Abstract
Background:
Skull base osteomyelitis (SBO) refers to infection that has spread beyond the external auditory canal to the base of the skull in advanced stages of otitis externa. Clinically, it may be difficult to differentiate SBO from severe otitis externa without bony involvement. This study was performed to determine the role of three phase bone scintigraphy (TPBS) and single photon emission tomography/computed tomography (SPECT/CT) in detecting SBO.
Materials and Methods:
We retrospectively analyzed records of 20 patients (14 M, 6 F) with otitis externa and suspected SBO. TPBS and SPECT/CT of the skull were performed. Findings were correlated with clinical, laboratory and diagnostic CT scan findings.
Results:
All patients were diabetic with elevated erythrocyte sedimentation rate. A total of 18 patients had bilateral and two unilateral symptoms. Cranial nerves were involved in eight patients and microbiological culture of ear discharge fluid positive in seven. Early images showed increased temporal vascularity in nine patients and increased soft-tissue uptake in 10, while delayed images showed increased bone uptake in 19/20 patients. Localized abnormal tracer uptake was shown by SPECT/CT in the mastoid temporal (15), petrous (11), sphenoid (3) and zygomatic (1) and showed destructive changes in five. Thus, TPBS was found positive for SBO in 10/20 patients and changed the management in four.
Conclusion:
Our study suggests that TPBS with SPECT/CT is a useful non-invasive investigation for detection of SBO in otitis externa.
Keywords: Bone scan, otitis externa, single photon emission computed tomography/computed tomography, skull base osteomyelitis
INTRODUCTION
Skull base osteomyelitis (SBO) is a rare, but life-threatening complication of otitis externa, occurring predominantly in diabetic or immune-compromised patients and indicates infection that has spread beyond the external auditory canal. The terms “invasive,” “necrotizing” or “malignant” otitis externa are also used.[1] It begins as a soft-tissue infection of the external auditory canal and spreads through the fissure of Santorini and the tympanomastoid suture to involve the cranial base and may cause cranial nerve palsies.[2] The infection preferentially spreads through venous channels and fascial planes within the temporal bone and along the dural sinuses of its middle and posterior fossa surfaces, eventually reaching the petrous apex. In the most severe cases, the infection crosses the midline to involve the contralateral temporal bone.[1,2,3]
The most frequent presenting symptom of this condition is unremitting deep otalgia that is typically worse at night and persists despite multiple courses of antibiotic treatment in primary and secondary care settings.[3] Otorrhea may be present and obliteration of the external auditory canal by edema and secretions may cause hearing loss.[4] A granulomatous polyp is sometimes present in the floor of the external auditory canal at the bony-cartilaginous junction and is virtually pathognomonic of this condition.[1] The most common pathogenic micro-organism to cause SBO is Pseudomonas aeruginosa that typically colonizes the external auditory canal after water exposure or trauma and becomes pathogenic predominantly in immune-compromised states.[5] Infrequently fungal or mixed bacterial and fungal infections may be involved; the most common fungal pathogen is aspergillus fumigatus.[3]
The diagnosis of SBO is based on the clinical findings and confirmed by laboratory tests and imaging studies. Laboratory tests include erythrocyte sedimentation rate, white and red blood cell counts, glucose and creatinine levels and microbial culture of ear secretions.[6] Histopathological examination of granulation tissue from the external auditory canal helps to exclude malignant (disease) processes.[7] Imaging modalities include computed tomography (CT), 99mtechnetium methylene diphosphonate (99mTc MDP) bone scintigraphy or magnetic resonance imaging (MRI). CT is sensitive to bone erosion and decreased skull base density, but at least one-third of bone mineral must be lost before radiologic changes become apparent.[8] Changes in soft-tissue are better detected on MRI, particularly dural enhancement and involvement of medullary bone spaces.[9] However, this investigation is generally not recommended as a first-line diagnostic imaging modality, given its lower sensitivity for imaging bone erosion compared with CT.
