Abstract
During the inhalation of anaesthesia use of low fresh gas flow (0.35-1 L/min) has some important advantages. There are three areas of benefit: pulmonary - anaesthesia with low fresh gas flow improves the dynamics of inhaled anaesthesia gas, increases mucociliary clearance, maintains body temperature and reduces water loss. Economic - reduction of anaesthesia gas consumption resulting in significant savings of > 75% and Ecological - reduction in nitrous oxide consumption, which is an important ozone-depleting and heat-trapping greenhouse gas that is emitted. Nevertheless, anaesthesia with high fresh gas flows of 2-6 L/min is still performed, a technique in which rebreathing is practically negligible. This special article describes the clinical use of conventional plenum vaporizers, connected to the fresh gas supply to easily perform low (1 L/min), minimal (0.5 L/min) or metabolic flow anaesthesia (0.35 L/min) with conventional Primus Draeger® anaesthesia machines in routine clinical practice.
Keywords: Closed-circuit anaesthesia, inhalation anaesthesia, quantitative closed circuit anaesthesia
INTRODUCTION
In order to ensure satisfactory gas exchange and dynamics during inhalation anaesthesia, a low fresh gas flow technique (0.35-1 L/min) is mandatory. There are several reasons:
Pulmonary: Anaesthesia with low fresh gas flow improves the flow dynamics of the inhaled air,[1] it also serves to increase mucociliary clearance, maintain body temperature and reduce fluid loss.[2]
Economical reasons: Reduction of anaesthesia gas consumption results in significant savings. Used routinely, low flow anaesthesia can result in savings of up to 75%.[3]
Ecological reasons: Reduction of greenhouse gas emissions through a reduction of unused surplus gas, a significant reduction in anaesthesia gas emissions is achieved, which can amount to up to 90%. This has positive effects: Workplace concentration of anaesthesia gas in the operating room is greatly reduced. This reduces exposure of anaesthesia and other operating room personnel to nitrous oxide. Reduction of anaesthesia gas emissions (nitrous oxide and inhalation anaesthetics) reduces the impact on the ozone layer. Nitrous oxide is now the dominant ozone-depleting and heat-trapping greenhouse gas substance emitted by humans.[4]
It is well-known that only low flow anaesthesia makes adequate use of rebreathing systems. Acceptance of these methods has grown greatly since the introduction of new inhalation anaesthetics and installation of gas monitoring in anaesthesia machines. Nevertheless, anaesthesia with high fresh gas flows between 2 L/min and 6 L/min are still performed, a technique in which rebreathing is practically negligible. This article describes the clinical practice of low (1 L/min), minimal (0.5 L/min) or metabolic flow anaesthesia (0.35 L/min).
REBREATHING SYSTEMS
The fresh gas flow rate determines whether a rebreathing system is classified as an open, semi-open, semi-closed or closed system. If fresh gas is fed into the rebreathing system in an amount exceeding 50-100% of the minute volume, the anaesthesia system is considered to be open. Practically no exhaled air is reused to ventilate the patient. The exhaled anaesthesia gas is removed from the circuit as surplus gas. Higher the fresh gas flow, lower is the rebreathed portion and larger the surplus gas portion [Figure 1].[4] A basic prerequisite for the conduct of low flow anaesthesia is use of rebreathing systems. Characteristic for this system is a carbon dioxide absorber removing CO2 from the breathing circuit and the common reaction of neutralization is:
Figure 1.
