Abstract
The advantages of video assisted thoracoscopic surgery (VATS) in children have led to its increased usage over the years. VATS, however, requires an efficient technique for one lung ventilation. Today, there is an increasing interest in developing the technique for lung isolation to meet the anatomic and physiologic variations in infants and children. This article aims to provide an updated and comprehensive review on one-lung ventilation strategies for infants and children undergoing VATS. Search of terms such as ‘One lung ventilation for infants and children’, ‘Video assisted thoracoscopic surgery for infants and children’, and ‘Physiologic changes during one lung ventilation for infants and children’ were used. The search mechanics and engines for this review included the following: Kandang Kerbau Hospital (KKH) eLibrary, PubMed, Ovid Medline, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews. During the search the author focused on significant current and pilot randomized control trials, case reports, review articles, and editorials. Critical decision making on what device to use based on the age, weight, and pathology of the patient; and how to use it for lung isolation are discussed in this article. Furthermore, additional information regarding the advantages, limitations, techniques of insertion and maintenance of each device for one lung ventilation in infants and children were the highlights in this article.
Keywords: Balloon-tipped bronchial blockers, double lumen endobronchial tubes, infants and children, one lung ventilation, univent tubes, video assisted thoracoscopic surgery
INTRODUCTION
Video assisted thoracoscopic surgery (VATS) is a less invasive approach for thoracoscopic surgery. It renders less postoperative pain, fewer operative complications, and shortened hospital stay. This makes VATS favourable for paediatric patients.[1]
Over the years, the indications for VATS in children have increased exponentially. The American Thoracic Society has suggested the use of VATS for stage II pulmonary empyema.[2] A total of 133 children with various thoracic diseases underwent VATS at a University Teaching Hospital in India, from June 2000 to December 2007.[3] From 2000 to 2005, Kandang Kerbau (KKH) Women's and Children's Hospital, Singapore, the Department of Paediatric Anaesthesia; accommodated 10-15 cases of lung isolation for VATS per year. From 2006-2012, lung separation cases for the said procedure increased to 25-33 cases per year.
However, a successful VATS requires well-executed one-lung ventilation (OLV). Currently, lung isolation in infants and children includes the use of single lumen endotracheal tube (ETT), balloon-tipped bronchial blockers (BB) such as Fogarty® embolectomy catheter and Arndt Endobronchial Blocker®, double lumen endobronchial tubes (EBT), and Univent tubes.
Presently, interests are gearing towards foregoing improvements in paediatric lung isolation technique with the following: (1) a ‘quiet’ surgical field with adequate exposure, (2) avoidance of contamination of the normal lung, and (3) prevention of detrimental complications for the young patients such as hypoxemia.[2]
Respiratory insult during OLV: Adults vs. Children
Ventilation (V) and perfusion (Q) are highest on the most dependent portion of the lungs for adults and children. This is due to pressure gradient and gravitational pull. Both factors (V and Q) should be well matched. However, during one lung ventilation during VATS, there are factors that can increase V/Q mismatch because of a decrease in functional residual capacity and tidal volume. General anaesthesia, suboptimal patient positioning, surgical retraction and mechanical ventilation contribute for V/Q mismatch.[3]
Hypoxic pulmonary vasoconstriction (HPV) minimize V/Q mismatch by diverting blood flow away from atelectatic underventilated lung. The HPV response is maximal at normal and decreased at either high or low pulmonary vascular pressure. Furthermore, one can attain maximal HPV when partial pressure in venous blood (PvO2) is normal and decreased response when either high or low PvO2. Therefore, the use of inhalational anaesthetic agents and other vasodilating drugs, together with high or low fraction of inspired oxygen (FiO2) will diminish HPV response.[4] This principle holds true for children and young adults.[5]
The impact of lateral decubitus position on V/Q mismatch on the other hand is different in infants as compared with teens and adults. Placing an adult in a lateral position with the healthy lung on the dependent position causes optimal oxygenation due to hydrostatic pressure gradient between the two lungs and gravitational pull.[6] On the other hand, infants have soft, easily compressible lungs. Their residual volume is closer to functional residual capacity. As such, ventilating the dependent healthy lung, infants can easily have decrease in lung compliance and increase in airway closure even during tidal breathing.[5,7]
Furthermore, the infant's small size results in the decrease in hydrostatic pressure gradient between dependent and nondependent lung. Therefore, there is a loss of the favourable response of increasing perfusion to the dependent ventilated side while reducing the perfusion in the pathologic lung, leaving infants susceptible to hypoxia during one lung ventilation while placed in lateral decubitus position.[8] With this in mind, access for ventilating and providing oxygen on the pathologic side must be maintained during OLV, in the midst of significant oxygen desaturation during operation.[5,6,7]
One lung ventilation techniques in infants and children
One lung ventilation for infants and children is achieved with single-lumen ETT, balloon-tipped bronchial blockers, double-lumen endobronchial tube, and a Univent tube.