Bone scintigraphy may be positive before radiological changes and is useful for early detection of the disease. Three phase bone scintigraphy (TPBS) and single photon emission computed tomography (SPECT) may better differentiate between uptake in soft-tissues and inner skull bony structures. While clinical assessment alone cannot differentiate SBO from severe otitis externa without extension to the adjacent bone, SPECT/CT provides precise anatomical localization. Precise detection of skull base involvement is needed to decide the duration of the treatment. Hardoff et al., have semi quantitatively analyzed planar and SPECT scintigraphy for differentiating necrotizing external otitis from severe external otitis.[10] The authors have previously published a case report demonstrating the utility of SPECT/CT in malignant otitis externa.[11] The aim of this study was to determine the role of TPBS and delayed phase SPECT/CT in the detection of SBO.
MATERIALS AND METHODS
Clinical records of 20 patients with otitis externa, clinically suspected of SBO and referred for bone scintigraphy between March 2008 and August 2010 were retrospectively studied. All 20 patients were diabetic (two patients of type I, 18 of type II) and eight were on insulin. All patients were initially treated at other hospitals and subsequently referred to our institute because of inadequate response to treatment. After local examination, microbial cultures were obtained from the external ear canal. TPBS with SPECT/CT imaging after the delayed phase was performed in all patients. Patients were treated with systemic antibiotics (levofloxacin), topical antibiotic application and surgical debridement of granulation tissue (when present), which was then sent for histopathological examination. Use of other antibiotics such as ceftazidime or piperacillin/tazobactum combination and the duration of antibiotic treatment were based on the findings of bone scintigraphy, microbiological cultures of ear swabs and histopathological tests.
Sequential dynamic images of the skull were acquired for 1 min (2 s/frame) in a 64 × 64 matrix under a dual detector gamma camera (ECam, Siemens, Erlangen, Germany or Infinia Hawkeye 4, GE Health-care, Milwaukee, USA) immediately after intravenous injection of 740-1,110 MBq 99mTc MDP. Blood pool and delayed static images were acquired in anterior, posterior, right and left lateral projections of the skull after 1 min and 3-4 h respectively. Delayed whole body images were also acquired at 3 h. Subsequently, SPECT images of the skull were obtained in a 360° circular orbit with 60 stops and 25 s/stop. The CT part of the SPECT/CT was acquired at 140 kVp voltage and 2.5 mA tube current. The raw data were reconstructed using the filtered back projection with a Butterworth filter in coronal, sagittal and transaxial planes. Fusion of the SPECT and CT images was carried out using the Volumetric Hawkeye software (GE Health-care, Milwaukee, USA).
The images were reviewed by two experienced nuclear medicine physicians (AB, BRM). Posterior views were used to evaluate the planar images. In each case, increased blood flow in the perfusion phase, blood pool and delayed phase uptake of the tracer were noted and visually compared to the non-affected side. SPECT/CT studies were analyzed in the trans-axial, coronal and sagittal planes. Areas of increased tracer uptake were identified from the maximum intensity projection SPECT image. After triangulation of the area of increased uptake, CT and fused SPECT/CT images were analyzed for anatomical localization of abnormally increased uptake of tracer and destructive bony lesions. Findings on the images were compared with clinical signs and symptoms as well as findings on the diagnostic CT scans.
RESULTS
Of the 20 patients, 14 were male and six female, with age ranging from 48 to 77 (mean 62 ± 15) years. Other patient characteristics are listed in Table 1.
Table 1.
Clinical details of the patients
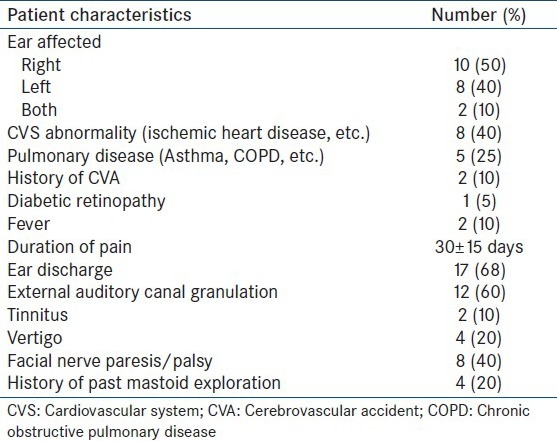
Swab culture from the ear discharge showed growth of P. aeruginosa in four patients and Staphylococcus aureus, diphtheroids and Aspergillus sp. in one patient each. One patient showed mixed growths of P. aeruginosa and S. aureus. Histopathological examination from tissue biopsy showed the presence of inflammatory cells in nine patients. Diagnostic CT scan of the skull revealed destructive bony changes in six patients and mastoid inflammation in seven. Two patients underwent MRI – while one showed involvement of the medial temporal lobe, the other showed features of a necrotizing inflammatory process. Two patients who underwent gallium-67 scintigraphy showed abnormal tracer uptake initially, with resolution on a follow-up scan after treatment.
