Abstract
Background:
Maintenance of adequate depth of anaesthesia in spine surgery is vital to prevent awareness, to reduce stress response and possible autonomic instability frequently associated with spine surgery. Dexmedetomidine, a α2-adrenoceptor agonist with analgesic and sedative adjuvant property has been found to reduce dose requirement of multiple anaesthetic agents both for induction and during the maintenance of anaesthesia.
Aim:
The aim of this study is to observe the effect of dexmedetomidine, on the requirement of propofol for induction and maintenance of adequate depth of anaesthesia during spine surgery.
Methods:
It was a prospective, randomised, double-blinded, parallel group, placebo controlled and open-lebel study in tertiary care hospital. A total of 70 patients aged 20-60 years, American Society of Anaesthesiologists GradeI and II, scheduled for elective spine surgery were randomly allocated into two groups. Each patient of Group D (n=35) received an initial loading dose of dexmedetomidine at 1 μg/kg over 10 min, started 15 min before induction of anaesthesia followed by an infusion at a rate of 0.2 μg/kg/h. Patients of Group P (n=35) received the same volume of 0.9% normal saline solution as placebo. Requirement of propofol at induction and during maintenance was calculated maintaining bispectral index between 40 and 60. P<0.05 was considered to be statistically significant.
Results:
Mean requirement of propofol was found to be lessened by 48.08% and 61.87% for induction and maintenance of anaesthesia respectively while using dexmedetomidine.
Conclusion:
Administration of dexmedetomidine significantly reduces the requirement of propofol while maintaining desired depth of anaesthesia without any significant complication.
Keywords: Bispectral index monitor, dexmedetomidine, propofol, spine surgery
INTRODUCTION
Spine surgery presents a number of challenges to the anaesthesiologists. Adequate depth of anaesthesia is essential for maintaining intra operative haemodynamic stability and prevention of recall after wards. Dexmedetomidine is a highly selective α2- adrenoreceptor agonist, possessing hypnotic, sedative, anxiolytic, sympatholytic and analgesic properties without producing significant respiratory depression.[1,2] Various studies had found that dexmedetomidine decreases the requirement of thiopentone[3,4] and volatile anaesthetic agents in perioperative period.[5,6,7] There is a paucity of clinical trials regarding the effect of dexmedetomidine on the requirement of propofol in maintaining adequate depth of anaesthesia with stable haemodynamic status perioperatively during the spine surgery. In view of the above observations, the present study was designed as randomised, double blinded, parallel group, placebo controlled and open-label manner to evaluate the effect of dexmedetomidine on propofol requirement for induction and maintenance of desired depth of anaesthesia on the basis of targeted bispectral index (BIS) value in spine surgery on prone patients under general anaesthesia.
METHODS
After obtaining the Institutional Ethics Committee clearance and written informed consent from each patient, 70 adult patients of American Society of Anaesthesiologists (ASA) Grade I and II aged 20-60 years undergoing elective spinal surgeries under general anaesthesia were included in this study. Patients above 60 years, patients with a higher degree A-V block, obstructive sleep apnoea and morbid obesity and on the chronic opioid analgesic, tricyclic antidepressant, clonidine, mono amine oxidase-inhibitor therapy were excluded from the study.
Sample size estimation was performed using power and sample size calculation software (version 2.1.30, DuPont and Plummer February 2003). Assuming power of the study to be 90% and probability of Type 1 error to be 5%, a total of 69 patients were found to be required to detect a statistically significant difference of mean dose of propofol consumption. Hence, a total of 70 patients (n=70) were incorporated in the study and were distributed randomly into two study groups, Groups P and D, each consisting of an equal number of patients of 35 (n=35).
A computer generated randomisation table was used to assign each patient to a placebo group (Group P, n=35) or dexmedetomidine group (Group D, n=35). A pharmacologist and/or a nursing staff of the institution not involved in this study, prepared injectable solution of either dexmedetomidine (study solution) or 0.9% saline (control solution). The investigator also remained blind regarding the content of these solutions prepared for the patients. The study solution intended for patients of Group D was prepared by dissolving one ampoule of dexmedetomidine (containing 200 μg, in a concentration of 100 μg/ml) in normal saline to make a solution of 50 ml to yield a final concentration of 4 μg/ml. For patients of Group P solution containing only 50 ml 0.9% saline was prepared.
