Abstract
The quantitative assessment of gene expression and related enzyme activity in vivo could be important for the characterization of gene altering diseases and therapy. The development of imaging techniques, based on specific reporter molecules may enable routine non-invasive assessment of enzyme activity and gene expression in vivo. We recently reported the use of commercially available S-Gal® as a β-galactosidase reporter for 1HMRI, and the synthesis of several S-Gal® analogs with enhanced response to β-galactosidase activity. We have now compared these analogs in vitro and have identified the optimal analog, C3-GD, based on strong T1 and T2 response to enzyme presence (ΔR1 and ΔR2 ∼ 1.8 times S-Gal®). Moreover, application is demonstrated in vivo in human breast tumor xenografts. MRI studies in MCF7-lacZ tumors implanted subcutaneously in athymic nude mice (n = 6), showed significant reduction in T1 and T2 values (each ∼ 13%) 2 h after intratumoral injection of C3-GD, whereas the MCF7 (wild type) tumors showed slight increase. Thus, C3-GD successfully detects β-galactosidase activity in vivo and shows promise as a lacZ gene 1HMR reporter molecule.
Keywords: β-galactosidase, 1H MRI, T1, T2, Signal enhancement, Fe-chelation, lacZ, Gene reporters
1. Introduction
One of the major challenges in the field of gene therapy has been the lack of methods to quantitatively assess the success of gene transfection and the longevity of gene expression [1]. Reporter genes are typically used to assess regulation and transfection of genes in biological pathways. Popular reporter genes are those that generate β-galactosidase (β-gal), β-glucuronidase, firefly luciferase, fluorescent proteins (e.g., green fluorescent protein), transferrin and ferritin [1–3]. Historically, the lacZ gene encoding β-gal has been the most extensively used reporter with applications ranging from basic science to translational and clinical trials for assaying protein expression and interactions [4,5].
There are various commercially available reagents (substrates) in regular use to detect β-gal expression in vitro by colorimetric stains and assays, such as nitrophenyl-β-d-galactopyranoside (generates yellow color) [6], 4-chloro-3-bromoindole-galactose (X-Gal®, generates a blue stain) [7] and 3,4-cyclohexenoesculetin β-d-galactopyranoside (S-Gal® - generates a black stain) [8]. The β-gal expression is also associated with cellular senescence [9] and X-Gal® has been used to assay senescence-associated β-gal in vitro and ex vivo [10].
In recent years, development of novel reporter molecules and techniques for non-invasive in vivo detection of β-gal activity, as a marker of lacZ transgene expression, has been reported including optical [11–13], photoacoustic [14], radionuclide [15,16] and MR imaging (1H [17–19] and/or 19 F [19–24]) methods. In a recent study, we demonstrated the ability to detect β-gal activity in lacZ-transfected breast cancer cells (MCF7) in vitro and in vivo using the commercially available S-Gal® by 1H MRI [25]. We also demonstrated the synthesis of several S-Gal® analogs for improved response to β-gal activity [26]. In both cases, β-gal cleaves the substrate molecule at the C-O bond between β-d-galactopyranoside and aglycone, to release the aglycone, which forms a paramagnetic chelate in the presence of Fe3+ generating T1/T2/T2*-weighted 1H MRI contrast. Fig. 1 illustrates the mechanism for 1H-MRI detection of β-gal activity, using C3-GD as a model reporter. In the present study, we have compared the S-Gal® analogs in vitro and shown application of the lead agent for detection of β-galactosidase activity in human breast tumor xenografts.
Fig. 1.
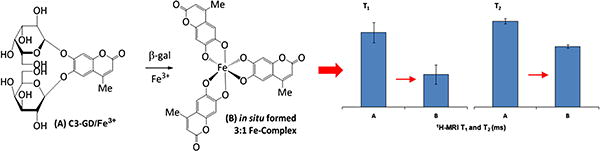
The illustration of mechanism for 1H-MRI detection of β-gal activity, using C3-GD as a model reporter.
