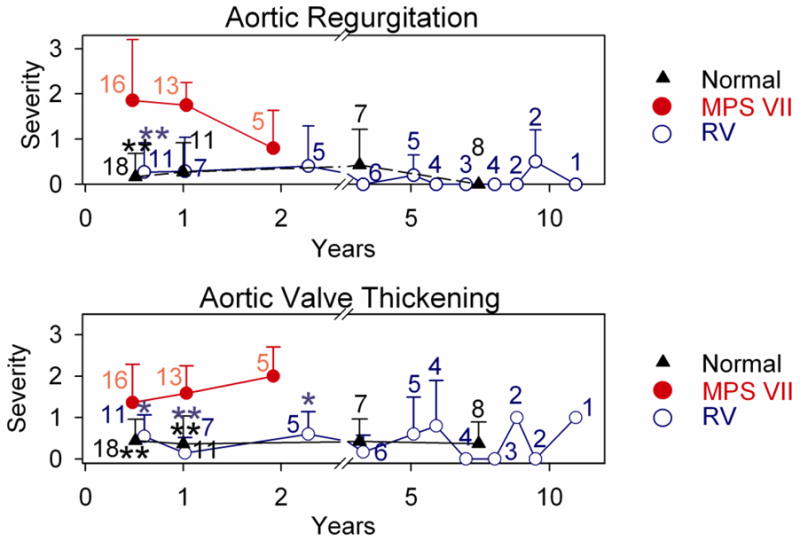
Quantitative analysis of MR at various ages is shown in Fig. 1I, where severity was scored as detailed in section 2.2. Phenotypically normal dogs were usually heterozygous for the GUSB mutation, and were only evaluated at some ages due to cost considerations. Untreated MPS VII dogs were not evaluated beyond 2 years as they died spontaneously or were euthanized for humane reasons, which were primarily related to severe bone and joint disease. None of the untreated or treated MPS VII dogs had overt signs of cardiac disease at any age of evaluation other than cardiac murmurs consistent with mitral regurgitation in some. At 6 months, untreated MPS VII dogs had moderate MR with a severity score of 1.7±1.2 (N=12), which was higher than the value of 0.5±0.8 in normal dogs (N=18; p<0.01 vs. MPS VII) and the score of 0.9±1.0 in treated MPS VII dogs (N=11), although the latter was not significantly different from the value in untreated MPS VII dogs. At 1 year, MR scores were even more elevated in untreated MPS VII dogs at 2.2±1.0 (N=13), which was now statistically higher than for both normal (0.8±0.8; N=11; p<0.05) and treated MPS VII (0.6±0.5; N=7; p<0.01) dogs. Similarly, the MR score of 2.2±0.5 in untreated MPS VII dogs at 2 years (N=5) was higher than the value of 0.8±0.8 in treated MPS VII dogs (p=0.03; N=5).
Of 5 treated MPS VII dogs that were followed for 6 years or longer, 4 were killed for bone and joint problems, and 1 was killed for a benign splenic hemangioma. None had overt signs of heart failure prior to death. Other treated MPS VII dogs are still alive or were sacrificed at various ages while in good health for collection of tissues. At 8 years, treated dogs had MR scores of 1.7±0.6 (N=3), which was not different from the value of 1.4±1.2 (N=8) in normal dogs at this age. The MR increased to a score of +2 in all treated dogs at 9 years or older, but a score of +3 was detected in some normal dogs from the colony at this age. Echocardiographic analysis demonstrated that untreated MPS VII dogs had substantial MV thickening at 0.5 to 2 years of age as shown in Fig. 2, which was significantly reduced in normal and treated MPS VII dogs (p<0.01). The increase in MV thickening at 8 years or older in RV-treated dogs was also observed in heterozygous normal dogs from the colony. The elevated MR and MV thickening scores in phenotypically normal aged dogs raises the possibility that abnormalities seen in aged treated MPS VII dogs reflects the normal aging process in this colony of dogs rather than MPS VII-related disease.
Fig. 2. Echocardiogram analysis of MV thickening.