Fifteen minutes before induction of anaesthesia, patients of both groups received identical premedication of injection glycopyrrolate 0.2 mg intravenous (i.v.). At the same time, infusion of the study or control solution was started for patients of Group D or Group P respectively initially at a rate of 1.5 ml/kg/h over 10 min (i.e., loading dose of dexmedetomidine at 1 μg/kg over the period of 10 min), followed by infusion of 0.05 ml/kg/h (i.e., maintenance of dexmedetomidine at a rate of 0.2 μg/kg/h) according to the study protocol. During the period of infusion of study/control solutions heart rate (HR), saturation of peripheral oxygen, systolic blood pressure (SBP), diastolic blood pressure, mean arterial pressure (MAP), electrocardiogram and consciousness level were closely monitored in the patients.
Pre-oxygenation with 100% oxygen for 3 min was performed for all patients. Injection fentanyl 2 μg/kg body weight was given i.v. 3 min before induction. Induction of anaesthesia was started in all patients by injection propofol, slow i.v. until loss of response to verbal command. Anaesthesia was maintained with nitrous oxide (N2O) 66% in oxygen and injection propofol as a continuous infusion via a separate syringe pump. Propofol was started initially at 5 mg/kg/h and then adjusted to maintain a BIS value in the range of 40-60 and its requirement was observed and recorded in each patient. Endotracheal intubation was performed following adequate muscle relaxation with injection atracurium 0.5 mg/kg i.v. Relaxation was maintained with continuous infusion of atracurium at a rate of 0.25-0.5 mg/kg/h with constant neuromuscular (train-of-four response) monitoring. Injection tramadol 1.5 mg/kg i.v. was given 30 min after intubation to each patient. Standard fluid regimen was followed during the intraoperative period, with blood loss replaced with either crystalloid in 3:1 fashion or with colloid in 1:1 fashion.
Comparison of MAP and HR were done in two groups – baseline (before receiving study/control solution – MAP1, HR1), at pre-induction (after completion of study/control solution - MAP2, HR2), at induction (1 min after administration of induction agent - MAPi, HRi), after intubation (1 min after laryngoscopy and intubation - MAPi2, HRi2), at skin incision (1 min after giving skin incision - MAPs, HRs) and during the intraoperative period at 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110 and 120 min and after 1 min of extubation. Depth of anaesthesia was monitored with BIS™ monitoring (Aspect Medical Systems, Inc.).
Additional boluses of injection propofol 20 mg i.v. were administered whenever BIS value approaching towards target higher value of 60. Injection fentanyl i.v. was administered intermittently to maintain MAP and HR within 20% of their pre-induction values. Patients were observed throughout the surgeries for any significant bradycardia (HR<45 bpm) and significant hypotension (MAP<20% of pre- induction value or SBP<90 mm of Hg). Bradycardia was scheduled to be treated with injection atropine 0.6 mg i.v. and significant hypotension was decided to be managed with vasoactive drugs.
At the end of surgery, N2O was discontinued after the appearance of spontaneous respiration. Residual neuromuscular blockade was reversed by injection neostigmine 0.05 mg/kg along with injection glycopyrrolate 0.01 mg/kg i.v.
All patients were shifted to post-anaesthetic care unit following adequate reversal with clear airway, adequate cough reflex and sustained head lifting for 5 s and train-of-four >0.9.
Statistical analysis
All raw data were subsequently entered into a Microsoft excel spread sheet and analysed using Microsoft excel 2003, statistical 6.0 and IBM SPSS software version 16.
Categorical data (e.g., sex distribution, ASA physical status) were analysed using the Pearson's Chi-square test with Yate's correction. Parametrical numerical data between groups were analysed using the Student's t-test. Within group variables at different time points were analysed using the Friedman's analysis of variance followed by Wilcoxon's matched – pairs signed rank test for post hoc analysis. A P<0.05 was considered to be statistically significant.