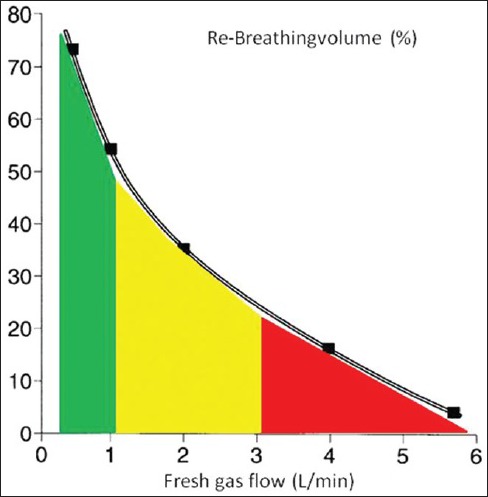
With partial rebreathing, the rebreathing system is semi-open. This corresponds to a fresh gas flow of 3-6 L/min. Further reduction of fresh gas flow (1-3 L/min) with a subsequent increase in the rebreathed portion is known as semi-closed. If however, the fresh gas flow is adjusted to compensate for just the amount of gas uptake by the patient, the expired air must be returned to the patient following CO2 elimination in its entirety for the next breath. Fresh gas is fed into the system only to replace gas absorbed by the patient and this is known as a closed rebreathing system
CO2 + Ca(OH)2 → CaCO3+ H2O + heat (13.7 kcal).
The continuous loss of gas from the system caused by patient oxygen consumption is compensated by the introduction of fresh gas into the breathing circuit.
OXYGEN, NITROUS OXIDE AND INHALATION ANAESTHETIC UPTAKE
During the anaesthesia, patient oxygen uptake corresponds to approximately the basal metabolic rate. The simplified Brody equation[5] allows an estimate of oxygen consumption [Table 1]. Nitrous oxide uptake follows an exponential function: During the initial minutes of anaesthesia, it is high. With increasing duration of anaesthesia, tissue compartments become saturated and uptake slows. A rough estimate of nitrous oxide uptake can be calculated by the Severinghaus equation[6] [Table 1]. The uptake of volatile anaesthetics decreases during anaesthesia as a function of tissue saturation. Inhalation anaesthetic uptake is described by the exponential equation derived by Lowe[7,8] [Tables 1, 2 and Figures 2].
Table 1.
Oxygen, nitrous oxide and volatile anaesthetic uptake

Table 2.
Gas uptake (ml/min) calculated according to the equation above after 30 min of anaesthesia for oxygen (ml*), nitrous oxide (ml**) and sevoflurane (ml***)

Figure 2.
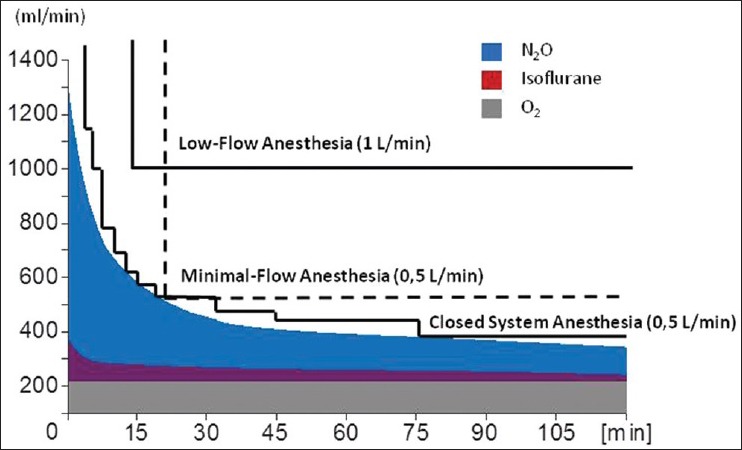
Total gas uptake of a 75 kg patient and different methods of low flow anaesthesia: Different grades of precision to adapting the fresh gas flow to the uptake
CLASSIFICATION OF ANAESTHESIA METHODS WITH LOW FRESH GAS FLOW
Fresh gas flows can be continuously reduced to any desired value down to the gas volume, which is actually metabolised by patient. For practical purposes, the following methods for low flow anaesthesia have been categorised. Low flow anaesthesia starts initially with high fresh gas flows between 4 L/min and 6 L/min and then reduced to 1.0 L/min after 10 min [Figure 2: Low flow anaesthesia]. Minimal Flow anaesthesia starts with an initial phase of 15-20 min of 4-6 L/min, followed by a reduction of fresh gas flow down to 0.5 L/min[9] [Figure 2].