Single-lumen endotracheal tube
Single-lumen endotracheal tube (ETT) provides the simplest means of lung isolation. Tube size selection and depth of insertion follow the standard computation based from age; supported by auscultation for breath sounds.[8,9] After tracheal intubation, the ETT can deliberately be advanced into bronchus to isolate the lungs. Difficulties arise when the left bronchus is to be intubated. In order to achieve blind left bronchial Intubation, suggested techniques are using a stylet to curve the distal end of the tracheal tube to the left,[9,10] and using a distally curved rubber bougie that is directed blindly to the left bronchus, followed by railroading the tube over the bougie.[10] Another technique for left lung intubation is when the bevel of the tube is rotated 180° while the head is turned to the right. The ETT is advanced into the bronchus until the right breath sound disappears.[11] The above mentioned techniques do not require a more advanced equipment unless there is a need to confirm tube placement with fiberoptic bronchoscopy (FOB). Single lumen ETT is preferred for emergencies such as contralateral tension pneumothorax.[5,12]
One of the challenges in using a single-lumen ETT is the inadequacy to provide a good seal in the bronchus. As a result, it may not be able to provide a collapsed lung for the operative site, or protect the normal lungs from contamination.[12,13]
In addition to this, the use of single-lumen ETT can easily cause hypoxemia if the short right side bronchus is intubated leading to upper lobe bronchus obstruction. Furthermore, if the patient is in hypoxia, converting to two lung ventilation is risky and technically difficult.[14]
Despite this, single-lumen ETT is still an acceptable option. In a case report by Paquet, they have successfully isolated the whole lung for lavage without the need for post procedural ventilation. They used two single-lumen ETT through the glottis. One tube is seated endobronchially to isolate the lung, to do lavage and the second seated at the trachea for ventilation.[15]
Double-lumen endobronchial tubes
Double-lumen endobronchial tube (EBT) can provide numerous advantages in executing successful one lung ventilation [Table 1]. The ease of positioning and securing the device can give reassurance especially for the general anaesthetists doing OLV.[16] Once positioned, the transparent tube can show if there is any isolated secretion between the right and left side of the lung. In addition, either side of the lung can be suctioned individually. The operated side of the lung can be easily ventilated as the need arises.[17] The bronchial cuff's high volume/low pressure properties reduce the risk of ischemic pressure damage to airway as seen by the very few reports of airway damage in adults and none in children.[18]