RESULTS
In our study, patients in both groups (Groups P and D) were comparable in respect to demographic data such as age, sex, body weight and ASA physical status. There was no difference in the mean duration and sites of spinal surgery performed between the groups [Table 1].
Table 1.
Demographic characteristics of the patients in two groups
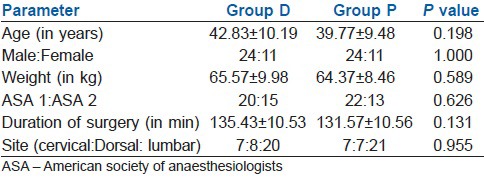
Mean induction dose of propofol was found to be significantly lesser in Group D (mean 66.86±12.549 mg) when compared with Group P (mean 124±16.033 mg) [Table 2]. Mean maintenance dose of propofol in Group D was 155.00±40.584 mg and that in Group P was 405.57±67.767 mg [Table 2]. That is in patients of Group D, mean requirement of propofol for induction and maintenance of anaesthesia were 48.08% and 61.87% lesser respectively, than the mean requirement in patients of Group P. And regarding the total requirement of propofol, mean requirement in Group D (222.43±46.863 mg) was 57.99% lesser than the requirement in Group P (529.57±76.026 mg) [Table 2].
Table 2.
Induction, maintenance and total dose of propofol requirement characteristics in two groups
MAP and HR were significantly decreased in Group D after administration of loading dose of dexmedetomidine. i.e., MAP2 and HR2 in Group D were significantly lower when compared with MAP1 (P<0.001). Whereas the above two parameters in Group P were not different statistically. Pre-induction value of MAP and HR were compared with MAP and HR after induction, laryngoscopy and intubation and skin incision in the same group with Wilcoxon signed rank test. In both groups, MAP and HR declined significantly after induction with propofol. This haemodynamic perturbation was although significant statistically, was not of clinical significance. MAP and HR after laryngoscopy and intubation and after skin incision rose in both groups, but the rise was more in Group P [Figures 1 and 2].
Figure 1.
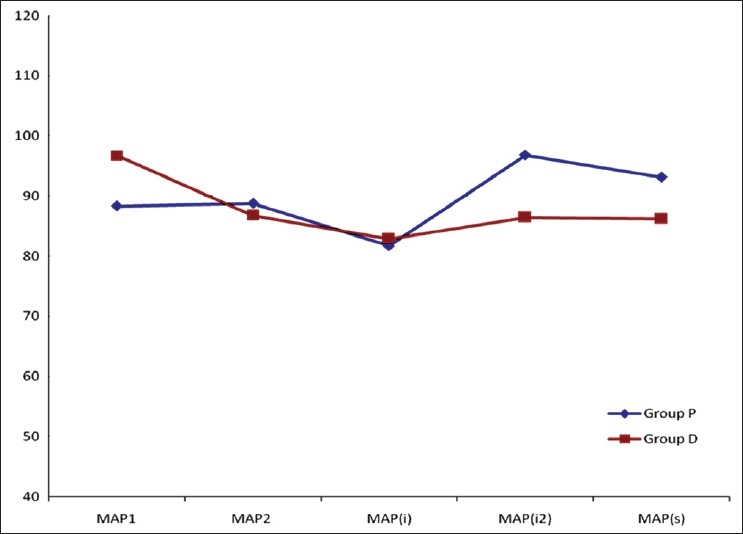
Comparison of haemodynamic parameter (mean arterial pressure) at various points of time (pre-operative, pre-induction, 1 min after induction, 1 min after laryngoscopy and intubation and at skin incision
Figure 2.