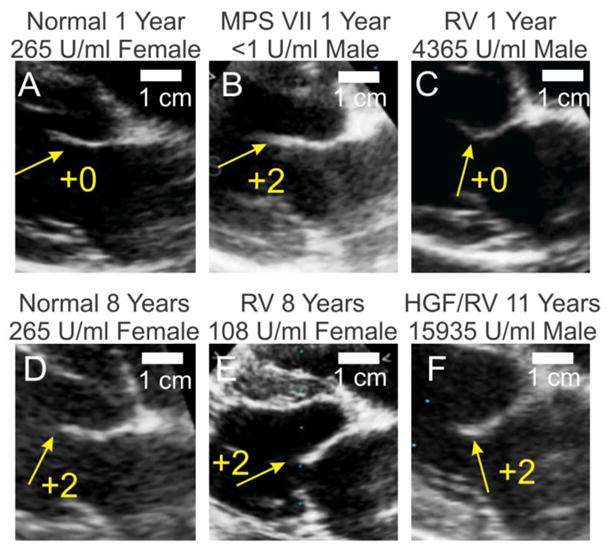
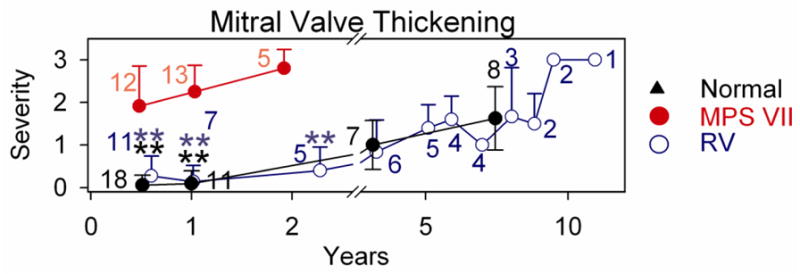
Standard 2-Dimensional echocardiographic imaging from the right parasternal window in the long axis at the mitral valve were used to assess mitral valve thickening. Shown here are frozen images obtained during diastole. A–F. Representative examples of echocardiograms. Panels are labeled as in Fig. 1. The anterior MV leaflet is identified with white arrows, and the score for MV thickening is shown from 0 (normal) to +4 (severe). Panel C has an image for M2165, panel D–E has images for M1332, and panel F has an image for M1287 G. Average MV thickening score. Dogs were scored for MV thickening from normal (0) to severe (+4), and statistics was performed as described in Fig. 1.
3.3 Gross appearance of the mitral valve
Components of the MV apparatus were evaluated at dissection. An untreated MPS VII dog had a thickened MV with nodules, and chordae tendinae (CT) with increased diameter, as shown in Fig. 3. Treated MPS VII dogs that were euthanized at 6 to 11 years of age had reduced thickening and nodules of the MV relative to that seen in untreated MPS VII dogs, although abnormalities were still present. Gross images of normal, untreated MPS VII, and treated MPS VII dogs at younger ages were reported previously [30].
Fig. 3. Gross photographs of MV.
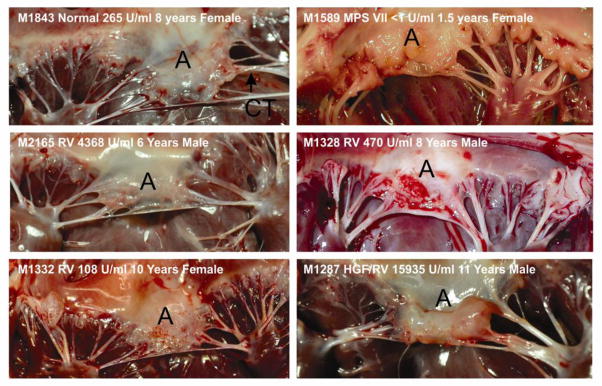
Photographs of the MV were obtained from a normal dog (Normal), an untreated MPS VII dog (MPS VII), or MPS VII dogs that received neonatal IV injection of RV alone (RV) or RV preceded by hepatocyte growth factor (HGF/RV). The animal number, the serum GUSB activity, age in years, and the gender are also indicated. The anterior leaflet (A) is identified.
