Abstract
Background
As efforts intensify to eliminate perinatal HIV transmission, understanding kinetics of maternal-to-child transfer of antiretrovirals during pregnancy and breastfeeding is critical. Antiretroviral levels in plasma, cord blood and breastmilk reflect exposure over short intervals. Hair concentrations reflect cumulative exposure and can uniquely quantify in-utero transfer of maternal medications to infants. We measured plasma and hair antiretroviral levels in HIV-infected Ugandan mothers and their infants at delivery and during breastfeeding to assess transfer.
Methods
HIV-infected pregnant women were randomized to lopinavir/ritonavir- or efavirenz-based therapy in a larger trial (PROMOTE). At 0, 8 and 12 weeks postpartum, plasma antiretroviral levels were measured in 117 mother-infant pairs; hair levels were assayed at 12 weeks. Ratios and correlations of infant:maternal concentrations were calculated.
Results
By 12 weeks, 90.4% of mothers reported exclusive breastfeeding. Hair and plasma levels over time suggest moderate (47%) to extensive (87%) in-utero transfer of lopinavir and ritonavir, respectively, but negligible transfer of either via breastfeeding. Moderate transfer of efavirenz occurs during pregnancy and breastfeeding (40% cumulative; 15% during breastfeeding). Despite differences in exposure, no infant seroconversions or correlations between infant hair/plasma antiretroviral levels and adverse effects were observed.
Conclusions
Using a unique approach combining hair and plasma data, we found that different antiretrovirals had distinct kinetics of mother-to-infant transfer. Efavirenz transfers during both pregnancy and breastfeeding, whereas lopinavir and ritonavir transfer only in-utero. Further study of the degree and timing of maternal-to-child transfer by antiretroviral will help optimize strategies that protect infants and minimize toxicities during periods of risk.
INTRODUCTION
The prevention of HIV transmission during pregnancy and breastfeeding is an urgent priority with the Global Plan for the Elimination of New HIV Infections among Children by 2015 (UNAIDS)1 report outlining an ambition to halt perinatal HIV transmission worldwide. Significant strides have been made in the resource-rich setting in nearly eliminating perinatal transmission, but over 330,000 children are newly infected around the globe annually2. “Option B” and “Option B+” of the World Health Organization (W.H.O.) guidelines recommend antepartum and postpartum or lifelong use of triple antiretroviral combinations for HIV-infected women, respectively, to minimize risks of infant infection and maximize maternal benefit3, but little is known about the timing and degree of transfer of maternal antiretrovirals to infants during periods of risk. Such information can be used to identify the optimal maternal antiretroviral regimen(s) to maximize infant protection and minimize toxicity during pregnancy and breastfeeding.
Limited studies have examined transplacental and breastmilk transfer of antiretrovirals using surrogate measures of drug exposure or assays that may not reflect long-term exposure in the infant4. For instance, antiretroviral concentrations in cord blood reflect maternal exposure over a short time period and do not accurately represent exposure in a newborn already capable of metabolism. Single plasma or urine levels of antiretrovirals in infants at delivery provide only a “snapshot” of recent exposure and do not reflect long-term exposure in-utero. Ex-vivo human placental perfusion models can only simulate in-vivo conditions and do not account for the effects of infant metabolism. Breastmilk concentrations of antiretrovirals similarly do not incorporate the effects of infant absorption and maturing infant metabolism. Finally, single infant plasma levels during breastfeeding represent only recent exposure and can demonstrate significant day-to-day variation5. Given the limitations of these standard measures, no study to date has been able to accurately quantify cumulative exposure to antiretrovirals in the infant during pregnancy and breastfeeding.
As a monitoring matrix, neonatal hair is highly effective in quantifying prenatal exposure to medications and other substances ingested by the mother, with feasibility and accuracy advantages over matrices such as cord blood, meconium or infant plasma4. Hair levels reflect drug uptake from the systemic circulation over weeks to months6, capturing cumulative exposure to medications. Infant hair growth starts at approximately ten weeks gestation and neonatal scalp hair predominantly reflects drug exposure during the third trimester7. Infant hair replaces neonatal hair (defined as developing antenatally and in early infancy) at approximately three months of life, so that analysis of drug concentrations in baby hair up to 12 weeks of life reflects a combination of drug exposure in-utero and cumulative drug exposure from breastfeeding, if present. Plasma levels of maternal drug in breastfeeding infants reflect exposure over the past 1-3 days4, so that combining hair and plasma level monitoring in infants out to 12 weeks of age is a unique approach to determine the timing of maternal-to-infant drug transfer both during pregnancy and breastfeeding.