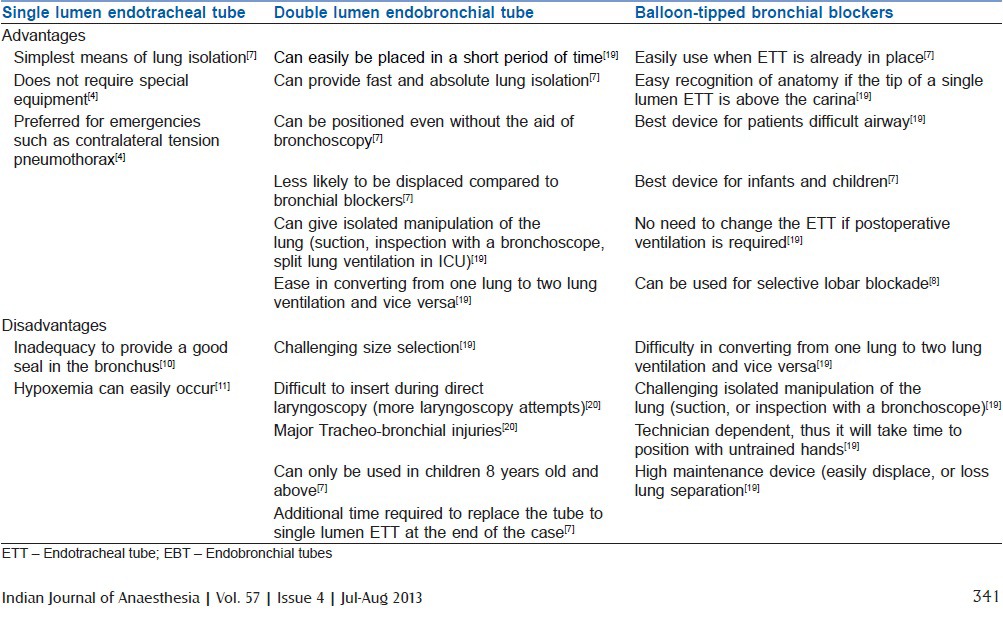
Table 1.
Advantages and disadvantages of single lumen ETT double lumen EBT and balloon-tipped bronchial blockers[4,7,10,11,19,20]
One of the limitations of the double-lumen EBT is the fact that the right main bronchus is shorter than the left and on rare occasions, the right upper lobe bronchus originates not in the right main bronchus but elsewhere. This makes using a double-lumen EBT more challenging.[21] However, the only absolute contraindication for right-sided double-lumen EBT use is the presence of an anomalous right upper lobe take-off from the trachea, which is estimated to occur in 1 in 250 cases.[22]
The technique for insertion of a double-lumen EBT in children is the same as in the adult.[23] The bronchoscopy-guided technique is recommended for placement and positioning of either right or left double lumen EBT. After placing the double-lumen EBT at the trachea, under direct laryngoscopy, the double lumen EBT will be advanced only to the point where the tracheal cuff is at the level just beyond the vocal cords. The tube is then rotated 90° to the indicated side. Then a fiberoptic bronchoscope with an outside diameter (OD) small enough to fit in the tube is used to guide the EBT lumen until it is seated at the appropriate depth.[5,24]
Brodsky suggested two ways in choosing a correct size for double-lumen EBT for children. One is by direct measurement of the bronchial width by chest radiograph, the other is by computing the diameter of the left bronchus (WLB) via known tracheal width (WT) using the formula WLB = (0.4 × WT) + 3.3. He suggested that these techniques are more accurate than relying on age, gender, height or weight.[17,18] In adults, studies have shown that smaller 35-37 F has no associated clinical intraoperative outcome compared to larger 39-41 F double lumen EBT.[18] Currently, the smallest size available is 26 F (Rusch, Duluth, GA. USA) which can be used for children 8 years old and above.[5,10,22]
Balloon-tipped bronchial blockers
Balloon-tipped bronchial blockers (BB) remain the ‘technique of choice’ in paediatric patients, under the age of 6 years.[16,25] This is because Univent 3.5 uncuffed version tube (recommended for 6-8 years old) and double-lumen EBT (recommended for 8-10 years old) diameters are big for the aforementioned age group.[25]
Balloon-tipped BB can advance down or alongside the single lumen ETT. This is done with the use of a fiberoptic bronchoscope (FOB) and checked by auscultation. Considering this, balloon-tipped bronchial blockers [Figure 1] are also favoured for the use in an intubated (orally or nasally) patient and patients with tracheostomy with sufficient internal diameter[25,26] [Table 1].