MV were difficult to cut at gross dissection for some untreated MPS VII dogs that were 2 years old or for treated MPS VII dogs that were 6 years or older, suggesting that they might be calcified. Indeed, radiographs demonstrated that 4 of 5 MV from treated MPS VII dogs that were collected at 6 years or older had calcification that was at least 2 mm in one dimension in the annulus of the MV (data not shown), while none of 8 MV samples from normal dogs collected at 6 to 9 years of age were calcified. Although only 1 of 4 MV from untreated MPS VII dogs that were collected at 2 years of age had overt calcification on radiographs, this lower incidence than in treated dogs may reflect their younger age.
3.4 GUSB stain of MV
A histochemical stain for GUSB activity was performed on frozen sections of MV from normal, untreated MPS VII, and RV-treated MPS VII dogs, as shown in Fig. 4. This demonstrated that MV from normal dogs were diffusely but weakly positive for GUSB activity, untreated MPS VII dogs had no activity, and RV-treated valves had detectable activity, which tended to be near the edges of the valves or near blood vessels.
Fig. 4. Histochemical stain of mitral valves (MV) for GUSB activity.
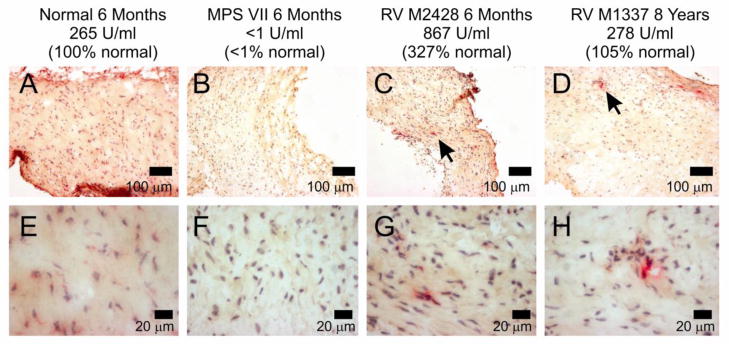
Frozen cross sections of MV leaflets of normal, untreated MPS VII, or RV-treated MPS VII dogs at the indicated age with the indicated serum GUSB activity were stained for GUSB activity (red), and counterstained with hematoxylin to identify nuclei (blue). The black arrows in the top panels for RV-treated dogs indicate regions with GUSB activity that are shown at higher power in the lower panels. The scale markers are indicated in each panel.
3.5 Echocardiographic evaluation of the aortic valve
The AV was evaluated with echocardiography, and scored from 0 (normal) to +4 (markedly abnormal) for AR and AV thickening. Fig. 5B shows a representative example of an untreated MPS VII dog that had +2 AR at 1 year of age, while no AR was detected in normal dogs at 1 year (Fig. 5A) and 8 years (Fig. 5D) of age, or in treated MPS dogs at 1 year (Fig. 5C) and 11 years (Fig. 5E). Quantitative analysis of echos from several animals in each group is shown in Fig. 5F. At 6 months of age, untreated MPS VII dogs had AR scores of 1.9±1.4 (N=12), which was higher than the value of 0.2±0.5 in normal (N=18; p<0.01) and 0.3±0.7 in treated MPS VII (N=11; p<0.01) dogs. AR scores were similar in untreated MPS VII dogs at 1 year at 1.8±0.5, although this was not statistically different from values in normal or treated MPS VII dogs at that age. AR scores were lower in MPS VII dogs at 2 years at 0.8±0.8 than at earlier ages in MPS VII dogs for unclear reasons. Treated MPS VII dogs continued to have low AR scores long-term, and the longest surviving dog (M1287) had a score of 0 at 11 years.
Fig. 5. Echocardiograms of AV.
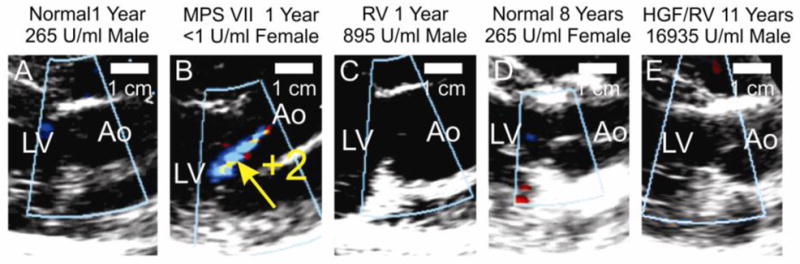
Color flow Doppler echocardiographic imaging from the right parasternal window in the long axis at the left ventricular outflow tract was used to assess aortic regurgitation during diastole. The genotype, age, treatment status, gender, and average serum GUSB activity are shown as in Fig. 1. The untreated MPS VII dog in panel B has a multicolored regurgitant jet that was scored +2 and is indicated with a yellow arrow; 0 represents normal and +4 represents severely abnormal AR. All other dogs shown in this figure had an AR score of 0. Panel C shows an image from M2421, while panel E shows an image for M1287. F and G. Quantification of AR and AV thickening. These parameters were performed and evaluated as described in Fig. 1.