Our group has pioneered the use of small hair samples to monitor antiretroviral adherence and exposure in the HIV treatment and prevention setting8-14. We have developed methods to extract and analyze protease inhibitors (PIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs)13,14 from hair. In HIV-infected individuals, we have demonstrated that hair levels of antiretrovirals are the strongest independent predictor of virologic success in large cohorts, surpassing single plasma levels and self-reported adherence as predictors of treatment outcome8-12. To determine the degree and timing of transfer of lopinavir/ritonavir (LPV/r) versus efavirenz (EFV) from women to their infants during pregnancy and breastfeeding, we collected plasma and hair samples from mother-infant pairs within a randomized clinical trial of HIV-infected pregnant and breastfeeding women receiving combination antiretroviral therapy (cART) in Uganda.
METHODS
Study population and protocol
The Prevention of Malaria and HIV disease in Tororo (PROMOTE) study is a recently-completed clinical trial in which HIV-infected pregnant Ugandan women were randomized to receive either LPV/r or EFV-containing cART from 12-28 weeks of gestation with continuation throughout one year of breastfeeding (NCT00993031)15. At 30 weeks gestation, the dose of LPV/r increased from 400mg/100mg twice daily to 600mg/150mg twice daily for all women. Infants received postpartum antiretroviral prophylaxis as per Ugandan Ministry of Health guidelines. At the beginning of the study, infants received one week of zidovudine. As of November 20, 2010, infants receive six weeks of daily nevirapine. All infants also received trimethoprim/sulfamethoxazole prophylaxis from six weeks of life until six weeks following breastfeeding cessation. Women were counseled to exclusively breastfeed for six months and continue breastfeeding until one year postpartum.
Between December 15, 2009 and September 27, 2012, 390 HIV-infected pregnant women were enrolled into the study and a total of 374 infants were born. A nested study performed pharmacokinetic (PK) monitoring by measuring plasma levels of both LPV and ritonavir (RTV) or EFV in women and their breastfeeding infants at 0, 8 and 12 weeks postpartum. Small hair samples (~20-30 strands) were cut from the occipital portion of the scalp from women and infants at 12 weeks postpartum and analyzed for antiretroviral levels. This report summarizes the analyses of antiretroviral concentrations from approximately the first 50 mother-infant pairs where the woman was randomized to LPV/r and the first 50 pairs where the woman was randomized to EFV.
Mothers and infants were monitored extensively for outcomes. Infants were tested for HIV-infection at birth, 24 weeks, and 58 weeks of life using HIV DNA-PCR (Roche Amplicor HIV-1 DNA test version 1.5, Branchburg, NJ, USA). Maternal and infant adverse events were assessed monthly using the Division of AIDS (DAIDS) Toxicity Table for Grading Severity of Adult and Pediatric Adverse Events16. Infants had monthly study visits and were assessed for respiratory or gastrointestinal morbidities, malnutrition, or poor feeding. Infant laboratory evaluations included serial complete blood count and liver enzymes. The Institutional Review Boards at the Makerere University College of Health Sciences in Kampala, Uganda and at the University of California, San Francisco (UCSF) both approved the study and all mothers gave informed consent for the collection of hair and plasma from themselves and their infants.
Laboratory procedures
Plasma concentrations
Methods to analyze plasma concentrations of LPV, RTV and EFV have been described17-19. Plasma concentrations of LPV and RTV are determined by a liquid chromatography/tandem mass spectrometry (LC/MS/MS) method. Single step extraction of LPV, RTV and the appropriate internal standard from plasma is accomplished by simple protein precipitation with acetonitrile. The lower limits of quantification (LLQ) for both LPV and RTV are 50 nanograms (ng)/milliliter (mL). The inter-assay precision as defined by coefficient of variation (CV) ranges from 3.45% to 7.05% and 6.61% to 8.91% for LPV and RTV, respectively.
Plasma concentrations of EFV are determined by a reversed phase high-performance liquid chromatographic (HPLC) method. EFV and the internal standard are first extracted from plasma by simple protein precipitation with acetonitrile. The LLQ for EFV with our assay is 50 ng/mL. The inter-assay precision (CV%) for EFV ranges from 4.66% to 4.76%. All plasma antiretroviral assays are validated according to the DAIDS Clinical Pharmacology Quality Control (CPQA) guidelines based on current FDA guidelines for bio-analytical methods.
Hair concentrations
Methods to analyze LPV, RTV and EFV levels in hair have been described previously11-13. A small thatch of hair (~20-30 strands) is cut as close as possible to the scalp and the distal portion labeled, with the relevant antiretroviral extracted and measured by LC/MS/MS. This method has been validated from 0.05 to 20 ng/mg hair for LPV (lopinavir-d8 used as the internal standard), from 0.01 to 4 ng/milligram (mg) for RTV (ritonavir-d6 used as the internal standard) and from 0.05 to 20 ng/mg for EFV (efavirenz-d4 used as the internal standard), with good linearity (R2 >0.99) and reproducibility (CV <15%) for all three hair assays.