Figure 1.
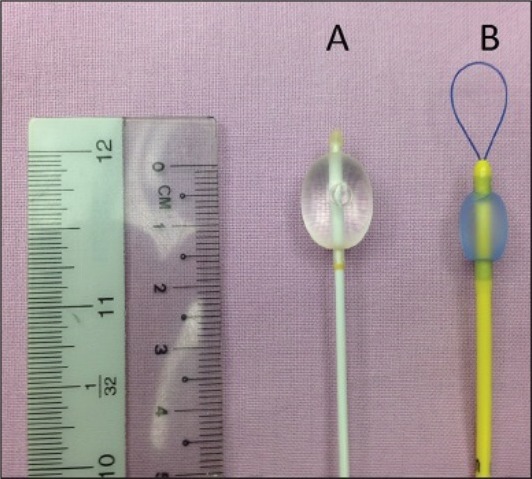
A – Biosensors® embolectomy catheter, B – Arndt Endobronchial Blocker®
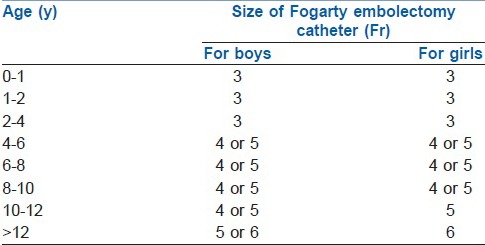
Over the years various balloon-tipped BB has been designed and used according to their purpose. As early as 1969, Vale and Lines already described the use of Fogarty® embolectomy catheter for lung isolation in small children and suggested appropriate size selection for this age group.[27,28] Tan and Tan-Kendrick, measured right and left bronchus of 250 children aged 2 days to 16 years old using computed tomograms (CT) of the thorax and correlated it with the patient's age and weight, in order to create a guide to size selection of the Fogarty catheter. From this study, they found out that the age, but not the weight, of the patient is a good predictor of the main bronchial diameters. They recommended the use of a 3 Fr Fogarty catheter up to the age of 4 years and a 5 Fr catheter for 5-12 years old [Table 2]. Their decision to use a size 5 Fr Fogarty instead of 4 Fr as a bronchial blocker for children older than 4 years old is due to small (1 mm) difference in maximum diameter between the two (5 Fr and 4 Fr). In addition to this, they perceived that the balloon pressure exerted by the larger catheter on the bronchial wall might be lower. Since embolectomy catheters can exert high pressure on the airway, it is worth knowing that by using incremental volumes of air in inflating the balloon until the seal is achieved will be helpful in preventing untoward effects of having high pressures.[29]
Table 2.
Fogarty catheter size for lung isolation in children by age from Tan and Tan-Kendrick[29]
The Arndt Endobronchial Blocker® (Cook, WEB, Critical care, Bloomington, IN, USA) [Figure 1] contains a flexible wire loop that passes from the proximal end and exits at the distal end. A special three-part swivel adaptor allows introduction of the FOB in one port, a balloon-tipped BB through the second port and a third port for ventilation circuit[30] [Figure 2]. The suggested first step is to insert endobronchial blocker through the blocker port of the Arndt Multiport Adapter. Advancing it until the guide loop is within the body of the adapter. Following it is the insertion of FOB through the bronchoscopy port until it passes through the loop [Figure 3]. Then the coupled FOB and endobronchial blocker is advanced to the side of the lung to be blocked. After correct identification of bronchus to be blocked, the FOB is distally pushed further, enough such that the Arndt Endobronchial Blocker® enters the bronchus.[31] Once it is certain that the blocker is in position, the FOB is slowly withdrawn. Then the cuff will be inflated under direct FOB visualization with incremental introduction of air appropriate for the size of the bronchial blocker. This is done until total bronchial blockade is achieved.[19,25,26] Lung exclusion is confirmed by direct vision of the inflated balloon into the bronchus and auscultation of lung separation.[32] Then the fiberoptic scope is removed before the bronchial scope port tightened to permit correct ventilation.[26]
Figure 2.