Anaesthesia, which employs closed systems, can be performed in two ways: If fresh gas flow is continuously adjusted with a dosing apparatus to meet individual patient gas uptake and the amount of oxygen, nitrous oxide and inhalation anaesthetic is replaced, which is actually absorbed by patient at any given time, the method is known as quantitative closed-loop anaesthesia. The Zeus IE, developed by Draeger® Lόbeck, Germany, is capable of automatically adjusting oxygen administration, carrier gas and volatile agents to deliver the right absorbed amount through direct injection of volatile agents as quantitative closed-loop anaesthesia.[10]
It can also be performed by manually adjusting the fresh gas flow volume, without exact knowledge of the uptake of patient (non-quantitative or nearly closed circuit anaesthesia [Figure 2: Closed system anaesthesia].
Oxygen as a carrier gas
High oxygen concentration in inhaled air not only increases patient safety, it also reduces the incidence of post-operative wound infections and the frequency of nausea and vomiting. The increased likelihood of atelectasis at high oxygen tensions as compared to ventilation at lower oxygen concentrations appears to have no clinical effect on post-operative gas exchange or ventilation function. For healthy patients, a limited period of ventilation of up to 6-8 h with high oxygen concentrations is considered to be without significant disadvantage. The use of recruitment manoeuvres and positive end-expiratory pressures serve to inhibit atelectasis formation.[11]
Some contraindications exist for ventilation with high oxygen concentrations in patients with increased bronchial secretions; bronchioles may become clogged and are consecutively cut-off from ventilation. A decreased tolerance to high oxygen concentrations can be assumed for patients with acid aspiration, severe inflammatory alterations of pulmonary tissues, premature neonates and patients undergoing chemotherapy with bleomycin and mitomycin. Laser surgery in regions where ventilation gases are present cannot be performed if high oxygen levels are used due to risk of fire or explosion. In most hospitals, these contraindications are rarely experienced, but they require the use of anaesthesia machines equipped with a second gas dosing apparatus for air.[11]
It remains questionable as to whether the oxygen concentration of 80% quoted in numerous publications truly represents a turning point in the balance between positive and negative effects of higher oxygen tensions.
Oxygen and medical air as a carrier gas mixture
If the anaesthesia machine is equipped with a gas dosing system designed for use with minimal flow anaesthesia, the Anaesthetist can adjust the composition of the carrier gas mixture to achieve the desired oxygen concentration in the breathing circuit. Economical anaesthesia, up to and including closed loop anaesthesia, is feasible.
If the anaesthesia machine uses a dosing apparatus, which does not allow low levels of gas flow for medical air, the anaesthetist must then decide whether he or she is willing to do without the economic advantages of low or minimal flow anaesthesia and instead use low inspiratory oxygen concentrations during anaesthesia. Such machines can, however, be used to perform closed-loop anaesthesia, which substitutes small amounts of pure oxygen following an initial high flow phase.
If medical air is not available or the anaesthesia machine is not equipped with a corresponding dosing apparatus, the only possible alternative is to avoid the use of nitrous oxide and use pure oxygen as the carrier gas as described above.