Statistical analyses
All analyses were conducted using Stata (version 11.2, College Station, TX) and SAS (version 9.2, SAS Institute, Cary, NC). Means and ranges of plasma and hair concentrations at each time point in women and infants by antiretroviral were calculated. Ratios of infant to maternal LPV, RTV and EFV concentrations in plasma at 0, 8 and 12 weeks postpartum were calculated and the mean/ranges of the individual ratios summarized. Ratios of infant to maternal LPV, RTV and EFV concentrations in hair at 12 weeks postpartum were calculated and the mean, 95% confidence intervals (CIs), and ranges of the individual ratios determined. Spearman correlation coefficients for antiretroviral plasma and hair concentrations between women and their infants were calculated at each time point. Finally, Spearman correlation coefficients were calculated for infant hair and plasma antiretroviral levels and infant adverse events, with separate analyses evaluating correlations for number of grade 1-2 events, number of grade 3-4 events, number of grade 3-4 anemia events, and number of grade 3-4 neutropenia events.
RESULTS
We report analyses of plasma and hair samples for antiretroviral concentrations from 51 mother-infant pairs where the mother received LPV/r and 56 pairs where the woman received EFV in the Ugandan PROMOTE trial at 0, 8 and 12 weeks postpartum. Women in this sample initiated antiretrovirals at a median of 22.6 weeks gestation. By 12 weeks postpartum, 99.1% of women in the overall sample reported any breastfeeding, 90.4% of women reported exclusively breastfeeding, and 93.9% reported predominantly breastfeeding. There were no statistically significant differences between rates of breastfeeding (any, exclusively or predominantly) among women on LPV/r and women on EFV in this sample. Due to changes in the Ugandan Ministry of Health guidelines for infant prophylaxis during the study period, 77 of infants in the overall study received 1 week of zidovudine and 297 of infants received 6 weeks of postpartum nevirapine. There were no statistically significant differences between rates of zidovudine versus nevirapine receipt among infants born of mothers on LPV/r and mothers on EFV in this sample. All of the infants in this sample remained HIV-uninfected at 12 weeks, 24 weeks and 58 weeks postpartum.
Infant/maternal plasma concentrations
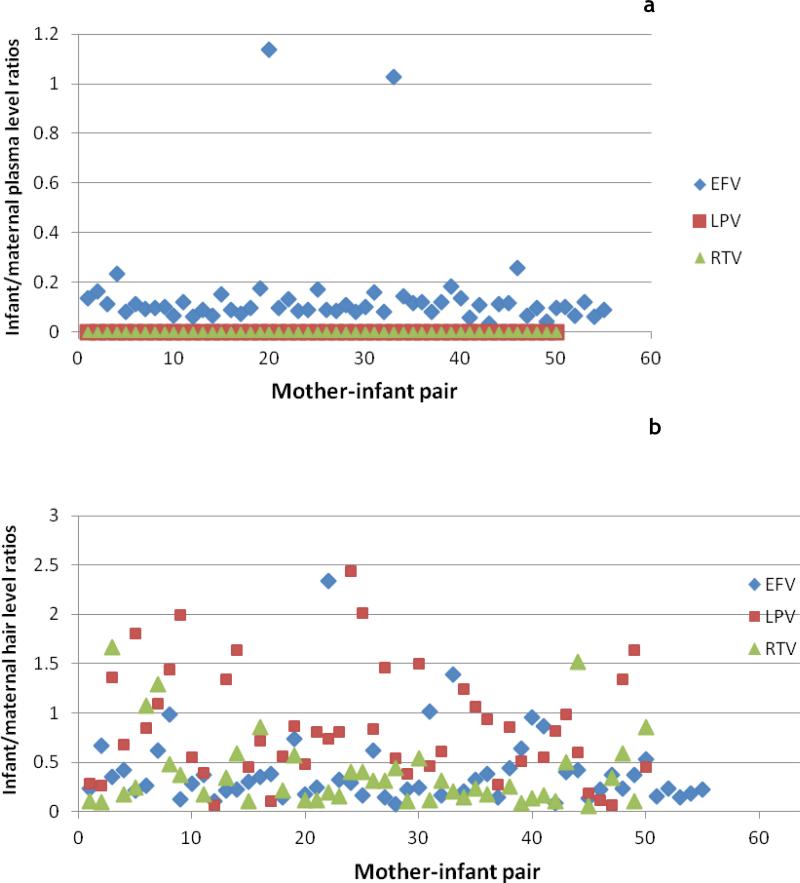
Table 1 shows mean plasma antiretroviral concentrations of each drug for mothers and infants and the percent of each group with detectable plasma levels at each of the three time points; Figure 1a shows the ratios of infant/maternal concentrations in plasma at 12 weeks by drug. At 0, 8 and 12 weeks postpartum, most mothers on LPV/r-based regimens had detectable plasma LPV levels (82%, 94% and 91%, respectively). In contrast, although 41% of infants had detectable LPV plasma concentrations at delivery, only one infant had detectable LPV levels at 8 weeks, and none had detectable LPV plasma levels by 12 weeks. For RTV, most women, but few infants, had detectable plasma levels at all time points. For EFV, 100% of both mothers and infants had detectable plasma levels at 0, 8 and 12 weeks postpartum. Correlation coefficients for LPV and RTV plasma levels between mothers and their breastfeeding infants were low at all time points, whereas correlation coefficients for EFV levels between women and infants at 0, 8 and 12 weeks postpartum were high and statistically significant (Table 2).