2. Materials and methods
2.1. General
3,4-Cyclohexenoesculetin β-d-galactopyranoside (S-Gal® sodium salt), and ferric ammonium citrate (FAC) were purchased from Sigma-Aldrich (St. Louis, MO). Monogalactopryanosides 7-O-(β-d-galactopyranosyl)-8-hydroxy-3, 4-cyclohexenocoumarin (C1-MGD), 7-O-(β-d-galactopyranosyl)-8-hydroxy-4-methylcoumarin (C2-MGD), 7-O-(β-d-galactopyranosyl)-6-hydroxy-4-methylcoumarin (C3-MGD), 7-O-(β-d-galactopyranosyl)-8-hydroxy-6-methoxycoumarin (C4-MGD) and digalactopryanosides 7,8-di-O-(β-d-galactopyranosyl)-3, 4-cyclohexenocoumarin (C1-GD), 7,8-di-O-(β-d-galactopyranosyl)-4-methylcoumarin (C2-GD), 6,7-di-O-(β-d-galactopyranosyl)-4-methylcoumarin (C3-GD), 7,8-di-O-(β-d-galactopyranosyl)-6-methoxycoumarin (C4-GD) (Fig. 2) were synthesized by us as described previously [26]. All MR studies were performed on a Varian INOVA 4.7 T horizontal-bore MR system (200 MHz for 1H) equipped with actively shielded gradients. T1 mapping was performed using a spin echo sequence with TE = 12 ms and varying TR (0.2–6 s), while T2 mapping was performed using a spin echo sequence with varying TE (12–200 ms) and TR = 6 s.
Fig. 2.
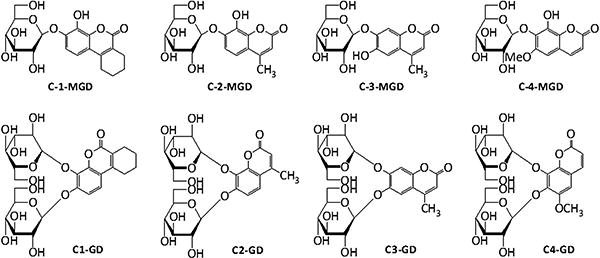
The structures of mono- and di-galactoside analogs of S-gal®.
2.2. Detection of β-gal activity in vitro
For in vitro measurements, each reporter molecule solution (15 mM in de-ionized water, 40 μL) was mixed with FAC (5 mM, 40 μL) in 2% agar solution with or without β-gal (5 units, E801A, Sigma-Aldrich, St. Louis, MO) and placed in a 4 × 4 cut section of a 96 well plate. The prepared phantom was placed on a heating block maintained at 37 °C for 30 mins for the enzymatic reaction to stabilize. T1 and T2 maps of the phantom were acquired using a 3 cm solenoid coil (home-built) at 37 °C. MRI parameters: FOV 40 mm × 40 mm, matrix size 128 × 128, slice thickness 1 mm.
2.3. In vivo detection of β-gal activity in MCF7 and MCF7-lacZ tumors
All in vivo studies were performed with approval from the UT Southwestern Medical Center Animal Care and Use Committee. MCF7 (wild-type 1.5 × 106) and MCF7-lacZ (2 × 106) cells were implanted subcutaneously in the thighs of athymic nude mice (N = 6) on contralateral sides. MRI studies were performed when the tumor size reached 0.75 cm3. Mice were anesthetized with 1.5% isoflurane in air and placed in a Litzcage volume coil (Doty Scientific Inc, Columbia, SC) for MRI. Animal body temperature was maintained at 37 °C using a circulating warm water pad. Baseline (pre-injection) T1 and T2 maps of tumor containing slices were acquired using MRI parameters: FOV 100 mm × 50 mm, matrix size 256 × 128, slice thickness 1 mm, 13 slices. Following baseline imaging, the mouse was removed from the scanner and 25 μL of a solution containing 15 mM C3-GD and 5 mM FAC in water was injected intra-tumorally in a fan pattern in a sagittal plane using a fine 32G Hamilton® needle into each tumor. After repositioning the mouse carefully, T1 and T2 maps were acquired 1 and 2 hours post injection. Multiple slices were acquired and those with injected contrast agent were identified based on the T2- and T1-weighted images (to delineate tumor boundary and locate the injected Fe3+ ions, respectively) for analysis. Tumor voxels from the injected slice of all animals were pooled with respect to imaging time point (baseline, 1 h and 2 h post injection) and statistical analysis with one-way ANOVA followed by Bonferroni's multiple comparison test was performed using GraphPad Prism (GraphPad Software Inc., La Jolla, CA).