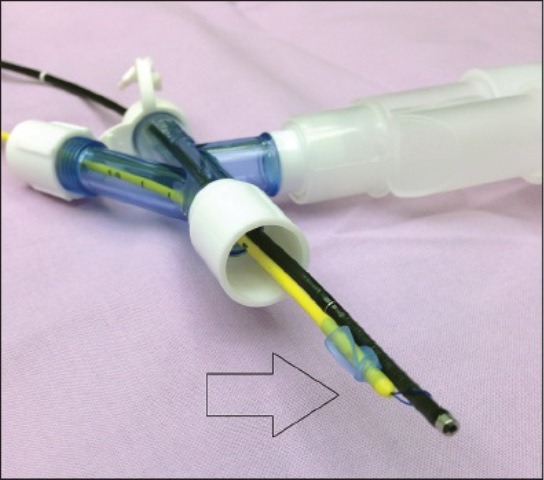
Arndt endobronchial blocker® wire loop is coupled with FOB to direct the blocker to the mainstem bronchus
Figure 3.
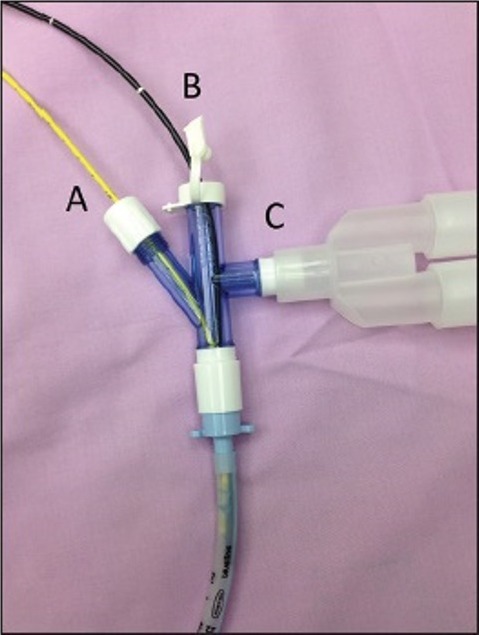
Arndt endobronchial blocker® by Cook, Multiport airway adapter: A – Blocker port, B – FOB port, C – Ventilation port
In the randomized trial of three bronchial blockers (Arndt, Cohen, and Fuji) versus double-lumen tubes by Narayanaswamy, he concluded that among the bronchial blockers, the Arndt Endobronchial Blocker® needed to be repositioned more often.[33] The cuff of the blocker is deflated and advanced 1 cm deeper to avoid proximal dislodgement before turning the patient into lateral decubitus position. Direct visual check with FOB whether the bronchial blocker has achieved optimal position can allay uncertainties. With the patient in the lateral decubitus position, the outer surface of the blocker balloon should be at least 5 mm below the tracheal carina. Withdrawing the wire loop will convert this 1.4 mm channel into a suction port to expedite lung collapse.
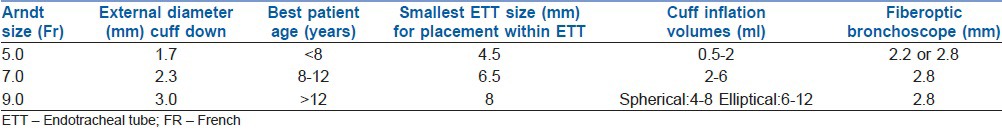
For children, the Arndt Endobronchial Blocker® is only suitable if the ETT to be used is greater than 4.5 mm internal diameter, as the available 5 Fr catheter has a diameter of 2.5 mm and requires a small bronchoscope of at least 2.2 mm for positioning [Table 3].[34,35]
Table 3.