Table 1.
Percent of plasma and hair concentrations detectable in mothers and their breastfeeding infants (with mean and range of concentrations) at each time point for lopinavir, ritonavir and efavirenz
| Antiretroviral | Plasma levels (ng/mL*), 0 weeks | Plasma levels (ng/mL), 8 weeks | Plasma levels (ng/mL), 12 weeks | Hair levels (ng/mg**), 12 weeks |
|---|---|---|---|---|
| Lopinavir (mothers) | 81.5% 5562 (171-15700) | 94.1% 7245 (1500-20200) | 91% 6836 (73-18900) | 100% 5.8 (0.92-12.8) |
| Lopinavir (infants) | 41% 877 (71-5500) | 2% 117 (117, 117) | 0% None detectable | 96% 5.1 (0.13-15.8) |
| Ritonavir (mothers) | 59.3% 238 (100-589) | 86.3% 358 (51-1170) | 80% 360 (68-833) | 100% 0.51 (0.06-1.35) |
| Ritonavir (infants) | 2% 112 (112-112) | 0% None detectable | 0% None detectable | 91% 0.15 (0.03-0.42) |
| Efavirenz (mothers) | 100% 2830 (79-10301) | 100% 2563 (75-13555) | 100% 2956 (72-12430) | 100% 6.3 (0.33-34.2) |
| Efavirenz (infants) | 100% 1695 (50-7881) | 100% 309 (50-1435) | 100% 297 (66-1106) | 100% 1.9 (0.34-11.0) |
ng/mL = nanograms/milliliter
ng/mg= nanograms/milligram
Figure 1.
a: Ratios of infant/maternal plasma concentrations at 12 weeks by drug (n=56 for EFV and n=51 for LPV/RTV); Figure 1b: Ratios of infant/maternal hair concentrations at 12 weeks by drug
Table 2.
Correlation coefficients between mother and infant lopinavir, ritonavir and efavirenz concentrations at each time point (rs*, p-value)
| Antiretroviral | Plasma levels, 0 weeks | Plasma levels, 8 weeks | Plasma levels, 12 weeks | Hair levels, 12 weeks |
|---|---|---|---|---|
| Lopinavir | −0.0247 (0.913) | −0.241 (0.130) | n/a** | 0.625 (<0.0001) |
| Ritonavir | n/a** | n/a** | n/a** | 0.220 (0.1609) |
| Efavirenz | 0.903 (< 0.0001) | 0.655 (< 0.0001) | 0.722 (<0.0001) | 0.527 (<0.0001) |
rs: Spearman correlation coefficient
n/a – Spearman coefficient non-estimable due to excessive ties at undetectable levels
Infant/maternal hair concentrations
At 12 weeks postpartum, almost all mothers and infants had detectable LPV, RTV and EFV concentrations in hair samples (Table 1). The mean concentration of LPV in hair for mothers was 5.8 ng/mg and the mean LPV hair levels in their infants was 5.1 ng/mg. The mean concentrations of EFV in maternal and infant hair samples were 6.3 ng/mg and 1.9 ng/mg, respectively. Correlation coefficients for LPV and EFV hair levels between mothers and their breastfeeding infants were high and statistically significant at 12 weeks postpartum; the correlation coefficient for RTV between mothers and infants was lower and not significant (Table 2). Individual ratios of infant to maternal hair concentrations at 12 weeks postpartum were calculated (Figure 1b) and the means of those ratios were 0.87 for LPV, 0.47 for RTV and 0.40 for EFV (Table 3).
Table 3.
Mean of individual ratios of infant/maternal lopinavir, ritonavir and efavirenz concentrations in hair and plasma at 12 weeks postpartum
| Antiretroviral | Infant/maternal hair | 95% confidence interval (CI) | Range of ratios | Infant/maternal plasma | 95% CI | Range of ratios |
|---|---|---|---|---|---|---|
| Lopinavir | 0.87 | 0.700 - 1.03 | 0.621- 2.44 | 0 | 0 | No peaks |
| Ritonavir | 0.47 | 0.247 - 0.694 | 0.059 - 4.45 | 0 | 0 | No peaks |
| Efavirenz | 0.40 | 0.303 - 0.488 | 0.058- 2.34 | 0.149 | 0.121-0.177 | 0.034- 1.14 |
Correlation between infant plasma and hair levels and adverse event
No infants in this sample acquired HIV infection by 58 weeks. The cumulative frequency of any infant adverse event (grade 1 or above) was 4.2%, 19.3% and 20.7% at 0, 8 and 12 weeks postpartum, respectively. Only 2.1%, 7.0% and 8.6% of infants in the sample had grade 3 or higher anemia at 0, 8 and 12 weeks postpartum, respectively, as defined by the DAIDS grading scale16. None of the infants were neutropenic at delivery, although approximately 9% of infants by 12 weeks of life had neutropenia of any grade. There was no significant correlation between infant plasma or hair levels of LPV, RTV or EFV at any time point with individual adverse events. Furthermore, there was no correlation between antiretroviral levels in infant plasma or hair with any grade 1-2 events, any grade 3-4 events, any grade 3-4 anemia events, or any grade 3-4 neutropenia events.