2.4. Histology
Following MRI, the tumors were excised and fixed in 4% formalin. These tumor sections were embedded in paraffin and then cut into 5 μm-thick sections. These sections were further stained with 4-chloro-3-bromoindole-galactoside (X-Gal®) and counter stained by nuclear fast red to reveal β-gal activity and nuclear locations respectively.
3. Results
3.1. Comparison of reporter molecules in vitro
T1 and T2 maps of the reporter molecules together with FAC in agar gel phantoms showed varying contrast in the presence of 5 units β-gal (Fig. 3). The largest responses in T1 were observed for C3-GD, C2-MGD and C3-MGD with decreases of 1.12 s, 1.24 s, and 1.17 s, respectively, which is greater than 1.03 s observed using S-Gal® (Table 1). Similarly, C3-GD and C3-MGD showed a decrease in T2 of 45 ms and 95 ms respectively, which is significantly higher than 23 ms observed using S-Gal® (Table 1). These values correspond to ΔR1 and ΔR2 about 1.8 times greater than S-Gal® for C3-GD and ΔR1 ∼ 2.8 and ΔR2 ∼ 6.5 times S-Gal® for C3-MGD. C3-GD was used for further in vivo studies due to better water solubility than C3-MGD.
Fig. 3.
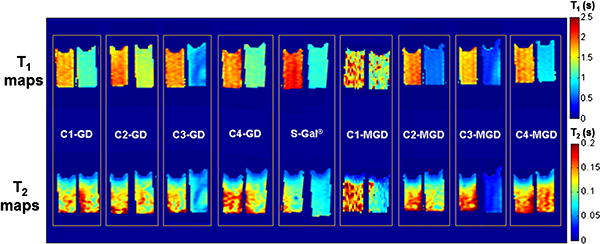
In vitro lacZ gene reporter activity of various analogs of S-gal ®. T1 (top row) and T2 (bottom row) maps of each sample containing 15 mM agent + 5 mM FAC in agarose without or with 5 units of β-gal enzyme (left and right sample in each box, respectively). Pronounced T1 and T2 effects are seen for the agents C3-GD and C3-MGD.
Table 1.
In vitro comparison of various analogs of S-gal® as MR gene reporter molecules: T1 and T2 (mean ± standard deviation) of the synthesized gene reporter molecules (7.5 mM) in the absence or presence of β-galactosidase (5 units) in 1% agar containing ferric ammonium citrate (FAC, 2.5 mM).
| Agent | T1 (s) |
(s-1) |
T2 (ms) |
(s-1) |
||
|---|---|---|---|---|---|---|
|
|
|
|||||
| w/o β-gal | w/β-gal | w/o β-gal | w/β-gal | |||
| C1-MGD | 1.79 ± 0.28 | 1.46 ± 0.19 | 0.13 | 0.13 ± 0.04 | 0.09 ± 0.03 | 3.42 |
| C2-MGD | 1.83 ± 0.13 | 0.58 ± 0.05 | 1.18 | 0.12 ± 0.05 | 0.10 ± 0.03 | 1.72 |
| C3-MGD | 1.68 ± 0.12 | 0.51 ± 0.13 | 1.37 | 0.13 ± 0.05 | 0.03 ± 0.01 | 22.09 |
| C4-MGD | 1.78 ± 0.09 | 0.84 ± 0.05 | 0.63 | 0.13 ± 0.05 | 0.13 ± 0.03 | 0.09 |
| S-Gal® | 2.07 ± 0.12 | 1.04 ± 0.05 | 0.48 | 0.09 ± 0.03 | 0.07 ± 0.01 | 3.41 |
| C1-GD | 1.76 ± 0.13 | 1.11 ± 0.09 | 0.33 | 0.12 ± 0.03 | 0.12 ± 0.04 | −0.84 |
| C2-GD | 1.81 ± 0.15 | 1.39 ± 0.09 | 0.17 | 0.12 ± 0.05 | 0.11 ± 0.03 | 0.76 |
| C3-GD | 1.83 ± 0.15 | 0.72 ± 0.12 | 0.84 | 0.11 ± 0.04 | 0.07 ± 0.01 | 5.97 |
| C4-GD | 1.85 ± 0.15 | 1.18 ± 0.06 | 0.31 | 0.13 ± 0.05 | 0.11 ± 0.04 | 1.67 |
Also listed are the corresponding differences in the relaxation rates due to β-galactosidase ( and , respectively). T1 and T2 values of the agents C3-GD and C3-MGD decreased the most in the presence of the enzyme.