Increased flow of the tracer in the perfusion phase was found in nine patients in the temporal region and increased soft-tissue uptake (in the blood pool phase) in 10. Delayed phase images at 3 h showed increased tracer uptake in the skull bones in 19 patients. Hybrid SPECT/CT of the skull localized areas of increased tracer uptake to the mastoid part of the temporal bone in 15 patients and petrous part in 11, sphenoid in three patients and zygomatic in one, with the CT detecting destructive changes in five patients (corroborated on the diagnostic CT findings). The TPBS findings in our study were suggestive of SBO in 10 patients and changed patient management in four when compared to the diagnostic CT scan alone. A representative scan of one of patients with findings indicative of SBO on TPBS is shown in Figure 1 and the corresponding transaxial SPECT/CT image in Figure 2. SPECT/CT images of two other patients are shown in Figures 3 and 4 to demonstrate different patterns of bony involvement in SBO.
Figure 1.
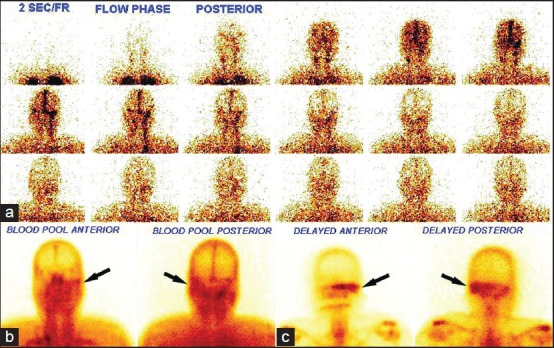
99mTechnetium methylene diphosphonate three phase bone scan showing symmetrical flow of tracer on both sides of the head in the perfusion phase, (a) increased soft-tissue tracer uptake in the left temporal region (arrows) in the blood pool phase (b) and increased tracer uptake in the left temporal and sphenoid region of the skull (arrows) in the delayed phase, (c) the patient was a 56-year-old man with type II diabetes, discharge from the left ear, fever and facial nerve palsy
Figure 2.
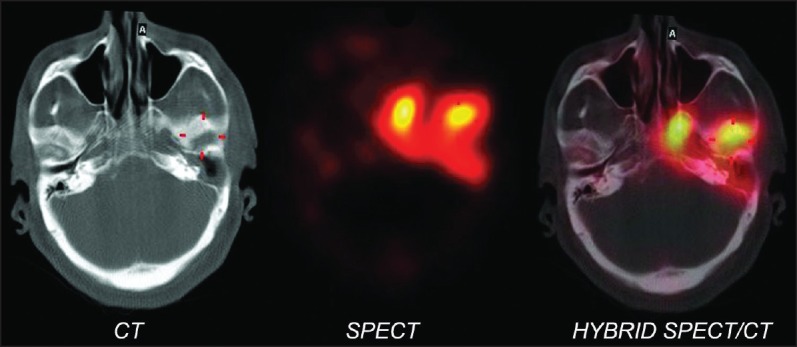
Hybrid single photon emission tomography/computed tomography of the skull (transaxial view) of the patient in Figure 1 shows increased tracer uptake in the petrous part of the left temporal and sphenoid bones with corresponding destructive bony changes
Figure 3.
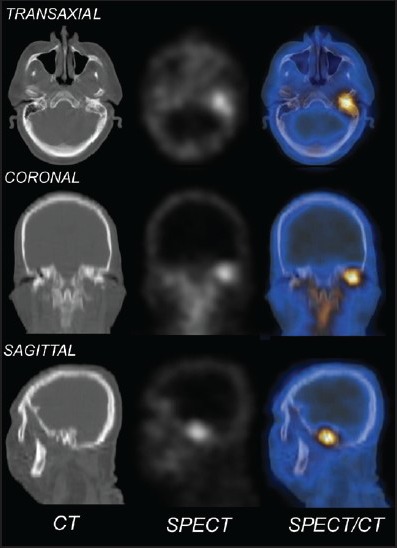
Computed tomography (CT), single photon emission computed tomography (SPECT) and SPECT/CT of the skull of a patient with SBO involving the left mastoid bone with corresponding destructive bony changes
Figure 4.