DISCUSSION
Using a novel approach of combining hair and plasma PK evaluations, we found that lopinavir, ritonavir and efavirenz all transfer from mother to infant in-utero, while only efavirenz transfers during breastfeeding. Using hair as a toxicologic matrix in neonates overcomes many of the methodologic limitations in prior studies studying maternal-to-infant transfer of antiretrovirals. Assays of antiretroviral levels in neonatal hair at 12 weeks of life reflect maternal to child transfer both in-utero and during breastfeeding, whereas plasma levels of antiretrovirals in infants can only indicate recent exposure. Therefore, a combination of hair and plasma antiretroviral monitoring in infants born to mothers taking antiretrovirals during pregnancy and breastfeeding provides a unique approach to determine the timing and degree of maternal-to-infant drug transfer during critical periods of risk.
Plasma and hair data in the Ugandan PROMOTE study suggest very different kinetics of transfer from mother to baby for LPV, RTV and EFV during pregnancy and breastfeeding. For LPV and RTV, infant plasma levels at delivery and hair levels at 12 weeks suggest significant transfer in-utero (approximately 87% and 47% transferring from mother to infant, respectively), but negligible transfer of either during breastfeeding. Moderate transfer of EFV occurs both during pregnancy and breastfeeding, with approximately one-third of transfer occurring postpartum (cumulative transfer 40% with 15% transfer during breastfeeding).
Prior studies of maternal-to-infant transfer of antiretrovirals in the antenatal and postpartum periods have been limited, provide conflicting findings, and use measures that may not accurately reflect cumulative infant exposure. Rates of transfer of LPV and RTV from mother-to-infant during pregnancy are variable depending on the study and metric used. One study showed no detectable LPV in umbilical cord blood from 11 mothers (despite detectable plasma LPV levels in 10/11) in contrast to detectable antiretroviral cord blood levels in women on other PIs or nevirapine20. Other studies examining umbilical cord antiretroviral levels showed either non-detectable cord concentrations for LPV21 or low cord:maternal ratios in the 0.2222- 0.2423 range. In contrast, ex-vivo human placental perfusion models have shown fetal transfer rates of LPV/RTV that eventually achieve levels well above the 50% inhibitory concentration for viral replication24, with transfer being highly dependent on placental albumin and p-glycoprotein efflux transporter concentrations25.
Our study provides findings that are biologically plausible with the model that placental transfer of LPV, a highly protein-bound molecule, occurs through passive diffusion of the unbound drug fraction24. Another study examining cord:maternal ratios of LPV demonstrated an increase in that ratio (2.7 fold) when the free fraction of LPV in cord blood was measured, rather than the total fraction26. Since hair levels of LPV in infants can only reflect what the infant was exposed to, it is likely that previous studies examining protein-bound LPV fractions in cord blood did not accurately reflect drug transfer to the infant. Moreover, because LPV and RTV are significantly less protein-bound during pregnancy than postpartum (38 to 50% less)26,27, rates of LPV/RTV placental transfer are predicted to be higher than transfer rates from breastmilk, an observation confirmed by our study.
Studies that examine LPV transfer during breastfeeding with surrogates of exposure (e.g. breastmilk levels) were more consistent with our findings. In the Breastfeeding, Antiretroviral and Nutrition (BAN) study, investigators measured LPV and RTV concentrations in breastmilk and in maternal and infant plasma samples at 6, 12 and 24 weeks postpartum in eight mother-infant pairs where mothers were on LPV/r-based regimens28. LPV and RTV breastmilk concentrations were ~11% that of concentrations in mothers’ plasma, but all infants had undetectable LPV and RTV plasma concentrations at all three time points, consistent with our data showing negligible absorption or transfer of these drugs during breastfeeding. In another study, no detectable levels of LPV or RTV were seen in milk samples from 60 HIV-infected mothers on LPV/r-based regimens29. A recent study of 66 HIV-infected women on various combination antiretroviral regimens in Malawi30 showed that breastmilk penetration for LPV was lower than that of other antiretrovirals, although higher breastmilk concentrations of LPV and RTV were observed in mothers at delivery than at later time points. Of note, infant plasma concentrations of LPV were generally low during breastfeeding and infant plasma concentrations of all antiretrovirals tended to decrease at later time points, suggesting maturation of infant metabolism.