3.2. Evaluation of reporter molecule C3-GD in vivo
T1 and T2 maps of athymic nude mice bearing wild-type (WT) and lacZ-transfected MCF7 tumors before and after the injection of C3-GD (15 mM) + FAC (5 mM) solution (25 μL vol.) are shown in Fig. 4. T1-weighted images showed small hyper-intensities, which were better perceived from the decrease in T1 values, as seen in the T1 maps of representative tumor slices (Fig. 3a). There was a small, but significant, difference between mean baseline T1 (1.84 ± 0.28 s; WT (n = 6) vs. 1.88 ± 0.22 s; lacZ (n = 6); p < 0.0001; unpaired t-test) of the two tumor types (Table 2). Following injection of reporter molecule solution (C3-GD + FAC), T1 decreased significantly in WT tumors (T1 = 1.72 ± 0.29 s, p < 0.0001) after 1 h, but recovered back by the end of 2 h (T1 = 1.97 ± 0.35 s, p < 0.0001) to exceed pre-injection values. In lacZ tumors, T1 decreased significantly by the end of 1 h (T1 = 1.69 ± 0.33 s, p < 0.0001) and showed continued decrease at 2 h (T1 = 1.63 ± 0.25 s, p < 0.0001).
Fig. 4.
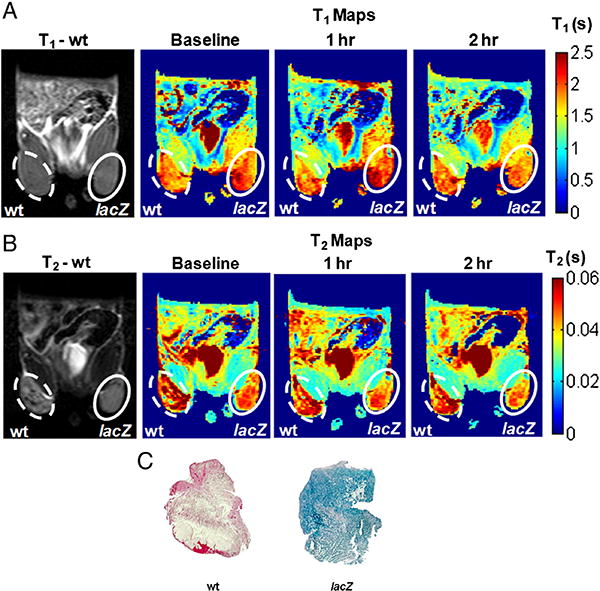
In vivo lacZ gene reporter activity of C3-GD. MRI of a representative nude mouse with wild type MCF7 tumor (left, dotted) and lacZ transfected MCF7 tumor (right, solid). A) Baseline T1 weighted image and T1 maps obtained before, 1 h after, and 2 h after direct intra tumoral injection of 15 mM C3-GD and 5 mM FAC (top row) and B) corresponding T2 weighted images and T2 maps (bottom row) showed decrease in the relaxation times in lacZ transfected tumors. C) X-gal and Nuclear fast staining of slices (whole mount) from the same wild type MCF7 (left) and MCF7-lacZ (right) tumors showed β-gal activity (intense blue stain from X-gal) for the MCF7-lacZ tumor section only.