AV thickening scores (Fig. 5G) were high at 6 months of age in untreated MPS VII dogs at 1.4±0.9 (N=12), which was higher than the value of 0.4±0.5 in normal dogs (N=18, p<0.01), and the value of 0.5±0.6 in treated MPS VII dogs (N=11; p<0.05). AV thickening scores remained high in untreated MPS VII dogs at 1 and 2 years of age, and were significantly higher than in age-matched dogs of the other groups. Treated MPS VII dogs maintained relatively low AV thickening scores for their lifetime, with a maximum average score of 1.0± 0.0 at 9 years of age (N=2), which was not significant vs. normal dogs at 8 years, when the score was 0.0±0.0 (N=8).
AV of treated MPS VII dogs were difficult to cut at gross dissection for dogs that were 6 years of age or older. Fig. 6B and 6C demonstrate that calcium was present in the AV annulus of 2 treated dogs that were sacrificed at 11 and 10 years of age, respectively, but was absent from a normal dog at 8 years.
Fig. 6. Radiographs of valve apparatus of the hearts.
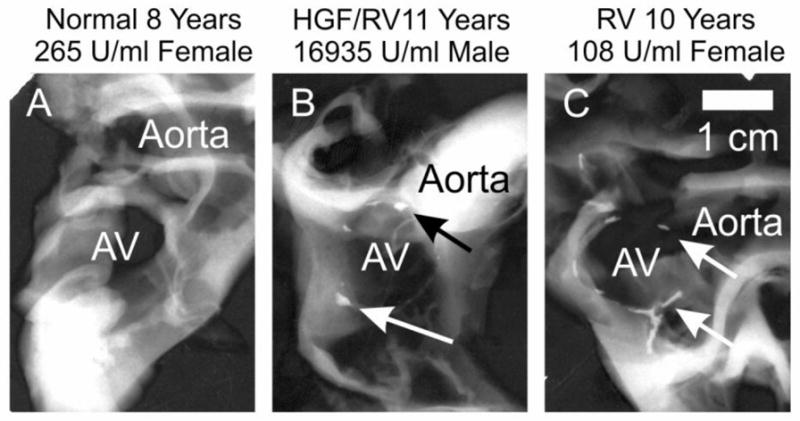
The region of the aorta, AV, and in some cases the MV were dissected from the heart, and radiographs were obtained to evaluate calcification. The position of the aorta is indicated. The middle of the AV annulus is indicated as AV. Arrows for the images in panel B (M1287) and panel D (M1332) indicate regions of calcification in the AV annulus.
4. Discussion
The goal of this study was to determine if neonatal gene therapy with an RV could prevent cardiac valve disease in MPS VII dogs long-term. Cardiac valve disease is a frequent finding in patients with various types of MPS including MPS VII, as discussed in the introduction. This study is an extension of our previous report [30] that neonatal gene therapy can reduce cardiovascular disease for up to 2 years.