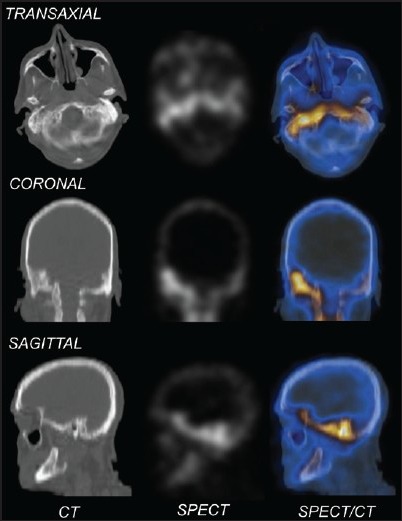
Computed tomography (CT), single photon emission computed tomography (SPECT) and SPECT/CT of the skull of a patient with skull base osteomyelitis involving the petrous temporal bone bilaterally (right > left) with corresponding bony changes
DISCUSSION
SBO is a rare, but serious complication and occurs due to contiguous spread of invasive or necrotizing infection from the ear canal. Delay in diagnosis, inadequate treatment, failure to respond to antibiotics, or infection by non-pseudomonal organisms in susceptible immune-compromised individuals are thought to be the major contributing factors.[12,13] Under-treatment is the major factor for recurrence. Bilateral or contralateral SBO is very rare and diabetics are more susceptible, comprising up to 94% of cases.[14] In two of our patients, involvement of the contralateral side was clinically manifested at the time of diagnosis. P. aeruginosa was the commonest causative pathogen in 80-98% of the cases.[15] All patients in our study were diabetic and Pseudomonas was cultured in four patients with scintigraphic evidence of SBO. No organisms were identified in three cases, possibly because of administration of topical antibiotics before culture.
In diabetes mellitus, poor vascular supply resulting from microvascular disease aggravates pseudomonal vasculitis, which further restricts tissue fusion. Diabetes mellitus is also associated with impaired polymorphonuclear cell function and a higher pH of cerumen in the aural canal. These factors, along with the sensitivity of P. aeruginosa to low pH, further restrict body defense against infection.[16] Our series showed five patients with fungal or mixed bacterial and fungal infections, with A. fumigatus being the most common fungal pathogen.
The facial nerve is the most commonly involved cranial nerve because it passes through stylomastoid foramen. As the infection spreads to the skull base, it may affect the lower cranial nerves (IX, X, XI and XII). Eight of the 10 scan-positive patients in our study had facial nerve involvement, which is broadly comparable to the 24-43% of cranial nerve involvement reported in other studies.[17] Initial evaluation of the extent and severity of extra-temporal soft-tissue involvement and SBO is usually carried out using the diagnostic CT imaging. However, since more than 30% of the affected bone needs to be demineralized to appear eroded on CT, early findings are limited to soft-tissue inflammation. An early feature is effacement of the fat planes in the sub-temporal triangle where the facial nerve exits the stylomastoid foramen.[18] While, advanced disease will show skull base bone destruction and abscess formation, CT cannot distinguish SBO from malignancy.[9]
MRI cannot detect bony destruction, but is better than CT in identifying soft-tissue abnormalities. On T2-weighted images sub-temporal extent of soft-tissue involvement is seen as low signal intensity, unlike normal inflammation, indicating a fibrotic, necrotizing pathologic process. Middle ear, mastoid and central skull base involvement is identified by high signal intensity on T2-weighted images. Dural enhancement and involvement of the medullary bone are the only significant findings exclusive to MRI; this investigation is therefore recommended when bony erosion of the skull base is seen on the CT scan.[9]
Bone scintigraphy with 99mTc MDP is positive in almost 100% of patients with SBO.[19] The high sensitivity is due to the fact that this radiotracer concentrates in areas with increased osteoblastic activity as found in infection, trauma and neoplasm. Conventionally, the diagnosis of active bony inflammation by TPBS is based on increased vascularity followed by increased soft-tissue and bony uptake of the tracer. Given its high sensitivity, 99mTc MDP imaging allows earlier diagnosis of osteomyelitis than CT, as it can detect as little as 10% of the bone demineralization.[20] Moreover, the slow progression of periosteal reaction and bone formation in diabetic patients renders radiographic changes less sensitive as compared to non-diabetics.[21] The sensitivity of bone scintigraphy for temporal bone osteomyelitis can be improved through identifying increased tracer uptake on delayed imaging between 4 h and 24 h after tracer injection.[10] In our series, a total of 10 patients were found positive for skull base involvement on SPECT/CT of which 6 were also positive on diagnostic CT. However, 99mTc MDP scintigraphy is not specific for infection and will also be positive in malignancy and non-infective bony inflammation; hence the need for tissue biopsy and bacterial culture.