Table 2.
In vivo lacZ gene reporter activity of C3-GD: T1 and T2 (mean ± standard deviation) obtained from ROI analysis of in vivo data (n = 6) from wild type and lacZ transfected MCF7 tumors.
| T1 (s) |
(s-1) |
T2 (ms) |
(s-1) |
|||
|---|---|---|---|---|---|---|
|
|
|
|||||
| MCF7-WT | MCF7-lacZ | MCF7-WT | MCF7-lacZ | |||
| Baseline | 1.84 ± 0.28 | 1.88 ± 0.22 | −0.01 | 56 ± 16 | 51 ± 8 | 1.75 |
| 1 hr post injection | 1.72 ± 0.29* | 1.69 ± 0.33* | 0.01 | 51 ± 16* | 44 ± 10* | 3.12 |
| 2 hr post injection | 1.97 ± 0.35* | 1.63 ± 0.25* | 0.51 | 57 ± 18 | 44 ± 9* | 5.18 |
| Change over 1 hr | −6.5% | −10.1% | - | −8.9% | −13.7% | - |
| Change over 2 hr | 7.1% | −13.3% | - | 1.7% | −13.7% | - |
Also listed are the corresponding differences in the relaxation rates (between wild type and lacZ tumor) due to β-galactosidase (ΔR1β-gal and ΔR2β-gal, respectively). MCF7-lacZ tumors showed a significant decrease in both T1 and T2 values at 2 h post injection compared to baseline.
Analysis of the same ROIs (as in T1 analysis) on T2 maps showed large decrease in T2 for MCF7-lacZ tumors when compared to baseline (Fig. 4b). There was a significant difference in the baseline T2 values of WT and lacZ transfected tumors (T2 = 56 ± 16 ms (WT) vs. T2 = 51 ± 8 ms (lacZ); p < 0.0001; unpaired t-test). Following administration of the solution of C3-GD + FAC, T2 decreased significantly in WT tumors (T2 = 51 ± 16 ms; p < 0.0001) at 1 h, but returned back to baseline value (T2 = 57 ± 18 ms; p > 0.05) at 2 h (Table 2). In lacZ tumors, T2 decreased significantly by the end of 1 h (T2 = 44 ± 10, p < 0.0001) and remained unchanged at 2 h (T2 = 44 ± 9, p < 0.0001). The X-gal and nuclear fast staining of tumor slices (Fig. 4) obtained following the MRI showed an intense blue stain representing β-gal activity only in the MCF7-lacZ tumor, and not in the MCF7 wild-type tumor (Fig. 4c).
4. Discussion
We have demonstrated the relative relaxation response of various S-Gal® analogs to β-galactosidase. 1H MR showed both T1 and T2 response in vitro and the lead agent was examined in vivo. The mono- and di-galactopyranosides showed differential enhancements in response to β-gal in the presence of ferric ions (Fe3+ from FAC). The aglycone products readily form paramagnetic iron chelates causing pronounced T1 and T2 shortening in MR images. The mono-galactopyranosides always showed better T1 and T2 contrast when compared to their associated di-galactopyranosides: e.g., C2-MGD and C3-MGD showed greater T1 & T2 shortening than C2-GD and C3-GD, respectively (Fig. 2). This can be attributed to the need for two reactions on the di-galactopyranoside before liberating the aglycone to form the iron chelate. The mono and di-galactopyranosides display a variety of hydrolytic rate constants all of which lead to reaction completion times that are greater than the 30 min incubation time used here (even after accounting for the higher enzyme amount used in [26]). Thus, differences between various analogs may reflect differences in product generation at the 30 min time point apart from the relaxivities of the chelation complexes. C3-MGD and C3-GD both demonstrated pronounced T1 and T2 shortening in the presence of 5 units of β-gal, but given its better water solubility, C3-GD was used for in vivo studies. Our goal in this study was to compare the various synthesized gene reporter molecules and identify lead candidates in vitro followed by in vivo testing of detectability of reporter activity. With that in mind, the intra-tumoral injection route was preferred over i.v. delivery to ensure consistent deposition of equal amount of the agent in lacZ transfected and control tumors. In comparison, an i.v. delivery would have resulted in heterogeneous deposition of the agent due to variations in vasculature and confounded the testing by the inability to distinguish between poor delivery and poor in vivo activity. We do note that solubility was not an issue for intratumoral delivery, but it could be important for potential systemic delivery applications in the future. At the concentrations used for direct intra-tumoral injections in this study, either agent could have been used and given the higher contrast effect and lower solubility, we can expect better performance of C3-MGD for applications that permit intra-tissue injection of the reporter probes. Given that the chelation complex is identical for C3-MGD and C3-GD, other practical concerns such as hydrolytic rates, cellular uptake and retention may dominate the effective observed contrast in vivo.