Low flow anaesthesia using an oxygen/nitrous oxide carrier gas
Induction of low flow anaesthesia is identical to conventional methods: Following pre-oxygenation, injection of an opioid and hypnotic agent and if desired, a muscle relaxant, an endotracheal tube or laryngeal mask is placed in position. Patient is connected to the rebreathing system.[12]
With the connection of the patient to the anaesthesia machine, an initial phase of high fresh gas flow (4-6 L/min) follows. During this initial phase, adequate denitrogenation and distribution of anaesthetic gases in the desired concentration throughout the system is achieved and the desired level of anaesthesia is attained. The duration of the initial phase is determined by the amount of flow reduction and the individual gas uptake (4-5 L/min, 6-8 min). After this time, oxygen and nitrous oxide levels reach 30% O2 and 65% N2O, respectively [Figure 3]. If isoflurane is used, the vaporizer is set to 1.5%; enflurane and sevoflurane require 2.5% setting and desflurane requires a 4-6% setting. After 6-8 min, an expiratory concentration corresponding to 0.8 minimum alveolar concentration (MAC) of the respective anaesthetic agent is attained [Figure 3]. At an additive nitrous oxide concentration of 50-60%, this value approximately corresponds to AD95, or the anaesthetic agent concentration at which 95% of patients tolerate a skin incision without noticeable reaction.
Figure 3.
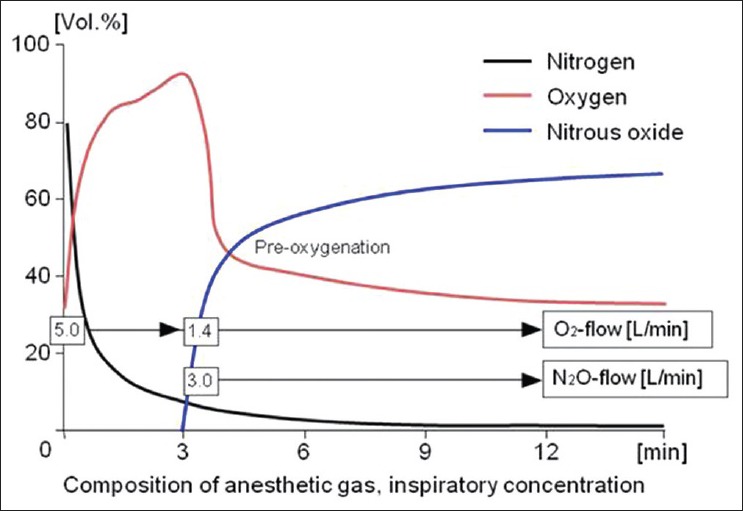
Initial high flow anaesthesia with oxygen and nitrous oxide to establish adequate nitrous oxide concentration
After 10 min, the total gas uptake of an adult patient is about 600 ml/min, so that at this time, the flow can be reduced to 1.0 L/min and low flow anaesthesia can be initiated [Figure 3]. An inspiratory oxygen concentration level of 30% can only be maintained if the fresh gas oxygen concentration is increased to 50% once the flow has been reduced.
Flow reduction also decreases the amount of anaesthetic agent, which is introduced into the fresh gas flow by the vaporizer. If the initially selected anaesthetic agent concentration of 0.8 × MAC is to be maintained, the vaporizer must be adjusted for 3.0%. For desflurane, no changes are necessary.[9]
Before reducing fresh gas flow rates to even lower levels, i.e., 0.5 L/min, to perform minimal flow anaesthesia, the initial phase should be extended to 15 min and for very large patients to 20 min.
Because the rebreathing fraction is increased compared with low flow anaesthesia, oxygen concentration should be increased to 60%; 50% represents an absolute minimum value.
An expiratory anaesthetic agent concentration of 0.8 × MAC can only be maintained if the fresh gas concentration of enflurane is increased to 2.5%, enflurane and sevoflurane to 3.5% and desflurane is increased 1% above the initial setting [Figure 4].
Figure 4.