Arndt endobronchial blocker® sizing lifted from Bird et al.[34]
Univent tube
The Univent tubes (TCB type, Fuji Systems, Tokyo, Japan) with movable bronchial blockers have an advantage of technical ease in positioning and placement when used to facilitate one lung ventilation. The manufacturer has two recommendations in inserting the univent tube. The first method is by inserting the tube orotracheally as with a conventional ETT and rotating it 90° so that the blocker lumen is on the thoracotomy side. After inflating the balloon and securing the tube, the bronchial blocker shaft will be pushed out of the tracheal tube pocket. The second method is FOB assisted, where in the bronchial blocker shaft will be pushed away under direct vision.[36] In the study by Hao, et al., they concluded that manual auscultation method for Univent intubation was feasible, less time consuming and relatively easy. In a difficult airway, lighted stylet-guided Univent tube placement took less time for correct placement. However, the study was conducted among adult population and with a small sample size.[37] On the other hand, compared to double-lumen EBT, Univent tubes may reduce the airway injury and improve the compliance during OLV. However, there is a higher incidence of intraoperative malposition.[38]
The univent tube with a 3.5 mm internal diameter has an external size of 7.5-8.0 mm while univent tube size 4.5 mm internal diameter has an outer diameter of 8.5-9 mm. Thus, the 3.5 mm tube can only be used for older children whose airway is small for the smallest double lumen tubes.[39,40]
CONCLUSIONS
To overcome the challenges of rendering one lung ventilation technique in infants and children coming for video assisted thorascopic surgery, one must be mindful of the respiratory insult caused by OLV under general anaesthesia, and positioning during operation. Although it is prudent to use a device one is technically familiar with, the anaesthetists must also be aware whether if it is appropriate for the patients’ age and weight. Furthermore, if the device is equipped with safety features such as ventilating both lungs in the event of hypoxia, and if it can provide efficient lung isolation intraoperatively.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Shah R, Reddy AS, Dhende NP. Video assisted thoracic surgery in children. J Minim Access Surg. 2007;3:161–7. doi: 10.4103/0972-9941.38910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ris HB, Krueger T. Video-assisted thoracoscopic surgery and open decortication for pleural empyema. Multimed Man Cardiothorac Surg. 2004;10:1510. doi: 10.1510/mmcts.2004.000273. [DOI] [PubMed] [Google Scholar]
- 3.Oak SN, Parelkar SV, Satishkumar KV, Pathak R, Ramesh BH, Sudhir S, et al. Review of video-assisted thoracoscopy in children. J Minim Access Surg. 2009;5:57–62. doi: 10.4103/0972-9941.58498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campos JH. Progress in lung separation. Thorac Surg Clin. 2008;15:71–83. doi: 10.1016/j.thorsurg.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Dimitriou G, Greenough A, Pink L, McGhee A, Hickey A, Rafferty GF. Effect of posture on oxygen and respiratory muscle strain in covalescent infants. Arch Dischild Fetal Neonatal. 2002;86:147–50. doi: 10.1136/fn.86.3.F147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommer N, Dietrich A, Schermuly RT, Ghofrani HA, Gaudermann T, Schulz R, et al. Regulation of hypoxic pulmonary vasoconstriction: Basic mechanisms. Eur Respir J. 2008;32:1639–51. doi: 10.1183/09031936.00013908. [DOI] [PubMed] [Google Scholar]