4.1. Mitral and aortic valve disease is reduced with neonatal gene therapy
We demonstrate here that untreated MPS VII dogs consistently have MR at 6 months of age or older, and that some develop MVP, suggesting that the strength of the valve might be reduced. It is also possible that irregularities of the leaflet margin could contribute to abnormal coaptation of the leaflets. MPS VII dogs that received neonatal IV injection of a gamma RV had a statistically significant reduction in their average MR and MV thickening scores at most ages at 2 years or less relative to untreated MPS VII dogs, and maintained relatively low scores through age 7 years. Although average scores increased to +2 or greater at 8 years of age and older, the presence of similar MR and MV thickening scores in heterozygous controls from the colony at a similar age make it difficult to determine if abnormalities in the treated dogs were due to MPS VII or to the aging process in dogs. The presence of MR in the normal dogs was likely due to the frequent development of MR in dogs as they age [35–36]. Despite this caveat, it is clear that neonatal gene therapy had a marked beneficial effect on echocardiographic manifestations of MPS VII. The improvement in echocardiographic parameters in the MV in treated MPS VII dogs was associated with an improvement in collagen structure in the MV, as discussed in the accompanying paper. This was likely due to the ability of some enzyme to diffuse into the valve, as the GUSB enzyme activity was 21% of normal in the MV as shown in the accompanying paper, and some GUSB enzyme activity was visible in the valve of treated MPS VII dogs with a histochemical stain, as shown here.
Similarly, treated MPS VII dogs had AR scores that were significantly lower than in untreated dogs at 0.5 years, and AV thickening scores that were lower than in untreated MPS VII dogs at all ages at 2 years or younger. Both AR and AV thickening scores were maintained at low levels for the duration of evaluation in all treated MPS VII dogs, which was up to 11 years in 1 dog. These data are encouraging that improvements in aortic valve disease can be maintained long-term.
4.2. Calcification of cardiac valves
Despite substantial functional and structural improvements in cardiac valves of treated MPS VII dogs relative to untreated MPS dogs, both the AV and the MV developed calcification in the annulus of the majority of the older treated MPS VII dogs, which was apparent at 6 years or older. This is similar to the calcification that was noted in a human MPS VII patient in the accompanying manuscript, while it was previously reported that the MV annulus developed calcification in humans with MPS I at a mean age of 10 years [37]. It is likely that calcification of the MV and AV annulus will pose clinically-relevant problems such as stenosis as patients live longer due to treatment.
4.3 Implications for treatment of MV disease
These data demonstrate that neonatal IV injection of a gamma RV that programs the liver to secrete GUSB can markedly improve the function of the MV for at least 7 years, and the AV for at least 11 years. The average serum GUSB activity was quite high at 2504±4946 U/ml, which was 9.5-fold the level found in homozygous normal dogs. Dogs with lower expression such as M1332 with 108 U/ml of serum GUSB activity (41% normal), lived for up to 10 years and had valves with similar echocardiographic scores as M1287, the HGF/RV-treated dog with very high average serum GUSB activity at 16,935 U/ml (64-fold normal) that lived to 11 years. Therefore, achieving 100 U/ml of serum GUSB activity had a beneficial effect on heart valves and is a reasonable target for humans should gene therapy trials be performed, although differences between species may affect the target serum GUSB activity. Other gene therapy approaches are also being tested in animal models, as we discussed recently [28], but to date the level of expression and clinical benefit have been most profound with the gamma RV than with other vectors in the MPS VII dog model (MEH, unpublished data). Gene therapy could be a simple and effective way to reduce cardiovascular manifestations of MPS if safety concerns can be addressed.
Similar results will likely be seen in patients that receive ERT, as the levels of expression in RV-treated dogs with low serum GUSB activity are likely similar to the amounts of enzyme that can be delivered with ERT, while the levels in the HGF/RV-treated dog M1287 likely exceed those that can be achieved with standard dose ERT [28]. Indeed, Dierenfeld et al. demonstrated that initiation of ERT in the newborn period in MPS I dogs reduced MV thickening at 1 year, and that this was more effective than was initiation at older ages [25]. This gene therapy approach can serve as a surrogate for the long-term effect of ERT, suggesting that ERT will reduce cardiovascular disease long-term if initiated in the newborn period.
Supplementary Material
Highlights.
Neonatal gene therapy with a gamma retroviral vector reduces cardiovascular disease in MPS VII dogs for up to a decade.
These results should predict that initiation of enzyme replacement therapy at birth will reduce cardiovascular disease long-term.
Acknowledgments
Funding was provided by the National Institutes of Health (DK054481 and P40 OD010939 awarded to MEH and HD061879 awarded to KPP), and the National MPS Society. Histology was supported by P30 DK52574.