Following intra-tumoral injection of the C3-GD/FAC combination in wild type tumors, we observed a decrease in T1 and T2 from baseline to 1 h followed by return to baseline values by 2 h. This initial decrease in T1 and T2 values can be attributed to the free ferric ions, which are eventually cleared. In the lacZ expressing tumors, paramagnetic complexes are formed due to the chelation of ferric ions by the β-gal-cleaved aglycone molecules. This causes a shortening of T1 and T2 when compared to baseline (pre-injection) measurements. Catechol moieties exhibit a high affinity for Fe3+ and the complex is expected to stabilize the ferric state preventing other changes under biological conditions [27,28]. The aglycone-Fe3+ complex is poorly soluble and is likely to precipitate in situ (source of the strong T2 contrast), further aiding the stability of the complex in vivo. The clearance of the nano-scale precipitate is expected to be slow compared to small molecular chelates, as seen previously with S-Gal® [25]. In similar experiments with S-Gal® we previously noted intense contrast immediately after injection due to T2 shortening, whereas after one hour, the contrast declined considerably, presumably due to clearance [25]. By comparison, the current experiments used only 8% the concentration of FAC and 20% as much substrate, yet achieved ∼13% reduction in baseline relaxation times over 2 h in the same tumor types. This indicates better retention and higher in vivo sensitivity to C3-GD in tumors compared to S-Gal®. The lower dose of FAC needed to achieve detectable contrast suggests greater potential feasibility for systemic delivery of the substrate/FAC combination for assessment of lacZ gene expression in vivo, though this has not been tested.
By analogy, alternative sugar moieties could be used to develop substrates to report other enzymes such as glucoronidases and glucosidases. The ability to observe both T1 and T2 contrast adds considerably to the confidence of identifying β-gal activity. Otherwise tissue heterogeneity may mask contrast. We chose to deliver the reporter system intra-tumorally in this proof of principle study to separate the effect of delivery from the activity on the observed contrast. In its existing state, this system can be useful for pre-clinical investigations of development and improvement of novel gene-therapy approaches. For potential human use, the system will need to be refined to the point where even endogenous Fe (up-regulated in cancer and a target for chelation therapy) can provide sufficient contrast. While intra-tumoral injections are sub-optimal for human use, we note that all the recent studies on gene-therapy in the clinic have used intra-tumoral/intra-organ injection of the adenoviral vector [29]. Most β-gal reporters to date also have required direct intra-tumoral injection, but systemic administration would be advantageous and remains a goal.
Acknowledgments
This research was supported in part by the NIH National Cancer Institute (R21 CA120774, R21 CA132096) and the Southwestern Small Animal Imaging Research Program (SW-SAIRP), which is supported in part by U24 CA126608 and P30 CA142543. NMR experiments were performed at the Advanced Imaging Research Center, an NIH BTRP facility (P41RR02584).