- 7.Hammer GB. Single-lung ventilation in infants and children. Pediatr Anesth. 2004;14:98–102. doi: 10.1046/j.1460-9592.2003.01197.x. [DOI] [PubMed] [Google Scholar]
- 8.Heaf DP, Helms P, Gordon MB, Turner HM. Postural effects on gas exchange in infants. N Engl J Med. 1983;28:1505–8. doi: 10.1056/NEJM198306233082505. [DOI] [PubMed] [Google Scholar]
- 9.Mansell A, Bryan C, Levison H. Airway closure in children. J Appl Physiol. 1972;33:711–4. doi: 10.1152/jappl.1972.33.6.711. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky J. Lung separation and the difficult airway. Br J Anaesth. 2009;103(Suppl 1):i66–75. doi: 10.1093/bja/aep262. [DOI] [PubMed] [Google Scholar]
- 11.Baraka A, Slim M, Dajani A, Lakkis S. One-lung ventilation of children; during surgical excision of hydatid cysts of the lung. Br J Anaesth. 1982;54:523–8. doi: 10.1093/bja/54.5.523. [DOI] [PubMed] [Google Scholar]
- 12.Baraka A, Dajani A, Maktabi M. Selective contralateral bronchial intubation in children with pneumothorax or bronchopleural fistula. Br J Anaesth. 1983;55:901–4. doi: 10.1093/bja/55.9.901. [DOI] [PubMed] [Google Scholar]
- 13.Rowe R, Andropoulos D, Heard M, Johnson K, DeCampli W, Idowu O. Anesthestic Management of patients undergoing thoracoscopy. J Cardiothorac Vasc Anesth. 1994;8:563–6. doi: 10.1016/1053-0770(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 14.Kubota H, Kubota Y, Toyoda Y, Ishida H, Asada A, Matsuura H. Selective blind endobronchial intubation in children and adults. Anesthesiology. 1987;67:587–9. doi: 10.1097/00000542-198710000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Paquet C, Karsli C. Technique of lung isolation for whole lung lavage in a child with pulmonary alveolar proteinosis. Anesthesiology. 2009;110:190–2. doi: 10.1097/ALN.0b013e318190bda2. [DOI] [PubMed] [Google Scholar]
- 16.Campos JH. Progress in lung separation. Thoracic Surg Clin. 2008;15:71–83. doi: 10.1016/j.thorsurg.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Brodsky JB, Lemmens HJ. Left double-lumen tubes: Clinical experience with 1,170 patients. J Cardiothorac Vasc Anesth. 2003;17:289–98. doi: 10.1016/s1053-0770(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 18.Brodsky JB, Adkins MO, Gaba DM. Bronchial cuff pressures of double lumen tubes. Anesth Analg. 1989;69:608–10. [PubMed] [Google Scholar]
- 19.Campos JH. Which device should be considered the best for lung isolation: Double-lumen endobronchial tubes versus bronchial blockers. Curr Opin Anesthesiol. 2007;20:27–31. doi: 10.1097/ACO.0b013e3280111e2a. [DOI] [PubMed] [Google Scholar]
- 20.Grocott HP, Darrow TR, Whiteheart DL, Glower DD, Smith MS. Lung isolation during port-access cardiac surgery: Double-lumen endotracheal tube versus single-lumen endotracheal tube with a bronchial blocker. J Cardiothorac Vasc Anesth. 2003;17:725–7. doi: 10.1053/j.jvca.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Benumof JL, Partrige BL, Salvatierra C. Margin of safety in positioning modern double-lumen endotracheal tubes. Anesthesiology. 1987;67:729–38. doi: 10.1097/00000542-198711000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Stene R, Rose M, Weinger MB, Benumof JL, Harrell J. Bronchial trifurcation at the carina complicating use of double-lumen tracheal tube. Anesthesiology. 1994;80:1162–4. doi: 10.1097/00000542-199405000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Brodsky JB, Mark JBD. A simple technique for accurate placement of double-lumen endobronchial tubes. Anesthesia Rev. 1983;10:26–30. [Google Scholar]