Our study is one of the first to examine efavirenz transfer from mother-to-infant during periods of risk using robust measures of short and long-term exposure. In one recent study, 25 pairs of maternal delivery and cord blood samples were collected from HIV-infected women on EFV and the median ratio of cord blood/maternal EFV concentration at delivery was 0.49 (range 0.37–0.74)31, consistent with our ratio of 0.40 for infant/maternal hair concentrations. In terms of EFV transfer during breastfeeding, a study of 13 mothers and their infants in Rwanda showed significant rates of transfer of maternal EFV into breastmilk and a significant correlation between EFV concentrations in breastmilk and infant plasma32. The newborn plasma concentrations of EFV was an average of 13.1% (range 5.6%-26.8%) of the maternal plasma concentrations, consistent with our plasma data showing 15% transfer during breastfeeding.
The clinical significance of high rates of LPV transfer and moderate rates of RTV and EFV transfer in-utero, compared to limited rates of LPV/r transfer and moderate rates of EFV transfer during breastfeeding, can only be determined in larger clinical trials. If maternal HIV viral load is the sole determinant of HIV transmission risk during pregnancy33, then significant antiretroviral transfer to the fetus in-utero may only serve to elevate rates of infant toxicity without protective benefits in settings of full adherence to maternal antiretrovirals and consistent virologic suppression. On the other hand, if some degree of antiretroviral exposure in-utero is necessary to decrease transmission risk, then using antiretrovirals during pregnancy with higher transplacental transfer may be indicated, most notably in cases of incomplete maternal antiretroviral adherence (or settings in which viral load monitoring is not feasible), whereby transferred antiretrovirals could act both as pre-exposure and post-exposure prophylaxis for the fetus.
The situation may be analogous during the postpartum period: the negligible transfer of LPV and RTV during breastfeeding may minimize infant toxicity, but may put infants at higher risk of HIV acquisition compared to other maternal antiretroviral regimens, especially in cases of maternal HIV viremia during lactation. However, moderate exposure to any antiretroviral, especially an NNRTI, may predispose to HIV viral resistance in the infant in the event of seroconversion34. Of the 13 newborns in the Rwandan study32, eight had EFV concentrations below the lower therapeutic level recommended for adults. However, no data about the minimum EFV concentrations necessary for effective protection in neonates have been published and the relationship between EFV plasma concentrations in infants and rates of viral resistance in those who seroconvert is not clear. There were no infant HIV seroconversions in our sample and our study was too small to adequately explore the relationship between infant drug concentrations and adverse events.
Assessing antiretroviral concentrations in small hair samples to quantify transfer of antiretrovirals from mother-to-infant during pregnancy and breastfeeding is the only method to assess cumulative exposure during these periods. Moreover, the recent report of a possible functional cure mediated by antiretroviral therapy in an infant born of an HIV-infected mother will trigger research studies where novel tools of assessing mother-to-infant drug transfer may be important35. Unlike phlebotomy, hair collection is noninvasive, providing obvious advantage in pediatric settings36, and does not require specific skills, sterile equipment, or specialized storage conditions. Hair can be stored for prolonged durations prior to analysis at room temperature and shipped without biohazardous precautions. These features may make this monitoring tool particularly promising in resource-poor settings.
In conclusion, using an innovative combination of maternal and infant hair and plasma concentration measurements, we demonstrate moderate to high transfer of RTV and LPV during pregnancy, with negligible transfer during breastfeeding, and moderate transfer of EFV during both periods. This work provides unique information given that studies to date examining maternal-to-infant transfer of antiretrovirals have provided conflicting findings using limited methodologies assessing short-term exposure. We suggest a novel method of antiretroviral exposure monitoring using hair samples in prevention of perinatal transmission settings. Given increasing access to antiretrovirals in HIV-infected pregnant and breastfeeding women worldwide, and the importance of identifying the most appropriate regimens to both prevent HIV transmission and minimize toxicity, further study of the implications of differing kinetics of maternal-to-child transfer by type of antiretroviral is needed.
ACKNOWLEDGEMENTS
We thank the women and infants in the PROMOTE study for their participation in this trial. We also thank the PROMOTE study staff and the midwives at Tororo District Hospital. We thank Ruth Greenblatt MD at UCSF for conceptual input into using hair measures of antiretrovirals as a mode of exposure monitoring. We also thank Patricia Lizak, Florence Marzan and David Gingrich of the UCSF Drug Research Unit, Department of Clinical Pharmacy for their technical support for antiretroviral plasma analyses.