References
- 1.Gilad AA, Winnard PT, Jr, van Zijl PC, Bulte JW. Developing MR reporter genes: promises and pitfalls. NMR Biomed. 2007;20(3):275–90. doi: 10.1002/nbm.1134. [DOI] [PubMed] [Google Scholar]
- 2.Contag CH, Ross BD. It's not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J Magn Reson Imaging. 2002;16(4):378–87. doi: 10.1002/jmri.10178. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman R. Green fluorescent protein imaging of tumour growth, metastasis, and angiogenesis in mouse models. Lancet Oncol. 2002;3(9):546–56. doi: 10.1016/s1470-2045(02)00848-3. [DOI] [PubMed] [Google Scholar]
- 4.Kruger A, Schirrmacher V, Khokha R. The bacterial lacZ gene: An important tool for metastasis research and evaluation of new cancer therapies. Cancer Metastasis Rev. 1999;17:285–94. doi: 10.1023/a:1006066706040. [DOI] [PubMed] [Google Scholar]
- 5.Braybrooke JP, Slade A, Deplanque G, Harrop R, Madhusudan S, Forster MD, et al. Phase I study of MetXia-P450 gene therapy and oral cyclophosphamide for patients with advanced breast cancer or melanoma. Clin Cancer Res. 2005;11(4):1512–20. doi: 10.1158/1078-0432.CCR-04-0155. [DOI] [PubMed] [Google Scholar]
- 6.Eustice DC, Feldman PA, Colberg-Poley AM, Buckery RM, Neubauer RH. A sensitive method for the detection of beta-galactosidase in transfected mammalian cells. Biotechniques. 1991;11(6):739–740. 742–733. [PubMed] [Google Scholar]
- 7.Horwitz JP, Chua J, Curby RJ, Tomson AJ, Darooge MA, Fisher BE, et al. Substrates for cytochemical demonstration of enzyme activity. I. Some substituted 3-indolyl-beta-d-glycopyranosides. J Med Chem. 1964;7:574–5. doi: 10.1021/jm00334a044. [DOI] [PubMed] [Google Scholar]
- 8.Heuermann K, Cosgrove J. S-Gal: an autoclavable dye for color selection of cloned DNA inserts. Biotechniques. 2001;30(5):1142–7. doi: 10.2144/01305pf01. [DOI] [PubMed] [Google Scholar]
- 9.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci. 1995;92(20):9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandyopadhyay D, Gatza C, Donehower LA, Medrano EE. Analysis of cellular senescence in culture in vivo: the senescence-associated beta-galactosidase assay. Current protocols in cell biology / editorial board, Juan S Bonifacino [et al.] 2005;Chapter 18(Unit 18 19) doi: 10.1002/0471143030.cb1809s27. [DOI] [PubMed] [Google Scholar]
- 11.Tung CH, Zeng Q, Shah K, Kim DE, Schellingerhout D, Weissleder R. In vivo imaging of beta-galactosidase activity using far red fluorescent switch. Cancer Res. 2004;64(5):1579–83. doi: 10.1158/0008-5472.can-03-3226. [DOI] [PubMed] [Google Scholar]
- 12.Josserand V, Texier-Nogues I, Huber P, Favrot MC, Coll JL. Non-invasive in vivo optical imaging of the lacZ and luc gene expression in mice. Gene Ther. 2007;14(22):1587–93. doi: 10.1038/sj.gt.3303028. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Mason RP. Imaging beta-galactosidase activity in human tumor xenografts and transgenic mice using a chemiluminescent substrate. PLoS One. 2010;5(8):e12024. doi: 10.1371/journal.pone.0012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Zemp RJ, Lungu G, Stoica G, Wang LHV. Photoacoustic imaging of lacZ gene expression in vivo. J Biomed Opt. 2007;12(2):020504. doi: 10.1117/1.2717531. [DOI] [PubMed] [Google Scholar]
- 15.Lee KH, Byun SS, Choi JH, Paik JY, Choe YS, Kim BT. Targeting of lacZ reporter gene expression with radioiodine-labelled phenylethyl-beta-D-thiogalactopyranoside. Eur J Nucl Med Mol Imaging. 2004;31(3):433–8. doi: 10.1007/s00259-003-1395-7. [DOI] [PubMed] [Google Scholar]