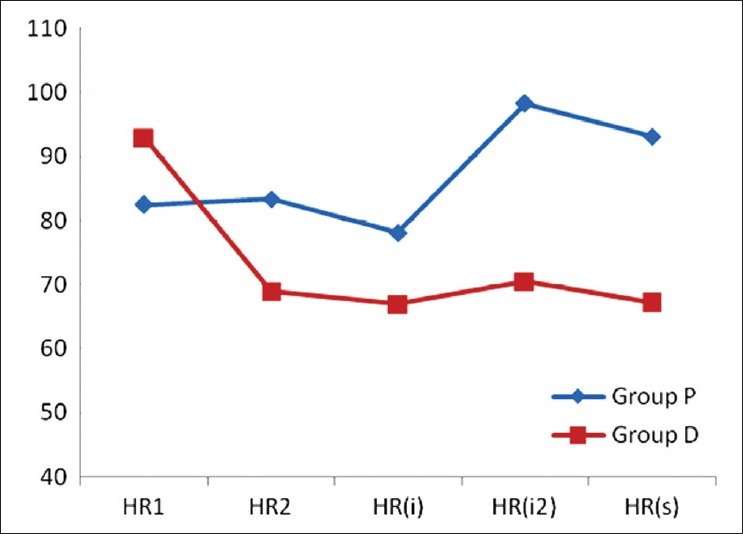
Comparison of haemodynamic parameter (heart rate) at various points of time (pre-operative, pre-induction, 1 min after induction, 1 min after laryngoscopy and intubation and at skin incision
For comparison of intra operative haemodynamics, in both groups, MAP and HR at 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110 and 120 min and after extubation were compared individually with MAP2 and HR2 respectively (i.e., pre-induction value of MAP and HR) with Wilcoxon signed rank test. In both groups, though they were found to be different statistically at various points of times, they were considered clinically insignificant [Figures 3 and 4].
Figure 3.
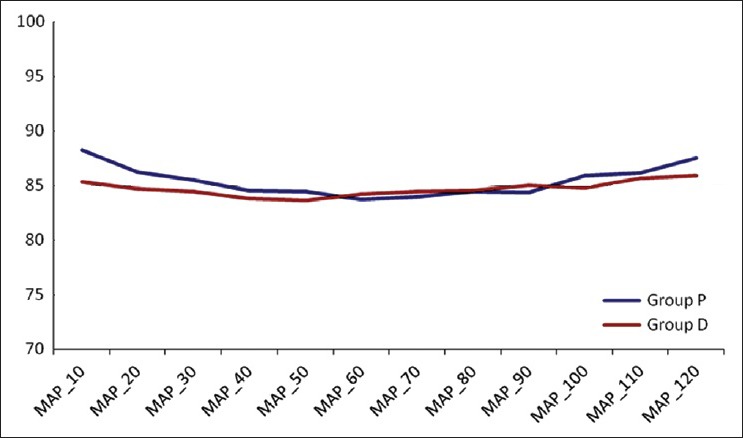
Comparison of mean arterial pressure in intraoperative period
Figure 4.
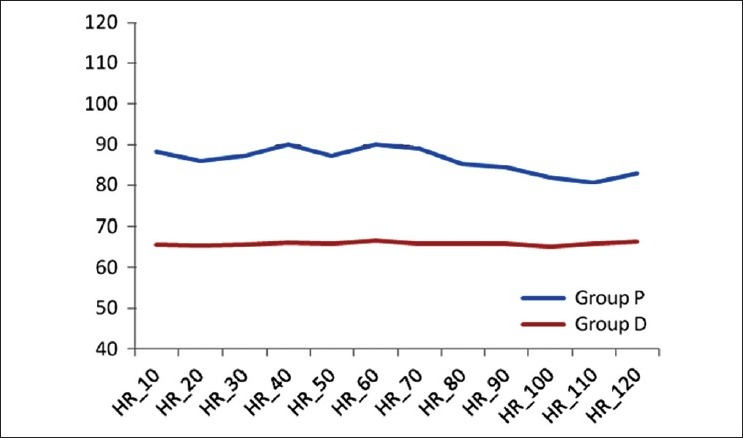
Comparison of heart rate in intraoperative period
None of the patients in both groups developed significant bradycardia and significant hypotension that required treatment any time during the study period.
DISCUSSION
Maintenance of adequate depth of anaesthesia throughout the intraoperative period is vital to prevent intraoperative awareness, to maintain haemodynamic stability and to reduce intraoperative stress response. In spine surgery, patient's pre-existing autonomic instability might lead to increased intraoperative haemodynamic perturbation. Blunting of these autonomic responses by maintaining adequate depth of anaesthesia is of utmost importance. Moreover, prone position for spine surgery itself requires maintenance of adequate depth of anaesthesia to avoid haemodynamic and airway related complications.