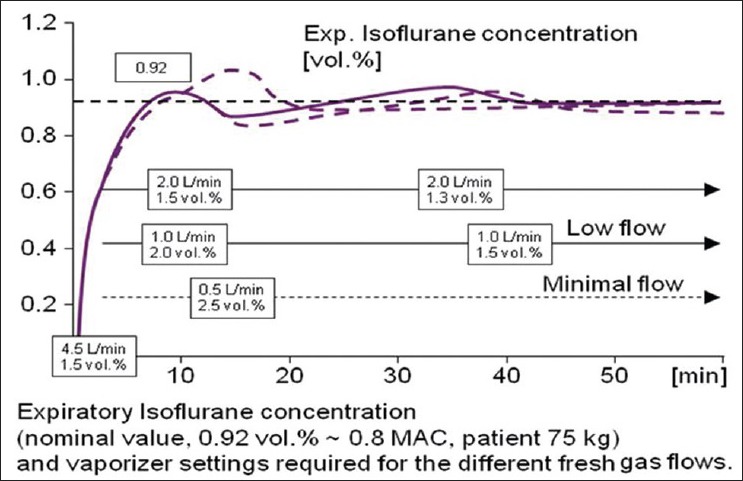
Isoflurane vaporizer setting values with high fresh gas flow (2 L/min), with low fresh gas flow (1 L/min) and minimal flow anaesthesia (0.5 L/min)
In low flow anaesthesia, the composition of anaesthetic gases changes continuously during the course of anaesthesia, making intermittent adjustments to fresh gas composition necessary.
Another specific characteristic of low flow anaesthesia is the large amount of latency with which changes in the anaesthetic gas concentration of the fresh gas result in changes in anaesthetic gas concentrations in the breathing circuit. The speed of the wash-in and wash-out processes can be described with the time constant of the systems. According to the Conway Equation: T = VS/(VF- VU) the time constant T is proportional to the system volume VS (machine and lung volume) and at a constant rate of uptake VU, inversely proportional to the amount of anaesthetic agent fed into the system at the time [Figure 5].[9] In low flow anaesthesia, changes in fresh gas composition require a significant amount of time to cause changes in the composition of the gases in the breathing circuit. These large time constants must be considered when performing low flow anaesthesia [Figure 5]. Anaesthesiologists discuss about the use of nitrous oxide as composition of the carrier gas (pro/contra discussion).[11] At the Marienhospital Vechta the use of nitrous oxide was stopped in 2003.
Figure 5.
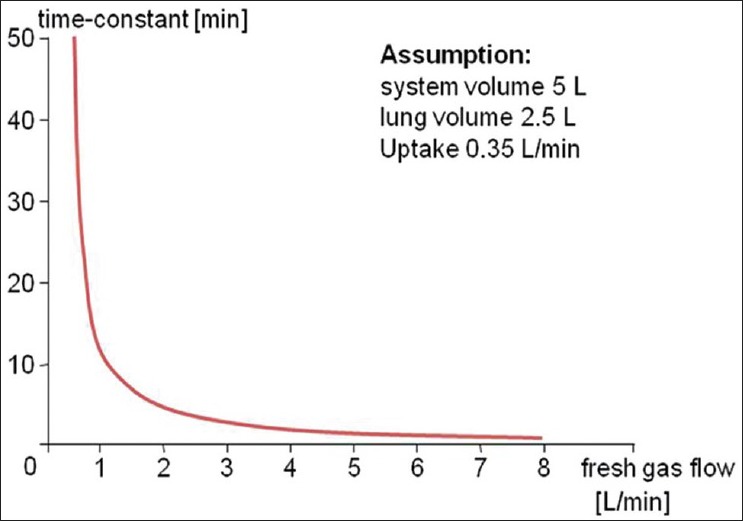
Time-constant using different fresh gas flow
Monitoring requirements for the safe conduct of low flow anaesthesia with nitrous oxide
The lower alarm of the inspiratory oxygen concentration should be set between 28% and 30%. The continuous monitoring of sufficient gas filling of the breathing circuit can be achieved by monitoring peak pressure or minute volume. The alarm threshold of the disconnection alarm should be set to a value 5 mbar below the peak pressure. The threshold for minute volume should be set at a value 0.5 L/min below the desired minute volume setting.