Footnotes
Mucopolysaccharidosis (MPS); glycosaminoglycan (GAG); mitral valve (MV); aortic valve (AV); mitral regurgitation (MR); aortic regurgitation (AR), hematopoietic stem cell transplantation (HSCT); enzyme replacement therapy (ERT); mannose 6-phosphate (M6P); intravenous (IV); retroviral vector (RV); β-glucuronidase (GUSB); transducing units (TU); hepatocyte growth factor (HGF); left atrium (LA); human α1-antitrypsin (hAAT); long terminal repeat (LTR); mitral valve prolapse (MVP); and chordae tendinae (CT).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Neufeld EF, Muenzer J. The Mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Basis of Inherited Disease. New York: McGraw Hill; 2001. pp. 3421–3452. [Google Scholar]
- 2.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 3.Braunlin EA, Harmatz PR, Scarpa M, Furlanetto B, Kampmann C, Loehr JP, Ponder KP, Roberts WC, Rosenfeld HM, Giugliani R. Cardiac disease in patients with mucopolysaccharidosis: presentation, diagnosis and management. J Inherit Metab Dis. 2011;34:1183–97. doi: 10.1007/s10545-011-9359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wippermann CF, Beck M, Schranz D, Huth R, Michel-Behnke I, Jüngst BK. Mitral and aortic regurgitation in 84 patients with mucopolysaccharidoses. Eur J Pediatr. 1995;154:98–101. doi: 10.1007/BF01991908. [DOI] [PubMed] [Google Scholar]
- 5.Fesslová V, Corti P, Sersale G, Rovelli A, Russo P, Mannarino S, Butera G, Parini R. The natural course and the impact of therapies of cardiac involvement in the mucopolysaccharidoses. Cardiol Young. 2009;19:170–178. doi: 10.1017/S1047951109003576. [DOI] [PubMed] [Google Scholar]
- 6.Dangel JH. Cardiovascular changes in children with mucopolysaccharide storage diseases and related disorders--clinical and echocardiographic findings in 64 patients. Eur J Pediatr. 1998;157:534–538. doi: 10.1007/s004310050872. [DOI] [PubMed] [Google Scholar]
- 7.Soliman OI, Timmermans RG, Nemes A, Vletter WB, Wilson JH, ten Cate FJ, Geleijnse ML. Cardiac abnormalities in adults with the attenuated form of mucopolysaccharidosis type I. J Inherit Metab Dis. 2007;30:750–757. doi: 10.1007/s10545-007-0586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaudet AL, DiFerrante NM, Ferry GD, Nichols BL, Jr, Mullins CE. Variation in the phenotypic expression of beta-glucuronidase deficiency. J Pediatr. 1975;86:388–394. doi: 10.1016/s0022-3476(75)80968-1. [DOI] [PubMed] [Google Scholar]
- 9.Sly WS, Quinton BA, McAlister WH, Rimoin DL. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973;82:249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- 10.Vogler C, Levy B, Kyle JW, Sly WS, Williamson J, Whyte MP. Mucopolysaccharidosis VII: postmortem biochemical and pathological findings in a young adult with beta-glucuronidase deficiency. Mod Pathol. 1994;7:132–137. [PubMed] [Google Scholar]
- 11.Schroeder L, Orchard P, Whitley CB, Berry JM, Tolar J, Miller W, Braunlin EA. Cardiac Ultrasound Findings in Infants with Severe (Hurler Phenotype) Untreated Mucopolysaccharidosis (MPS) Type I. JIMD Rep. 2013 doi: 10.1007/8904_2012_208. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arn P, Wraith JE, Underhill L. Characterization of Surgical Procedures in Patients with Mucopolysaccharidosis Type I: Findings from the MPS I Registry. J Pediatr. 2009;154:859–864. doi: 10.1016/j.jpeds.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Grigull L, Sykora KW, Tenger A, Bertram H, Meyer-Marcotty M, Hartmann H, Bültmann E, Beilken A, Zivicnjak M, Mynarek M, Osthaus AW, Schilke R, Kollewe K, Lücke T. Variable disease progression after successful stem cell transplantation: prospective follow-up investigations in eight patients with Hurler syndrome. Pediatr Transplant. 2011;15:861–869. doi: 10.1111/j.1399-3046.2011.01595.x. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe N, Anagnostopoulos PV, Azakie A. Aortic stenosis in a patient with Hurler’s syndrome after bone marrow transplantation. Cardiol Young. 2011;21:349–50. doi: 10.1017/S1047951110002015. [DOI] [PubMed] [Google Scholar]