Propofol is a suitable i.v. anaesthetic agent for induction and maintenance of anaesthesia during the spine surgery. Important advantage of propofol in general anaesthesia is the rapid emergence.[8] Although known for its remarkable safety, various recent literatures based mainly on intensive care unit studies have raised questions regarding prolonged infusion of propofol. Recent evidences had also suggested about the potential for intraoperative complications even with short-term infusions.[9,10,11,12] Moreover, rapid recovery, the main advantage of propofol could be jeopardised following prolonged high dose infusion.[13] Hence, the idea was to use propofol with another adjuvant having sedative properties that could reduce the requirement of propofol.
Dexmedetomidine, a novel α2 agonist with multifaceted beneficial actions such as sedative analgesic and anxiolytic properties, has been found to reduce anaesthetic drug requirements in the intraoperative period.[14] Its sympatholytic effect had shown to decrease MAP and HR by reducing norepinephrine release. They had also shown to decrease BIS value in the intraoperative period when used as an adjuvant with other drugs given as continuous i.v. infusion.[15]
Few of recently done studies have found definite role of dexmedetomidine in reducing dose requirement of propofol for induction and during maintenance of anaesthesia. The studies were done using mainly motor, sensory or autonomic responses for monitoring depth of anaesthesia.[16,17] However, one recent study where depth of anaesthesia was measured with responses to stimuli in children for short surgical procedures had found that dose response curve for propofol was not altered with concomitant use of dexmedetomidine.[18] Our present study was designed primarily to find out the role of dexmedetomidine on propofol dose requirement, for induction as well as for maintenance of a desired depth of anaesthesia in spine surgery, which requires precise haemodynamic stability to avoid intraoperative blood loss. BIS monitoring was preferred to sensory or motor responses as it is a standard and Food and Drug Administration approved monitor for depth of anaesthesia in perioperative period and also more convenient for personnel and for the institutional operating theatre setup.
In our study, it was found that mean induction dose of propofol and mean dose requirement for maintenance were significantly lower in Group D patients (48.08%, 61.87% respectively) when compared with Group P patients. Total mean requirement of propofol, in Group D patients was found 57.99% lower than the requirement in Group P patients, which was also remained significant.
Most of the previous studies that had found positive adjuvant effect of dexmedetomidine did not consider haemodynamic status in their observations. Here in our study, although not a primary outcome measurement, haemodynamic changes during direct laryngoscopy aided intubation and following skin incision were found to be much higher in patients of Group P than Group D, which suggests a significant role of dexmedetomidine in preventing haemodynamic response to laryngoscopy as corroborated to the previous study.[19] Intraoperative haemodynamic parameters were found to be similar in both groups. Although some data of intra group variables were found to be statistically significant, no clinical correlation in terms of treatment of adversities was faced in any point of time during study periods of these patients.
Limitations
BIS being the only mode of measuring depth of anaesthesia for the patients remained a limitation of the study. Use of N2O might confound the interpretation of BIS. Study may not be adequately powered to detect significant vvariation in haemodynamic parameters at various points of time in two groups. Comparison might be confounded as all kinds of spine procedures were enrolled for this study. A single kind of procedure at single spinal level would become more appropriate for comparison of data. And lastly, delayed recall in patients of either group was not interviewed as it was beyond the scope of the study protocol.
Future research directions
Future studies regarding the role of dexmedetomidine on propofol kinetics may be undertaken with total i.v. anaesthesia and target control closed loop propofol infusion that might be more precise regarding the current topic.
CONCLUSION
In conclusion, infusion of dexmedetomidine in peri operative period significantly reduced the requirement of propofol for induction and maintenance of adequate depth of anaesthesia with stable haemodynamic parameters.