Funding: This work was supported by the National Institute of Child Health and Human Development (NICHD)/ National Institutes of Health (NIH) (P01 HD059454, Havlir). Additional funding is provided by the National Institute of Allergy and Infectious Diseases (NIAID))/NIH (RO1 AI098472, Gandhi). The plasma level analysis was supported by UCSF Center for AIDS Research (P3O AI022763) Pharmacology Core. Lopinavir/ritonavir for study participants was provided by Abbvie (formerly Abbott Laboratories).
Footnotes
Conflict of interests: None of the authors have a commercial or other association that might pose a conflict of interest
Conference presentations: 4th International Conference on HIV and Pediatrics, Washington DC, July 20-21, 2012 (oral abstract O-14); XIX International AIDS Conference, Washington DC, July 22-27, 2012 (abstract TUPE057)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.UNAIDS [October 2012];Global Plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. 2011 http://www.who.int/hiv/pub/me/monitoring_framework/en/index.html.
- 2.UNAIDS [October 2012];Together we will end AIDS. 2012 http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/20120718_togetherwewillendaids_en.pdf.
- 3.W.H.O. [October 2012];Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. 2012 Apr; http://www.who.int/hiv/pub/mtct/programmatic_update2012/en/index.html.
- 4.Gray T, Huestis M. Bioanalytical procedures for monitoring in utero drug exposure. Anal Bioanal Chem. 2007 Aug;388(7):1455–1465. doi: 10.1007/s00216-007-1228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson GD. Using pharmacokinetics to predict the effects of pregnancy and maternal-infant transfer of drugs during lactation. Expert Opin Drug Metab Toxicol. 2006 Dec;2(6):947–960. doi: 10.1517/17425255.2.6.947. [DOI] [PubMed] [Google Scholar]
- 6.Beumer J, Bosman I, Maes R. Hair as a biological specimen for therapeutic drug monitoring. In J Clin Pract. 2001;55:353–357. [PubMed] [Google Scholar]
- 7.Gareri J, Koren G. Prenatal hair development: implications for drug exposure determination. Forensic Sci Int. 2010 Mar 20;196(1-3):27–31. doi: 10.1016/j.forsciint.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi M, Ameli N, Bacchetti P, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011 May;52(10):1267–1275. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi M, Ameli N, Bacchetti P, et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS. 2009 Feb 20;23(4):471–478. doi: 10.1097/QAD.0b013e328325a4a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi M, Ameli N, Gange S, et al. Concentrations of Efavirenz in Hair Correlate Strongly with 24-hour Intensive Pharmacokinetic Measurements and with Virologic Outcomes.. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010; paper 604. [Google Scholar]
- 11.Gandhi M, Greenblatt RM, Bacchetti P, et al. A Single-Nucleotide Polymorphism in CYP2B6 Leads to >3-Fold Increases in Efavirenz Concentrations in Plasma and Hair Among HIV-Infected Women. J Infect Dis. 2012 Nov;206(9):1453–1461. doi: 10.1093/infdis/jis508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Zyl GU, van Mens TE, McIlleron H, et al. Low lopinavir plasma or hair concentrations explain second line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr. 2011 Apr;56(4):333–339. doi: 10.1097/QAI.0b013e31820dc0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008 Nov;22(21):3401–3409. doi: 10.1002/rcm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Yang Q, Yoon K, et al. Microanalysis of the antiretroviral nevirapine in human hair from HIV-infected patients by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011 Oct;401(6):1923–1933. doi: 10.1007/s00216-011-5278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achan J, Kakuru A, Ikilezi G, et al. Antiretroviral Agents and Prevention of Malaria in HIV-Infected Ugandan Children. N Engl J Med. 2012;367:2110–2118. doi: 10.1056/NEJMoa1200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DAIDS Grading Scale [October 2012];Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. Version 1.0, December, 2004; Clarification August 2009. http://www.scribd.com/doc/57431404/DAIDS-Grading-Scale.