Continuous monitoring of the anaesthetic agent concentration according to applicable technical norms should also be considered mandatory as well as continuous monitoring of inspiratory and expiratory CO2 concentrations.[13]
Low flow anaesthesia without nitrous oxide
In clinical practice, conduction of inhalation anaesthesia without nitrous oxide is simple: The loss of analgesic effect can be compensated by a moderate increase in the additive dose of opioids, while the reduction in hypnotic effect can be countered with an increase in the concentration of the inhaled anaesthetic agent by only 0.2-0.25% of the MAC value of the respective agent used. For adults, the desired expiratory isoflurane concentration is 1.2%, for sevoflurane 2.2% and for desflurane 5%.
Because only oxygen and the anaesthetic agent are absorbed, total gas uptake is noticeably reduced and de-nitrogenation is no longer necessary. The initial phase of low flow anaesthesia, when high fresh gas flow rates are used, can be kept much shorter. Its duration is solely determined by the wash-in characteristics of the inhalation anaesthetic agent used. By eliminating nitrous oxide uptake, the breathing system filling is improved following the reduction of fresh gas flow– even down to 0.5 L/min, than in low flow anaesthesia using a nitrous oxide/oxygen carrier gas mix. When using anaesthesia machines with very airtight, compact breathing systems, the conduction of non-quantitative anaesthesia with closed systems is possible, where fresh gas flow can be reduced to patient oxygen uptake. This can be estimated with the help of the Brody Equation and is about 250-350 ml/min for adults of normal weight. At this low flow rate, however, the limits of the vaporizer's capability to emit sufficient amounts of agent into the fresh gas stream are reached, which, as a rule, are approximately 3 × MAC of the respective anaesthetic agents. The new volatile anaesthetics with reduced solubility, sevoflurane and desflurane, are much better suited for use with fresh gas flows corresponding to the rate of patient oxygen uptake.[11]
Indications for low flow anaesthesia
The described variations of low flow anaesthesia do not represent a separate entity of anaesthesia methodology, but rather describe techniques, which should be used with rebreathing systems. Whenever inhalation anaesthesia is indicated and an anaesthesia machine with a rebreathing system is used, fresh gas flow should be reduced in order to avoid the completely unnecessary emission of surplus anaesthetic gases to a minimum level in line with the available technical and monitoring resources. Guidelines for indications according to particular surgical procedures or patient morbidity cannot be formulated. Short duration mask anaesthesia and methods, which do not provide a sufficiently airtight airway seal, e.g., bronchoscopy with a rigid bronchoscope require higher fresh gas flow.
Contraindications for low flow anaesthesia
If outgassing of alcohol during anaesthesia of intoxicated patients is not to be inhibited, a fresh gas flow of at least 1 L/min should be used in order to provide a continuous wash-out effect.
During anaesthesia on patients with ketoacidosis, such as diabetic, an increase in acetone concentrations in the blood may occur. In such cases, fresh gas flow should be kept at least 1.0 L/min in order to prevent the undesirable build-up of acetone in the breathing system.
Accumulation of carbon monoxide in breathing systems is generally clinically insignificant. Even with long duration minimal flow anaesthesia, the carboxy-haemoglobin concentration in the blood increases only slightly. Same is the situation with Compound A; Mazze et al. published results of an investigation on nephrotoxicity of Compound A in primates demonstrating that only at a load of at least 800 ppmh nephrotoxic effects occurred.[14] Accepting this threshold for nephrotoxic load with Compound A, absolutely no flow restriction would be justified. Even long lasting Minimal Flow Anaesthesia with sevoflurane could be performed safely, although Compound A peak concentrations were found to reach 50-60 ppm with this technique. Therefore, sevoflurane was approved for clinical use without any fresh gas flow restriction in all countries of the European Common Market.