ACKNOWLEDGMENT
The authors would like to thank Dr. Tapas Ghose, Assistant Prof., SDLD, IPGME and R, Kolkata.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–33. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl. Br J Anaesth. 1992;68:126–31. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- 4.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aho M, Erkola O, Kallio A, Scheinin H, Korttila K. Dexmedetomidine infusion for maintenance of anesthesia in patients undergoing abdominal hysterectomy. Anesth Analg. 1992;75:940–6. [PubMed] [Google Scholar]
- 6.Vickery RG, Sheridan BC, Segal IS, Maze M. Anesthetic and hemodynamic effects of the stereoisomers of medetomidine, an alpha 2-adrenergic agonist, in halothane-anesthetized dogs. Anesth Analg. 1988;67:611–5. [PubMed] [Google Scholar]
- 7.Bekker A, Sturaitis M, Bloom M, Moric M, Golfinos J, Parker E, et al. The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesth Analg. 2008;107:1340–7. doi: 10.1213/ane.0b013e3181804298. [DOI] [PubMed] [Google Scholar]
- 8.Shafer A, Doze VA, Shafer SL, White PF. Pharmacokinetics and pharmacodynamics of propofol infusions during general anesthesia. Anesthesiology. 1988;69:348–56. doi: 10.1097/00000542-198809000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Burow BK, Johnson ME, Packer DL. Metabolic acidosis associated with propofol in the absence of other causative factors. Anesthesiology. 2004;101:239–41. doi: 10.1097/00000542-200407000-00035. [DOI] [PubMed] [Google Scholar]
- 10.Salengros JC, Velghe-Lenelle CE, Bollens R, Engelman E, Barvais L. Lactic acidosis during propofol-remifentanil anesthesia in an adult. Anesthesiology. 2004;101:241–3. doi: 10.1097/00000542-200407000-00036. [DOI] [PubMed] [Google Scholar]
- 11.Hatch DJ. Propofol-infusion syndrome in children. Lancet. 1999;353:1117–8. doi: 10.1016/S0140-6736(99)90018-1. [DOI] [PubMed] [Google Scholar]
- 12.Bray RJ. Propofol infusion syndrome in children. Paediatr Anaesth. 1998;8:491–9. doi: 10.1046/j.1460-9592.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 13.Pascoe PJ, Ilkiw JE, Frischmeyer KJ. The effect of the duration of propofol administration on recovery from anesthesia in cats. Vet Anaesth Analg. 2006;33:2–7. doi: 10.1111/j.1467-2995.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghodki PS, Thombre SK, Sardesai SP, Harnagle KD. Dexmedetomidine as an anesthetic adjuvant in laparoscopic surgery: An observational study using entropy monitoring. J Anaesthesiol Clin Pharmacol. 2012;28:334–8. doi: 10.4103/0970-9185.98329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 16.Dutta S, Karol MD, Cohen T, Jones RM, Mant T. Effect of dexmedetomidine on propofol requirements in healthy subjects. J Pharm Sci. 2001;90:172–81. doi: 10.1002/1520-6017(200102)90:2<172::aid-jps8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Ngwenyama NE, Anderson J, Hoernschemeyer DG, Tobias JD. Effects of dexmedetomidine on propofol and remifentanil infusion rates during total intravenous anesthesia for spine surgery in adolescents. Paediatr Anaesth. 2008;18:1190–5. doi: 10.1111/j.1460-9592.2008.02787.x. [DOI] [PubMed] [Google Scholar]
- 18.Hammer GB, Sam WJ, Chen MI, Golianu B, Drover DR. Determination of the pharmacodynamics interaction of propofol and dexmedetomidine during esophagogastroduodenoscopy in children. Paediatr Anaesth. 2009;19:138–44. doi: 10.1111/j.1460-9592.2008.02823.x. [DOI] [PubMed] [Google Scholar]
- 19.Bajwa SJ, Kaur J, Singh A, Parmar S, Singh G, Kulshrestha A, et al. Attenuation of pressor response and dose sparing of opioids and anaesthetics with pre-operative dexmedetomidine. Indian J Anaesth. 2012;56:123–8. doi: 10.4103/0019-5049.96303. [DOI] [PMC free article] [PubMed] [Google Scholar]