- 24.Merli G, Guarino A, Della Rocca G, Frova G, Petrini F, Sorbello M, et al. Recommendations for airway control and difficult airway management in thoracic anesthesia and lung separation procedures. Minerva Anestesiol. 2009;75:59–78. [PubMed] [Google Scholar]
- 25.Hammer GB, Fitzmaurice BG, Brodsky JB. Methods for single lung ventilation in pediatric patients. Anesth Analg. 1999;89:1426–9. doi: 10.1097/00000539-199912000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Rothenberg SS. Thoracoscopy in infants and children: State of the art. J Pediatr Surg. 2005;40:303–6. doi: 10.1016/j.jpedsurg.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Vale R. Selective bronchial blocking in a small child. Case Report. Br J Anaesth. 1969;41:453–4. doi: 10.1093/bja/41.5.453. [DOI] [PubMed] [Google Scholar]
- 28.Lines V. Selective bronchial blocking in a small child. Br J Anaesth. 1969;41:893. doi: 10.1093/bja/41.5.453. [DOI] [PubMed] [Google Scholar]
- 29.Tan GM, Tan-Kendrick AP. Bronchial diameters in children – use of the Fogarty catheter for lung isolation in children. Anaesth Intensive Care. 2002;30:615–8. doi: 10.1177/0310057X0203000512. [DOI] [PubMed] [Google Scholar]
- 30.Campos JH. Update on lung separation techniques: Double-lumen tubes and bronchial blockers. Revista Mexicana de Anestesiologia. 2011;34:S270–7. [Google Scholar]
- 31.Campos JH, Kerstine KH. A comparison of left sided broncho-cath with the torque control blocker univent and the wire guided blocker. Anesth Analg. 2003;96:283–9. doi: 10.1097/00000539-200301000-00056. [DOI] [PubMed] [Google Scholar]
- 32.Larson CE, Gasior TA. A device for endobronchial blocker placement during one lung anesthesia. Anesth Analg. 1990;71:311–2. doi: 10.1213/00000539-199009000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Narayanaswamy M, McRae K, Slinger P, Dugas G, Kanellakos GW, Roscoe A, et al. Choosing a lung isolation device for thoracic surgery: A randomized trial of three bronchial blockers versus double-lumen tubes. Anesth Analg. 2009;108:1097–101. doi: 10.1213/ane.0b013e3181999339. [DOI] [PubMed] [Google Scholar]
- 34.Bird GT, Hall M, Nel L, Davies E, Ross O. Case Report: Effectiveness of Arndt endobronchial blockers in pediatric scoliosis surgery: A case series. Pediatr Anesth. 2007;17:289–94. doi: 10.1111/j.1460-9592.2006.02121.x. [DOI] [PubMed] [Google Scholar]
- 35.Wald SH, Mahajan A, Kaplan MB, Atkinson JB. Experiencewith the Arndt paediatric bronchial blocker. Br J Anaesth. 2004;94:92–4. doi: 10.1093/bja/aeh292. [DOI] [PubMed] [Google Scholar]
- 36.Inoue H, Shohtsu A, Ogawa J, Kawada S, Koide S. New device for one-lung anesthesia: Endotracheal tube with movable blocker. J Thorac Cardiovasc Surg. 1982;83:940–1. [PubMed] [Google Scholar]
- 37.Weng H, Xu ZY, Liu J, Ma D, Liu DS. Placement of the Univent tube without fiberoptic bronchoscope assistance. Anesth Analg. 2010;110:508–14. doi: 10.1213/ANE.0b013e3181c5ed18. [DOI] [PubMed] [Google Scholar]
- 38.Zheng H, Duan Y, Geng WM, Liu W, Gao GK, Wang C. A comparison of double-lumen endotracheal tube with univent blocker during thoracic surgical anesthesia. Zhonghua Yi Xue Za Zhi. 2012;92:2481–4. [PubMed] [Google Scholar]
- 39.Hammer GB, Brodsky Jb, Redepath JH, Cannon WB. The Univent tube for single-lung ventilation in paediatric patients. Paediatr Anaesth. 1998;8:55–7. doi: 10.1046/j.1460-9592.1998.00691.x. [DOI] [PubMed] [Google Scholar]
- 40.Takenaka I, Aoyama K, Kadoya T. Use of the Univent bronchial- blocker tube for unanticipated difficult endotracheal intubation. Anesthesiology. 2000;93:590–1. doi: 10.1097/00000542-200008000-00060. [DOI] [PubMed] [Google Scholar]