CONCLUSION
In clinical practice, conduct of inhalation anaesthesia with 0.35 L/min, 0.5 L/min and 1 L/min fresh gas flow without nitrous oxide is simple. Low flow anaesthesia techniques are beneficial for the patient by improving pulmonary dynamics of the anaesthetic gases, increasing mucocilliary clearance, maintaining body temperature and reducing fluid loss. Reduction of anaesthesia gas consumption results in significant savings up to 75%, decrease of greenhouse gas emissions and lower impact on the ozone layer.
ACKNOWLEDGMENT
In memory of our friend and teacher Prof. Dr. Med. Jan Baum (Died on November 13th, 2009).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kleemann PP. Humidity of anaesthetic gases with respect to low flow anaesthesia. Anaesth Intensive Care. 1994;22:396–408. doi: 10.1177/0310057X9402200414. [DOI] [PubMed] [Google Scholar]
- 2.Aldrete JA, Cubillos P, Sherrill D. Humidity and temperature changes during low flow and closed system anaesthesia. Acta Anaesthesiol Scand. 1981;25:312–4. doi: 10.1111/j.1399-6576.1981.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 3.Suttner S, Boldt J. Low-flow anaesthesia. Does it have potential pharmacoeconomic consequences? Pharmacoeconomics. 2000;17:585–90. doi: 10.2165/00019053-200017060-00004. [DOI] [PubMed] [Google Scholar]
- 4.Baum JA. Low-flow anesthesia: Theory, practice, technical preconditions, advantages, and foreign gas accumulation. J Anesth. 1999;13:166–74. doi: 10.1007/s005400050050. [DOI] [PubMed] [Google Scholar]
- 5.Brody JS. New York: Reinhold; 1945. Bioenergetics and Growth, with Special Reference to the Efficiency Complex in Domestic Animals. [Google Scholar]
- 6.Severinghaus JW. The rate of uptake of nitrous oxide in man. J Clin Invest. 1954;33:1183–9. doi: 10.1172/JCI102991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg IS, Mostert JW, Lanzl EF, Lowe HJ. A pharmacokinetic model of closed-circuit inhalation anesthesia. Ann Biomed Eng. 1978;6:231–49. doi: 10.1007/BF02409345. [DOI] [PubMed] [Google Scholar]
- 8.Lowe HJ, Mostert JW. Quantitative closed circuit anaesthesia. Anaesthesia. 1974;29:110–1. [PubMed] [Google Scholar]
- 9.Baum J. 2nd ed. Oxford, Boston: Butterworth-Heinemann; 2001. Low Flow Anaesthesia: The Theory and Practice of Low Flow, Minimal Flow and Closed System Anaesthesia. [Google Scholar]
- 10.De Cooman S, Lecain A, Sosnowski M, De Wolf AM, Hendrickx JF. Desflurane consumption with the Zeus during automated closed circuit versus low flow anesthesia. Acta Anaesthesiol Belg. 2009;60:35–7. [PubMed] [Google Scholar]
- 11.Baum JA. The carrier gas in anaesthesia: Nitrous oxide/oxygen, medical air/oxygen and pure oxygen. Curr Opin Anaesthesiol. 2004;17:513–6. doi: 10.1097/00001503-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Hönemann CW, Hahnenkamp K, Möllhoff T, Baum JA. Minimal-flow anaesthesia with controlled ventilation: Comparison between laryngeal mask airway and endotracheal tube. Eur J Anaesthesiol. 2001;18:458–66. doi: 10.1046/j.1365-2346.2001.00868.x. [DOI] [PubMed] [Google Scholar]
- 13.Baum JA, Aitkenhead AR. Low-flow anaesthesia. Anaesthesia. 1995;50(Suppl):37–44. doi: 10.1111/j.1365-2044.1995.tb06189.x. [DOI] [PubMed] [Google Scholar]
- 14.Mazze RI, Friedman M, Delgado-Herrera L, Galvez ST, Mayer DB. Renal toxicity of compound a plus sevoflurane compared with isoflurane in non-human primates. Anesthesiology. 1998;89:A490. [Google Scholar]