- 15.Braunlin EA, Stauffer NR, Peters CH, Bass JL, Berry JM, Hopwood JJ, Krivit W. Usefulness of bone marrow transplantation in the Hurler syndrome. Am J Cardiol. 2003;92:882–886. doi: 10.1016/s0002-9149(03)00909-3. [DOI] [PubMed] [Google Scholar]
- 16.Gompf RE, Shull RM, Breider MA, Scott JA, Constantopoulos GC. Cardiovascular changes after bone marrow transplantation in dogs with mucopolysaccharidosis I. Am J Vet Res. 1990;51:2054–2060. [PubMed] [Google Scholar]
- 17.Sammarco C, Weil M, Just C, Weimelt S, Hasson C, O’Malley T, Evans SM, Wang P, Casal ML, Wolfe J, Haskins M. Effects of bone marrow transplantation on the cardiovascular abnormalities in canine mucopolysaccharidosis VII. Bone Marrow Transplant. 2000;25:1289–1297. doi: 10.1038/sj.bmt.1702448. [DOI] [PubMed] [Google Scholar]
- 18.Braunlin EA, Berry JM, Whitley CB. Cardiac findings after enzyme replacement therapy for mucopolysaccharidosis type I. Am J Cardiol. 2006;98:416–418. doi: 10.1016/j.amjcard.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 19.Brands MM, Frohn-Mulder IM, Hagemans ML, Hop WC, Oussoren E, Helbing WA, van der Ploeg AT. Mucopolysaccharidosis: Cardiologic features and effects of enzyme-replacement therapy in 24 children with MPS I, II and VI. J Inherit Metab Dis. 2013;36:227–234. doi: 10.1007/s10545-011-9444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sifuentes M, Doroshow R, Hoft R, Mason G, Walot I, Diament M, Okazaki S, Huff K, Cox GF, Swiedler SJ, Kakkis ED. A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab. 2007;90:171–180. doi: 10.1016/j.ymgme.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Braunlin E, Rosenfeld H, Kampmann C, Johnson J, Beck M, Giugliani R, Guffon N, Ketteridge D, SáMiranda CM, Scarpa M, Schwartz IV, Leão Teles E, Wraith JE, Barrios P, Dias da Silva E, Kurio G, Richardson M, Gildengorin G, Hopwood JJ, Imperiale M, Schatz A, Decker C, Harmatz P. MPS VI Study Group, Enzyme replacement therapy for mucopolysaccharidosis VI: long-term cardiac effects of galsulfase (Naglazyme(®)) therapy. J Inherit Metab Dis. 2013;36:385–94. doi: 10.1007/s10545-012-9481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furujo M, Kubo T, Kosuga M, Okuyama T. Enzyme replacement therapy attenuates disease progression in two Japanese siblings with mucopolysaccharidosis type VI. Mol Genet Metab. 2011;104:597–602. doi: 10.1016/j.ymgme.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 23.Leal GN, de Paula AC, Morhy SS, Andrade JL, Kim CA. Advantages of early replacement therapy for mucopolysaccharidosis type VI: echocardiographic follow-up of siblings. Cardiol Young Mar. 2013;5:1–7. doi: 10.1017/S1047951113000152. [DOI] [PubMed] [Google Scholar]
- 24.McGill JJ, Inwood AC, Coman DJ, Lipke ML, de Lore D, Swiedler SJ, Hopwood JJ. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age--a sibling control study. Clin Genet. 2010;77:492–8. doi: 10.1111/j.1399-0004.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 25.Dierenfeld AD, McEntee MF, Vogler CA, Vite CH, Chen AH, Passage M, Le S, Shah S, Jens JK, Snella EM, Kline KL, Parkes JD, Ware WA, Moran LE, Fales-Williams AJ, Wengert JA, Whitley RD, Betts DM, Boal AM, Riedesel EA, Gross W, Ellinwood NM, Dickson PI. Replacing the enzyme alpha-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I. Sci Transl Med. 2010;2:60ra89. doi: 10.1126/scitranslmed.3001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponder KP, Haskins ME. Gene therapy for mucopolysaccharidosis. Expert Opin Biol Ther. 2007;7:1333–1345. doi: 10.1517/14712598.7.9.