Soft-tissue and bone infections can be detected with gallium-67 citrate (67GaC) imaging. While, this tracer lacks specificity for the detection of SBO, the uptake normalizes once the infection has resolved.[22] In our series, two patients showed increased tracer uptake on the 67GaC scan, with resolution of the lesions on the follow-up scan after 3 months. This scan may be repeated to monitor antibiotic response until it is normal. However, the cost, time, radiation dose (5 mGy for a bone scan versus 12 mGy for a gallium scan) and physical characteristics make 67GaC scan relatively unattractive for clinical practice.
Of the 10 patients diagnosed by 99mTc MDP TPBS as negative for active bony inflammation of the skull base, one showed normal blood flow, blood pool and delayed planar and SPECT/CT tracer uptake while the other nine showed increased tracer uptake with mucosal hypertrophy in the mastoid and the petrous temporal bones in the delayed phase, without any bone abnormality.
SBO requires aggressive management with a combination of anti-Pseudomonal antibiotics for prolonged periods of time, approximately for 8-12 weeks. In this study, appropriate localization of increased tracer uptake to the skull base on SPECT/CT before radiological evidence of bone destruction changed the management in 4/20 patients.
One limitation of this study is the non-availability of the culture report of discharged ear liquid of some of the patients. Only one patient underwent TPBS after 3 months, which showed persistence of uptake in the delayed phase of the scan with the blood pool phase becoming normal. It is therefore not possible to comment on the role of this investigation in disease monitoring at present. A technical limitation of our study was the non-diagnostic CT of our SPECT/CT equipment, which may cause degradation of the anatomical resolution of the images. This non-diagnostic CT is mainly used for attenuation correction and localization of abnormal foci of tracer uptake.
CONCLUSION
Our study suggests that TPBS with SPECT/CT is a useful non-invasive imaging modality for the detection of SBO in patients with otitis externa.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Chandler JR. Malignant external otitis. Laryngoscope. 1968;78:1257–94. doi: 10.1288/00005537-196808000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bernheim J, Sade J. Histopathology of the soft parts in 50 patients with malignant external otitis. J Laryngol Otol. 1989;103:366–8. doi: 10.1017/s0022215100108977. [DOI] [PubMed] [Google Scholar]
- 3.Sreepada GS, Kwartler JA. Skull base osteomyelitis secondary to malignant otitis externa. Curr Opin Otolaryngol Head Neck Surg. 2003;11:316–23. doi: 10.1097/00020840-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Martel J, Duclos JY, Darrouzet V, Guyot M, Bébéar JP. Malignant or necrotizing otitis externa: Experience in 22 cases. Ann Otolaryngol Chir Cervicofac. 2000;117:291. [PubMed] [Google Scholar]
- 5.Wright DN, Alexander JM. Effect of water on the bacterial flora of swimmers’ ears. Arch Otolaryngol. 1974;99:15–8. doi: 10.1001/archotol.1974.00780030019003. [DOI] [PubMed] [Google Scholar]
- 6.Ceruse P, Colleaux B, Truy E, Disant F, Morgon AH, Lahneche B. Malignant external otitis. Apropos of 7 recent cases. Ann Otolaryngol Chir Cervicofac. 1993;110:332–6. [PubMed] [Google Scholar]