- 17.Chi J, Jayewardene AL, Stone JA, Motoya T, Aweeka FT. Simultaneous determination of five HIV protease inhibitors nelfinavir, indinavir, ritonavir, saquinavir and amprenavir in human plasma by LC/MS/MS. J Pharm Biomed Anal. 2002;30(3):675–684. doi: 10.1016/s0731-7085(02)00357-6. [DOI] [PubMed] [Google Scholar]
- 18.Villani P, Feroggio M, Gianelli L, et al. Antiretrovirals: simultaneous determination of five protease inhibitors and three nonnucleoside transcriptase inhibitors in human plasma by a rapid high-performance liquid chromatography--mass spectrometry assay. Ther Drug Monit. 2001;23(4):380–388. doi: 10.1097/00007691-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Keil K, Frerichs VA, DiFrancesco R, Morse G. Reverse phase high-performance liquid chromatography method for the analysis of amprenavir, efavirenz, indinavir, lopinavir, nelfinavir and its active metabolite (M8), ritonavir, and saquinavir in heparinized human plasma. Ther Drug Monit. 2003 Jun;25(3):340–346. doi: 10.1097/00007691-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Ivanovic J, Nicastri E, Anceschi MM, et al. Transplacental transfer of antiretroviral drugs and newborn birth weight in HIV-infected pregnant women. Curr HIV Res. 2009 Nov;7(6):620–625. doi: 10.2174/157016209789973628. [DOI] [PubMed] [Google Scholar]
- 21.Marzolini C, Rudin C, Decosterd LA, et al. Transplacental passage of protease inhibitors at delivery. AIDS. 2002 Apr 12;16(6):889–893. doi: 10.1097/00002030-200204120-00008. [DOI] [PubMed] [Google Scholar]
- 22.Gingelmaier A, Kurowski M, Kastner R, et al. Placental transfer and pharmacokinetics of lopinavir and other protease inhibitors in combination with nevirapine at delivery. AIDS. 2006 Aug 22;20(13):1737–1743. doi: 10.1097/01.aids.0000242820.67001.2c. [DOI] [PubMed] [Google Scholar]
- 23.van Hoog S, Boer K, Nellen J, Scherpbier H, Godfried MH. Transplacental passage of nevirapine, nelfinavir and lopinavir. Neth J Med. 2012 Mar;70(2):102–103. [PubMed] [Google Scholar]
- 24.Gavard L, Gil S, Peytavin G, et al. Placental transfer of lopinavir/ritonavir in the ex vivo human cotyledon perfusion model. Am J Obstet Gynecol. 2006 Jul;195(1):296–301. doi: 10.1016/j.ajog.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Ceccaldi PF, Gavard L, Mandelbrot L, et al. Functional role of p-glycoprotein and binding protein effect on the placental transfer of lopinavir/ritonavir in the ex vivo human perfusion model. Obstet Gynecol Int. 2009;2009:726593. doi: 10.1155/2009/726593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fayet-Mello A, Buclin T, Guignard N, et al. Free and total plasma levels of lopinavir during pregnancy, at delivery and in postpartum: implication for dosage adjustments in pregnant women. Antivir Ther. 2012 Aug 22; doi: 10.3851/IMP2328. [DOI] [PubMed] [Google Scholar]
- 27.Kiser J, Mawhinney S, Kinzie K, et al. Total and Unbound Lopinavir/Ritonavir Pharmacokinetics in a Concentration-guided Study of HIV-infected Women throughout Pregnancy and Post-partum.. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. Feb. 2009; Paper #946. [Google Scholar]
- 28.Corbett A, Martinson F, Rezk N, et al. Lopinavir/Ritonavir Concentrations in Breast Milk and Breast-feeding Infants.. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. Feb. 2009; Paper 947. [Google Scholar]
- 29.Rezk NL, White N, Bridges AS, et al. Studies on antiretroviral drug concentrations in breast milk: validation of a liquid chromatography-tandem mass spectrometric method for the determination of 7 anti-human immunodeficiency virus medications. Ther Drug Monit. 2008 Oct;30(5):611–619. doi: 10.1097/FTD.0b013e318186e08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palombi L, Pirillo MF, Andreotti M, et al. Antiretroviral prophylaxis for breastfeeding transmission in Malawi: drug concentrations, virological efficacy and safety. Antivir Ther. 2012 Aug 21; doi: 10.3851/IMP2315. [DOI] [PubMed] [Google Scholar]
- 31.Cressey TR, Stek A, Capparelli E, et al. Efavirenz pharmacokinetics during the third trimester of pregnancy and postpartum. J Acquir Immune Defic Syndr. 2012 Mar 1;59(3):245–252. doi: 10.1097/QAI.0b013e31823ff052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider S, Peltier A, Gras A, et al. Efavirenz in human breast milk, mothers’, and newborns’ plasma. J Acquir Immune Defic Syndr. 2008 Aug 1;48(4):450–454. doi: 10.1097/QAI.0b013e31817bbc21. [DOI] [PubMed] [Google Scholar]
- 33.Mayaux MJ, Dussaix E, Isopet J, et al. Maternal virus load during pregnancy and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohort studies. SEROGEST Cohort Group. J Infect Dis. 1997 Jan;175(1):172–175. doi: 10.1093/infdis/175.1.172. [DOI] [PubMed] [Google Scholar]
- 34.Zeh C, Weidle PJ, Nafisa L, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS Med. 2011 Mar;8(3):e1000430. doi: 10.1371/journal.pmed.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persaud D, Gay H, Ziemniak C, et al. Functional HIV Cure after Very Early ART of an Infected Infant.. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013; paper #48LB. [Google Scholar]
- 36.ter Heine R, Beijnen JH, Huitema AD. Bioanalytical issues in patient-friendly sampling methods for therapeutic drug monitoring: focus on antiretroviral drugs. Bioanalysis. 2009 Oct;1(7):1329–1338. doi: 10.4155/bio.09.124. [DOI] [PubMed] [Google Scholar]