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponder KP, Melniczek JR, Xu L, Weil MA, O’Malley TM, O’Donnell PA, Knox VW, Aguirre GD, Mazrier H, Ellinwood NM, Sleeper M, Maguire AM, Volk SW, Mango RL, Zweigle J, Wolfe JH, Haskins ME. Therapeutic neonatal hepatic gene therapy in mucopolysaccharidosis VII dogs. Proc Natl Acad Sci USA. 2002;99:13102–13107. doi: 10.1073/pnas.192353499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith LJ, Martin JT, O’Donnell P, Wang P, Elliott DM, Haskins ME, Ponder KP. Effect of neonatal gene therapy on lumbar spine disease in mucopolysaccharidosis VII dogs. Mol Genet Metab. 2012;107:145–52. doi: 10.1016/j.ymgme.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Haskins ME, Melniczek JR, Gao C, Weil MA, O’Malley TM, O’Donnell PA, Mazrier H, Ellinwood NM, Zweigle J, Wolfe JH, Ponder KP. Transduction of hepatocytes after neonatal delivery of a Moloney murine leukemia virus based retroviral vector results in long-term expression of beta-glucuronidase in mucopolysaccharidosis VII dogs. Mol Ther. 2002;5:141–153. doi: 10.1006/mthe.2002.0527. [DOI] [PubMed] [Google Scholar]
- 30.Sleeper MM, Fornasari B, Ellinwood NM, Weil MA, Melniczek J, O’Malley TM, Sammarco CD, Xu L, Ponder KP, Haskins ME. Gene therapy ameliorates cardiovascular disease in dogs with mucopolysaccharidosis VII. Circulation. 2004;110:815–820. doi: 10.1161/01.CIR.0000138747.82487.4B. [DOI] [PubMed] [Google Scholar]
- 31.Metcalf JA, Linders B, Wu S, Bigg P, O’Donnell P, Sleeper MM, Whyte MP, Haskins M, Ponder KP. Upregulation of elastase activity in aorta in mucopolysaccharidosis I and VII dogs may be due to increased cytokine expression. Mol Genet Metab. 2010;99:396–407. doi: 10.1016/j.ymgme.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponder KP, O’Malley TM, Wang P, O’Donnell PA, Traas AM, Knox VW, Aguirre GA, Ellinwood NM, Metcalf JA, Wang B, Parkinson-Lawrence EJ, Sleeper MM, Brooks DA, Hopwood JJ, Haskins ME. Neonatal gene therapy with a gamma retroviral vector in mucopolysaccharidosis VI cats. Mol Ther. 2012;20:898–907. doi: 10.1038/mt.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traas AM, Wang P, Ma X, Tittiger M, Schaller L, O’donnell P, Sleeper MM, Vite C, Herati R, Aguirre GD, Haskins M, Ponder KP. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol Ther. 2007;15:1423–31. doi: 10.1038/sj.mt.6300201. [DOI] [PubMed] [Google Scholar]
- 34.Ray J, Bouvet A, DeSanto C, Fyfe JC, Xu D, Wolfe JH, Aguirre GD, Patterson DF, Haskins ME, Henthorn PS. Cloning of the canine beta-glucuronidase cDNA, mutation identification in canine MPS VII, and retroviral vector-mediated correction of MPS VII cells. Genomics. 1998;48:248–253. doi: 10.1006/geno.1997.5189. [DOI] [PubMed] [Google Scholar]
- 35.Fox PR. Pathology of myxomatous mitral valve disease in the dog. J Vet Cardiol. 2012;14:103–126. doi: 10.1016/j.jvc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Borgarelli M, Haggstrom J. Canine degenerative myxomatous mitral valve disease: natural history, clinical presentation and therapy. Vet Clin North Am Small Anim Pract. 2010;40:651–663. doi: 10.1016/j.cvsm.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Renteria VG, Ferrans VJ, Roberts WC. The heart in the Hurler syndrome: gross, histologic and ultrastructural observations in five necropsy cases. Am J Cardiol. 1976;38:487–501. doi: 10.1016/0002-9149(76)90468-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.