- 7.Devaney KO, Boschman CR, Willard SC, Ferlito A, Rinaldo A. Tumours of the external ear and temporal bone. Lancet Oncol. 2005;6:411–20. doi: 10.1016/S1470-2045(05)70208-4. [DOI] [PubMed] [Google Scholar]
- 8.Gold S, Som PM, Lucente FE, Lawson W, Mendelson M, Parisier SC. Radiographic findings in progressive necrotizing “malignant” external otitis. Laryngoscope. 1984;94:363–6. doi: 10.1288/00005537-198403000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Grandis JR, Curtin HD, Yu VL. Necrotizing (malignant) external otitis: Prospective comparison of CT and MR imaging in diagnosis and follow-up. Radiology. 1995;196:499–504. doi: 10.1148/radiology.196.2.7617867. [DOI] [PubMed] [Google Scholar]
- 10.Hardoff R, Gips S, Uri N, Front A, Tamir A. Semiquantitative skull planar and SPECT bone scintigraphy in diabetic patients: Differentiation of necrotizing (malignant) external otitis from severe external otitis. J Nucl Med. 1994;35:411–5. [PubMed] [Google Scholar]
- 11.Chakraborty D, Bhattacharya A, Kamaleshwaran KK, Agrawal K, Gupta AK, Mittal BR. Single photon emission computed tomography/computed tomography of the skull in malignant otitis externa. Am J Otolaryngol. 2012;33:128–9. doi: 10.1016/j.amjoto.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Lancaster J, Alderson DJ, McCormick M. Non-pseudomonal malignant otitis externa and jugular foramen syndrome secondary to cyclosporin-induced hypertrichosis in a diabetic renal transplant patient. J Laryngol Otol. 2000;114:366–9. doi: 10.1258/0022215001905580. [DOI] [PubMed] [Google Scholar]
- 13.Grobman LR, Ganz W, Casiano R, Goldberg S. Atypical osteomyelitis of the skull base. Laryngoscope. 1989;99:671–6. doi: 10.1288/00005537-198907000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Cohen D, Friedman P. The diagnostic criteria of malignant external otitis. J Laryngol Otol. 1987;101:216–21. doi: 10.1017/s0022215100101562. [DOI] [PubMed] [Google Scholar]
- 15.Corey JP, Levandowski RA, Panwalker AP. Prognostic implications of therapy for necrotizing external otitis. Am J Otol. 1985;6:353–8. [PubMed] [Google Scholar]
- 16.Handzel O, Halperin D. Necrotizing (malignant) external otitis. Am Fam Physician. 2003;68:309–12. [PubMed] [Google Scholar]
- 17.Mani N, Sudhoff H, Rajagopal S, Moffat D, Axon PR. Cranial nerve involvement in malignant external otitis: Implications for clinical outcome. Laryngoscope. 2007;117:907–10. doi: 10.1097/MLG.0b013e318039b30f. [DOI] [PubMed] [Google Scholar]
- 18.Murray ME, Britton J. Osteomyelitis of the skull base: The role of high resolution CT in diagnosis. Clin Radiol. 1994;49:408–11. doi: 10.1016/s0009-9260(05)81827-6. [DOI] [PubMed] [Google Scholar]
- 19.Franco-Vidal V, Blanchet H, Bebear C, Dutronc H, Darrouzet V. Necrotizing external otitis: A report of 46 cases. Otol Neurotol. 2007;28:771–3. doi: 10.1097/MAO.0b013e31805153bd. [DOI] [PubMed] [Google Scholar]
- 20.Strashun AM, Nejatheim M, Goldsmith SJ. Malignant external otitis: Early scintigraphic detection. Radiology. 1984;150:541–5. doi: 10.1148/radiology.150.2.6228955. [DOI] [PubMed] [Google Scholar]
- 21.Seldin DW, Heiken JP, Feldman F, Alderson PO. Effect of soft-tissue pathology on detection of pedal osteomyelitis in diabetics. J Nucl Med. 1985;26:988–93. [PubMed] [Google Scholar]
- 22.Stokkel MP, Boot CN, van Eck-Smit BL. SPECT gallium scintigraphy in malignant external otitis: Initial staging and follow-up. Case reports. Laryngoscope. 1996;106:338–40. doi: 10.1097/00005537-199603000-00018. [DOI] [PubMed] [Google Scholar]