- 16.Celen S, Deroose C, de Groot T, Chitneni SK, Gijsbers R, Debyser Z, et al. Synthesis and evaluation of 18 F- and 11C-labeled phenyl-galactopyranosides as potential probes for in vivo visualization of LacZ gene expression using positron emission tomography. Bioconjug Chem. 2008;19(2):441–9. doi: 10.1021/bc700216d. [DOI] [PubMed] [Google Scholar]
- 17.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18(3):321–5. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 18.Chang YT, Cheng CM, Su YZ, Lee WT, Hsu JS, Liu GC, et al. Synthesis and characterization of a new bioactivated paramagnetic gadolinium(III) complex [Gd(DOTA-FPG)(H2O)] for tracing gene expression. Bioconjug Chem. 2007;18(6):1716–27. doi: 10.1021/bc070019s. [DOI] [PubMed] [Google Scholar]
- 19.Yu JX, Kodibagkar VD, Hallac RR, Liu L, Mason RP. Dual (19)F/(1)H MR gene reporter molecules for in vivo detection of beta-galactosidase. Bioconjug Chem. 2012;23(3):596–603. doi: 10.1021/bc200647q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui W, Otten P, Li Y, Koeneman KS, Yu J, Mason RP. Novel NMR approach to assessing gene transfection: 4-fluoro-2-nitrophenyl-beta-D-galactopyranoside as a prototype reporter molecule for beta-galactosidase. Magn Reson Med. 2004;51(3):616–20. doi: 10.1002/mrm.10719. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Otten P, Ma Z, Cui W, Liu L, Mason RP. Novel NMR platform for detecting gene transfection: synthesis and evaluation of fluorinated phenyl beta-D-galactosides with potential application for assessing LacZ gene expression. Bioconjug Chem. 2004;15(6):1334–41. doi: 10.1021/bc049936d. [DOI] [PubMed] [Google Scholar]
- 22.Yu JX, Ma Z, Li Y, Koeneman KS, Liu L, Mason RP. Synthesis and evaluation of a novel gene reporter molecule: detection of β-galactosidase activity using 19 F NMR of a fluorinated vitamin B6 conjugate. Med Chem. 2005;1(3):255–62. doi: 10.2174/1573406053765495. [DOI] [PubMed] [Google Scholar]
- 23.Kodibagkar VD, Yu J, Liu L, Hetherington HP, Mason RP. Imaging beta-galactosidase activity using 19F chemical shift imaging of LacZ gene-reporter molecule 2-fluoro-4-nitrophenol-beta-D-galactopyranoside. Magn Reson Imaging. 2006;24(7):959–62. doi: 10.1016/j.mri.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Yu JX, Kodibagkar VD, Liu L, Mason RP. A 19F-NMR approach using reporter molecule pairs to assess beta-galactosidase in human xenograft tumors in vivo. NMR Biomed. 2008;21(7):704–12. doi: 10.1002/nbm.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui W, Liu L, Kodibagkar VD, Mason RP. S-Gal®, A novel 1H MRI reporter for β-galactosidase. Magn Reson Med. 2010;64(1):65–71. doi: 10.1002/mrm.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu JX, Gulaka PG, Liu L, Kodibagkar VD, Mason RP. Novel Fe3+ Based 1H MRI β -galactosidase reporter molecules. Chem Plus Chem. 2012;77:370–8. doi: 10.1002/cplu.201100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu ZD, Hider RC. Design of iron chelators with therapeutic application. Coord Chem Rev. 2002;232(1–2):151–71. [Google Scholar]
- 28.Zhou T, Ma Y, Kong X, Hider RC. Design of iron chelators with therapeutic application. Dalton Trans. 2012;41(21):6371–89. doi: 10.1039/c2dt12159j. [DOI] [PubMed] [Google Scholar]
- 29.Duarte S, Carle G, Faneca H, de Lima MC, Pierrefite-Carle V. Suicide gene therapy in cancer: where do we stand now? Cancer Lett. 2012;324(2):160–70. doi: 10.1016/j.canlet.2012.05.023. [DOI] [PubMed] [Google Scholar]


